Abstract
Sounds provide important information about the spatial environment, including the location of approaching objects. Attention to sounds can be directed through automatic or more controlled processes, which have been well studied in the visual modality. However, little is known about the neural underpinnings of attentional control mechanisms for auditory signals. We studied healthy adults who underwent event‐related FMRI while performing a task that manipulated automatic and more controlled auditory orienting by varying the probability that cues correctly predicted target location. Specifically, we examined the effects of uninformative (50% validity ratio) and informative (75% validity ratio) auditory cues on reaction time (RT) and neuronal functioning. The stimulus‐onset asynchrony (SOA) between the cue and the target was either 100 or 800 ms. At the 100 ms SOA, RT was faster for valid than invalid trials for both cue types, and frontoparietal activation was greater for invalid than valid trials. At the 800 ms SOA, RT and functional activation depended on whether cues were informative or uninformative, and whether cues correctly or incorrectly predicted the target location. Contrary to our prediction, activation in a frontoparietal network was greater for uninformative than informative cues across several different comparisons and at both SOAs. This finding contrasts with similar research of visual orienting, and suggests that the auditory modality may be more biased toward automatic shifts of attention following uninformative cues. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: cueing, attention, orienting, FMRI, auditory
INTRODUCTION
Attention to sounds can be directed to different spatial locations automatically following an unexpected noise, or intentionally, such as when listening to a private conversation. In the auditory modality, this has been traditionally manipulated by varying how informative a spatial cue is in predicting a subsequent target's location [Mondor, 1999; Mondor and Bryden, 1992; Spence and Driver, 1994]. Several neuroimaging studies have directly compared these modes of attentional control for visual information [Kim et al., 1999; Kincade et al., 2005; Mayer et al., 2004; Peelen et al., 2004; Rosen et al., 1999], but not for sounds despite behavioral [Mondor, 1999; Mondor and Zatorre, 1995; Spence and Driver, 1994, 1998; Tassinari et al., 2002] and electrophysiological [Schröger and Eimer, 1996; Tata and Ward, 2005] evidence suggesting that distinct neuronal systems may mediate these different forms of auditory orienting.
In both the auditory and visual modality, peripherally presented, uninformative cues (i.e., predicting target location at chance) can be used to initiate automatic or exogenous shifts of attention. In contrast, the conditions necessary for generating controlled or endogenous shifts of attention are not as well defined in the auditory modality as they are for the visual modality. A centrally presented cue (e.g., an arrow) that correctly predicts a target's upcoming location on a majority of trials (75–90%) is typically used to evoke controlled/endogenous shifts of attention to visual stimuli [Müller and Rabbitt, 1989; Yantis and Jonides, 1990]. However, a centrally presented cue that contains spatial information is not readily available in the auditory modality. Although linguistic cues can be used to evoke controlled shifts of auditory attention [Mayer and Kosson, 2004; Quinlan and Bailey, 1995; Wu et al., 2007], to date they remain understudied, and likely engage cognitive and neuronal resources related to language processing. Instead, the vast majority of behavioral [Mondor, 1999; Mondor and Zatorre, 1995; Spence and Driver, 1994, 1998; Tassinari et al., 2002] and electrophysiological [Schröger and Eimer, 1996; Tata and Ward, 2005] studies have utilized lateralized tone pips to direct auditory spatial attention, manipulating whether the cues are informative or uninformative to alter how attention is allocated.
Early research was unable to reliably demonstrate orienting effects in the auditory modality [Spence and Driver, 1994], which was most likely the result of using target detection rather than localization or discrimination tasks [Rhodes, 1987; Schmitt et al., 2000; Spence and Driver, 1994], or the result of low angles of cue‐target eccentricity [Spence and Driver, 1994]. The effectiveness of auditory cues in producing shifts of attention to aural targets has since been reported in localization [Mondor and Amirault, 1998; Mondor and Zatorre, 1995; Spence and Driver, 1994; Ward, 1994] and discrimination tasks [Mondor and Breau, 1999; Mondor et al., 1998; Spence and Driver, 1994]. When lateralized auditory cues predict a subsequent target's location in a majority of trials (70–90%) during a localization task, participants intentionally use the spatial information provided by the informative cues to make controlled shifts of attention [Mondor and Zatorre, 1995; Spence and Driver, 1994]. This results in shorter RTs for valid trials for extended stimulus‐onset asynchronies (SOAs), whereas invalid cues produce costs (i.e., prolonged RTs) because attention needs to be reoriented to the target's location. In contrast, when cues are uninformative and predict target location only at chance levels (e.g., 50%), a biphasic reaction time (RT) pattern emerges due to the automatic allocation of attentional resources. RTs are faster for validly than invalidly cued trials at short SOAs (100–200 ms), followed by a reversal in cueing effects (i.e., faster RTs for invalidly than validly cued trials) called inhibition of return (IOR) at longer SOAs [Mondor, 1999; Mondor and Breau, 1999; Spence and Driver, 1998; Tassinari et al., 2002]. This biphasic response time pattern suggests that it is imperative to study attentional orienting at both short and longer SOAs to truly capture the different orienting responses that are present following informative and uninformative cues.
Frontal and posterior parietal cortices have long been implicated in spatial attention [Mesulam, 1981; Posner and Peterson, 1990]; however, their roles in different modes of attentional control have only recently been elucidated. One influential model of visual orienting [Corbetta and Shulman, 2002] implicates the bilateral intraparietal sulcus (IPs) and frontal eye fields (FEFs) in controlled shifts of attention. In contrast, automatic visuals shifts are thought to be mediated by a right‐hemisphere inferior‐frontal and temporal–parietal juncture (TPJ) network. Although the exact neural mechanisms involved in controlled and automatic visual orienting remain debated, most studies agree that there is substantial neuronal overlap between the two [Kim et al., 1999; Peelen et al., 2004; Rosen et al., 1999], with the potential for increased frontoparietal involvement during more controlled orienting processes [Kincade et al., 2005; Mayer et al., 2004]. To date, similar mechanisms of attentional control have not been directly compared within the auditory modality. Rather, the few neuroimaging studies of auditory orienting have only examined reorienting (i.e., compared invalid to valid trials) during informative cueing [Mayer et al., 2006; Wu et al., 2007], voluntary spatial, and nonspatial shifts of auditory attention following target detection [Shomstein and Yantis, 2006], or reorienting and IOR during more automatic cueing processes [Mayer et al., 2007].
The primary aim of this study was to investigate the neural networks that mediate automatic (i.e., uninformative cueing paradigm) and controlled (i.e., informative cueing paradigm) shifts of auditory attention at both short (100 ms) and longer (800 ms) SOAs using event‐related FMRI. The terms “automatic” and “controlled” orienting are used rather than exogenous and endogenous orienting to reflect paradigmatic differences commonly used to evoke different modes of attentional control in visual and auditory modalities. Based on similar studies of exogenous and endogenous orienting in the visual modality [Kincade et al., 2005; Mayer et al., 2004], we hypothesized that frontoparietal activation would be greater in the controlled (i.e., informative cues) than the automatic (i.e., uninformative cues) condition due to intentional allocation of attention to the cues. We also predicted that both informative and uninformative cues would result in faster RTs for valid than invalid trials at the 100 ms SOA, and greater FEF [Paus, 1996], supplementary FEF [Grosbras et al., 1999] and inferior parietal activation for invalid than valid trials. At the 800 ms SOA, we predicted that IOR would be present during uninformative cueing, whereas facilitation would be seen for informative cues. We also predicted that both would activate common fronto‐oculomotor networks (i.e., FEFs and supplementary FEF), but in opposite directions for invalidly and validly cued trials [Mayer et al., 2007].
EXPERIMENTAL PROCEDURES
Subjects
Twenty‐seven (14 males, 13 females) strongly right‐handed (mean Edinburgh Handedness Inventory score = 96.8% ± 8.4%) adult volunteers (mean age = 26.2 ± 3.8) were included in the study. None of the study participants were taking psychoactive prescriptive medications or had a history of neurological, psychiatric, or substance abuse disorders. Informed consent was obtained from all subjects according to institutional guidelines at the University of New Mexico. Of the 27 subjects, one subject was identified as an outlier on behavioral measures (three standard deviations) and two subjects exhibited excessive motion through visual inspection (e.g., greater than one voxel displacement on several images); these subjects were subsequently discarded from final behavioral and functional analyses.
