Abstract
Objectives: The aim of this study was to investigate the relationship between medial cortical activation and the presence of self and consciousness in healthy subjects and patients with vegetative state and minimally conscious state using functional magnetic resonance imaging (fMRI). Experiment design: We first conducted two fMRI experiments in healthy subjects to identify brain regions specifically associated with self‐perception through the use of different auditory stimuli that had different grades of self‐relatedness. We then applied these regions as functional localizers to examine the relationship between neural activity changes during self‐relatedness and consciousness level in the patients with disorders of consciousness (DOC). Principal observations: We demonstrated recruitment of various anterior medial cortical regions including the anterior cingulate cortex (ACC) in healthy subjects during auditory perception of self‐related stimuli. We further showed that patients with DOC showed signal changes in the ACC during auditory perception of self‐related stimuli. Finally, it was shown that these signal changes correlate with the level of consciousness in the patients with DOC. Conclusion: The degree of consciousness in patients with DOC was correlated with neural activity in the ACC induced by self‐related stimuli. Our results not only shed light on the pathophysiology of DOC, but may also suggest a useful neural, and thus diagnostic, marker of the dysfunction of consciousness in vegetative patients. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: minimally conscious state, vegetative state, own name, anterior cingulate cortex, self
INTRODUCTION
Disorders of consciousness (DOC) are defined by a loss or severe deficit in consciousness that concerns predominantly the awareness of the environment and of the self [e.g., the vegetative state (VS) and minimally conscious state (MCS)] [Laureys et al., 2007]. However, recent studies have indicated that some special brain activity associated with self‐relatedness seems to be somehow maintained in these patients. For instance, clinical observations have demonstrated that exposure to self‐related stimuli, such as the individuals own name, may induce corresponding responses in the DOC patients' brain [Di et al., 2007; Kotchoubey et al., 2004; Mazzini et al., 2001; Perrin et al., 2006; Schnakers et al., 2008; Signorino et al., 1995].
A recent imaging study in DOC patients by Owen et al. [ 2006] demonstrated neural activity during the imagination of tennis playing and spatial navigation, each of which requires some functioning awareness of the self. In addition, two single case reports reported neural activity in anterior midline regions during presentation of self‐related stimuli to VS [Staffen et al., 2006] and MCS patients [Laureys et al., 2004]. Whether these findings are applicable at the group level and involve regions that are specific for self‐relatedness remains unclear however. Most importantly, the neural mechanisms by which neural activity during self‐relatedness are linked to the deficits of consciousness in DOC have not yet been revealed. This is not only scientifically, but also clinically relevant since, if neural activity during self‐relatedness is correlated with the degree of consciousness, the former may serve as a neural, and thus diagnostic, marker of the latter.
Although the self remains to be investigated in DOC, there have been numerous studies in healthy subjects investigating the neural mechanisms underlying the self. Imaging studies in healthy subjects have demonstrated the involvement of anterior and posterior cortical midline regions in self‐related processing, with the anterior cingulate cortex (ACC) and the ventro‐ and dorsomedial prefrontal cortex (VMPFC, DMPFC) featuring most prominently [D'Argembeau et al., 2005; Jackson et al., 2006; Keenan et al., 2000; Kelley et al., 2002; Mitchell et al., 2005; Northoff and Bermpohl, 2004; Northoff et al., 2006; Ochsner et al., 2005; Schmitz et al., 2004; Vogeley et al., 2004; Moran et al., 2009]. Of particular relevance in the context of this article, brain activity in such midline regions has been observed in healthy individuals upon hearing their own name [Kampe et al., 2003; Perrin et al., 2005; Staffen et al., 2006]. Interestingly, most of these studies rely on evaluation of self‐ and nonself‐related stimuli, thereby presupposing consciousness of one's self, that is, self‐consciousness. It not known, however, exactly how self and consciousness are linked together.
For instance, a region like the ACC has been associated with both consciousness [Devinsky et al., 1995] and the self [Northoff et al., 2006]. The neural processes mediating such linkage between self and consciousness remain unclear though. This may not only be scientifically relevant, but also clinically since many patients with MCS are often misdiagnosed as VS (Andrews et al., 1996); the evaluation of the neural activity during the own name may then help to better differentiate VS and MCS.
The aim of this study was to investigate the relationship between medial cortical activation and the presence of self and consciousness in both healthy subjects and DOC patients using functional magnetic resonance imaging (fMRI). We thus delineated those medial cortical regions that showed neural activity changes in healthy subjects in response to auditory self‐related stimuli. These regions were then used as functional localizers to investigate neural activity changes during auditory self‐related stimuli in patients with DOC, and the relationship between these signal changes and the patients' level of consciousness.
SUBJECTS AND METHODS
Subjects
Seventeen healthy volunteers participated in the study (10 female; age range 20–27 years; 16 right handed). None had a history of neurological or psychiatric disorders or were taking medication. All healthy subjects participated in both Experiments 1 and 2. Eleven patients with DOC were studied (two female; age range 21–61 years). Among them, seven patients were diagnosed with VS and four with MCS. The patients were imaged 2–48 months after injury (Table I). All subjects were native Chinese speakers. Informed written consent was obtained from the healthy participants and the family members of the patients. The study was approved by the local Ethics Committees of the Institute of Psychology at Chinese Academy of Sciences and Zhejiang University School of Medicine.
