Abstract
Considerable studies reported that females are more susceptible to affective disturbances such as depression, anxiety disorder, and phobia compared to males. Based on the close relation between emotional sensitivity and liability to affective disturbances (Hofer et al. [2006]: NeuroImage 32, 854–862; Spearing [2001]: Bipolar disorder, 2nd ed. Bethesda (MA): National institute of Mental Health), this study investigated the neural mechanism underlying the females' liability to affective disturbances by hypothesizing that females are more susceptible to negative emotions than males. Event‐related potentials (ERPs) were recorded for highly negative (HN), moderately negative (MN), and neutral images in Experiment 1, and for highly positive, moderately positive, and neutral images in Experiment 2, whereas subjects (15 males and 15 females) performed a standard/deviant distinction task, irrespective of the emotional valence of deviants in both experiments. In addition to the prominent emotional reactions evoked by HN stimuli in both genders, Experiment 1 displayed conspicuous emotional responses of females to MN stimuli across N2 and P3 components, which were absent in males. In contrast, Experiment 2 demonstrated neither significant valence effect, nor significant valence by gender interaction effect at these components. Thus, although both genders are sensitive to HN stimuli, females, instead of males, are particularly susceptible to negative stimuli of lesser salience, and this female specific susceptibility does not exist to the positive stimuli. Therefore, females must be more susceptible to negative emotions in life settings, which may be one important mechanism underlying their higher prevalence of affective disturbances. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
INTRODUCTION
A growing body of studies has revealed a higher prevalence of affective disturbances such as depression, anxiety disorder, and phobia in females than in males [Nolen‐Hoeksema, 1990; Scheibe et al., 2003; Simon et al., 2006]. In early studies on epidemiology of depression, one of the most significant findings is associated with the greater rates of women versus men that suffer from unipolar depression [Boyd and Weissman, 1981; Nolen‐Hoeksema, 1987, 1900]. It was reported that the greater prevalence of depression in women emerges at approximately 10 years of age and persists until midlife [Kessler et al., 1993]. For different cultures and ethnic groups, women in reproductive age are approximately twice more likely to suffer from depressive syndromes than men [Nolen‐Hoeksema, 1987; Weissman et al., 1996]. Furthermore, a recent study by Scheibe et al. [2003] revealed that depressed females experienced more anxiety and anger than their male counterparts, and they also reported higher symptom severity on self‐report measures than males [Scheibe et al., 2003]. More recently, Simon et al. [2006] indicated that the generalized anxiety disorder (GAD), another prevalent affective disturbance, occurs at a much younger age in women than in men [Simon et al., 2006]. Additionally, women are also reported more susceptible to other affective disturbances such as phobias, dysthymia, and panic disorder relative to males [Altemus, 2006].
Therefore, it is clear that females are more susceptible to various affective disturbances than males. The reasons for this, however, are not clear enough. It has been proposed that gender differences in response to social rejection stress may be the reason underlying gender differences in depression [Stroud et al., 2002]. By measuring salivary cortisol responses to different types of stressors and the self‐reported affect ratings, Stroud et al. [2002] found that females were more physiologically responsive to social rejection stressors than males. The stronger reaction to rejection stress was suggested to contribute to the greater rates of depression in females than in males. However, this study was unable to observe a similar gender difference during post‐stress mood ratings. As depression is an affective disorder characterized by enduring experience of intense negative emotions such as sadness and pessimism [Nolen‐Hoeksema, 1991; Spearing, 2001], females' stronger reaction to social rejection challenges alone, without corresponding gender differences in emotional ratings, seems insufficient to account for females' greater rates of depression. More importantly, females were also demonstrated to be more susceptible to various affective disturbances, such as anxiety disorder, phobia, and panic disturbances other than depression [Altemus, 2006], it is likely that there exists a general mechanism that underlies the females' increased susceptibility to diverse affective disturbances.
In life settings, it appears unsurprising to see more emotional involvement in females than in males. For example, it was reported that females experience negative emotions such as anger, fear, and sadness more frequently and intensely than do males across nations and cultures [Brebner, 2003]. It is also common that females manifest prominent emotional responses to some unpredictable stimuli which, however, evoke less emotional responses in males [Li et al., 2008]. More noticeably, there are a growing number of neuroimaging studies that demonstrated greater neural activations in females versus males during negative mood induction [Hofer et al., 2006; Schirmer et al., 2004; Wrase et al., 2003]. In an fMRI study, Wrase et al. [2003] observed stronger anterior and medial cingulated gyrus activations in females versus males during the presentation of negative pictures. Similarly, adopting brain mapping measures, a recent study demonstrated that females elicited stronger neural activations in bilateral superior temporal gyrus and in cerebellar vermis during the perception of negative emotions relative to males [Hofer et al., 2006]. All this evidence implies that females may be more susceptible to emotionally negative events compared to males.