Procedures
Subjects performed the auditory spatial cueing task while undergoing FMRI on a 1.5 Tesla Marconi‐Picker scanner at the Veterans Affairs Medical Center in Albuquerque. Subjects rested supine in the scanner with their head secured by chin and forehead straps, and foam padding to limit head motion in the head coil. Two 100 ms monaural pure tone pips with a 10 ms linear onset–offset ramp, served as the cue and target. The first tone pip (1,000 Hz) served as a spatial cue, which correctly (i.e., valid trials) predicted the location of a second target tone pip (2,000 Hz) on 50% of the cued experimental trials in the automatic‐uninformative condition and on 75% of the trials for the controlled‐informative condition (see Fig. 1). On the remainder of the trials, the cue incorrectly (i.e., invalid trial) predicted the target location. In both experimental conditions, targets were equally likely to occur in the right or left headphone.
Figure 1.
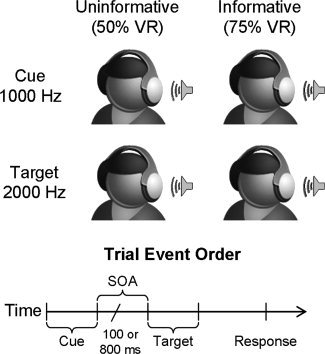
A cartoon representation of the current task. In both the informative and uninformative conditions, a lateralized tone pip was first presented as a cue (1000 Hz tone) in either the right or left headphone. This was followed by a variable SOA of either 100 or 800 ms, and the appearance of a target (2000 Hz tone) in one of the two possible locations. In the uninformative condition, the ratio of valid to invalid trials (validity ratio; VR) was 50% so that cues predicted target location at chance levels. In the informative condition, the validity ratio was increased to 75% to increase the likelihood of controlled orienting processes.
The stimuli were delivered directly into the subjects' pinnae through 3.2 m of plastic tubing, which passed through headphones and separate earplugs to attenuate scanner noise. The SOA between the cue and the target was either 100 or 800 ms, and both SOA and trial order (valid, invalid) were randomly varied across trials. To minimize neuronal activation associated with eye movements, subjects were instructed to maintain fixation throughout the task on a white central fixation cross presented on a black background. Subjects viewed the fixation cross through an Avotech vision goggle system. Although eye movements were not directly recorded in this study, previous work using eye‐tracking devices have demonstrated that healthy subjects are capable of maintaining visual fixation during visual orienting tasks within the scanner [Gitelman et al., 2000; Mesulam et al., 2001] and during auditory orienting tasks outside of the scanner [Spence and Driver, 1994]. Subjects were informed prior to the start of each condition, whether the cues would predict target location for the majority of the trials (informative) or at chance levels (uninformative). Subjects were (1) required to verbally demonstrate 100% proficiency in verbally identifying the target and cue tone pip before entering the scanner and (2) performed a brief practice version of the task prior to being scanned. Presentation software was used to control stimulus presentation, synchronization of stimulus events with the MRI scanner, and the collection of accuracy and RT data for offline analyses.
A nonferrous key‐press device was positioned directly under the subject's right hand to record responses. Subjects identified the target location by making a key press with their right middle finger for targets appearing in the right headphone and right index finger for targets appearing in the left headphone. RT was measured from the onset of the target stimulus to the completion of a key press response. Each subject's median RT (for correct trials only) was obtained for each trial type and served as the dependent measure for all behavioral analyses. Two imaging series were collected with uninformative cues, followed by three imaging series with informative cues. Uninformative cues were always collected first to avoid the establishment of a validity bias in this condition [Carter et al., 1995; Whitehead et al., 1997]. Therefore, there were a total of 64 valid and 64 invalid trials presented when the cue information was uninformative, and a total of 168 valid and 54 invalid trials when the cue information was informative. A larger number of valid informative trials were collected due to the higher validity ratio (75%) needed to induce controlled orienting.
The intertrial interval (ITI) was randomly jittered within each imaging series to allow for the best sampling of the hemodynamic response [Burock et al., 1998]. This was accomplished by randomly sorting the 2.0 s epochs (equivalent to repetition time) that could either contain one of the cueing trials or only the fixation cross. The additional constraint of a minimal ITI of 3.0 s was also applied to the data to reduce the likelihood of nonlinear summing of overlapping hemodynamic responses [Glover, 1999] so that the resulting trial length was either 4, 6, 8, or 10 s. The distribution of ITIs was randomized throughout the entire experimental protocol. This procedure also allowed for the establishment of a baseline resting state in the regression model, which corresponded to the neuronal activation associated with maintaining fixation and from the ambient scanner noise resulting from the switching of the gradients.
Functional MR Imaging
At the beginning of the scanning session, high‐resolution anatomic images were collected [echo time (TE) = 4.5 ms, repetition time (TR) = 15 ms, 25° flip angle, number of excitations (NEX) = 1, slice thickness = 1.2, FOV (field of view) = 25.6 cm, resolution = 256 × 256]. Echo‐planar images were collected using a single‐shot, gradient‐echo echo‐planar pulse sequence [TE = 37.3 ms; TR = 2,000 ms; FOV = 25.6 cm; matrix size = 64 × 64]. A total of 225 sequential echo‐planar images were collected per time series. Twenty‐one contiguous sagittal 6‐mm thick slices were selected to provide coverage of the entire brain (voxel size: 4 mm × 4 mm × 6 mm). A clustered volume acquisition technique [Hall et al., 1999] was not adopted for EPI data collection as it increases the total data acquisition time while decreasing the temporal sampling rate, which equates to fewer collected trials (i.e., reduced signal to noise) and lower temporal resolution of the hemodynamic response function [Seifritz et al., 2006].
Image Processing and Statistical Analyses
Functional images were generated using Analysis of Functional NeuroImages (AFNI) software package [Cox, 1996]. Time series images were spatially registered in both two and three‐dimensional space to minimize effects of head motion and corrected for time‐slice acquisition differences. A deconvolution analysis was used to generate one impulse response function (IRF) for each condition on a voxel‐wise basis. In addition to these regressors of interest, a polynomial term was included for each run in the equation to remove a constant term and linear drift. Each IRF was derived relative to the baseline state (fixation) and based on the first six TRs poststimulus onset. An estimate of percent signal change was obtained for the images acquired 4.0–8.0 s poststimulus onset from the cue, corresponding to the peak of the hemodynamic response function [Cohen, 1997]. Anatomical and functional images were then interpolated to volumes with 1 mm3 voxels, coregistered, converted to a standard stereotaxic coordinate space [Talairach and Tournoux, 1988], and blurred using a 4‐mm Gaussian full‐width half‐maximum filter.
Two condition (uninformative, informative) × cue validity (valid, invalid) repeated‐measure ANOVAs were performed to separately examine differences between the predicted reorienting response for informative and uninformative cues at the 100 ms SOA, and reorienting (informative) and IOR (uninformative) at the 800 ms SOA. In addition, a supplementary condition × SOA ANOVA was conducted to compare functional activation between informative and uninformative cues during valid trials only to control the effects of reorienting responses on brain activation. This analysis also enabled us to indirectly contrast the present results with a previous study that compared automatic and controlled visual orienting [Mayer et al., 2004]. A significance threshold of P < 0.005 was applied to all functional data in combination with a minimum cluster size threshold of 0.480 ml, corresponding to the volume of five original voxels, to minimize false positives [Forman et al., 1995]. This resulted in a corrected significance level of P < 0.05 for all voxels.
RESULTS
Behavioral Data
Task accuracy (mean error rate = 4.45 ± 5.9; 98.7%) was near ceiling across all participants and was therefore not subjected to further analyses. Repeated‐measure ANOVAs with cue type (uninformative, informative) and cue validity (valid, invalid) as the within‐subject factors were performed separately for each SOA (see Fig. 2A). At the 100 ms SOA, the expected main effect of cue validity was found, F 1,23 = 47.9, P < 0.001, with slower RTs for invalidly (mean = 828.36 ms) than validly (mean = 594.37 ms) cued trials. There was also a significant cue type × validity interaction, F 1,23 = 19.8, P < 0.001. Follow‐up analyses indicated that the cue validity effect (invalid–valid trials; see Fig. 2B) was significantly greater in the informative (mean = 288.8 ms) than the uninformative (mean = 179.9 ms) condition, t 1,23 = −4.4, P < 0.001.
Figure 2.
