Abstract
In this study, a combined repetitive transcranial magnetic stimulation/electroencephalography (rTMS/EEG) method was used to explore the acute changes of cortical oscillatory activity induced by intermittent short trains of high‐frequency (5‐Hz) rTMS delivered over the left primary motor cortex (M1). We evaluated the electrophysiological reaction to magnetic stimulation during and 2–4 s after 20 trains of 20‐pulses rTMS, using event‐related power (ERPow) that reflects the regional oscillatory activity of neural assemblies, and event‐related coherence (ERCoh) that reflects the interregional functional connectivity of oscillatory neural activity. These event‐related transformations were for the upper α (10–12 Hz) and β (18–22 Hz) frequency ranges, respectively. For the α band, threshold rTMS and subthreshold rTMS induced an ERPow increase during the trains of stimulation mainly in frontal and central regions ipsilateral to stimulation. For the β band, a similar synchronization of cortical oscillations for both rTMS intensities was seen. Moreover, subthreshold rTMS affected α‐band activity more than threshold rTMS, inducing a specific ERCoh decrease over the posterior regions during the trains of stimulation. For β band, the decrease in functional coupling was observed mainly during threshold rTMS. These findings provide a better understanding of the cortical effects of high‐frequency rTMS, whereby the induction of oscillations reflects the capacity of electromagnetic pulses to alter regional and interregional synaptic transmissions of neural populations. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: motor cortex, connectivity, oscillations, cerebral cortex, EEG‐TMS combination, GABAergic modulation
INTRODUCTION
In recent years, the excitability and function of human cerebral cortex has been variously investigated by repetitive transcranial magnetic stimulation (rTMS) as a useful noninvasive tool in healthy subjects. Repetitive TMS can promote changes in the excitability of neuronal circuits, which outlast the period of stimulation (i.e. plasticity), and which are not limited to the stimulated cortex but spread across neuronal circuits of functionally related cortical and subcortical brain areas [Siebner and Rothwell, 2003]. In cognitive neuroscience, TMS is normally used to experimentally induce focal “virtual lesions” in order to study the behavioral consequences (i.e. reaction times) of TMS when subjects carry out a given task. This approach can help to test various models of the complex interplay across areas of a cognitive network [Theoret and Pascual‐Leone, 2003; Walsh and Pascual‐Leone, 2003]. However, there is a dearth of knowledge regarding the mode of action of a wide variety of rTMS applications. To better understand the effects of TMS‐induced oscillatory activity, we combined TMS with simultaneous electroencephalographic (EEG) recording. We mapped the activation and interaction of cortical oscillatory patterns elicited by intermittent short trains of high‐frequency rTMS at intensities below or at resting motor thresholds (RMTs) over the left primary motor cortex (M1).
Most of the previous rTMS studies involving delivery of short trains of stimulation have failed to demonstrate lasting modulation in corticospinal excitability, as measured by changes in the size of EMG responses evoked by suprathreshold TMS pulses in muscles contralateral (MEPs) to the site of such stimulation [Di Lazzaro et al., 2002b; Maeda et al., 2000; Peinemann et al., 2004; Quartarone et al., 2005]. This may be because the modulatory effect of rTMS may be short‐lived and might have been missed, as data collection started more than 10 s after application of the pulse train [Maeda et al., 2000]. Modugno et al. [2001] found a significant short‐lasting (i.e. 1 s) inhibition of corticospinal excitability using short trains of high‐frequency rTMS.
The effect of high‐frequency rTMS has not only been investigated at the level of muscle responses. Recently, a combination of rapid‐rate (3–10 Hz) rTMS with positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) has been used to provide direct evidence of neurophysiological alteration in cortical and subcortical activations evoked by short trains of rTMS over left M1 [Bestmann et al., 2003, 2004; Strafella et al., 2003; Takano et al., 2004].
The TMS‐related changes in EEG cortical oscillatory activities in the “resting” brain have not been widely studied [Fuggetta et al., 2005; Jing and Takigawa, 2000; Oliviero et al., 2003; Paus et al., 2001; Strens et al., 2002]. Oliviero et al. [2003] applied 5‐Hz train of 50 pulses at active motor threshold (AMT) over left M1 and found a significant decrease in cortico‐cortical interhemispheric coherence in the upper α band (10.7–13.6 Hz) between motor and premotor cortex, which lasted for a few minutes after stimulation.
In the present study, we aimed to explore both the acute and short‐lasting effects of intermittent short trains of TMS by analyzing EEG oscillations during and immediately after each train of rTMS. We evaluated EEG responses with ERPow and ERCoh transformations, which reflect the regional neural activity and the interregional functional coupling between cortical areas, respectively. In another experiment (Manganotti and Fuggetta, submitted), we applied a parallel procedure to study the effects of 1‐Hz rTMS of the same group of subjects on the same day.
MATERIALS AND METHODS
Subjects
Eleven healthy volunteers (five males, six females), aged 19 years and 11 months to 23 years and 5 months (mean 21.61 years, SD 1.01 years) and with no history of neurological disorder or head injury participated in the study. All subjects were right‐handed as assessed by the Edinburgh handedness inventory [Oldfield, 1971]. All subjects gave written informed consent for the study in accordance with the declaration of Helsinki, and the study was approved by the Local Ethics Committee of the Department and Hospital.
Experimental Design
Each subject underwent a 90‐min session consisting in total of four experimental conditions. To minimize plasticity effects in the excitability of the stimulated left M1, each of the four experimental blocks was separated by a break of 5 min. The order of presentation of the four blocks was counterbalanced across participants. Three 15‐min conditions of 5‐Hz rTMS over the left M1 were applied with respect to the individual RMT: (1) 80%; (2) 100%; and (3) sham. Finally, a 15‐min block of (4) 5‐Hz repetitive peripheral electrical stimulation was performed. Low‐intensity subthreshold stimulation was used to ensure purely local effects and avoid evoked muscle twitches that could modify central processing through altered afferent input from each twitch [Strens et al., 2002]. Moreover, we applied threshold level rTMS to study the spread of cortical activation of rTMS at distal cortical areas. Additional unwanted activation of the auditory, cutaneous, or somatosensory cortex can confound the results of the present study due to concomitant sensory stimulation. Thus, a sham rTMS condition was carried out to control for the air and bone‐conducted auditory stimuli that could contaminate EEG oscillations in the target motor system.
