Abstract
Background:
Inhibitory dysfunction is a key behavioral and cognitive phenotype of attention‐deficit hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD). Both disorders show neuropsychological deficits and fronto‐striatal dysfunction during tasks of motor response inhibition and cognitive flexibility. This study investigates differences and commonalities in functional neural networks mediating inhibitory control between adolescents with ADHD and those with OCD to identify disorder‐specific neurofunctional markers that distinguish these two inhibitory disorders.
Methods:
Event‐related fMRI was used to compare brain activation between 20 healthy boys, 18 (Stop task) or 12 boys (Switch task) with ADHD, and 10 boys with OCD during a tracking Stop task that measures inhibition and stopping failure and during a visual–spatial switching task measuring cognitive flexibility.
Results:
Both patient groups shared brain dysfunction compared to healthy controls in right orbitofrontal (successful inhibition) and left dorsolateral prefrontal cortices (failed inhibition). Right inferior prefrontal dysfunction, however, was disorder‐specific to ADHD during both tasks. Left inferior prefrontal dysfunction during the Switch task was significant in children with ADHD relative to controls, but only reached a trend in patients with OCD. Patients with ADHD furthermore showed disorder‐specific dysfunction in left basal ganglia and cingulate gyrus during the Switch task.
Conclusions:
Patients with ADHD compared to those with OCD have both common and distinct dysfunctions during inhibitory control. The most consistently reported functional abnormality in children with ADHD in right inferior prefrontal cortex during inhibitory control appears to be disorder‐specific when compared to patients with OCD and may be a specific neurofunctional biomarker of ADHD. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: attention deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), fMRI, inhibition, error detection, switching, orbitofrontal cortex, inferior prefrontal cortex
INTRODUCTION
Abnormalities in inhibitory networks appear to be a trans‐diagnostic deficit in both attention‐deficit hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD). ADHD is characterized by behavioral features of inattention, impulsiveness, and hyperactivity (DSM IV). ADHD has been associated with neuropsychological [Rubia et al., 2001, 2007a; Willcutt et al., 2005] and fronto‐striatal neurofunctional deficits in inhibitory functions including motor response inhibition and cognitive switching [Dickstein et al., 2006; Durston et al., 2003; Rubia et al., 1999, 2001, 2005a, 2008, 2009b; Smith et al., 2006].
OCD is characterized by poor inhibition over intrusive, unwanted obsessive thoughts and compulsions (DSM IV). Patients with OCD also have deficits in tasks of inhibitory control, including motor response inhibition and cognitive switching [Chamberlain et al., 2006; Penades et al., 2007] and structural [Huyser et al., 2009; Menzies et al., 2007, 2008] and functional abnormalities in inhibitory fronto‐striatal networks [Menzies et al., 2008; Woolley et al., 2008].
Therefore, it has been argued that the underlying etiopathophysiology for both disorders is an abnormality in fronto‐striatal inhibitory neural networks [Dickstein et al., 2006; Huyser et al., 2009; Menzies et al., 2008; Rubia et al., 1999, 2005a, 2008]. A shared pathophysiology, however, is at odds with a relatively clear symptomatic distinction of the two disorders, with compulsivity and impulsivity often considered as situated at opposite ends of a behavioral spectrum [Carlsson, 2000]. Only about 8% of children with ADHD meet OCD criteria, while up to 30% of children with OCD meet criteria for ADHD [Geller et al., 1996, 2000]. It remains to be clarified whether there is overlap or differences in the inhibitory networks that are affected in these two disorders. Such a difference at the neurofunctional level would provide disorder‐specific biomarkers that could assist with differential diagnosis and treatment. No functional imaging study, to our knowledge, however, has compared these two disorders during inhibition or any other functions.
The aim of this study was therefore to use fMRI to investigate the differences and commonalities in the functional activation abnormalities between noncomorbid children with ADHD and noncomorbid children with OCD when compared to a healthy comparison group during two tasks of cognitive control: a tracking Stop task measuring successful and failed motor response inhibition; and a visual–spatial switching task, requiring the inhibition of previously relevant and predominant stimulus‐response associations to facilitate newly relevant ones, thus measuring besides motor and conflict inhibition also attention control as well as the facilitation of relevant stimulus‐response mappings [Derrfuss et al., 2005; Yeung et al., 2006]. To minimize the confounding impact of concurrent anxiety, ritualizing or the potential need to inhibit obsessions or compulsions during fMRI, we studied adolescents with treated OCD who had minimal residual symptoms.
Based on our previous findings of inferior prefrontal dysfunction [Rubia et al., 1999, 2001, 2005a; Smith et al., 2006] and disorder‐specificity of this inferior prefrontal dysfunction in ADHD compared to children with CD during tasks of cognitive control [Rubia et al., 2008, 2009a, b], we hypothesized that this brain dysfunction would also be disorder‐specific to children with ADHD when compared to those with OCD. Based on our previous fMRI findings of orbitofrontal dysfunction in the same group of patients with OCD compared to 10 age‐matched controls [Woolley et al., 2008], we hypothesized that children with OCD would show disorder‐specific orbitofrontal dysfunction when compared to children with ADHD.
METHOD
Subjects
Patients were 10 right‐handed, male adolescents with OCD and 18 (Stop task) or 12 patients (Switch task) with ADHD, between 9 and 16 years (see Table I), recruited from clinics, parent support groups, and advertisement. Clinical diagnosis of combined hyperactive‐impulsive subtype of ADHD without the diagnosis of OCD and OCD without clinical ADHD symptoms (DSM IV) [American Psychiatric Association, 1994] was established through interviews with a child psychiatrist using the standardized Maudsley diagnostic interview (MDI) [Goldberg, 2002]. Exclusion criteria for both patient groups were drug and substance abuse and a history of a general or specific learning disability or comorbidity with any other major psychiatric disorder, as assessed using the Child Behavior Checklist [Achenbach and Edelbrook, 1983] and the MDI. The exception was comorbidity with CD for the ADHD group, which was present in one patient.
Table I.