Table I.
Patients' basic information table
| Sex/Age, years | Cause | Time of fMRI (months after insult) | Lesions on MRI | GCS | At the time of fMRI | 3 months later | |||
|---|---|---|---|---|---|---|---|---|---|
| CRS‐R | Diagnosis | CRS‐R | Diagnosis | ||||||
| VS patients | |||||||||
| 1 | M/58 | Anoxic | 4 | Right temporal gyrus (diffuse cortical atrophy) | 9 | 7 | VS | 7 | VS |
| 2 | M/61 | Traumatic | 8 | Left temporal frontal cortex | 7 | 4 | VS | 4 | VS |
| 3 | M/29 | Anoxic | 48 | Diffuse cortical atrophy | 8 | 6 | VS | 4 | VS |
| 4 | M/42 | Traumatic | 2 | Diffuse left hemisphere/right temporal and parietal cortex | 7 | 4 | VS | 4 | VS |
| 5 | F/52 | Traumatic | 2 | Right frontal and left temporal cortex | 9 | 6 | VS | 6 | VS |
| 6 | M/21 | Traumatic | 4 | Right temporal, frontal and parietal cortex | 9 | 6 | VS | 11 | MCS |
| 7 | M/38 | Traumatic | 4 | Left occipital cortex and bilateral basal ganglia | 7 | 6 | VS | 9 | MCS |
| MCS patients | |||||||||
| 1 | M/30 | Traumatic | 2 | Right temporal and frontal cortex | 10 | 10 | MCS | 21 | MCS |
| 2 | F/24 | Traumatic | 3 | Bilateral frontal subdural hematoma, brain stem | 9 | 7 | MCS | 20 | MCS |
| 3 | M/38 | Traumatic | 6 | Bilateral temporal and frontal cortex | 8 | 5 | MCS | 6 | MCS |
| 4 | M/30 | Traumatic | 26 | Left temporal and bilateral frontal cortex | 10 | 13 | MCS | 14 | MCS |
GCS, Glasgow Coma Scale; CRS‐R, Coma Recovery Scale‐Revised.
Behavioral Assessments
The patients were assessed by means of the Glasgow Coma Scale (GCS) [Teasdale and Jennett, 1974] and the Coma Recovery Scale‐Revised (CRS‐R) [Giacino et al., 2004] at the beginning of the study. They were diagnosed according to the CRS‐R and reassessed with the CRS‐R 3 months later. Two patients with VS (VS 6 and VS 7) recovered to MCS 3 months later (Table I). The CRS‐R comprises six subscales addressing auditory, visual, motor, oromotor/verbal, communication, and arousal, respectively (see Supporting Information Table I for patient test scores).
Stimuli
Five auditory stimuli with different grades of self‐relatedness were used: the subjects own name (SON) presented by a familiar voice (SON‐FV); SON presented by an unknown voice (SON‐UV); a friend's name (Chinese) presented by an unknown voice (FN‐UV); an unknown person's name (Chinese) presented by an unknown voice (UN‐UV); and an English name presented by an unknown voice (EN‐UV). The names used as stimuli were carefully chosen to control the degree of familiarity. Specifically, the names of subjects' classmates of the same gender were used as FN‐UV, while the unknown names were chosen from a pool of the most commonly used names in China. For the unknown names, the first 200 disyllabic names, 100 female and 100 male, from the pool were selected and then rated for familiarity by 100 undergraduate students (5 = extremely common, 4 = very common, 3 = common, 2 = not common, 1 = very rare). Those names that scored 4–5 were then used as the unknown names for the experiment, the female names for female subjects and male names for male subjects. All stimuli were presented at 80 dB with a mean duration of 741 ± 84 ms (S.E.). After scanning, all subjects expressed that they could hear the names clearly.
Experimental Design
Because of the inherent difficulties in scanning DOC patients, a three‐stage experimental design involving both the patients and a group of healthy subjects was adopted. This allowed the regions involved in self‐related processing to be established in the healthy subjects in a manner that took into account various potential confounding factors, with these regions of interest (ROIs) then being applied to the DOC patients to identify self‐related neural responses in them. This procedure meant that time required to scan the patients could be minimized whilst maximizing the quality of data obtained from them (see Fig. 1).
Figure 1.
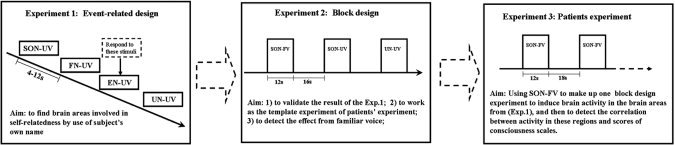
Schemata for experimental design. Experiment 1, in healthy subjects, allowed the regions involved in self‐related processing specifically to be identified. These results were then validated in the same experimental context as would be used in the scanning of DOC patients (i.e., in a block rather than event‐related design) in Experiment 2. The regions of interest identified in Experiment 1 were then applied to the analysis of the data obtained in Experiment 3, in which the patient was presented with their own name only.