Finally, to determine if the effects of threshold rTMS were purely central in origin or if peripheral muscle twitches affected central processing through changed afferent input, we also performed peripheral repetitive electrical stimulation of the right hand. Stimulation parameters were identical for each experimental condition, which included a total of 400 stimuli delivered in 20 trains of 20 stimuli at 5 Hz frequency.
Subjects were tested (by one examiner individually) in a quiet room with the ambient light on. They were seated in a comfortable armchair with their elbows flexed at 90°, hands pronated in a relaxed position, with eyes open while watching a computer screen positioned at a viewing distance of about 57 cm. Cyan or black crosses, subtending a visual angle of 2° (width) × 2° (height), were presented continuously at the centre of a grey screen. Every trial began with the onset of a cyan cross displayed for 15 s, during which interval blinking and some movement were permitted at rest. This cue was followed by a black cross, where subjects were instructed to keep their eyes open, avoid blinking, and to fixate on a stationary, centrally located cross, displayed for a total of 19 s, subdivided into three epochs of 5‐s prestimulation, 4‐s of repetitive stimulation, and 10‐s poststimulation.
Because event‐related changes in ongoing EEG need time to develop and to recover, particularly where α‐band rhythms are concerned, the inter‐trial‐interval (ITI) was 30 s long for all experimental conditions. Ideally the ITI should be about 10 s for TMS, since a previous study has found that short trains of up to 20 pulses at 5 Hz to the left M1 at motor threshold can influence measures of corticospinal excitability for more than 2 s after the end of the train [Modugno et al., 2001]. Figure 1 shows an example of the order of presentation of stimuli.
Figure 1.
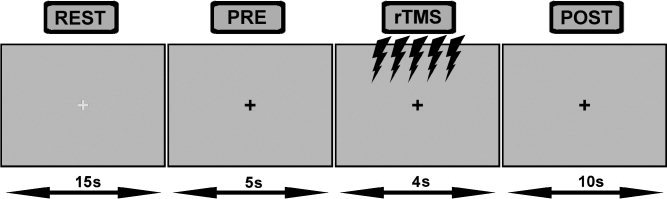
Experimental design: the study design consisted of four experimental conditions (rTMS 80%, rTMS 100%, Sham rTMS, and Repetitive peripheral electric stimulation, respectively) that comprised four blocks of epochs of EEG and EMG measurements recorded continuously immediately before, during, and immediately after repetitive 5‐Hz stimulation (Pre, rTMS, Post, respectively). The order execution of four conditions was counterbalanced among individuals.
The participants were naive to rTMS prior to the study and were unfamiliar with the differences between sham and active rTMS regarding its acoustic and tactile artifacts. The magnetic or electric pulses were triggered by the computer in all conditions and their timings marked in a separate channel of the multichannel EEG recording system.
TMS Procedure
TMS was carried out with a high‐power Magstim‐Rapid stimulator (Magstim, Whitland, Dyfed, UK). The magnetic stimulus had a biphasic waveform with a pulse width of about 300 μs. In this study, stimulus intensities are expressed as a percentage of the subject's RMT. TMS was delivered through a figure‐of‐eight shaped coil (70‐mm standard coil; Magstim), oriented so that the induced electric current flowed in a posterior–anterior direction over the left M1. The coil was placed tangentially with respect to the scalp, with the handle pointing backwards and laterally at a 45° angle away from the midline, approximately perpendicular to the line of the central sulcus to achieve the lowest motor threshold [Brasil‐Neto et al., 1992].
Motor‐evoked potentials (MEPs) were recorded from the right thenar eminence (TE) muscle using Ag/AgCl surface electrodes in a belly‐tendon montage. We determined the optimal position for activation of the right TE by moving the coil in 0.5‐cm steps around the presumed motor hand area of the left motor cortex. The site where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs with the steepest negative slope in the target muscle was marked as the “hot spot.” This procedure was repeated to locate the right motor cortex hand area. The individual RMT intensity was determined by reducing the stimulus intensity in 1% steps. It was defined as the first stimulus intensity that failed to produce an MEP of at least 50 μV in at least five out of 10 consecutive trials [Rossini et al., 1994]. The intensity of rTMS was then set to 80 or 100% of individual RMT. The sham condition was performed with an intensity of 80% RMT with the coil tilted at 90° to the skull in order to avoid real stimulation of the motor cortex.
Peripheral Electrical Stimulation Procedure
Peripheral electrical stimulation was carried out with the Brainquick electromyography system (Micromed, Treviso, Italy). The electrical stimulus consisted of a pulse of 10‐ms duration, applied at three times the individual perception threshold, defined as the minimum stimulus intensity that produced a subjective report of sensation in five out of 10 subsequent trials. The electrical stimulation was delivered through two‐ring electrodes around the right TE muscle, positioned near the bipolar MEP electrodes. The mean intensity of stimulation was 20 mA (SD: 5 mA).
Data Acquisition
Continuous EEG was recorded with a 30‐channel MR‐compatible system (Micromed), using an anterior to Fz electrode as a reference and a posterior to Fz electrode as the ground (see Fig. 2).
Figure 2.
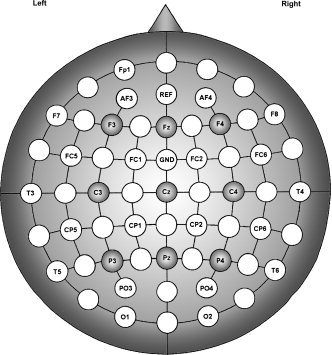
Electrode montage and placement according to the 10/20 system with additional electrodes for a total of 30. The filled circles indicate the nine electrodes of interest on which the EEG signal and statistical analyses of event‐related power and event‐related coherence transformations were based.
An EEG/MR‐compatible amplifier (Micromed, Mogliano Veneto, Italy) was connected via a fibreoptic cable to a standard PC running SystemPlus Software (Micromed). Overheating of electrodes in the vicinity of the stimulating coil [Roth et al., 1992] was minimized by using TMS‐compatible Ag/AgCl‐coated electrodes (8‐mm diameter, 0.5 mm thickness) with 2‐mm slits to interrupt eddy currents. Electrode impedance was below 10 kΩ. The activities in the right (TE) muscle and in the right eye vertical electroculogram (vEOG) were bipolarly registered from surface electrodes in two EMG channels. The bandwidth of the amplifiers was 0.01–512 Hz. All data were sampled at a frequency of 1,024 Hz.
