Abstract
Impaired performance in verbal fluency tasks is an often replicated finding in schizophrenia. In functional neuroimaging studies, this dysfunction has been linked to signal changes in prefrontal and temporal areas. Since schizophrenia has a high heritability, it is of interest whether susceptibility genes for the disorder, such as NRG1, modulate verbal fluency performance and its neural correlates. Four hundred twenty‐nine healthy individuals performed a semantic and a lexical verbal fluency task. A subsample of 85 subjects performed an overt semantic verbal fluency task while brain activation was measured with functional magnetic resonance imaging (MRI). NRG1 (SNP8NRG221533; rs35753505) status was determined and correlated with verbal fluency performance and brain activation. For the behavioral measure, there was a linear effect of NRG1 status on semantic but not on lexical verbal fluency. Performance decreased with number of risk‐alleles. In the fMRI experiment, decreased activation in the left inferior frontal and the right middle temporal gyri as well as the anterior cingulate gyrus was correlated with the number of risk‐alleles in the semantic verbal fluency task. NRG1 genotype does influence language production on a semantic level in conjunction with the underlying neural systems. These findings are in line with results of studies in schizophrenia and may explain some of the cognitive and brain activation variation found in the disorder. More generally, NRG1 might be one of several genes that influence semantic language capacities. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: NRG1, fMRI, schizophrenia, verbal fluency, ACC
INTRODUCTION
Schizophrenia is a common and severe disorder with an onset in early adulthood [Owen et al., 2003]. Its etiology, symptom clusters, and course are heterogeneous [Kirov et al., 2005] and are known to be under genetic control. A meta‐analysis of 12 twin studies estimated heritability in liability to schizophrenia at 81% [Sullivan et al., 2003]. Only recently the first vulnerability genes for schizophrenia have been reported. These include among others neuregulin 1 (NRG1) [Li et al., 2006; Stefansson et al., 2002; Zhao et al., 2004], DISC1 and DISC2 [Hennah et al., 2003, 2005; Hodgkinson et al., 2004; Porteous et al., 2006], dysbindin [Schwab et al., 2003; Straub et al., 2002; Van Den Bogaert et al., 2003], and RGS4 (Chen et al., 2004; Morris et al., 2004; Williams et al., 2004].
NRG1 was identified in an Icelandic sample by Stefansson et al. [ 2002] and associations between this gene and schizophrenia were repeatedly found in independent populations [Munafo et al., 2006; Tosato et al., 2005]. The core at‐risk haplotype consists of two microsatellite markers and five single nucleotide polymorphisms (SNPs). Of all studied markers, SNP8NRG221533 (rs35753505), which is located in the 5′‐untranslated region of NRG1, is the most commonly reported single marker [Munafo et al., 2006]. Even though some authors found strong associations of SNP8NRG221533 with schizophrenia [Li et al., 2006; Stefansson et al., 2003], findings are not unequivocal [Munafo et al., 2006; Thiselton et al., 2004; Walss‐Bass et al., 2005; Zhao et al., 2004], and two studies [Addington et al., 2007; Bakker et al., 2004] observed overtransmission of the opposite allele reported in the original study [Stefansson et al., 2002]. There is plausible evidence for the importance of NRG1 in the etiology of schizophrenia. NRG1 is involved in a number of neurodevelopmental functions [Corfas et al., 2004], such as neuronal migration [Anton et al., 1997; Ghashghaei et al., 2006], myelination [Chen et al., 2006; Nave and Salzer, 2006], neurotransmitter receptor expression and function [Hahn et al., 2006; Liu et al., 2001; Ozaki et al., 1997], and various other cerebral processes [Falls, 2003; Harrison and Law, 2006]. Thus it may be promising to study possible associations between NRG1 and putative underlying endophenotypes.
Cognition is known to be generally impaired in schizophrenia [Glahn et al., 2007; Heinrichs and Zakzanis, 1998]. Among the different cognitive domains, verbal memory, word fluency, and attention are constructs with the highest effect sizes when compared with healthy control subjects. Between 61% and 78% of patients perform below the median of aggregated patient‐control samples in these domains [Heinrichs and Zakzanis, 1998]. Most importantly, these impairments are independent of psychotic episodes and are found in risk groups and relatives of schizophrenia patients. These domains are, thus, under potential genetic influence and of interest to test as putative endophenotype markers in conjunction with susceptibility genes.
The concept of cognitive endophenotypes has already been successfully applied in several studies in schizophrenia, particularly using a COMT polymorphism [Egan et al., 2001; Stefanis et al., 2005]. With respect to NRG1, however, only little research has been conducted so far: NRG1 has been found to correlate with personality dimensions [Krug et al., 2008a, b] and to have an influence on personality and an influence on verbal IQ and brain activation in the Hayling task in a high‐risk population [Hall et al., 2006] and has been reported to have an effect on brain volume in patients with childhood‐onset schizophrenia [Addington et al., 2007]. In addition, it could be shown that NRG1 has an influence on white matter density and integrity in healthy individuals [McIntosh et al., 2007]. On a behavioral level, NRG1 status has been shown to influence performance on the continuous performance task (CPT) and its neural correlates [Krug et al., 2008a, b; Stefanis et al., 2007].
The cognitive domain investigated in the current study covers word production utilizing a traditional semantic verbal fluency paradigm. Despite extensive research in healthy populations [for a review on word production see Indefrey and Levelt, 2004], the neural correlates have only been sparsely studied in patients with schizophrenia, mostly corresponding to performance during letter fluency tasks [for an overview of letter fluency see Spence et al., 2000; for semantic fluency see Kircher et al., in press; Ragland et al., 2007]. For both fluency versions, BOLD‐responses in healthy subjects have been reported predominantly in prefrontal and temporal regions of the perisylvian language network, yet the data are heterogeneous. Compared with healthy control subjects, hyperactivity of the left superior temporal cortex has been reported during letter fluency besides comparable left prefrontal activation patterns [for an overview see Spence et al., 2000]. Other neuroimaging studies failed to show such temporal response patterns but stressed the relevance of impaired executive functions associated with the lateral and medial (i.e. anterior cingulate) prefrontal cortices. Again, the results ranged from hypo‐ to hyperactivations in patients compared with control subjects during letter fluency [Boksman et al., 2005; Curtis et al., 1998; Dye et al., 1999; Fletcher et al., 1996; Frith et al., 1995; Fu et al., 2005; Spence et al., 2000; Yurgelun‐Todd et al., 1996]. Suggestions have been made to describe the BOLD‐response patterns in a more systematic way, implying an interaction between prefrontal and temporal structures during expressive language tasks [Fletcher et al., 1996; Ford et al., 2002; Frith et al., 1995; Yurgelun‐Todd et al., 1996].
