Abstract
Although heterosexual and homosexual individuals clearly show differences in subjective response to heterosexual and homosexual sexual stimuli, the neurobiological processes underlying sexual orientation are largely unknown. We addressed the question whether the expected differences in subjective response to visual heterosexual and homosexual stimuli may be reflected in differences in brain activation pattern. Twenty‐four healthy male volunteers, 12 heterosexuals and 12 homosexuals, were included in the study. BOLD signal was measured while subjects were viewing erotic videos of heterosexual and homosexual content. SPM02 was used for data analysis. Individual sexual arousal was assessed by subjective rating. As compared to viewing sexually neutral videos, viewing erotic videos led to a brain activation pattern characteristic for sexual arousal in both groups only when subjects were viewing videos of their respective sexual orientation. Particularly, activation in the hypothalamus, a key brain area in sexual function, was correlated with sexual arousal. Conversely, when viewing videos opposite to their sexual orientation both groups showed absent hypothalamic activation. Moreover, the activation pattern found in both groups suggests that stimuli of opposite sexual orientation triggered intense autonomic response and may be perceived, at least to some extent, as aversive. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: sexual arousal, sexual orientation, functional magnetic resonance imaging
INTRODUCTION
Although there are evident differences in subjective response to heterosexual and homosexual stimuli in heterosexual and homosexual individuals [Mavissakalian et al., 1975], the neurobiological processes underlying sexual orientation are largely unknown. Single reports have been published investigating structural differences of the brain with respect to sexual orientation, including the hypothalamus [Byne et al., 2001; LeVay, 1991] and anterior commissure [Allen and Gorski, 1992]. However, the role of sexual orientation has not been at the focus of neural functional studies published to date.
Several functional imaging studies using positron emission computed tomography (PET) have examined brain response to sexual stimulation, identifying brain regions activated with sexual arousal. Tiihonen and colleagues observed increased regional cerebral blood flow (rCBF) in the right prefrontal cortex during orgasm [Tiihonen et al., 1994]. Stoleru et al. [1999] found increased rCBF in response to visual sexual stimulation in the inferior temporal cortex, the right caudate nucleus, and paralimbic areas, such as the right inferior frontal cortex, right insula, and left anterior cingulate gyrus. In another PET study, Rauch et al. [1999] used a paradigm of script‐driven imaginary and recollection of sexually arousing events to measure rCBF during sexual arousal and observed increases in the brain stem and claustrum. Redoute et al. [2000] identified the claustrum, striatum, and posterior hypothalamus, as well as paralimbic areas, such as the anterior cingulate gyrus and orbitofrontal cortex, to be activated with visual sexual stimulation. In a similar experimental setting using PET and visual sexual stimulation, Bocher et al. [2001] found increased rCBF in the occipitotemporal cortex, right inferior prefrontal cortex, and the midbrain. Holstege et al. [2003] measured rCBF during orgasm and found activation in the mesodiencephalic junction, an area known to contain centers that are involved in rewarding behaviors. They also found activation in the striatum, claustrum, insula, and right prefrontal cortex, corresponding to the activations found with visual sexual stimulation in the above mentioned studies by Stoleru et al., Redoute et al., and Bocher et al.
In a number of studies, functional magnetic resonance imaging (fMRI) has been used to examine brain response to sexual arousal, with the advantage of high spatial resolution and superior temporal correlation between external stimuli and brain response. Park and colleagues used fMRI to examine brain activation in response to visual sexual stimulation in males and females. They found activation in the thalamus, striatum, and inferior temporal lobe as well as paralimbic areas, such as the inferior frontal lobe, the cingulate gyrus, and the insula in both males and females [Park et al., 2001a, b]. Also using visual sexual stimulation, Karama and colleagues found activation in the orbitofrontal cortex, anterior cingulate cortex, insular cortex, occipitotemporal cortex, amygdala, and ventral striatum with visual sexual stimulation in males and females. However, significant activation in the hypothalamus and thalamus was only observed in males [Karama et al., 2002]. In a subsequent study, Beauregard et al. [2001] observed activation in the hypothalamus, right amygdala, and right anterior temporal lobe with visual sexual stimulation in males. These activations were suppressed and instead activation in the right superior frontal lobe and right anterior cingulate gyrus was found when subjects voluntarily attempted inhibition of sexual arousal. Arnow et al. [2002] examined brain response to visual sexual stimulation in males and observed strong activation in the right insula and claustrum, similar to that reported previously [Redoute et al., 2000; Stoleru et al., 1999]. Activation was also found in the hypothalamus, striatum, cingulate gyrus, and temporal, frontal, and occipital cortices [Arnow et al., 2002]. In another fMRI study investigating brain response to visual sexual stimulation in males, Mouras et al. [2003] found activations in parietal, occipital, and frontal cortices and the cerebellum. Hamann et al. [2004] compared brain response to visual sexual stimulation in males and females and found activation in parietal, temporal, and occipital cortices as well as the anterior cingulate and striatum in both males and females, whereas only males showed significant activation in the hypothalamus and amygdala.
