Abstract
Traumatic brain injury (TBI) is a major cause of impairment and functional disability in children and adolescents, including deterioration in fine as well as gross motor skills. The aim of this study was to assess deficits in sensory organization and postural ability in a young group of TBI patients versus controls by using quantitative force‐platform recordings, and to test whether balance deficits are related to variation in structural properties of the motor and sensory white matter pathways. Twelve patients with TBI and 14 controls (aged 8–20 years) performed the Sensory Organisation Test (SOT) protocol of the EquiTest® (Neurocom). All participants were scanned using Diffusion Tensor Imaging (DTI) along with standard anatomical scans. Quantitative comparisons of DTI parameters (fractional anisotropy, axial and radial diffusivity) between TBI patients and controls were performed. Correlations between DTI parameters and SOT balance scores were determined. Findings revealed that the TBI group scored generally lower than the control group on the SOT, indicative of deficits in postural control. In the TBI group, reductions in fractional anisotropy were noted in the cerebellum, posterior thalamic radiation, and corticospinal tract. Degree of white matter deterioration was highly correlated with balance deficits. This study supports the view that DTI is a valuable tool for assessing the integrity of white matter structures and for selectively predicting functional motor deficits in TBI patients. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: traumatic brain injury, DTI, balance, children, neuroplasticity
INTRODUCTION
Traumatic brain injury (TBI) is the leading cause of acquired disability in children and adolescents (Kraus and McArthur,1996; Sosin et al.,1996), often due to traffic accidents, and resulting in significant deficits in cognition. Moreover, motor performance is impaired for years following the insult, often including balance deficits that interfere with successful performance on daily tasks. Although children and adolescents with TBI often regain some of their lost postural control after therapy, complaints remain prevalent years after injury (Rossi and Sullivan,1996). The extensive diffuse nature of axonal injury is often regarded as the primary cause of balance deficits in TBI.
Diffuse axonal injury (DAI) results from the rotational acceleration/deceleration forces occurring at the time of injury (Adams et al.,1980; Gentry,1994) and is characterized by microscopic lesions scattered throughout the white matter (WM), occurring particularly in the corpus callosum, upper brain stem, and internal capsule (Levin et al.,1997). Whereas conventional MRI is quite insensitive to axonal injury in TBI, diffusion tensor imaging (DTI) has proven to be more appropriate for assessment of diffuse WM damage (Levin,2003). More specifically, it determines the magnitude and directionality of water diffusion along WM tracts as characterized by the diffusion tensor and its derived diffusion parameters, such as fractional anisotropy (FA), mean diffusivity (MD), and diffusion eigenvalues (Ge et al.,2005; Le Bihan et al.,2001).
A number of recent DTI studies in TBI adults have demonstrated changes in diffusion anisotropy, particularly in the corpus callosum, internal capsule, and centrum semiovale, sometimes with significant correlations to clinical and behavioral parameters (e.g. Kraus et al.,2007; Kennedy et al.,2009; Salmond et al.,2006; Sidaros et al.,2008). On the contrary, DTI applications on TBI children are still very limited but potentially promising. Recent DTI studies in TBI children have provided evidence for associations between FA and performance on neurocognitive and behavioral measures. To the best of our knowledge, DTI studies in TBI children have not yet tested whether deficits in postural control are related to variation in structural properties of WM pathways.
This study determined deficits in sensory organization and postural ability in TBI children versus controls by using quantitative force‐platform recordings. DTI was used to assess the structural integrity of principal motor and sensory tracts/regions. It was hypothesized that the degree of microstructural WM damage in principal sensorimotor structures, as assessed by FA, would predict balance performance in TBI patients.
MATERIALS AND METHODS
Participants
Twenty‐six children and adolescents participated in the study, including 12 subjects with TBI (mean age 14 years 8 months, SE 1 year 1 month, range 8–20 years of age, seven boys and five girls) and 14 control subjects (mean age 12 years 10 months, SE 8 months, range 9–17 years of age; seven boys and seven girls). The TBI patients were classified as ‘moderate‐to‐severe’ based on several factors: the Glasgow Coma Scale score after resuscitation (a subgroup of five children had a GCS of 8 or below), the anatomical features of the injury based on inspection by an expert neuroradiologist (see later), and the injury mechanism (traffic accidents and falls), or combinations thereof. The demographic and clinical characteristics of the TBI group are shown in Table I. All TBI patients were assessed at least 6 months post‐injury, when neurological recovery was stabilized. The interval between injury and scanning (age of injury) was on average 3 years, 6 months (SE 9 months). Their age at injury was on average 10 years, 6 months (SE 3 years, 2 months). Participants were excluded if they had pre‐existing developmental or intellectual disabilities, a progressive disease, or were taking medication.