In this study, the performance on and the neural correlates of a verbal fluency task was linked to genetic NRG1 status in large groups of healthy subjects. The SNP8NRG221533 (rs35753505) in the NRG1 gene which has most consistently been found to be associated with schizophrenia [Li et al., 2006] was analyzed as marker. Based on meta‐analytical findings [e.g. Bokat and Goldberg, 2003], it was hypothesized that performance on the fluency tasks correlates with the number of NRG1 risk‐alleles. Differences between risk groups in brain activation were expected in key regions underlying verbal fluency performance in healthy and schizophrenia subjects, such as left lateral and medial prefrontal and temporal cortices.
METHODS AND MATERIALS
Overall Sample
Subjects
Subjects were recruited at RWTH Aachen University, the majority of them were students. Four hundred twenty‐nine subjects (218 men, 211 women) were enrolled into the study. The inclusion criteria were age 18–55 years and no psychiatric disorder according to ICD‐10. The subjects had a mean age of 24.7 years (SD = 5.97), were all right handed [as tested with the Edinburgh Laterality Scale [Oldfield, 1971] and had 15.53 (2.63) years of education. Their fathers were educated for 14.99 (6.73) and their mothers for 13.36 (4.29) years on average. The German MWT‐B multiple choice vocabulary test (Lehrl et al., 1995] was used as assessment for verbal intelligence estimation. Scores were converted into IQ estimates. Characteristics of this sample are given in Table I. All subjects were of Western‐ or Middle European descent. After a complete description of the procedure subjects provided written informed consent to participate in the study. The protocol was approved by the local ethics committee according to the declaration of Helsinki. After participants provided consent, the cognitive tests were assessed and 30 ml venous EDTA blood was taken.
Table I.
Sociodemographic data of the behavioural sample, standard deviations in parentheses (n = 429)
| Variable | T/T | T/C | C/C | F | P |
|---|---|---|---|---|---|
| Sex ratio (men/women) | 89/86 | 106/98 | 23/27 | χ = 0.57 | NS |
| Age | 24.97 (6.5) | 24.3 (5.11) | 25.38 (7.22) | 0.92 | NS |
| Education | 15.43 (2.6) | 15.74 (2.5) | 15.02 (3.2) | 1.7 | NS |
| Estimated verbal IQ | 111.1 (11.7) | 110.4 (11.8) | 110.7 (13.8) | 0.17 | NS |
NS = nonsignificant (all P > 0.1).
fMRI sample
Eighty‐five of the 429 individuals who participated in the behavioral part participated in the fMRI study. Subjects were chosen based on four criteria: First, subjects had to be native German speakers for the verbal fluency task during fMRI scanning. Second, subjects had to be eligible for scanning (no tattoos, etc.), third they had to be willing to be scanned, and fourth we had to ensure that we had enough subjects in each genotype group. Written informed consent was obtained from all subjects after thorough description of the study. The protocol was approved by the local ethics committee. After analyzing the fMRI data, five subjects had to be excluded from further analyses for not following instructions. The characteristics of this sample are given in Table II.
Table II.
Sociodemographic variables of subjects in fMRI study, standard deviations in parentheses (n = 80)
| Variable | T/T | T/C | C/C | F | P |
|---|---|---|---|---|---|
| Sex ratio (men/women) | 22/12 | 16/3 | 16/11 | χ2 = 2.7 | NS |
| Age | 23.59 (3.7) | 23.53 (2.5) | 22.78 (2.5) | 1.07 | NS |
| Education | 15.9 (3.0) | 16.0 (2.5) | 15.2 (2.1) | 0.77 | NS |
| Estimated verbal IQ | 113.0 (11.7) | 109.5 (12.2) | 112.4 (13.9) | 0.49 | NS |
NS = nonsignificant (all P > 0.1).
Genetic Analysis
The SNP8NRG221533 (rs35753505) was genotyped using Applied Biosystems 7900HT Fast Real‐Time PCR System and TaqMan‐probes designed by Applied Biosystems (Foster City, CA). Primers and VIC/FAM‐probe sequences for SNP8NRG221533 detection were as follows: forward‐5′‐TTTAAGGCATCAGTTTTCAATAGCTTTTTTATGT‐3′; reverse‐5′‐AGACAGATGTCTCAAGAGACTGGAA‐3′; 5′‐VIC‐CATGTATCTTTATTTTGCCAAAT‐3′; 5′‐FAM‐CATGTAT CTTTATTTTACCAAAT‐3′. Sequence information was obtained from the homepage of deCODE Genetics (http://decode.com/nrg1/markers/SNPS.htm). Hardy‐Weinberg equilibrium (HWE) was assessed using Haldanés exact test (Elston, 1977]. Genotype distribution was in accordance with HWE (P = 0.57).
Behavioral Task
Verbal fluency
The participants' verbal fluency was measured with two widely used tests [Daum et al., 1996]. Only one category and one letter was used due to time limitations. In the semantic verbal fluency task, as many countries as possible had to be named within 1 minute. In the letter fluency task as many words as possible beginning with the letter b had to be produced within 1 minute. Criteria for hits were either to name a country (only once!) or name any non‐name word beginning with the letter “b.” Subjects were instructed to name each instance only once. In the lexical task, subjects were additionally instructed not to commit any perseveration errors such as “bird” and “birdcage.” Errors in the semantic task occurred mostly due to naming of provinces or states instead of countries (such as Alaska, America, or Tuscany). Number of correctly named countries and words were recorded. To control for differences in other cognitive domains, other cognitive tests were assessed: These were the d2 attention task (Brickenkamp, 2002], the letter‐number‐span [Gold et al., 1997], the spatial span [Wechsler, 1997], and the TMT‐B [Reitan and Wolfson, 1985].
