Abstract
Transcranial magnetic stimulation (TMS) has become a common tool for the brain mapping of a wide variety of cognitive functions. Because TMS over cortical regions of interest other than motor cortex often does not produce easily observable effects, the ability to calibrate TMS intensity for stimulation over nonmotor regions can be problematic. Previous studies reported no correlation between motor thresholds (MT) over the motor cortex and phosphene thresholds (PT) over the visual cortex. However, different thresholding methods, lighting, and eye‐closure conditions were used to determine MT and PT. We investigated the correlation between resting MT (rMT), active MT (aMT), and PT in 27 dark‐adapted healthy volunteers. All thresholds were measured with eyes‐open in the dark and determined by gradually reducing stimulation intensity downward. All subjects had aMT and rMT; 21 subjects had measurable PT. rMT was 70.4% ± 9.8% (mean ± SD of maximum stimulator output); aMT was 61.1% ± 7.9%; PT was 82.2% ± 10.1%. A significant positive correlation was found between aMT and PT (r = 0.53; P = 0.014) with a trend toward correlation between rMT and PT (r = 0.43; P = 0.052). Our results suggest that sensitivity to TMS over visual and motor cortices may be correlated under similar thresholding procedures. They also provide a rationale for the use of easily obtained aMT to calibrate TMS intensities in brain mapping studies that employ TMS in cortical regions besides motor cortex. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: motor threshold (MT), phosphene threshold (PT), cortical excitability, transcranial magnetic stimulation (TMS)
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a noninvasive method for brain stimulation that has become an important modality for mapping brain–behavior relationships in cognitive neuroscience [Robertson et al., 2003]. In contrast to correlational neuroimaging methods, such as fMRI or PET, TMS interacts with ongoing brain activity around the region of cortex where induced current under the coil is produced. Consequently, TMS can be used to directly evaluate the critical and causal significance of the stimulated areas. In conjunction with behavioral tasks, TMS studies can directly demonstrate causal relationships between brain areas and tasks. For example, TMS over the occipital lobe can disrupt Braille reading in congenitally blind individuals [Cohen et al., 1997], TMS over Broca's area can interfere with imitation of hand postures [Heiser et al., 2003], and TMS over the posterior parietal cortex can disrupt feedforward error correction in visually guided aiming [Desmurget et al., 1999].
TMS effects depend on factors, such as cortical target, TMS coil geometry, pulse waveform, and stimulation parameters, such as intensity, frequency, and number of pulses. While the nature of the brain mapping experiment can determine cortical targets of interest, the choice of stimulation parameters is not always straightforward. Even if all technical factors that determine the topography and strength of the magnetic field are identical, individual differences in the intrinsic responsiveness, or excitability, of each subject's brain to stimulation will introduce unwanted variability in TMS effects [Robertson et al., 2003]. To assure comparability between experimental conditions, TMS intensities should be ideally calibrated such that TMS pulses produce a constant neurophysiological effect across subjects.
In addition to being variable across subjects, excitability also varies across different cortical regions and in different contexts within a given subject [Robertson et al., 2003]. Over the motor cortex, excitability can be quantified using a measurable motor evoked potential response (MEP) in a contralateral muscle. The motor threshold (MT) is commonly defined as the minimum TMS intensity that elicits a MEP above a minimal size and is routinely determined in each individual subject prior to an experiment. Experimental stimulation intensities can then be set at a percentage of this MT, which assures that a suprathreshold TMS intensity used over the motor cortex in one subject will be equivalently suprathreshold for another. Over the occipital cortex, excitability can be assessed using TMS‐induced phosphenes as a region‐specific response measure. Analogous to MT, a phosphene threshold (PT) can be defined as the minimum TMS intensity that elicits perception of phosphenes. The PT then becomes a valid reference intensity for TMS studies of visual perception [Boroojerdi et al., 2002; Gothe et al., 2002; Hotson and Anand, 1999; Kammer, 1999; Stewart et al., 2001a].
The uncertainty in calibrating intensities across subjects for brain regions beyond the motor and visual cortices potentially increases the variance of TMS effects, reduces statistical power of TMS studies, and represents a major limitation when considering applications of TMS as a brain mapping tool across multiple cortical regions [Robertson et al., 2003]. The assumption that a relevant proportion of TMS threshold measures between different neocortical regions could reflect a shared component of within‐individual responsiveness to TMS has been criticized by previous studies that demonstrated no correlation between MT and PT [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b].
However, none of those studies used similar methods to determine both thresholds (see Table I). For example, while PT is usually measured under dark‐adapted conditions, MT is not. The approach toward threshold (upwards, downwards, or variable) is not consistent between or within studies [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b]. Furthermore, other factors may have led to additional confounds in previous studies. For example, paired pulses of TMS, which enhance phosphene perception, have been used over the visual cortex in contrast to single TMS pulses over the motor cortex [Antal et al., 2003b; Boroojerdi et al. 2002] or different machines were used for PT and MT determinations [Antal et al., 2003b]. Since MTs can be affected by these factors [Leon‐Sarmiento et al., 2005; Tranulis et al., 2006], the possibility exists that these different methodological factors could have limited the detection of a correlation between MT and PT.