Some studies have been published that investigate brain response to visual sexual stimulation in patients with sexual disorders. Montorsi et al. [2003] used fMRI to investigate the influence of apomorphine on brain response in males with erectile dysfunction in comparison to healthy controls. They found similar activation in frontal, parietal, occipital, and temporal cortices in both groups. However, only the patient group showed activation in the frontobasal cortex and cingulate gyrus, a difference that could partially be revoked by application of apomorphine. In a PET study with males suffering from erectile dysfunction, Hagemann et al. [2003] found activation in the inferior frontal cortex and anterior cingulate with visual sexual stimulation and increased activity in the right prefrontal cortex after application of apomorphine. In another PET study, Stoleru et al. [2003] examined brain response in males with hypoactive sexual desire disorder. Compared to healthy controls, these patients showed a maintained activity of the medial orbitofrontal cortex with visual sexual stimulation. Yang [2004] examined brain response in depressed males with sexual dysfunction and, compared to healthy controls, found reduced activity in the temporal cortex, thalamus, hypothalamus, and striatum.
On the basis of the evidence from these previous studies, we used fMRI to examine the influence of sexual orientation on brain response to visual sexual stimuli in healthy males. We addressed the question whether the expected differences in subjective response to visual heterosexual and homosexual stimuli may be reflected in differences in brain activation pattern, particularly in brain regions known to be responsive to sexual arousal.
SUBJECTS AND METHODS
Subjects and Experimental Design
Twenty‐four healthy male volunteers aged 22–46 years (mean ± standard deviation 33.4 ± 7.2 years) participated in the study. All subjects gave informed consent to participate, the study was approved by the ethics committee of the Faculty of Medicine, University of Duisburg‐Essen, Germany. Prior to inclusion, the sexual orientation of each subject was assessed using the Kinsey heterosexual–homosexual rating scale [Kinsey et al., 1948]. Twelve heterosexual subjects (ages 23–46 years, mean ± standard deviation 34.8 ± 7.6 years) with scores 0 or 1 (8 with score 0 and 4 with score 1, mean score 0.33) and 12 homosexual subjects (ages 22–43 years, mean ± standard deviation 32.0 ± 6.8 years) with scores 5 or 6 (5 with score 6 and 7 with score 5, mean score 5.42) were included. The functional imaging sessions were run in a block design and consisted of seven epochs of sexually arousing visual stimuli and seven epochs of sexually neutral visual stimuli, arranged in an alternating pattern. The epoch length was 38.5 s. All subjects underwent two consecutive functional imaging sessions, one with sexually arousing stimuli of heterosexual content versus sexually neutral stimuli and one with sexually arousing stimuli of homosexual content versus sexually neutral stimuli. Each subject therefore went through one session with visual sexual stimuli corresponding to their respective sexual orientation (COR) and one session with stimuli opposite to their sexual orientation (OPP), both contrasted to sexually neutral visual stimuli. The sessions were counterbalanced between subjects for order of presentation. As sexually arousing stimuli film excerpts of heterosexual couples or male homosexual couples engaged in explicit sexual activity were used. The content included explicit view of genitals. Mostly genital sexual intercourse was presented but also some parts of oral sexual practice. The sexually neutral stimuli were videos showing couples during regular nonsexual activity such as gardening, working together, or talking. The heterosexual stimuli had been validated and used in previous neuroimaging studies [Karama et al., 2002]. The homosexual material was evaluated for arousal and emotional valence in a further group of age matched volunteers. The homosexual stimuli consisted of film excerpts of males in explicit genital and oral sexual activity in a similar arrangement as in the heterosexual stimuli, the corresponding sexually neutral stimuli were film excerpts showing dressed persons during nonerotic activity, equivalent to the heterosexual stimuli. Individual sexual arousal was assessed immediately after functional imaging by subjective rating, using a rating scale based on the Acute Sexual Experience Scale (ASES), with a range of 0 (lowest) to 10 (highest) [Kruger et al., 2003].
Data Acquisition
All images were acquired using a 1.5 T magnetic resonance (MR) scanner (Sonata, Siemens, Erlangen, Germany) with a standard head coil. Blood oxygenation level dependent (BOLD) contrast images were acquired using an echoplanar technique (TR 3,500 ms, TE 55 ms, flip angle 90°, FOV 220–240 mm, matrix 64) with 36 transversal slices of 3 mm thickness and a slice gap of 0.3 mm. Additionally, a 3D FLASH sequence (TR 10 ms, TE 4.5 ms, flip angle 30°, FOV 240 mm, matrix 512, slice‐thickness 1.5 mm) was acquired for individual coregistration of functional and structural images.
Imaging Data Analysis
For data analysis Statistic Parametric Mapping (SPM02) software (Wellcome Department of Cognitive Neurology, London, UK) was used. The first three scans of each session were eliminated from data analysis to account for T1 relaxation effects. Prior to statistical analysis, images were realigned using sinc interpolation and normalized to the Montreal Neurological Institute (MNI) space, a template created from 152 brain data sets in Talairach coordinates [Talairach and Tournoux, 1988]. Trilinear interpolation was applied for normalization. The images were smoothed with an isotropic Gaussian kernel of 9 mm. A voxel‐by‐voxel comparison according to the general linear model was used to calculate differences of activation between active and resting condition. The model consisted of a boxcar function convolved with the hemodynamic response function and the corresponding temporal derivative. High‐pass filtering with a cut off of 128 s and low‐pass filtering with the hemodynamic response function was applied. For analysis of group‐specific and shared neural activation, single‐subject contrast images were entered into a random effects model. Significant signal changes for each contrast were assessed by means of t statistics on a voxel‐by‐voxel basis [Friston et al., 1995]. The resulting set of voxel values for each contrast represents a statistical parametric map of the t statistics. For visualization of neural activity, the activation maps were overlaid on the high‐resolution T1‐weighted single subject images from the SPM02 canonical image set.