On the basis of our previous work showing differential sensitivity of the brain to negative stimuli of varying valences [Yuan et al., 2007a, b], and the reported gender differences in emotional processing [Brebner, 2003; Campanella et al., 2004; Hofer et al., 2006; Li et al., 2008; Schirmer et al., 2004; Wrase et al., 2003], this study hypothesizes that females are more susceptible to negative emotions than are males. Specifically, by manipulating the valence strength of emotional stimuli, we expect to see that: 1, both genders show pronounced emotional reactions to highly negative (HN) stimuli, as predicted by the established emotional negativity bias [Cacioppo and Gardner, 1999; Huang and Luo, 2007; Li et al., 2008]; 2, when the valence strength of negative stimuli decreases to a moderate level, the emotional reactivity remains conspicuous in females whereas that of males is unapparent, or even absent if subjects are engaged in a distracting task. 3, males and females display similar, and probably, fewer affective responses to positive stimuli of varying valences during the same task, as positive stimuli, regardless of valence strength, are known to be smaller in adaptive values than negative stimuli for both genders [Huang and Luo, 2007; Yuan et al., 2007a, b]. If these hypotheses are confirmed, it would be apparent that females are more susceptible to negative emotions than males, and negative emotions are easier to be triggered in females than in males in real life settings. This, to a large extent, may contribute to the females' greater rates of various affective disturbances.
As emotional responses are often triggered by unpredictable stimuli during nonemotional activities in natural settings [Delplanque et al., 2005; Li et al., 2008; Yuan et al., 2007a, b], this study used an implicit emotional task that did not require subjects to evaluate valence. Instead, subjects were instructed to make a standard/deviant distinction by pressing different keys, irrespective of the emotional valence of the deviants, consequently to allow emotional responses in the laboratory setting to more closely resemble nature [Li et al., 2008; Yuan et al., 2007a]. Rather than requiring a single response for the deviants, two responses were designed to mask the true purpose of the experiment, thus to avoid a “relevance‐for‐task” effect that was repeatedly reported to obscure the effect of valence on event‐related potentials (ERPs) [Carretie′ et al., 1996, 2001]. As a cultural bias for the International Affective Picture System (IAPS) has been reported in Chinese subjects [Huang and Luo, 2004], the pictures used to elicit emotional responses in this study were from the native Chinese Affective Picture System (CAPS1) [Bai et al., 2005, Yuan et al., 2007a, b]. The CAPS was established in a similar way to IAPS to better fit our native subjects in emotional inducement [Bai et al., 2005; Huang and Luo, 2007; Yuan et al., 2007a, b]. Moreover, according to Lang's [1995] theory of emotional dimensions, valence (ranging from unpleasant to pleasant) and arousal (ranging from calm to excited) are the two primary dimensions that should be considered in emotional studies. Thus, the emotional studies that address valence effect need to control for arousal influences, which were indicated to confound the valence effects on ERPs non specifically [Carretie′ et al., 1997; Johnson, 1993; Lang, 1995]. Therefore, in this study, emotional pictures were selected in such a way that the arousal level was matched across the three valence conditions in either experiment.
MATERIALS AND METHODS
Participants
Thirty paid undergraduate students participated in the experiments (15 females, 18–22 years of age, M = 20.6; 15 males, 18–23 years of age, M = 21.1). They were right‐handed, free of any reported affective disorders, and had normal or corrected to normal vision. The IRB of the School of Psychology approved the experimental procedures of the study. Each participant signed an informed consent prior to the experiment.
Stimuli
This study included two modified oddball experiments. Each experiment consisted of six blocks of 100 trials, with each block including 70 standard and 30 deviant (grouped into three conditions) pictures. All deviant pictures were taken from the CAPS.
In Experiment 1, a natural scene of cup served as the frequent standard picture and 30 pictures grouped as either HN, moderately negative (MN), or neutral served as the deviants. In Experiment 2, a natural scene of a bench served as the frequent standard picture, and 30 pictures grouped as either highly positive (HP), moderately positive (MP), or neutral served as the deviants. The sequence of standard and deviant pictures was randomized in either experiment. In Experiment 1, the three groups of deviant pictures differed significantly in valence from one another [mean: HN = 1.85, MN = 3.52, neutral = 5.46; F(2,87) = 266.19, P < 0.001; Max (HN) = 2.20, Min (MN) = 2.98] but were similar in arousal (mean: HN = 6.08, MN = 5.88, neutral = 5.86; F(2,87) = 1.49, P = 0.23). Similarly, the deviant pictures presented in Experiment 2 were significantly different in valence [mean: HP = 7.41, MP = 6.60, neutral = 5.41; F(2,87) = 96.16, P < 0.001; Max (MP) = 6.96, Min (HP) = 7.00] but were similar in arousal (mean: HP = 5.58, MP = 5.40, neutral = 5.37; F(2,87) = 1.29, P = 0.28). All pictures were identical in size and resolution (15 cm × 10 cm, 100 pixels per inch). In addition, the luminance of the pictures was matched across valence conditions during either experiment, and the contrast of the monitor was set to a constant value across experiments and subjects.
Procedures
Subjects were seated in a quiet room at approximately 150 cm from a computer screen with the horizontal and vertical visual angles below 6°. Prior to the experiment, all subjects were told that the purpose of the study was to investigate their ability to make a fast response selection, and their ability to inhibit the prepotent response to the frequent picture when the deviant appears. At the end of each of the six blocks, accuracy rates for both standard and deviant stimuli were given to the subjects as a feedback of their performance. Each trial was initiated by a 300 ms presentation of a small black cross on the white computer screen for subjects to fix their gaze on the center of the display throughout the block; subsequently, a blank screen whose duration varied randomly between 500 and 1500 ms was followed by the onset of picture stimulus. Each subject was instructed to press the “F” key on the keyboard as accurately and quickly as possible if the standard picture appeared, and to press the “J” key if the deviant picture appeared. The stimulus picture was terminated by a key pressing, or was terminated when it elapsed for 1,000 ms. Therefore, each subject was informed that their responses must be made under 1,000 ms. Each response was followed by 1,000 ms of a blank screen. Pretraining with 10 practice trials was used before formal experiment to familiarize subjects with the procedure, and the standard picture in pretraining was the same as that in the subsequent experiment whereas the deviants for pretraining were neutral pictures that were not selected for the formal experiment. During an interview session conducted immediately after the experiment, each subject was debriefed with respect to the characteristics of the deviant stimuli. On the basis of their reports of the detection of emotional contents, they were further required to estimate the percentage of deviant images that were emotionally relevant amongst all deviant stimuli. All subjects achieved 100% accuracy on 10 practice trials prior to the formal experiment. Each subject participated in both experiments, with the order of the two experiments counterbalanced between subjects.