Experiment 1
An event‐related fMRI design was employed to determine those regions that were activated in response to self‐related stimuli (SON‐UV) when compared to stimuli that was not self‐related (FN‐UV and UN‐UV). In studies of the self, familiarity is one of the main confounding factors. This is because subjects are, by definition, familiar with self‐related stimuli [Gillihan and Farah, 2005]. In addition, an overlap has been observed between brain regions activated by self‐related and friend‐related stimuli [Seger et al., 2004]. As such, the FN‐UV condition was included in addition to the UN‐UV condition to preclude the effect of familiarity.
Stimuli were presented in a pseudo‐random order with no concurrent occurrences of SON‐UV. To control for attentional effects during the experiment, subjects were asked to respond, via a key press, to English names spoken by an unknown voice (EN‐UV) [Kampe et al., 2003]. In all other trials the subjects listened passively (with eyes open) without giving any response, since the initiative movements and subsequent evaluative‐reflective processes may generate conscious awareness of the self, which may in turn confound those neural activity changes that are specifically related to the self, independent of consciousness [Jardri et al., 2007]. Sixty each of SON‐UV, UN‐UV, and FN‐UV, along with 24 EN‐UV, were presented in four runs, with stimulus onset randomized between 4 and 12 s (Fig. 1).
Experiment 2
The same cohort of healthy subjects underwent a second scanning session in which the same SON‐UV and UN‐UV stimuli as in Experiment 1 were presented in a block design. In addition, the subject's own name spoken by a familiar voice (the voice of a classmate) was presented (SON‐FV). This condition was included to allow the effect of the familiarity of the voice in which stimuli were presented in the neural responses to SON‐FV, as used in the DOC patients, to be controlled for [von Kriegstein et al., 2005]. Experiment 2 also allowed the results of Experiment 1 to be validated, and, importantly, provided sample independence for subsequent statistical analyses [Kriegeskorte et al., 2009; Poldrack and Mumford, 2009]. Five blocks of each of the three stimuli were presented (15 blocks in total). Each 12‐s block consisted of six stimulus presentations, with an intertrial interval of 16 s. During the experiment, participants listened passively to the stimuli.
Experiment 3
A block design was used in the fMRI experiment for the patients with DOC. To stimulate as much neural activity as possible [Holeckova et al., 2006], we thus focused on the SON presented by a familiar voice (SON‐FV). Focusing on this stimulus only allowed us to maximize the number of presentations of maximally self‐related stimuli. SON‐FV was presented seven times within each 12‐s block. Six blocks in total were presented, with an interval of 18 s between each block. During the experiment, patients listened passively to the stimuli.
MRI Scanning
In healthy subjects, MR images were acquired on a GE 3.0T Sigma Excite scanner. Functional images were acquired using a T2*‐weighted echo‐planar imaging (EPI) sequence (TR/TE/θ = 2,000 ms/30 ms/90°, FOV = 240 × 240 mm, matrix = 64 × 64, slice‐thickness = 4 mm, gap = 0 mm). Each volume had 31 axial slices, covering the whole brain. Following the functional scans, a fast SPoiled GRass (SPGR) sequence was used to obtain the 3D whole brain images (TR = 8.5 ms, TE = 3.4 ms, flip angle = 12°, FOV = 280 × 280 mm, Matrix = 256 × 256, slice‐thickness = 1 mm) for functional image registration and localization. For patients, data were collected using a 1.5‐T General Electrics Sigma Horizon MRI system (GE Medical Systems, Milwaukee, WI). For the EPI sequence, TR = 3,000 ms, TE = 60 ms, matrix = 64 × 64). See Di et al. [ 2007] for more information on data acquisition.
Data Analysis
Functional images were processed using the AFNI software package [Cox, 1996]. The data from all three experiments underwent a preprocessing procedure that included two‐ and three‐dimensional head‐motion corrections, masking for the removal of the skull, and spatial smoothing using a kernel of 4 mm full‐width at half‐maximum. Scan data from Experiments 1 and 2 were converted to Talairach space and resampled to 1‐mm isotropic voxels. To protect against Type I error, a P < 0.005 and a cluster volume exceeding 280 mm3 were considered to show a positive response (α level of 0.01). These thresholds were calculated with the AlphaSim program in AFNI with multiple testing FWE corrections.
Experiment 1
Following the above preprocessing procedures, the data from Experiment 1 were submitted to deconvolution analysis to obtain a map of the estimated coefficients for the three stimuli (SON‐UV, UN‐UV, and FN‐UV). Group analyses were performed to produce the following three contrasts using paired t tests: SON‐UV versus UN‐UV, SON‐UV versus FN‐UV, and FN‐UV versus UN‐UV. The contrast between SON‐UV and UN‐UV showed the brain areas involved in both self‐related processing and familiarity‐processing, while the contrast between FN‐UV and UN‐UV showed the brain areas just involved in familiarity‐processing. The contrast between SON‐UV and FN‐UV was then used to identify those brain areas involved solely in self‐related processing.