Data Analysis
To characterize TMS‐induced effects, EEG data were analyzed with commercial software (Vision Analyzer; BrainVision, Munich, Germany) to enable computation of: (i) event‐related power (ERPow) and (ii) event‐related coherence (ERCoh) measures.
Since high‐voltage and high‐frequency artifacts contaminated the EEG signal within the first 20 ms following the magnetic pulse, analysis of the EEG trace for analyses started at 30 ms after each magnetic pulse (see Fig. 3).
Figure 3.
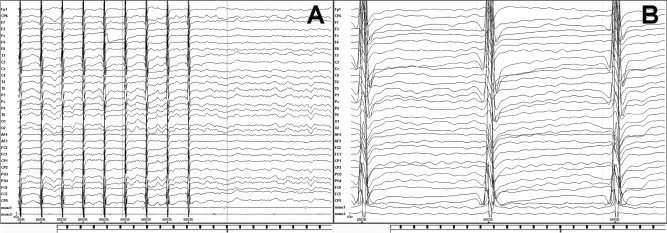
Raw data showing EEG and EMG recording of a subject in which rTMS pulses were delivered to left M1 at 80% RMT. Panel A represents 3 s of recorded raw data. Panel B shows 0.5 s of recorded raw data. We analyzed the EEG signal starting 30 ms from each pulse during rTMS trains.
For all conditions, analyses of EEG signals were computed for three epochs: baseline period (4,125–2,125 ms before each train of stimulation), first epoch (30–155 ms after each stimulation), and second epoch (2,125–4,125 ms after each train of stimulation). Raw data for each period was segmented into epochs of 500 ms (containing 512 data points). For analyzing the EEG signal during repetitive stimulation, we programmed a macro (i.e. TMS artifact Remove) that joined together epochs of 125 ms (containing 128 data points) between TMS pulses into a continuous raw EEG trace. This made it possible to segment the derived continuous EEG track into 500‐ms epochs (512 data points). However, appending together EEG segments of 125 ms, we obtained edge artifacts in the EEG raw data, exactly in the junction between the segments. Hence, we found that the multiple of 8‐Hz frequency bands (i.e. 8, 16, 24 Hz) were liable to erroneous spectral estimates with discrete fast Fourier transformation (FFT), but intermediate frequency bands were reliable. For this reason, we restricted the computation of ERPow and ERCoh to the two frequency bands of upper α (10–12 Hz) and β band (18–22 Hz), respectively. The two frequency ranges chosen have been previously shown to be particularly sensitive to movement‐related changes in cortical oscillatory activity in humans [Manganotti et al., 1998; Salmelin and Hari, 1994; Tiihonen et al., 1989]. Several studies support the notion that activities in the upper and lower α bands are likely to be functionally distinct; the upper α is more specific for motor areas, while the lower α is more specific for sensory areas [Manganotti et al. 1998; Pfurtscheller et al., 2000]. It has been documented in a previous study that rTMS over left M1 changed cortical coupling mainly in the upper α band [Oliviero et al., 2003]. In addition, the 18–22 Hz range chosen could be a harmonic of upper α and/or an additional responsive frequency representative of motor areas. Finally, Paus et al. [2001] demonstrated that single‐pulse TMS specifically induces a highly synchronous oscillation in the 21–25 Hz frequencies of the β range.
EEG signals were then filtered (0.1–50 Hz, slope 24 dB/octave), while EMG signals were bandpass‐filtered (30–300Hz, slope 48 dB/octave). A notch filter (50 Hz) was also applied to all channels.
An automatic epoch inspection‐rejection procedure was applied to avoid residual TMS, muscle, or EOG artifacts. Two criteria were used for automatic epoch rejection: the difference criterion that allowed 300 μV of maximal absolute difference of two values in the segment; the amplitude criterion which eliminated artifacts outside the range of ±200 μV. The F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4 channels were then selected for inspection. For all experimental conditions, periods which showed movement‐related activity or relaxation of the muscles prior to and following each train of stimulation were excluded to avoid confound of using data changes due to movement preparation or termination. Thus, we analyzed only steady‐state stimulation and rest periods. A mean of 30 s of clean data were extracted from each block pre‐ and poststimulation recording, and a mean of 40 s were extracted during the stimulation condition. These data lengths were sufficient to achieve reliable spectral estimates. In two previous studies that combined EEG with rTMS, 110 s of clean data were used from each recording to achieve reliable spectral estimates with low 95% confidence limits [Oliviero et al., 2003; Strens et al., 2002].
Event‐Related Power
A discrete FFT of three epochs of 512 data points (500 ms) each was computed for all electrodes and then averaged across epochs under the same conditions. Power spectra were estimated for all frequency bins between 0 and 30 Hz (2 Hz maximum bin width). Recordings were Hamming‐windowed to control for spectral leakage. Broad‐band power changes were obtained by averaging the power values of upper α (10–12 Hz) and β (18–22 Hz) frequency ranges chosen for analysis.
To reduce the effects of intersubject and interelectrode variability in absolute spectral power values, the event‐related relative changes of EEG power at each electrode (ERPowx) was quantified using an accepted event‐related desynchronization/synchronization (ERD/ERS) procedure [Leocani et al., 1997; Pfurtscheller and Aranibar, 1977; Pfurtscheller and Lopes da Silva, 1999], according to Eq. (1).
| (1) |
The ERPow (or ERD/ERS) transformation was defined as the percentage decrease/increase of instant power density at the “event” compared with a “pre‐event” baseline. Therefore, ERPow decreases (“cortical activation state”) are expressed as negative values, while ERPow increases (“cortical idling state”) are expressed as positive values.
Event‐Related Coherence
Coherence was calculated by selecting a combination of the C3 electrode (the nearest channel to the individual “hot‐spot”) with all pairs from the FFT power spectrum.