Table I.
Review of Correlational TMS Studies Reporting No Significant Correlation Between MT and PT
| Articles | Subjects (# with PT) | PT Mode | Eye/Light Conditions | Approach to Threshold | Coil Position (Handle) | Stimulator | |||
|---|---|---|---|---|---|---|---|---|---|
| M1 | V1 | M1 | V1 | M1 | V1 | ||||
| Stewart et al., 2001b | 15 (15) | SP | NI | blindfolded | from above, 2% decrements | from 60% MSO, 5% increments or decrements | latero‐medial | upwards | Magstim 200; monophasic |
| Boroojerdi et al., 2002 | 8 (8) | PP | NI | blindfolded, dark room | NI | from below, 1% increments | NI | upwards | Magstim SuperRapid; biphasic |
| Gerwig et al., 2003 | 32 (30) | SP | NI | blindfolded; periodic light exposure | from above, 2% decrements | from below, 5% increments, then 2% at random | posterior | horizontal | Dantec MagPro; biphasic |
| Antal et al., 2003b | 11 (11)1 | PP | NI | eyes closed, dark room | NI | first, from above, 5% decrements; then, from below, 2% increments | latero‐medial | upwards | M1: Magstim 200; monophasic |
| M1/V1: Magpro; biphasic | |||||||||
| Present study | 27 (21) | SP | light‐proof goggles2 | light‐proof goggles3 | from above, 1% decrements | from above, 1% decrements | latero‐medial | upwards | Magstim SuperRapid; biphasic |
SP = single‐pulse, PP = paired‐pulse, NI = no information, MSO = maximum stimulator output.
MT and PT determined on different days.
15 minutes of dark adaptation.
45 minutes of dark adaptation.
The aim of the present study was to investigate the relationship between MT and PT with psychophysically similar thresholding procedures over motor and visual cortices. Specifically, we sought to determine all thresholds under both dark‐adapted conditions and a uniform systematic downward approach toward threshold with single TMS pulses from the same TMS coil. A finding of a significant MT and PT correlation would suggest some level of common excitability across these two cortical regions. In addition, a significant result would provide a rationale to the practice of calibrating TMS intensities over different cortical regions using MT.
SUBJECTS AND METHODS
Subjects
We recruited and obtained informed consent from 27 healthy subjects excluding those with a previous history of neurological or psychiatric disorders, who did not take any regular medications, and who did not have exclusions relevant to TMS. Study procedures were approved by the UCLA Medical Institutional Review Board.
TMS Procedures
All subjects were dark‐adapted for the study by donning lightproof goggles at the start of the experimental session. Goggles were adjusted to ensure that no light was visible. Goggles were designed not to produce pressure on eyelids and preserve normal blinking. The room was darkened. Participants were continually reminded to keep eyes open and to fixate forward throughout each thresholding procedure. Since different examiners may constitute a significant source of variability [Chaudry et al., 1991], we had the same two investigators present at every session who supervised a consistent application of methods. Each investigator conducted the same thresholding procedure throughout this study. One investigator performed all motor thresholding procedures. A second investigator was only interested in measuring PT for a vision study that required participants to see phosphenes, and performed all subsequent phosphene thresholding procedures. The second investigator was blinded to the purpose of this study since no correlation was expected to be found. In addition, the thresholding procedures were spread out over the course of several weeks, and no correlation analysis was computed until all subjects for the vision study were enrolled.
Thresholding procedures were conducted on the same day in the following order: (1) measurement of resting motor threshold (rMT), (2) active motor threshold (aMT), and (3) phosphene threshold (PT). Goggles were worn for at least 15 min before rMT thresholding procedures began. Adaptation to dark was present for ∼45 min by the time PT procedures began. To ensure parallel procedures, all thresholds were measured by consistently starting from a clearly suprathreshold intensity and gradually reducing stimulation intensity in steps of 1% until threshold intensity was obtained. No thresholds were approached from below. Participants wore a tight lycra cap, on which grids were drawn over the region of the left primary motor cortex and left occipital visual areas.
We used a Magstim SuperRapid biphasic stimulator with a figure‐8 coil (14 cm width) for all motor and PT. All following thresholds are expressed in percent of maximum stimulator output (MSO) (peak field strength 2 T).
Motor Thresholding Procedures
For rMT and aMT, the figure‐8 coil was held tangentially to the skull and mediolaterally with the handle pointing backwards and at a 45° angle from the sagittal midline [Brasil‐Neto et al., 1992]. Thus, the induced current pointed forward in a roughly perpendicular manner to the fictitious line of the central sulcus.
Surface EMG electrodes were placed over the right first dorsal interosseus (FDI) muscle. EMG was sampled at 1 kHZ, amplified, and 1‐1 kHz bandpass filtered. MEP sizes were measured as peak‐to‐peak amplitudes. Aliasing of higher frequency components in the EMG signal, where power is minimal, is unlikely to affect thresholding results.