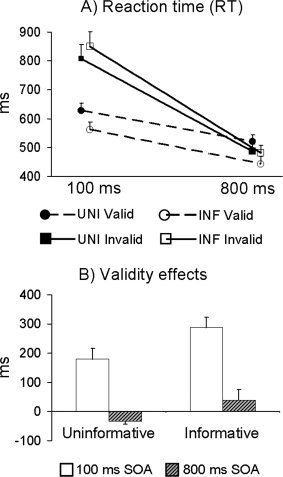
Panel A displays the reaction time data for both uninformative (UNI; filled symbols) and informative (INF; unfilled symbols) conditions for validly (circles) and invalidly (squares) cued trials. Panel B displays the validity effects (invalid–valid cues) for both conditions at either the 100 (white bars) or 800 (grey bars with black stripes) ms stimulus onset asynchrony (SOA). Results indicated that facilitation (valid < invalid) was present for both conditions at the 100 ms SOA, although the magnitude of the validity effect was larger during the informative cueing condition. At the 800 ms SOA, inhibition of return (invalid < valid) was observed during uninformative cueing, whereas facilitation was still present for the informative condition.
For the 800 ms SOA, there was a main effect of cue type, F 1,23 = 11.7, P < 0.005, with faster RTs for informative (mean = 462.24 ms) than uninformative (mean = 504.38 ms) cued trials. However, the cue type × SOA interaction was also significant, F 1,23 = 48.2, P < 0.001. Follow‐up simple effects tests, examining the cue‐validity effect, revealed the expected effects of facilitation (mean = 38.0 ms) during informative cueing and IOR (mean = −33.9 ms) during uninformative cueing, t 1,23 = −6.9, P < 0.001 (see Fig. 2B).
Functional Data
Identical cue type (uninformative, informative) × validity (valid, invalid) repeated‐measure ANOVAs were performed on the functional data separately for the 100 and 800 ms trials. These analyses identified areas that were preferentially activated by either condition or cue validity, or that depended on the combined effects of these two factors.
100 ms SOA
A main effect of cue type (Fig. 3A; Table I) showed that activation was greater for uninformative than informative trials in the right superior frontal and precentral gyrus (BA 6), right SMA proper extending into the cingulate gyrus (BA 6/24/31), left precentral gyrus (BA 6) and right cerebellar tonsil (Lobule IX). Contrary to our prediction, no regions demonstrated greater activation for informative than uninformative cues.
Figure 3.
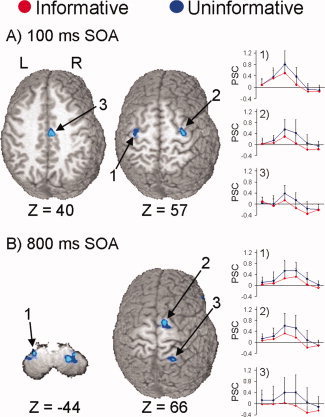
Selected regions that showed a main effect of condition at the 100 (Panel A) and 800 ms (Panel B) stimulus onset asynchronies (SOA). Panel A displays two slices corresponding to 40 and 57 mm superior (Z direction) to the origin of Talairach space. Preferential activation for uninformative cues is displayed in blue coloring and informative cues in red coloring. Key areas of activation include the (1) left precentral gyrus, (2) right superior frontal and precentral gyrus, and (3) the right supplementary motor area extending to the cingulate cortex. To the right of these brain slices, the graphs display the impulse response function for selected regions based on the first six images post stimulus onset (x‐axis, time; y‐axis, mean percent signal change) for both the conditions. Panel B (800 ms SOA) corresponds to slices −44 and 66 mm in Talairach space, and includes the (1) left cerebellar tonsil, (2) right SMA and superior frontal gyrus, and (3) right superior parietal lobule.
Table I.
Regions showing a significant main effect of cue type at the 100 or 800 ms SOA
| Region | Side | Uninformative > Informative | ||||
|---|---|---|---|---|---|---|
| BA | X | Y | Z | Vol | ||
| SOA 100 | ||||||
| Frontal Lobe | ||||||
| Superior and precentral | R | 6 | 21 | −8 | 63 | 1.185 |
| SMA and cingulate | R | 6/24/31 | 7 | −12 | 44 | 0.803 |
| Precentral | L | 6 | −28 | −15 | 55 | 0.856 |
| Cerebellum | ||||||
| Tonsil (Lobule IX) | R | 7 | −40 | −46 | 0.485 | |
| SOA 800 | ||||||
| Frontal Lobe | ||||||
| Superior and SMA | R | 6 | 12 | −4 | 65 | 0.879 |
| Inferior and insula | R | 45/13 | 43 | 23 | 10 | 1.454 |
| Parietal Lobe | ||||||
| Superior and postcentral | R | 7 | 23 | −50 | 63 | 0.481 |
| Cerebellum | ||||||
| Tonsil (Lobule VIII) | R | 27 | −50 | −44 | 1.423 | |
| L | −27 | −46 | −45 | 0.562 | ||
Side refers to the hemisphere showing activation, where L and R refer to left and right hemisphere. The Brodmann area (BA), the center of mass in Talairach coordinates (X, Y, Z) and volume (Vol) in milliliters are specified for each area of activation. All regions showed greater activation when cues were uninformative.
Analyses of the validity main effect (Fig. 4A; Table II) identified a large‐scale bilateral network for covert reorienting of attention (i.e., invalid > valid trials) that was independent of the mode of attentional control at the 100 ms SOA. Exemplary regions (see Table II for complete listing) from this network included the bilateral medial frontal gyrus (pre‐SMA and SMA proper) extending into the anterior cingulate gyrus (BAs 6/32), bilateral middle and precentral gyrus (BA 6) including the FEFs, the bilateral inferior/middle frontal and precentral gyrus (BAs 6/9), bilateral precuneus (BA 7), right inferior and superior parietal cortex (BAs 7/40), and left superior parietal cortex (BA 7). Although greater activation for valid than invalid trials was observed in several clusters, this was due to greater deactivation during invalid trials, with little or no change in baseline activation during valid trials.
Figure 4.
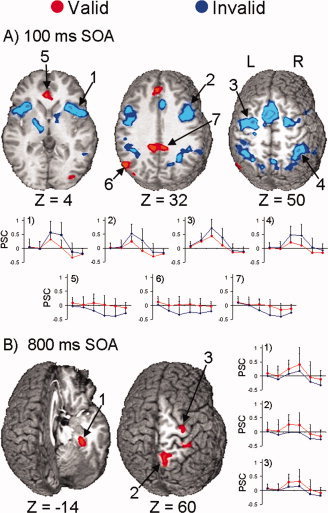
Selected regions that showed a main effect of cue validity at the 100 (Panel A) or 800 (Panel B) ms stimulus‐onset asynchrony (SOA). Both panels show select foci that exhibited greater activation in response to valid (red colorings) or invalid trials (blue colorings). Panel A (100 ms SOA) displays three slices corresponding to 4, 32, and 50 mm superior (Z direction) to the origin of Talairach space. The impulse response functions (x‐axis, time; y‐axis, mean percent signal change) for the (1) right insula, (2) right inferior and middle frontal gyrus, (3) left FEF, and (4) right posterior parietal lobe are presented, all of which showed greater activation for invalid than valid trials. The next three graphs show the percent signal change in the (5) bilateral anterior cingulate gyrus, (6) left middle temporal and angular gyrus, and (7) bilateral posterior cingulate gyrus, which showed greater activation for valid than invalid trials due to deactivation during the invalid trials. Panel B (800 ms SOA) shows two slices corresponding to −14 and 60 mm superior to the origin of Talairach space, and includes activation within the (1) right parahippocampal and fusiform gyrus, (2) right precuneus, and (3) right prefrontal gyrus. The graphs to the right show that activation was greater for validly than invalidly cued trials in these regions.
Table II.