Multiple univariate ANOVA group comparisons for age and IQ
| Controls | ADHD | OCD | F | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Stop task | N = 20 | N = 18 | N = 10 | df = 2,43 | ||||
| Age (years) | 14.5 | 1.1 | 13.9 | 1.1 | 14.3 | 1.7 | 1 | n.s. |
| IQ estimate | 104 | 15 | 93 | 9 | 102 | 20 | 3 | n.s. |
| Switch task | N = 20 | N = 12 | N = 10 | df = 2,39 | ||||
| Age (years) | 14.5 | 1.1 | 13.7 | 1.6 | 14.3 | 1.7 | 1 | n.s. |
| IQ estimate | 104 | 15 | 99 | 13 | 102 | 20 | 0.5 | n.s. |
All patients with ADHD were medication‐naïve; they scored above threshold on the hyperactivity scale of the Strength and Difficulty Questionnaire (SDQ) [Goodman et al., 1997] and above the 5th percentile on the Raven's Standard Progressive Matrices Intelligence Questionnaire [IQ; i.e. converted IQ estimate over 75; Raven, 1960; Table I].
Patients with OCD were treated and in partial remission. A pretreatment Children's Yale‐Brown Obsessive Compulsive Scale [CY‐BOCS; Scahill et al., 1997; mean total score = 20.5, range 12–33] was repeated before scanning (mean total score 11, range 2–21) and confirmed significant symptomatic improvement (46% for obsessions and 49% for compulsions). Some residual symptoms were present in all subjects at scanning (mean total CY‐BOCS score 11, range 2–21), and so all can be characterized as treatment responders. Predominant symptom subtypes were washing and checking. To avoid potential state effects of anxiety and depression, additional exclusion criteria were scores above 15 on the Birleson depression questionnaire [Birleson, 1981] and above 19 on the Revised Children Manifest Anxiety scale [R‐CMAS; Reynolds and Richmond, 1978]. They also underwent SDQ ratings for hyperactivity at initial assessment. The majority of patients (n = 8) were being treated with a selective serotonin reuptake inhibitor (SSRI; mean 5 months, range 2–12). Five patients had completed a course of cognitive behavioral therapy (mean eight sessions, range 4–10). Their IQ estimates were above 75.
Control subjects were 20 right‐handed male healthy adolescents between 10 and 17 years with no history of any other mental or neurological disorder. They had no history of neurotropic medication or drug and substance abuse and an IQ estimate of over 75 (Table I).
Written informed consent/assent was given for all participants and the study was approved by the local Ethical Committee.
Data from the 10 of the patients with ADHD and 17 of the control children have been reported previously in case–control studies of the Stop and Switch tasks [Rubia et al., 2005a; Smith et al., 2006] and the patients with OCD have previously been compared to a subgroup of 10 of the control children [Woolley et al., 2008].
One‐way ANOVAs showed that groups did not differ significantly in age and IQ (Table I). As expected, patients with ADHD scored significantly higher than patients with OCD on the SDQ ratings for hyperactivity [Mean SDQ (SD): ADHD, 9 (1); OCD, 5 (3); t = 5, df = 26, P < 0.0001].
fMRI Paradigms
Subjects practiced both tasks once prior to scanning. Rapid event‐related fMRI was used with randomized trials to maximize efficiency.
Stop Task
The visual tracking Stop task requires withholding of a motor response to a go stimulus when it is followed unpredictably by a stop signal [Rubia et al., 2003, 2005a, 2007b, 2008]. The basic task is a choice reaction time task (left and right pointing arrows: go signals) with a mean ITI of 1.8 s. In 20% of trials, pseudorandomly interspersed, and at least three repetition times apart for adequate separation of the hemodynamic response, go signals are followed (about 250 ms later) by arrows pointing upwards (stop signals), and subjects have to inhibit their motor responses. A tracking algorithm changes the time interval between go‐signal and stop‐signal onsets according to each subject's inhibitory performance to ensure that the task is equally challenging for each individual and to provide 50% successful and 50% unsuccessful inhibition trials (see Fig. 1a).
Figure 1.
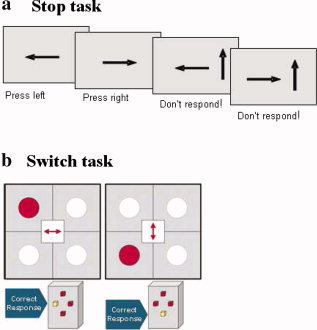
Schematic illustration of the fMRI tasks. (a) Tracking Stop task. The interval between horizontal (go signal) and vertical arrows (stop signal) in the stop trials becomes shorter/longer in steps of 50 ms depending on each subject's performance on previous trials ensuring 50% of correctly inhibited and 50% failed stop trials for each subject. (b) Visual–spatial Switch task. Subjects have to respond according to the arrow direction in the middle of the grid and switch their response according to the horizontal dimension (indicate whether the dot is left or right of the grid) or the vertical dimension (indicate whether the dot is above or below the grid). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In fMRI analysis, brain activation to the 50% successful stop trials and the 50% unsuccessful stop trials is contrasted with that of go trials (i.e. successful/unsuccessful stop–go trials).
Switch Task
A modified version of the Meiran Switch task was used, requiring cognitive switching between two spatial dimensions, with minimal working memory load, and is described in detail elsewhere [Rubia et al., 2006; Smith et al., 2004, 2006]. A target dot appeared at one of four corners of a grid with an arrow in the middle of the grid (mean ITI was 2.4 s). If the central arrow was horizontal, the subject had to indicate whether the target was on the left or right side of the grid (left or right button); if the central arrow was vertical, subjects had to indicate whether the target was in the lower or upper half of the grid (up or down button). During switch trials (21%; N = 32) the central arrow changed position, which occurred after every four to six repeat trials (79%; N = 120), i.e. at least four repetition times apart for adequate separation of the hemodynamic response (Fig. 1b).
The task therefore measures motor response inhibition (of the previously valid stimulus‐response association during the response switch), interference/conflict inhibition from previous trials, the attention switch between the horizontal and vertical dimension as well as the facilitation of relevant stimulus‐response mappings [Derrfuss et al., 2005; Yeung et al., 2006].
The event‐related fMRI analysis subtracted activation associated with repeat trials from activation associated with switch trials (switch–repeat).