Experiment 2
The preprocessed data from Experiment 2 were submitted to deconvolution analysis to obtain a map of the estimated coefficients for the three stimuli. Because of severe head movement (>2 mm), data from two healthy subjects were discarded. The brain areas recruited by SON‐UV versus FN‐UV in Experiment 1 were used as the ROIs for further localization of functional activation in relation to the signal changes during SON‐FV in Experiment 2. Since previous studies in healthy subjects associated predominantly the anterior cortical midline regions with self‐relatedness [Northoff et al., 2006], we focused on those medial regions identified in Experiment 1—the caudal anterior cingulate cortex (cACC), the anterior anterior cingulate cortex (aACC), and the supplementary motor area (SMA). The three ROIs were then applied to the data from Experiment 2 to calculate signal changes in these regions during SON‐FV, SON‐UV, and UN‐UV. This served to produce three contrasts (SON‐FV vs. SON‐UV, SON‐FV vs. UN‐UV, and SON‐UV vs. UN‐UV) in all three ROIs (paired t‐tests, two‐tailed). This step allowed the exclusion of the possibility that UN‐UV could induce brain activity in the three ROIs. To verify that UN‐UV itself did not induce brain activity in the three ROIs, separate one‐sample t‐tests were carried out for each region to identify any signal changes.
Experiment 3
The preprocessed data from patients were submitted to deconvolution analysis to obtain a map of the estimated coefficients for SON‐FV, the maps of estimated coefficients were not transferred into the Talairach space; instead, the patient's functional EPI images were coregistered to the 3D structural images from each patient, normalized to a standard T1‐weighted template. The inverse of these normalization parameters was then used to warp the independent ROIs (activated brain areas in medial regions during self‐relatedness) from healthy subjects onto the non‐normalized structural image for each patient [Coleman et al., 2007]. The ROIs obtained in Experiments 1 and 2 were applied to each patient to extract the mean signal change for SON‐FV in all three ROIs. Finally, a one‐way ANOVA was used to identify differences in signal changes in each of the three ROIs between VS and MCS patients.
Signal intensities as calculated in the ROIs in DOC patients were also correlated with the scores from the various behavioral scales (see above) using Pearson correlation analysis (two‐tailed). The data were analyzed using SPSS version 13.0 (SPSS, Chicago, IL). Correlation analysis was performed in order to link neural signal changes during self‐relatedness with measures of consciousness level, such that the predictive character of the former for the latter could be established. To confirm the result of ROI analysis, we also determined those brain areas that were active during SON‐FV in a voxelwise way across the whole brain for each patient without regard to these ROIs. Finally, the scores of the CRS‐R at the time of scanning and 3 months later were compared using a paired sample t‐test (two‐tailed).
RESULTS
Patients' Behavioral Data
The 11 patients with DOC were diagnosed as being either in a vegetative state (VS; n = 7) or in a minimally conscious state (MCS; n = 4) based on clinical assessment using the Coma Recovery Scale Revised (CRS‐R) at the time of fMRI. Two of the seven patients with VS (VS 6 and VS 7) recovered to MCS 3 months later, while the other five remained unconscious (Table I). At the time of fMRI, CRS‐R scores significantly correlated with those of the GCS (P < 0.01, r = 0.815). After 3 months, the total score of the CRS‐R did not show significant change when compared with the scores at the time of fMRI [P = 0.076, t = 1.982 (df = 10, two‐tailed); Supporting Information Table I].
Signal Changes During Self‐Relatedness in Healthy Subjects
Experiment 1
Comparing subjects' own name with a familiar name (SON‐UV > FN‐UV) (P = 0.05, FWE corrected) yielded activity in the caudal ACC (cACC), anterior ACC (aACC), SMA, bilateral superior temporal gyrus, bilateral insula, and right pallidum (Table II and Fig. 2A,B). The control contrast comparing presentation of one's own name versus an unknown name (SON‐UV > UN‐UV; P = 0.05, FWE corrected) also induced activity in aACC, cACC and SMA, but no significant difference between FN‐UV and UN‐UV was found in the three regions (Table II and Supporting Information Figs. 1 and 2). This suggests that the cACC, SMA, and aACC show the most specific signal changes during self‐relatedness. We thus defined these as ROIs for subsequent analyses.
Table II.