Table I.
Summary of demographic and injury characteristics for the TBI group
| TBI patient #; Age/gender/handedness | GCS | Acute scan within 24 h after injury Lesion location/pathology | MRI scan at examination Lesion location/pathology |
|---|---|---|---|
| TBI 1; 15,7/F/RH | 8 | (R) FL subdural hematoma | (R) FL, splenium corpus callosum shearing injuries |
| TBI 2; 9,9/F/RH | 5 | (R) TL/PL subdural hematoma and (R) cerebellar contusion | (R) TL/PL Subdural hematoma and (R) cerebellar contusion |
| TBI 3; 13,1/M/LH | Lesion and location not specified in available records | Hemosiderin deposits (R) thalamus and (R) posterior limb of the internal capsule, shearing injuries corpus callosum | |
| TBI 4; 16,6/M/RH | 7 | Enlarged (R) lateral ventricle, (R) hematoma occipital horn lateral ventricle, hyperdensity (L) thalamus, (LH) shearing injuries | Enlarged (R) lateral ventricle, (RH) atrophy, hemosiderin deposits (splenium corpus callosum, (R) corona radiata), asymmetry cerebral peduncles, (L) contusion pons |
| TBI 5; 14,1/F/RH | 3 | (R) FL/PL/OL contusion; contusion basal ganglia (thalamus, (R) nucleus caudatus) | Atrophy (R) FL/TL, nucleus caudatus, lentiformis, thalamus, internal capsule, atrophy (R) amygdala, hippocampus, cerebellum; asymmetry lemniscus, cerebral peduncles, pons; shearing injuries corpus callosum |
| TBI 6; 16,8/M/RH | 8 | (L) TL contusion, (L) FL punctiform contusion, (R) contusion mesencephalon, (L) FL hemorrhagic injuries and (L) thalamus | Enlarged ventricles, (L) FL hemosiderin deposits |
| TBI 7; 19,1/M/RH | Lesion and location not specified in available records | Shearing injury splenium corpus callosum | |
| TBI 8; 20,4/M/RH | (R) FL hematoma, enlarged ventricles | Atrophy RH, (R) contusion superior frontal gyrus, atrophy (R) nucleus caudatus, (R) nucleus lentiformis, injured corpus callosum | |
| TBI 9; 17,2/M/RH | Contusions (L) FL, TL, (R) PL, subdural hematoma | Contusion (L) PL inferior, (L) LH hemosiderin deposits, (R) TL contusion, atrophy (L) lateral TL, atrophy (L) FL | |
| TBI 10; 11,5/F/RH | (R) FL subdural hematoma, hemorrhagic injuries thalamus, fornix, corpus callosum | (R) FL subdural hematoma | |
| TBI 11; 18,7/F/RH | Enlarged ventricles, atrophy PL, FL, RH WM, atrophy (L) hippocampus, shearing injuries | Enlarged ventricles, atrophy LH, (L) PL inferior contusion, injuries superior frontal sulcus, contusion RH, orbitofronal contusion, (R) nucleus lentiformis contusion, asymmetry cerebral peduncles, atrophy (R) cerebellum | |
| TBI 12; 8,9/M/RH | Hemorrhagic injuries RH/LH, FL/TL contusion, (L) FL subdural hematoma | (L) FL contusion and subdural hematoma, (R) TL contusion and subdural hematoma |
Anatomy codes: WM, white matter; GM, grey matter; RH, right hemisphere; LH, left hemisphere; FL, frontal lobe; TL, temporal lobe; PL, parietal lobe; OL, occipital lobe; R, right; L, left. Other codes: TBI, traumatic brain injury; GCS, Glasgow Coma Scale score; MRI, magnetic resonance imaging; RH, right‐handed; LH, left‐handed; M, male; F, female.
General motor performance was assessed using the Movement Assessment Battery for Children (M‐ABC), including measures of manual dexterity, ball skills, and static and dynamic balance (Henderson and Sugden,1992; Smits‐Engelsman,1998). The TBI group scored on average worse than the control group on the total score of the M‐ABC, t(24) = 4.00, p < 0.001 (Mean TBI = 15.32, Mean Control = 5.64), balance subscore, t(24) = 4.70, p < 0.001 (Mean TBI = 7.55, Mean Control = 2.46), and manual dexterity subscore, t(24) = 3.87, p < 0.001 (Mean TBI = 5.63, Mean Control = 1.54), confirming the former's lower mean motor performance levels.
