Abstract
Our lab has long been interested in the development of methods for the creation of enantioenriched all-carbon quaternary stereocenters. Historically, our interest has centered on palladium-catalyzed allylic alkylation, though recent efforts have moved to include the study of iridium catalysts. Whereas palladium catalysts enable the preparation of isolated stereocenters, the use of iridium catalysts allows for the direct construction of vicinal stereocenters via an enantio-, diastereo-, and regioselective allylic alkylation. This account details the evolution of our research program from inception, which focused on the first iridium-catalyzed allylic alkylation to prepare stereodyads containing a single quaternary center, to our most recent discovery that allows for the synthesis of vicinal quaternary centers.
Keywords: iridium, asymmetric catalysis, enantioselective, allylic alkylation, quaternary stereocenters, umpolung
Graphical Abstract
1. Introduction
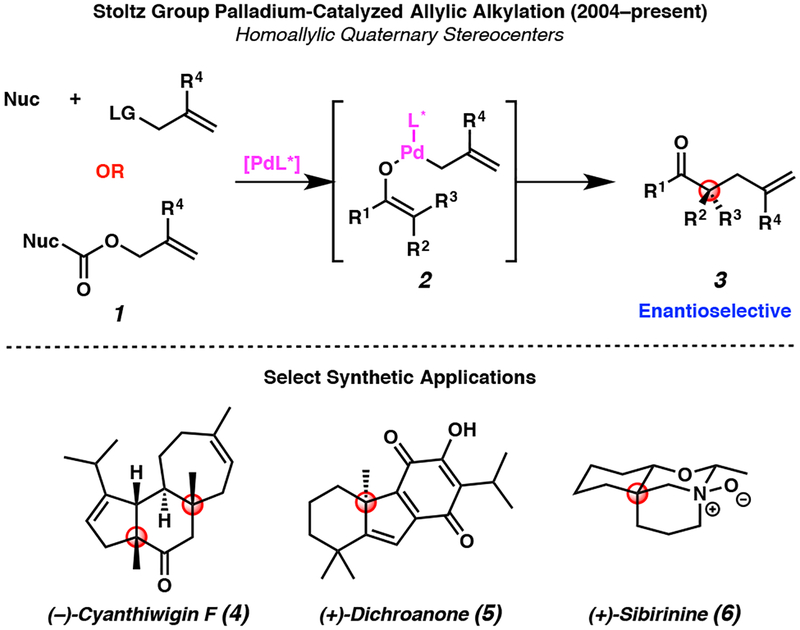
To enable our ongoing research program focused on the synthesis of complex natural products, we became interested in developing robust methods for the synthesis of stereochemically rich building blocks containing quaternary stereocenters. As a result, our group has disclosed a range of technologies for the enantioselective preparation of all-carbon quaternary stereocenters.1 Perhaps most notably, we have developed a general asymmetric palladium-catalyzed allylic alkylation method to provide access to a wide variety of products 3 bearing a homoallylic quaternary stereocenter (Figure 1, top).2 Over the past decade, we have expanded this methodology to a broad array of substrates 13 and applied this chemistry to facilitate expedient total syntheses of a variety of natural products, including (−)-cyanthiwigin F (4),4 (+)-dichroanone (5),5 and (+)-sibirinine (6, Figure 1, bottom).6
Figure 1.
Stoltz group contributions to palladium-catalyzed allylic alkylation methodology and application in natural product total synthesis
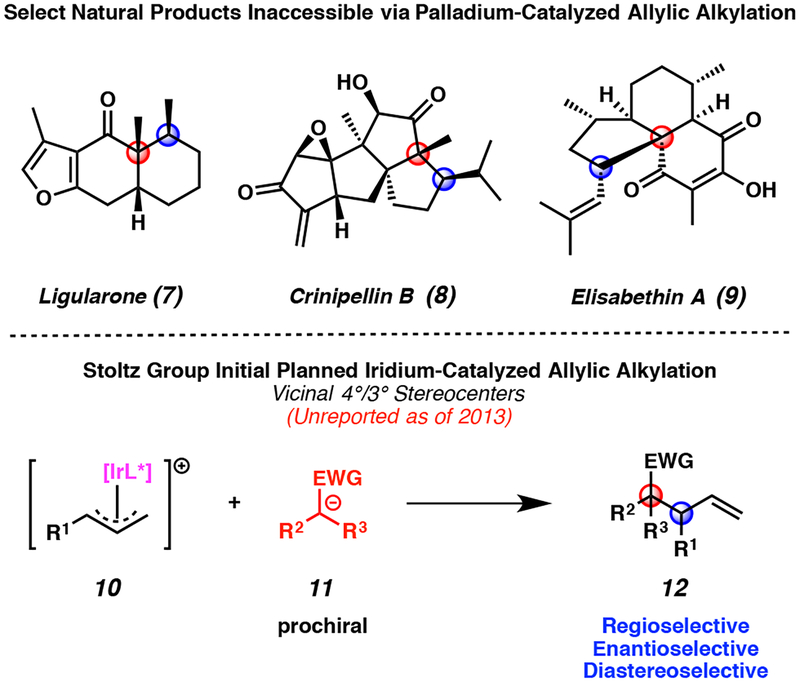
Despite its extensive substrate tolerance, our palladium-catalyzed allylic alkylation technology is limited to the synthesis of isolated stereocenters. Thus, to date this methodology is not amenable to the direct preparation of vicinal stereocenters, which are found in a variety of synthetic targets such as ligularone (7), crinipellin B (8), and elisabethin A (9, Figure 2, top). Inspired by these diverse and numerous stereodyad-containing natural products, we sought to expand our stereoselective allylic alkylation research program to include iridium-catalyzed processes that do enable the construction of such vicinal stereocenters 12 (Figure 2, bottom).
Figure 2.
Stereodyad-containing natural products as inspiration for the development of novel iridium-catalyzed allylic alkylation technology
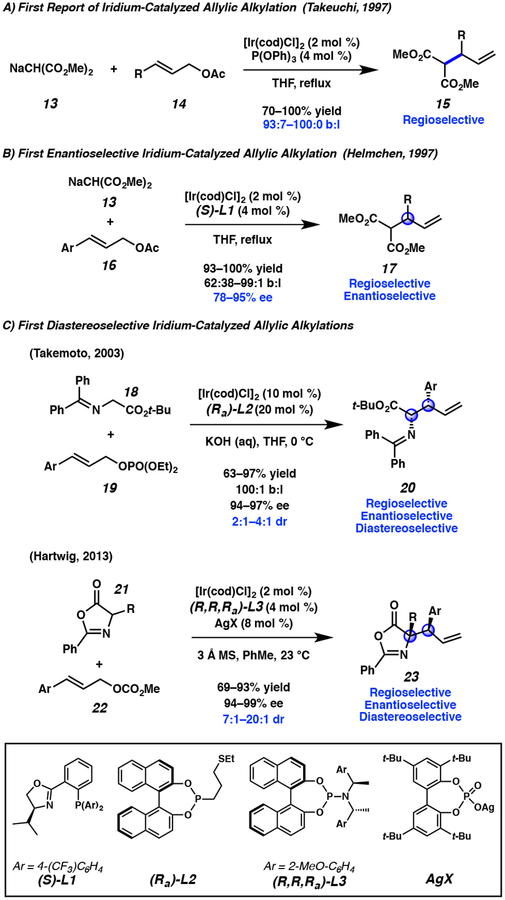
While the aforementioned palladium-catalyzed allylic alkylation has been under exploration since the 1960s,7 iridium-catalyzed allylic alkylation is a relatively new area of research. Takeuchi reported the first example of iridium-catalyzed allylic alkylation in 1997 using malonate nucleophiles 13, demonstrating that iridium catalysts can provide high branched selectivity (branched to linear ratio, b:l) in contrast to palladium catalysts that favor the synthesis of linear products (Figure 3A).8,9 Shortly thereafter, Helmchen reported that the reaction could be rendered enantioselective with the inclusion of chiral phosphinooxazoline ligand L1 (Figure 3B).10 In 2003, Takemoto disclosed the first report of a diastereoselective iridium-catalyzed allylic alkylation reaction, wherein prochiral nucleophiles 18 were utilized to form vicinal trisubstituted and tertiary stereocenters 20 (Figure 3C, top).11 It was then a decade later before the next report of diastereoselective iridium-catalyzed allylic alkylation was disclosed, which this time enabled the preparation of vicinal tertiary and tetra-substituted stereocenters 23 (Figure 3C, bottom).12
Figure 3.