ERP Recording and Analysis
The EEG was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (Brain Products), with the references on the left and right mastoids [average mastoid reference, Luck, 2005] and a ground electrode on the medial frontal aspect. The vertical electrooculograms (EOGs) were recorded supra‐ and infra‐orbitally at the left eye. The horizontal EOG was recorded from the left versus right orbital rim. The EEG and EOG were amplified using a DC of ∼100 Hz bandpass and continuously sampled at 500 Hz/channel. All inter‐electrode impedance was maintained below 5 kΩ. Averaging of ERPs was computed off‐line; trials with EOG artifacts (mean EOG voltage exceeding ±80 μV) and those contaminated with artifacts due to amplifier clipping, peak‐to‐peak deflection exceeding ±80 μV were excluded from averaging.
EEG activity for correct response in each valence condition was overlapped and averaged separately. ERP waveforms were time‐locked to the onset of stimuli and the average epoch was 1,200 ms, including a 200 ms prestimulus baseline. As shown by ERPs' grand average waveforms and topographical map (see Fig. 1 and 2), prominent central and frontal N2 components, and broadly distributed P3 components were elicited during all the three valence conditions in both experiments, irrespective of gender. Moreover, Experiment 1 exhibited clear ERP differences during HN, MN, and neutral conditions across genders, and these differences were largest at central and frontal sites. Conversely, Experiment 2 showed no obvious ERP differences between the three valence conditions in both groups. Thus, the following 15 electrode sites [Fz, FC3, FC4, FCz, FC1, FC2, C1, C2, Cz, C3, C4 (11 frontal and central sites), CP3, CP4, CPz, and Pz (four central‐parietal and parietal sites)] were selected for statistical analysis of the P3 component (350–450 ms), and N2 (230–290 ms) was analyzed at the 11 frontal and central sites. In addition, because clear N1 and P2 activity was observed mainly at central and more frontal sites in both experiments, early N1 (80–130 ms) and P2 (130–190 ms) components were analyzed at the 11 anterior sites, too. A repeated measures analysis of variance (ANOVA) on the amplitude and latency of each component was conducted with Valence (three levels: HN, MN, and neutral for Experiment 1; HP, MP, and neutral for Experiment 2) and electrode sites as within‐subjects factors, and gender as between‐subjects factor. As this study focused on the effect of gender on affective reactions to deviant stimuli of varying valences, our analyses mainly focused on gender by valence interaction effect. The degrees of freedom of the F‐ratio were corrected according to the Greenhouse‐Geisser method.
Figure 1.
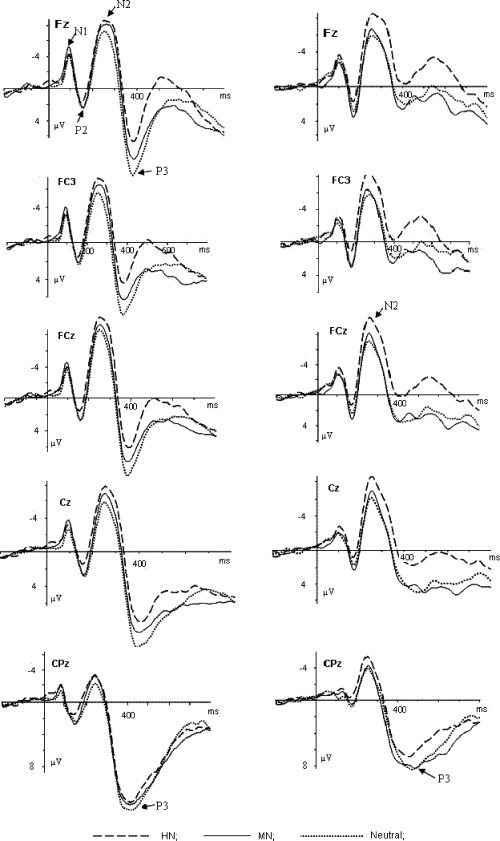
The averaged ERPs during HN (dashed lines), MN (solid lines), and neutral (dotted lines) conditions at Fz, FC3, FCz, Cz, and CPz in Experiment 1. The left column: averaged ERPs for females; the right column: averaged ERPs for males.
Figure 2.
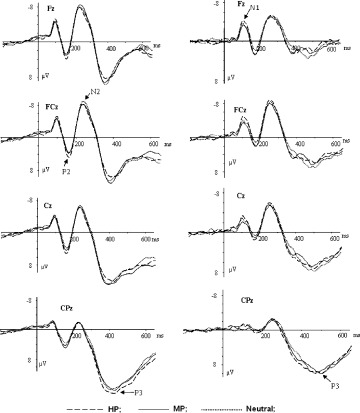
The averaged ERPs during HP (dashed lines), MP (solid lines), and neutral (dotted lines) conditions at Fz, FCz, Cz, and CPz in Experiment 2. The left column: averaged ERPs for females; the right column: averaged ERPs for males.