Regions showing a significant main effect of cue validity at the 100 or 800 ms SOA
| Region | Cue Vadility | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Side | BA | Invalid > Valid | Valid > Invalid | |||||||
| X | Y | Z | Vol | X | Y | Z | Vol | |||
| 100 ms SOA | ||||||||||
| Frontal Lobe | ||||||||||
| SMA and anterior cingulate | M | 6/32 | 1 | 8 | 50 | 14.575 | ||||
| Anterior cingulate | R | 17 | 29 | −8 | 0.666 | |||||
| M | 24/32 | −3 | 33 | 10 | 1.310 | |||||
| Medial | M | 6/9 | 1 | 42 | 35 | 0.951 | ||||
| Precentral and middle | L | 6 | −31 | −6 | 53 | 8.312 | ||||
| R | 6 | 28 | −3 | 56 | 4.957 | |||||
| Precentral | L | 4 | −32 | −19 | 55 | 0.967 | ||||
| Inferior, middle and precentral | R | 6/9 | 41 | 11 | 28 | 6.275 | ||||
| L | 6/9 | −44 | 3 | 31 | 5.854 | |||||
| Anterior insula and superior temporal | R | 13/22 | 40 | 13 | 3 | 6.416 | ||||
| Temporal Lobe | ||||||||||
| Middle and superior | R | 13/22 | 55 | −43 | 8 | 0.713 | ||||
| L | 13/22 | −55 | −45 | 13 | 2.873 | |||||
| Middle and angular | L | 39 | −45 | −66 | 27 | 1.816 | ||||
| Parietal Lobe | ||||||||||
| Precuneus | R | 7 | 16 | −66 | 44 | 4.249 | ||||
| L | 7 | −14 | −67 | 49 | 0.782 | |||||
| Posterior parietal | R | 7/40 | 38 | −48 | 42 | 10.648 | ||||
| Precuneus and superior | L | 7 | −38 | −44 | 42 | 8.932 | ||||
| Superior temporal and supramariginal | R | 40 | 57 | −47 | 26 | 0.562 | ||||
| Posterior cingulate | M | 31 | −1 | −40 | 33 | 1.872 | ||||
| Subcortical | ||||||||||
| Thalamus and putamen | R | 10 | −3 | 9 | 1.087 | |||||
| L | −14 | −10 | 7 | 2.160 | ||||||
| Occipital Lobe | ||||||||||
| Middle | R | 18/19 | 35 | −81 | 5 | 0.704 | ||||
| Cerebellum | ||||||||||
| Uvula and pyramis | L | −11 | −70 | −30 | 0.566 | |||||
| Tonsil (Lobule IX) | L | −11 | −40 | −40 | 1.900 | |||||
| Lobule (VI/VIII), dentate, culmen and tonsil | R | 19 | −56 | −33 | 3.594 | |||||
| L | −29 | −56 | −37 | 4.000 | ||||||
| 800 ms SOA | ||||||||||
| Frontal Lobe | ||||||||||
| Superior and SMA | R | 6 | 10 | −16 | 67 | 0.643 | ||||
| Precentral | R | 6 | 32 | −17 | 62 | 0.519 | ||||
| Temporal | ||||||||||
| Parahippocampal and fusiform | R | 36 | 28 | −32 | −13 | 0.591 | ||||
| Parietal Lobe | ||||||||||
| Precuneus | R | 7 | 11 | −55 | 57 | 1.571 | ||||
Side refers to the hemisphere showing activation, where M, L, and R refer to midline, left, and right hemisphere. The Brodmann area (BA), the center of mass in Talairach coordinates (X, Y, Z) and volume (Vol) in milliliters are specified for each area of activation.
Figure 5A and Table III show that activation in several regions depended on the interaction of cue type and cue validity. Follow‐up simple‐main effects analyses of these interactions (P < 0.005) revealed four different activation patterns secondary to reorienting. The first pattern consisted of regions (left inferior parietal lobule/supramarginal gyrus (BA 40) and the right claustrum) that demonstrated greater activation for invalid than valid trials for informative trials only. The second pattern consisted of regions that also modulated reorienting following informative cues, but where effects were due to deactivation during invalid trials. This included the left superior and middle frontal gyrus (BA 6), the left anterior/superior temporal gyrus (BA 38), and the left parahippocampal/fusiform gyrus (BAs 36/37). The third pattern consisted of the left anterior insula, which modulated reorienting in both cueing conditions. The fourth pattern involved the bilateral pons, which uniquely modulated exogenous reorienting.
Figure 5.
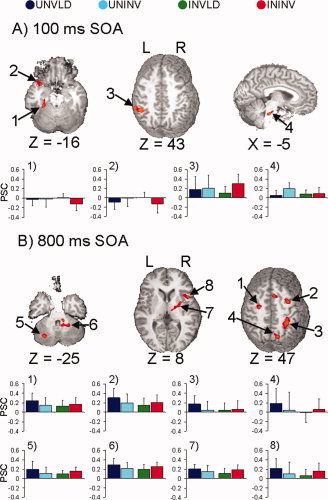
Selected regions that were dependent on both cue type and cue validity for the 100 ms (Panel A) and 800 ms (Panel B) stimulus‐onset asynchronies (SOA). In both panels, impulse response functions are graphed (x‐axis, time; y‐axis, percent signal change) for valid and invalid uninformative (UNVLD, dark blue; UNINV, cyan) and informative (INVLD, green; ININV, red) conditions. Panel A (100 ms SOA) displays two slices −16 and 43 mm superior (Z direction) and one slice −5 mm to the right of the origin in Talairach space. The graphs in Panel A show the activation patterns in the (1) left parahippocampal and fusiform gyrus, (2) anterior aspects of the left superior temporal gyrus, (3) left IPL, and (4) bilateral pons amongst the four conditions. Panel B (800 ms SOA) corresponds to slices −25, 8, and 47 mm superior to the origin. The first row of graphs display activation patterns in the four conditions that were preferentially associated with IOR, including the left and right precentral and middle frontal gyrus (Graphs 1 and 2), the right inferior parietal lobe (Graph 3), and the right precuneus (Graph 4). The last row of graphs display activation patterns in regions that modulated both modes of control, but exhibited opposite patterns of cue‐validity effects, including the left cerebellar tonsil and right culmen (Graphs 5 and 6) and the right insula (Graph 7). Data from the right putamen (Graph 8) is also displayed.
Table III.
Regions showing a cue type x cue validity interaction at the 100 or 800 ms SOA
| Region | Side | Cue type × Cue validity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BA | X | Y | Z | Vol | Cue validity | Cue type | ||||
| UNI | INF | Valid | Invalid | |||||||
| SOA 100 | ||||||||||
| Frontal Lobe | ||||||||||
| Superior and middle | L | 6 | −18 | 20 | 53 | 0.658 | VLD > INV | UNI > INF | ||
| Insula | L | 13 | −34 | 16 | 7 | 0.585 | INV > VLD | INV > VLD | ||
| Temporal Lobe | ||||||||||
| Superior | L | 38 | −38 | 11 | −20 | 0.655 | VLD > INV | |||
| Parahippocampal and fusiform | L | 36/37 | −26 | −33 | −14 | 0.664 | VLD > INV | UNI > INF | ||
| Parietal Lobe | ||||||||||
| Inferior and supramarginal | L | 40 | −44 | −44 | 40 | 1.772 | INV > VLD | |||
| Subcortical and Brainstem | ||||||||||
| Claustrum | R | 24 | 13 | 14 | 0.573 | INV > VLD | ||||
| Pons | M | 3 | −30 | −27 | 0.561 | INV > VLD | UNI > INF | |||
| SOA 800 | ||||||||||
| Frontal Lobe | ||||||||||
| Precentral | L | 6 | −38 | −7 | 55 | 0.488 | ||||
| Superior and middle | L | 6 | −20 | −5 | 62 | 1.236 | VLD > INV | UNI > INF | ||
| Superior and SMA | R | 6 | 14 | 10 | 52 | 0.618 | VLD > INV | UNI > INF | ||
| Precentral and middle | R | 6 | 32 | −4 | 53 | 1.357 | VLD > INV | UNI > INF | ||
| L | 6 | −25 | −10 | 48 | 0.812 | VLD > INV | UNI > INF | |||
| Insula and inferior | R | 13/7 | 40 | 14 | 5 | 1.426 | VLD > INV | INV > VLD | UNI > INF | |
| Parietal Lobe | ||||||||||
| Inferior and superior | R | 40/7 | 33 | −42 | 48 | 1.554 | VLD > INV | UNI > INF | ||
| Precuneus | R | 7 | 15 | −71 | 44 | 0.697 | VLD > INV | |||
| Subcortical | ||||||||||
| Thalamus | L | −15 | −22 | 0 | 0.568 | VLD > INV | UNI > INF | |||
| Putamen | R | 24 | −5 | 3 | 1.082 | INV > VLD | UNI > INF | |||
| Cerebellum | ||||||||||
| Culmen/dentate (Lobule III and VI) | R | 14 | −45 | −25 | 0.878 | VLD > INV | INV > VLD | UNI > INF | ||
| Tonsil/pyramis (Lobule VII and VIII) | L | −28 | −63 | −36 | 2.424 | VLD > INV | INV > VLD | |||
| Tonsil (Lobule VIII) | R | 21 | −59 | −38 | 0.744 | VLD > INV | UNI > INF | |||
| Tonsil (Lobule IX) | L | −14 | −39 | −41 | 0.871 | VLD > INV | UNI > INF | |||
Side refers to the hemisphere showing activation, where M, L, and R refer to midline, left, and right hemisphere. The Brodmann area (BA), the center of mass in Talairach coordinates (X, Y, Z) and volume (Vol) in milliliters are specified for each area of activation. The results of follow‐up tests are reported on the right side of the table for each SOA. Follow‐up analyses for the effect of cue validity (valid versus invalid) were tested separately for uninformative and informative cues. Likewise, the effect of attention condition (uninformative versus informative) was tested separately for valid and invalid trials. For each region, the table reports the condition that showed the greatest activation for a particular simple‐main effect test. VLD, valid; INV, invalid; UNI, uninformative cues; INF, informative cues.
