Abstract
Previous research showed that the processing of overt threat cues formed by evolutionary experience such as snake or angry face induced automatic increased responses of the emotion‐related system consisting of the amygdala, the anterior cingulate, and the orbitofrontal cortex. The present study used functional magnetic resonance imaging (fMRI) to investigate brain circuits involved in perception of threat cues that lack obvious emotion contents but are potentially dangerous in a particular social situation. Subjects were scanned while watching images showing a person in either a safe or a potentially dangerous situation and being asked to detect threat signals or to evaluate the degree of threat. We found that, in contrast with gender identification, threat detection and evaluation were underpinned by a neural network, shared by both male and female subjects, consisting of the medial and lateral frontal cortex, superior parietal lobes, posterior middle temporal cortex, and cerebellum. In addition, detection of threat cues was associated with stronger posterior parietal activation for males than females. Our findings suggest that neural processing of evolutionary unprepared threat cues in social environments does not necessarily involve the emotion‐related neural system and is influence by evolutionary pressure on sex differences. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: threat cue, emotion, fMRI, gender
INTRODUCTION
The perception of danger is a primitive cognitive ability that is crucial for humans to survive in both natural and social environments. It is also an ability that may differ across males and females. Evolutionary pressure biases men toward hunting and women toward gathering [Ardrey, 1976; Dahlberg, 1981]. Hunters are more likely to be confronted with dangerous situations than gatherers, and this may lead to disparities in the processing of threat signals in males and females. Indeed, relative to males, females have a lower threshold for fear when faced with the same level of objective physical danger [Campbell et al., 2001] and show greater perceived risks when faced with a potential dangerous social situation [Harris and Miller, 2000] or when assessing physical risks to health [Weber et al., 2002]. These differences may partially reflect the lesser physical strength of females [Hines and Fry, 1994; Thompson et al., 1992], along with an evolutionary bias for females to behave in a more cautious and less aggressive fashion [Campbell, 1999; Hines and Fry, 1994]. However, despite the importance of understanding responses to threat, and gender differences in this ability, there has been minimal study of the underlying neural substrates. The present article sets out to rectify this.
Previous research has examined specific physiological responses to the perception of images containing overt threat cues formed by evolutionary experience. For instance, images of snakes or spiders produce enhanced skin conductance responses [Globisch et al., 1999; Öhman and Soares, 1993] and associated increased activity in the amygdala, the anterior cingulate, and ventromedial prefrontal cortex [Carlsson et al., 2004; Carretie et al., 2005]. These responses are most pronounced in snake‐ or spider‐fearful subjects. Angry or fearful faces also elicit increased activity in the amygdala and anterior cingulate [Morris et al., 1996; Pissiota et al., 2003]. In addition, males and females appear to show differential neural activity to images associated with potential danger, though the direction of these effects do not necessarily fit with the idea that females are more sensitive to threat signals. For example, pictures of attacks by humans or animals have been shown to induce greater activation in the amygdala in males than females [Schienle et al., 2005]. In contrast, fearful or angry faces elicited a more persistent or stronger response in the amygdala for females than males [McClure et al., 2004; Williams et al., 2005].
All these studies used threat cues such as snake or angry faces that induced automatic increased responses of the emotion‐related system (i.e., the amygdala, the anterior cingulate, and the orbitofrontal cortex showed increased activation to dangerous signals even when no explicit tasks were assigned to the dangerous stimuli). It has been suggested that these threat cues are evolutionary‐prepared because of the requirement for predatory defense, and fear is most likely to occur to these threats that are dangerous to pretechnological man and induces a specific fear neural system in the brain [Mineka and Öhman, 2002; Öhman and Mineka, 2001]. However, people are often confronted with threat cues in contemporary social environments that lack obvious emotion contents but are potentially dangerous in a particular social situation. For example, a car or a gun may produce injury on people when these objects are related to people in a particular way. Deliberative cognitive processes are possibly required for detection and evaluation of such threat signals. To date, we have known little about the neural substrates underlying the cognitive processes of these “evolutionary unprepared” threat cues.
The current work first examined if there exists a neural network supporting the processing of evolutionary unprepared threat cues that is independent of the fear or emotion‐related system. We used stimulus displays that consisted of a person with a neutral facial expression in either a safe situation (e.g., walking besides a stationary car) or a potentially dangerous situation (e.g., walking in front of a moving car, Fig. 1). These stimulus displays did not include any overt threat cues such as angry faces or snakes and spiders that induce automatic emotional responses. Participants were asked either to judge whether the person in the situation was in potential danger (Experiment 1) or to evaluate the degree of danger the person in the situation was in (mild vs. severe, Experiment 2). These tasks were contrasted with a baseline condition of judging the gender of the person in the stimuli, to control for low‐level visual feature processing, any automatic emotional responses elicited by the attended stimuli, and the processing of threat irrelevant social information such as people's gender. Experiment 2 complemented Experiment 1 in two aspects. First, in Experiment 1, the contrast between the threat detection task and the controlled condition revealed neural substrates of initial threat processing (i.e., detection of the presence of threat cues). Experiment 2 asked subjects to evaluate the degree of danger to examine if the same neural circuit is involved in threat detection and evaluation. Second, because the stimuli were identical in the threat detection task and in the controlled task, the contrast used in Experiment 1 identified neutral activity related to the ongoing task demand (i.e., threat detection). In Experiment 2, the stimulus displays showed a person in a safe situation in the controlled task, whereas in a dangerous situation in the evaluation task. We compared danger versus no‐danger stimuli to further examine if the emotion‐related system was involved in evaluation of threat cues.
Figure 1.

Illustration of the stimulus displays used in the current study. Left = dangerous and right = safe image. Colorful stimuli were used in both experiments.
In addition, we investigated whether the neural substrates underlying the perception of evolutionary unprepared threat cues differ between males and females. The division of labor formed by evolutionary pressure may lead to males being confronted with dangerous situations more frequently than females, and this may contribute to differential neural responses to threat cues. One possibility is that, in males, spatial processes often used in hunting and tracking are associated to threat signals [Silverman and Eals, 1992; Silverman et al., 2000], so that males engage more in spatial processing than females when detecting or evaluating these signals. This would lead to the selective recruitment, in males, of brain regions linked to spatial processing (e.g., in the parietal lobe).