To measure rMT, single pulses of TMS were delivered over the left motor cortex, while the right FDI was kept relaxed. Trials where baseline EMG, in an interval 100 ms prior to TMS pulse, showed visible EMG activity (>20 μV) were discarded. TMS pulses were delivered, while the coil was moved systematically, first at and then, between grid points. The location that evoked the largest and most reliable MEP amplitudes was designated the motor hotspot.
Starting suprathreshold intensities induced clearly distinguishable MEP's with every TMS pulse. Intensities were then lowered by 1% decrements. The lowest intensity with the coil at the motor hotspot for which peak‐to‐peak MEP amplitudes greater than 50 μV occurred in at least 5 out of 10 trials was designated the rMT.
For aMT, subjects were asked to squeeze a small cylinder with a light steady pinch grip, while FDI activation was monitored online, using EMG to ensure a constant average level of activity around 100 μV. With the coil held over the same hotspot, TMS intensity was then lowered by 1% increments from rMT. The lowest intensity for which peak‐to‐peak MEPs greater than 100 μV above baseline EMG occurred in 5 out of 10 trials was designated the aMT. Throughout rMT and aMT procedures, subjects were frequently asked to keep their eyes open, while looking forward in the dark.
Phosphene Thresholding Procedures
All PT procedures were done with the FDI muscles relaxed. To elicit phosphenes, the coil was positioned with the handle pointing upwards, parallel to the subject's spine [Antal et al., 2003b; Boroojerdi et al., 2002; Stewart et al., 2001b]. The initial position of the coil was midline, 2 cm above the inion. Single pulses of TMS were delivered over occipital cortex, while the coil was moved over a 1 × 1 cm2 interval grid marked on the lycra cap. The coil was moved systematically over the left visual cortex to induce the perception of phosphenes in the right visual field. All phosphene percepts were initially determined at 100% MSO. The phosphene localization procedure was designed to maximize the likelihood that reliable phosphenes were detected: during our first pass over the grid, participants were asked to attend to the whole visual field and report the presence or absence of any induced visual phenomena after each TMS pulse. If they did not report a reliable phosphene, each site on the grid was tested three times at 100% MSO. After a 10‐min‐break, the concept of a phosphene was explained again, and the whole procedure was repeated all over again. After this screening procedure, if no phosphene was reported, we felt it reasonable to stop.
Once a valid phosphene was reported, the coil was moved until a bright, reliable phosphene was reported in a paracentral location approximately within the central 8° of the visual field. Phosphene location was reported by participants either by indicating the approximate position of the phosphene percept in visual space by pointing out in front of themselves or pointing on the front of the goggles, whichever they felt more comfortable doing. Uncertain responses were classified as absent phosphenes, but often suggested that either coil location and/or intensity was close to parameters that would induce reproducible phosphenes. Positive responses were then qualified as either central or peripheral in nature. The quality of the visual phenomena was further assessed with open questions (textured, colored, shaped and so on). Candidate phosphenes were validated by moving the coil laterally to ensure that the perceived phosphene shifted location in a predictable manner, validating the retinotopic nature of the visual percept. The location that evoked the brightest and most reliable phosphene was designated the visual hotspot. Then, from the suprathreshold intensity, intensities were lowered by 1% increments. The lowest stimulation intensity at which stable phosphenes were perceived in at least 5 of 10 stimulations was recorded as the PT.
TMS‐induced phosphene perception can be improved by training in individuals over time or by a period of dark adaptation [Boroojerdi et al., 2000a]. Most studies using phosphenes for calibration purposes have not typically trained participants to see phosphenes before a thresholding session, however our phosphene screening procedure gave multiple opportunities for subjects to become familiar with the concept and appearance of TMS induced phosphenes. We dark‐adapted subjects for 45 min, during which we were able to record motor thresholds on all subjects, while increasing chances of reliable phosphene detection. Also, because we approached PT by decreasing TMS intensities, a phosphene was reported on every trial for the early part of thresholding procedures allowing participants to recognize a reliable precept of phosphenes before determining PT. This descending approach to phosphene threshold was selected to reduce the risk of participants having artificially high thresholds as they were not waiting for a phosphene to appear, but rather for one that they could already see to disappear.
Data Analysis
rMT, aMT, and PT were compared using one‐way repeated measures ANOVA with least significant difference post‐hoc contrasts. Pearson correlation coefficients were computed for each pair of threshold comparisons. Significance was set at P < 0.05 to assess differences from the null hypotheses.
RESULTS
Resting and aMT were measurable in all 27 participants. rMT ranged between 51 and 87% MSO (mean = 70.4; SD = 9.8); aMT ranged between 47 and 77% MSO (mean = 61.1; SD = 7.9). 21 out of 27 participants saw reproducible phosphenes and had a measurable PT ranging from 59 and 99% MSO (mean = 82.2; SD = 10.1) over the mean target site of 1 cm lateral and 3 cm above the inion. In most participants, phosphenes were small, diffuse, white flashes in the paracenter of the visual field that tended to grow smaller and dimmer with lower stimulator intensities. Four participants perceived colored static phosphenes.
Subjects that did not perceive phosphenes revealed a slightly higher aMT and rMT (aMT 65.3 ± 5.3 SD, rMT 74.7 ± 8.9) compared with subjects who did perceive phosphenes (aMT 59.9 ± 8.2 SD; rMT 69.2 ± 9.9), but the differences were not significant (aMT, P = 0.14; rMT P = 0.24).