Activated brain areas in healthy subjects
| Brain areas | Side | Volume (mm3) | Talairach coordinate | t value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| SON‐UV vs. UN‐UV | ||||||
| Anterior cingulate cortex | B | 3,667 | −10 | 9 | 37 | 6.508 |
| Supplementary motor area | B | 1,075 | 14 | 10 | 59 | 6.211 |
| Post cingulate cortex | B | 7,905 | 2 | −40 | 22 | 8.829 |
| Temporoparietal | R | 1,752 | 57 | −31 | 22 | 7.593 |
| Temporoparietal | L | 1,692 | −46 | −36 | 26 | 6.442 |
| Super temporal gyrus | R | 1,423 | 50 | 6 | −10 | 11.055 |
| Super temporal gyrus | L | 595 | −49 | −1 | 2 | 6.552 |
| Insula | L | 1,228 | −34 | 11 | 2 | 5.568 |
| Insula | R | 495 | 39 | 2 | 8 | 5.431 |
| SON‐UV vs. FN‐UV | ||||||
| Candual part of anterial cingulate cortex | B | 1,519 | 8 | 12 | 35 | 6.156 |
| Supplementary motor area | B | 825 | 7 | 5 | 58 | 7.113 |
| Anterial part of anterial cingulate cortex | B | 327 | 0 | 24 | 24 | 5.129 |
| Super temporal gyrus | L | 1,890 | −48 | 14 | −3 | 6.203 |
| Super temporal gyrus | R | 569 | 43 | −17 | 2 | 5.029 |
| Insula | R | 312 | 38 | 2 | 8 | 4.507 |
| Insula | L | 420 | −34 | 24 | −2 | 4.228 |
| Pallidum | R | 285 | 17 | 0 | −2 | 7.15 |
| FN‐UV vs. UN‐UV | ||||||
| Post cingulate cortex | B | 10,632 | 2 | −61 | 25 | 6.642 |
| Ventromedial prefrontal cortex | B | 385 | −2 | 30 | 2 | 4.578 |
| Medial prefrontal cortex | B | 540 | 2 | 46 | 14 | 5.744 |
| Temporoparietal | L | 2,002 | −39 | −53 | 15 | 5.287 |
| Temporoparietal | R | 1,327 | 46 | −54 | 21 | 9.89 |
Talairach coordinates represent the voxel showing the maximal activation within each cluster (P = 0.01, FWE corrected).
B, bilateral; L, left; R, right.
Figure 2.
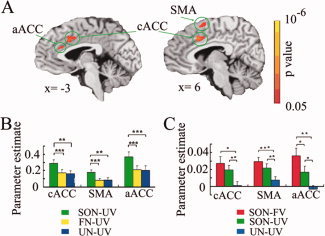
fMRI results in healthy subjects. (A) Brain activations during SON‐UV compared to FN‐UV (P = 0.01, FWE corrected). (B) Parameter estimates (means ± S.E.) for SON‐UV, FN‐UV, and UN‐UV in the cACC, SMA, and aACC in the event‐related experiment (Experiment 1). (C) Parameter estimates (means ± S.E.) for SON‐FV, SON‐UV, and UN‐UV in the same three ROIs for the block‐design experiment (Experiment 2). * denotes P < 0.05; ** denotes P < 0.01; *** denotes P < 0.001.
Experiment 2
Applying the three main regions from Experiment 1 as functional localizers for the findings in this experiment, both presentation of subjects' own name by a familiar voice (SON‐FV) and by an unknown voice (SON‐UV) resulted in significantly stronger signal changes in cACC, aACC, and SMA when compared to an unknown name (UN‐UV) (paired t‐test, P < 0.05). Moreover, a significant difference between signal changes during SON‐FV and SON‐UV (paired t‐test, P < 0.05), which mirrors the effect of the familiarity of the voice, was obtained in the aACC, but not in either the cACC or SMA (Fig. 2C and Supporting Information Fig. 3). UN‐UV did not induce brain activities in the three regions [cACC: t = 0.339, P = 0.740; SMA: t = 1.843, P = 0.087; aACC: t = −0.686, P = 0.504 (df = 14, two‐tailed)] (Supporting Information Fig. 4).
Signal Changes During Self‐Relatedness in Patients With DOC
Applying the three ROIs from Experiments 1 and 2 in healthy subjects as functional localizers in the DOC patients yielded the following results on a single subject level (Fig. 3A): All of the MCS, and six of the seven VS patients showed signal changes during auditory presentation of their own name by a familiar voice (SON‐FV) in at least one of the three regions (Supporting Information Table II); when MCS patients were then compared with VS patients, stronger signal changes were observed in the cACC [F = 6.710, P = 0.029, df = (1,9)], but not in both aACC and SMA (Fig. 3B).
Figure 3.
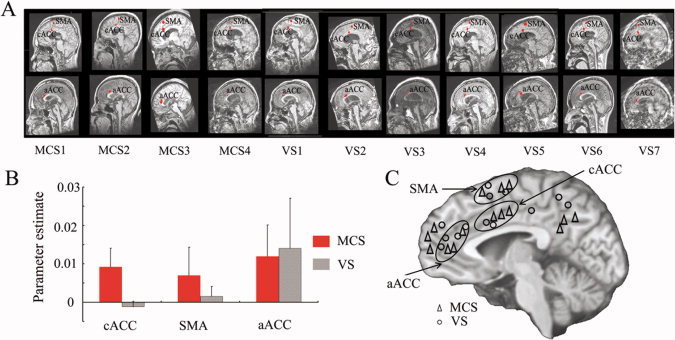
Patient fMRI results. (A) ROIs defined for cACC, SMA, and aACC in the patients with DOC. (B) Parameter estimates for the VS and MCS patients respectively in the three ROIs (mean ± S.E.). (C) Schematic representation of the midline structures activated during SON‐FV in the patients. Those activated areas (labeled with points) in the same circle were regarded as the activations in the same anatomical localization.
To confirm the observation of signal changes in anterior midline regions during the presentation of self‐related stimuli, we conducted a separate a posteriori whole brain analysis in DOC patients that no longer relied on the ROIs defined above. At the subject level, signal changes induced by presentation of one's own name were identified in similar regions as our predefined ROIs, as well as in further anterior and posterior midline regions (P = 0.05 FDR corrected; Fig. 3C, Supporting Information Fig. 5 and Supporting Information Table III).