Analysis of Performance Data
Repeated measures t‐tests were used to measure the Switch effect on reaction time and on accuracy across all subjects. Multiple univariate ANOVAs were used to compare the main variables of the Stop and Switch task performance between the three groups: (1) Stop task variables: mean reaction time (MRT) to go trials; stop signal reaction time (SSRT), calculated by subtracting the mean stop signal delay (SSD: the average time between go and stop signal, at which the subject managed to inhibit to 50% of trials) from the mean reaction time (MRT) to go trials, i.e. MRT − SSD; (2) Switch task: Mean reaction time to all trials, Switch error and Switch reaction time costs (Switch MRT/errors–Repeat MRT/errors). P‐values were adjusted for multiple testing using the False Discovery Rate [Benjamini and Hochberg, 1995].
fMRI Image Acquisition
Gradient‐echo echoplanar MR imaging (EPI) data were acquired on a GE Signa 1.5T Horizon LX System (General Electric, Milwaukee, WI) at the Maudsley Hospital, London. Consistent image quality was ensured by a semiautomated quality control procedure. A quadrature birdcage head coil was used for RF transmission and reception. In each of 16 noncontiguous planes parallel to the anterior–posterior commissural, 196 (Stop task) or 154 (Switch task) T2*‐weighted MR images depicting blood oxygen level dependent (BOLD) contrast covering the whole brain were acquired with TE = 40 ms, TR = 1.8 s (Stop), 2.4 s (Switch), flip angle = 90°, in‐plane resolution = 3.1 mm, slice thickness = 7 mm, slice‐skip = 0.7 mm. This EPI dataset provided almost complete brain coverage.
fMRI Image Analysis
The method of fMRI analysis used [XBAM, http://www.brainmap.co.uk; Brammer et al., 1997] makes no normality assumptions, which are usually violated in fMRI data, but instead uses median statistics to control outlier effects and permutation rather than normal theory based inference. Furthermore, the most common test statistic is computed by standardizing for individual difference in residual noise before embarking on second‐level, multisubject testing using robust permutation‐based methods. This allows a mixed effects approach to analysis—an approach that has recently been recommended following a detailed analysis of the validity and impact of normal theory‐based inference in fMRI in large number of subjects [Thirion et al., 2007].
fMRI data were realigned to minimize motion‐related artifacts [Bullmore et al., 1999] and smoothed using a Gaussian filter (full‐width half maximum, 7.2 mm). Time‐series analysis of individual subject activation was performed using XBAM, with a wavelet‐based resampling method previously described [Bullmore et al., 2001]. Briefly, we first convolved each experimental condition with two Poisson model functions (delays of 4 and 8 s). We then calculated the weighted sum of these two convolutions that gave the best fit (least‐squares) to the time series at each voxel. A goodness‐of‐fit statistic (the SSQ‐ratio) was then computed at each voxel consisting of the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the sum of squares due to the residuals (original time series minus model time series). The appropriate null distribution for assessing significance of any given SSQ‐ratio was established using the wavelet‐based data resampling method [Bullmore et al., 2001] and applying the model‐fitting process to the resampled data. This process was repeated 20 times at each voxel and the data combined over all voxels, resulting in 20 null parametric maps of SSQ‐ratio for each subject, which were combined to give the overall null distribution of SSQ‐ratio. The same permutation strategy was applied at each voxel to preserve spatial correlation structure in the data. Activated voxels, at a <1 level of Type I error, were identified through the appropriate critical value of the SSQ‐ratio from the null distribution. Individual SSQ‐ratio maps were then transformed into standard space, first by rigid body transformation of the fMRI data into a high‐resolution inversion recovery image of the same subject, and then by affine transformation onto a Talairach template [Talairach and Tourneaux, 1988].
Group Analysis
A group activation map was produced for each experimental condition by calculating the median observed SSQ‐ratio over all subjects at each voxel in standard space and testing them against the null distribution of median SSQ‐ratios computed from the identically transformed wavelet resampled data [Brammer et al., 1997]. The voxel‐level threshold was first set to 0.05 to give maximum sensitivity and to avoid Type II errors. Next, a cluster‐level threshold was computed for the resulting 3D voxel clusters such that the final expected number of Type I error clusters was <1 per whole brain. The necessary combination of voxel and cluster level thresholds was not assumed from theory, but rather was determined by direct permutation for each data set, giving excellent Type II error control [Bullmore et al., 1999]. Cluster mass rather than a cluster extent threshold was used to minimize discrimination against possible small, strongly responding foci of activation [Bullmore et al., 1999]. In all group activation analyses, less than one false positive activation locus was expected for P < 0.05 at voxel level and P < 0.01 at cluster level.
ANOVA Group Effect Analysis
For the between‐group comparisons, one‐way ANOVA analyses with group as factor were conducted using randomization‐based tests for voxel or cluster‐wise differences as described in detail [Bullmore et al., 1999, 2001]. For these between‐group comparisons, less than 1 false activated cluster was expected at a P‐value of <0.05 for voxel and <0.03 for cluster comparisons. Then the standardized BOLD response values (SSQ ratios) for each participant were extracted for the mean activation of each of the significant clusters of the three‐group ANOVA analysis, and post‐hoc Bonferroni‐corrected t‐tests (correcting for multiple comparisons) were conducted to identify between‐group differences.
Correlation Between Brain Activation and Symptoms in Patients
Statistical measures of BOLD response for each patient participant was extracted in each of the significant clusters of between‐group activation differences. Within patients with ADHD, brain activation in these clusters was correlated with symptom measures on the SDQ scores for hyperactivity/inattention. For patients with OCD, brain activation was correlated with SY‐BOCS measures for obsession and compulsion symptoms and percentage improvement in these measures. P‐values were corrected for multiple testing using the Bonferroni correction.
RESULTS
Task Performance
All subjects achieved a mean of about 50% in the Stop task, suggesting that the Stop algorithm was successful (see Table II). For the Switch task, repeated measures t‐test within all subjects showed that there was a highly significant Switch effect on reaction times [MRT Switch (SD) = 836 (144); MRT Repeat (SD) = 736 (129); t = 9, df = 41; P < 0.001] and errors [Switch error percentage (SD) = 7 (7); Repeat error percentage (SD) = 3 (7); t = 4, df = 41, P < 0.001], showing that the switch trials were more difficult for all subjects than repeat trials.