Timeline for the development of iridium-catalyzed allylic alkylation prior to the Stoltz group’s entry into the field
Despite these seminal reports, when our group entered the field in 2013, there were no examples of an iridium-catalyzed allylic alkylation reaction to form a single all-carbon quaternary stereocenter, let alone a stereodyad containing an all-carbon quaternary stereocenter.13 Therefore, we were highly intrigued by the possibility of developing new iridium-catalyzed allylic alkylation technology that would allow for the preparation of sterically congested enantioenriched quaternary stereocenters, and thus open the door to the synthesis of a range of new natural product targets (Figure 2, top).
2. Synthesis of Vicinal Tertiary and All-Carbon Quaternary Stereocenters via Enantio- and Diastereoselective Iridium-Catalyzed Allylic Alkylation
2.1. Cyclic Nucleophiles14,15
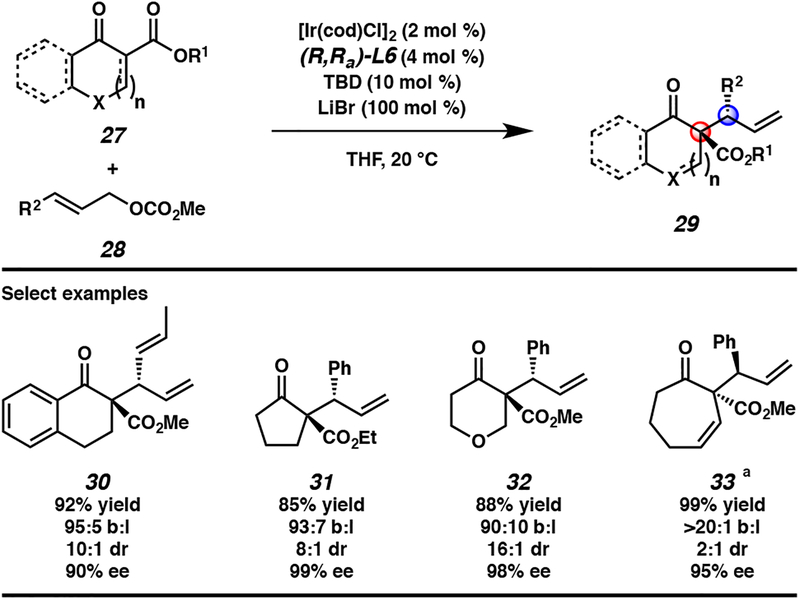
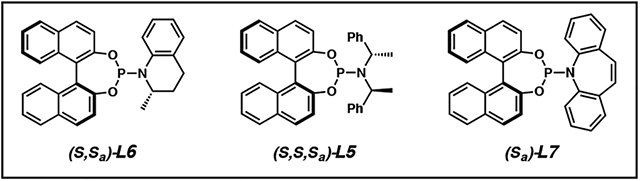
In our quest to develop the first iridium-catalyzed allylic alkylation reaction to form vicinal tertiary and all-carbon quaternary stereocenters, we initially selected cyclic prochiral enolates as our nucleophiles in order to obviate the need to control enolate geometry during the allylic alkylation reaction.1,16 Thus, our preliminary exploration into this research area commenced with tetralone 24. We rapidly found that the standard phosphoramidite ligands L4 and L5, which were at the time typically utilized in iridium-catalyzed allylic alkylation with enolate equivalents, were not amenable to the synthesis of a quaternary stereocenter, as neither provided high levels of diastereoselectivity (Table 1, entries 1 and 2). Inspired by the work of the You group on the allylic alkylation of heterocycles,17 we evaluated the effect of MeTHQPhos (L6) as the ligand and found that it provided product 26 with a high degree of regio-, diastereo-, and enantioselectivity (entry 3).
Table 1.
Development of conditions for the iridium-catalyzed allylic alkylation reaction of cyclic nucleophiles forming vicinal tertiary and all-carbon quaternary stereocentersa
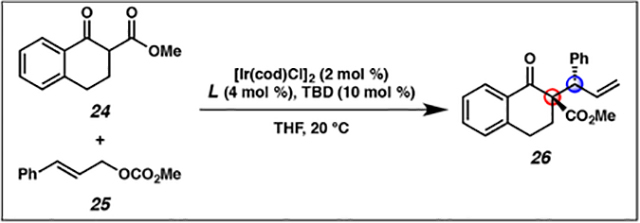 | |||||
|---|---|---|---|---|---|
| Entry | L | Additive (X mol %) |
b:lb | drb | ee (%)c |
| 1 | L4 | NaH (200) | >95:5 | 1:1 | 99 |
| 2 | L5 | NaH (200) | >95:5 | 1:2 | 32 |
| 3 | L6 | NaH (200) | 95:5 | >20:1 | 98 |
| 4 | L6 | - | 80:20 | 11:1 | 96 |
| 5 | L6 | LiCl (100) | 88:12 | 14:1 | 98 |
| 6 | L6 | LiBr (100) | 95:5 | >20:1 | >99 |
 | |||||
Reactions performed with 0.1 mmol of 25, 0.2 mmol of 24 in THF (0.1M) and allowed to proceed to complete consumption of 25.
Determined by 1H NMR and UHPLC-MS analysis of the crude mixture.
Determined by chiral HPLC analysis of the major diastereomer.
TBD = 1,3,5-triazabicyclo[4.4.0]dec-5-ene.
At this point in our optimization efforts, we realized that the reaction conditions could be simplified if the exogenous base was removed and the carbonate leaving group was instead relied on as the stoichiometric base required for enolate formation. Toward this end, sodium hydride was excluded from the reaction, but we observed diminished selectivity (Table 1, entry 4). Previous literature reports documented the beneficial effects of halide salts on selectivity in iridium-catalyzed allylic alkylation reactions.18 When this strategy was explored in our reaction conditions, LiCl provided only minor enhancement in selectivity (entry 5). However, LiBr led to a pronounced enhancement in both conversion and selectivity, providing us with our optimized reaction conditions (entry 6).
Upon investigating the substrate scope of the developed transformation, we found the reaction amenable to a wide variety of substitution on both the allylic electrophile and the nucleophile (Scheme 1).14 Though, as a general trend, branched regioselectivity increases with greater carbocation stability on the allylic electrophile, thus electron-rich aromatics 28 (R2 = EDG-Ar) provide higher branched to linear ratios. Additionally, the reaction is not limited to aryl substitution on the allylic electrophile, as both heteroaryl and alkenyl substitution provide the corresponding products in good yields and selectivities (e.g., 30). A key limitation to this initial report is that alkyl-substituted allylic electrophiles are not tolerated and instead proceed with poor conversions and selectivities. With respect to nucleophile 27, we found that the aryl ring of tetralone 24, used as the optimization substrate, could be removed without diminishing reactivity or stereoselectivity of the reaction (e.g., as seen in products 31 and 32). Finally, we observed that unsaturated nucleophiles 27 are tolerated, allowing for access to products bearing a 1,5-diene (e.g., 33) for subsequent functionalization, though the addition of LiBr was not required in these reactions.15
Scheme 1.
Enantio- and diastereoselective iridium-catalyzed allylic alkylation of cyclic nucleophiles 27, aReaction performed with (S,Sa)-L6 without LiBr.
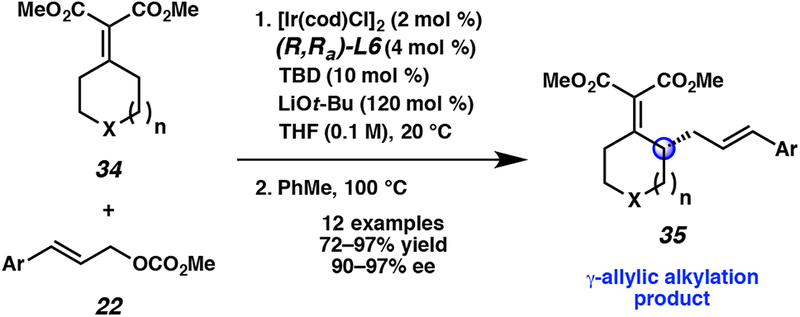
Upon further optimization, we found that the reactions of unsaturated nucleophiles (e.g., 34) progressed with a higher degree of selectivity when LiOt-Bu was utilized as a base additive in place of LiBr.15 These allylic alkylation conditions followed by a subsequent thermal Cope rearrangement, allow unsaturated compounds 34 to formally undergo allylic alkylation at the γ-position to produce compounds such as 35 with a high degree of enantioselectivity (Scheme 2).