Correlation Between Consciousness and Signal Changes Induced by Self‐Related Stimuli in Patients With DOC
To link neural activity changes during self‐relatedness to the degree of consciousness, we correlated the former with the scores for the consciousness scales (detailed above) taken at the same time of scanning. Total scores from the CRS‐R, as well as its four subscales (auditory, motor, oromotor/verbal, and communication), all correlated positively with the signal changes in the cACC induced by auditory presentation of the subjects own name (SON‐FV) (total: r = 0.800, P = 0.003) (Fig. 4 and Supporting Information Fig. 6A). Similar correlations were observed with the GCS score (Supporting Information Fig. 7). To ensure that those points that lie at the extreme of the sample were not driving a spurious correlation result, an additional robust regression using an iteratively reweighted least squares algorithm that gave less weighting to those values that lie at the outer extremes of the sample was carried out in MATLAB. This robust regression also indicated that brain activity in cACC is correlated with total scores of CRS‐R (t = 3.679, P = 0.0051) (Supporting Information Fig. 8), as well as indicating a significant correlation between brain activity in cACC and scores of GCS (t = 4.7117, P = 0.0011). In contrast to the cACC, signal changes in the aACC and SMA did not correlate with the consciousness scales scores.
Figure 4.
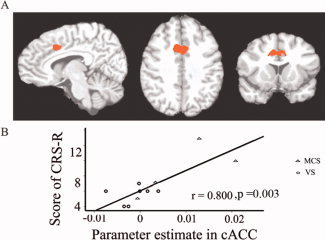
Correlation between neural activity in cACC and consciousness. (A) The cACC as a ROI defined from Experiment 1, which was used as the template to obtain the cACC location for each patient. (B) Correlations between the parameter estimates in cACC and the CRS‐R scores at the time of fMRI.
In addition, the signal changes during self‐relatedness were correlated with the CRS‐R scores obtained 3 months after scanning. This yielded significant correlation of the CRS‐R total scores and its subscales (visual, motor, oromotor/verbal, communication, and arousal) with the cACC signal changes during auditory presentation of one's own name (SON‐FV; Supporting Information Fig. 6B,C). Although the correlation between intensity in cACC and difference of CRS‐R (3 months later vs. time of scanning) did not reach statistically significance (r = 0.520, P = 0.101), it did for some subscales (subscale‐2, visual; and subscale‐6, arousal; Supporting Information Fig. 6D).
DISCUSSION
We here investigated the relationship between self and consciousness in patients with DOC. Patients with DOC showed signal changes during self‐relatedness in exactly those medial cortical regions in which healthy subjects showed the strongest responses to self‐related stimuli. Furthermore, the signal changes in the ACC during self‐relatedness were correlated with the degree of consciousness in DOC patients. Taken together, our results demonstrate that patients with DOC show neural activity changes in the ACC when exposed to self‐related stimuli.
Healthy subjects showed activation in various medial cortical regions during the presentation of their own name when compared to the presentation of another person's name (Fig. 1, Supporting Information Figs. 1 and 2). This is in line with previous studies that showed the involvement of cortical midline structures during presentation of self‐related stimuli [Northoff et al., 2006]. Our results extend these findings by demonstrating the involvement of these medial cortical regions during auditory presentation of stimuli, as distinguished from the visually presented stimuli used in the majority of the above cited studies (see Johnson et al. [ 2002] for an exception). This result was then confirmed using the presentation of the own name in a different voice. Experiments 1 and 2 thus allowed us to reliably determine the involvement of anterior medial cortical regions in auditory self‐related processing.
The anterior midline regions showing specific involvement in self‐related processing served as functional localizer in DOC patients. Our DOC patients showed some signal changes, which varied somewhat across individuals, in these midline regions during auditory presentation of their own name. This is in accordance with previous studies that, whilst not testing for self‐relatedness, showed activity in anterior midline regions in DOC [de Jong et al., 1997; Kassubek et al., 2003; Owen et al., 2002]. Our results show on both the single subject and group level that DOC patients display neural activity changes in midline regions like the ACC when exposed to self‐related stimuli. Hence, our findings complement recent observations of neural activity changes during consciousness of the environment [Coleman et al., 2007; Owen and Coleman, 2008; Owen et al., 2006] with regard to consciousness of the self. One may consequently assume that the here observed neural activity changes during the presentation of self‐related stimuli may underlie the clinical observations of some preserved self‐functions in these patients [Di et al., 2007; Kotchoubey et al., 2004; Mazzini et al., 2001; Perrin et al., 2006; Schnakers et al., 2008; Signorino et al., 1995].
Our most important finding is the correlation between the degree of consciousness and neural activity changes in a particular subregion of the ACC—the caudal ACC—during self‐related processing. Greater activity within the cACC during the presentation of self‐related stimuli predicted a higher degree of consciousness at the time of scanning. Unlike other midline regions, the cACC seems to be crucially involved in linking self and consciousness in DOC. This is in accordance with the double role of the ACC in both self and consciousness, as observed in healthy and lesion subjects [Devinsky et al., 1995; Feinstein et al., 2004; Luo et al., 2009; Paus, 2001]. Our findings complement these observations by showing that the altered neural activity in the cACC during self‐relatedness may be crucial in determining the degree of consciousness in vegetative patients. This not only gives some insight into the neural mechanisms of DOC, but may also be clinically relevant in providing a neural, and thus diagnostic, marker of the deficit of consciousness. Whether neural activity during self‐relatedness may even serve as prognostic or predictive markers of therapeutic recovery of consciousness in DOC needs to be investigated in future studies.