fMRI Task
Task and stimuli
The task has been used previously in two other studies of ours successfully [Whitney et al., in press; Kircher et al., 2008]. The procedure, the selection of stimulus words and pretests are described there in detail. The subjects of the current study are different from the previous ones. Stimuli were presented with Presentation software package (Neurobehavioral Systems Inc., San Francisco, CA). Words were presented in white color on a black background. This semantic verbal fluency task used a block design with two alternating conditions: subjects had to read aloud single German nouns presented to them (high‐level baseline condition) or, in response to a German noun, they had to name one member of the category this noun represented (e.g. say “dog” when the word “animal” was presented; semantic verbal fluency condition). Hits were any members of the category just presented. Errors were any nonmember of the given category (e.g. when “tree” was presented and “rose” was answered). There were four baseline blocks and four semantic verbal fluency blocks. All stimuli represented categories and were only presented once. Each block consisted of 10 stimuli (resulting in 80 stimuli total) and was 34‐second long. At the beginning of each block, an instruction slide was shown for 2,000 ms (semantic verbal fluency: “generate a category member”; baseline: “read the word”). Then, a fixation cross appeared in the centre of the screen for 500 ms which was followed by the stimulus word for 3,000 ms. Subjects were required to respond within this time frame. Appearance of the #‐symbol for 6,000 ms indicated the end of each block.
Data Acquisition
Imaging was performed on a 3‐T Tim Trio MR scanner (Siemens Medical Systems) in the Institute of Neuroscience and Biophysics—Medicine, Research Centre Jülich. Functional images were collected with echo planar imaging (EPI) sensitive to BOLD contrast (T2*, 64 × 64 matrix, FoV 200 mm × 200 mm, 36 slices, 3 mm thickness, TR = 2.25 seconds, TE = 30 ms, flip angle = 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior‐posterior commissural line (AC‐PC). One hundred fifty‐seven functional images were collected, and the initial three images excluded from further analysis to remove the influence of T1 stabilization effects.
Data Analyses
Since there were enough data points in all three groups, statistical analyses of genotype effects in the behavioral as well as fMRI data were performed in accordance with a codominant model (regression analysis), thus checking if the groups showed differences in a linear fashion (either an increasing effect with increasing number of C or T‐alleles).
fMRI Data Analysis
FMRI data analyses were calculated using SPM5. After realignment, unwarping and stereotaxic normalization (2 mm × 2 mm × 2 mm), a 6‐mm full‐width‐at‐half‐maximum (FWHM) Gaussian smoothing kernel was applied to increase the signal‐to‐noise ratio and compensate for intersubject anatomical variation. The volume of interest was restricted to grey matter voxels by use of an inclusive mask created from the segmentation of the standard brain template (SPM2).
Semantic verbal fluency related brain activation was analyzed for each subject contrasting the semantic verbal fluency condition with high‐level baseline. In a first step, a one sample t‐test was calculated for the whole sample (n = 85) to investigate general semantic verbal fluency activation. To investigate genotype effects, resulting contrasts were entered in a multiple regression using genotype as a covariate. The multiple regression was calculated both ways to detect increasing brain activation depending on increasing number of T‐alleles or C‐alleles. To correct for multiple comparisons within a search volume we applied a cluster extent threshold determined by Monte Carlo simulations [Slotnick et al., 2003]. For a threshold at the voxel level at P = 0.001 and spatial properties as present in this study, 10,000 simulations resulted in an extent threshold of 26 resampled voxels. This procedure prevented a false‐positive rate above 5% due to multiple testing. Brain activations were plotted on the anatomical SPM template.
RESULTS
Whole Group Results
Genetic analysis
Resulting distribution of SNP8NRG221533 (rs35753505) was 50 with C/C genotype (23 males), 204 with T/C genotype (106 males), and 175 with T/T genotype (89 males). The resulting groups and variables are displayed in Table I (behavioral sample) and Table II (fMRI sample).
Behavioral data
Group comparisons were performed assessing univariate ANCOVAs with genotype and gender as factors between subjects and age as a covariate. Age was assessed as a covariate, but it showed no statistical influence on the results. The variables showed no signs of deviation from normal distribution (e.g. in terms of skewness). The results of NRG1 genotype are displayed in Table III.
Table III.
Results of neuropsychological paradigms
| Variable | T/T | T/C | C/C | F | P |
|---|---|---|---|---|---|
| Attention | 193.6 (37.0) | 194.2 (33.5) | 188.3 (36.0) | 0.57 | NS |
| L‐N‐S | 16.62 (2.51) | 16.47 (2.46) | 16.58 (2.77) | 0.17 | NS |
| TMT‐B | 61.81 (20.19) | 59.73 (14.34) | 61.63 (23.31) | 0.68 | NS |
| Spatial span | 18.96 (2.85) | 19.23 (2.96) | 18.7 (3.29) | 0.78 | NS |
| Sem. verb. fl. | 32.4 (9.2) | 31.1 (9.0) | 28.6 (10.4) | 3.4 | .034 |
| Lex. verb. fl. | 16.5 (4.7) | 16.9 (4.7) | 17.1 (3.7) | 0.59 | NS |
Numbers represent correct responses, standard deviations in parentheses (whole sample, n = 429). Results of dependent variables of T/C polymorphism in NRG1 as entered in ANOVA, standard deviations are in parentheses.
An effect of NRG1 genotype emerged for semantic verbal fluency (F 1,422 = 3.4, P = 0.034) only. Carriers of the NRG1 C/C variant named fewer countries than carriers of the T/T variant, while carriers of the T/C variant performed intermediate, not significantly deviating from either of the two other groups as calculated in post hoc comparisons. This result was further confirmed in a regression analysis where a linear effect of NRG1 on semantic verbal fluency emerged (subjects carrying no risk‐alleles performed highest, subjects with two risk‐alleles performed lowest with subjects having one risk‐alleles performed in between; β = 0.122 P = 0.011). None of the above effects was present for the lexical fluency results.
There was also a main effect of gender on semantic verbal fluency (F 1,422 = 45.5, P < 0.0001) but not on lexical verbal fluency (F 1,422 = 1.9, P > 0.16). Males could name more countries.