The coherence values were calculated for each frequency bin from 0 to 30 Hz (2 Hz of maximum bin width) according to Eq. (2), using commercial software (Vision Analyzer; BrainVision).
| (2) |
Equation (2) is the extension of the Pearson's correlation coefficient to complex number pairs. In this equation, f denotes the spectral estimate of two EEG signals x and y for a given frequency bin (λ). The numerator contains the cross‐spectrum for x and y(fxy), the denominator the respective autospectra for x(fxx) and y(fyy). For the frequency λ, the coherence value (Cohxy) is obtained by squaring the magnitude of the complex correlation coefficient R, and is a real number between 0 and 1. Because coherence is the cross‐correlation of two power spectra divided by the respective powers, it is already normalized by power within each subject. To reduce the effect of intersubject and interelectrode pair variations in absolute coherence values introduced by the reference electrodes [Fein et al., 1988; Rappelsberger and Petsche, 1988], event‐related relative coherence (ERCohxy) was obtained by subtracting the resting value (Cohxy rest) from the corresponding activation conditions (Cohxy activation), according to Eq. (3).
| (3) |
Therefore, coherence magnitude increments were expressed as positive values and coherence decrements were expressed as negative values [Manganotti et al., 1998]. Group ERCoh changes for each electrode were obtained by averaging the individual values from 0 to 30 Hz frequency ranges with 2‐Hz bins. To obtain broad‐band ERPow and ERCoh values for statistical analyses, the cortical oscillatory activity values were averaged over 2‐Hz frequency bins in the chosen α (10–12 Hz) and β (18–22 Hz) frequency bands.
Statistical Analysis
Spectral values for both ERPow and coherence changes were submitted to repeated analyses of variance (ANOVAs) for the upper α (10–12 Hz) and β (18–22 Hz) frequency ranges, respectively. Three‐way ANOVAs were performed with factors: “experimental condition” (5‐Hz rTMS at 80 or 100% RMT, Sham 5‐Hz rTMS at 80% RMT and peripheral electrical stimulation at 5 Hz); “epoch” (first epoch during a stimulation train, and second epoch poststimulation); and “electrode” (F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4). To further investigate which electrodes explain for the reported significant three‐way interactions, we also tested for possible significant two‐way interactions of “experimental condition” × “epoch of time” for each of the nine electrodes analyzed. For both ERPow and ERCoh transformations, post‐hoc paired t tests (adjusted for multiple comparisons using the Bonferroni method) were used to investigate significant main effects and interactions. For all statistical tests, a P < 0.05 was considered significant.
RESULTS
Event‐Related Power
Power changes in the α band
Figure 4 shows the grand average of ERPow for α band (10–12 Hz) as a function of the three factors: experimental condition, epoch, and electrode. The ANOVA showed the following statistically significant main effects and interactions: “electrode” with F(8, 80) = 4.341, P < 0.001; “experimental condition × electrode” with F(24, 240) = 1.884, P < 0.01; and “epoch × electrode” with F(8, 80) = 5.476, P < 0.001.
Figure 4.
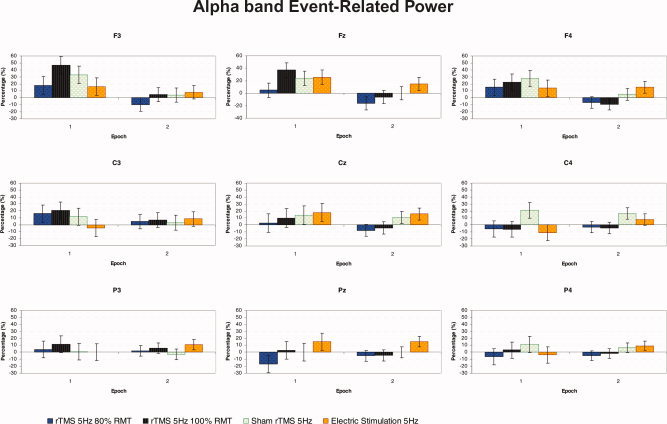
Grand average of event‐related power transformation for upper α (10–12 Hz) band of nine electrodes analyzed, as a function of the experimental condition and epoch of time (n = 11). Repetitive TMS at 100% RMT induced an increase in amplitude in EEG oscillations mainly for F3 electrode compared with C4 electrode. At epoch 1, during the trains of stimulation, there was an increase in power for F3, Fz, and F4 frontal electrodes compared with the second epoch after each train of repetitive stimulation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Post‐hoc comparisons for the significant interaction “experimental condition × electrode” showed that for the Pz electrode there was a significant increase of power in the repetitive peripheral electric stimulation condition compared with the decrease of EEG oscillations in the rTMS 80% RMT condition (15.2 vs. −10.9%).
For rTMS at 100% RMT, there was a significant increase of power for F3 compared with C4 (25.6 vs. −5.1%). For peripheral repetitive electrical stimulation there was a significant increase of power at F3 and Fz sites compared with C4 (11.9, 20.4 vs. −1.5%, respectively) and for Cz relative to C3 (16.8 vs. 1.9%).
Considering the post‐hoc comparisons for the significant epoch × electrode interaction, there was a significant increase of power at frontal electrodes during the first epoch compared with the second epoch after each train of repetitive stimulation [F3 (28.3 vs. 1.8%), Fz (22.9 vs. −1.9%), and F4 (19.8 vs. 1.0%)]. Moreover, during the intermittent trains of stimulation, synchronization was observed at the F3, Fz, and F4 sites relative to C4 (28.3, 22.9, and 19.8 vs. −0.5%, respectively). A significant increase of power was also observed at Cz and Fz electrodes compared with Pz (11.1, and 22.9 vs. 0.2%, respectively) (see Fig. 4).
Power changes in the β band
Figure 5 shows the grand average of ERPow for the β band (18–22 Hz) as a function of the three factors: epoch of time, experimental condition, and electrode.
Figure 5.