Interestingly, we found that signal changes within the cACC also correlated with the degree of consciousness measured 3 months after the scanning session, as well as with the change in score in that period for certain subscales of the CRS‐R. There were two patients with VS that showed significant brain activity in cACC at the time of fMRI; these two patients also improved to MCS 3 months later. Although these patients were diagnosed as VS on the basis of their inability to give a motor response [Owen et al., 2006], they may have been actually already in a MCS state because of their apparent ability to induce neural activity during their own name that possibly presupposes some degree of consciousness.
Based on our data, we assume that self‐related stimuli could induce some automatic processing, thus raising the question for the possible distinction between implicit and explicit self‐related processing. There is indeed some empirical evidence for such automatic implicit self‐related processing. An EEG study demonstrated the induction of a special ERP waveform during listening to the own name when the participants were sleeping and thus in an unconsciousness state [see Perrin et al., 1999 and Pratt et al., 1999]. Moreover, a recent fMRI study demonstrated that activity changes in the midline regions did not depend so much on the task (they used a passive and active task with regard to self‐referential stimuli), but rather on the degree of self‐relatedness of the stimuli. This task‐independence of midline signal changes indicates that it is the degree of the self‐relatedness of the stimuli rather than the mode or task, be it passive or active, which determines neural activity in these regions. Taken together, these data support the assumption of possible automatic processing of self‐related stimuli in the midline regions, corresponding with what we assume to occurring in vegetative patients when they hear their own name.
Some limitations in our study need to be mentioned. The main limitation is that we did not adopt a control condition for self‐relatedness in DOC patients, and therefore cannot be sure whether the observed signal changes are specifically associated with self‐relatedness. The chosen methodology did, however, allow us to maximize the number of self‐related trials possible within the limited scanning time possible with DOC patients, allowing us to obtain robust fMRI results. The fact that we observed signal changes in these regions to be specific for self‐relatedness in our two carefully designed experiments in healthy subjects does, however, make it likely that the signal changes in DOC patients mirror self‐relatedness as distinguished from nonself‐relatedness. Second, we did not directly measure self‐consciousness in our subjects. This was due to the obvious difficulty in doing so in DOC patients. Finally, our assumption of the close linkage between self and consciousness must be considered preliminary and hypothetical. This is because our assumption relies only on the observation of a correlation between self and consciousness in DOC patients, rather than on a direct manipulation of consciousness or self‐relatedness, which would then allow a determination of the interaction between the two. Nonetheless, our findings at least suggest that neural activity in the cACC may be crucial in linking self and consciousness together. This in turn may provide the ground for future imaging studies on self and consciousness in healthy subjects. Patients with akinetic mutism would also be of particular interest in this context since they show lesions in the ACC, including the area we here designated as cACC [Devinsky et al., 1995; Paus, 2001].
CONCLUSION
We here demonstrate for the first time the linkage of neural activity in the ACC during self‐relatedness with the degree of consciousness in DOC. Patients with DOC were able to induce signal changes during self‐relatedness in medial cortical regions like the ACC. Most importantly, signal changes during self‐relatedness in a caudal subregion of the ACC were correlated with the DOC patients' level of consciousness. Our data thus demonstrate the potential diagnostic value of neural activity in the ACC during self‐relatedness for the degree of consciousness in DOC. This may not only contribute to better understand the neural mechanisms underlying DOC, but may also be clinically relevant in providing possible neural and thus diagnostic and therapeutic markers of the deficit in consciousness in vegetative patients.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
SL is Senior Research Associate at the Fonds de la Recherche Scientifique (FRS).