Sensory Organisation Test
Posturography was used to determine sensory organization (which sensory stimuli a subject primarily relies on) and muscle coordination (how well postural adjustments are realized). The EquiTest System (NeuroCom International, Clackamas, Oregon) provided objective assessment of balance control and postural stability under dynamic test conditions, designed to reflect the challenges of daily life. As part of the EquiTest System, the Sensory Organisation Test (SOT) protocol systematically disrupted the sensory selection processes (i.e. somatosensory, visual inputs, or both) while measuring a subject's ability to maintain equilibrium. Six sensory conditions evaluated the relative contributions of vision, vestibular, and somatosensory input in balance function (see Fig. 1). In condition 1, all three sensory systems were operational while the participant stood on a fixed platform with eyes open, and a baseline measure of stability was obtained. Condition 2 was the same as condition 1, except that the eyes were closed. Condition 3 was similar to condition 1 but the visual surround moved to track the participant's sway, which provided inaccurate orientation cues. In condition 4, the subject stood with the eyes open and the visual surround fixed but the platform moved in response to his/her sway such that the ankle joints did not bend in response to the sway, providing inaccurate proprioceptive input to the brain. Condition 5 was identical to condition 4 except that the eyes were now closed, such that only the vestibular system was fully operational. Condition 6 was the same as condition 4 except that the visual surround moved in response to the participant's sway, and thus both vision and proprioception were compromised, leaving only the vestibular system as a reliable source. The test protocol consisted of three repetitions of each condition. An equilibrium score was calculated for these 6 sensory conditions. The subject's sway was calculated from the maximum anterior and posterior center of gravity displacements, occurring over the 20‐s trial period. Maximum displacement without losing balance was assumed to be within a range of 12.5° (6.25° anterior, 6.25° posterior). The equilibrium scores were then expressed as percentages, 0 indicating sway exceeding the limits of stability and 100 indicating perfect stability. The test series involved 18 trials overall. A composite equilibrium score, describing a person's overall level of performance during all the SOT trials was also calculated, with higher scores being indicative of better balance performance. This task typically lasted 15 min.
Figure 1.
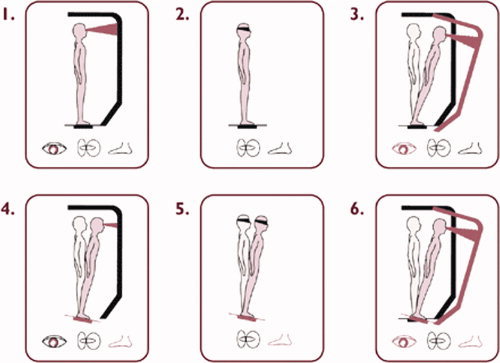
Schematic representation of the six conditions of the Sensory Organisation Test of the EquiTest System. Reproduced with permission from NeuroCom International, Clackamas, Oregon. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
MRI Data Acquisition
MR examination took place without sedation on a 3T scanner (Intera, Philips, Best, the Netherlands) with an eight channel phased‐array head coil. A DTI SE‐EPI (diffusion weighted single shot spin‐echo echoplanar imaging), with a data acquisition matrix = 112 × 112; field of view (FOV) = 220 × 220 mm2; TR = 7916 ms, TE = 68 ms, parallel imaging factor 2.5, 68 contiguous sagittal slices (slice thickness = 2.2 mm; voxel size = 2 × 2 × 2.2 mm3) covering the entire brain and the brainstem, was acquired. A pair of diffusion gradients was applied along 45 non‐collinear directions with a b‐value of 800 s/mm2. Additionally, one set of images with no diffusion weighting (b = 0 s/mm2) was acquired.
A T1‐weighted coronal 3D‐TFE (182 contiguous coronal slices covering the whole brain and brainstem; FOV = 250 mm; TE = 4.6 ms; TR = 9.7 ms; slice thickness = 1.2 mm; matrix size = 256 × 256; voxel size = 0.98 × 0.98 × 1.2 mm3; scan duration = 4:21 min) was consequently acquired for anatomical detail. These structural MRI scans were investigated by an expert neuro‐radiologist to indicate location and type of pathology (e.g., gliosis, shearing, and haemorrhage) (see Table I).