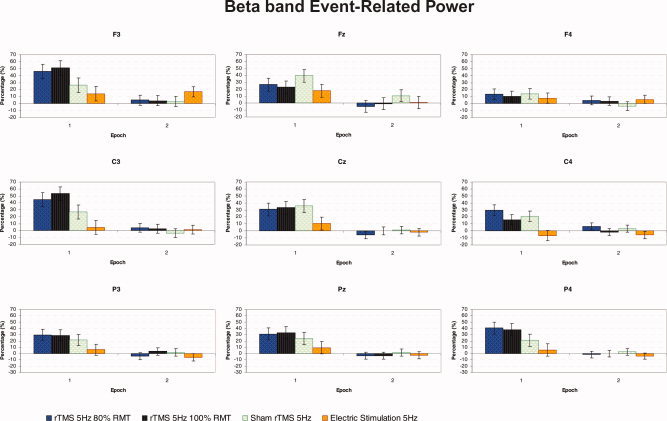
Grand average of event‐related power transformation for β (18–22 Hz) band of nine electrodes analyzed, as a function of the experimental condition and epoch of time (n = 11). The F3, C3, and P4 were the most sensitive electrode to experimental manipulations. At epoch 1, during repetitive stimulation, for the C3 electrode there was a significant increase in amplitude of EEG oscillations with rTMS at 100% RMT compared with repetitive peripheral electric stimulation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The ANOVA showed the following statistically significant main effects and interactions: “epoch” with F(1, 10) = 49.014, P < 0.001; “electrode” with F(8, 80) = 2.608, P < 0.05; “experimental condition × epoch” with F(3, 30) = 3.510, P < 0.05; “epoch × electrode” with F(8, 80) = 3.331, P < 0.005; and “condition × epoch × electrode” with F(2, 240) = 1.596, P < 0.05. This shows that the stimulation‐specific effects differed across the recorded sites. The ANOVAs for each of the nine electrodes analyzed show that F3, C3, and P4 were the most sensitive electrodes to experimental manipulations, with a significant two‐way interaction of “experimental condition” × “epoch of time” for F3 with F(3, 30) = 3.630, P < 0.05; C3 with F(3, 30) = 4.854, P < 0.01; and P4 with F(3, 30) = 5.080, P < 0.01, respectively.
Post‐hoc analyses of the three‐way “epoch × experimental condition × electrode” interaction revealed that for the first epoch only, there was a significant synchronization in cortical β oscillations over all electrodes for rTMS 80% RMT, rTMS 100% RMT, and sham rTMS compared with epoch 2 after each train of magnetic or peripheral electric stimulation.
Post‐hoc analyses also enabled us to compare the modulation of regional oscillatory activity between the four experimental conditions. The C3 electrode displayed a significant increase in ERPow at epoch 1 during rTMS at 100% RMT in comparison with repetitive peripheral electric stimulation (44.6 vs. 4.7%, respectively). There was a significant increase of β oscillatory activity at C4 for both real and sham rTMS at 80% RMT relative to peripheral electric stimulation (29.9 and 21.1 vs. −6.5%, respectively). For the P4 electrode there was a significant difference in β activity between rTMS at 100% RMT and peripheral electric stimulation (37.6 vs. 5.4%).
The post‐hoc pairwise comparisons between different electrodes also revealed a significant increase in β power at C3 relative to C4 during the first epoch of threshold rTMS (53.4 vs. 10.1%) (see Fig. 5).
Event‐Related Coherence
Coherence changes in the α band
Figure 6 shows the grand average of ERCoh for α band (10–12 Hz) for each of eight electrodes coupled with “C3” as a function of “epoch of time,” “experimental condition,” and “electrode.”
Figure 6.
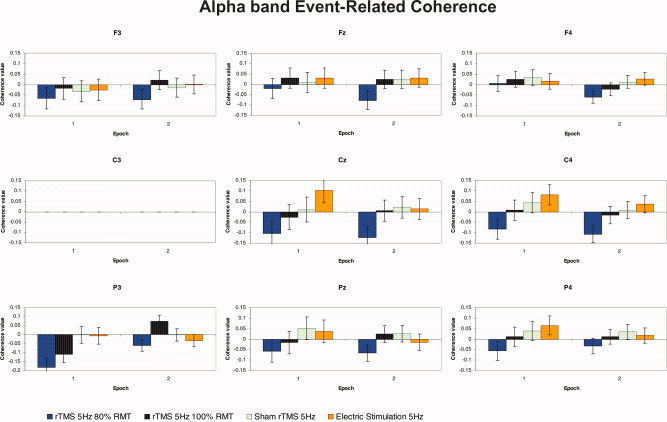
Grand average of event‐related coherence transformation for upper α (10–12 Hz) band of nine electrodes analyzed referenced to C3 electrode, as a function of the experimental condition and epoch of time (n = 11). At epoch 1, during the trains of stimulation, subthreshold more than threshold rTMS produced a significant decrease in functional coupling in the posterior P3‐C3 pair of electrodes in contrast with the absence of increase in connectivity for both control conditions of sham rTMS and repetitive peripheral electrical stimulation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
With regard to the averaged coherence between the 8 electrodes referenced to C3, the ANOVA showed statistically significant interactions for “epoch × electrode” with F(8, 80) = 3.278, P < 0.005, and “experimental condition × epoch × electrode” with F(24, 240) = 2.245, P < 0.005. Furthermore, the ANOVAs for each of the nine couples of electrodes analyzed showed that the P3‐C3 pair of electrodes was the most sensitive to experimental manipulations with a significant two‐way interaction of “experimental condition” × “epoch of time” evident with F(3, 30) = 5.177, P < 0.01.
Post‐hoc analyses of the three‐way “experimental condition × epoch × electrode” interaction revealed that there was a significant decrease in functional coupling between the P3 and C3 electrodes at epoch 1 for real rTMS at 80% RMT compared with sham as well as repetitive electric stimulation (−0.182 vs. −0.002, −0.006, respectively). A significant “rebound” of coupling was then observed between P3 and C3 in the second epoch, after threshold rTMS, compared with a decline in connectivity between these sites following subthreshold rTMS (0.073 vs. −0.059).
We compared the modulation of interregional coherence between the two epochs of time. For the P3‐C3 pair of electrodes there was a significant decrease in cortico‐cortical coherence during rTMS at 80% RMT when compared with the second epoch after each train of stimulation (−0.182 vs. −0.059). A similar decrease in coupling was seen during rTMS 100% RMT between these, which was followed by an increase in coherence during epoch 2 (−0.109 vs. 0.073).
Post‐hoc tests also revealed statistical differences between the different electrodes during the first epoch with rTMS at 80% RMT. Thus, the decline in coherence between the P3‐C3 pair was significant relative to the coherence changes between the F4‐C3 and C3‐C3 pairs during subthreshold rTMS (−0.182 vs. 0.006 and 0, respectively). In addition, we found a decrease in coherence between P3 and C3 with respect to P4‐C3 during threshold rTMS in epoch 1 (−0.108 vs. 0.012). No significant differences were found across pairs of electrodes for the sham rTMS condition or repetitive peripheral electrical stimulation conditions (see Fig. 6).
Coherence changes in the β band
Figure 7 shows the average ERCoh for β band (18–22 Hz) for each of eight electrodes coupled with “C3” as a function of the three factors: epoch of time, experimental condition, and electrode.