RESULTS
Behavioral Performance
The false responses or responses exceeding time limits were rare, as nearly all subjects achieved 100% accuracy rates for both standard and deviant stimuli in both experiments. A two‐way ANOVA on RT data in Experiment 1 (Valence as within‐subjects factor whereas Gender as between‐subjects factor) demonstrated no significant valence effect, or valence by gender interaction effect [F(2,56) = 0.96, P = 0.37; F(2,56) = 0.48, P = 0.58]. The averaged RTs in females were 518.30 ± 53.01 ms for HN, 519.50 ± 59.86 ms for MN, and 504.62 ± 63.77 ms for neutral conditions, whereas those in males were 553.20 ± 76.72 ms for HN, 554.08 ± 78.56 ms for MN and 551.19 ± 58.80 ms for neutral conditions. Additionally, the main effect of gender was not significant, either [F(1,28) = 2.93, P = 0.098]. Similarly, the two‐way ANOVA on RT data in Experiment 2 (valence as within‐subjects factor whereas gender as between‐subjects factor) showed no significant valence effect, or valence by gender interaction [F(2, 56) = 1.14, P = 0.33; F(2,56) = 0.56, P = 0.55]. The averaged RTs were 549.87 ± 72.52 ms for HP, 550.544 ± 74.80 ms for MP, and 547.86 ± 53.62 ms for neutral conditions in males and were 523.64 ± 43.33 ms for HP, 526.17 ± 47.99 ms for MP, and 511.29 ± 54.96 ms for neutral conditions in females. Also, the main effect of gender was nonsignificant in this experiment [F(1, 28) = 2.04, P = 0.16]. Thus, behavioral response to the deviant pictures was not affected by differences in valence in both genders. This suggests that the experimental design did preoccupy subjects in standard/deviant categorization task, thus effectively masked the true objective of the study.
ERP Analysis
Experiment 1
As illustrated in Figure 1, N1 and P2 activity was observed during all three valence conditions in Experiment 1. Nevertheless, no gender by valence interaction effects were observed at these components, thus, N1 and P2 components were not further addressed. In N2 component, the repeated measures ANOVA conducted on the amplitudes demonstrated highly significant main effects for Valence and Electrode [F(2,56) = 12.56, P < 0.001; F(10,280) = 11.68, P < 0.001], and also a marginal effect of valence by gender interaction [F(2,56) = 2.98, P < 0.07]. The pairwise comparison for the valence main effect showed that HN condition elicited larger amplitudes than MN [F(1, 29) = 10.86, P < 0.01], and neutral [F(1,29) = 24.28, P < 0.001] conditions while the latter two conditions did not show significant amplitude differences [F(1,29) = 1.94, P = 0.19]. Larger amplitude was elicited in medial frontal‐central sites (FC1, FCz, and FC2) than other sites. The simple effects analyses on the interaction effect between valence and gender demonstrated a significant valence effect in females [F(2,28) = 8.48, P < 0.01]. Larger amplitudes were elicited during HN condition than during MN condition [F(1,14) = 5.12, P < 0.05], which, in turn, elicited larger amplitudes than neutral condition [F(1,14) = 9.06, P < 0.01]. Males also showed a significant valence effect [F(2,28) = 4.82, P < 0.05], and HN condition elicited larger amplitudes than MN and neutral conditions [F(1,14) = 5.36, P < 0.05; F(1,14) = 11.86, P < 0.01]. However, in contrast with females, males showed no significant amplitude differences between MN and neutral conditions [F(1,14) = 0.18, P = 0.68]. Neither other significant main effects or interactions were found for N2 amplitudes, and nor were the main effects or interactions for N2 latency.
The repeated measures ANOVA on P3 amplitudes showed significant main effects at valence and electrode sites [F(2,56) = 16.67, P < 0.001; F(14,392) = 25.22, P < 0.001], and also significant gender by valence interaction effect [F(2,56) = 3.97, P < 0.05] and electrode by valence interaction effect [F(28,784) = 7.35; P < 0.001]. Central‐parietal and parietal sites recorded larger P3 amplitudes than central and frontal sites, and the amplitude differences during HN, MN, and neutral conditions were largest at central and frontal sites (Fig. 1 and 2). HN condition elicited smaller amplitudes than MN and neutral conditions [F(1,29) = 12.14, P < 0.01; F(1,29) = 37.79, P < 0.001]. In addition, the amplitude differences during MN and neutral conditions were also significant [F(1,29) = 4.32, P < 0.05]. Simple effects analyses of the interaction effect between gender and valence demonstrated a significant valence effect in females [F(2,28) = 16.81, P < 0.001], and HN condition elicited smaller amplitudes than MN [F(1,14) = 9.03, P < 0.01], which, in turn, elicited significantly smaller amplitude than the neutral condition [F(1,14) = 8.32, P < 0.02]. Males also showed a significant valence effect [F(2,28) = 4.44, P < 0.05], with amplitudes smaller in HN condition than in MN [F(1,14) = 4.69, P < 0.05], and neutral conditions [F(1,14) = 10.68, P < 0.01]. However, in contrast with females, male subjects showed no significant amplitude differences between MN and neutral conditions [F(1,14) = 0.154, P = 0.701]. Significant gender effect was also observed [F(1,28) = 5.46, P < 0.05], and the amplitudes elicited in females were larger than those in males across the valence conditions. As the main effect of gender signals gender differences in ERP response to rare deviants in general, and it has been found that novelty processing is modulated by gender non‐specifically [Nagy et al., 2003]. Thus, this effect probably stems from gender differences in novelty processing and would not be discussed later. Lastly, a main effect of latency at electrode sites was significant [F(14,392) = 7.20, P < 0.001], and posterior sites elicited longer latency than anterior sites. No other main or interaction effects were seen at this component.