DTI Processing
The DTI data were analyzed and processed in ExploreDTI (Leemans et al.,2009), using the following multi‐step procedure: (a) Subject motion and eddy‐current induced geometrical distortions were corrected (Leemans and Jones,2009), (b) the diffusion tensors and subsequently the FA, axial (λ||) and radial (λ⊥) diffusivity were calculated using a non‐linear regression procedure (Mori et al.,2008), (c) the DTI data were coregistered to MNI space for analyzing potential group differences (Leemans et al.,2005; Van Hecke et al.,2007,2008), (d) a standard deterministic streamline tractography approach was used, as described in Basser et al. (2000), to reconstruct the WM fiber pathways of interest, (e) the probabilistic cytoarchitectonic atlas, developed by Eickhoff et al. (2005), and a digitized version of the original Talairach atlas (Lancaster et al.,2000,2007), both mapped in MNI space as provided by the FSL toolbox, were used to perform the correlation analysis (Smith et al.,2004).
ROIs Definition
Preserving balance requires multisensory integration to drive motor commands (Nashner and Peters,1990). An automated observer‐independent approach was used here to define several WM pathways that are mainly concerned with sensory afferent input on one hand—such as the medial lemniscus, the posterior thalamic radiation, the middle cerebellar peduncle, and the pons—and motor functions on the other hand, including the corticospinal tract, the superior cerebellar peduncle, the cerebral peduncle, and the anterior limb of the internal capsule. Pathways carrying both input and output fibers, such as the inferior cerebellar peduncle, the posterior limb of the internal capsule, the brainstem, and the cerebellum, were also analyzed for diffusion parameters. Tracts and regions were reconstructed and presented on a 3D T1‐image for a single representative subject and fractional anisotropy maps of the white‐matter atlas of Mori et al. (2005) in Figures 2 and 3, respectively.
Figure 2.
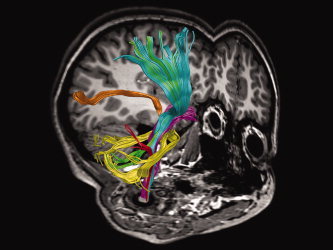
Sensory and motor tracts used in the study. Tracts were reconstructed and displayed on a 3D T1‐image for a typical control subject: Corticospinal tract (cyan), inferior cerebellar peduncle (red), middle cerebellar peduncle (yellow), medial lemniscus (magenta), posterior thalamic radiation (orange), and superior cerebellar peduncle (green). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
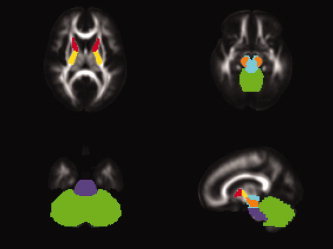
Example sensory and motor regions of interest masks depicted on fractional anisotropy maps of the white‐matter atlas of Mori et al. (2005): Anterior limb of the internal capsule (red), posterior limb of the internal capsule (yellow), cerebellum (green), brainstem (blue), pons (violet), cerebral peduncle (orange). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Statistical Analysis
Analysis of the SOT equilibrium scores was assessed by a 2 × 6 (Group x Sensory condition) ANOVA with repeated measures on the last factor. For the composite score of the SOT, two‐sample t‐tests compared the TBI group with the age‐ and gender‐matched control group. These behavioural parameters were also used to assess relationships between measures of WM integrity and balance using partial Pearson correlations (with the effect of age removed) within the TBI group.
The primary analyses carried out on the dependent measures extracted from the DTI data (FA, axial and radial diffusivity) were two‐sample t‐tests with group membership (TBI and control) as the between subjects comparison. Data were confirmed to have a normal distribution using the Shapiro‐Wilks Normality Test. Bonferroni corrections for multiple comparisons were made (hence p < 0.004 was considered significant following correction for the between‐group comparisons regarding the DTI parameters and p < 0.007 for the correlation analyses).