Table II.
Multiple univariate ANOVAs for the main variables of the Stop and Switch tasks by group
| Measure | Controls | ADHD | OCD | F | P value (corr.) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Stop task | N = 20 | N = 18 | N = 10 | df = 2, 48 | ||||
| MRT go (ms) | 800 | 174 | 764 | 115 | 843 | 160 | 0.7 | n.s. |
| PI (%) | 51 | 7 | 50 | 9 | 52 | 11 | 0.2 | n.s. |
| SSRT (ms) | 256 | 165 | 279 | 197 | 283 | 193 | 0.1 | n.s. |
| Switch task | N = 20 | N = 12 | N = 10 | df = 2, 39 | ||||
| MRT all (ms) | 749 | 140 | 785 | 114 | 859 | 112 | 3 | n.s. |
| Switch RT cost (ms) | 118 | 63 | 59 | 76 | 113 | 61 | 3 | n.s. |
| Switch error cost (%) | 3 | 6 | 4 | 7 | 5 | 4 | 0.3 | n.s. |
MRT go = mean reaction time to go trials (ms); PI = probability of inhibition in percentage; SSRT = stop signal reaction time (calculated as MRT to go trials − stop signal delay); Switch RT cost: MRT to switch trials − MRT to repeat trials; Switch error cost (in percentage): Switch errors − Repeat errors (in percentage).
Multiple univariate ANOVAs for both tasks showed no group differences in any of the performance variables (see Table II).
Brain Activation
Motion
All subjects were within acceptable limits for head movement (below 1.5 mm). Repeated measures ANOVAs showed no significant group or group × motion effects in the extent of three‐dimensional motion in x, y, and z translation and rotation for any of the two tasks.
Stop task
Successful Stop–Go trials.
Within‐group activations are shown in Figure 2a. The ANOVA analysis showed a significant interaction effect in the activation of ventromedial orbitofrontal cortex (Table III, Fig. 3a). Post‐hoc t‐tests showed that both patient groups compared to healthy comparison subjects had reduced BOLD response in this region (ADHD < controls; P < 0.001; OCD < controls: P < 0.05), but did not differ from each other.
Figure 2.
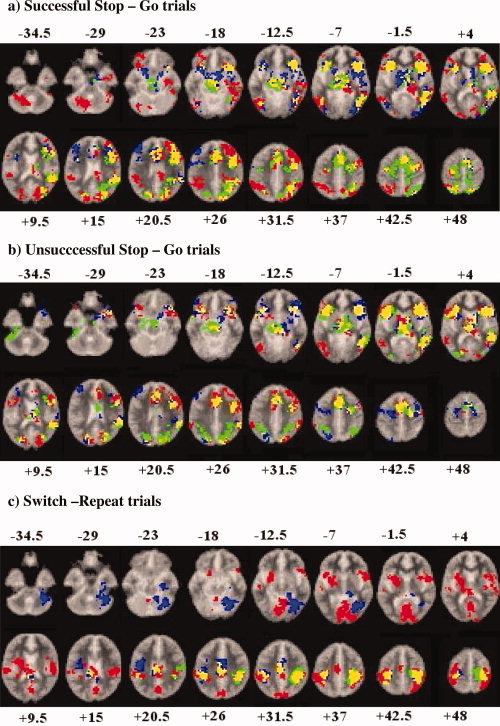
Axial slices for the group activation maps for the three groups at P < 0.05 for voxel and P > 0.01 for cluster levels for the contrasts of (a) Stop task: successful Stop–Go trials; (b) Stop task: unsuccessful Stop–Go trials; and (c) Switch task: switch–repeat trials. Red = controls; green = ADHD; blue = OCD. Overlapping brain regions: yellow, overlap between ADHD and controls; magenta, overlap between OCD and controls; cyan, overlap between ADHD and OCD; white, overlap between all groups. Talairach z‐coordinates are indicated for slice distance (in mm) from the intercommissural line. The right side of the picture corresponds to the right side of the brain.
Table III.
ANOVA differences in brain activation between adolescents with ADHD and OCD and healthy comparison adolescents
| Subject contrast | Brain regions of activation (Brodmann area; BA) | Talairach coordinates (x;y;z) | Voxels | Cluster P value |
|---|---|---|---|---|
| Stop–Go | ||||
| C > ADHD, OCD | R orbitofrontal/anterior cingulate (BA 11/10/47/32/24) | 18;52;−13 | 101 | 0.02 |
| Failed Stop–Go | ||||
| C, OCD > ADHD | R middle/inferior prefrontal (BA 44/45/9/46) | 51;22;20 | 22 | 0.027 |
| C > ADHD, OCD | L medial frontal/anterior cingulate (BA 8/9/32) | −14;33;26 | 29 | 0.027 |
| Switch–Repeat | ||||
| C > ADHD | R inferior frontal/insula/putamen/sup. temporal (BA 44/45/47/38) | 50;11; −2 | 47 | 0.01 |
| C > ADHD, OCDa | L inferior frontal/premotor/insula (BA 44/45/6) | −40;15;4 | 48 | 0.02 |
| C, OCD > ADHD | L putamen/caudate/anterior/posterior cingulate/parietal (BA 24/32/23/31/40) | −18;4;9 | 141 | 0.002 |
P‐value for ANOVAs is <0.05 for voxel activation and <0.03 for cluster activation. Those P‐values were selected to yield less than one false positive cluster per brain map.
Reduced in patients with OCD at a trend‐level.
Figure 3.