Repeated measures ANOVA revealed significant differences between thresholds [F(2,40) = 2642, P < 0.001]. Post‐hoc contrasts revealed significant differences between each pair of thresholds: (a) rMT vs. aMT: mean difference ± SD = 9.33 ± 3.98, P < 0.001, (b) rMT vs. PT: 13.76 ± 9.59, P < 0.001, and (c) aMT vs. PT: 22.33 ± 9.02, P < 0.0001.
There was a strong positive correlation between rMT and aMT (r = 0.92; P < 0.001). A significant positive correlation was also found between aMT and PT (r = 0.53; P = 0.014; Fig. 1a). A trend toward a correlation was present between rMT and PT (r = 0.43; P = 0.052, Fig. 1b).
Figure 1.
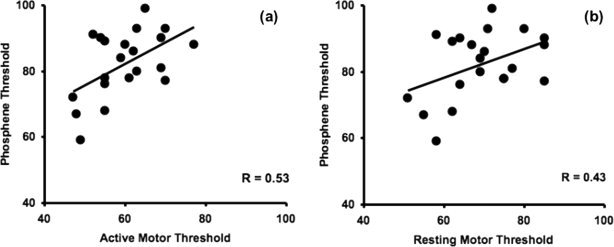
(a) Relationship between phosphene thresholds (PT) and active motor thresholds (rMT). PT and aMT are significantly correlated (P = 0.014). (b) Relationship between PT and resting motor thresholds (rMT) (P = 0.052). All thresholds are in percent of maximum stimulator output.
DISCUSSION
In contrast to previous reports [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b], we found a significant correlation between aMT and PT with a trend toward significance between rMT and PT. While the aMT and PT correlation was modest, with aMT accounting for 27.8% of the group variance in PT, our finding is the first to suggest such a relation between TMS thresholds in visual and motor cortex. Such a correlation is consistent with the idea that there is an element of global excitability specific to each subject if the thresholding procedures are similar for motor and visual cortex. While it may be useful to determine TMS intensities with relevant region‐ and task‐specific thresholds [Robertson et al., 2003], the present data suggest that aMT may help guide stimulation intensity over the visual cortex, and perhaps over other nonmotor regions. More clearly, as our finding is at variance with other studies using different methodologies, these data underscore the sensitivity of motor or PT to the details of the thresholding protocol, lighting conditions, and eyes‐open or eyes‐closed state. This advocates for brain mapping studies that use TMS to provide detailed statements of procedures, methodology, and TMS factors used when measuring reference thresholds and establishing experimental intensities over various brain regions.
The use of different approaches toward threshold (up, down, or other) and different states of visual input (eyes open or closed/blindfolded), employed in previous studies during MT and PT determinations, may have limited the ability to find a correlation between the two measures. Our emphasis on systematic parallel methodology for measuring MT and PT may have been critical in producing our positive findings.
All prior studies reported slightly different protocols for approaching PT. Stewart et al. [2001b] determined PT by decreasing or increasing TMS intensity in 5% increments from a starting point of 60% MSO. Two studies approached PT from below by increasing intensities in 1 or 2% increments [Antal et al., 2003b; Boroojerdi et al., 2002]. Gerwig et al. [2003] established PT by first increasing intensities by 5% increments to then randomly increasing and decreasing intensities by additional 2% increments.
In contrast, details about MT procedures were relatively sparse. One study reported approaching MT downward by reducing intensities by 2% increments [Stewart et al., 2001b]. Other studies provided few details about the approach to MT determination [Antal et al., 2003b; Boroojerdi et al., 2002; Stewart et al., 2001b]. Notably, while a downward approach to threshold has been recommended for MT in recent guidelines [Rothwell et al., 1999], none of the previous studies reported a downward approach to PT [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b].
Since both MEPs and phosphene perception show substantial trial‐to‐trial variability, all MT and PT thresholding procedures in the present and prior studies have used a statistical endpoint of at least 50% detection of behavioral outcome (MEP or phosphene perception) out of 6–10 test pulses. However, such statistical endpoints may produce different thresholds depending on whether it is systematically approached from below or from above. Consequently, it has been proposed to define MT as the mean of two thresholds, one from above and one from below [Mills and Nithi, 1997]. However, this method is time‐consuming and no more reliable than measuring thresholds by consistently approaching it downward [Tranulis et al., 2006]. However, MTs obtained with different techniques in the same subject can differ by as much as 8% of MSO [Mills and Nithi, 1997]. Although comparable data regarding differences in PT when approached differently are not available, we explicitly adopted a consistently downward threshold search for both MT and PT.
Previous studies investigating MT and PT correlations performed PT determinations with either closed eyes [Antal et al., 2003b] or blindfolds [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b]. In contrast, lighting conditions during MT determination procedures were either done with eyes open [Stewart et al., 2001b] or not reported [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b]. In either case, it is unlikely that explicit attention was taken to ensure comparable visual exposure and eyelid state during both MT and PT procedures.