MATERIALS AND METHODS
Subjects
Twenty‐four adults (12 females, mean (±SD) age 21.42 ± 1.08 years; 12 males, mean age 21.25 ± 1.76 years) participated in both Experiments 1 and 2. The female and male subjects were matched on educational level (1–5 years university). All participants were native Chinese speakers and had no neurological or psychiatric history. All were right‐handed, and had normal or corrected‐to‐normal vision. Informed consent was obtained from all participants prior to scanning. This study was approved by a local ethic committee at the Department of Psychology, Peking University.
Stimuli and Procedure
The stimuli were presented through an LCD projector onto a rear‐projection screen located at a subject's head. The screen was viewed with an angled mirror positioned on the head‐coil. Visual stimuli consisted of photos taken from two males and two females in nine different scenes. The person in each scene showed neutral facial expression but could be in either a safe (i.e., walking besides a stationary car or facing an apple) or a potentially dangerous situation (i.e., walking in front of a moving car or facing a gun), as illustrated in Figure 1. Such situations can easily be judged as safe or dangerous based on everyday experience. All the photos were flipped horizontally to produce a set of symmetrical stimulus displays, resulting totally in 144 stimulus displays. Half of the displays showed a person in a potentially dangerous situation and the others showed the same person in a safe situation. These photos were first evaluated by 20 independent subjects to verify that they indeed represented potentially dangerous situations (the mean ratings were 7.31 ± 1.79 and 1.36 ± 0.84, respectively for dangerous and safe stimuli on a 11‐point scale, where 0 = very safe, 10 = very dangerous, t(19) = 14.16, P < 0.001). All the photos were classified into safe and dangerous situations according to the original design. Each stimulus display subtended visual angles of 16.2° × 13.5° (width and height) at a viewing distance of 90 cm.
In Experiment 1, subjects were presented with all the photos and asked to judge whether or not the person in the situation was in potential danger (threat detection task) or to judge the gender of the person in the identical stimulus displays (control task). In Experiment 2, subjects were presented with only the photos showing dangerous situations and asked to judge if the person was in mild danger or extreme danger (threat evaluation task) or were presented with the photos showing safe situations and asked to judge the gender of the person in the stimulus displays (control task).
A box‐car design was used. Experiments 1 and 2 consisted of one scan containing six 60‐s sessions. Each session began with the presentation of instructions for 6 s followed by 24 trials. Each trial began with the presentation of a stimulus display for 250 ms followed a fixation cross (0.36° × 0.36°) of 2,000 ms at the center of the screen. The short stimulus duration was used to avoid salient eye movements. The instructions defined either a control task to identify the gender of the person in stimulus displays or the tasks to identify issues associated with threat in the situation. In each experiment, the control task and the tasks associated with threat processing appeared alternately. Subjects pressed one of two buttons with the left or right index finger (counterbalanced across subjects) to respond to each stimulus according to the instructions. The order of the tasks (threat detection and threat evaluation) was counterbalanced across subjects. Anatomical images were obtained from each subject after the functional scanning. Two‐sample t‐tests were conducted to compare the difference in behavioral performance between males and females.
fMRI Measurement
Scanning was performed on a 3 T Siemens Trio system using a standard head coil at Beijing MRI Center for Brain Research. Thirty‐two transversal slices of functional images that covered the whole brain were acquired using a gradient‐echo echo‐planar pulse sequence (64 × 64 × 32 matrix with 3.4 × 3.4 × 4.4 mm3 spatial resolution, TR = 2000 ms, TE = 30 ms, FOV = 220 mm, flip angle = 90°). Anatomical images were obtained using a standard 3D T1‐weighted sequence (256 × 256 × 176 matrix with 0.938 × 0.938 × 1.3‐mm spatial resolution, TR = 1,600 ms, TE = 3.93 ms).
fMRI Data Analysis
SPM2 (the Wellcome Department of Cognitive Neurology, UK) was used for data analysis. The functional images were realigned to the first scan to correct for the head movement between scans. The anatomical image was coregistered with the mean functional image produced during the process of realignment. All images were normalized to a 2 × 2 × 2 mm3 Montreal Neurological Institute (MNI) template in Talairach space [Talairach and Tournoux, 1998] using bilinear interpolation. Functional images were spatially smoothed using a Gaussian filter with a full‐width at half maximum (FWHM) parameter set to 8 mm. The image data were modeled using a box‐car function and were high‐pass filtered and corrected for global means. For each subject, the fixed effect model was used to define the contrasts between threat detection and gender identification (Experiments 1) and between threat evaluation and gender identification (Experiment 2). Random effect analyses were then conducted across the group of subjects based on statistical parameter maps from each individual subject to allow population inferences. Areas of significant activation were identified at the voxel level for values exceeding a P value of 0.001 (uncorrected) and at the cluster level for values exceeding a P value of 0.05 (corrected for multiple comparisons). Conjunction analysis was conducted to assess the common brain areas activated in threat detection (Experiment 1) and threat evaluation (Experiment 2). To examine differential brain activations in the two experiments, we also conducted interaction analysis. This was done by first making contrast of threat detection versus gender identification and contrast of threat evaluation versus gender identification for each subject. A paired t‐test was then conducted to compare the two contrasts. Between subjects, two‐tailed two‐sample t‐tests were conducted to compare the difference in threat detection and evaluation between males and females, similar to the prior work [Grön et al., 2000]. Here, the threshold at the cluster level was also set to P < 0.05 (corrected for multiple comparisons). The SPM coordinates for standard brain from MNI template were converted to Talairach coordinates using a nonlinear transform method (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
Region of interest (ROI) analysis was conducted to compare fMRI signal changes in the brain areas in males and females that showed gender differences in danger perception. The ROIs were defined as spheres with a radius of 3 mm centered at the peak voxel in the activation cluster shown in the SPM two‐sample t‐tests. Percent fMRI signal changes in the ROI related to threat detection was defined as (fMRI signals associated with threat detection minus fMRI signals associated with gender identification)/(the mean of MRI signals associated with threat detection and gender identification) and calculated using the fMRI raw data. The percent signal changes were then compared using two‐tailed two‐sample t‐tests between male and female subjects.