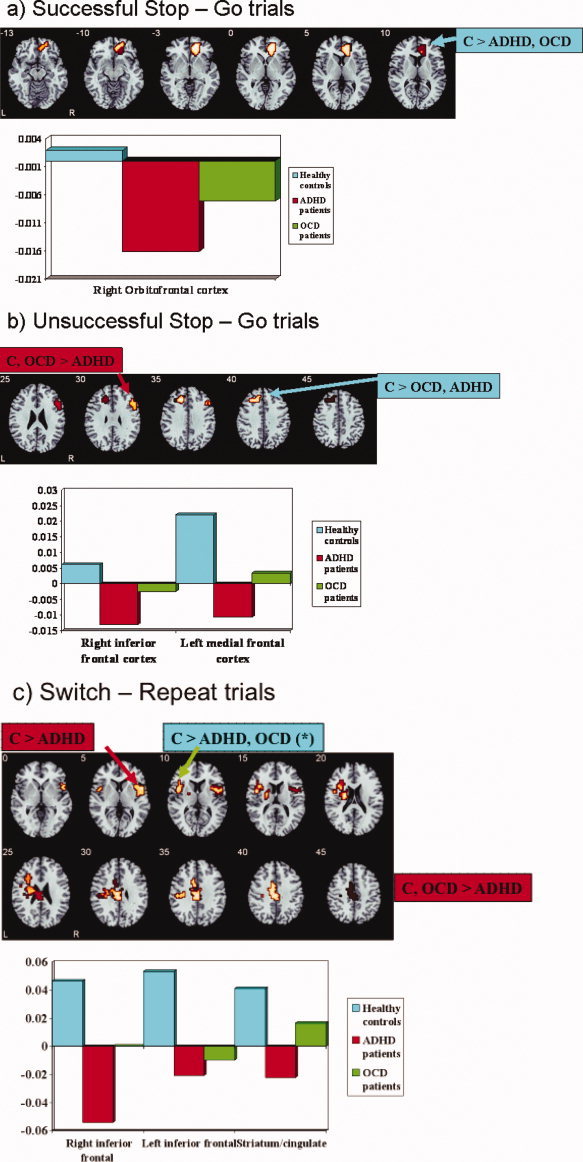
Axial sections showing the ANOVA results for the between‐group differences in brain activation at P < 0.05 for voxel and P < 0.03 for cluster levels for the contrast of (a) Successful Stop–Go trials. The activation cluster in right ventromedial orbitofrontal cortex was reduced in both patients with ADHD and OCD compared to the control group. (b) Unsuccessful Stop–Go trials. The cluster in right inferior prefrontal cortex was reduced only in patients with ADHD compared to both controls and patients with OCD. Activation in left dorsolateral prefrontal cortex was reduced in both patient groups compared to controls. (c) Switch–repeat trials. Right inferior prefrontal activation was reduced in patients with ADHD compared to controls. Left inferior prefrontal activation was reduced in both patient groups compared to controls, but this reached only a trend in patients with OCD. The cluster in left caudate/putamen/cingulate was reduced only in patients with ADHD when compared to both controls and patients with OCD. Talairach z‐coordinates are indicated for slice distance (in mm) from the intercommissural line. The right side of the picture corresponds to the right side of the brain. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In patients with OCD, BOLD response in the orbitofrontal cortex was positively correlated with the percentage improvement scores on the CY‐BOCS obsession symptoms [r = 0.7, P < 0.01; P (Bonferroni‐corrected) < 0.045]. No other correlations were significant. In patients with ADHD, no correlations between hyperactivity scores and BOLD response were observed.
Failed Stop–Go trials.
Group activations are shown in Figure 2b. ANOVA analysis showed significant between‐group interaction effects in right middle/inferior frontal gyrus (BA 46/44) and in left medial frontal gyrus reaching into anterior cingulate gyrus (BA 8/9/32) (Table III, Fig. 3b). Post‐hoc t‐tests showed that patients with ADHD showed significantly reduced BOLD response in right inferior prefrontal gyrus compared to controls (P < 0.0001) and to patients with OCD (P < 0.05); patients with OCD did not differ from controls in this measure. Both patient groups showed reduced BOLD response in medial frontal gyrus compared to control boys (ADHD < controls: P < 0.001; OCD < controls: P < 0.05), but did not differ from each other in this measure.
No significant correlations were observed between the BOLD response in these brain activation clusters and behavioral symptoms in patients.
Switch task
Group activations are shown in Figure 2c. There was a significant interaction effect for the comparison of switch–repeat trials in right inferior prefrontal cortex and insula reaching into putamen, in a cluster in left inferior prefrontal, premotor cortex, and insula and in a predominantly left‐hemispheric cluster comprising caudate, putamen, insula, and anterior and posterior cingulate reaching laterally into left inferior parietal lobe (see Table III, Fig. 3c). Post‐hoc comparisons showed that compared to controls, right inferior prefrontal activation was reduced in patients with ADHD (P < 0.001), but not in those with OCD (P < 0.1). Both patient groups did not differ from each other in this measure. Left inferior prefrontal activation was reduced significantly in patients with ADHD compared to controls (P < 0.02) and, at a trend level, also in patients with OCD (P < 0.08). Both patient groups did not differ from each other in this activation cluster. The cingulate/putamen reduction was specific to ADHD compared to both control (P < 0.001) and patients with OCD [P < 0.04). Patients with OCD did not differ from controls in this measure.
No significant correlations were observed between the BOLD response in these brain activation clusters and behavioral symptoms in patients.
DISCUSSION
Despite comparable task performance, children with noncomorbid ADHD show both similarities and differences in their brain activation abnormalities compared to patients with noncomorbid OCD when performing cognitive control tasks. During both inhibitory tasks, patients with ADHD showed disorder‐specific dysfunction in right inferior prefrontal cortex. Patients with ADHD furthermore showed disorder‐specific underactivation in a cluster comprising caudate, putamen, and anterior and posterior cingulate gyri during the Switch task. Both disorders, however, shared brain dysfunction compared to the healthy comparison group in right ventromedial orbitofrontal cortex during successful inhibition and in left medial prefrontal cortex during inhibition failures.
Our hypothesis of a disorder‐specific dysfunction in ADHD children in inferior prefrontal cortex was confirmed during both tasks. A more ventrolateral location of bilateral inferior prefrontal cortex was reduced during the Switch task and a more dorsolateral right hemispheric location during inhibition failures in the Stop task. Bilateral inferior prefrontal activation has consistently been associated with cognitive switching [Derrfuss et al., 2005; Rubia et al., 2006; Smith et al., 2004, 2006]. Given that right inferior prefrontal cortex is crucial to both motor response inhibition [Garavan et al., 1999; Rubia et al., 2003, 2007b] and cognitive switching [Derfuss et al., 2005; Konishi et al., 2002; Rubia et al., 2006; Smith et al., 2004, 2006], it has been argued that right inferior prefrontal cortex is likely to mediate the inhibition of previously learned stimulus‐response associations necessary for the cognitive switch, with left inferior prefrontal cortex mediating the initiation of the switch response and/or the updating of cognitive set [Derrfuss et al., 2005; Konishi et al., 2002; Rubia et al., 2006].