In a post hoc exploratory approach, we further tested for interactions of genotype by gender. There were no significant interactions in either one of the verbal fluency tasks (F 2,422 = 1.6, P > 0.2 for semantic verbal fluency and F 2,422 = 1.9, P > 0.15 for lexical verbal fluency, respectively).
NRG1 did not have an effect on any of other cognitive domains.
fMRI Results
Behavioral data
Recorded speech of all subjects was analyzed. Five subjects had to be excluded from further analyses for not following instructions. They did not name category members but freely associated about the word stimulus. Remaining data were analyzed and checked for errors in the semantic verbal fluency task. Means of the three NRG1 groups on errors were 3.81 (SD = 2.67) for T/T status, 2.94 (2.05) for T/C status, and 4.11 (2.98) for C/C status. There was no significant influence of genotype on errors (F 2,77 = 0.91, NS).
fMRI data
Because we used an overt word production task which could lead to head movement, this was checked for each subject. All subjects had tolerable head movement smaller than one voxel size, similar to our previous findings [Kircher et al., in press].
Analysis of the contrast semantic verbal fluency—reading aloud in the entire group of 80 fMRI subjects revealed activations as expected mainly in the left inferior frontal gyrus (BA 47), left middle temporal gyrus (BA19), and the cingulate gyrus (BA 24). Activated regions are listed in Table IV.
Table IV.
Brain activation for semantic verbal fluency condition—reading aloud for the whole fMRI sample (n = 80), P = 0.05 corr, cluster extend 20 voxels
| Region | Side | X | Y | Z | k E | Max. SPM (T) |
|---|---|---|---|---|---|---|
| Middle temporal gyrus, BA 19 | L | −30 | −56 | 14 | 63 | 7.2 |
| Temporal lobe, subgyral | L | −36 | −33 | −3 | 55 | 6.85 |
| Hypothalamus | R | 4 | −6 | −5 | 21 | 6.58 |
| Inferior frontal gyrus, BA 47 | L | −28 | 21 | −4 | 23 | 5.54 |
| Cingulate gyrus, BA 24 | L | −8 | 21 | 41 | 32 | 5.34 |
Coordinates are listed in Talairach and Tournoux atlas space [Talairach and Tournoux, 1988]. BA is the Brodmann area nearest to the coordinate and should be considered approximate.
Regression analyses revealed a linear influence of C‐alleles on brain activation for the contrast verbal fluency > high level baseline. Regions that were activated in this manner are displayed in Figure 1 and listed in Table V. Brain activation decreased with the number of C‐alleles in the cingulate gyrus (BA 24), the left middle and left inferior temporal gyri (BA 37 and 47, respectively) the left precuneus, and the right middle frontal gyrus (BA 46). No increasing brain activation with respect to an increasing number of C‐alleles was observed.
Figure 1.
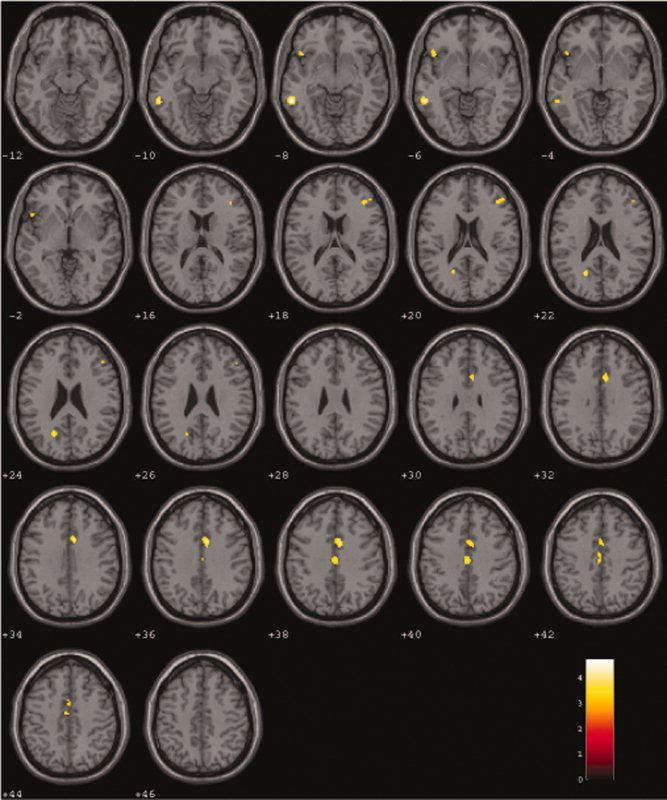
Regression analysis of BOLD response (NRG1 C/C < T/C < T/T) during semantic verbal fluency (SVF) versus reading. During semantic verbal fluency versus reading BOLD response increased with the number of T‐alleles in a linear fashion (P < 0.001, corrected by Monte Carlo simulations; cluster extend = 26 voxels). Numbers on colored bar represent t‐values.
Table V.
Regression of semantic verbal fluency contrast (semantic verbal fluency—reading) with number of T alleles as predictor (activation in C/C < T/C < T/T; P < 0.001 extend 26 voxels)
| Region | Side | X | Y | Z | k E | Max. SPM (T) |
|---|---|---|---|---|---|---|
| Regression T/T < T/C < C/C | ||||||
| Middle temporal gyrus, BA 37 | L | −57 | −43 | −5 | 64 | 4.7 |
| Inferior frontal gyrus, BA 47 | L | −48 | 17 | −3 | 26 | 3.66 |
| Middle frontal gyrus, BA 46 | R | 40 | 34 | 15 | 30 | 3.46 |
| Cingulate gyrus, BA 24 | R | 6 | 9 | 33 | 106 | 4.18 |
| Cingulate gyrus, BA 24 | 0 | −18 | 38 | 53 | 3.68 | |
| Precuneus, BA 31 | L | −20 | −61 | 25 | 27 | 3.67 |
| Regression C/C < T/C < T/T | ||||||
| No significant results |
Coordinates are listed in Talairach and Tournoux atlas space [Talairach and Tournoux, 1988]. BA is the Brodmann area nearest to the coordinate and should be considered approximate.
Entering the number of errors during the fMRI verbal fluency task as a covariate did not change the results.