Although PT does not change significantly under brief exposure to different lighting conditions [Kammer and Beck, 2002], longer periods of dark adaptation reduce PT and increase the yield of TMS induced phosphenes [Boroojerdi et al., 2000a; Marg and Rudiak, 1994]. We determined PT after 45 min of darkness, a time point by which most dark adaptation has taken place [Boroojerdi et al., 2000a]. Even though dark adaptation may continue beyond this time point, PT determinations at the same point in time allows reasonable comparisons between subjects. Since time of dark adaptation was not set in most other studies comparing MT and PT [Antal et al., 2003b; Boroojerdi et al., 2002; Gerwig et al., 2003; Stewart et al., 2001b], our data suggest that aMT may correlate mainly with a sufficiently dark‐adapted PT.
Even if no light is present, having eyes open or closed differentially influences cortical network activation and cortical excitability. An fMRI study demonstrated that activation patterns differ between eyes‐open and eyes‐closed conditions in darkness [Marx et al., 2004]. Whereas visual cortex showed greater activation and geniculate nucleus smaller activation under the eyes‐open condition, visuomotor structures (e.g., prefrontal and parietal cortices, frontal eye fields, cerebellar vermis, thalamus, and basal ganglia) revealed greater activation under the eyes‐closed condition. In addition, TMS studies have reported that, after dark adaptation, motor cortex excitability is increased [Leon‐Sarmiento et al., 2005], an effect comparable to the decreased phosphene threshold after light deprivation [Boroojerdi et al., 2001], possibly due to GABAergic and glutamatergic mechanisms from corticocortical networks connecting motor and visual areas [Bullier et al., 1996; Fadiga et al., 2000; Leon‐Sarmiento et al., 2005]. Thus, it seems apparent that the degree of light exposure and having eyes open or closed critically affects both brain activation patterns and cortical excitabilty. However, unlike PT [Boroojerdi et al., 2001], the time course of how MT might vary with light deprivation is not known and it is unclear to what degree 15 min of dark‐adaptation might have had, if any, on our MT measurements. Because of the possibility of different time‐courses for dark‐adaptation effects, we chose to measure MT and PT consistently at 15 and 45 minutes respectively after donning goggles rather than randomize the order of MT/PT determinations between subjects.
As expected and consistent with prior literature, we found significant differences between group mean rMTs, aMTs, and PTs. Within each subject, thresholds were consistently highest for PT and lowest for aMT. Because muscle contraction reduces variability of the spinal excitability by ensuring suprathreshold activation of spinal motor neurons, similar to other reports, we found that variability of aMT was lower than rMT [Antal et al., 2003b; Nitsche et al., 2005]. Voluntary muscle contraction raises motor cortical excitability (lower aMT), while reducing the spinal contribution toward variability [Kiers et al., 1993]. In parallel, it is possible that raised visual cortical excitability (lower PT) with dark‐adaptation [Boroojerdi et al., 2000a] may also reduce the variability of PT which is yet, to our knowledge, to be systematically examined. Thus, we note that our correlation was identified between aMT and dark‐adapted PT, both of which represent conditions of increased excitability and possibly of reduced variability or measurement noise. Kiers et al. [1993] suggested that during a relaxed muscle state, changes in cortical excitability in different regions may be relatively independent whereas these changes may be positively correlated during voluntary muscle contraction. By changing tonic levels of variability specific to each modality tested, i.e. for motor responses, removing spinal inhibition by muscle contraction; for visual percepts, increasing cortical excitability by dark adaptation, noise in measurements can be reduced.
Further, aMT and the PT we measured are likely related to the cortical elements or circuitry specific to the coil position and orientation used. Differences in magnetic stimulation parameters between PT and MT procedures also potentially limit correlations within studies. In one study, different stimulator models which generate different waveform types were used for PT and MT determination [Antal et al., 2003b]. Two studies used paired‐pulse TMS over the occipital lobe, while single‐pulse TMS was used for MT [Antal et al., 2003b; Boroojerdi et al., 2002]. For MT determinations, coil orientation was consistently oriented perpendicular to the central sulcus; for PT determinations, the coil handle was held upward in three studies [Antal et al., 2003b; Boroojerdi et al., 2002; Stewart et al., 2001b] and laterally in one study [Gerwig et al., 2003]. To control for these factors, we employed the same stimulator and applied single‐pulse TMS for all thresholding procedures. Although recent studies suggest a lateral preference for current orientation for phosphene induction [Kammer, 1999], we used the cranio‐caudal direction used in most previous MT and PT studies [Antal et al., 2003b; Boroojerdi et al., 2002; Stewart et al., 2001b].
Whether cortico‐cortical or/and cortico‐spinal axons are activated in the hand area depend on the orientation of the TMS‐applied magnetic field and the shape of the coil [Di Lazzaro et al., 2003]. For example, with a biphasic stimulator, both biphasic pulses may activate different descending volleys depending on the stimulus intensity and the direction of current flow. When the current is characterized by a lateromedial direction, such as was the case in this study, both the cortico‐spinal and cortico‐cortical axons are activated. Although it has been suggested that the level of muscle contraction does not affect the amplitude of the D‐wave induced by lateromedial magnetic stimulation, the level of excitability of the pyramidal tract neurons lead to an increase in the size and number of the I‐wave volleys [Di Lazzaro et al., 2003]. Consequently, the finding that resting and active muscle states may recruit different axonal elements may also partially explain why aMT, but not rMT was found correlated to PT. Similarly, PT is also sensitive to coil orientation with a preference for an induced lateromedial current direction [Kammer et al., 2001a], which suggests that the PT we determined is specific for our biphasic current and vertical coil orientation.