Figure 7.
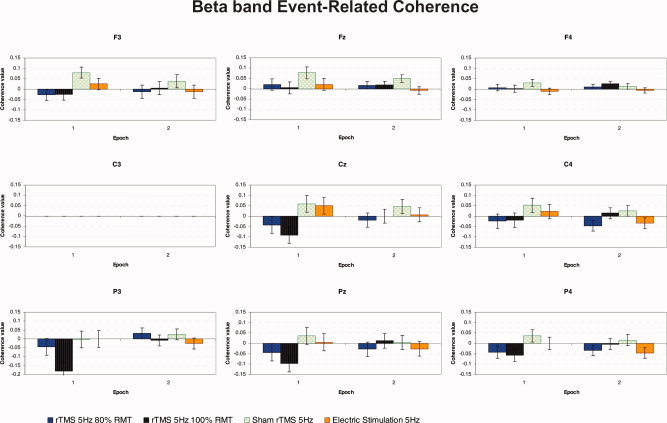
Grand average of event‐related coherence transformation for β (18–22 Hz) band of nine electrodes analyzed referenced to C3 electrode, as a function of the experimental condition and epoch of time (n = 11). During each train of repetitive stimulation rTMS 100% RMT induced a significant decrease of functional coupling mainly for the posterior P3‐C3 electrodes pair compared with F3‐C3, and Fz‐C3 frontal couple of electrodes. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The ANOVA showed that the main factor “electrode” was statistically significant, with F(8, 80) = 3.315, P < 0.005. Moreover, the interaction “experimental condition × epoch” was also significant, with F(3, 30) = 4.165, P < 0.05, as was the interaction between “epoch” × “electrode,” with F(8, 80) = 4.166, P < 0.001.
Post‐hoc pairwise comparisons of the two‐way “experimental condition × epoch” interaction revealed a significant decrease in EEG coherence for rTMS at 100% RMT compared with sham rTMS (−0.052 vs. 0.041, respectively) for all electrodes during the first epoch only. Furthermore, there was a significant decrease in overall electrode coupling at rTMS 100% RMT in epoch 1 with respect to epoch 2 (−0.051 vs. 0.007).
Post‐hoc comparisons of the two‐way “epoch of time × electrode” interaction demonstrated that there was a general decrease of functional coupling between P3 and C3 during repetitive stimulation (epoch 1) when compared with coherence values between electrodes at the second epoch (−0.057 vs. 0.005, respectively). Furthermore, a decrease of functional connectivity between P3 and C3 was seen in epoch 1, in contrast to a nonspecific increase in coupling between the F3‐C3 and Fz‐C3 pairs (−0.057 vs. 0.012 and 0.030, respectively) across all experimental conditions (see Fig. 7).
DISCUSSION
The combination of TMS with EEG represents a powerful tool to study the effects of TMS‐induced cortical reactions with high temporal resolution, thus providing useful information about the neurophysiological processes underlying TMS. The main finding of this study is the acute modulation of cortical oscillations during short trains of high‐frequency rTMS over the left M1 in healthy subjects, which to our knowledge has not been reported previously.
Repetitive TMS Effects on Regional Oscillatory Activity of Neural Assemblies
The effect of short trains of rTMS was restricted to the time between the magnetic pulses, but did not extend to the second epoch, 2–4 s posttrain for either upper α (10–12 Hz) or β (18–22 Hz) rhythms. Thus a transient increase in power of EEG oscillations during rTMS probably lasted for a very short time only after the train of stimulation, as previously investigated with single‐pulse TMS at a variety of intensities over left M1 [Fuggetta et al., 2005]. These findings are also in agreement with those of Modugno et al. [2001] who used a similar protocol of short trains of high‐frequency rTMS and also found a suppression of EMG responses evoked by a suprathreshold TMS within 2 s of stimulation. Previous studies that investigated the effects of low‐ and high‐frequency rTMS on cortico‐cortical connectivity in a similar manner to our study reported no changes in α EEG power after the trains of pulses [Oliviero et al., 2003; Strens et al., 2002]. However, in the present study, we analyzed very short periods of EEG data immediately after (2–4 s) the rTMS rather than long periods (110 s) of spectral analysis in the first epoch after rTMS as in those studies [Oliviero et al., 2003; Strens et al., 2002].
ERPow in the upper α band during trains of rTMS at threshold showed an increase mainly in the F3 electrode ipsilateral to stimulation side compared with contralateral C4 electrode. Moreover, there was an increase in power for all frontal electrodes analyzed during rTMS compared with the poststimulation epoch. For the β band, the F3 and C3 electrodes ipsilateral to the site of stimulation were the most reactive electrodes to rTMS. The C3 electrode showed a significant increase in β power with rTMS at 100% RMT compared with the control condition of repetitive peripheral electrical stimulation.
These results suggest a modulatory effect of rTMS on cortical oscillations that spread from the stimulated primary motor cortex to frontal motor regions, both of which are strongly interconnected (i.e. “network effects”). In a similar experimental paradigm, with short trains of low‐frequency (1 Hz) rTMS over the left M1 (100% RMT), a likewise effect was seen, with an immediate increase of oscillations over the F3 electrode (Manganotti and Fuggetta, submitted). Additional evidence for connections between the M1 and ipsilateral premotor is provided by the conditioning effect of low‐frequency rTMS stimulation over left premotor cortex on MEPs evoked by M1 stimulation [Gerschlager et al., 2001]. Furthermore, combined TMS and fMRI studies have proven that cortical effects of TMS may not be localized to the site of stimulation, but may spread between M1 and left dorsal premotor cortex during high‐frequency short trains of rTMS [Bestmann et al., 2003, 2004]. Our results are in agreement with those of Takano et al. [2004], which indicated a significant increase of activation in the stimulated left M1, using PET, which was induced by a 30‐s train of subthreshold high‐frequency (5 Hz) rTMS (150 pulses). However, Bestmann et al. [2004] failed to find MRI‐detectable activity following the stimulation of the left M1, using short subthreshold rTMS pulses. The authors proposed that this might be a consequence of insufficient sensitivity for detecting MRI signal changes at the stimulation site unless the background physiological “noise” level is exceeded [Bestmann et al., 2004].