After having identified the involvement of several brain areas (i.e., the parietal and prefrontal cortex bilaterally and the superior medial frontal cortex) in threat detection and that male participants showed stronger parietal activation linked to threat detection than female participants, we performed a psychophysiological interaction (PPI) analysis [Friston et al., 1997] to identify brain regions in this network that showed significantly stronger covariation with the right parietal cortex during threat detection relative to gender identification. The coordinates of the peak voxel from the random effect analysis comparing threat detection with gender identification in Experiment 1 were used to serve a landmark for the individual seed voxels. An ROI of a sphere with 6 mm diameter in the right parietal cortex was searched around the peak voxel. The time series of each ROI were then extracted, and PPI regressor was calculated as the element‐by‐element product of the mean‐corrected activity of this ROI and a vector coding for the differential task effect of threat detection versus gender identification. The PPI regressors reflected the interaction between psychological variable (threat detection vs. gender identification) and the activation time course of the right parietal cortex. The individual contrast images reflecting the effects of the PPI on other brain areas from 10 participants of the male or female groups, who showed reliable right parietal activation associated with threat detection, were subsequently subject to a one‐sample t‐test. The results of the group analysis identified brain regions that showed increased activity to threat detection when the activity in the right parietal cortex was high. The threshold at the voxel level was set to P < 0.001 (uncorrected) for the identification of brain areas that showed significant functional connectivity with the ROI.
RESULTS
Behavioral Performance
In Experiment 1, response accuracy for threat detection did not differ between females (89.8% ± 5.1%) and males (85.5% ± 7.7%) (t = 1.587, P < 0.127). However, females (836 ± 97 ms) responded faster than males (954 ± 160 ms) when asked to identify if the person in the situation was in danger (t = 2.170, P < 0.041). Responses to gender identification did not differ between females (95.6% ± 3.2%, 707 ± 111 ms) and males (95.1% ± 4.8%, 783 ± 164 ms, t = 0.351 and 1.324, both P < 0.199), but were faster to those to threat detection (females: t = 5.053, P < 0.001; males: t = 6.916, P < 0.001).
In Experiment 2, females and males did not differ in the number of stimuli judged as extremely dangerous (52.8% ± 14.3% vs. 47.7% ± 12.7%, t = 0.918, P < 0.369), or the time taken to make this judgment (872 ± 155 vs. 883 ± 108 ms, t = 0.186, P < 0.855). Performance of gender identification did not differ between males (703 ± 146 ms, 92.3% ± 1.1%) and females (747 ± 83 ms, 95.9% ± 4.3%) (t = 0.90 and 1.05, both P < 0.31). Responses to gender identification were faster to those to threat evaluation for both females (t = 3.834, P < 0.003) and males (t = 3.575, P < 0.004).
fMRI Results
Experiment 1: Threat detection versus gender identification
To identify brain circuits involved in threat detection, we analyzed the neural activity for the male and female subjects separately. In the contrast between threat detection and gender identification, males showed activation increases in the superior parietal cortex and the intraparietal sulcus bilaterally (Fig. 2a and Table I). Increased activity was also evident in the lateral prefrontal cortex, including the middle and inferior frontal gyrus bilaterally, and the superior medial prefrontal cortex. Activation was also observed in the left posterior middle temporal gyrus and the right hemisphere of the cerebellum.
Figure 2.
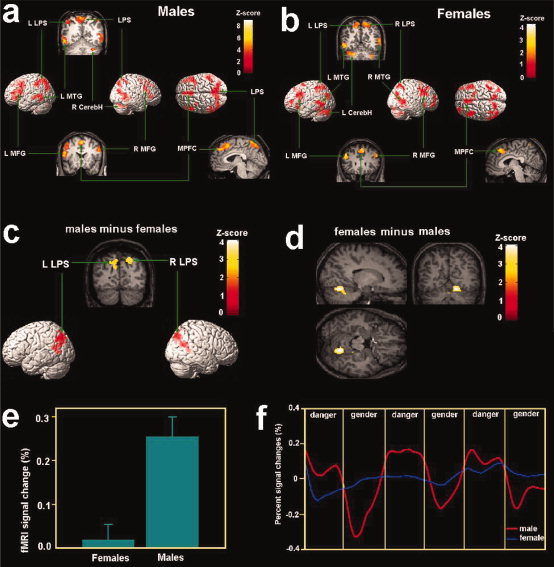
Brain activation associated with danger detection in Experiment 1 shown in the random effect analysis. (a) Brain activations associated with danger detection in males; (b) brain activations associated with danger detection in females; (c) stronger posterior parietal activity was observed in the posterior parietal cortex in males than females during danger detection; (d) stronger cerebellar activity was observed in the cerebellum in females than males during danger detection; (e) percent signal changes of activity in the right parietal cortex was larger for males than females; (f) The time course of signal changes of the ROI in the right parietal cortex. The x‐axis of the time course is for the whole session of Experiment 1. Each local segment of the time course contains one 60‐s epoch in the scan corresponding to danger detection or gender identification. The time courses were averaged from raw fMRI signals. LPS = the superior parietal lobe, MFG = middle frontal gyrus; MPFC = medial prefrontal cortex, MTG = middle temporal gyrus; CerebH = cerebellum.
Table I.