Our correlation is limited to those volunteers who saw phosphenes. We found no significant differences in either aMT or rMT in the six subjects that did not see phosphenes versus those who did see phosphenes. While some studies report that all their subjects discerned phosphenes [Boroojerdi et al., 2002; Rauschecker et al., 2004; Stewart et al., 2001b], other studies found a similar percentage of subjects lacked phosphene perception [Antal et al., 2003a, b; Boroojerdi et al., 2000b; Meyer et al., 1991; Sparing et al., 2005]. It is not certain that subjects who do not see phosphenes simply have higher than measurable thresholds [Chronicle and Mulleners, 2004]. For example, it may be differences in cortical anatomy or intrinsic differences in posterior cortical organization that could account some subjects from not perceiving phosphenes. Meister et al. [2003] proposed that the difference in phosphene perception may be found in different regions of visual cortical excitability: people who see phosphenes were associated with greater BOLD activation in primary striate and early extrastriate visual cortex, while those who do not perceive phosphenes were associated with higher BOLD activation in higher extrastriate areas [Meister et al., 2003].
Although we matched many psychophysical aspects of MT and PT procedures, we recognize that our procedures were not purely parallel. MT was objectively quantified by MEP amplitudes, while PT depended on subjective reporting. While the problem of phosphene perception is by nature a subjective one, future studies might more closely match MT procedures by asking subjects to report on their perception of muscle twitches following TMS over the motor cortex. Scalp–brain distances also differ over motor and visual cortices and without MRI scans, we could not adjust thresholds for scalp–brain distances as has been proposed [Stokes et al., 2005]. We did not quantitatively control force or baseline EMG amplitude for aMT, but rather monitored baseline EMG qualitatively online, while subjects squeezed with a pinch grip estimated at 10–15% of maximal voluntary force, a level above which further MEP facilitation is minimal [Mills, 1999]. While this procedure is comparable to some routine assessments of aMT, it also likely introduces greater noise in the aMT measurement than if controlled. Future studies might control baseline force or EMG more closely during aMT assessment. In addition, follow‐up correlational studies that use two fully blinded investigators to measure thresholds may help further clarify this issue. With these limitations accounted for, it is possible our mild correlation between aMT and dark adapted PT could have been further strengthened.
Physiologic differences and similarities exist between motor and visual cortices that may affect correlations between PT and MT. For example, paired‐pulse TMS studies over the motor cortex show differential excitatory or inhibitory effects dependent on the interstimulus interval (ISI); in contrast, phosphene detection is facilitated with paired‐pulse TMS over visual cortices, independently of ISI [Sparing et al., 2005]. Pharmacologic studies with TMS have shown that MT can be affected by drugs that block voltage‐gated sodium and calcium channels (e.g., carbamazepine, phenytoin, and lamotrigine) [Boroojerdi, 2002; Chen et al., 1997b; Manganotti et al., 1999; Ziemann et al., 1996b], while being unaffected by drugs that block GABA receptors (e.g., lorazepam, diazepam, vigabatrin) [Boroojerdi, 2002; Inghilleri et al., 1996; Ziemann et al., 1996a, b]. In contrast, these drug classes did not affect PT [Boroojerdi, 2002]. These differences continue to underscore the use of region‐specific thresholds when possible as reference thresholds for TMS studies.
On the other hand, a common excitability across the brain has been proposed in discussions of neurological disorders with either increased or decreased excitability (e.g., exemplified by epilepsy as a disorder of hyperexcitability) [Saugstad, 2005], and some neurophysiologic features of motor and visual cortices have been found to be similar. For example, both MT and PT increase with hyperventilation [Sparing et al., 2007]; cortical excitability, as measured by either MT or PT, is reduced by 1 Hz rTMS over each area [Boroojerdi et al., 2000b; Chen et al., 1997a]; and, rapid plasticity paradigms such as ischemic deafferentation over the motor cortex or dark‐adaptation over the visual cortices are both dependent on GABA and NMDA receptors [Boroojerdi et al., 2001; Buetefisch et al., 2000; Ziemann et al., 2004]. Specifically, some features of synaptic plasticity [long‐term potentiation (LTP) and long‐term depression (LTD)], within both primary motor and visual cortex appear similar [Bear, 1996; Boroojerdi et al., 2001; Buetefisch et al., 2000; Gilbert, 1998; Sanes and Donoghue, 2000; Ziemann et al., 2004]. In the motor cortex, LTP is dependent on NMDA activation and reduced GABA‐ergic inhibition [Hess and Donoghue, 1994; Otsuki et al., 1998], while being unaffected by sodium and calcium channel modulation [Buetefisch et al., 2000; Otsuki et al., 1998]. Similarly, the direction of GABA‐ergic inhibition and NMDA receptor activation, essential for LTP, have been linked with visual plasticity as well [Artola and Singer, 1987; Bear, 1996; Quinlan et al., 1999], while being independent of sodium channel blockade [Boroojerdi et al., 2001]. This evidence of shared neurophysiologic features between motor and visual cortices may suggest a rationale for our correlation.