The acute TMS‐related synchronization effect may confirm the idea that magnetic pulses reset the ongoing cortical oscillatory activity and trigger the stimulated neurons to oscillate at the frequencies of the motor cortex, as previously suggested by other studies [Fuggetta et al., 2005; Paus et al., 2001]. It has been hypothesized that TMS might induce a synchronous activation of neurons in cortical and subcortical structures via the modulation of the unilateral reciprocal cortex–thalamus–cortex pathways, through which cortical oscillations are generated [Fuggetta et al., 2005]. Further studies in patients with deficient thalamo‐cortical interactions may be valuable in investigating the role of the thalamus in TMS‐induced oscillations.
In the α band, the increase of synchrony of neural populations over the motor and prefrontal regions ipsilateral to rTMS was larger than the mild changes in synchronization seen during repetitive peripheral electrical stimulation that were mostly distributed in central and parietal areas (Cz and Pz electrodes). Furthermore, we found a general increase in synchronization of β oscillations for C3, C4, and P4 electrodes, respectively, at both intensities of rTMS compared with peripheral electric stimulation. These results suggest that the specific effects of rTMS were central in origin and not due to reafferent feedback from peripheral muscle twitches evoked by the threshold TMS pulses.
Among the main findings, mild synchronization of EEG oscillations was observed during sham rTMS in most cortical regions (F3, Fz, C3, Pz, C4, and P4 electrodes) and in both frequency bands analyzed. It is known that the coil‐click sound elicits auditory‐evoked potentials, namely the N1‐P2 complex, with the maxima over the central and parietotemporal regions [Nikouline et al., 1999; Tiitinen et al., 1999]. According to previous studies, waves phase‐locked to the auditory stimulus can contaminate the estimate of the μ (10 Hz) ERD [Kalcher and Pfurtscheller, 1995]. In fact, ERP waveforms may fall in the μ frequency range, appearing as ERS after each tone. However, in our previous study with single‐pulse TMS [Fuggetta et al., 2005], we did not obtain an increase of EEG oscillations following the coil‐click in the entire α (8–13 Hz) and β (13–30 Hz) frequencies during the sham condition. In the present study, the synchronization of oscillations we obtained for sham condition extended to both frequency ranges analyzed could be a cumulative effect of the rapid sequence of auditory coil‐click sounds produced during each train of high‐frequency rTMS. These findings emphasize the need to carefully control for external influences on cortical oscillations due to concomitant auditory stimulation. Similar to our study, additional confounds related to unwanted activation of the auditory system caused by TMS have been seen in some recent neuroimaging studies in which the motor system was investigated [Bestmann et al., 2004; Takano et al., 2004].
Repetitive TMS Effects on the Interregional Functional Connectivity of Oscillatory Neural Activity
High‐frequency rTMS produced a significant decrease of coherence during repetitive stimulation, mainly in the posterior region ipsilateral to the stimulation site (coupling between P3 and C3), with different effects of stimulus intensity on the two frequency bands analyzed. In the α band specifically, rTMS at 80% RMT resulted in a larger decline in functional coupling in the posterior area than threshold stimulation. This was in contrast to the absence of connectivity changes during control conditions of sham rTMS and repetitive peripheral electrical stimulation. For the β range, rTMS at 100% RMT produced a significant decrease of connectivity in the parietal region ipsilateral to stimulation when compared with a nonspecific increase in functional coupling between F3 and C3 and between Fz and C3 that may have been induced by the sham rTMS condition. These results demonstrate that the source and direction of interregional functional coupling of oscillatory activity could be dissociated for different intensities of rTMS as well as for real rTMS and control experimental conditions, which produced different modifications of central processing.
This study therefore suggests that upper α and β frequencies could have different sources and functional meanings in response to repetitive TMS. The dissimilar effect of intensity on cortico‐cortical coherence in the two frequency bands is probably related to different sensitivities of the frequency ranges analyzed. Our results for upper α band are in agreement with those of Oliviero et al. [2003], who showed that a train of high‐frequency (5‐Hz) rTMS over the left M1 at AMT caused a poststimulation decrease in ipsilateral cortico‐cortical coherence between the ipsilateral M1 and more frontal areas. In contrast to their findings, we also demonstrated a decrease in connectivity between the M1 and parietal region ipsilateral to stimulation site. This topographical difference between the two studies is likely to be due to the different periods of EEG chosen for analysis, since we investigated the effect of the coherence during the rTMS, while Oliviero et al. [2003] analyzed the period up to 110 s after rTMS, thus rendering the results not directly comparable. With a similar experimental paradigm to the present study, but delivering short trains of low‐frequency (1‐Hz) rTMS over left M1 (with data analyzed in an identical way), we similarly found a decrease in cortico‐cortical coherence poststimulation over frontal regions (Manganotti and Fuggetta, submitted).
As proposed by Oliviero et al. [2003], it is likely that 5‐Hz rTMS might reduce the efficacy of inhibitory cortico‐cortical projections from primary motor cortex to other regions or through effects on local GABAergic interneurons. Other similar studies, involving short trains of high‐frequency rTMS, have documented a consistent attenuation of short‐latency intracortical inhibition with an increase in MEP size evoked by paired‐pulse TMS in healthy subjects [Di Lazzaro et al., 2002b; Takano et al., 2004]. Two studies provided further evidence for the cortical origin of the effects of rTMS by recording corticospinal volleys directly using epidural electrodes. Responses to single‐pulse TMS were recorded in conscious human subjects via implanted epidural stimulators for the control of pain [Di Lazzaro et al., 2002a, b]. Subthreshold 5‐Hz stimulation (50 total stimuli at AMT) did not modulate the size and number of descending corticospinal volleys (i.e. the later I3, I4 I waves) evoked by each TMS pulse, and had no effect on MEPs, even though short‐interval intracortical inhibition (SICI) was reduced. This is consistent with the hypothesis that reduced SICI is due to effects at the motor cortex [Di Lazzaro et al., 2002b]. An additional measure of spinal excitability, the H‐reflex, was similarly unchanged after rTMS [Modugno et al., 2001]. These studies support the view that the conditioning effect of a low‐intensity high‐frequency rTMS train on connectivity, as seen in the present study, is mainly a cortical effect. Since the SICI is thought to reflect the excitability of interneurons subserving short‐lasting GABAergic inhibition [Ziemann, 1999], it was argued that low‐intensity rTMS at 5 Hz can selectively decrease the excitability of the intracortical inhibitory GABAergic pathways in the stimulated M1 [Di Lazzaro et al., 2002b]. Takano et al. [2004] also found an increase of synaptic activity, measured with PET, and a decrease of intracortical inhibition, measured by SICI, which outlasted 8 min of rTMS, suggesting a shift in the balance between intracortical inhibitory and facilitatory circuits, mediated by a decrease in the efficacy of GABAA‐ergic inhibitory synapse.