Brain areas showing increased activity in danger detection relative to gender identification in Experiment 1
| Region | Voxel no. | BA | X | Y | Z | Z value | P value |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Medial/left superior parietal cortex | 1,453 | 7 | −10 | −57 | 54 | 4.76 | 0.01 |
| Right superior parietal cortex | 89 | 7 | 38 | −66 | 40 | 4.05 | 0.05 |
| Left inferior parietal cortex | 251 | 40 | −48 | −41 | 43 | 4.54 | 0.01 |
| Dorsal medial prefrontal cortex | 477 | 8 | 0 | 16 | 49 | 4.63 | 0.01 |
| Left inferior frontal gyrus | 234 | 46 | −44 | 39 | 5 | 4.51 | 0.01 |
| Left superior/middle frontal gyrus | 482 | 6/44 | −38 | 6 | 40 | 4.37 | 0.01 |
| Right middle/inferior frontal gyrus | 612 | 9/45 | 42 | 24 | 14 | 4.10 | 0.01 |
| Left posterior middle/inferior temporal gyrus | 161 | 21/37 | −52 | −62 | 3 | 3.98 | 0.01 |
| Cerebellum | 545 | 34 | −70 | −20 | 3.80 | 0.01 | |
| Females | |||||||
| Right superior parietal gyrus | 433 | 7 | 22 | −64 | 51 | 4.44 | 0.01 |
| Left superior parietal gyrus | 327 | 7 | −16 | −62 | 51 | 3.39 | 0.01 |
| Dorsal medial prefrontal cortex | 451 | 8 | 6 | 33 | 44 | 3.91 | 0.01 |
| Left middle/inferior frontal gyrus | 584 | 9/46 | −44 | 27 | 26 | 4.03 | 0.01 |
| Right middle/inferior frontal gyrus | 721 | 9/46 | 50 | 30 | 26 | 3.53 | 0.01 |
| Left posterior middle/inferior temporal gyrus | 490 | 21 | −53 | −52 | 3 | 3.89 | 0.01 |
| Right posterior middle/inferior temporal gyrus | 857 | 21/39 | 55 | −65 | 16 | 3.85 | 0.01 |
| Cerebellum | 1,277 | −24 | −61 | −24 | 4.05 | 0.01 | |
Voxel no. = number of voxels in a cluster; BA = Brodmann area.
The P‐values at the cluster‐level were corrected for multiple comparison.
Similar to males, females showed increased activation related to threat detection in bilateral superior parietal cortex (Fig. 2b and Table I). Activation also peaked in the frontal lobe including the middle and inferior frontal gyrus bilaterally, the superior medial prefrontal cortex. Unlike males, the middle temporal gyrus showed activation increases in both hemispheres, extending through the angular gyrus (BA 39). In addition, for females, cerebellar activation was evident in the left hemisphere.
Two‐sample t‐tests based on the contrast between threat detection and gender identification from each subject were conducted to uncover gender differences in neural activity related to threat detection. Relative to females, males showed significantly stronger activation in the posterior parietal cortex including the inferior parietal sulcus and bilateral precuneus close to the parieto‐occipital sulcus (centered at 20/−72/44, Z = 3.41, P < 0.01; Fig. 2c). The opposite contrast, however, showed increased activation in the cerebellum in females relative to males (14/−61/−10, Z = 3.47, Fig. 2d) at a more liberal threshold of uncorrected P < 0.05. To assess if the gender difference observed in the above two‐sample t‐tests arose from the disparity in gender identification between males and females, another two‐sample t‐test was conducted by contrasting imaging data related to the gender identification task. There was no differential activation between males and females, indicating that the baseline condition was comparable for the two sexes. To further validate the gender difference in the parietal cortex, an ROI analysis was conducted to compare fMRI signals computed from the raw imaging data for both sexes. Percent signal changes were calculated in the ROI centered at the peak voxel (20/−72/44) in the right posterior parietal cortex that showed gender differences in the two‐sample t‐tests. fMRI signal change was greater in males than in females in this area associated with threat detection [t(22) = 4.287, P < 0.001, Fig. 2e]. Figure 2f shows the time course of the fMRI signal change in this area. The percent signal change in this brain area varied systematically as a function of the task (threat detection vs. gender identification) for males but not for females.
Given the differential activation of the right parietal cortex between the two sexes, we examined if the functional connectivity between the right parietal cortex and other brain areas in the network was different between males and females. The PPI analysis confirmed that, in the male participants, the medial prefrontal cortex (BA8/9, −4/54/30, Z = 3.36) showed stronger covariation with the activation in the right parietal cortex during threat detection than during gender identification, suggesting stronger functional connectivity between the right parietal cortex and the medial prefrontal cortex during threat detection. However, similar PPI analysis performed on the female participants did not find significant functional connectivity between the right parietal cortex and other brain areas in the network. Figure 3a,b shows the result of the group analysis and activation profiles for a representative male participant during threat detection and gender identification. Results of the same analysis from female participants are shown in Figure 3c,d.
Figure 3.
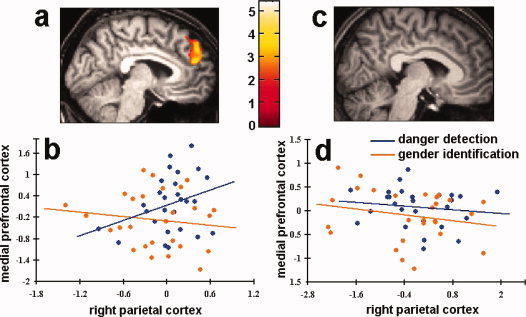
Functional connectivity between the right parietal cortex and the medial prefrontal cortex. (a) The results of the group analysis of the male participants. The medial prefrontal area showed increased activity when the right parietal cortex showed increased activation; (b) regression of the medial prefrontal activation on the right parietal activation for a representative male participant during danger detection and gender identification. The activity of the right parietal cortex and the medial prefrontal cortex were correlated during danger detection but not gender identification; (c) the results of the group analysis of the female participants did not show activation in other brain areas in the network; (d) regression of the medial prefrontal activation on the right parietal activation for a representative female participant during danger detection and gender identification. The activity of the right parietal cortex and the medial prefrontal cortex did not show correlation during both danger detection and gender identification.
Experiment 2: Threat evaluation versus gender identification
The judgment of whether the person in the stimuli was in mild danger or extreme danger was contrasted with judgments of the gender of the person to identify brain circuits involved in threat evaluation. For males, activation increases were observed in the right superior parietal cortex (Fig. 4 and Table II) and left inferior parietal cortex, the middle and inferior frontal gyrus bilaterally, the superior medial prefrontal cortex, the left posterior middle temporal gyrus, and the temporal‐occipital junction. Increased activation was also found in both the right and left hemispheres of the cerebellum.
Figure 4.