Power analyses and effect sizes
For the fMRI results, effect sizes of partial η2 = 0.194 resulted. Interestingly, this effect is of about the same size as previously reported in Krug et al., 2008a, b. The power to find an effect of this size is 1 − β = 0.971. This effect was found for the left middle temporal gyrus. Effect sizes for the other clusters are somewhat smaller as can be seen in the t values reported. For the behavioral task in the fMRI paradigm, an effect of partial η2 = 0.029 led to an observed power of 1 − β = 0.201.
For the initial sample, the effect in the semantic verbal fluency task was partial η2 = 0.016 and an observed power of 1 − β = 0.64 while for the lexical verbal fluency task partial η2 = 0.003 and an observed power of 1 − β = 0.145 resulted.
DISCUSSION
In this study, the effect of NRG1 status on semantic verbal fluency and its neuronal correlates was investigated in a large sample of healthy subjects. A significant influence of NRG1 status on the behavioral level and a linear decrease in brain activation measured with fMRI in the lateral frontal, temporal, and anterior cingulate cortex during a semantic verbal fluency task was found. NRG1 genotype modulates semantic language production capacity on a behavioral and neural level.
Behavioral Tasks
Genotype status was correlated with performance in the semantic verbal fluency task in a linear fashion but not with lexical fluency. Hereby, an increase in the number of risk‐alleles for schizophrenia corresponded to a decrease in verbal fluency performance. This result can be interpreted within the framework of deficient semantic processing that is typically found in schizophrenia, particularly in patients with positive formal thought disorder [for an overview see Goldberg and Weinberger, 2000]. One of the most robust findings in schizophrenia research describes a pronounced deficit in the generation of category lists (semantic verbal fluency) compared with phonological word lists [letter fluency; for a review see Bokat and Goldberg, 2003] and has been taken, along with results of semantic priming tasks [Kuperberg et al., 2007; Spitzer et al., 1993], as evidence for a selective deficit of semantic rather than word retrieval processes per se. Our results imply that this finding in schizophrenia patients during word production can be detected at the stage of a genetic predisposition for the disorder. On the other hand, it is possible that the effect in the initial sample found in the semantic verbal fluency task is due to sample size. The estimated effect in the semantic verbal fluency task is about half the size of the effect in the fMRI paradigm while power is threefold. It is of note that NRG1 did not have an effect on the cognitive domains also assessed in this study, so it is unlikely, that the results on semantic verbal fluency could be mediated by these domains.
fMRI Task
The results of the regression analysis of the fMRI data implicate a decrease in BOLD‐response with increasing number of risk‐alleles for schizophrenia during verbal fluency compared with reading aloud. Activations predominantly comprised areas which have been associated with semantic processing such as the left inferior frontal (IFG) and middle temporal gyrus (MTG) as well as the anterior cingulate cortex (ACC) [for reviews see Bookheimer, 2002; Cabeza and Nyberg, 2000; Heim, 2005; Indefrey and Levelt, 2004]. Despite differences on the neural level, verbal fluency performance was comparable for T/T, T/C, and C/C carriers during fMRI measurement (see Table IV). The results support a genetic influence onto brain activity during verbal fluency in core components of the semantic language network (IFG, MTG) as well as executive (ACC) and, presumably, complementary components (MFG, precuneus) which are not explainable by behavioral performance.
Word Retrieval in Healthy Subjects
During our verbal fluency task, we found differential activation in a key network known to be implicated in semantic verbal fluency tasks. Here, category members in response to a verbal cue are produced, an operation requiring access to a word's conceptual representation as well as executive and control functions to select the appropriate response [Bookheimer, 2002]. In healthy individuals, the middle temporal gyrus is relevant for conceptually driven word retrieval during e.g., picture naming and word generation [for a review see Indefrey and Levelt, 2004; Warburton et al., 1996], semantic word processing [e.g. Friederici et al., 2000; Gold et al., 2006], and has been implicated in semantic aspects of the mental lexicon [Heim, 2005]. In contrast, the left inferior frontal gyrus is implicated in semantic search and selection among items, retrieving the word from semantic memory and keeping the retrieved information in verbal working memory for subsequent manipulation [for reviews see Bookheimer, 2002; Cabeza and Nyberg, 2000; Indefrey and Levelt, 2004; Thompson‐Schill et al., 1997]. The anterior cingulate gyrus has been consistently associated with interrelated control function such as initiation, inhibition, attention, and selection during a variety of tasks [for an extensive review see Cabeza and Nyberg, 2000] and has been linked to the suppression of competitive and inappropriate responses during verbal retrieval in particular [e.g. Fletcher et al., 1996]. Apart from the core components of semantic processing, neuroimaging studies of letter or category verbal fluency have also documented an involvement of the right middle frontal gyrus [Amunts et al., 2004; Fu et al., 2002] but these findings are less frequent [see Indefrey and Levelt, 2004].
Influence of Schizophrenia Risk Genes on Brain Activity in the Semantic Network
Previous investigations have shown that both, patients with schizophrenia and those at genetic risk exhibit a selective semantic deficit during word retrieval. Brain regions correlated with this impairment include most of the neural components that we have identified in the regression analysis of the present study: hypoactivations of the left IFG [Curtis et al., 1998], ACC [Fletcher et al., 1996; Fu et al., 2005], and left posterior MTG [Fu et al., 2005] during letter as well as semantic verbal fluency have been reported in previous schizophrenia and at‐risk studies [Hall et al., 2006; Ragland et al., 2007]. For all these investigations, similar behavioral performance was apparent despite differential brain activation [see also Kircher et al., in press]. These findings imply that although no differences emerged on the behavioral level, brain activation can be altered not only in patients but also in subjects with a predisposition for the disorder.
In the present study, alterations in performance were found between the different carriers of the main group but not in the fMRI sample. This result could be explained in statistical terms because of the smaller sample size resulting in lower power. Alternatively, the task in the scanner was somewhat easier, thus causing adaptation in neural function in the at‐risk carriers, which could compensate the somewhat lower processing capacity on the behavioral level.