A more dorsolateral inferior prefrontal cortex focus was underactivated in patients with ADHD during inhibition failures. We have previously found that inferior prefrontal underactivation in children with ADHD is more typically observed during successful inhibition trials [Rubia et al., 1999, 2005a, 2008], although others have also observed reduced inferior prefrontal activation during failed stop trials [Pliszka et al., 2006]. It has been argued that failed inhibition trials may be attempted successful stop trials that are too late to be successful and therefore share common brain activation with inhibition trials [Pliszka et al., 2006; Rubia et al., 2008]. There is also evidence that the more dorsal lateral inferior prefrontal cortex, which was underactivated in patients with ADHD in this study, in its connection to anterior cingulate plays an important role in performance monitoring [Ridderinkhof et al., 2004; Rubia et al., 2003, 2007b]. Subjects receive indirect feedback during failed stop trials, as they see the stop stimuli appearing after their erroneous response to go stimuli. Errors are likely to trigger enhanced performance monitoring processes, mediated by anterior cingulate and inferior prefrontal cortex [Ridderinkhof et al., 2004; Ullsperger and von Cramon, 2004].
Underactivation of bilateral inferior prefrontal cortex in the context of tasks of inhibitory and cognitive control is the most consistent finding in the fMRI literature of ADHD and has been observed during motor response inhibition [Pliszka et al., 2006; Rubia et al., 1999, 2001, 2005a, 2008], cognitive flexibility [Silk et al., 2005; Smith et al., 2006], and other cognitive control tasks [Rubia et al., 2001, 2009a, b; Smith et al., 2006; for meta‐analysis, see Dickstein et al. (2006)]. Furthermore, both ventrolateral as well as dorsolateral inferior prefrontal dysfunctions have been shown to be disorder‐specific underactivations in children with ADHD compared to children with conduct disorder [Rubia et al., 2008, 2009a, b]. The finding of disorder‐specificity of these inferior prefrontal brain dysfunctions when compared to patients with OCD further reinforces the potential role of this brain abnormality as a specific neurofunctional biomarker for ADHD.
We could not confirm the hypothesis of disorder‐specific orbitofrontal dysfunction in OCD compared to children with ADHD, as both patient groups underactivated this region during successful inhibition. Interestingly, however, the activation in the orbitofrontal cortex was only significantly correlated with behavioral symptoms in patients with OCD, where it correlated with the degree of obsessive symptom improvement. The orbital and dorsolateral prefrontal dysfunction findings in patients with OCD are in line with a meta‐analysis of structural and functional findings in adult OCD [Menzies et al., 2008]. Furthermore, the ventromedial orbitofrontal underactivation was in the same location where reduced gray matter density was observed in adult patients with OCD that correlated with prolonged SSRT during a Stop task in adult patients with OCD [Menzies et al., 2007]. The location of the orbitofrontal dysfunction for ADHD was more ventromedial and orbital compared to the more lateral inferior prefrontal location we have previously observed in these patients during the same task [Rubia et al., 1999, 2005a, 2008]. Current fMRI hardware and software, however, have improved in detecting orbitofrontal activation that may have been missed in previous fMRI studies due to magnetic susceptibility artifacts in this region. As mentioned, the gray matter density of the same location of orbitofrontal cortex correlated with the SSRT measure of the Stop task in both healthy and patients with OCD [Menzies et al., 2007]. Structural imaging studies in ADHD show that the orbitofrontal cortex is smaller in cortical thickness and reduced in its structural connectivity with other brain regions [Makris et al., 2007, 2008]. The ventromedial orbitofrontal cortex has consistently been implicated in impulse control [Best et al., 2002; Potenza et al., 2003] and has been found to be underactivated in other disorders of impulsiveness during inhibitory tasks such as impulsive aggression, bipolar disorder, and pathological gamblers [Best et al., 2002; Blumberg et al., 2003; Potenza et al., 2003]. We have recently, under improved fMRI hardware and software, observed underactivation of a ventromedial orbitofrontal location in patients with ADHD during a task of temporal discounting, where patients displayed a more impulsive choice pattern [Rubia et al., 2009c].
The shared reduction in medial prefrontal cortex activation—a region known to be important for performance monitoring [Ridderinkhof et al., 2004; Rubia et al., 2003; Ullsperger et al., 2004]—during failed inhibition is in line with previous underactivation findings in both patient groups during error monitoring and enhanced attention allocation [Rubia et al., 2009b; Woolley et al., 2008].
The disorder‐specific dysfunction in children with ADHD in anterior and posterior cingulate gyri, caudate, and putamen during the Switch task is in line with previous dysfunction findings in children with ADHD compared to healthy controls during tasks of cognitive control [Durston et al., 2003; Shafritz et al., 2004; Silk et al., 2005; Rubia et al., 1999, 2007c, 2008, 2009b]. Although we have previously observed caudate underactivation in the same OCD group when compared to 10 controls alone [Woolley et al., 2008], this dysfunction did not emerge for patients with OCD in the three‐group ANOVA comparison of this study. Basal ganglia dysfunction thus appears to be more pronounced in patients with ADHD than in those with OCD.