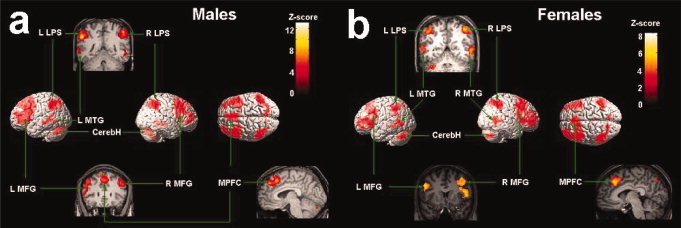
Brain activation associated with danger evaluation in Experiment 2 shown in the random effect analysis. (a) Brain activations associated with danger evaluation in males; (b) brain activations associated with danger evaluation in females. LPS = the superior parietal lobe, MFG = middle frontal gyrus; MPFC = medial prefrontal cortex, MTG = middle temporal gyrus; CerebH = cerebellum.
Table II.
Brain areas showing increased activity in danger evaluation relative to gender identification in Experiment 2
| Region | Voxel no. | BA | X | Y | Z | Z value | P value |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Right superior parietal gyrus | 845 | 7/40 | 51 | −40 | 50 | 4.47 | 0.01 |
| Left inferior parietal gyrus | 1022 | 40 | −55 | −43 | 38 | 5.47 | 0.01 |
| Dorsal medial prefrontal cortex | 1231 | 8 | −8 | 32 | 55 | 4.86 | 0.01 |
| Left middle/inferior frontal gyrus | 2484 | 9/44 | −48 | 17 | 21 | 5.14 | 0.01 |
| Right middle/superior frontal gyrus | 1214 | 8/9 | 38 | 18 | 40 | 4.69 | 0.01 |
| Right inferior frontal gyrus | 442 | 9/10 | 38 | 53 | 15 | 4.96 | 0.01 |
| Left posterior middle/inferior temporal gyrus | 196 | 21/37 | −59 | −54 | 6 | 3.90 | 0.01 |
| Cerebellum | 703 | −16 | −78 | −30 | 4.64 | 0.01 | |
| 75 | 26 | −71 | −18 | 3.82 | 0.01 | ||
| Females | |||||||
| Right superior parietal gyrus | 530 | 7 | 40 | −50 | 52 | 4.03 | 0.01 |
| Left superior parietal gyrus | 230 | 7/40 | −53 | −41 | 41 | 3.74 | 0.01 |
| Dorsal medial prefrontal cortex | 340 | 8 | −8 | 31 | 37 | 4.08 | 0.01 |
| Left inferior frontal gyrus | 644 | 9/46 | −48 | 32 | 11 | 4.25 | 0.01 |
| Left middle frontal gyrus | 213 | 8/9 | −51 | 13 | 32 | 4.02 | 0.01 |
| Right superior frontal gyrus | 1196 | 8/9 | 42 | 10 | 38 | 4.13 | 0.01 |
| Right inferior frontal gyrus | 1409 | 45/46 | 40 | 33 | 4 | 4.58 | 0.01 |
| Left posterior middle/inferior temporal gyrus | 203 | 21/37 | −51 | −60 | 3 | 4.08 | 0.05 |
| Right posterior middle/inferior temporal gyrus | 149 | 21/37 | 60 | −60 | 5 | 3.98 | 0.05 |
| Cerebellum | 427 | −28 | −63 | −25 | 4.45 | 0.01 | |
| 267 | 38 | −68 | −32 | 4.24 | 0.05 | ||
Voxel no. = number of voxels in a cluster; BA = Brodmann area.
The P‐values at the cluster‐level were corrected for multiple comparison.
Females similarly showed activation increases in bilateral superior parietal cortex (Fig. 4 and Table II), the left middle and inferior frontal gyrus, the right superior and middle frontal gyrus, the superior medial prefrontal cortex, bilateral posterior middle temporal gyrus, and the temporal‐occipital junction. Females also showed increased activation in both hemispheres of the cerebellum.
Two‐sample t‐tests were also conducted to examine gender differences in neural activity related to threat evaluation. However, no differential activity in any brain areas was observed between males and females.
Comparison across experiments 1 and 2
The conjunction analysis identified brain activations common for both threat detection (Experiment 1) and threat evaluation (Experiment 2). For male subjects, the conjunction analysis showed activations in the superior parietal lobe (−12/−72/46, Z = 4.15), left prefrontal cortex (−52/26/15, Z = 4.03), right posterior temporal cortex (53/−45/−8, Z = 3.92), medial prefrontal cortex (−2/44/35, Z = 3.82), right prefrontal cortex (57/20/21, Z = 3.59), right posterior temporal cortex (−55/−55/−2, Z = 3.78), and the cerebellum (30/−61/−23, Z = 3.95). For female subjects, the conjunction analysis showed activations in the right posterior temporal cortex (60/−58/6, Z = 4.25), superior parietal lobe (22/−62/56, Z = 4.16), left posterior temporal cortex (−53/−62/3, Z = 4.12); left prefrontal cortex (−46/26/26, Z = 3.72), medial prefrontal cortex (4/31/43, Z = 3.64), right prefrontal cortex (46/10/42, Z = 3.58), and the cerebellum (−24/−64/−24, Z = 4.52).
To compare the magnitudes of the activation increases related to threat detection and evaluation, paired t‐tests were conducted by contrasting brain activation associated with threat evaluation (Experiment 2) and threat detection (Experiment 1) for males and females, respectively. For males, threat evaluation elicited stronger activity in the superior parietal cortex bilaterally, the superior and inferior frontal cortex bilaterally, and the medial prefrontal cortex (Table III). For females, threat evaluation showed increased activity in the right superior parietal cortex and the right superior/middle/inferior frontal cortex as compared with threat detection. The reverse contrast did not show any increased activity in the network associated with threat detection compared with threat evaluation.
Table III.