800 ms SOA
Analyses of the main effect of cue type at the 800 ms SOA (Fig. 3B; Table I) showed that activation was again greater for uninformative than informative cues. These regions included the right superior frontal gyrus and SMA proper (BA 6), right inferior frontal gyrus extending into the insula (BA 45/13), right superior parietal cortex extending into the postcentral gyrus (BA 7), and bilateral cerebellar tonsil (Lobule VIII).
In contrast to the finding of greater activation for invalid than valid trials at the 100 ms SOA, Figure 4B and Table II show that at 800 ms greater activation was found for valid than invalid trials in a right‐lateralized network including the superior frontal gyrus and SMA proper (BA 6), precentral gyrus (BA 6), parahippocampal and fusiform gyrus (BAs 20/36), and precuneus (BA 7).
Figure 5B and Table III show that activation in several regions also depended on the interaction of cue type and cue validity. Follow‐up tests of these interactions revealed three different patterns of activation. The first pattern consisted of regions that uniquely modulated IOR (valid > invalid trials) during uninformative cueing, and included the left superior and middle frontal gyrus (BA 6), right superior frontal gyrus and SMA (BA 6), bilateral precentral and middle frontal gyrus extending into the FEFs (BA 6), right inferior and superior parietal lobe (BAs 40/7), right precuneus (BA 7), left thalamus, and bilateral cerebellar tonsil. In the majority of these areas, activation during valid trials was also greater for uninformative than informative cues, but only on valid trials. The second pattern consisted of regions that modulated both IOR (uninformative cues) and reorienting (informative cues) in opposite directions. Specifically, activation was greater for valid than invalid trials (IOR) during uninformative trials and greater for invalid than valid trials during informative trials. These regions included the right insula/inferior frontal gyrus (BAs 13/47), the right culmen and dentate nucleus, and left lobules VII and VIII of the cerebellum. The third pattern of activation was restricted to the right putamen, which exhibited significantly greater activation for invalid than valid trials, but only for informative cues.
Validly Cued Trials
A 2 × 2 repeated‐measure ANOVA with cue type and SOA as the repeated factors was conducted to examine differences between the uninformative and informative conditions during valid cues only. This analysis was performed to control for potential confounding effects of attentional reorienting following invalid trials; it also permitted a qualitative comparison to our results from a similar study of visual orienting [Mayer et al., 2004]. Only the main effect of cue type is reported here to eliminate redundancy with previous analyses. Figure 6 and Table IV show increased activation for the uninformative than informative condition in the bilateral middle frontal and precentral gyrus (BA 6), the right SMA proper and superior frontal gyrus (BA 6), the right insula and inferior frontal gyrus (BAs 13 and 45), the right superior temporal gyrus (BA 13), the bilateral inferior parietal lobe (BA 40), and the right cerebellar tonsil.
Figure 6.
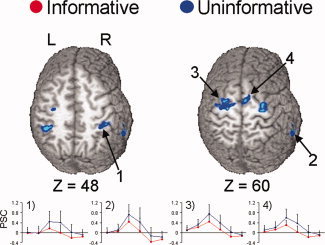
Activation associated with mode of attentional control for validly cued trails only. Similar to previous results, activation was only greater for uninformative (blue colorings) than informative (red colorings) cued trials. Activations on slices 48 and 60 mm superior to the origin of Talairach space are displayed, and impulse response functions (x‐axis, time; y‐axis, percent signal change) from the (1) right inferior parietal lobe, (2) right superior temporal sulcus, (3) left middle frontal and precentral gyrus and (4) right supplementary motor area are shown.
Table IV.
Regions showing greater activation during uninformative than informative valid‐cued trials
| Region | Side | Uninformative > Informative | ||||
|---|---|---|---|---|---|---|
| BA | X | Y | Z | Vol | ||
| Frontal Lobe | ||||||
| Middle and precentral | R | 6 | 30 | −12 | 59 | 0.693 |
| L | 6 | −23 | −9 | 58 | 2.266 | |
| SMA and superior | R | 6 | 12 | −4 | 64 | 1.636 |
| Insula and inferior | R | 13/45 | 40 | 19 | 9 | 1.322 |
| Temporal Lobe | ||||||
| Superior | R | 13 | 58 | −43 | 19 | 1.287 |
| Parietal Lobe | ||||||
| Inferior | R | 40 | 39 | −37 | 43 | 1.471 |
| L | 40 | −37 | −40 | 49 | 0.597 | |
| Cerebellum | ||||||
| Tonsil (Lobule VII and Crus 2) | R | 23 | −50 | −44 | 2.649 | |
The analyses included only validly cued trials. Side refers to the hemisphere showing activation, where L and R refer left and right hemisphere. The Brodmann area (BA), the center of mass in Talairach coordinates (X, Y, Z) and volume (Vol) in milliliters are specified for each area of activation.
DISCUSSION
As expected, we replicated previous behavioral findings of faster RTs for validly than invalidly cued trials for both short and long SOAs when auditory peripheral cues were informative [Mondor and Zatorre, 1995; Spence and Driver, 1994]. In contrast, facilitation (100 ms SOA) and IOR (800 ms SOA) were observed when cues were uninformative [Mondor, 1999; Mondor and Breau, 1999; Spence and Driver, 1998; Tassinari et al., 2002]. Collectively, the RT results indicated that participants allocated attentional resources using the experimenter‐defined modes of controlled and automatic orienting. Previous behavioral findings [Mondor, 1999] were also extended by showing that cue‐validity effects were greater for informative than uninformative cues at the 100 ms SOA, suggesting that participants purposefully and rapidly utilized cue‐target spatial information to both their detriment and benefit.
In support of our conclusions about the behavioral results, the FMRI results uncovered three important findings about the neural modulation of auditory orienting following informative and uninformative cues. First, contrary to our prediction, activation in frontal, parietal, and cerebellar regions was greater in the uninformative cue condition, which elicited more automatic orienting, than in the informative cue condition, which engaged more controlled orienting. This finding contrasts with previous reports of automatic and controlled orienting in the visual modality [Kim et al., 1999; Kincade et al., 2005; Mayer et al., 2004; Peelen et al., 2004; Rosen et al., 1999]. Second, activation was greater for invalid than valid trials at the 100 ms SOA and for valid than invalid trials at the 800 ms SOA. This finding demonstrated the dominance of reorienting (invalid > valid) and IOR mechanisms (valid > invalid) at short and long cue‐target intervals, respectively. Third, the above results were qualified by our finding of several brain‐activation patterns that depended on the combined effects of whether the cue was valid or invalid, and whether attention was being directed by informative or uninformative cues. The implications of these results are now more fully discussed.
To begin with, one key finding was that the magnitude of functional activation in response to validly or invalidly cued trials was highly dependent on the amount of time that elapsed between cues and targets. At the 100 ms SOA, invalidly cued trials produced greater activation than validly cued trials in a large‐scale, bilateral frontoparietal–temporal reorienting network. This result is consistent with and extends previous studies that separately examined reorienting to informative or uninformative cues [Mayer et al., 2006, 2007]. The bilateral activation of this network stands in contrast to imaging studies of visual attention, which typically report a right hemisphere bias for attentional reorienting following invalid cues [Arrington et al., 2000; Corbetta et al., 2000; Thiel et al., 2004]. Auditory reorienting at shorter SOAs may engage neuronal resources bilaterally because resolving the spatial location of information requires a complex integration of sound characteristics and head positioning information from both ears [Spence and Driver, 1994] compared with the direct cortical mapping of visual space.
In contrast, at the 800 ms SOA greater activation was found for validly than invalidly cued trials in the right superior frontal, right medial frontal gyrus, right parahippocampal/fusiform gyrus, and right precuneus in both the informative and uninformative cue conditions. Again, the RT correlates of these functional findings were a reduced validity effect relative to the 100 ms SOA in the informative cue condition and the emergence of IOR during the uninformative condition. The more automatic processing of auditory than visual cues may have contributed to the reduced cue validity effects on RT and functional activation, even in the informative cue condition at the 800 ms SOA [Mayer et al., 2006; Mondor, 1999; Mondor and Zatorre, 1995]. Alternatively, the reduced cue‐validity effects may reflect the operation of both controlled and automatic processes during the informative cue condition. As noted in the introduction, a true analog of visual “endogenous” orienting does not exist in the auditory modality, and the use of lateralized tone pips in the informative condition may have partially evoked a more “exogenous” response even though the validity ratio was high in the informative cue condition [Mayer et al., 2006].