For each group of subjects, the heterosexual group (HET) and the homosexual group (HOM), two contrasts were applied: erotic stimuli corresponding to the subjects' sexual orientation minus neutral stimuli (COR contrast) and erotic stimuli opposite to the subjects' sexual orientation minus neutral stimuli (OPP contrast). Areas of significant neural activation for these contrasts were identified by whole‐brain analysis with a statistical threshold of P < 0.001, uncorrected for multiple comparisons, with a spatial extent of at least five adjacent voxels.
In addition, brain regions known to be responsive to sexual arousal were examined by a priori analysis. On the basis of the results of previous neuroimaging studies, the following brain regions were defined as regions of interest (ROI): hypothalamus, amygdala, claustrum, striatum, insula, anterior cingulate gyrus, and orbitofrontal cortex [Arnow et al., 2002; Beauregard et al., 2001; Hamann et al., 2004; Karama et al., 2002; Park et al., 2001b; Redoute et al., 2000; Stoleru et al., 1999]. The hypothalamic ROI was defined as a sphere of 8 mm diameter centered on the Talairach coordinates 0, 0, and −9. To examine differences in neural response between HET and HOM, subtraction analysis using the statistical activation maps of each subject generated by the COR and OPP contrasts, was performed. To compare response to heterosexual stimuli, the activation maps of HOM viewing heterosexual stimuli were subtracted from the activation maps of HET viewing heterosexual stimuli (HET COR minus HOM OPP). Conversely, to compare response to homosexual stimuli, the activation maps of HET viewing homosexual stimuli were subtracted from the activation maps of HOM viewing homosexual stimuli (HOM COR minus HET OPP). To compare response to the COR and OPP contrasts, subtraction analysis of the COR and the OPP contrasts (HET COR minus HOM COR and vice versa; HET OPP minus HOM OPP and vice versa) was performed. Finally, a simple regression analysis of the COR contrasts with the individual rating of sexual arousal as a regressor (covariate) for each subject's activation map was performed. For the comparative analyses, the regression analysis, and the inclusive masking analysis, the statistical threshold was lowered to P < 0.05, uncorrected for multiple comparisons.
RESULTS
In both the heterosexual group (HET) and the homosexual group (HOM) individual sexual arousal was greatly dependent on the stimulus type. Both groups rated COR as more arousing than OPP (Fig. 1). In HET the ratings ranged from 1.6 to 9.2 (mean 6.0) for COR and from 0.2 to 6.7 (mean 2.5) for OPP, in HOM the ratings ranged from 2.8 to 8.9 (mean 6.5) for COR and from 0.0 to 3.6 (mean 1.6) for OPP. All subjects rated the COR stimuli as more arousing than the OPP stimuli. A two‐factor analysis of variance (ANOVA; individual sexual orientation, stimulus type) showed a highly significant main effect of stimulus type (F = 34.49, P < 0.00001). There was no significant difference between HET and HOM in sexual arousal (F = 0.10, P > 0.75) and no interaction was found between sexual orientation and stimulus type (F = 1.07, P > 0.30).
Figure 1.
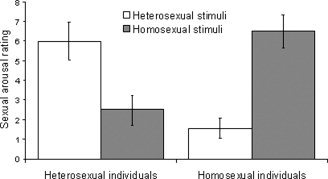
Mean sexual arousal ratings of heterosexual and homosexual subjects. Individual sexual arousal was assessed by subjective rating, using a rating scale based on the ASES, with 0 = lowest and 10 = highest. Each subject rated the heterosexual and the homosexual stimuli immediately after functional imaging. Error bars indicate standard error of the mean.
The whole‐brain analysis of the functional images revealed neural activation in various parts of the brain in HET and HOM when viewing COR and OPP. HET showed an extensive bilateral occipito‐parieto‐temporal cluster of activation for the COR contrast (Fig. 2A). Additionally, HET showed activation in the right anterior and left rostral cingulate gyrus, left caudate nucleus, right insula, right superior frontal gyrus, and left medial frontal gyrus, as well as bilaterally in the cerebellum and the right midbrain. In HOM, the COR contrast led to a similar activation pattern (Fig. 2B) with a large bilateral occipito‐parieto‐temporal cluster of activation. Furthermore, activation was found in the right medial frontal gyrus and the left inferior frontal gyrus, and bilateral cerebellum. In Table I a complete list of clusters of significant activation for the COR contrasts in HET and HOM in the whole‐brain analysis is given.
Figure 2.