Scheme 2.
Iridium-catalyzed allylic alkylation/Cope rearrangement sequence
2.2. Acyclic Nucleophiles19
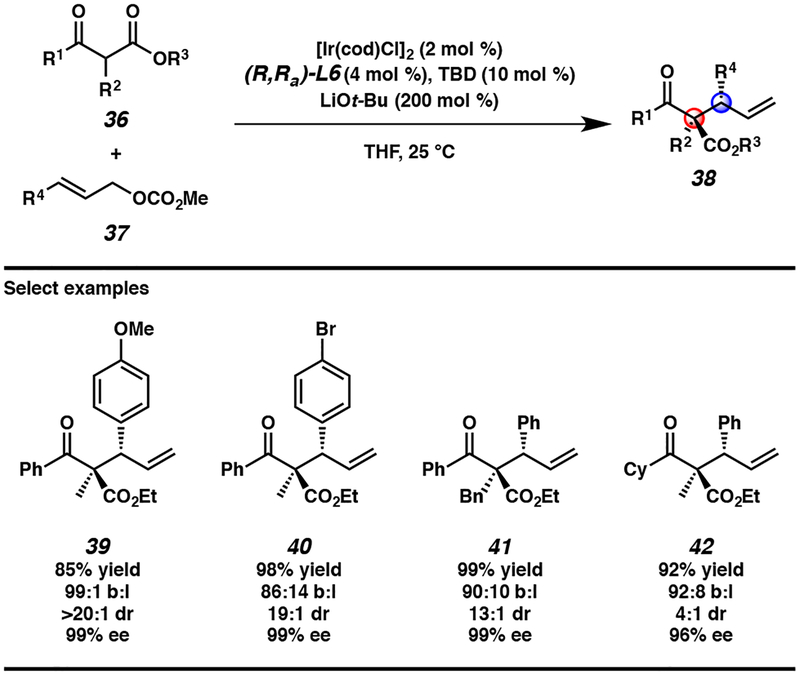
In looking to expand our iridium-catalyzed allylic alkylation reaction from cyclic enolate nucleophiles to acyclic variants, we fortuitously discovered that our conditions for cyclic nucleophiles provide satisfactory reactivity and selectivity for acyclic nucleophile 36 (Scheme 3). With further optimization though, we ultimately found LiOt-Bu to be the optimal additive as it led to shorter reaction times. With respect to the substrate scope of this transformation, we again observed that reaction regioselectivity is directly affected by the electronics of the aryl functionality on allylic electrophile 37, however, enantio- and diastereoselectivities remain consistently excellent. Moreover, heteroaryl-substituted allylic electrophiles are tolerated, as are various substituents at the α-position of acyclic β-keto ester nucleophile 36. However, in contrast to the cyclic variants, we noted pronounced decreases in the diastereomeric ratio of product 38 when ketone 36 was not an aryl ketone (e.g., 39 versus 42).
Scheme 3.
Enantio- and diastereoselective Iridium-catalyzed allylic alkylation of acyclic nucleophiles 36
2.3. Alkyl-Substituted Electrophiles20
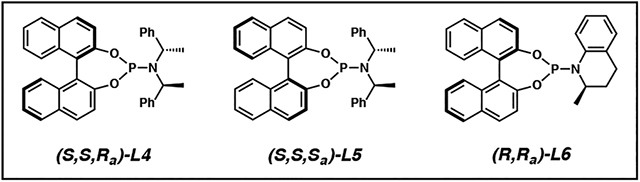
After disclosing methods for the synthesis of vicinal tertiary and all-carbon quaternary stereocenters using cyclic,14 acyclic,19 and extended enolate15 nucleophiles in iridium-catalyzed allylic alkylation, we noted that none of these protocols tolerated the use of alkyl-substituted electrophiles. In fact, at the time, no transition metal-catalyzed process enabled the construction of an alkyl-substituted stereodyad between neighboring tertiary and quaternary carbon atoms. Realizing that many synthetic targets require the installation of this specific stereodyad, we became intrigued with the possibility of further developing our enantio- and diastereoselective iridium-catalyzed allylic alkylation chemistry to allow for the use of allylic electrophiles bearing an sp3-hybridized substituent.
Based on our prior efforts in optimizing iridium-catalyzed allylic alkylation reactions, we anticipated that we would need to explore new combinations of additives and ligands in order to effect selectivity in the reaction between tetralone nucleophile 24, utilized in our seminal report14 (Table 1), and alkyl-substituted electrophile 43 (Table 2). Indeed, when we employed our previously reported conditions for cyclic or acyclic nucleophiles,14,19 we observed excellent yields and good diastereoselectivities but poor branched to linear ratios (Table 2, entries 1 and 2). Given the trends established in our earlier work where regioselectivity increased with increasing carbocation stability of the iridium π-allyl cation, these findings were not surprising as the methyl group is less stabilizing than an aryl substituent. We hypothesized that decreasing carbocation stability results in slow equilibration between iridium π-allyl diastereomers. Thus, we sought to employ LiCl as an additive, which has been proposed to facilitate iridium π-allyl equilibration and therefore improve regio- and enantioselectivity in iridium-catalyzed allylic alkylation reactions.18 While we did not see a marked effect by adding LiCl alone (entry 3), we postulated that by rendering all anions in solution congruent, the effect of the chloride additive would be more pronounced. This hypothesis led us to replace the methyl carbonate leaving group of crotyl electrophile 43 with chloride, and in doing so we noted a significant increase in reaction regioselectivity, though the diastereoselectivity was now only moderate (entry 4).
Table 2.
Optimization of iridium-catalyzed allylic alkylation reaction of alkyl-substituted electrophile 43a
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | L | Base (200 mol %) |
Additive (mol %) |
LG | yield (%)b | b:lc | drc | ee (%)d |
| 1 | L6 | - | LiBr (100) | OCO2Me | 100 | 55:45 | 6.4:1 | - |
| 2 | L6 | LiOt-Bu | - | OCO2Me | 85 | 34:66 | 5.3:1 | - |
| 3 | L6 | LiOt-Bu | LiCI (100) | OCO2Me | 69 | 50:50 | 7.2:1 | - |
| 4 | L6 | LiOt-Bu | LiCI (100) | CI | 94 | 86:14 | 4.8:1 | - |
| 5 | L6 | Proton sponge | LiCI (100) | CI | 100 | 93:7 | 7.9:1 | 66 |
| 6 | L7 | Proton sponge | LiCI (100) | CI | 46 | 95:5 | 6.0:1 | 96 |
| 7 | L7 | Proton sponge | LiCI (400) | Cl | 78 | 94:6 | 6.7:1 | 97 |
Reactions performed on 0.1 mmol of 43, 0.2 mmol of 24 in THF (0.1 M) for 18 h.
1H NMR yield of the mixture of diastereomers based on internal standard.
Determined by 1H NMR analysis of the crude reaction mixture.
Determined by chiral SFC analysis.
Proton sponge = 1,8-bis(dimethylamino)naphthalene.
We next sought to improve the diastereoselectivity of the reaction by investigating additional bases other than LiOt-Bu, as we had found in our prior work that bases had a significant effect on selectivity.14,15,19 After an extensive screen of bases, we identified that the bulky amine base, proton sponge, allowed our transformation to proceed in good yield, regio- and diastereoselectivity, but poor enantioselectivity (entry 5). In order to increase the enantioselectivity of our desired reaction, we moved to employ bulkier phosphoramidite ligand L7, which led to excellent enantioselectivity, though at the expense of yield (entry 6). Ultimately, we discovered that we could improve the reaction yield, with no effect on selectivity, by increasing the amount of the inexpensive LiCl additive to 400 mol % (entry 7). This extensive fine-tuning of reaction parameters is included here as an illustrative example of how altering one reaction partner (e.g., the substituent on the electrophile) in an iridium-catalyzed allylic alkylation reaction necessitates complete reoptimization of the system.