Limitations
We only studied one SNP of the NRG1 gene (SNP8NRG221533; rs35753505). This polymorphism was selected for the present study as it gave the best uncorrected single marker association for NRG1 in the study of Stefansson et al. [ 2002]. As this SNP was only one of the markers of the “core at‐risk haplotype” in the study of Stefansson et al. [ 2002], it might be possible to miss information compared with the haplotype based on a complete set of markers. On the other hand, testing for more than one SNP would have resulted in an increased chance for false positives. A second limitation is that we did not use exactly the same semantic verbal fluency task in the whole sample as in the fMRI study. The standardized behavioral task was taken from a widely used cognitive test battery. For the fMRI study we used another widely applied task adapted for functional imaging, in line with many other previous studies on verbal fluency [Alario et al., 2006; Tremblay and Gracco, 2006]. As we investigated healthy probands, we cannot definitely conclude that the effect we observed is also acting in patients with schizophrenia or if the effect is limited to healthy probands. Yet, all studies on verbal fluency found differences between schizophrenia patients and control subjects in the areas implicated in our study. Since these are the first reported correlations of NRG1 (SNP8NRG221533; rs35753505) with semantic verbal fluency, they should be confirmed in independent samples of healthy probands as well as in schizophrenia patients.
In summary, we found a linear effect of NRG1 on task performance in semantic verbal fluency, but not in lexical verbal fluency in a large sample of 429 healthy individuals. There was also a linear decrease in brain activation during a semantic verbal fluency task in the left lateral and frontal cortices as well as the anterior cingulate in a subsample of 80 healthy individuals. This decrease was correlated with the number of risk‐alleles. The data point to an influence of NRG1 onto brain activation during semantic verbal fluency, even though this decrease in activation does not necessarily surface on the behavioral level and seems to be task or effort dependent. It is noteworthy that NRG1 influences neural activation in healthy individuals in a way that is very similar to activation patterns in patients with schizophrenia.
All authors report no potential conflict of interest.
REFERENCES
- Addington AM,Gornick MC,Shaw P,Seal J,Gogtay N,Greenstein D,Clasen L,Coffey M,Gochman P,Long R,Rapoport JL ( 2007): Neuregulin 1 (8p12) and childhood‐onset schizophrenia: Susceptibility haplotypes for diagnosis and brain developmental trajectories. Mol Psychiatry 12: 195–205. [DOI] [PubMed] [Google Scholar]
- Alario FX,Chainay H,Lehericy S,Cohen L ( 2006): The role of the supplementary motor area (SMA) in word production. Brain Res 1076: 129–143. [DOI] [PubMed] [Google Scholar]
- Amunts K,Weiss PH,Mohlberg H,Pieperhoff P,Eickhoff S,Gurd JM,Marshall JC,Shah NJ,Fink GR,Zilles K ( 2004): Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—The roles of Brodmann areas 44 and 45. Neuroimage 22: 42–56. [DOI] [PubMed] [Google Scholar]
- Anton ES,Marchionni MA,Lee KF,Rakic P ( 1997): Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124: 3501–3510. [DOI] [PubMed] [Google Scholar]
- Bakker SC,Hoogendoorn ML,Selten JP,Verduijn W,Pearson PL,Sinke RJ,Kahn RS ( 2004): Neuregulin 1: Genetic support for schizophrenia subtypes. Mol Psychiatry 9: 1061–1063. [DOI] [PubMed] [Google Scholar]
- Bokat CE,Goldberg TE ( 2003): Letter and category fluency in schizophrenic patients: A meta‐analysis. Schizophr Res 64: 73–78. [DOI] [PubMed] [Google Scholar]
- Boksman K,Theberge J,Williamson P,Drost DJ,Malla A,Densmore M,Takhar J,Pavlosky W,Menon RS,Neufeld RW ( 2005): A 4.0‐T fMRI study of brain connectivity during word fluency in first‐episode schizophrenia. Schizophr Res 75: 247–263. [DOI] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 51–88. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R ( 2002): Der Aufmerksamkeits‐Belastungstest d2. Göttingen Hogrefe. [Google Scholar]
- Cabeza R,Nyberg L ( 2000): Imaging cognition. II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Chen S,Velardez MO,Warot X,Yu ZX,Miller SJ,Cros D,Corfas G ( 2006): Neuregulin 1‐erbB signaling is necessary for normal myelination and sensory function. J Neurosci 26: 3079–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X,Dunham C,Kendler S,Wang X,O'Neill FA,Walsh D,Kendler KS ( 2004): Regulator of G‐protein signaling 4 (RGS4) gene is associated with schizophrenia in Irish high density families. Am J Med Genet B Neuropsychiatr Genet 129: 23–26. [DOI] [PubMed] [Google Scholar]
- Corfas G,Roy K,Buxbaum JD ( 2004): Neuregulin 1‐erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 7: 575–580. [DOI] [PubMed] [Google Scholar]
- Curtis VA,Bullmore ET,Brammer MJ,Wright IC,Williams SC,Morris RG,Sharma TS,Murray RM,McGuire PK ( 1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Daum I,Graber S,Schugens MM,Mayes AR ( 1996): Memory dysfunction of the frontal type in normal ageing. Neuroreport 7: 2625–2628. [DOI] [PubMed] [Google Scholar]
- Dye SM,Spence SA,Bench CJ,Hirsch SR,Stefan MD,Sharma T,Grasby PM ( 1999): No evidence for left superior temporal dysfunction in asymptomatic schizophrenia and bipolar disorder. PET study of verbal fluency. Br J Psychiatry 175: 367–374. [DOI] [PubMed] [Google Scholar]
- Egan MF,Goldberg TE,Kolachana BS,Callicott JH,Mazzanti CM,Straub RE,Goldman D,Weinberger DR ( 2001): Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston RCF ( 1977): Testing for Hardy‐Weinberg equilibrium in small samples. Biometrics 33: 536–542. [Google Scholar]
- Falls DL ( 2003): Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res 284: 14–30. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Frith CD,Grasby PM,Friston KJ,Dolan RJ ( 1996): Local and distributed effects of apomorphine on fronto‐temporal function in acute unmedicated schizophrenia. J Neurosci 16: 7055–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM,Mathalon DH,Whitfield S,Faustman WO,Roth WT ( 2002): Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry 51: 485–492. [DOI] [PubMed] [Google Scholar]