Brain areas showing stronger activity in danger evaluation (Experiment 2) relative to danger detection (Experiment 1)
| Region | Voxel no. | BA | X | Y | Z | Z value | P value |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Right superior parietal gyrus | 749 | 7/40 | 48 | −47 | 41 | 4.71 | 0.01 |
| Left superior parietal gyrus | 592 | 7/40 | −48 | −56 | 52 | 5.65 | 0.01 |
| Dorsal medial prefrontal cortex | 588 | 8 | 2 | 29 | 39 | 4.31 | 0.01 |
| Left superior frontal gyrus | 97 | 9 | −36 | 26 | 36 | 3.97 | 0.05 |
| Left middle frontal gyrus | 411 | 46 | −30 | 45 | 14 | 4.58 | 0.01 |
| Left inferior frontal gyrus | 111 | 47 | −46 | 15 | −2 | 4.03 | 0.05 |
| Right superior frontal gyrus | 646 | 8 | 42 | 25 | 44 | 4.54 | 0.01 |
| Right inferior frontal gyrus | 191 | 10 | 44 | 52 | −4 | 4.60 | 0.01 |
| Females | |||||||
| Right superior parietal gyrus | 445 | 7/40 | 57 | −46 | 43 | 3.37 | 0.05 |
| Right superior frontal gyrus | 352 | 8 | 28 | 19 | 34 | 4.49 | 0.05 |
| Right middle frontal gyrus | 363 | 9/46 | 40 | 46 | 23 | 3.76 | 0.05 |
| Right inferior frontal gyrus | 917 | 45 | 42 | 31 | 2 | 4.07 | 0.01 |
Voxel no. = number of voxels in a cluster; BA = Brodmann area.
The P‐values at the cluster‐level were corrected for multiple comparison.
DISCUSSION
The current study provides evidence that a neural network that is independent of the emotion‐related fear system in the human brain can mediate detection and evaluation of evolutionary unprepared threat cues. To investigate the processing of evolutionary unprepared threat cues in social environments, we presented displays showing people in safe or potentially dangerous situations that did not contain evolutionary prepared threats (e.g., sakes) and overt emotional contents (e.g., angry faces). We assessed the neural activity when subjects either detected the presence of potential danger or evaluated degree of danger to a subject in a display by comparing threat detection and evaluation tasks with a gender identification task that controlled for the processing of low‐level visual features and threat irrelevant social information. Because the stimuli and motor responses in Experiment 1 were identical for these comparisons, any differential activity caused by automatic emotional responses to stimuli should be eliminated. Even the contrast between potentially dangerous and safe situations in Experiment 2 did not show any activation increase in the neural system usually associated with emotional responses (e.g., the amygdala, anterior cingulate cortex, and orbitalfrontal cortex) [Carlsson et al., 2004; Carretie et al., 2005; Morris et al., 1996; Pissiota et al., 2003]. Thus, our findings indicate that detection and evaluation of evolutionary unprepared threats in social situations do not necessarily involve the emotion‐related neural system and that the processing of threat signals can be independent of the affective network in the absence of overt threat cues (such as fearful faces or snakes). The lack of increased activity of the emotion‐related neural system was evident even when explicit tasks were assigned to the evolutionary unprepared threat cues in the stimuli, suggesting that the processing of such threat cues is significantly different from that of overt threat cues that automatically activated the emotion‐related neural system. One account for the absence of the activity of the affective network was that our stimuli did not activate the emotion‐related system because the stimuli had no overt emotion contents such as angry face or snakes. Alternatively, the activity of other brain areas related to the detection or evaluation tasks might dampen the activity to the evolutionary unprepared threat cues in brain structures such as amygdala. Such modulation of the amygdala activity has been observed in the previous research that found increased frontal activity and decreased activity in the amygdala associated with determining whether affect pictures goes with the word “angry” or “afraid” [Hariri et al., 2000, 2003].
Across both male and female subjects, we found evidence for a shared neural network engaged in detection and evaluation of evolutionary unprepared threat cues. This network includes the medial and lateral frontal lobes, superior parietal lobes, posterior middle temporal cortex, and the cerebellum. Parietal activity associated with the processing of these threat cues was evident in the superior parietal cortex and the intraparietal sulcus (BA 7/40) in both hemispheres. Previous studies have shown evidence that these brain areas in the dorsal pathway play an important role in the processing of spatial information [Colby and Goldberg, 1999]. Particularly, the posterior superior and inferior parietal cortices mediate the formation of spatial representations of body locations with respect to the subject's environment [the intraparietal sulcus, Their and Andersen, 1996] and visual‐spatial judgments [BA7/40, Fink et al., 2000], while the mesial superior parietal cortex (BA7) is linked to representing location information from a third person's perspective [Vogeley et al., 2004]. Applying the prior results on spatial‐cognition in the parietal cortex, we suggest that the functional role of the parietal activation in our study was to analyze the spatial information in the stimulus displays for identification of the evolutionary unprepared threat cues. For instance, the spatial relationship between the person and the car (i.e., whether the person is in front of or besides the car and the distance between the person and the car) is pivotal for judging if the person in Figure 1 is in danger. Interestingly, recent monkey studies showed that both stimulation of the intraparietal sulcus and air pruff simulating noxious threats evokes facial or shoulder movement specific for a defense reaction [Cooke and Graziano, 2003], suggesting that the intraparietal sulcus plays a role in visuospatial encoding of noxious threats. Our fMRI results reinforce this by showing that this brain area is also involved in perception of threat cues in social environments.
Detection and evaluation of these threat cues were also linked to the lateral prefrontal lobes in both hemispheres, the posterior middle/inferior temporal gyrus (in the left hemisphere for males and bilaterally for females), and the cerebellum. Numerous imaging studies have demonstrated that these brain regions are involved in memory retrieval. Particularly, the retrieval of semantic knowledge is linked to the left frontal and temporal gyrus [Martin et al., 1995; Thompson‐Schill et al., 1997; Tulving et al., 1994; Wiggs et al., 1999] and the retrieval of information from episodic memory is associated with the right frontal gyrus and the cerebellum [Donohue et al., 2005; Henson et al., 1999; Wiggs et al. 1999]. These results support a hemispheric encoding retrieval asymmetry model that differentiates the functions of the left and right hemispheres in memory retrieval [Tulving et al., 1994]. Retrieval of information from both semantic knowledge and episodic memory is critical for threat detection and evaluation. To decide that a person walking in front of a moving car is in potential danger requires semantic knowledge that people can be hit when they walk in this position and recall of traffic accidents observed in media or movies. The posterior parietal lobes may also contribute to episodic memory retrieval when processing threat signals by guiding attention to the appropriate internal representation in memory [Wagner et al., 2005].