Although other work [Wu et al., 2007] suggests that the modulation of attention to centrally presented linguistic cues (i.e., words) may be more similar to endogenous visual cues (e.g., centrally presented arrows), to our knowledge, neither cognitive‐behavioral nor imaging studies have directly compared attention to binaurally presented linguistic cues with lateralized tone pips using a high validity ratio, indicating that this is a relatively unstudied phenomenon. Only one behavioral study has compared both linguistic and lateralized cues, but did so under different experimental conditions [Quinlan and Bailey, 1995]. Specifically, this study used different SOAs for peripheral (Experiments 2 and 3) than linguistic (Experiment 4) cues, and never directly compared response times across the different experimental sessions. However, another imaging study reported similar frontal, temporal, and parietal activation for lateralized and centrally presented auditory stimuli, regardless of eccentricity [Zimmer et al., 2006]. Although this finding suggests that eccentricity may not play a large role in determining neuronal activation, cue eccentricity is only one aspect of controlled orienting. Consequently, additional imaging and behavioral studies are needed that directly address the question of how different parameters of attentional cueing contribute to evoking controlled auditory orienting to better understand potential parallels with endogenous orienting, which is commonly discussed in the visual attention literature.
Brain activation patterns that depended on the interaction of cue type and cue validity also differed between the two SOAs. At the 100 ms SOA, all activation patterns for both cue types were dominated by reorienting effects. Two activation patterns emerged during the informative cueing condition, one related to greater activation (left inferior parietal, right claustrum) and the other to greater deactivation (left superior frontal, parahippocampal, fusiform, and superior temporal gyrus) during invalidly than validly cued trials. Both patterns were consistent with the observed larger cue‐validity effect on RTs during informative cueing, and may reflect the neuronal cost of intentionally allocating attention to invalidly cued targets. In contrast, unique activation following uninformative invalid cues was observed only in the medial pons, potentially corresponding to auditory brainstem regions such as the cochlear nucleus and superior olivary complex [Hesselmann et al., 2001]. This finding suggests that more automatic orienting mechanisms immediately following uninformative cues may preferentially engage brainstem auditory pathways when attention is reoriented.
At the 800 ms SOA, three activation patterns emerged from the interaction, all of which were characterized by their regulation of continued reorienting during informative cues and/or the emergence of IOR during uninformative cues. Both reorienting and IOR were modulated by the right insula, inferior frontal gyrus, and bilateral cerebellum (lobules III, VI), whereas only the right putamen, which presumably mediates cognitive switching [Kimura et al., 2004] uniquely modulated reorienting. The activation of cerebellar Lobules III and VI during both reorienting (informative cue condition) and IOR (uninformative cue condition) at the 800 ms SOA suggests that the cerebellum may act to inhibit a response when reorienting attention as well as inhibit the allocation of attention or the implementation of occulomotor programs during IOR. Although a recent meta‐analysis suggested that cerebellar activation is more dependent on the sensory processing of auditory information rather than an attention mechanism per se [Petacchi et al., 2005], cerebellar pathology, such as autism and Williams syndrome, can produce attentional dysfunction [Courchesne et al., 2001; Lincoln et al., 2002]. Moreover, our results and those of others also suggest that the cerebellum may play a more direct role in modulating attention. Specifically, the cerebellum has been implicated in imaging studies of visual reorienting [Lepsien and Pollmann, 2002], sound discrimination [Belin et al., 2002], spatial localization [Zatorre et al., 2002], and auditory reorienting and IOR [Mayer et al., 2007]. Indeed, a recent study of visual and auditory shifts of attention reported that the posterior cerebellum was activated for both the auditory and visual modalities [Salmi et al., 2007]. Salmi et al. [2007] attributed the increase in cerebellar activity to sustained attentional processes resulting from their blocked FMRI design. However, current results and those of others [Lepsien and Pollmann, 2002] indicate that cerebellar activity is present in event‐related FMRI studies of visual and auditory orienting as well.
In contrast, IOR was uniquely modulated by a more distributed network, including traditional attentional areas such as the bilateral FEFs, right posterior parietal lobule, right precuneus, left thalamus, and bilateral posterior cerebellum. This is partially consistent with previous imaging studies of visual and auditory attention, which consistently implicate both frontal oculomotor and posterior parietal regions in the generation of IOR [Lepsien and Pollmann, 2002; Mayer et al., 2007]. Transcranial magnetic stimulation studies, however, suggest that frontal oculomotor regions may play a more dominant role than parietal regions in IOR [Ro et al., 2003].
Contrary to our predictions, we also found that activation was greater for uninformative than informative cues in the middle and lateral–frontal cortex at both SOAs, and in the right superior parietal cortex at the 800 ms SOA. The superior parietal lobe has been implicated in spatial and nonspatial shifts of auditory [Shomstein and Yantis, 2006] and visual attention [Yantis et al., 2002], and in attentional shifting between the two sensory modalities [Shomstein and Yantis, 2004]. Therefore, the increase in activity in the right superior parietal lobe may have been the result of the uninformative cues resulting in a more pronounced shift of attentional resources. Right superior parietal activation has also been reported in a study examining mechanisms of attentional capture to deviant auditory stimuli [Watkins et al., 2007], suggesting that this region may modulate processing of both spatial and nonspatial stimuli that automatically capture attention, irrespective of signal modality.
The analyses that were restricted to only the valid trials verified that the increased activation observed during uninformative cueing was not the result of differences in attentional reorienting. Specifically, activation was greater for uninformative than informative validly cued trials in the bilateral IPL, bilateral middle‐frontal gyrus, and several right‐lateralized fronto‐temporal cortical areas. These findings contrast with an attention study using centrally presented visual cues, in which right inferior parietal and middle‐inferior frontal activation was greater for validly cued trials that were informative (90% validly cued) than those that were uninformative (60% validly cued) [Vossel et al., 2006]. Likewise, equivalent activation [Kim et al., 1999; Peelen et al., 2004], a greater volume of activation [Rosen et al., 1999], or even unique activation of the FEFs and posterior parietal lobes [Kincade et al., 2005; Mayer et al., 2004] have been reported for controlled compared with automatic orienting in the visual modality.
Our findings of increased posterior parietal lobe activation during the uninformative cueing conditions is consistent with studies of saccadic eye movements [Mort et al., 2003], a study comparing auditory and visual shifts of attention [Salmi et al., 2007], and a prominent theory of visual attention [Corbetta and Shulman, 2002]. Specifically, lesion studies in primates and humans suggest that posterior parietal lesions impair exogenously cued saccades, but lesions to the FEF and supplementary eye fields do not [Gaymard et al., 1998; Tehovnik et al., 2000]. Other neuroimaging data suggest that automatically triggered saccades are associated with greater activity in the angular gyrus and IPL, whereas internally generated cued saccades are associated with greater FEF activity [Mort et al., 2003]. A recent FMRI study [Salmi et al., 2007] reported that superior parietal lobe activation was greater for visual than auditory shifts of attention, whereas inferior parietal lobe/TPJ and ventral prefrontal cortex activation was greater for auditory than visual shifts of attention. Although not directly testable, the authors attributed these results to modality specific differences in attentional control across the two sensory modalities based on a prominent theory of spatial attention [Corbetta and Shulman, 2002; Fox et al., 2006].
In this model, automatic orienting is thought to be mediated by a ventral network consisting of the TPJ and regions of ventral frontal cortex. In contrast, controlled orienting is thought to be mediated by more dorsal parietal structures and the FEFs. Collectively, current and previous [Salmi et al., 2007] results suggest that automatic reorienting processes may dominate in the auditory modality, even when cues are informative, whereas controlled cueing may dominate in the visual modality. However, additional research that directly compares automatic and controlled orienting in the two primary sensory modalities is needed before this preliminary hypothesis can be confirmed. Moreover, our finding of greater right ventral and bilateral superior frontal activation for uninformative than informative cues, especially when IOR was present, is somewhat inconsistent with the Corbetta model. This suggests that new models of attentional orienting are needed that account for different control mechanisms and input modalities, as well as the redundancy of some neural systems in modulating different attention mechanisms [Mesulam, 1981, 1999].