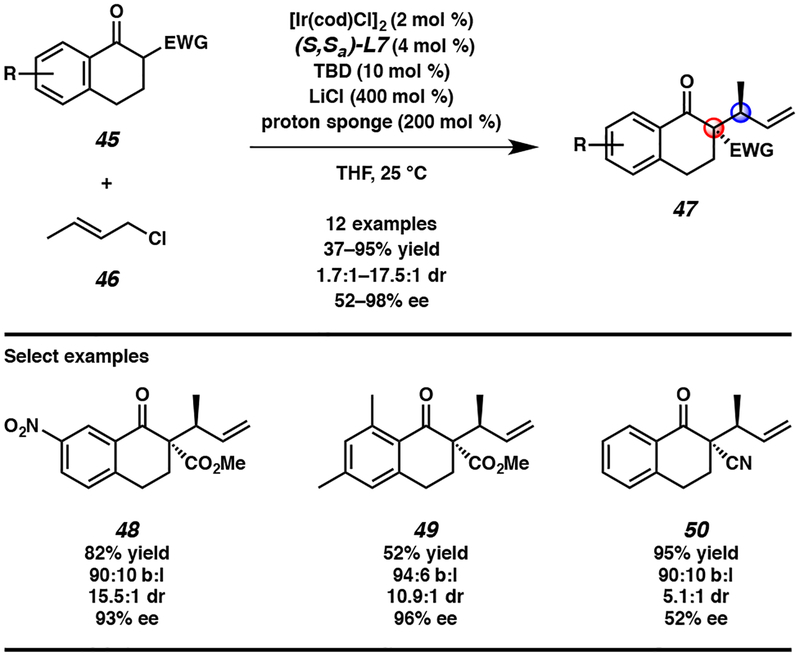
The optimized conditions allow for a wide range of substituted tetralone nucleophiles 45 to undergo a highly selective iridium-catalyzed allylic alkylation reaction with crotyl chloride (46, Scheme 4). However, at this time, the nucleophile scope is limited to β-keto ester-based tetralones. We postulate that this limitation is due to both pKa restrictions of the nucleophile that prevent the use of tetralones bearing other α-electron withdrawing groups (e.g., nitrile, ketone; 50 versus 44) as well as the necessity of sp2-hybridized bulk at the carbonyl α′ position to induce selectivity. With respect to the electrophile, longer chain alkyl-substituted electrophiles result in diminished yields and selectivities, likely due to increased sterics. Limitations aside, this transformation represents the first transition metal-catalyzed allylic alkylation reaction forming vicinal tertiary and all-carbon quaternary stereocenters between prochiral enolates and an alkyl-substituted electrophile.20 We envision that with further exploration of new catalytic systems that the substrate scope of this transformation can be expanded to additional alkyl-substituted electrophiles in the future.
Scheme 4.
Enantio- and diastereoselective iridium-catalyzed allylic alkylation reactions with crotyl chloride (46)
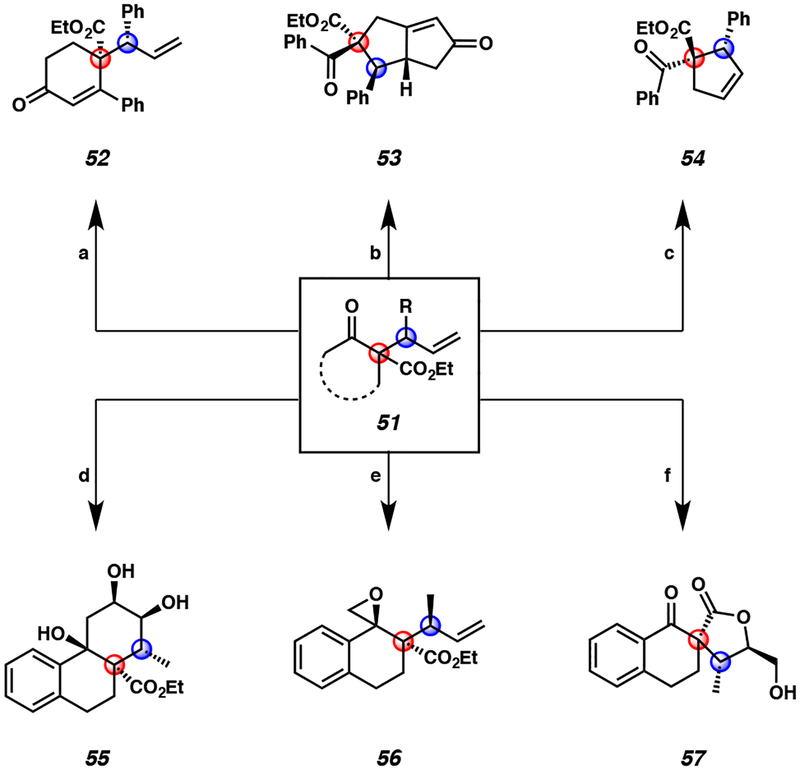
In order to demonstrate the synthetic utility of our enantio- and diastereoselective iridium-catalyzed allylic alkylation methodology, we carried out series of product transformations on allylic alkylation products 51 (Figure 4). Notably, all of these derivatizations proceed with excellent diastereoselectivity to facilitate the synthesis of complex building blocks, demonstrating the ease with which complexity can be added to these high-value products.
Figure 4.
Select examples of diverse product transformations of enantio- and diastereoselective iridium-catalyzed allylic alkylation products 51a
aConditions: (a) pyrrolidine, AcOH, t-BuOMe, reflux, 95% yield. (b) Co2(CO)8, CH2Cl2, then Me3NO×2H2O, >20:1 dr, 99% yield. (c) HG-II (10 mol %), CH2Cl2, 40 °C, 96% yield. (d) i) allylmagnesium chloride, THF, −78 °C, 71% yield, ii) HG-II, CH2Cl2, 81% yield, iii) K2OsO4, NMO, THF/H2O, 59% yield. (e) Me3S(O)I, NaH, DMSO, 82% yield. (f) K2OsO4, NMO, THF/H2O, 65% yield.
3. Umpoled Iridium-Catalyzed Allylic Alkylation Reactions
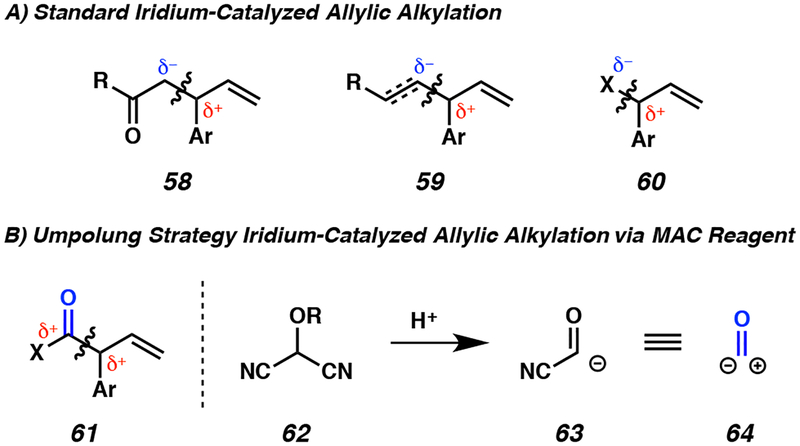
After four years of expanding the limits of enantio- and diastereoselective iridium-catalyzed allylic alkylation, we became aware of another limitation in the field. Specifically, we noted that over the past two decades of iridium-catalyzed allylic alkylation research since the seminal report,8 the technology had been widely developed for standard reactivity patterns between electrophilic π-allyl species and nucleophilic enolate equivalents (58), carbanion equivalents (59), or heteroatoms (60, Figure 5A).13,21 However, the application of an umpolung strategy in iridium-catalyzed allylic alkylation to stitch together two formally electrophilic species remained underexplored (61, Figure 5B, left). At the time, only two examples of reverse-polarity nucleophiles had been reported for this chemistry,22 though neither were in the carboxylic acid oxidation state which would allow for direct access to either enantioenriched amides, esters, or carboxylic acids.
Figure 5.
Iridium-catalyzed allylic alkylation strategies
3.1. Tertiary Allylic Stereocenters23
With this gap in the literature noted we set forth to develop an iridium-catalyzed allylic alkylation method for accessing carboxylic acid derivatives and we identified masked acyl cyanide (MAC) reagents 62 as potential nucleophiles (Figure 5B, left). These MAC reagents which were developed by Nemoto and Yamamoto,24a–e and popularized by Rawal,24f–h can expel an equivalent of cyanide when exposed to acid therefore allowing them to function as acyl cyanide nucleophiles (63, Figure 5B, right). As acyl cyanides are highly electrophilic, the MAC reagent truly functions as a carbon monoxide synthon (64). Thus, we envisioned that if the MAC reagent could react with an iridium-π allyl species to generate an alkylation product, we could use extensive literature precedent to unmask the MAC adduct and further transform the transient acyl cyanide to carboxylic acid derivatives 61 upon treatment with heteroatom nucleophiles (e.g., H2O, RNH2, ROH).24,25 As a result, the MAC reagent would function as each an amide, ester, and carboxylic acid synthon.