One further region engaged in processing threat signals was the medial prefrontal cortex (BA 8). Activation of these brain regions has been observed when people make judgments about humans relative to objects [Mitchell et al., 2002] or animals [Mason et al., 2004] and when people perceive humans but not animals [Han et al., 2005]. These results indicate a key role of this area in the processing of person knowledge. In addition, the medial prefrontal cortex has been associated with drawing inductive reasoning based on others' knowledge states [Goel et al., 1995] and deductive reasoning about contexts within a social content [Canessa et al., 2005]. Since subjects in our study were asked to make judgments about people in particular social contexts, the medial prefrontal cortex could be recruited to represent person knowledge. Detection of these threat cues required more inspection of social contexts (and took longer time) than judging a person's gender. The medial prefrontal cortex might also be involved in making inference about potential danger based on the analysis of the contexts, providing a third cognitive component in processing threat signals in addition to spatial analysis and memory retrieval.
While our fMRI results showed evidence for a shared neural network in males and females associated with threat detection and evaluation, we also found evidence for differential activity. Specifically, we found stronger activity in the posterior parietal cortex including the inferior parietal sulcus (BA 7), with an extension into the precuneus bilaterally, in males than in females. The ROI analysis showed that fMRI signal change in the right posterior parietal cortex varied systematically as a function of the task (threat detection vs. gender identification) for males but not for females. These differences were evident during detection (Experiment 1) but not evaluation (Experiment 2) of the threat cues. Cells in the posterior parietal cortex (BA7) receive input from areas (e.g. V3) that are implicated in spatial or motion analysis [Baizer et al., 1991] and play an important role in multimodal representation of space [Andersen et al., 1997]. The precuneus also acts in concert with the lateral posterior parietal cortex in elaborating spatial relations in egocentric and allocentric space for the control of body movement and other higher‐order spatial processes, including attention shifts in space [Cavanna and Trimble, 2006]. Therefore, the increased posterior parietal activity observed in males relative to females is consistent with males having enhanced processing of spatial relationships between people and objects (e.g., car, gun) in the stimulus displays when asked to detect threat. This fMRI result also fits with the hunter‐gatherer theory of spatial abilities [Silverman and Eals, 1992; Silverman et al., 2000], which posits that males should out‐perform females in spatial skills (i.e., orienting oneself in relation to objects and places or assessing spatial relations between objects and places) that would facilitate successful hunting. Our results suggest that these enhanced spatial abilities may also be recruited when responding to the presence of threat signals. The spatial processing involved, however, appears to be different from the visuospatial navigation ability that is associated with stronger activity in right inferior parietal cortex (BA 40) in females than in males [Grön et al., 2000].
The precuneus also shows activation during tasks requiring memory retrieval. In particular, the increased activity of precuneus has been associated with retrieval of information from episodic memory [Cavanna and Trimble, 2006]. Given that males report being exposed to violence more than females [Barkin et al., 2001], it may be proposed that, when detecting the presence of threat signals in the stimuli, past experience of exposure played a stronger role for males relative to females. The additional spatial analysis and memory retrieval might take more time and result in longer reaction times to threat detection in males than females. Another interpretation, however, is that females were superior at responding to threat signals relative to males, and therefore had less need to recruit explicit memory retrieval and spatial processing in order to decide that a given situation was dangerous. This would fit with work showing that females are more cautious and sensitized to danger, relative to males [Campbell, 1999; Hines and Fry, 1994], and with the behavioral evidence for females having faster RTs to detect threat in Experiment 1 here. The one area that showed the opposite pattern across the genders, with more activation for females compared to males, was the cerebellum. However, the cerebellum activity was only observed with a relatively lax threshold, and this result needs to be confirmed in future work.
Interestingly, the increased posterior parietal activity in males relative to females was evident only when the task required discrimination between dangerous and safe situations, and no gender difference was observed when subjects were asked to evaluate the degree of danger in scenes already selected as being dangerous. This suggests that males and females differed in their responsiveness and the processes they brought to bear when they had to detect threat. However, having decided there was threat present, males and females engaged in similar explicit processes to judge the degree of danger. Hence, there was increased neural activity in both genders, in regions activated in detecting threat.
Finally, the PPI analysis showed stronger functional connectivity between the right parietal cortex and the medial prefrontal cortex during threat detection than gender identification. But this was evident in male but not female participants. It appears that the stronger parietal activity linked to threat detection in males than females also resulted in stronger functional connectivity between the right parietal cortex and other brain areas. If the medial prefrontal cortex plays a role in inference about potential danger based on the analysis of the contexts, as suggested above, it had to receive information from other brain areas to accomplish the process of inference. This can explain why the parietal cortex exhibited significantly strong connectivity with the medial prefrontal cortex but not with other brain areas.
CONCLUSION
Our brain imaging results demonstrate the existence of a neural network engaged in danger perception in the absence of overt threat cues (e.g., snakes or angry faces) formed by evolutionary experience. The components of this network for the processing of evolutionary unprepared threat cues in social situations did not overlap with the brain areas belonging to the emotion‐related system, indicating that the neural network underlying the cognitive processing of the threat cues can be independent of the brain circuits underlying emotional responses to overt threat cues such as fearful faces or snakes. The same network was engaged in both detection and evaluation of the threat cues but showed increased activity in the latter condition, implying that there was likely more processing of similar perceptual signals when the level of danger had to be judged in a known dangerous situation. Our brain imaging results suggest that the processing of these threat cues involves at least three cognitive processes, i.e., perceptual analysis of the spatial relations between people and objects in a scene, retrieval of information from semantic and episodic memory, and inference processes based on the context. Males and females recruited a common neural network for the detection and evaluation of the threat cues, suggesting common processes of danger perception. Nevertheless, for detecting threat, males showed stronger activation in the posterior parietal cortex relative to females, suggesting that males engaged more in coding spatial relationships between people and objects in the environment, and that their judgments could involve retrieval of dangerous situations from long‐term memory. However, it should be noted that our speculations of the functional role of each subcomponent of the neural circuit underlying the neural processing of threat cues in social environments were drawn on the basis of previous brain imaging evidence for the cognitive function of the brain areas such as parietal lobule, lateral frontal cortex, and precuneus. Such reverse inference does not demonstrate these speculations. However, the discussions based on reverse inference help to address possible functional roles of each subcomponent of the neural circuit that will stimulate further research.