The findings of unimpaired performance in tasks of motor inhibition and cognitive switching in adolescents with OCD and ADHD are not in line with previous deficit findings in children with ADHD and in adults with OCD [Chamberlain et al., 2006; Penades et al., 2007; Rubia et al., 2007a; Willcutt et al., 2005]. However, lack of performance deficits could be accounted for in a variety of ways. The subject numbers of this study were relatively small for neuropsychological analyses, and group differences may have emerged with larger sample sizes, especially in the Stop tasks, where patients had numerically, albeit nonsignificantly, larger SSRT values. Furthermore, we tested adolescents with ADHD and these are less impaired and often grow out of their neuropsychological deficits compared to the younger child age group who is typically tested in neuropsychological studies. The adolescents with OCD included in this study were in partial remission or at a low current level of symptoms, which could explain the relatively good performance. Lastly, fMRI adaptations of cognitive tasks lose behavioral sensitivity and our task designs were purposely easier than offline task versions, i.e. slower and with more predictable timing of target events. This was necessary due to fMRI requirements of hemodynamic separability of target from nontarget trials as well as to assure that patients were able to perform the task reasonably well to obtain sufficient numbers of target events to allow comparability with controls. It has been shown consistently that brain activation is more sensitive than behavioral performance, and reduced brain activation despite good task performance has repeatedly been observed in patients with OCD and ADHD [Dickstein et al., 2006; Menzies et al., 2008; Nakao et al., 2005; Pliszka et al., 2006; Rubia et al., 1999, 2001, 2005a, 2007c, 2008, 2009a– c; Smith et al., 2006].
Although the sample sizes for the ADHD and control groups were considerably large for fMRI studies, the sample size of the children with OCD was relatively small. A further limitation of this study is the difference between the two disorders in clinical severity and medication status. While patients with ADHD were fully symptomatic and medication‐naïve, most of the patients with OCD were medicated with SSRIs and in partial remission. While this has the advantage of keeping confounding state symptoms of anxiety, depression, and ritualizing in the OCD group minimal, a comparison with fully symptomatic patients with OCD may elicit more severe and disorder‐specific brain dysfunctions in patients with OCD.
Although SSRIs appear to have little effect on inhibitory performance measures [Anderson et al., 2008], there is some evidence to suggest that serotonin agonists enhance activation in right inferior prefrontal cortex during tasks of inhibitory control. In healthy volunteers, SSRIs as well as the antidepressant serotonin agonist Mirtazapine have shown to enhance right inferior prefrontal activation during the go/no‐go task [Andersen et al., 2002; Del‐Ben et al., 2005; Vollm et al., 2006]. Indirect evidence for a modulatory effect of serotonin on the activation of inferior prefrontal cortex during inhibitory control tasks comes from tryptophan depletion studies that diminish serotonin levels in the brain and have found a reduction in activation in right and left inferior prefrontal cortex during tasks of motor and interference inhibition [Lamar et al., 2009; Rubia et al., 2005b]. In adult OCD, SSRI medication has been shown to reduce symptom‐related overactivation in frontal and striatal brain regions, but to increase task‐relevant parietal and cerebellar brain activation during interference inhibition [Nakao et al., 2005]. A study in children with OCD, however, found that SSRIs reduced enhanced activation compared to controls in insula and putamen during a motor task [Lázaro et al., 2008]. Despite some controversy, there is therefore some evidence to imply that SSRI medication in patients with OCD may have had a mitigating effect on brain dysfunction that may possibly be more pronounced in medication‐naïve adolescents with OCD.
In conclusion, to our knowledge, this study is a first step toward delineating the underlying neurofunctional differences between these two diagnostic disorders in relation to a commonly affected behavioral and neuropsychological phenotype that is motor and cognitive inhibitory dyscontrol. The disorder‐specific dysfunction in patients with ADHD in left and right inferior prefrontal cortices—that have previously been observed to be disorder‐specific compared to patients with CD—, if replicated, may be a specific neurofunctional biomarker for ADHD that could be clinically relevant for the development of a more objective phenomenological differentiation and disorder‐specific tailored treatment.
None of the authors has any interests to declare.
REFERENCES
- Achenbach T, Edelbrook CS ( 1983): Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, Vermont: University of Vermont. [Google Scholar]
- American Psychiatric Association ( 1994): Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson IM, Clark L, Elliott R, Kulkarni B, Williams SR, Deakin JF ( 2002): 5‐HT(2C) receptor activation by m‐chlorophenylpiperazine detected in humans with fMRI. Neuroreport 13: 1547–1551. [DOI] [PubMed] [Google Scholar]
- Anderson IM, McKie S, Elliott R, Williams SR, Deakin JFW ( 2008): Assessing human 5‐HT function in vivo with pharmacoMRI. Neuropharmacology 55: 1029–1037. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y ( 1995): Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300. [Google Scholar]
- Best M, Williams JM, Coccaro EF ( 2002): Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci USA 99: 8448–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birleson P ( 1981): The validity of depressive disorder in childhood and the development of a self‐rating scale—A research report. J Child Psychol Psychiatry 22: 73–88. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS ( 2003): A functional magnetic resonance imaging study of bipolar disorder: State‐ and trait‐related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60: 601–609. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, Woodruff PW, Rabe‐Hesketh S ( 1997): Generic brain activation mapping in functional magnetic resonance imaging: A nonparametric approach. Magn Reson Imaging 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M ( 2001): Colored noise and computational inference in neurophysiological (fMRI) time series analysis: Resampling methods in time and wavelet domains. Hum Brain Mapp 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson ML ( 2000): On the role of cortical glutamate in obsessive–compulsive disorder and attention‐deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand 102: 401–413. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ ( 2006): Motor inhibition and cognitive flexibility in obsessive–compulsive disorder and trichotillomania. Am J Psychiatry 163: 1282–1284. [DOI] [PubMed] [Google Scholar]
- Del‐Ben CM, Deakin JFW, Mckie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM ( 2005): The effect of Citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: An fMRI study. Neuropsychopharmacology 30: 1724–1734. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP ( 2006): The neural correlates of attention deficit hyperactivity disorder: An ALE meta‐analysis. J Child Psychol Psychiatry 47: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang YH, Ulug AM, Casey BJ ( 2003): Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 53: 871–878. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA ( 1999): Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Griffin S, Jones J, Lefkowitz TR ( 1996): Comorbidity of juvenile obsessive–compulsive disorder with disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry 35: 1637–1646. [DOI] [PubMed] [Google Scholar]
- Geller D, Biederman J, Faraone SV, Frazier J, Coffey BJ, Kim G, Bellordre CA ( 2000): Clinical correlates of obsessive compulsive disorder in children and adolescents referred to specialized and non‐specialized clinical settings. Depress Anxiety 11: 163–168. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Murrey R, editors ( 2002): Maudsley Handbook of Practical Psychiatry. Oxford: Oxford University Press. [Google Scholar]