CONCLUSION
Using procedurally similar approaches toward thresholding, we report a significant correlation between aMT over motor cortex and dark‐adapted PT over visual cortex. This correlation provides evidence that a shared, significant global contribution to cortical responsiveness to TMS might be present over different cortical regions. Our data suggest that TMS thresholds are sensitive to details of thresholding procedure, lighting conditions, and eyes‐open/eyes‐closed state and invite a re‐evaluation of methods of threshold determination when comparisons are being made across regions. Since aMT are easily measured, our data suggest the possibility of using aMT as a guide to calibrate TMS intensities during TMS mapping studies over the visual cortex and possibly other nonmotor cortical regions. In addition, our correlation provides a rationale for current guidelines that calibrate TMS intensity for repetitive TMS applications based on MT regardless of cortical target [Wassermann, 1998].
REFERENCES
- Antal A, Kincses TZ, Nitsche MA, Paulus W ( 2003a): Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res 150: 375–378. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Lampe C, Paulus W ( 2003b): No correlation between moving phosphene and motor thresholds: A transcranial magnetic stimulation study. Neuroreport 15: 297–302. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W ( 1987): Long‐term potentiation and NMDA receptors in rat visual cortex. Nature 330: 649–652. [DOI] [PubMed] [Google Scholar]
- Bear MF ( 1996): Progress in understanding NMDA‐receptor‐dependent synaptic plasticity in the visual cortex. J Physiol (Paris) 90: 223–227. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B ( 2002): Pharmacologic influences on TMS effects. J Clin Neurophysiol 19: 255–271. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Bushara KO, Corwell B, Immisch I, Battaglia F, Muellbacher W, Cohen LG ( 2000a): Enhanced excitability of the human visual cortex induced by short‐term light deprivation. Cereb Cortex 10: 529–534. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG ( 2000b): Reduction of human visual cortex excitability using 1 Hz transcranial magnetic stimulation. Neurology 54: 1529–1531. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG ( 2001): Mechanisms underlying rapid experience‐dependent plasticity in the human visual cortex. Proc Natl Acad Sci USA 98: 14698–14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Meister IG, Foltys H, Sparing R, Cohen LG, Toepper R ( 2002): Visual and motor cortex excitability: A transcranial magnetic stimulation study. Clin Neurophysiol 113: 1501–1504. [DOI] [PubMed] [Google Scholar]
- Brasil‐Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M ( 1992): Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol 9: 132–136. [PubMed] [Google Scholar]
- Buetefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG ( 2000): Mechanisms of use‐dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: 3661–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J, Schall JD, Morel A ( 1996): Functional areas in occipito‐frontal connections in the monkey. Behav Brain Res 76: 89–97. [DOI] [PubMed] [Google Scholar]
- Chaudry V, Cornblath DR, Mellits ED, Avila O, Freimer ML, Glass JD ( 1991): Inter‐ and intra‐examiner reliability of nerve conduction measurements in normal subjects. Annal Neurol 30: 841–843. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. ( 1997a): Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology 48: 1398–1403. [DOI] [PubMed] [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M. ( 1997b): Effects of phenytoin on cortical excitability in humans. Neurology 49: 881–883. [DOI] [PubMed] [Google Scholar]
- Chronicle EP, Mulleners WM ( 2004): Controversies in headache [Letter to the Editor]. Cephalalgia 24: 317–318. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual‐Leone A, Corwell B, Faiz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M ( 1997): Functional relevance of cross‐modal plasticity in blind humans. Nature 389: 180–183. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST ( 1999): Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2: 563–567. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Mazzone P, Insola A, Ranieri F, Tonali PA ( 2003): Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol Res 25: 143–150. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Gallese V, Rizzolatti G ( 2000): Visuomotor neurons: Ambiguity of the discharge or ‘motor’ perception? Int J Psychophysiol 35: 165–177. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Kastrup O, Meyer B‐U, Niehaus L ( 2003): Evaluation of cortical excitibilty by motor and phosphene thresholds in transcranial magnetic stimulation. J Neurol Sci 215: 75–78. [DOI] [PubMed] [Google Scholar]
- Gilbert CD ( 1998): Adult cortical dynamics. Physiol Rev 78: 467–485. [DOI] [PubMed] [Google Scholar]
- Gothe J, Brandt SA, Irlbacher K, Roericht S, Sabel BA, Meyer BU ( 2002): Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain 125: 479–490. [DOI] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC ( 2003): The essential role of Broca's area in imitation. Eur J Neurosci 17: 1123–1128. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP ( 1994): Long‐term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol 71: 2543–2547. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Anand S ( 1999): The selectivity and timing of motion processing in human temporo‐parieto‐occipital cortex: A transcranial magnetic stimulation study. Neuropsychologia 37: 169–179. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M ( 1996): Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res 109: 467–472. [DOI] [PubMed] [Google Scholar]