Experiment 2
The ERP averages of Experiment 2 were displayed in Figure 2. Similar to those in Experiment 1, clear N1 and P2 components were observed during each of the three valence conditions in Experiment 2, and again, there were no significant interaction effects between valence and gender at these early components (Fig. 2). In N2 component, the repeated measures ANOVA on the amplitudes demonstrated a significant main effect of electrode sites [F(10,280) = 14.27, P < 0.001], and larger amplitude was elicited in medial frontal‐central sites (FC1, FCz, and FC2) than in other sites. However, the main effect of valence [F(2,56) = 0.48, P = 0.51], as well as the interaction effect between valence and gender [F(2,56) = 0.28, P = 0.63], was nonsignificant. No other main or interaction effects were observed at this component. Moreover, the repeated measures ANOVA on P3 amplitudes demonstrated no significant main effect of valence [F(2,56) = 1.81, P > 0.1] or significant interaction effect between valence and gender[F(2,56) = 2.09, P>0.1]. In addition, there were significant main effects of amplitudes at gender [F(1,28) = 5.31, P < 0.05] and electrode sites [F(14,392) = 37.98, P < 0.001].Females elicited larger amplitudes than did males across valence conditions, and P3 amplitudes were larger at posterior‐parietal sites than at central and more frontal sites. No other significant main effects or interactions were found at this component.
Therefore, although both genders elicited prominent emotional reactions during HN condition, females, instead of males, exhibited increased brain responses to MN stimuli than to neutral stimuli across N2 and P3 components in Experiment 1. On the other hand, this gender difference was absent during the processing of positive stimuli of varying valences. Specifically, males and females were similar in emotional reactions during HP condition, and so were their emotional reactions during MP condition (Experiment 2). The MN minus neutral difference wave in females displayed conspicuous negative deflections at 250–500 ms interval, which covers both N2 and P3 components (Fig. 3). Additionally, the voltage amplitudes of the difference wave distributed broadly over central and frontal cortical areas (Fig. 3b).
Figure 3.
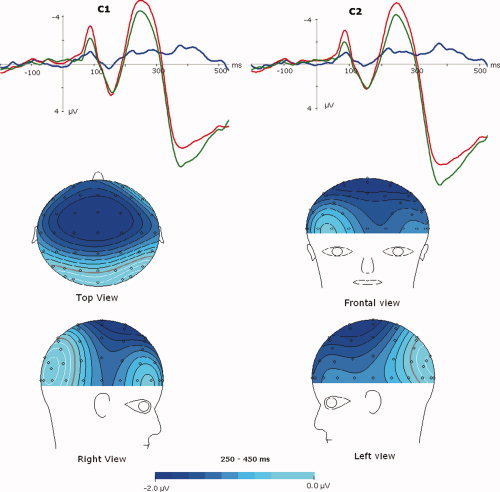
The averaged ERPs for MN (dotted lines) and neutral (solid lines) conditions in Experiment 1, and the MN minus neutral difference waveform (bold lines) at C1 and C2 in females. Bottom: topographical maps of voltage amplitudes for the MN‐neutral difference waveform during 250–450 ms post stimulus. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Post‐Experiment Debriefing
Experiment 1
In the post‐experiment interview session, all subjects reported that they detected some emotionally salient scenes during the deviant condition, and they just ignored the emotionality of these stimuli and focus on the standard/deviant distinction. Typically, female subjects reported that a number of deviant pictures were aversive; whereas males reported that some deviant images were unpleasant. Moreover, in response to the question “what percentage of the deviant stimuli in your impression were unpleasant?,” the averaged estimation was higher in females than in males [F(1,28) = 40.7, P < 0.001]. The averaged estimation value was 61.5% in females and 38.9% in males. This suggests that females must have elicited more affective reactions than males in the experiment.
Experiment 2
The post‐experiment debriefing indicates that all subjects in this experiment focused on the standard/deviant distinction, and it took more time for them to respond to deviant versus standard stimuli. However, to our surprise, rare subjects, irrespective of gender, reported that they detected the appearance of deviant images that were emotionally arousing in this experiment.