- Friederici AD,Opitz B,von Cramon DY ( 2000): Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cereb Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Frith CD,Friston KJ,Herold S,Silbersweig D,Fletcher P,Cahill C,Dolan RJ,Frackowiak RS,Liddle PF ( 1995): Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 167: 343–349. [DOI] [PubMed] [Google Scholar]
- Fu CH,Morgan K,Suckling J,Williams SC,Andrew C,Vythelingum GN,McGuire PK ( 2002): A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: Greater anterior cingulate activation with increased task demand. Neuroimage 17: 871–879. [PubMed] [Google Scholar]
- Fu CH,Suckling J,Williams SC,Andrew CM,Vythelingum GN,McGuire PK ( 2005): Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. Am J Psychiatry 162: 485–494. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT,Weber J,Pevny L,Schmid R,Schwab MH,Lloyd KC,Eisenstat DD,Lai C,Anton ES ( 2006): The role of neuregulin‐ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA 103: 1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC,Almasy L,Blangero J,Burk GM,Estrada J,Peralta JM,Meyenberg N,Castro MP,Barrett J,Nicolini H,Raventós H, Escamilla MA ( 2007): Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 144: 242–249. [DOI] [PubMed] [Google Scholar]
- Gold BT,Balota DA,Jones SJ,Powell DK,Smith CD,Andersen AH ( 2006): Dissociation of automatic and strategic lexical‐semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci 26: 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM,Carpenter C,Randolph C,Goldberg TE,Weinberger DR ( 1997): Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 54: 159–165. [DOI] [PubMed] [Google Scholar]
- Goldberg TE,Weinberger DR ( 2000): Thought disorder in schizophrenia: A reappraisal of older formulations and an overview of some recent studies. Cogn Neuropsychiatry 5: 1–19. [Google Scholar]
- Hahn CG,Wang HY,Cho DS,Talbot K,Gur RE,Berrettini WH,Bakshi K,Kamins J,Borgmann‐Winter KE,Siegel SJ,Gallop RJ, Arnold SE ( 2006): Altered neuregulin 1‐erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12: 824–828. [DOI] [PubMed] [Google Scholar]
- Hall J,Whalley HC,Job DE,Baig BJ,McIntosh AM,Evans KL,Thomson PA,Porteous DJ,Cunningham‐Owens DG,Johnstone EC,Lawrie SM ( 2006): A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci 9: 1477–1478. [DOI] [PubMed] [Google Scholar]
- Harrison PJ,Law AJ ( 2006): Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol Psychiatry 60: 132–140. [DOI] [PubMed] [Google Scholar]
- Heim S ( 2005): The structure and dynamics of normal language processing: Insights from neuroimaging. Acta Neurobiol Exp (Wars) 65: 95–116. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW,Zakzanis KK ( 1998): Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 12: 426–445. [DOI] [PubMed] [Google Scholar]
- Hennah W,Tuulio‐Henriksson A,Paunio T,Ekelund J,Varilo T,Partonen T,Cannon TD,Lonnqvist J,Peltonen L ( 2005): A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol Psychiatry 10: 1097–1103. [DOI] [PubMed] [Google Scholar]
- Hennah W,Varilo T,Kestila M,Paunio T,Arajarvi R,Haukka J,Parker A,Martin R,Levitzky S,Partonen T,Meyer J, Lönnqvist J, Peltonen L, Ekelund J ( 2003): Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex‐dependent effects. Hum Mol Genet 12: 3151–3159. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA,Goldman D,Jaeger J,Persaud S,Kane JM,Lipsky RH,Malhotra AK ( 2004): Disrupted in schizophrenia 1 (DISC1): Association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet 75: 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P,Levelt WJ ( 2004): The spatial and temporal signatures of word production components. Cognition 92: 101–144. [DOI] [PubMed] [Google Scholar]
- Kircher T,Whitney C,Krings T,Huber W,Weis S ( 2008): Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res 101: 242–255. [DOI] [PubMed] [Google Scholar]
- Kirov G,O'Donovan MC,Owen MJ ( 2005): Finding schizophrenia genes. J Clin Invest 115: 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A,Markov V,Eggermann T,Krach S,Zerres K,Stöcker T,Shah NJ,Schneider F,Nöthen MM,Treutlein J,Rietschel M,Kircher T ( 2008a): Genetic variation in the schizophrenia‐risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. Neuroimage 42: 1569–1576. [DOI] [PubMed] [Google Scholar]
- Krug A,Markov V,Leube D,Zerres K,Eggermann T,Nothen MM,Skowronek MH,Rietschel M,Kircher T ( 2008b) Genetic variation in the schizophrenia‐risk gene neuregulin1 correlates with personality traits in healthy individuals. Eur Psychiatry 23: 344–349. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR,Deckersbach T,Holt DJ,Goff D,West WC ( 2007): Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Arch Gen Psychiatry 64: 138–151. [DOI] [PubMed] [Google Scholar]
- Lehrl S,Triebig G,Fischer B ( 1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335–345. [DOI] [PubMed] [Google Scholar]
- Li D,Collier DA,He L ( 2006): Meta‐analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet 15: 1995–2002. [DOI] [PubMed] [Google Scholar]
- Liu Y,Ford B,Mann MA,Fischbach GD ( 2001): Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci 21: 5660–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM,Moorhead TW,Job D,Lymer GK,Munoz Maniega S,McKirdy J,Sussmann JE,Baig BJ,Bastin ME,Porteous D,Evans KL,Johnstone EC,Lawrie SM,Hall J ( 2007): The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry 13: 1054–1059. [DOI] [PubMed] [Google Scholar]
- Morris DW,Rodgers A,McGhee KA,Schwaiger S,Scully P,Quinn J,Meagher D,Waddington JL,Gill M,Corvin AP ( 2004): Confirming RGS4 as a susceptibility gene for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 125: 50–53. [DOI] [PubMed] [Google Scholar]
- Munafo MR,Thiselton DL,Clark TG,Flint J ( 2006): Association of the NRG1 gene and schizophrenia: A meta‐analysis. Mol Psychiatry 11: 539–546. [DOI] [PubMed] [Google Scholar]