- Kammer T ( 1999): Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: Their topographic relationship. Neuropsychologia 37: 191–198. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S ( 2002): Phosphene thresholds evoked by transcranial magnetic stimulation are insensitive to short‐lasting variations in ambient light. Exp Brain Res 145: 407–410. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Erb M, Grodd W ( 2001a): The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin Neurophysiol 112: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J ( 1993): Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89: 415–423. [DOI] [PubMed] [Google Scholar]
- Leon‐Sarmiento FE, Bara‐Jimenez W, Wassermann EM ( 2005): Visual deprivation effects on human motor cortex excitability. Neurosci Lett 389: 17–20. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Bongiovanni LG, Zanette G, Turazzini M, Fiaschi A ( 1999): Cortical excitability in patients after loading doses of lamotrigine: A study with magnetic brain stimulation. Epilepsia 40: 316–321. [DOI] [PubMed] [Google Scholar]
- Marg E, Rudiak D ( 1994): Phosphenes induced by magnetic stimulation over the occipital brain: description and probable site of stimulation. Optom Vis Sci 71: 301–311. [DOI] [PubMed] [Google Scholar]
- Marx E, Deutschlander A, Stephan T, Dieterich M, Wiesmann M, Brandt T ( 2004): Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. Neuroimage 21: 1818–1824. [DOI] [PubMed] [Google Scholar]
- Meister IG, Weidemann J, Dambeck N, Foltys H, Sparing R, Krings T, Thron A, Boroojerdi B ( 2003): Neural correlates of phosphene perception. Suppl Clin Neurophysiol 56: 305–311. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Diehl RR, Steinmetz H, Britton TC, Benecke R ( 1991): Magnetic stimuli applied over motor cortex and visual cortex: Influence of coil position and field polarity on motor responses, phosphenes and eye movements. Electroencephalogr Clin Neurophysiol 43: 121–134. [PubMed] [Google Scholar]
- Mills KR ( 1999). Magnetic Stimulation of the Human Nervous System. New York: Oxford University Press. [Google Scholar]
- Mills KR, Nithi K ( 1997): Corticomotor threshold to magnetic stimulation: Normal values and repeatability. Muscle Nerve 20: 570–576. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, et al. ( 2005): Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki K, Morimoto K, Sato K, Yamada N, Kuroda S ( 1998): Effects of lamotrigine and conventional antiepileptic drugs on amygdala‐ and hippocampal‐kindled seizures in rats. Epilepsy Res 31: 101–112. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF ( 1999): Rapid, experience‐dependent expression of synaptic NMDA receptors in visual cortex in vivo. Natl Neurosci 2: 352–357. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Bestmann S, Walsh V, Thilo KV ( 2004): Phosphene threshold as a function of contrast of external visual stimuli. Exp Brain Res 157: 124–127. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual‐Leone A ( 2003): Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cogn Neurosci 15: 948–960. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini PM, Paulus W ( 1999): Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neuropysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 97–103. [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP ( 2000): Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415. [DOI] [PubMed] [Google Scholar]
- Saugstad LF ( 2005): The “new‐old” way of thinking about brain disorder, cerebral excitability—The fundamental property of nervous tissue. Med Hypotheses 64: 142–492. [DOI] [PubMed] [Google Scholar]
- Sparing R, Dambeck N, Stock K, Meister IG, Huetter D, Boroojerdi B ( 2005): Investigation of the primary visual cortex using short‐interval paired‐pulse transcranial magnetic stimulation (TMS). Neurosci Lett 382: 312–316. [DOI] [PubMed] [Google Scholar]
- Sparing R, Dafotakis M, Buelte D, Meister IG, Noth J ( 2007): Excitability of human motor and visual cortex before, during and after hyperventilation. J Appl Physiol 102: 405–411. [DOI] [PubMed] [Google Scholar]
- Stewart L, Ellison A, Walsh V, Cowey A. ( 2001a): The role of transcranial magnetic stimulation (TMS) in studies of vision, attention and cognition. Acta Psychol 107: 275–291. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. ( 2001b): Motor and phosphene thresholds: A transcranial magnetic stimulation correlation study. Neuropsychologia 39: 415–419. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB ( 2005): Simple metric for scaling motor threshold based on scalp‐cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol 94: 4520–4527. [DOI] [PubMed] [Google Scholar]
- Tranulis C, Gueguen B, Pham‐Scottez A, Vacheron MN, Cabelguen G, Costantini A, Valero G, Calinovski A ( 2006): Motor threshold in transcranial magnetic stimulation: Comparison of three estimation methods. Neurophysiol Clin 36: 1–7. [DOI] [PubMed] [Google Scholar]
- Wassermann EM ( 1998): Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W ( 1996a): The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 109: 127–135. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W ( 1996b): Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magnetic stimulation study. Ann Neurol 40: 367–378. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D ( 2004): Learning modifies subsequent induction of long‐term potentiation‐like and long‐term depression‐like plasticity in human motor cortex. J Neurosci 24: 1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]