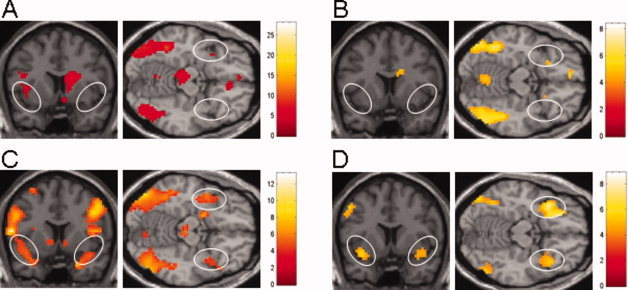
Regional maps of activation contrasts for sexual visual stimuli corresponding to the individual's sexual orientation (COR: A, B) and for sexual visual stimuli opposite to the individual's sexual orientation (OPP: C, D); both versus sexually neutral visual stimuli in heterosexual males (HET: A, C) and homosexual males (HOM: B, D). Statistical threshold P < 0.001 (uncorrected) for a minimum of five adjacent voxels. The right hemisphere is on the left of the coronal images and on the top of the axial images. Color bars indicate maximal Z values. (A) Bilateral occipito‐temporal, right insular and left caudate nucleus activation, absent anterior insular/inferior frontal activation in HET for the COR contrast. (B) Bilateral occipito‐temporal activation, absent anterior insular/inferior frontal activation in HOM for the COR contrast. (C) Bilateral occipito‐temporo‐parietal activation, strong bilateral anterior insular/inferior frontal activation in HET when viewing OPP. (D) Bilateral occipito‐temporal, right frontal and left caudate nucleus activation together with strong bilateral anterior insular/inferior frontal activation in HOM for the OPP contrast.
Table I.
Voxels with peak Z value within regions of significant neural activation for the COR contrasts (P < 0.001, uncorrected)
| Region | Talairach coordinates | Z value | ||
|---|---|---|---|---|
| x | y | z | ||
| HET COR | ||||
| Right anterior cingulate gyrus | 18 | 42 | −3 | 4.42 |
| Left rostral cingulate gyrus | −18 | 3 | 30 | 3.34 |
| Left caudate nucleus | −15 | 6 | 18 | 4.02 |
| Right insula/inferior frontal gyrus | 36 | 15 | −3 | 3.64 |
| Right superior frontal gyrus | 24 | 51 | 12 | 3.68 |
| Left medial frontal gyrus | −9 | 36 | −15 | 3.68 |
| Right occipital cortex | 36 | −81 | 3 | 6.76 |
| Left occipital cortex | −51 | −63 | −6 | 5.03 |
| Right parietal cortex | 60 | −27 | 24 | 4.66 |
| Left parietal cortex | −69 | −30 | 21 | 4.48 |
| Right temporal cortex | 42 | −48 | −15 | 5.04 |
| Left temporal cortex | −42 | −51 | −21 | 3.35 |
| Right cerebellar hemisphere | 21 | −66 | −51 | 4.14 |
| Left cerebellar hemisphere | −12 | −72 | −48 | 4.93 |
| Right midbrain | 6 | −27 | −15 | 3.94 |
| HOM COR | ||||
| Right medial frontal gyrus | 6 | 48 | −15 | 3.63 |
| Left inferior frontal gyrus | −24 | 33 | −9 | 3.54 |
| Right occipital cortex | 42 | −81 | 0 | 4.60 |
| Left occipital cortex | −39 | −75 | 3 | 4.02 |
| Right parietal cortex | 60 | −24 | 27 | 4.36 |
| Left parietal cortex | −24 | −48 | 39 | 3.73 |
| Right temporal cortex | 42 | −51 | −12 | 4.25 |
| Left temporal cortex | −48 | −42 | −18 | 3.98 |
| Right cerebellar hemisphere | 24 | −51 | −48 | 4.04 |
| Left cerebellar hemisphere | −21 | −60 | −48 | 3.64 |
When viewing the OPP contrast, HET showed a bilateral occipito‐parieto‐temporal cluster of activation similar to that of the COR contrast (Fig. 2C). In addition, activation was observed in the right amygdala, right caudate nucleus, right and left insula, left putamen, the right and left inferior and middle frontal gyrus, and bilateral midbrain. HOM showed an occipito‐parietal cluster of activation for the OPP contrast (Fig. 2D), together with activation in the left caudate nucleus, right medial, superior, and middle frontal gyrus and bilaterally in the anterior insula/inferior frontal gyrus. Table II lists all clusters of significant activation for the OPP contrasts in HET and HOM in the whole‐brain analysis.
Table II.