REFERENCES
- Andrews K, Murphy L, Munday R, Littlewood C ( 1996): Misdiagnosis of the vegetative state: Retrospective study in a rehabilitation unit. BMJ 313: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MR, Rodd JM, Davis MH, Johnsrude IS, Menon DK, Pickard JD, Owen AM ( 2007): Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain 130 ( Part 10): 2494–2507. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25: 616–624. [DOI] [PubMed] [Google Scholar]
- de Jong BM, Willemsen AT, Paans AM ( 1997): Regional cerebral blood flow changes related to affective speech presentation in persistent vegetative state. Clin Neurol Neurosurg 99: 213–216. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behavior. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Di HB, Yu SM, Weng XC, Laureys S, Yu D, Li JQ, Qin PM, Zhu YH, Zhang SZ, Chen YZ ( 2007): Cerebral response to patient's own name in the vegetative and minimally conscious states. Neurology 68: 895–899. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Castillo GN, Paulus MP ( 2004): From sensory processes to conscious perception. Conscious Cogn 13: 323–335. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J ( 2004): The JFK coma recovery scale‐revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85: 2020–2029. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ ( 2005): Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull 131: 76–97. [DOI] [PubMed] [Google Scholar]
- Holeckova I, Fischer C, Giard MH, Delpuech C, Morlet D ( 2006): Brain responses to a subject's own name uttered by a familiar voice. Brain Res 1082: 142–152. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J ( 2006): Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44: 752–761. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Bubrovszky M, Despretz P, Pruvo JP, Steinling M, Thomas P ( 2007): Self awareness and speech processing: An fMRI study. Neuroimage 35: 1645–1653. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP ( 2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Frith U ( 2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Els T, Spreer J, Herpers M, Krause T, Moser E, Lucking CH ( 2003): Activation of a residual cortical network during painful stimulation in long‐term postanoxic vegetative state: A 15O‐H2O PET study. J Neurol Sci 212: 85–91. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler MA, Gallup GG, Pascual‐Leone A ( 2000): Self‐recognition and the right prefrontal cortex. Trends Cogn Sci 4: 338–344. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Herb E, Maurer P, Birbaumer N ( 2004): Reliability of brain responses to the own name in healthy subjects and patients with brain damage In: Moore NC, Arikan MK, editors. Brainwaves and Mind: Recent Advances. New York: Kjellberg; pp 75–80. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI ( 2009): Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Perrin F, Faymonville ME, Schnakers C, Boly M, Bartsch V, Majerus S, Moonen G, Maquet P ( 2004): Cerebral processing in the minimally conscious state. Neurology 63: 916–918. [DOI] [PubMed] [Google Scholar]
- Laureys S, Perrin F, Bredart S ( 2007): Self‐consciousness in non‐communicative patients. Conscious Cogn 16: 722–741. [DOI] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Cheng X, Mondillo K, McCaffrey D, Holroyd T, Carver F, Coppola R, Blair J ( 2009): Visual awareness, emotion, and gamma band synchronization. Cereb Cortex 19: 1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Zaccala M, Gareri F, Giordano A, Angelino E ( 2001): Long‐latency auditory‐evoked potentials in severe traumatic brain injury. Arch Phys Med Rehabil 82: 57–65. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN ( 2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM ( 2009): Modulation of cortical midline structures by implicit and explicit self‐relevance evaluation. Soc Neurosci 4: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR ( 2008): Functional neuroimaging of the vegetative state. Nat Rev Neurosci 9: 235–243. [DOI] [PubMed] [Google Scholar]
- Owen AM, Menon DK, Johnsrude IS, Bor D, Scott SK, Manly T, Williams EJ, Mummery C, Pickard JD ( 2002): Detecting residual cognitive function in persistent vegetative state. Neurocase 8: 394–403. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD ( 2006): Detecting awareness in the vegetative state. Science 313: 1402. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Perrin F, Garcia‐Larrea L, Mauguiere F, Bastuji H ( 1999): A differential brain response to the subject's own name persists during sleep. Clin Neurophysiol 110: 2153–2164. [DOI] [PubMed] [Google Scholar]
- Perrin F, Maquet P, Peigneux P, Ruby P, Degueldre C, Balteau E, Del Fiore G, Moonen G, Luxen A, Laureys S ( 2005): Neural mechanisms involved in the detection of our first name: A combined ERPs and PET study. Neuropsychologia 43: 12–19. [DOI] [PubMed] [Google Scholar]
- Perrin F, Schnakers C, Schabus M, Degueldre C, Goldman S, Bredart S, Faymonville ME, Lamy M, Moonen G, Luxen A, Maquet P, Laureys S ( 2006): Brain response to one's own name in vegetative state, minimally conscious state, and locked‐in syndrome. Arch Neurol 63: 562–569. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA ( 2009): Independence in ROI analysis: Where is the voodoo? Soc Cogn Affect Neurosci 4: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt H, Berlad I, Lavie P ( 1999): ‘Oddball’ event‐related potentials and information processing during REM and non‐REM sleep. Clin Neurophysiol 110: 53–61. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara‐Baccus TN, Johnson SC ( 2004): Metacognitive evaluation, self‐relevance, and the right prefrontal cortex. Neuroimage 22: 941–947. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Majerus S, Ledoux D, Damas P, Boly M, Vanhaudenhuyse A, Bruno MA, Moonen G, Laureys S ( 2008): Voluntary brain processing in disorders of consciousness. Neurology 71: 1614–1620. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP ( 2004): Cortical activations during judgments about the self and an other person. Neuropsychologia 42: 1168–1177. [DOI] [PubMed] [Google Scholar]
- Signorino M, D'Acunto S, Angeleri F, Pietropaoli P ( 1995): Eliciting P300 in comatose patients. Lancet 345: 255–256. [DOI] [PubMed] [Google Scholar]
- Staffen W, Kronbichler M, Aichhorn M, Mair A, Ladurner G ( 2006): Selective brain activity in response to one's own name in the persistent vegetative state. J Neurol Neurosurg Psychiatry 77: 1383–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B ( 1974): Assessment of coma and impaired consciousness. A practical scale. Lancet 2: 81–84. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Kleinschmidt A, Sterzer P, Giraud AL ( 2005): Interaction of face and voice areas during speaker recognition. J Cogn Neurosci 17: 367–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