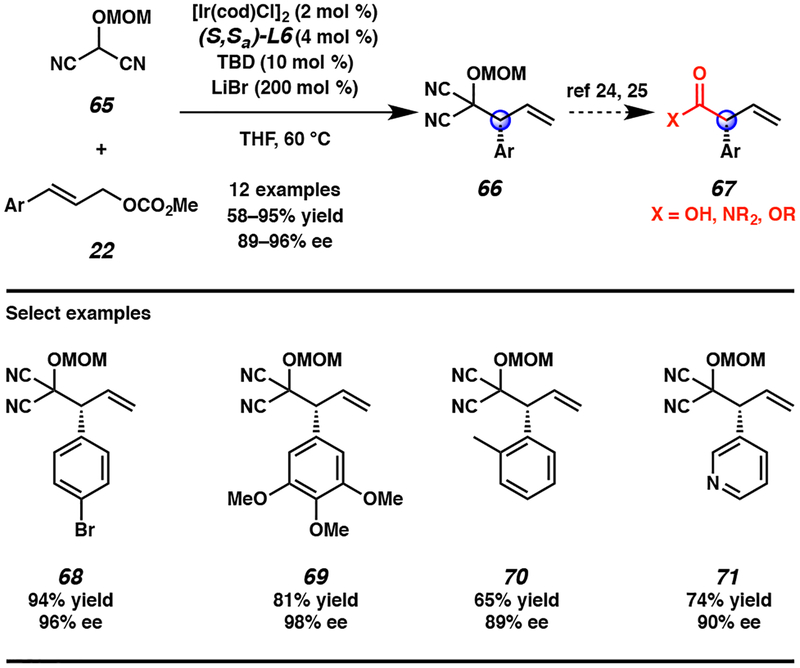
Employing our previously disclosed conditions for the iridium-catalyzed allylic alkylation of cyclic nucleophiles to form vicinal tertiary and all-carbon quaternary stereocenters,14 we were able to rapidly establish that the MAC reagent was a competent nucleophile in the iridium-catalyzed reaction given the judicious choice of protecting group on the hydroxyl moiety. Specifically, we found that a methyoxymethyl (MOM) group (65) was required in order to access products 66 in high yields (Scheme 5). We hypothesize that this protecting group is optimal due to its small steric profile as well as its potential to coordinate and be activated by the LiBr additive. Using these optimized conditions, we discovered that a range of cinnamyl-derived electrophiles, including heteroaryl-substituted allylic electrophiles, react in high yields and excellent enantioselectivities in up to gram scale to provide allylic alkylation products 66, which are amenable to the synthesis of highly desirable, enantioenriched vinylated α-aryl carboxylic acid derivatives.23
Scheme 5.
First report of MAC reagent 65 in an asymmetric transition metal-catalyzed reaction
3.2. Quaternary Allylic Stereocenters26
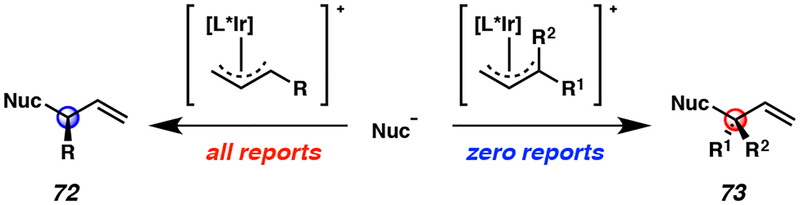
After having developed the iridium-catalyzed allylic alkylation chemistry for MAC nucleophile 65,23 we sought to further leverage the utility of the MAC reagents to access an even more challenging class of compounds – acyclic α-quaternary carbonyl derivatives. However, our proposed allylic alkylation strategy had one major caveat, namely, enantioenriched quaternary allylic stereocenters had never been synthesized via iridium-catalyzed allylic alkylation reactions (Figure 6).27
Figure 6.
Limitations in enantioselective iridium-catalyzed allylic alkylation prior to 2017
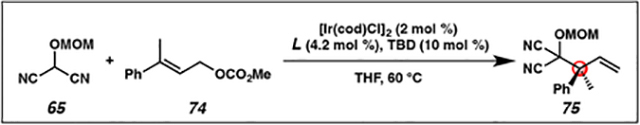
Over the past twenty years, enantioselective iridium-catalyzed allylic alkylation reactions have been exclusively limited to those synthesizing tertiary allylic stereocenters 72 (Figure 6, left).13,21 In contrast, use of a 1,1-disubstituted π-allyl to forge quaternary allylic stereocenters 73 was unreported (Figure 6, right). In order to access such a geminal-disubstituted π-allyl species, a trisubstituted allylic electrophile would need to be utilized; however, this class of electrophile was predicted to be unreactive in iridium-catalyzed allylic alkylation chemistry. Literature reports have demonstrated that the reaction rates of these processes decrease with increasing substitution on the olefin of the electrophile.28 Therefore, we anticipated that our preliminary experiments into this research area would focus on identifying a method in which to unlock reactivity from a heretofore unreactive trisubstituted allylic electrophile 74 (Table 3).
Table 3.
Optimization of the enantioselective synthesis of acyclic α-quaternary carbocyclic acid derivatives 75
 | |||||
|---|---|---|---|---|---|
| Entry | L | 65:74 | Additive (200 mol %) |
Yieldb | ee (%)c |
| 1 | L6 | 2:1 | LiBr | 0 | - |
| 2 | L5 | 2:1 | LiBr | 0 | - |
| 3 | L7 | 2:1 | LiBr | 13 | 79 |
| 4 | L7 | 2:1 | BEt3 | 34 | 93 |
| 5 | L7 | 1:2 | BEt3 | 99 | 94 |
 | |||||
Reactions performed on 0.1 mmol scale.
1H NMR yield based on internal standard.
Determined by chiral HPLC analysis.
As we anticipated, application of our standard conditions for the iridium-catalyzed allylic alkylation of cyclic nucleophiles failed to invoke reactivity from trisubstituted allylic electrophile 74, instead returning starting material (Table 3, entry 1). We rationalized that we would need to explore other phosphoramidite ligand frameworks in order to identify a more reactive catalyst (entries 2 and 3). Ultimately, we identified that phosphoramidite L7, developed by Carreira, was uniquely effective in providing desired product 75, though in only modest yield (entry 3).29 In an effort to increase the yield and selectivity of the transformation, we performed an extensive investigation into alternative additives that have proven successful in promoting iridium-catalyzed allylic alkylation reactions. However, it was not until we identified the necessity of a strong Lewis acid co-catalyst to promote oxidative addition via facilitating the ionization of the carbonate leaving group from allylic electrophile 74, that we saw improved reactivity. Specifically, we discovered that the addition of Lewis acidic triethylborane to the reaction provided access to desired product 75 in nearly triple the conversion and excellent enantioselectivity (entry 4). Finally, we found that we could further improve reaction yield by altering the nucleophile to electrophile stoichiometry such that an excess of electrophile is present (entry 5). Of note, the E-olefin isomer of allylic electrophile 74 is required, as the Z-trisubstituted allylic electrophile gave significantly decreased yields and selectivities.
While optimizing the reaction parameters for this novel transformation, we noted the importance of the guanidine base, 1,3,5-triazabicyclo[4.4.0]dec-5-ene (TBD), on reactivity. Typically, TBD is utilized as a substoichiometric base additive to promote the formation of an active iridicycle catalyst.30 However, Carreira has reported that phosphoramidite L7 does not form an iridicycle and thus a base additive is not required when employing this ligand in iridium-catalyzed allylic alkylation reactions.31 We postulate that in the case of our developed reaction, TBD may be serving as a labile placeholder ligand to prevent the formation of an inactive catalyst or as a base to promote the formation of a novel active iridicycle. Studies are currently ongoing to elucidate the unique effect of TBD under our reaction conditions.