Voxels with peak Z value within regions of significant neural activation for the OPP contrasts (P < 0.001, uncorrected)
| Region | Talairach coordinates | Z value | ||
|---|---|---|---|---|
| x | y | z | ||
| HET OPP | ||||
| Right amygdala | 21 | 0 | −18 | 4.34 |
| Right caudate nucleus | 9 | 6 | 0 | 3.78 |
| Right insula | 45 | 3 | 0 | 5.14 |
| Left insula | −36 | 3 | 6 | 3.37 |
| Left putamen | −12 | 9 | −3 | 3.21 |
| Right inferior frontal gyrus | 45 | 39 | 9 | 3.55 |
| Right middle frontal gyrus | 36 | 0 | 60 | 4.81 |
| Left inferior frontal gyrus | −54 | 18 | 12 | 3.96 |
| Left middle frontal gyrus | −51 | 6 | 42 | 4.59 |
| Right occipital cortex | 48 | −75 | −12 | 4.86 |
| Left occipital cortex | 45 | −75 | −9 | 4.99 |
| Right parietal cortex | 66 | −30 | 33 | 5.28 |
| Left parietal cortex | −33 | −48 | 57 | 5.00 |
| Right temporal cortex | 42 | 3 | −12 | 4.16 |
| Left temporal cortex | −39 | 9 | −18 | 3.50 |
| Right midbrain | 3 | −27 | −6 | 3.70 |
| Left midbrain | −6 | −21 | −9 | 4.19 |
| HOM OPP | ||||
| Left caudate nucleus | −9 | 15 | 6 | 3.26 |
| Right medial frontal gyrus | 9 | 57 | 3 | 3.73 |
| Right superior frontal gyrus | 15 | 57 | 27 | 3.91 |
| Right middle frontal gyrus | 24 | 27 | −15 | 4.30 |
| Right insula/inferior frontal gyrus | 39 | 15 | −12 | 4.65 |
| Left insula/inferior frontal gyrus | −39 | 15 | −12 | 3.83 |
| Right occipital cortex | 45 | 84 | 6 | 4.61 |
| Left occipital cortex | −54 | −72 | −6 | 4.19 |
| Right parietal cortex | 63 | −24 | 21 | 4.72 |
| Left parietal cortex | −66 | −30 | 24 | 4.19 |
In the comparison analysis as well as the regression analysis, evaluable neural activation in the ROI was only found when the threshold was set at a lenient P < 0.05 (uncorrected for multiple comparisons). At this threshold, the hypothalamus, the orbitofrontal cortex, and the parietal cortex were the only ROI that showed significant neural activation (Table III). Given the low level of statistical significance, further a priori analysis was therefore restricted to the hypothalamus. Table IV lists the results of hypothalamic activation for the comparison analysis and the regression analysis for the COR contrasts. Compared to HOM, HET revealed clear hypothalamic response to heterosexual erotic stimuli (HET COR minus HOM OPP). Conversely, compared to HET, HOM showed hypothalamic activation when viewing homosexual erotic stimuli (HOM COR minus HET OPP) (Fig. 3A, B). Similarly, in the regression analysis, both HET and HOM showed hypothalamic activation for the COR contrasts (Fig. 3C, D). We also extended the ROI slightly anteriorly covering the preoptic area (0, 6, −9). The sphere diameter was maintained. Using this further anterior ROI on the second level analysis of HET COR, a cluster of activation at 6, 6, −6 (Z = 3.4), on the second level analysis of HOM COR a cluster of activation at 5, 6, −9 (Z = 2.9) was revealed. Again the Z‐score was lower in HOM than in HET.
Table III.
Coordinates of voxel with peak Z value of cluster of activation for the comparison of the COR condition (P < 0.05, uncorrected)
| Contrast | Talairach coordinates | Cluster Z value | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| HET COR– HOM COR | 3 | −3 | 9 | 2.59 | Hypothalamus |
| 9 | 39 | −18 | 2.04 | Orbitofrontal | |
| HOM COR– HET COR | ±34 | −57 | 0 | 4.02 | Parietal |
| −6 | 48 | −18 | 2.04 | Orbitofrontal | |
Table IV.
Coordinates of voxel with peak Z value, Z value and k of cluster, and ROI of hypothalamic activation for the comparison and the regression analysis (P < 0.05, uncorrected)
| Contrast | Talairach coordinates | Cluster Z value | Cluster k | ROI Z value | ROI k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| HET COR–HOM OPP | −3 | −3 | 9 | 2.84 | 76 | 2.71 | 7 |
| HOM COR–HET OPP | 0 | 3 | −9 | 1.91 | 7 | 1.91 | 4 |
| Regression HET COR | −3 | 0 | −12 | 2.31 | 52 | 2.28 | 6 |
| Regression HOM COR | −3 | 0 | −12 | 1.89 | 9 | 1.88 | 4 |
ROI was defined as an 8 mm sphere centered on the coordinates 0, 0, −9.
Figure 3.
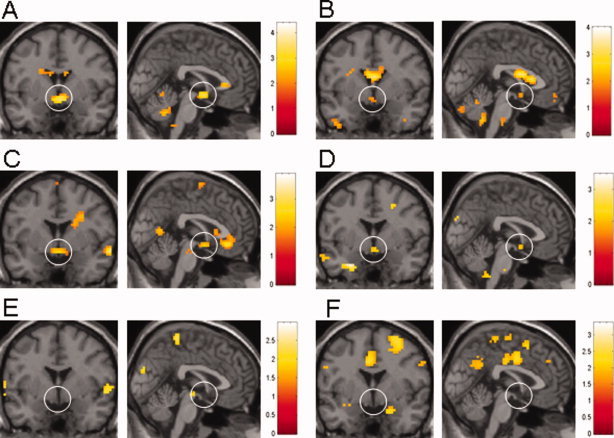
Coronal and sagittal view images showing activation maps of the comparison analysis (A, B, E, F) and regression analysis (C, D) of hypothalamic activation in heterosexual males (A, C, E) and homosexual males (B, D, F). The statistical threshold was set P < 0.05 (uncorrected). The right hemisphere is on the left of the coronal images. Color bars indicate maximal Z values. The activations in the ventricular system and corpus callosum seen in A, B, and C represent artifacts. (A) HET COR minus HOM OPP contrast showing strong hypothalamic activation. (B) HOM COR minus HET OPP contrast showing some hypothalamic activation. (C) HET COR contrast with subjective rating of sexual arousal as a regressor revealing strong hypothalamic activation. (D) HOM COR contrast with subjective rating of sexual arousal as a regressor again showing some hypothalamic activation. (E) HET OPP minus HOM COR contrast with absent hypothalamic activation. (F) Absent hypothalamic activation also in HOM COR minus HET OPP contrast.