DISCUSSION
Though clear N1 and P2 components were evoked during each valence condition in Experiment 1, no interaction effects between gender and valence were found at these components. This suggests that the early visual and perceptual processes, as indexed by N1 and P2 respectively, were similar between genders [Mangun, 1995; Thorpe et al., 1996], and gender differences in affective reactions to negative stimuli of varying valences may occur at later time points. Central‐frontal N2 are accepted to index attentional orientation to novel, potentially salient events [Nagy et al., 2003; Yuan et al., 2007a]. Experiment 1 showed an interaction effect between gender and valence on N2 amplitudes. As predicted by the established emotional negativity bias [Cacioppo and Gardner, 1999; Huang and Luo, 2007; Li et al., 2008], both genders elicited greater amplitudes during HN versus neutral conditions, probably because HN stimuli are biologically important [Yuan et al., 2007a, b]. Despite similar emotional reactions in females and males during HN condition, only females exhibited increased neural activity for MN stimuli than for neutral stimuli at this component. This suggests that females, instead of males, detected the emotional negativity of MN stimuli, and probably allocated more attentional resources to MN stimuli than to neutral stimuli at this stage. Therefore, females displayed clear emotional reactions to mild negative stimuli at the early stage. This contrasts with the absence of an attentional bias for MN stimuli in males (see Fig. 1), suggesting that males, different from females, elicited no affective reactions to MN stimuli at this stage.
The gender differences in affective reactions to negative stimuli of varying valences, however, were most noticeable during P3 interval. This study observed a significant gender and valence interaction effect on P3 amplitudes in Experiment 1. Previous literature has revealed that the P3 signals cognitive evaluation of stimuli's meaning. The cognitive evaluation is a deliberate controlled process in which the significance of emotional information is evaluated. Probably, the stimuli with different emotional values could be discriminated clearly during this stage [Ito et al., 1998; Yuan et al., 2007a]. In this study, despite similar emotional effects elicited in males and females during HN condition, females, instead of males, exhibited increased negativity during MN versus neutral conditions at P3 interval. Thus, at the higher cognitive stage, although males and females showed similar emotional reactivity to HN stimuli, a gender‐related dimorphism arises as the emotional salience of negative stimuli decreases to a moderate level. Females still displayed conspicuous emotional responses to MN stimuli (Figs. 1 and 3) which, however, elicited fewer responses in males.
Thus, whether at early attentional or later evaluative stages, females exhibited conspicuous affective reactions to MN stimuli. Conversely, being engaged in the standard/deviant distinction, male subjects exhibited little emotional reactivity to MN stimuli throughout the information processing stream, without additional resources devoted for emotional processing of MN stimuli whose valence intensity is distinct from that of neutral stimuli [Bai et al., 2005; Yuan et al., 2007a]. Therefore, females exhibited prominent emotional reactivity during both HN and MN conditions, whereas males exhibited emotional responses only during HN condition. Apparently, females elicited more emotional reactions than males in the present experiment (presumably, twice as often as those in male subjects), which is in agreement with the results of post experiment debriefing that suggests more emotional involvement in females versus males.
On the other hand, Experiment 2 demonstrated neither significant main effect of valence nor significant valence by gender interaction effect. This indicates that both HP and MP stimuli elicited similar neural activity as neutral stimuli in this experiment, where subjects were engaged in the standard/deviant distinction. Therefore, positive stimuli, irrespective of valence strength, elicited fewer affective reactions across genders, probably because positive stimuli are harmless to individual survival and smaller in adaptive values [Yuan et al., 2007a]. More importantly, there were no gender differences in affective reactions to positive stimuli of varying valences, suggesting that both groups manifested similar, and fewer responses to the emotional loads of positive stimuli during HP and MP conditions. Obviously, females are not more susceptible to mild positive stimuli than males. This, together with results of Experiment 1, suggests that females are not more reactive to moderately emotional stimuli in general; instead, they are only more susceptible to negative stimuli of lesser saliency compared to males.
In this study, the onset sequence of standard and deviant pictures was randomized in both experiments. As well, deviant pictures of which valence condition to be presented was determinedly randomly throughout the experiments. Thus, the occurrence of emotional stimuli in each condition (HN and MN in experiment 1, HP and MP in experiment 2) could not be predicted before stimulus onset. On the other hand, as the presentation of deviant stimuli was rare (30%), and deviant stimuli was composed of three valence conditions in either experiment, the occurrence of emotional events in each condition is rare (10%). This manipulation made emotional responses in current experiments closely resemble those in natural settings, where emotional reaction often occurs unpredictably and is triggered by some accidental stimulations during activity irrelevant to affective assessment [Delplanque et al., 2005; Yuan et al., 2007a].
Because both genders exhibited similar reactions to emotional loads of MP stimuli, and MN images elicited conspicuous emotional responses only in females during a task where emotional activity closely resemble nature, it is highly likely that females are more susceptible to negative emotions than males in life settings. As indicated by this study, females, instead of males, are particularly liable to negative emotions because of their increased susceptibility to mild negative events in life situations. Obviously, the majority of negative life events we meet are MN rather than extremely negative. For instance, the occurrence of daily hassles must be more frequent than that of traffic accidents. Thus, females must be suffering from negative emotions more frequently relative to males in life settings, which coincides with the behavioral report that females experience more frequent negative emotions across cultures [Brebner, 2003]. In previous studies, gender differences in neural response to emotional stimuli were suggested to be associated with the gender‐related prevalence of affective disturbances [Hofer et al., 2006; Kemp et al., 2004]. Similarly, with this gender‐specific vulnerability to negative events of lesser saliency, females would be more prone to affective disturbances relative to males, as frequent and intense negative emotions were implicated in the occurrence of affective disturbances [Hofer et al., 2006; Nolen‐Hoeksema, 1991; Spearing, 2001; Kemp et al., 2004]. Thus, it is not surprising to see higher prevalence of various affective disturbances in females when compared with those in males.