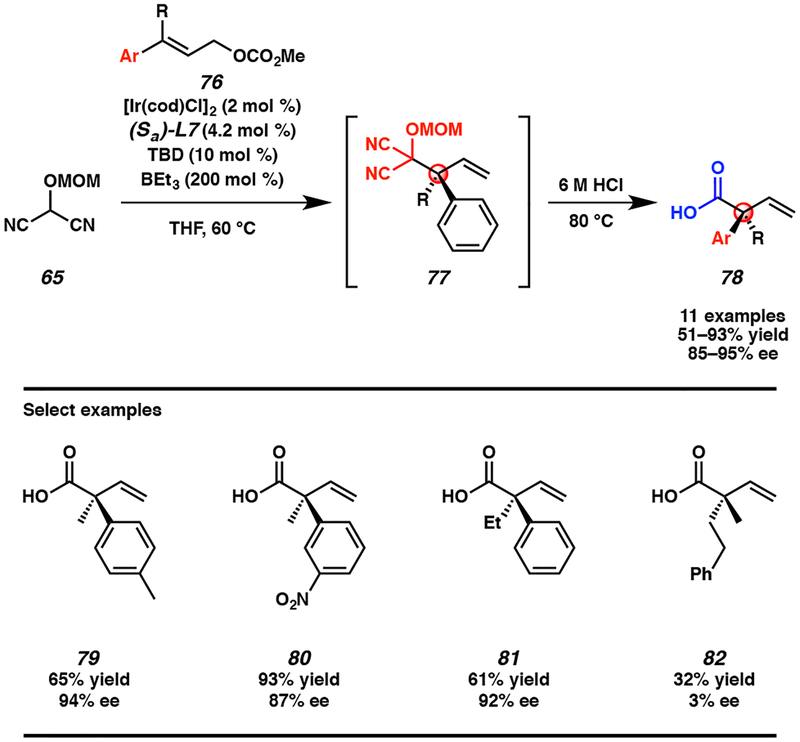
In looking to how this new reaction could be even further improved, we envisioned that hydrolysis of the MAC functionality of allylic alkylation product 77 could be carried out in the same reaction vessel as the iridium-catalyzed reaction to provide direct access to the corresponding carboxylic acid in a one-pot, two-step procedure (Scheme 6).25e Moreover, we hypothesized that carboxylic acid products 78 could be isolated in high purity after a simple acid/base work up alone, with no need for column chromatography. We were pleased to find that our hypothesis was valid and with our optimized conditions a range of acyclic α-quaternary carboxylic acids 78 were prepared with varying substitution at the α-position. Specifically, both electron withdrawing and donating groups are well tolerated at the para and meta positions of the aryl substituent on electrophile 76, though diminished yields are observed for bis-meta-substituted arenes and no reactivity is observed with ortho-substituted aryl groups. With respect to the alkyl substituent (R), lengthening the n-alkyl group beyond an ethyl moiety results in poor yields, as does the use of branched alkyl substituents. Also of note, bis-alkyl-substituted allylic electrophiles do not currently fare well in this chemistry (cf., 82) and might represent an interesting avenue of future exploration.
Scheme 6.
Enantioselective synthesis of acyclic α-quaternary carboxylic acids 78
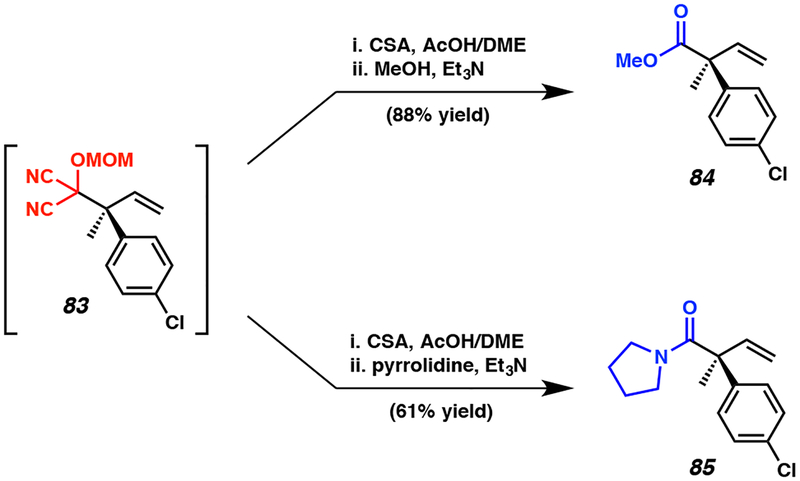
As MAC adducts can be transformed into essentially any carboxylic acid derivative, we sought to develop additional onepot methods to access α-quaternary esters and amides.25e Toward this end, we were pleased to find that MAC adduct 83 could be advanced in a one-pot fashion to a variety of esters (e.g., 84), as well as amides (e.g., 85) in moderate to high yields following cleavage of the MOM group and interception of the transient acyl cyanide by the appropriate nucleophile (Figure 7).
Figure 7.
Enantioselective synthesis of acyclic α-quaternary esters 84 and amides 85
4. Synthesis of Vicinal All-Carbon Quaternary Centers via Enantioselective Iridium-Catalyzed Allylic Alkylation32
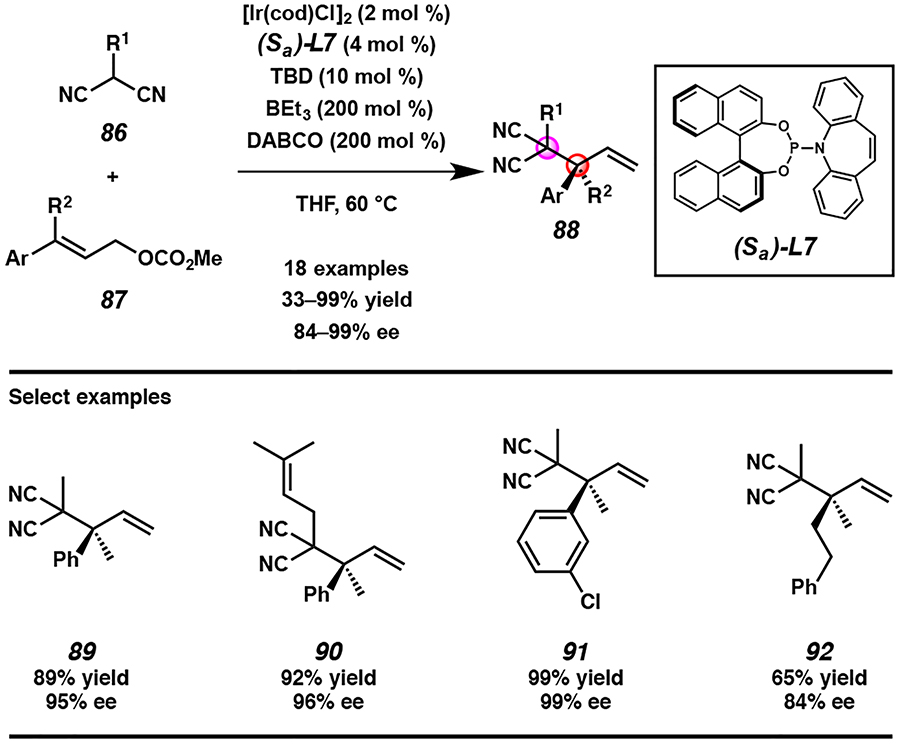
Noting that our preparation of enantioenriched α-quaternary carboxylic acid derivatives progresses through intermediate 77, bearing vicinal tetra-substituted and quaternary centers, led us to imagine that we could utilize this iridium-catalyzed allylic alkylation methodology to synthesize highly congested vicinal all-carbon quaternary centers. In designing a nucleophile for this desired reaction, we postulated that the most facile reaction development would be achieved if the malononitrile functionality of the previously utilized MAC nucleophile 65 was kept intact and the α-substitution were not more sterically demanding than the MOM group. Indeed, our previously reported conditions for the synthesis of α-quaternary carboxylic acid derivatives using MAC reagent 65 translated directly to the reaction of methylmalononitrile (86, R1 = Me) with trisubstituted allylic electrophile 87, though the enantioselectivity was low (Scheme 7). As our early work demonstrated that basic additives have pronounced effects on selectivity, we explored the effect of base on our current reaction. Ultimately, DABCO was revealed to be uniquely effective for this transformation, providing products 88 bearing vicinal all-carbon quaternary centers in moderate to good yields and excellent enantioselectivities for a variety of substituted malononitrile nucleophiles 86 and trisubstituted allylic electrophiles 87.
Scheme 7.