In contrast, neither HET nor HOM showed significant hypothalamic response in the comparison analysis for the OPP contrasts (Fig. 3E, F) and no significant hypothalamic activation was found for the OPP contrasts in the regression analysis (data not shown). HET showed considerably stronger hypothalamic activation for the COR contrast than HOM, with larger cluster size and higher Z value on the cluster level as well as higher number of activated voxels and higher Z value in the ROI analysis.
Direct comparison of the COR contrasts as well as the OPP contrasts in both groups (HET COR minus HOM COR; HET OPP minus HOM OPP and vice versa) did not reveal significant differences in any of the ROI. At a threshold of P < 0.001 (uncorrected) differences were restricted to cortical areas outside the predefined ROI, mainly the occipital cortex. At a threshold of P < 0.05 (uncorrected) no difference in hypothalamic activation was found (data not shown).
DISCUSSION
The neurobiological processes underlying sexual orientation are largely unknown and a neural functional correlate for sexual orientation has not been identified to date. In our study, we examined the influence of male sexual orientation on brain response to visual sexual stimulation by correlating subjective sexual arousal with brain activation pattern. The two types of visual sexual stimuli we used were videos of heterosexual and of homosexual content and hence each subject underwent two functional imaging sessions, one with stimuli corresponding to their sexual orientation (COR) and one with stimuli opposite to their sexual orientation (OPP). We found that subjective sexual arousal ratings for COR and OPP were equivalent in the heterosexual group (HET) and the homosexual group (HOM). In both groups sexual arousal was greatly dependent on the stimulus type. All subjects in both groups significantly rated COR as more sexually arousing than OPP. However, some subjects rated the OPP stimuli sexually arousing to some degree. This limited cross‐over arousal is in accordance with the fact that we not only included individuals with Kinsey Scale scores 0 and 6 (exclusively heterosexual/homosexual), but also with scores 2 and 5 (predominantly heterosexual/homosexual, only incidentally homosexual/heterosexual). In a previous study comparing response to visual sexual stimuli, heterosexual and homosexual males were reported to differ in subjective sexual arousal to COR and OPP, with both groups showing high sexual arousal with COR, but only homosexuals also showing high sexual arousal with OPP. The authors speculate that the presence of a man and a woman in the heterosexual stimuli allows the subject to focus on the figure of his preference and hence that erotic films with heterosexual couples are not suitable for assessing sexual orientation [Mavissakalian et al., 1975]. However, the majority of the homosexual subjects (5 of 6) in this study had a Kinsey Scale score of 5 and only one subject had a score of 6, whereas in our study only 7 of 12 homosexual subjects had a Kinsey Scale score of 5, reflecting the known variation in sexual orientation value in the general population [Le Vay, 1996; McConaghy, 1999], which may at least in part explain the observed discrepancies in the response to OPP. The sexual arousal in our group for the OPP stimulus was slightly lower in the group with a Kinsey score of 6 (1.56) than in the group with a Kinsey score of 5 (1.81). Additionally, all but one subject with a score of 6 rated the stimulus lower than those with a score of 5, but because of the small number in those two groups these results are not significant and have to be addressed in a further study.
In the first part of the study we analyzed brain response to COR stimuli in HET and HOM. In both groups, we found significant correlation between sexual arousal and neural activation in the hypothalamus, a key brain region in sexual function [Kupfermann, 1991]. This result is in line with the findings of several other neuroimaging studies that have shown correlation of hypothalamic activation with sexual arousal and penile tumescence in heterosexual males [Arnow et al., 2002; Hamann et al., 2004; Karama et al., 2002; Redoute et al., 2000]. Our results suggest that in heterosexual and homosexual males sexual arousal is uniformly associated with neural activation in the hypothalamus, independent of sexual orientation. Activation of the other brain areas defined as regions of interest, including the amygdala, claustrum, striatum, insula, anterior cingulate gyrus, and orbitofrontal cortex, with sexual arousal has also been well established in a number of neuroimaging studies [Arnow et al., 2002; Beauregard et al., 2001; Hamann et al., 2004; Karama et al., 2002; Park et al., 2001b; Redoute et al., 2000; Stoleru et al., 1999]. We were able to reproduce most of these findings in HET, whereas HOM showed only limited activation of these regions with sexual arousal. HET showed clear activation of the right anterior cingulate gyrus, a brain area that has mainly been attributed to visceral responses [Devinsky et al., 1995]. In HOM no significant activation of the cingulate gyrus was found. Similarly, activation of the right insular region, which is thought to be involved in somatosensory processing, possibly including recognition of erection [Arnow et al., 2002; Augustine, 1996; Oppenheimer et al., 1992], was found in HET. Moreover, activation of the right caudate nucleus, a finding that has been interpreted as control of motor expression in sexual arousal [Karama et al., 2002], was only observed in HET. Both HET and HOM showed activation of the orbitofrontal cortex which is thought to be involved in the representation of rewards [Francis et al., 1999] and processing emotionally charged stimuli [Lane et al., 1997]. However, negative emotional stimuli have also been described to be mediated via limbic structures, such as amygdala, hippocampus, and the orbitofrontal to insular region. Similarily, both groups showed activation of occipital, temporal, and parietal cortices, brain areas associated with attention, processing of visual stimuli, and motor‐sensory function. However, despite equal subjective sexual arousal ratings, HOM showed