However, females' higher prevalence of affective disturbances is a complex phenomenon whose development is modulated by both biological and sociocultural variables [Altemus, 2006; Kornstein, 2000 and 2003]. It has been suggested that culturally determined behaviors and experiences such as dieting and sexual abuse may promote development of affective disorders such as depression and anxiety disorder in women [Altemus, 2006]. In addition, females are subject to more fluxes in reproductive hormones across life span than males [Altemus, 2006; Roca et al., 2003], and the brain neurochemical systems that modulate anxiety and fear are functionally altered during pregnancy and lactation [Altemus, 2006; Altemus et al., 2004]. All these factors may contribute to the higher prevalence of affective disturbances in females. Therefore, the present finding that females are more susceptible to negative events of lesser saliency should not be considered as the sole mechanism underlying this phenomenon. Nevertheless, the subjects recruited in the present study were college students during their early adulthood (18–23 years old) whose ages were matched across genders, and all subjects were physiologically and psychologically healthy and free of history of affective disorders. As well, no female subjects were in pregnancy, lactation or had traumatic experiences such as sex abuse. Thus, the female susceptibility to negative emotions should be regarded as a general mechanism underlying the liability of females to various affective disturbances throughout the life development, rather than only during some specific periods [e.g. pregnancy, lactation, or menopausal periods; Kessler et al., 1993; Nolen‐Hoeksema and Girgu, 1994, Angold et al., 1999].
CONCLUSION
This study observed that, in addition to pronounced affective reactions of both genders to HN stimuli, females, instead of males, are particularly susceptible to negative stimuli of lesser saliency. In contrast, males and females reacted similarly to emotionally positive stimuli, irrespective of valence intensity. This indicates that females are more susceptible to negative emotions, which may be an important mechanism underlying their increased liability to affective disturbances.
Footnotes
1The standardized CAPS was developed in Key Laboratory of Mental Health, Chinese Academy of Sciences to avoid the cultural bias of emotional inducement found in Chinese participants when IAPS was used. The CAPS introduced a number of pictures characterized by oriental natural scenes and faces. The development method of this native emotional picture system is similar to that of IAPS. For the CAPS development, originators first collected over 2,000 pictures of various contents, and finally kept 852 pictures most of which are typical of Chinese cultures for the normative ratings. Chinese college students (gender‐matched) were recruited to rate the valence, arousal, and dominance by a self‐report 9‐point rating scale for the 852 pictures of the system. The pretest for this system showed that CAPS is reliable across individuals in emotional inducement (the between‐subjects reliability scores were 0.982 for valence and 0.979 for arousal). More details about CAPS are accessible in Bai et al. [2005].
REFERENCES
- Altemus M ( 2006): Sex differences in depression and anxiety disorders: Potential biological determinants. Horm Behav 50: 534–538. [DOI] [PubMed] [Google Scholar]
- Altemus M,Fong J,Yang R,Damast S,Luine V,Ferguson D ( 2004): Changes in CSF neurochemistry during pregnancy. Biol Psychiatry 56: 386–392. [DOI] [PubMed] [Google Scholar]
- Angold A,Costello E,Erkanli A,Wothman C ( 1999): Pubertal changes in hormone levels and depression in girls. Psychol Med 29: 1043–1053. [DOI] [PubMed] [Google Scholar]
- Bai L,Ma H,Luo YJ, et al. ( 2005): The development of native Chinese affective picture system. Chin Mental Health J 19: 719–722. [Google Scholar]
- Boyd JH,Weissman MM ( 1981): Epidemiology of affective disorders. A reexamination and future directions. Arch Gen Psychiatry 38: 1039–1046. [DOI] [PubMed] [Google Scholar]
- Brebner J ( 2003): Gender and emotions. Personality Individual Diff 34: 387–394. [Google Scholar]
- Cacioppo JT,Gardner WL ( 1999): Emotion. Annu Rev Psychol 50: 191–214. [DOI] [PubMed] [Google Scholar]
- Campanella S,Rossignol M,Mejias S, et al. ( 2004): Human gender differences in an emotional visual oddball task: An event‐related potentials study. Neurosci Lett 367: 14–18. [DOI] [PubMed] [Google Scholar]
- Carreti'e L,Iglesias J,Garcia T,Ballesteros M ( 1996): N300, P300 and the emotional processing of visual stimuli. Electroencephalogr Clin Neurophysiol 103 298–303. [DOI] [PubMed] [Google Scholar]
- Carretie L,Iglesias J,Garcia T ( 1997): A study on the emotional processing of visual stimuli through event‐related potentials. Brain Cogn 34: 207–217. [DOI] [PubMed] [Google Scholar]
- Carreti'e L,Mercado F,Tapia M ( 2001): Emotion, attention, and the ‘negativity bias’, studied through event‐related potentials. Int J Psychophysiol 41: 75–85. [DOI] [PubMed] [Google Scholar]
- Delplanque S,Silvert L,Hot P,Sequeira H ( 2005): Event‐related P3a and P3b in response to unpredictable emotional stimuli. Biol Psychol 68: 107–120. [DOI] [PubMed] [Google Scholar]