Synthesis of vicinal all-carbon quaternary centers 88 via enantioselective iridium-catalyzed allylic alkylation
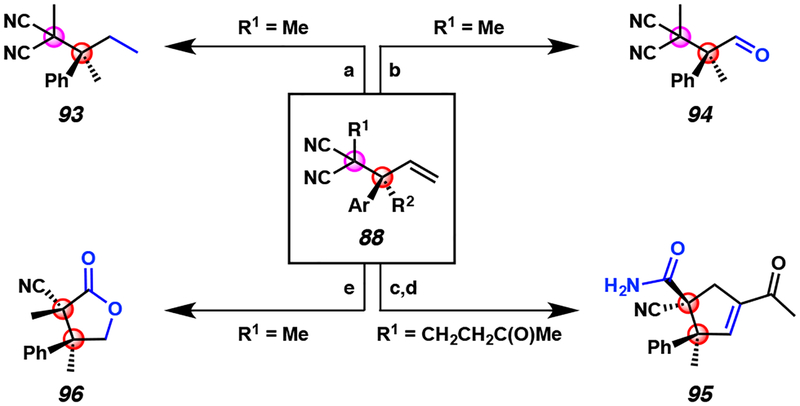
As we are inspired and driven by the application of our methods in complex molecule synthesis, we sought to demonstrate the feasibility of chemoselective manipulation of allylic alkylation product 88 (Figure 8). The olefin functionality of 88 can either be chemoselectively reduced or oxidized via ozonolysis. Furthermore, multistep procedures can be utilized to prepare densely-functionalized compounds bearing two contiguous all-carbon quaternary stereocenters, such as 95 and 96.
Figure 8.
Product derivatizations of iridium-catalyzed allylic alkylation products 88 bearing vicinal all-carbon quaternary centers
aConditions: (a) RhCl(PPh3)3, H2 (balloon), benzene, 23 °C, 18 h, 92% yield. (b) O3, pyridine, CH2Cl2, −78 °C, 4 min, 93% yield. (c) i. O3, pyridine, CH2Cl2, −78 °C, 4 min, ii. p-TsOH, benzene, reflux, 18 h, 47% yield. (d) NaOH, EtOH/H2O (1:1), 60 °C, 18 h, 38% yield. 1:11 dr. (e) i. O3, MeOH,−78 °C, 0.5 h, ii. NaBH4, 0 °C, 3 h, 65% yield, 1:2.5 dr.
5. Summary and Future Outlook
Building on our group’s longstanding interest in the synthesis of enantioenriched quaternary stereocenters, we have sought to expand the limits of iridium-catalyzed allylic alkylation chemistry to encompass the synthesis of sterically congested all-carbon quaternary stereocenters. Initially, we focused on the development of enantio- and diastereoselective iridium-catalyzed allylic alkylation technology for the construction of vicinal tertiary and all-carbon quaternary stereocenters. Our work has enabled the use of both cyclic and acyclic nucleophiles, as well as alkyl-substituted allylic electrophiles in this methodology. We then shifted our attention to umpolung strategy iridium-catalyzed allylic alkylation chemistry and developed MAC reagents as nucleophiles. Continued study of the umpolung chemistry led to the synthesis of allylic quaternary stereocenters via the use of trisubstituted allylic electrophiles in conjunction with a Lewis acid co-catalyst. Most recently, we have discovered iridium-catalyzed allylic alkylation technology that provides access to vicinal all-carbon quaternary centers.
Despite these advances by our group, as well as beautiful work from other groups in the area,13,21 this emerging field is not without limitations. While there have been extensive advances to widen the substrate scope with respect to the nucleophile partner, the allylic electrophile remains largely limited to aryl- and alkenyl-substitution, aside from our reported method for the use of crotyl chloride as an electrophile.20 Most importantly though, no general method for iridium-catalyzed allylic alkylation exists. As demonstrated vide supra, even minor changes to either the nucleophile or electrophile partner necessitate complete reoptimization of the reaction parameters. Ideally, a single catalyst system will be developed in the future which enables highly selective iridium-catalyzed allylic alkylation reactions for any combination of nucleophile and electrophile.
In looking forward, we envision that the field will shift its attention to the development of enantio- and diastereoselective iridium-catalyzed allylic alkylation for the synthesis of vicinal all-carbon quaternary stereocenters; a highly challenging motif to access that has also become a holy grail for many other areas of synthetic methodology. We hope that our recent work on the synthesis of vicinal quaternary centers will inspire and enable these studies. Ultimately, we look forward to a time when the broader synthetic community embraces iridium-catalyzed allylic alkylation as an enabling and reliable technology for the synthesis of complex targets.
Acknowledgment
The authors thank the many past and present co-workers whose efforts have made our contributions in iridium-catalyzed allylic alkylation possible: Prof. Wen-Bo Liu, Dr. Corey M. Reeves, Dr. Scott C. Virgil, Dr. Noriko Okamoto, Eric J. Alexy, Dr. Allen Y. Hong, and Dr. Kristy Tran.
Funding Information
Support for our program has been made available from the NIH-NIGMS (R01GM080269) and Caltech. Additionally, J.C.H. thanks the Camille and Henry Dreyfus postdoctoral program in Environmental Chemistry, and S.E.S. thanks the NIH-NIGMS for a predoctoral fellowship (F31GM120804).
Biosketches
(Middle)
Samantha E. Shockley was born in Birmingham, AL in 1990. She received her B.S. degree in Chemistry from the University of Chicago in 2012 where she conducted research for Professor Richard F. Jordan. After graduating from the College, she worked with Professor Martin Banwell at the Australian National University as a U.S. Fulbright scholar. She is now pursuing her graduate studies at the California Institute of Technology under the guidance of Professor Brian M. Stoltz. Her graduate research focuses on the development of enantioselective transition metal catalysis and natural product total synthesis.
(Right)
J. Caleb Hethcox was born in Montgomery, AL in 1988. He graduated from Auburn University in 2010 where he conducted research with Professor Michael E. Squillacote. In 2015, he completed his Ph.D. in the laboratory of Professor Stephen F. Martin at the University of Texas at Austin, where he studied total synthesis and enantioselective halolactonization reactions. Currently, he is the Henry and Camille Dreyfus Postdoctoral Fellow in Environmental Chemistry in the laboratory of Professor Brian M. Stoltz at the California Institute of Technology. His research interests include the development and study of asymmetric transition metal catalyzed reaction and their applications in natural product synthesis.
(Left)
Brian M. Stoltz was born in Philadelphia, PA in 1970 and obtained his B.S. degree from the Indiana University of Pennsylvania in Indiana, PA. After graduate work at Yale University in the laboratories of John L. Wood and an NIH postdoctoral fellowship at Harvard in the Corey laboratories, he took a position at the California Institute of Technology. A member of the Caltech faculty since 2000, he currently is a Professor of Chemistry. His research interests lie in the development of new methodology for general applications in synthetic chemistry.

References
- (1).Liu Y; Han S-J; Liu W-B; Stoltz BM Acc. Chem. Res 2015, 48, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Behena DC; Mohr JT; Sherden NH; Marinescu SC; Harned AM; Tani K; Seto M; Ma S; Novák Z; Krout MR; McFadden RM; Roizen JL; Enquist JA Jr.; White DE; Levine SR; Petrova KV; Iwashita A; Virgil SC; Stoltz BM Chem. Eur. J 2011, 17, 14199–14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For select examples, see:Reeves CM; Eidamshaus C; Kim J; Stoltz BM Angew. Chem. Int. Ed 2013, 52, 6718–6721;Korch KM; Eidamshaus C; Behenna DC; Nam S; Horne D; Stoltz BM Angew. Chem. Int. Ed 2015, 54, 179–183;Numajiri Y; Jiménez-Osés G; Wang B; Houk KN; Stoltz BM Org. Lett 2015, 17, 1082–1085;Craig RA II; Loskot SA; Mohr JT; Behenna DC; Harned AM; Stoltz BM Org. Lett 2015, 17, 5160–5163.