considerably lesser hypothalamic activation than HET. Interestingly, as a lower magnitude of hypothalamic activation with visual sexual stimulation has also been reported for (heterosexual) women in comparison to (heterosexual) men [Hamann et al., 2004; Karama et al., 2002], neural response to visual sexual stimulation in homosexual males shows some similarities to that in heterosexual females. Our results therefore may be an initial indication that neural activation during sexual arousal in homosexual males not only shares characteristics with that of heterosexual males but also with that of heterosexual females. However, particularly the necessary use of different stimuli for the two groups reduces the significance of this finding and it remains unclear whether the observed differences correspond to an actual neural functional correlate for male sexual orientation or are, at least partially, a result of differences in the experimental setting. Furthermore, the z value of hypothalamic activation was relatively low. Therefore, differences may be overestimated or equalized. To clarify this point, a further study leading to a greater number of individuals to enter the statistical analysis should be performed. Overall, our findings are in line with the results of previous studies and partly fit into a neurobehavioral model of sexual arousal put forward by Redoute et al. [2000] and supported by Mouras et al. [2003] that proposes cognitive, motivational, emotional, and autonomic components in sexual arousal response. We found activation in the right orbitofrontal gyrus in both groups (HET and HOM) with both contrasts (COR and OPP), which fits into the proposed role of this brain area to represent the cognitive component, appraising the presented stimuli for their emotional content and sexual attractiveness. We also found activation in the superior parietal cortex in all four contrasts, which has been interpreted as part of a neural network mediating motor imaginary. Activation in the right insula has been suggested to represent part of the emotional component, which in our study was observed in HET for both contrasts and in HOM for the OPP contrast. Finally, we found hypothalamic activation in both groups for the COR contrasts, which represents part of the proposed autonomic component of sexual arousal response.
In the second part of the study brain response to OPP stimuli was examined. As the OPP contrasts correspond to stimuli with erotic content opposite to the individual's sexual orientation, sexual arousal was not considered the primary effect of these stimuli. Accordingly, when viewing OPP stimuli, HET and HOM did not show significant hypothalamic activation, consistent with low sexual arousal as assessed by subjective rating. Also, in both groups activation in the cingulate gyrus was absent. On the contrary, both HET and HOM showed robust bilateral anterior insular/inferior frontal activation which was more pronounced than during the COR stimuli. This finding may, at least partly, be associated with visceral activity as well as aversive emotional stimuli such as disgust and fear [Critchley et al., 2002; Phillips et al., 1997; Wicker et al., 2003]. Moreover, HET showed right‐sided amygdala activation when viewing OPP, which has been associated with evaluation of the emotional content of visual stimuli and processing of aversive emotional stimuli on a low level of conscious awareness in some studies but also positive emotional content can be linked here [Beauregard et al., 2001; Morris et al., 1998]. Finally, in both groups activation of orbitofrontal as well as occipital, parietal, and in HET, temporal cortices were observed, which correspond to attention and processing of visual stimuli and motor‐sensory function. The activation pattern found with OPP suggests that in both groups these stimuli were perceived as highly emotionally charged and that they led to a more general autonomic response rather than an autonomic response specific for sexual arousal, such as penile tumescence. It further might indicate that particularly HET perceived OPP stimuli, at least to some extent, as aversive. As we did not record individual ratings of aversiveness, we lack a subjective correlate for this activation and cannot further clarify this point, particularly as the insular region has been reported to also mediate sexual emotional stimuli [Arnow et al., 2002; Hamann et al., 2004; Karama et al., 2002; Park et al., 2001b; Stoleru et al., 1999]. As our groups rated sexual arousal during the OPP condition as lower than during the COR condition, the stronger activation in the insular region is more likely to be related to distinct emotional processes rather than sexual emotions. This finding is in accordance with findings published by Mavissakalian et al. [1975] who found that heterosexual males rated stimuli of male homosexual content as clearly aversive.
Regarding the limitations of this study, the limited statistical power of our data which had particular impact on the ROI analysis has to be mentioned. Undisclosed heterogeneity among the subjects in their reception of the stimuli in conjunction with a relatively small number of subjects may be a possible explanation. Another limitation is that the assessment of sexual arousal was carried out by self‐report only. We did not record any objective autonomic variables such as penile tumescence, heart rate, or electrodermal activity as markers for sexual arousal. However, the sexually arousing stimuli had been assessed previously for their desired effect. As another limitation, habituation to the stimuli must be considered. Particularly the amygdala have been reported to rapidly habituate to repeated emotional stimuli [Breiter et al., 1996], which may in fact explain the lack of amygdala activation for the COR contrasts in both groups. The effect of habituation may be reduced by using an event‐related rather than a block design for the experiment and by counterbalancing stimulus types, which could have been applied in this study. However, we felt that the subjective rating of sexual arousal may be more accurate when the stimuli were presented in a block design fashion rather than a counterbalanced random presentation.