REFERENCES
- Andersen RA, Snyder LH, Bradley DC, Xing J ( 1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 202–220. [DOI] [PubMed] [Google Scholar]
- Ardrey R ( 1976): The Hunting Hypothesis. New York: Atheneum. [Google Scholar]
- Barkin S, Kreiter S, Durant RH ( 2001): Exposure to violence and intentions to engage in moralistic violence during early adolescence. J Adolesc 24: 777–789. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R ( 1991): Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neursci 11: 168–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A ( 1999): Staying alive: Evolution, culture, and women's intra‐sexual aggression. Behav Brain Sci 22: 203–252. [DOI] [PubMed] [Google Scholar]
- Campbell A, Muncer S, Bibel D ( 2001): Women and crime: An evolutionary approach. Aggress Violent Behav 6: 481–497. [Google Scholar]
- Canessa N, Gorini A, Cappa SF, Piattelli‐Palmarini M, Danna M, Fazio F, Perani D ( 2005): The effect of social content on deductive reasoning: An fMRI study. Hum Brain Mapp 26: 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Ohman A ( 2004): Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear‐relevant (but nonfeared) stimuli. Emotion 4: 340–353. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Mercado F, Tapia M ( 2005): Cortical response to subjectively unconscious danger. Neuroimage 24: 615–623. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioral correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME ( 1999): Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Graziano MSA ( 2003): Defensive movements evoked by air puff in monkeys. J Neurophysiol 90: 3317–3329. [DOI] [PubMed] [Google Scholar]
- Dahlberg F ( 1981): Woman the Gatherer. New Haven: Yale University Press. [Google Scholar]
- Donohue SE, Wendelken C, Crone EA, Bunge SA ( 2005): Retrieving rules for behavior from long‐term memory. Neuroimage 26: 1140–1149. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse‐Ruyken M, Ziemons K, Zilles K, Freund HJ ( 2000): Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54: 1324–1331. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Büchel G, Fink GR, Morris J, Rolls E, Dolan RF ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Öhman A ( 1999): Fear appear fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology 36: 66–75. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M ( 1995): Modeling other minds. Neuroreport 6: 1741–1746. [DOI] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW ( 2000): Brain activation during human navigation: Gender‐different neural networks as substrate of performance. Nat Neurosci 3: 404–408. [DOI] [PubMed] [Google Scholar]
- Han S, Jiang Y, Humphreys GW, Zhou T, Cai P ( 2005): Distinct neural substrates for the perception of real and virtual visual worlds. Neuroimage 24: 928–935. [DOI] [PubMed] [Google Scholar]
- Harris MB, Miller KC ( 2000): Gender and perception of danger. Sex Roles 43: 843–863. [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC ( 2000): Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 11: 43–48. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR ( 2003): Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53: 494–501. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ ( 1999): Right prefrontal cortex and episodic memory retrieval: A functional fMRI test of the monitoring hypothesis. Brain 122: 1367–1381. [DOI] [PubMed] [Google Scholar]
- Hines NJ, Fry DP ( 1994): Indirect modes of aggression among women of Buenos Aires, Argentina. Sex Roles 30: 213–236. [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG ( 1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105. [DOI] [PubMed] [Google Scholar]
- Mason MF, Banfield JF, Macrae SN ( 2004): Thinking about actions: The neural substrates of person knowledge. Cereb Cortex 14: 209–214. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS ( 2004): A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry 55: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Mineka S, Öhman A ( 2002): Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biol Psychiatry 52: 927–937. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN ( 2002): Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA 99: 15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJA ( 1996): Differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S ( 2001): Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev 108: 483–522. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares F ( 1993): On the automaticity of phobic fear: Conditioned skin conductance responses to masked phobic stimuli. J Abnorm Psychol 102: 121–132. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans Ö, Michelgard A, Appel L, Langström B, Flaten MA, Fredrikson M ( 2003): Amygdala and anterior cingulated cortex activation during affective startle modulation: A PET study of fear. Eur J Neurusci 18: 1325–1331. [DOI] [PubMed] [Google Scholar]
- Schienle A, Axel S, Rudolf S, Bertram W, Dieter V ( 2005): Gender differences in the processing of disgust‐ and fear‐inducing pictures: An fMRI study. Neuroreport 16: 277–280. [DOI] [PubMed] [Google Scholar]
- Silverman I, Eals M ( 1992): Sex differences in spatial abilities: Evolutionary theory and data In: Barkow JH, Cosmider L, Tooby J, editors. The Adapted Mind. Oxford: Oxford University Press; pp 533–549. [Google Scholar]
- Silverman I, Choi J, Machewn A, Fisher M, Moro J, Olshansky E ( 2000): Evolved mechanisms underlying wayfinding: Further studies on the hunter‐gatherer theory of spatial sex differences. Evol Hum Behav 21: 201–213. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1998): Co‐Planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Their P, Andersen RA ( 1996): Electrical microstimulation suggests two different forms of representation of head‐centered space in the intraparietal sulcus of rhesus monkeys. Proc Natl Acad Sci USA 93: 4962–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CY, Bankstone WB, LaPierre RL ( 1992): Parity and disparity among three measures of fear of crime: A research note. Devi Behav An Interdiscipl J 13: 373–389. [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S ( 1994): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai F, Zilles K, Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Weber EU, Blais A, Betz NE ( 2002): A domain‐specific risk‐attitude scale: Measuring risk perceptions and risk behaviors. J Behav Dec Making 15: 263–290. [Google Scholar]
- Wiggs CL, Weisberg J, Martin A ( 1999): Neural correlates of semantic and episodic memory retrieval. Neuropsychologia 37: 103–118. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA ( 2005): Distinct amygdala‐autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 28: 618–626. [DOI] [PubMed] [Google Scholar]