- (4).Enquist JA Jr.; Stoltz BM Nature 2008, 453, 1228–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).McFadden RM; Stoltz BM J. Am. Chem. Soc 2006, 128, 7738–7739. [DOI] [PubMed] [Google Scholar]
- (6).Numajiri Y; Pritchett BP; Chiyoda K; Stoltz BM J. Am. Chem. Soc 2015, 137, 1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tsuji J; Takahashi H; Morikawa M Tetrahedron Lett. 1965, 6, 4387–4388. [Google Scholar]
- (8).Takeuchi R; Kashio M Angew. Chem. Int. Ed 1997, 36, 263–265. [Google Scholar]
- (9).For examples where palladium-catalyzed allylic alkylation favors branched products, see:Prétôt R; Pfaltz A Angew. Chem. Int. Ed 1998, 37, 323–325;Hayashi T; Kawatsura M; Uozumi YJ Am. Chem. Soc 1998, 120, 1681–1687;You S-L; Zhu X-Z; Luo Y-M; Hou X-L; Dai L-XJ Am. Chem. Soc 2001, 123, 7471–7472;Zheng W-H; Sun N; Hou X-L Org. Lett 2005, 7, 5151–5154;Zheng W-H; Zheng B-H; Zhang Y; Hou X-LJ Am. Chem. Soc 2007, 129, 7718–7719;Liu W; Chen D; Zhu X-Z; Wan X-L; Hou X-LJ Am. Chem. Soc 2009, 131, 8734–8735;Fang P; Ding C-H; Hou X-L; Dai L-X Tetrahedron: Asymmetry 2010, 21, 1176–1178;Chen J-P; Ding C-H; Liu W; Hou X-L; Dai L-XJ Am. Chem. Soc 2010, 132, 15493–15495.
- (10).Janssen JP; Helmchen G Tetrahedron Lett. 1997, 38, 8025–8026. [Google Scholar]
- (11).Kanayama T; Yoshida K; Miyabe H; Takemoto Y Angew. Chem. Int. Ed 2003, 42, 2054–2056. [DOI] [PubMed] [Google Scholar]
- (12).Chen W; Hartwig JF J. Am. Chem. Soc 2013, 135, 2068–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).For a review on diastereoselective iridium-catalyzed allylic alkylation, see:Hethcox JC; Shockley SE; Stoltz BM ACS Catal. 2016, 6, 6207–6213.
- (14).Liu W-B; Reeves CM; Virgil SC; Stoltz BM J. Am. Chem. Soc 2013, 135, 10626–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Liu W-B; Okamoto N; Alexy EJ; Hong AY; Tran K; Stoltz BM J. Am. Chem. Soc 2016, 138, 5234–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Douglas CJ; Overman LE Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Das JP; Marek I Chem. Commun 2011, 47, 4593–4623; [DOI] [PubMed] [Google Scholar]; (c) Quasdorf KW; Overman LE Nature 2014, 516, 181–191; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Corey EJ; Guzman-Perez A Angew. Chem. Int. Ed 1998, 37, 388–401; [DOI] [PubMed] [Google Scholar]; (e) Christoffers J; Mann A Angew. Chem. Int. Ed 2001, 40, 4591–4597; [DOI] [PubMed] [Google Scholar]; (f) Trost BM; Jiang C Synthesis 2006, 369–396; [Google Scholar]; (g) Denissova I; Barriault L Tetrahedron 2003, 59, 10105–10146; [Google Scholar]; (h) Ling T; Rivas F Tetrahedron 2016, 72, 6729–6777. [Google Scholar]
- (17).(a) Liu W-B; He H; Dai L-X; You S-L Synthesis 2009, 2076–2082; [Google Scholar]; (b) Liu W-B; Zheng C; Zhuo C-X; Dai L-X; You S-LJ Am. Chem. Soc 2012, 134, 4812–4821. [DOI] [PubMed] [Google Scholar]
- (18).(a) Bartels B; Helmchen G Chem. Commun 1999, 741–742; [Google Scholar]; (b) Alexakis A; Polet D Org. Lett 2004, 6, 3529–3532; [DOI] [PubMed] [Google Scholar]; (c) Polet D; Alexakis A; Tissot-Croset K; Corminboeuf C; Ditrich K; Chem. Eur. J 2006, 12, 3596–3609. [DOI] [PubMed] [Google Scholar]
- (19).Liu W-B; Reeves CM; Stoltz BM J. Am. Chem. Soc 2013, 135, 17298–17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hethcox JC; Shockley SE; Stoltz BM Angew. Chem. Int. Ed 2016, 55, 16092–16095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).For selected reviews of asymmetric iridium-catalyzed allylic alkylation, see:Helmchen G; Dahnz A; Dübon P; Schelwies M; Weihofen R Chem. Commun 2007, 675–691;Hartwig JF; Pouy MJ Top. Organomet. Chem 2011, 34, 169–208;Liu W-B; Xia JB; You S-L Top. Organomet. Chem 2011, 38, 155–208.
- (22).(a) Förster S; Tverskoy O; Helmchen G Synlett 2008, 2803–2806; [Google Scholar]; (b) Breitler S; Carreira EM J. Am. Chem. Soc 2015, 137, 5296–5299. [DOI] [PubMed] [Google Scholar]
- (23).Hethcox JC; Shockley SE; Stoltz BM Org. Lett 2017, 19, 1527–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).(a) Nemoto H; Kubota Y; Yamamoto YJ Org. Chem 1990, 55, 4515–4516; [Google Scholar]; (b) Nemoto H; Li X; Ma R; Suzuki I; Shibuya M Tetrahedron Lett. 2003, 44, 73–75; [Google Scholar]; (c) Nemoto H; Kawamura T; Miyoshi NJ Am. Chem. Soc 2005, 127, 14546–14547; [DOI] [PubMed] [Google Scholar]; (d) Nemoto H; Ma R; Kawamura T; Kamiya M; Shibuya MJ Org. Chem 2006, 71, 6038–6043; [DOI] [PubMed] [Google Scholar]; (e) Nemoto H; Kawamura T; Kitasaki K; Yatsuzuka K; Kamiya M; Yoshioka Y Synthesis 2009, 1694–1702; [Google Scholar]; (f) Yang KS; Nibbs AE; Türkmen YE; Rawal VH J. Am. Chem. Soc 2013, 135, 16050–16053; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yang KS; Rawal VH J. Am. Chem. Soc 2014, 136, 16148–16151; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kagawa N; Nibbs AE; Rawal VH Org. Lett 2016, 18, 2363–2366. [DOI] [PubMed] [Google Scholar]
- (25).(a) Kociołek K; Leplawy MT Synthesis 1977, 778–780; [Google Scholar]; (b) Kubota Y; Nemoto H; Yamamoto YJ Org. Chem 1991, 56, 7195–7196; [Google Scholar]; (c) Nemoto H; Ma R; Ibaragi T; Suzuki I; Shibuya M Tetrahedron 2000, 56, 1463–1468; [Google Scholar]; (d) Yamatsugu K; Kanai M; Shibasaki M Tetrahedron 2009, 65, 6017–6024; [Google Scholar]; (e) Yang KS; Nibbs AE; Türkmen YE; Rawal VH J. Am. Chem. Soc 2013, 135, 16050–16053; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yang KS; Rawal VH J. Am. Chem. Soc 2014, 136, 16148–16151; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kagawa N; Nibbs AE; Rawal VH Org. Lett 2016, 18, 2363–2366. [DOI] [PubMed] [Google Scholar]
- (26).Shockley SE; Hethcox JC; Stoltz BM Angew. Chem. Int. Ed 2017, 56, 11545–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).At the time of publication, a singular example of an iridium-catalyzed allylic alkylation reaction producing a product bearing an allylic all-carbon stereocenter has been reported with 11% yield and 21% ee:Onodera G; Watabe K; Matsubara M; Oda K; Kezuka S; Takeuchi R Adv. Synth. Catal 2008, 350, 2725–2732.
- (28).(a) Madrahimov ST; Hartwig JF J. Am. Chem. Soc 2012, 134, 8136–8147; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Madrahimov ST; Li Q; Sharma A; Hartwig JF J. Am. Chem. Soc 2015, 137, 14968–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Defieber C; Ariger MA; Moriel P; Carreira EM Angew. Chem. Int. Ed 2007, 46, 3139–3143. [DOI] [PubMed] [Google Scholar]
- (30).For examples of the formation of active iridicycles, see:Jiang X; Beiger JJ; Hartwig JF J. Am. Chem. Soc 2017, 139, 87–90;Liu W-B; Zheng C; Zhuo C-X; Dai L-X; You S-LJ Am. Chem. Soc 2012, 134, 4812–4821.
- (31).Rössler SL; Krautwald S; Carreira EM J. Am. Chem. Soc 2017, 139, 3603–3606. [DOI] [PubMed] [Google Scholar]
- (32).Hethcox JC; Shockley SE; Stoltz BM Angew. Chem. Int. Ed 2018, doi: 10.1002/ange.201804820. [DOI] [PMC free article] [PubMed] [Google Scholar]