Taken together, our results demonstrate that brain response of heterosexual males to heterosexual stimuli is comparable to that of homosexual males to homosexual stimuli. In both groups only erotic stimuli corresponding to the individual's sexual orientation lead to a brain activation pattern characteristic for sexual arousal, suggesting a uniform neurofunctional correlate for sexual arousal, independent of individual sexual orientation. However, our results also indicate that despite equal sexual arousal ratings, homosexual males show a lower magnitude of hypothalamic activation in their response to visual sexual stimulation, a characteristic shared by heterosexual females. This finding may be an initial indication that sexual arousal in homosexual males shows similarities to that of both heterosexual males and heterosexual females. Finally, brain response to stimuli of opposite sexual orientation is also similar in heterosexual and homosexual males, with absent characteristic sexual arousal activation. Moreover, the observed brain activation pattern suggests that in both groups visual sexual stimuli opposite to the individual's sexual orientation are not only less sexually arousing than stimuli corresponding to their sexual orientation, but also trigger intense autonomic and emotional response.
REFERENCES
- Allen LS, Gorski RA ( 1992): Sexual orientation and the size of the anterior commissure in the human brain. Proc Natl Acad Sci USA 89: 7199–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, Lue TF, Atlas SW ( 2002): Brain activation and sexual arousal in healthy, heterosexual males. Brain 125 (Part 5): 1014–1023. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, Mishani E, Bonne O ( 2001): Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O‐H2O PET study in heterosexual men. Neuroimage 14 (1 Part 1): 105– 117. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR ( 1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB ( 2001): The interstitial nuclei of the human anterior hypothalamus: An investigation of variation with sex, sexual orientation, and HIV status. Horm Behav 40: 86–92. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ ( 2002): Volitional control of autonomic arousal: A functional magnetic resonance study. Neuroimage 16: 909–919. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118 (Part 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E ( 1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10: 453–459. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Hagemann JH, Berding G, Bergh S, Sleep DJ, Knapp WH, Jonas U, Stief CG ( 2003): Effects of visual sexual stimuli and apomorphine SL on cerebral activity in men with erectile dysfunction. Eur Urol 43: 412–420. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K ( 2004): Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci 7: 411–416. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AA ( 2003): Brain activation during human male ejaculation. J Neurosci 23: 9185–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M ( 2002): Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey AC, Pomeroy WR, Martin C ( 1948): Sexual Behavior in the Human Male. Philadelphia: W.B. Saunders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TH, Haake P, Haverkamp J, Kramer M, Exton MS, Saller B, Leygraf N, Hartmann U, Schedlowski M ( 2003): Effects of acute prolactin manipulation on sexual drive and function in males. J Endocrinol 179: 357–365. [DOI] [PubMed] [Google Scholar]
- Kupfermann I ( 1991): Hypothalamus and the limbic system: Peptidergic neurons,homeostasis, and emotional behavior In: Kandel ER, Schwartz GE, Jessell TM, editors. Principles of Neural Science. Norwalk: Appleton and Lange. [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ ( 1997): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933. [DOI] [PubMed] [Google Scholar]
- Le Vay S ( 1996): Queer Science. The Use and Abuse of Research into Homosexuality. Oxford: MIT Press. [Google Scholar]
- LeVay S ( 1991): A difference in hypothalamic structure between heterosexual and homosexual men. Science 253: 1034–1037. [DOI] [PubMed] [Google Scholar]
- Mavissakalian M, Blanchard EB, Abel GC, Barlow DH ( 1975): Responses to complex erotic stimuli in homosexual and heterosexual males. Br J Psychiatry 126: 252–257. [DOI] [PubMed] [Google Scholar]
- McConaghy N ( 1999): Unresolved issues in scientific sexology. Arch Sex Behav 28: 285–318. [DOI] [PubMed] [Google Scholar]
- Montorsi F, Perani D, Anchisi D, Salonia A, Scifo P, Rigiroli P, Zanoni M, Heaton JP, Rigatti P, Fazio F ( 2003): Apomorphine‐induced brain modulation during sexual stimulation: A new look at central phenomena related to erectile dysfunction. Int J Impot Res 15: 203–209. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ ( 1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Mouras H, Stoleru S, Bittoun J, Glutron D, Pelegrini‐Issac M, Paradis AL, Burnod Y ( 2003): Brain processing of visual sexual stimuli in healthy men: A functional magnetic resonance imaging study. Neuroimage 20: 855–869. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC ( 1992): Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW ( 2001a): Blood‐oxygenation‐level‐dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology 57: 1189–1194. [DOI] [PubMed] [Google Scholar]
- Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW ( 2001b): A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res 13: 73–81. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS, ( 1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, Macklin ML, Fischman AJ, Pitman RK ( 1999): Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res 91: 1–10. [DOI] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, Le Bars D, Forest MG, Pujol JF ( 2000): Brain processing of visual sexual stimuli in human males. Hum Brain Mapp 11: 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, Lavenne F, Le Bars D, Vernet‐Maury E, Rada H, Collet C, Mazoyer B, Forest MG, Magnin F, Spira A, Comar D ( 1999): Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 28: 1–21. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Redoute J, Costes N, Lavenne F, Bars DL, Dechaud H, Forest MG, Pugeat M, Cinotti L, Pujol JF ( 2003): Brain processing of visual sexual stimuli in men with hypoactive sexual desire disorder. Psychiatry Res 124: 67–86. [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotactic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tiihonen J, Kuikka J, Kupila J, Partanen K, Vainio P, Airaksinen J, Eronen M, Hallikainen T, Paanila J, Kinnunen I, Huttunen J ( 1994): Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neurosci Lett 170: 241–243. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G ( 2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Yang JC ( 2004): Functional neuroanatomy in depressed patients with sexual dysfunction: Blood oxygenation level dependent functional MR imaging. Korean J Radiol 5: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