- Hofer A,Siedentopf CM,Ischebeck A, et al. ( 2006): Gender differences in regional cerebral activity during the perception of emotion: A functional MRI study. NeuroImage 32: 854–862. [DOI] [PubMed] [Google Scholar]
- Huang YX,Luo YJ ( 2004): Native assessment of international affective picture system. Chin Mental Health J 9: 631–634. [Google Scholar]
- Huang YX,Luo YJ ( 2007): Attention shortage resistance of negative stimuli in an implicit emotional task. Neurosci Lett 412: 134–138. [DOI] [PubMed] [Google Scholar]
- Ito TA,Larsen JT,Smith NK,Cacioppo JT ( 1998): Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. J Pers Soc Psychol 75: 887–900. [DOI] [PubMed] [Google Scholar]
- Johnson R Jr. ( 1993): On the neural generators of the P300 component of the event‐ related potential. Psychophysiology 30: 90–97. [DOI] [PubMed] [Google Scholar]
- Kemp AH,Silberstein RB,Armstrong SM,Nathan PJ ( 2004): Gender differences in the cortical electrophysiological processing of visual emotional stimuli. NeuroImage 16: 632–646. [DOI] [PubMed] [Google Scholar]
- Kessler RC,McGonagle KA,Swartz M,Heath AC,Eaves LJ ( 1993): Sex and depression in the National Comorbidity Survey. I. Lifetime prevalence, chronicity and recurrence. J Affect Disord 29: 85–96. [DOI] [PubMed] [Google Scholar]
- Kornstein SG ( 2003): Gender, depression, and antidepressant treatment. Prim Psychiatry 10: 58–61. [Google Scholar]
- Kornstein SG,Schatzberg AF,Thase ME, et al. ( 2000): Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157: 1445–1452. [DOI] [PubMed] [Google Scholar]
- Lang PJ ( 1995): The emotion probe, studies of motivation and attention. Am Psychol 5: 372–385. [DOI] [PubMed] [Google Scholar]
- Li H,Yuan JJ,Lin CD ( 2008): The neural mechanism underlying the female advantage in identifying negative emotions: An event‐related potential study. NeuroImage 40: 1921–1929. [DOI] [PubMed] [Google Scholar]
- Luck SJ ( 2005): An introduction to event‐related potentials and their neural origins In: Luch SJ, editor. An Introduction to the Event‐Related Potential Technique. Cambridge, MA: MIT; p 107. [Google Scholar]
- Mangun GR ( 1995): Neural mechanisms of visual selective attention. Psychophysiology 32: 4–18. [DOI] [PubMed] [Google Scholar]
- Nagy E,Potts GF,Loveland KA ( 2003): Sex‐related ERP differences in deviance detection. Int J Psychophysiol 48: 285–292. [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema S ( 1987): Sex differences in unipolar depression: Evidence and theory. Psychol Bull 101: 259–282. [PubMed] [Google Scholar]
- Nolen‐Hoeksema S ( 1990): Sex Differences in Depression. Stanford, CA: Stanford University Press. [Google Scholar]
- Nolen‐Hoeksema S ( 1991): Responses to depression and their effects on the duration of depressed mood. J Abnorm Psychol 100: 569–582. [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema S,Girgus J ( 1994): The emergence of gender differences in depression during adolescence. Psychological Bull 115: 424–443. [DOI] [PubMed] [Google Scholar]
- Roca CA,Schmidt PJ,Altemus M,Deuster P,Danaceau MA,Putnam K,Rubinow DR ( 2003): Differential menstrual cycle regulation of hypothalamic‐pituitary‐adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 88: 3057–3063. [DOI] [PubMed] [Google Scholar]
- Scheibe S,Preuschhof C,Cristi C,Bagby RM ( 2003): Are there gender differences in major depression and its response to antidepressants? J Affect Disord 75: 223–235. [DOI] [PubMed] [Google Scholar]
- Schirmer A,Zysset S,Kotz SA,Cramon DY ( 2004): Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage 21: 1114–1123. [DOI] [PubMed] [Google Scholar]
- Simon NM,Zalta AK,Worthington JJ,Hoge EA, et al. ( 2006): Preliminary support for gender differences in response to fluoxetine for generalized anxiety disorder. Depress Anxiety 23: 373–376. [DOI] [PubMed] [Google Scholar]
- Spearing M ( 2001): Bipolar Disorder, 2nd ed. Bethesda (MA): National institute of Mental Health. [Google Scholar]
- Stroud LR,Salovey P,Epel ES ( 2002): Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiatry 52: 318–327. [DOI] [PubMed] [Google Scholar]
- Thorpe S,Fize D,Marlot C ( 1996): Speed of processing in the human visual system. Nature: 381: 520–522. [DOI] [PubMed] [Google Scholar]
- Weissman MM,Bland RC,Canino GJ, et al. ( 1996): Cross‐national epidemiology of major depression and bipolar disorder. JAMA 276: 293–299. [PubMed] [Google Scholar]
- Wrase J,Klein S,Gruesser SM,Hermann D,Flor H,Mann K,Braus DF,Heinz A ( 2003): Gender differences in the processing of standardized emotional visual stimuli in humans: A functional magnetic resonance imaging study. Neurosci Lett 348, 41–45. [DOI] [PubMed] [Google Scholar]
- Yuan JJ,Zhang QL,Chen AT,Li H, et al. ( 2007a): Are we sensitive to valence differences in emotionally negative stimuli? Electrophysiological evidence from an ERP study. Neuropsychologia 45: 2764–2771. [DOI] [PubMed] [Google Scholar]
- Yuan JJ,Li H,Chen AT,Luo YJ ( 2007b): Neural correlates underlying humans' differential sensitivity to emotionally negative stimuli of varying valences: An ERP study. Prog Nat Sci 17: 115–121. [Google Scholar]