- Nave KA,Salzer JL ( 2006): Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol 16: 492–500. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen MJ,O'Donovan M,Gottesman I ( 2003): Schizophrenia In: Mc Guffin P,Owen M,Gottesmann I, editors. Psychiatric Genetics & Genomics. Oxford: Oxford University Press: pp 247–266. [Google Scholar]
- Ozaki M,Sasner M,Yano R,Lu HS,Buonanno A ( 1997): Neuregulin‐beta induces expression of an NMDA‐receptor subunit. Nature 390: 691–694. [DOI] [PubMed] [Google Scholar]
- Porteous DJ,Thomson P,Brandon NJ,Millar JK ( 2006): The genetics and biology of DISC1—An emerging role in psychosis and cognition. Biol Psychiatry 60: 123–131. [DOI] [PubMed] [Google Scholar]
- Ragland JD,Moelter ST,Bhati MT,Valdez JN,Kohler CG,Siegel SJ,Gur RC,Gur RE ( 2007): Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophr Res 99: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R,Wolfson D ( 1985): The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson: Neuropsychology Press. [Google Scholar]
- Schwab SG,Knapp M,Mondabon S,Hallmayer J,Borrmann‐Hassenbach M,Albus M,Lerer B,Rietschel M,Trixler M,Maier W,Wildenauer DB ( 2003): Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib‐pair families with linkage and in an additional sample of triad families. Am J Hum Genet 72: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD,Moo LR,Segal JB,Hart J Jr ( 2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res 17: 75–82. [DOI] [PubMed] [Google Scholar]
- Spence SA,Liddle PF,Stefan MD,Hellewell JS,Sharma T,Friston KJ,Hirsch SR,Frith CD,Murray RM,Deakin JF,Grasby PM ( 2000): Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 176: 52–60. [DOI] [PubMed] [Google Scholar]
- Spitzer M,Braun U,Hermle L,Maier S ( 1993): Associative semantic network dysfunction in thought‐disordered schizophrenic patients: Direct evidence from indirect semantic priming. Biol Psychiatry 34: 864–877. [DOI] [PubMed] [Google Scholar]
- Stefanis NC,Trikalinos TA,Avramopoulos D,Smyrnis N,Evdokimidis I,Ntzani EE,Ioannidis JP,Stefanis CN ( 2007): Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol Psychiatry 62: 784–792. [DOI] [PubMed] [Google Scholar]
- Stefanis NC,van Os J,Avramopoulos D,Smyrnis N,Evdokimidis I,Stefanis CN ( 2005): Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: Tuning rather than improving performance Am J Psychiatry 162: 1752–1754. [DOI] [PubMed] [Google Scholar]
- Stefansson H,Sarginson J,Kong A,Yates P,Steinthorsdottir V,Gudfinnsson E,Gunnarsdottir S,Walker N,Petursson H,Crombie C,Ingason A,Gulcher JR,Stefansson K,St Clair D ( 2003): Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet 72: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H,Sigurdsson E,Steinthorsdottir V,Bjornsdottir S,Sigmundsson T,Ghosh S,Brynjolfsson J,Gunnarsdottir S,Ivarsson O,Chou TT,Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H,Stefansson K ( 2002): Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE,Jiang Y,MacLean CJ,Ma Y,Webb BT,Myakishev MV,Harris‐Kerr C,Wormley B,Sadek H,Kadambi B,Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D,Kendler KS ( 2002): Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 71: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF,Kendler KS,Neale MC ( 2003): Schizophrenia as a complex trait: Evidence from a meta‐analysis of twin studies. Arch Gen Psychiatry 60: 1187–1192. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Thiselton DL,Webb BT,Neale BM,Ribble RC,O'Neill FA,Walsh D,Riley BP,Kendler KS ( 2004): No evidence for linkage or association of neuregulin‐1 (NRG1) with disease in the Irish study of high‐density schizophrenia families (ISHDSF). Mol Psychiatry 9: 777–783; image 729. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL,D'Esposito M,Aguirre GK,Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato S,Dazzan P,Collier D ( 2005): Association between the neuregulin 1 gene and schizophrenia: A systematic review. Schizophr Bull 31: 613–617. [DOI] [PubMed] [Google Scholar]
- Tremblay P,Gracco VL ( 2006): Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage 33: 947–957. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaert A,Schumacher J,Schulze TG,Otte AC,Ohlraun S,Kovalenko S,Becker T,Freudenberg J,Jonsson EG,Mattila‐Evenden M,Sedvall GC, Czerski PM, Kapelski P, Hauser J, Maier W, Rietschel M, Propping P,Nöthen MM,Cichon S ( 2003): The DTNBP1 (dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet 73: 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walss‐Bass C,Escamilla MA,Raventos H,Montero AP,Armas R,Dassori A,Contreras S,Liu W,Medina R,Balderas TG,Levinson D, Pereira R, Pereira M, Atmella I, Nesmith L, Leach R,Almasy L ( 2005): Evidence of genetic overlap of schizophrenia and bipolar disorder: Linkage disequilibrium analysis of chromosome 18 in the Costa Rican population. Am J Med Genet B Neuropsychiatr Genet 139: 54–60. [DOI] [PubMed] [Google Scholar]
- Warburton E,Wise RJ,Price CJ,Weiller C,Hadar U,Ramsay S,Frackowiak RS ( 1996): Noun and verb retrieval by normal subjects. Studies with PET. Brain 119: 159–179. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1997): Wechsler Memory Scale: Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation: Harcourt Brace & Co. [Google Scholar]
- Whitney C,Weis S,Krings T,Huber W,Kircher T : Task‐dependent modulations of prefrontal and hippocampal activity during intrinsic word production. J Cogn Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM,Preece A,Spurlock G,Norton N,Williams HJ,McCreadie RG,Buckland P,Sharkey V,Chowdari KV,Zammit S,Nimgaonkar V, Kirov G, Owen MJ,O'Donovan MC ( 2004): Support for RGS4 as a susceptibility gene for schizophrenia. Biol Psychiatry 55: 192–195. [DOI] [PubMed] [Google Scholar]
- Yurgelun‐Todd DA,Waternaux CM,Cohen BM,Gruber SA,English CD,Renshaw PF ( 1996): Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry 153: 200–205. [DOI] [PubMed] [Google Scholar]
- Zhao X,Shi Y,Tang J,Tang R,Yu L,Gu N,Feng G,Zhu S,Liu H,Xing Y,Zhao S, Sang H, Guan Y,St Clair D,He L ( 2004): A case control and family based association study of the neuregulin1 gene and schizophrenia. J Med Genet 41: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