Several potential limitations of this study should be noted. First, it is possible that the increased activation in the uninformative condition was due to always presenting the uninformative cue condition first. This aspect of the study design was deliberately employed to reduce the likelihood of a validity bias in this condition [Carter et al., 1995; Whitehead et al., 1997]. However, we believe that an ordering effect explanation of the results is not compelling for several reasons. First, reorienting effects (i.e., invalid > valid) on RTs were larger in the informative than uninformative condition at both SOAs, suggesting that cueing effects were not reduced by the passage of time. Moreover, a study of visual attention that used a similar ordering of attention conditions reported greater activation for controlled (e.g., informative) than automatically (e.g., uninformative) cued trials [Mayer et al., 2004]. A second possible limitation is that the number of informative valid trials was greater than the number of uninformative valid trials due to the differential validity ratio, which was an essential feature of the study design. This is an unlikely explanation of the results, however, because one would predict greater activation for the condition with a larger number of informative cues (i.e., due to the effect of controlled attention), which was not found. Third, we did not use pure‐tone audiometry to verify subjects' normal hearing threshold; however, we did ensure that subjects were 100% proficient in distinguishing cue and target tones before starting the scanning experiment.
This study also cannot disambiguate the brain regions responsible for the processing of auditory cues from those involved in the identification of the target, as has previously been done in the visual modality [Corbetta et al., 2000; Woldorff et al., 2004]. This was because of the limited temporal resolution of the hemodynamic response at shorter SOAs, which were used to enable a comparison of informative and uninformative cues during time epochs when both were likely to produce facilitation (SOAs < 200 ms) and when uninformative cues were likely to produce IOR (SOAs > 400 ms). Moreover, distinguishing individual hemodynamic response functions during cue‐target SOAs of 3 s or less is severely compromised by nonlinear summing [Glover, 1999]. Although the use of longer SOAs enables separation of cue‐target intervals, this would change the study focus from investigating mechanisms that rapidly engage, disengage, and reorient attention to studying more sustained attentional processes, wherein the increased role of expectations and working memory demands are difficult to disentangle from attention mechanisms per se. Hence, in studies of attention such as ours, separating activation due to processing cues and targets would be better addressed by techniques with superior temporal resolution, such as magnetoencephalography and electroencephalography.
Summary
In summary, the neural modulation of automatic and controlled shifts of auditory attention was fundamentally different from similar studies of visual attention. Auditory reorienting at shorter SOAs was mediated by a bilateral frontoparietal network, irrespective of whether cues were informative or uninformative, which contrasted with the right lateralized reorienting network commonly reported for visual reorienting. Frontoparietal activation was also greater during automatic orienting after uninformative cues compared with controlled shifts of attention following informative cues. Finally, the neural control of auditory orienting partially depended on the combined effects of cue type and SOA. At the shorter SOA, classic attention networks (e.g., left middle‐frontal and inferior parietal cortex) modulated controlled reorienting, auditory processing regions (i.e., pons) modulated automatic reorienting, and somatosensory integration areas (i.e., insula) mediated reorienting in both attentional contexts in the same way. At the longer SOA, the insula and anterior cerebellum, modulated orienting for both informative and uninformative cues, but cue validity effects were in opposite directions, reflecting the operation of reorienting and IOR following informative and uninformative cues, respectively. In addition, IOR was uniquely modulated by classic attention networks (i.e., frontoparietal regions), whereas reorienting was uniquely controlled by the putamen.
Collectively, our results and those of others [Salmi et al., 2007; Shomstein and Yantis, 2006; Yantis et al., 2002] suggest that although a supramodal network may support attentional control in both primary sensory modalities, the way that this information is processed is highly dependent on the modality in which the information originates, the temporal proximity between two attended events, and behavioral expectancies about events. At shorter SOAs, auditory cues may promote a more automatic orienting response than visual cues, resulting in large reorienting effects due to the reflexive allocation of attention resources. At longer SOAs, this may be followed by increased activation for valid trials due to the presence of either an inhibitory bias or reduced reorienting effects. More automatic control mechanisms might be engaged by auditory information because it is processed more rapidly [Schröger et al., 2000] and as such, may serve as a warning system to alert organisms of changes in the environment [Posner et al., 1976], followed by a more volitional and thorough analysis of the environment via the visual system. This simple formulation, while requiring more extensive testing and evaluation, may offer a parsimonious explanation for the differential orienting effects observed between the two sensory modalities.
Acknowledgements
The authors give special thanks to Daniel Sheltraw, M.D., Ph.D., Alison Lindsay, Kim Paulson, and Carolyn Albers for technical support.
REFERENCES
- Arrington CM,Carr TH,Mayer AR,Rao SM ( 2000): Neural mechanisms of visual attention: object‐based selection of a region in space. J Cogn Neurosci 12: 106–117. [DOI] [PubMed] [Google Scholar]
- Belin P,McAdams S,Thivard L,Smith B,Savel S,Zilbovicius M,Samson S,Samson Y ( 2002): The neuroanatomical substrate of sound duration discrimination. Cogn Neuropsycho 40: 1956–1964. [DOI] [PubMed] [Google Scholar]
- Burock MA,Buckner RL,Woldorff MG,Rosen BR,Dale AM ( 1998): Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuro Rep 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Carter CS,Krener P,Chaderjian M,Northcutt C,Wolfe V ( 1995): Asymmetrical visual‐spatial attentional performance in ADHD: Evidence for a right hemispheric deficit. Biol Psychiatry 37: 789–797. [DOI] [PubMed] [Google Scholar]
- Cohen M ( 1997): Parametric analysis of fMRI data using linear systems methods. Neuroimage 6: 93–103. [DOI] [PubMed] [Google Scholar]
- Corbetta M,Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M,Kincade JM,Ollinger JM,McAvoy MP,Shulman GL ( 2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297. [DOI] [PubMed] [Google Scholar]
- Courchesne E,Karns CM,Davis HR,Ziccardi R,Carper RA,Tigue ZD,Chisum HJ,Moses P,Pierce K,Lord C,Lincoln AJ,Pizzo S,Schreibman L,Haas RH,Akshoomoff NA,Courchesne RY ( 2001): Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 57: 245–254. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Forman SD,Cohen JD,Fitzgerald M,Eddy WF,Mintun MA,Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD,Corbetta M,Snyder AZ,Vincent JL,Raichle ME ( 2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B,Ploner CJ,Rivaud S,Vermersch AI,Pierrot‐Deseilligny C ( 1998): Cortical control of saccades. Exp Brain Res 123: 159–163. [DOI] [PubMed] [Google Scholar]
- Gitelman DR,Parrish TB,LaBar KS,Mesulam MM ( 2000): Real‐time monitoring of eye movements using infrared video‐oculography during functional magnetic resonance imaging of the frontal eye fields. Neuroimage 11: 58–65. [DOI] [PubMed] [Google Scholar]
- Glover GH ( 1999). Deconvolution of impulse response in event‐related BOLD fMRI. Neuroimage 9: 416–429. [DOI] [PubMed] [Google Scholar]
- Grosbras MH,Lobel E,Van de Moortele PF,LeBihan D,Berthoz A ( 1999): An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex 9: 705–711. [DOI] [PubMed] [Google Scholar]
- Hall DA,Haggard MP,Akeroyd MA,Palmer AR,Summerfield AQ,Elliott MR,Gurney EM,Bowtell RW ( 1999): “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V,Wedekind C,Kugel H,Schulte O,Krug B,Klug N,Lackner KJ ( 2001): Functional magnetic resonance imaging of human pontine auditory pathway. Hear Res 158: 160–164. [DOI] [PubMed] [Google Scholar]
- Kim YH,Gitelman DR,Nobre AC,Parrish TB,LaBar KS,Mesulam MM ( 1999): The large‐scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage 9: 269–277. [DOI] [PubMed] [Google Scholar]
- Kimura M,Minamimoto T,Matsumoto N,Hori Y ( 2004): Monitoring and switching of cortico‐basal ganglia loop functions by the thalamo‐striatal system. Neurosci Res 48: 355–360. [DOI] [PubMed] [Google Scholar]