- Goodman R ( 1997): The strengths and difficulties questionnaire: A research note. J Child Psychol Psychiatry 38: 581–586. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F ( 2009): Paediatric obsessive compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev 33: 818–830. [DOI] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y ( 2002): Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA 99: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Cutter W, Rubia K, Brammer M, Cleare A, Murphy D ( 2009): Serotonin and prefrontal functioning in older adults: An fMRI investigation of interference inhibition during acute tryptophan depletion. Neurobiol Aging 30: 1135–1146. [DOI] [PubMed] [Google Scholar]
- Lázaro L, Caldú X, Junqué C, Bargalló N, Andrés S, Morer A, Castro‐Fornieles J ( 2008): Cerebral activation in children and adolescents with obsessive–compulsive disorder before and after treatment: A functional MRI study. J Psychiatr Res 42: 1051–1059. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ ( 2007): Cortical thinning of the attention and executive function networks in adults with attention‐deficit/hyperactivity disorder. Cereb Cortex 17: 1364–1375. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou M, Hodge SM, Vaera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ ( 2008): Attention and executive systems abnormalities in adults with childhood ADHD: A DT‐MRI study of connections. Cereb Cortex 18: 1210–1220. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen C‐H, del Campo N, Sahakian BJ, Robbins TW, Bullmore E ( 2007): Neurocognitive endophenotypes of obsessive–compulsive disorder. Brain 130 3223–3236. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET ( 2008): Integrating evidence from neuroimaging and neuropsychological studies of obsessive–compulsive disorder: The orbitofronto‐striatal model revisited. Neurosci Biobehav Rev 32: 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M ( 2005): A functional MRI comparison of patients with obsessive–compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res 139: 101–114. [DOI] [PubMed] [Google Scholar]
- Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C ( 2007): Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 22: 404–410. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud‐Clikeman M, Franklin C, Perez R, Xiong JJ ( 2006): Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long‐term treatment. Am J Psychiatry 163: 1052–1060. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung H‐C, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC ( 2003): An fMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry 160: 1990–1994. [DOI] [PubMed] [Google Scholar]
- Raven J ( 1960): Guide to the Standard Progressive Matrices. London: HK Lewis. [Google Scholar]
- Reynolds CR, Richmond BO ( 1978): What I think and feel—Revised measure of children's manifest anxiety. J Abnorm Child Psychol 6: 271–280. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Carter CS ( 2004): Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward‐based learning. Brain Cogn 56: 129–140. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore E ( 1999): Hypofrontality in attention deficit hyperactivity disorder ADHD during higher‐order motor control: A study using fMRI. Am J Psychiatry 156: 891–896. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksannen H, Overmeyer S, Newman S ( 2001): Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br J Psychiatry 179: 138–143. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E ( 2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20: 351–358. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Toone B, Taylor E ( 2005a): Medication‐naïve adolescents with attention‐deficit hyperactivity disorder show abnormal brain activation during inhibition and error detection. Am J Psychiatry 162: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lee F, Cleare A, Tunstall N, Fu C, McGuire P ( 2005b): Acute tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event related fMRI. Psychopharmacology 179: 791–801. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer MJ ( 2006): Progressive increase of fronto‐striatal brain activation from childhood to adulthood during event related tasks of cognitive control. Hum Brain Mapp 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer M, Taylor E ( 2007a): Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery for impulsiveness. Child Neuropsychol 13: 276–304. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M ( 2007b): Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Hum Brain Mapp 28: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E ( 2007c): Temporal lobe dysfunction in medication‐naive boys with attention‐deficit/hyperactivity disorder. Biological Psychiatry 62: 999–1006. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith A, Mohammad M, Scott S, Giampietro V, Taylor E, Brammer ME ( 2008): Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention‐deficit/hyperactivity disorder. Am J Psychiatry 165: 889–897. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Halari R, Matukura F, Mohammad M, Taylor E, Brammer M ( 2009a): Disorder‐specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure attention‐deficit/hyperactivity disorder during sustained attention. Am J Psychiatry 166: 83–94. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith A, Mohammad M, Scott S, Brammer M ( 2009b): Shared and disorder‐specific prefrontal abnormalities in boys with pure attention‐deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry 50: 669–678. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E ( 2009c): Impulsiveness as a disturbance of timing: Brain dysfunction in ADHD during temporal processes and normalisation with a dopaminergic agonist. Philos Trans R Soc Lond B Biol Sci 346: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwigginHardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF ( 1997): Children's Yale–Brown obsessive compulsive scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36: 844–852. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA ( 2004): The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry 161: 1990–1997. [DOI] [PubMed] [Google Scholar]
- Silk T, Vance A, Rinehart N, Egan G, O'Boyle M, Bradshaw JL, Cunnington R ( 2005): Fronto‐parietal activation in attention‐deficit hyperactivity disorder, combined type: Functional magnetic resonance imaging study. Br J Psychiatry 187: 282–283. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K ( 2004): The neural correlates of switching set as measured in fast, event‐related functional magnetic resonance imaging. Hum Brain Mapp 21: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K ( 2006): Task‐specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication‐naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 163: 1044–1051. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Brain. New York: Thieme. [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB ( 2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY ( 2004): Neuroimaging of performance monitoring: Error detection and beyond. Cortex 40( 4/5): 593–604. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, McKie S, Elliott R, Deakin JFW, Anderson IM ( 2006): Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: An fMRI study in healthy volunteers. Eur J Neurosci 23: 552–560. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF ( 2005): Validity of the executive function theory of attention‐deficit/hyperactivity disorder: A meta‐analytic review. Biol Psychiatry 57: 1336–1346. [DOI] [PubMed] [Google Scholar]
- Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K ( 2008): Brain activation in paediatric obsessive–compulsive disorder during tasks of inhibitory control. Br J Psychiatry 192: 25–31. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD ( 2006): Between‐task competition and cognitive control in task switching. J Neurosci 26: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]