In addition to effects on cortico‐cortical interactions, the possibility of a subcortical contribution to the effects of rTMS (with cortico‐subcortico‐cortical reentry loops) cannot be ignored [Strafella et al., 2003]. Changes in extracellular dopamine concentration were detected in the putamen in healthy subjects, using PET following rTMS (10‐pulse trains at 10 Hz and 90% RMT every 10 s) delivered to the left M1. High‐frequency rTMS over the cerebral cortex could therefore cause dopamine release in subcortical structures, supporting a role for corticostriatal projections of the stimulated area in dopaminergic transmission [Strafella et al., 2003].
We conclude that our results underscore the ability of rTMS to modulate synaptic activity in interconnected regions even at intensities below RMT. Short trains of high‐frequency subthreshold rTMS could act on the synaptic activity of intracortical inhibitory circuits that may be involved in the generation of regional and interregional cortical oscillations, thus inducing a decrease of coupling between brain areas. Nonetheless, this study demonstrates a new approach to the investigation of cortical rhythms in clinical populations, such as epileptic and depressed patients, or to the examination of TMS effects on electrophysiological indices of cognitive processes in healthy subjects [Taylor et al., 2006].
Acknowledgements
This research is dedicated to the memory of Emma Turchetto of Gatto, the grandmother of GF. We thank two anonymous reviewers for helpful comments on earlier drafts of the manuscript; Louise Doyle and Neil Muggleton for revisions to the language of the manuscript. This article represents part of GF's dissertation for the degree of Doctor of Philosophy in the University of Verona (Italy).
REFERENCES
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J ( 2003): Subthreshold high‐frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI‐TMS. Neuroimage 20: 1685–1696. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J ( 2004): Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19: 1950–1962. [DOI] [PubMed] [Google Scholar]
- Brasil‐Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M ( 1992): Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol 9: 132–136. [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, Saturno E, Dileone M, Tonali PA, Rothwell JC ( 2002a): Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res 144: 549–553. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC ( 2002b): Short‐term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res 147: 108–113. [DOI] [PubMed] [Google Scholar]
- Fein G, Raz J, Brown FF, Merrin EL ( 1988): Common reference coherence data are confounded by power and phase effects. Electroencephalogr Clin Neurophysiol 69: 581–584. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Fiaschi A, Manganotti P ( 2005): Modulation of cortical oscillatory activities induced by varying single‐pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. Neuroimage 27: 896– 908. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC ( 2001): Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57: 449–455. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M ( 2000): Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 1620–1631. [DOI] [PubMed] [Google Scholar]
- Kalcher J, Pfurtscheller G ( 1995): Discrimination between phase‐locked and non‐phase‐locked event‐related EEG activity. Electroencephalogr Clin Neurophysiol 94: 381–384. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M ( 1997): Event‐related coherence and event‐related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self‐paced movements. Electroencephalogr Clin Neurophysiol 104: 199–206. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual‐Leone A ( 2000): Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133: 425–430. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M ( 1998): Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62. [DOI] [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC ( 2001): Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res 140: 453–459. [DOI] [PubMed] [Google Scholar]
- Nikouline V, Ruohonen J, Ilmoniemi RJ ( 1999): The role of the coil click in TMS assessed with simultaneous EEG. Clin Neurophysiol 110: 1325–1328. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Strens LH, Di LV, Tonali PA, Brown P ( 2003): Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res 149: 107–113. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP ( 2001): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86: 1983–1990. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR ( 2004): Long‐lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 115: 1519–1526. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A ( 1977): Event‐related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 42: 817–826. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G ( 2000): Functional dissociation of lower and upper μ rhythms in relation to voluntary limb movement. Clin Neurophysiol 111: 1873–1879. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'Angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P ( 2005): Distinct changes in cortical and spinal excitability following high‐frequency repetitive TMS to the human motor cortex. Exp Brain Res 161: 114–124. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P, Petsche H ( 1988): Probability mapping: Power and coherence analyses of cognitive processes. Brain Topogr 1: 46–54. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH ( 1994): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92. [DOI] [PubMed] [Google Scholar]
- Roth BJ, Pascual‐Leone A, Cohen LG, Hallett M ( 1992): The heating of metal electrodes during rapid‐rate magnetic stimulation: A possible safety hazard. Electroencephalogr Clin Neurophysiol 85: 116–123. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R ( 1994): Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J ( 2003): Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp Brain Res 148: 1–16. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A ( 2003): Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126: 2609–2615. [DOI] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P ( 2002): The effects of subthreshold 1 Hz repetitive TMS on cortico‐cortical and interhemispheric coherence. Clin Neurophysiol 113: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, Siebner HR ( 2004): Short‐term modulation of regional excitability and blood flow in human motor cortex following rapid‐rate transcranial magnetic stimulation. Neuroimage 23: 849–859. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF ( 2006): Combining correlation and interference methods in the human brain. Focus on “Cortico‐cortical interactions in spatial attention: A combined ERP/TMS study.” J Neurophysiol 95: 2731–2732. [DOI] [PubMed] [Google Scholar]
- Theoret H, Pascual‐Leone A ( 2003): Transcranial magnetic stimulation and the study of cognition In: Hugdahl K, editor. Experimental Methods in Neuropsychology. Boston: Kluwer Academic; pp 173–195. [Google Scholar]
- Tiihonen J, Kajola M, Hari R ( 1989): Magnetic μ rhythm in man. Neuroscience 32: 793–800. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Virtanen J, Ilmoniemi RJ, Kamppuri J, Ollikainen M, Ruohonen J, Naatanen R ( 1999): Separation of contamination caused by coil clicks from responses elicited by transcranial magnetic stimulation. Clin Neurophysiol 110: 982–985. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual‐Leone A ( 2003): Neurochronometrics of Mind: Transcranial Magnetic Stimulation in Cognitive Science. Cambridge: MIT Press. [Google Scholar]
- Ziemann U ( 1999): Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalogr Clin Neurophysiol Suppl 51: 127–136. [PubMed] [Google Scholar]