- Kincade JM,Abrams RA,Astafiev SV,Shulman GL,Corbetta M ( 2005): An event‐related functional magnetic resonance imaging study of voluntary and stimulus‐driven orienting of attention. J Neurosci 25: 4593–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsien J,Pollmann S ( 2002): Covert reorienting and inhibition of return: An event‐related fMRI study. J Cogn Neurosci 14: 127–144. [DOI] [PubMed] [Google Scholar]
- Lincoln A,Lai Z,Jones W ( 2002): Shifting attention and joint attention dissociation in Williams syndrome: Implications for the cerebellum and social deficits in autism. Neurocase 8: 226–232. [DOI] [PubMed] [Google Scholar]
- Mayer AR, and Kosson DS ( 2004): The effects of auditory and visual linguistic distracters on target localization. Neuropsychol 15: 248–257. [DOI] [PubMed] [Google Scholar]
- Mayer AR,Dorflinger JM,Rao SM,Seidenberg M ( 2004): Neural networks underlying endogenous and exogenous visual‐spatial orienting. Neuroimage 23: 534–541. [DOI] [PubMed] [Google Scholar]
- Mayer AR,Harrington D,Adair JC,Lee R ( 2006): The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage 30: 938–949. [DOI] [PubMed] [Google Scholar]
- Mayer AR,Harrington DL,Stephen J,Adair JC,Lee RR ( 2007): An event‐related fMRI study of exogenous facilitation and inhibition of return in the auditory modality. J Cogn Neurosci 19: 455–467. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10: 309–325. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1999): Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc London B Biol Sci 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM,Nobre AC,Kim YH,Parrish TB,Gitelman DR ( 2001): Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Mondor TA ( 1999): Predictability of the cue‐target relation and the time‐course of auditory inhibition of return. Percept Psychophys 61: 1501–1509. [DOI] [PubMed] [Google Scholar]
- Mondor TA,Amirault KJ ( 1998): Effect of same‐ and different‐modality spatial cues on auditory and visual target identification. J Exp Psychol Hum Percept Perform 24: 745–755. [DOI] [PubMed] [Google Scholar]
- Mondor TA,Breau LM ( 1999): Facilitative and inhibitory effects of location and frequency cues: Evidence of a modulation in perceptual sensitivity. Percept Psychophys 61: 438–444. [DOI] [PubMed] [Google Scholar]
- Mondor TA,Bryden MP ( 1992): Orienting of auditory spatial attention: Effects of a lateralized tone cue. Cogn Neuropsychol 30: 743–752. [DOI] [PubMed] [Google Scholar]
- Mondor TA,Zatorre RJ ( 1995): Shifting and focusing auditory spatial attention. J Exp Psychol Hum Percept Perform 21: 387–409. [DOI] [PubMed] [Google Scholar]
- Mondor TA,Breau LM,Milliken B ( 1998): Inhibitory processes in auditory selective attention: Evidence of location‐based and frequency‐based inhibition of return. Percept Psychophys 60: 296–302. [DOI] [PubMed] [Google Scholar]
- Mort DJ,Perry RJ,Mannan SK,Hodgson TL,Anderson E,Quest R,McRobbie D,McBride A,Husain M,Kennard C ( 2003): Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18: 231–246. [DOI] [PubMed] [Google Scholar]
- Müller HJ,Rabbitt PM ( 1989): Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Acta Psychologica 15: 315–330. [DOI] [PubMed] [Google Scholar]
- Paus T ( 1996): Location and function of the human frontal eye‐field: A selective review. Cogn Neuropsychol 34: 475–483. [DOI] [PubMed] [Google Scholar]
- Peelen MV,Heslenfeld DJ,Theeuwes J ( 2004): Endogenous and exogenous attention shifts are mediated by the same large‐scale neural network. Neuroimage 22: 822–830. [DOI] [PubMed] [Google Scholar]
- Petacchi A,Laird AR,Fox PT,Bower JM ( 2005): Cerebellum and auditory function: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI,Peterson SE ( 1990): The attentional system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Posner MI,Nissen MJ,Klein RM ( 1976): Visual dominance: An information‐processing account of its origins and significance. Psychol Rev 83: 157–171. [PubMed] [Google Scholar]
- Quinlan PT,Bailey PJ ( 1995): An examination of attentional control in the auditory modality: Further evidence for auditory orienting. Percept Psychophys 57: 614–628. [DOI] [PubMed] [Google Scholar]
- Rhodes G ( 1987): Auditory attention and the representation of spatial information. Percept Psychophys 42: 1–14. [DOI] [PubMed] [Google Scholar]
- Ro T,Farne A,Chang E ( 2003): Inhibition of return and the human frontal eye fields. Exp Brain Res 150: 290–296. [DOI] [PubMed] [Google Scholar]
- Rosen AC,Rao SM,Caffarra P,Scaglioni A,Bobholz JA,Woodley SJ,Hammeke TA,Cunningham JM,Prieto TE,Binder JR ( 1999): Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci 11: 135–152. [DOI] [PubMed] [Google Scholar]
- Salmi J,Rinne T,Degerman A,Salonen O,Alho K ( 2007): Orienting and maintenance of spatial attention in audition and vision: Multimodal and modality‐specific brain activations. Brain Struct Funct 212: 181–194. [DOI] [PubMed] [Google Scholar]
- Schmitt M,Postma A,de Haan E ( 2000): Interactions between exogenous auditory and visual spatial attention Q. J Exp Psychol A 53: 105–130. [DOI] [PubMed] [Google Scholar]
- Schröger E,Eimer M ( 1996): Effects of lateralized cues on the processing of lateralized auditory stimuli. Biol Psychol 43: 203–226. [DOI] [PubMed] [Google Scholar]
- Schröger E,Giard MH,Wolff C ( 2000): Auditory distraction: Event‐related potential and behavioral indices. Clin Neurophysiol 111: 1450–1460. [DOI] [PubMed] [Google Scholar]
- Seifritz E,Di Salle F,Esposito F,Herdener M,Neuhoff JG,Scheffler K ( 2006): Enhancing BOLD response in the auditory system by neurophysiologically tuned fMRI sequence. Neuroimage 29: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Shomstein S,Yantis S ( 2004): Control of attention shifts between vision and audition in human cortex. J Neurosci 24: 10702–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S,Yantis S ( 2006): Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci 26: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C,Driver J ( 1994): Covert spatial orienting in audition: Exogenous and endogenous mechanisms. J Exp Psychol Hum Percept Perform 20: 555–574. [Google Scholar]
- Spence C,Driver J ( 1998): Auditory and audiovisual inhibition of return. Percept Psychophys 60: 125–139. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988). Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tassinari G,Campara D,Benedetti C,Berlucchi G ( 2002): The contribution of general and specific motor inhibitory sets to the so‐called auditory inhibition of return. Exp Brain Res 146: 523–530. [DOI] [PubMed] [Google Scholar]
- Tata MS,Ward LM ( 2005): Spatial attention modulates activity in a posterior “where” auditory pathway. Cogn Neuropsychol 43: 509–516. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ,Sommer MA,Chou IH,Slocum WM,Schiller PH ( 2000): Eye fields in the frontal lobes of primates. Brain Res Rev 32: 413–448. [DOI] [PubMed] [Google Scholar]
- Thiel CM,Zilles K,Fink GR ( 2004): Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: An event‐related fMRI study. Neuroimage 21: 318–328. [DOI] [PubMed] [Google Scholar]
- Vossel S,Thiel CM,Fink GR ( 2006): Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage 32: 1257–1264. [DOI] [PubMed] [Google Scholar]
- Ward LM ( 1994): Supramodal and modality‐specific mechanisms for stimulus‐driven shifts of auditory and visual attention. Can J Exp Psychol 48: 242–259. [DOI] [PubMed] [Google Scholar]
- Watkins S,Dalton P,Lavie N,Rees G ( 2007): Brain mechanisms mediating auditory attentional capture in humans. Cereb Cortex 17: 1694–1700. [DOI] [PubMed] [Google Scholar]
- Whitehead R,MacKenzie T,Schliebner S,Bachorowski JA ( 1997): Effects of cue validity upon performance in the attention cueing paradigm. Percept Mot Skills 84: 787–798. [DOI] [PubMed] [Google Scholar]
- Woldorff MG,Hazlett CJ,Fichtenholtz HM,Weissman DH,Dale AM,Song AW ( 2004): Functional parcellation of attentional control regions of the brain. J Cogn Neurosci 16: 149–165. [DOI] [PubMed] [Google Scholar]
- Wu CT,Weissman DH,Roberts KC,Woldorff MG ( 2007): The neural circuitry underlying the executive control of auditory spatial attention. Brain Res 1134: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S Jonides J ( 1990): Abrupt visual onsets and selective attention: Voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform 16: 121–134. [DOI] [PubMed] [Google Scholar]
- Yantis S,Schwarzbach J,Serences JT,Carlson RL,Steinmetz MA,Pekar JJ,Courtney SM ( 2002): Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ,Bouffard M,Ahad P,Belin P ( 2002): Where is ‘where’ in the human auditory cortex? Nat Neurosci 5: 905–909. [DOI] [PubMed] [Google Scholar]
- Zimmer U,Lewald J,Erb M,Karnath HO ( 2006): Processing of auditory spatial cues in human cortex: An fMRI study. Cogn Neuropsychol 44: 454–461. [DOI] [PubMed] [Google Scholar]