RESULTS
Group Differences in Postural Control
Group × Sensory condition ANOVAs with repeated measures on the last factor were conducted on the SOT. The control group scored higher than the group with TBI for all posturography SOT conditions, F(1,22) = 5.00, p < 0.05. Moreover, a significant main effect of sensory condition was found, F(5,110) = 138.1, p < 0.001. Post hoc (Tukey) testing exhibited significant differences between condition 1 and 4 on one hand and condition 5 and 6 on the other hand (p < 0.05). Finally, the interaction between group and sensory condition was also significant, F(5,110) = 3.66, p < 0.01 (see Fig. 4). Post hoc (Tukey) testing showed that the TBI group performed significantly worse than the controls on condition 5 (eyes closed, sway referenced surface). The mean composite SOT score (average across all six conditions) also differed significantly between the TBI patients (M = 58.8; range, 32.0–80.0) and the controls (M = 69.4; range, 58.2–75.7), t(22) = −2.20, p < 0.001. The lower scores of the subjects with TBI indicate poorer balance (larger anterior/posterior body sway) than the control subjects.
Figure 4.
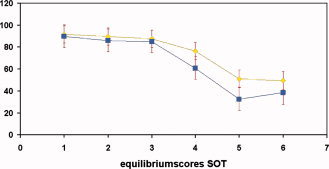
Graph depicting the significant interaction effect between sensory condition and group. The TBI group performed significantly worse than the controls on condition 5 (eyes closed, sway referenced surface). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Group Differences in DTI Parameters
A distinct pattern of significant differences between both groups was observed throughout the brain. Table II lists the diffusion parameters for the different fiber tracts studied in the TBI and control group.
Table II.
Diffusion parameters, mean, and standard error for each ROI for both groups
| Fractional anisotropy | Axial diffusivity [× 10‐3 mm2/s] | Radial diffusivity [× 10‐3 mm2/s] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | TBI | Control | TBI | Control | TBI | Control | |||||||||
| M | SE | M | SE | p | M | SE | M | SE | p | M | SE | M | SE | p | |
| Middle cerebellar peduncle | 0.406 | 0.023 | 0.403 | 0.012 | 0.895 | 1.316 | 0.026 | 1.231 | 0.014 | 0.006 | 0.608 | 0.021 | 0.588 | 0.009 | 0.359 |
| Inferior cerebellar peduncle | 0.386 | 0.026 | 0.403 | 0.017 | 0.579 | 1.244 | 0.037 | 1.270 | 0.024 | 0.542 | 0.634 | 0.023 | 0.640 | 0.011 | 0.823 |
| Superior cerebellar peduncle | 0.349 | 0.029 | 0.438 | 0.017 | 0.011 | 2.142 | 0.170 | 1.649 | 0.040 | 0.006 | 1.189 | 0.127 | 0.781 | 0.030 | 0.003 |
| Cerebral peduncle | 0.492 | 0.026 | 0.597 | 0.012 | 0.001 | 1.461 | 0.043 | 1.503 | 0.031 | 0.423 | 0.594 | 0.042 | 0.507 | 0.008 | 0.039 |
| Anterior limb of the internal capsule | 0.372 | 0.028 | 0.469 | 0.013 | 0.003 | 1.384 | 0.127 | 1.131 | 0.012 | 0.042 | 0.816 | 0.135 | 0.519 | 0.010 | 0.026 |
| Posterior limb of the internal capsule | 0.517 | 0.020 | 0.606 | 0.006 | <0.001 | 1.325 | 0.026 | 1.359 | 0.016 | 0.269 | 0.534 | 0.022 | 0.468 | 0.004 | 0.004 |
| Cerebellum | 0.172 | 0.004 | 0.206 | 0.003 | <0.001 | 1.146 | 0.034 | 0.995 | 0.007 | <0.001 | 0.830 | 0.031 | 0.681 | 0.004 | <0.001 |
| Brainstem | 0.341 | 0.016 | 0.390 | 0.006 | 0.005 | 1.391 | 0.038 | 1.331 | 0.011 | 0.126 | 0.710 | 0.030 | 0.643 | 0.007 | 0.028 |
| Pons | 0.370 | 0.025 | 0.426 | 0.010 | 0.035 | 1.369 | 0.036 | 1.365 | 0.018 | 0.902 | 0.677 | 0.029 | 0.624 | 0.012 | 0.087 |
| Corticospinal tract | 0.252 | 0.009 | 0.306 | 0.004 | <0.001 | 1.250 | 0.027 | 1.108 | 0.012 | <0.001 | 0.751 | 0.021 | 0.635 | 0.008 | <0.001 |
| Medial lemniscus | 0.454 | 0.034 | 0.546 | 0.012 | 0.012 | 1.343 | 0.047 | 1.383 | 0.034 | 0.486 | 0.621 | 0.046 | 0.542 | 0.009 | 0.084 |
| Posterior thalamic radiation | 0.430 | 0.023 | 0.526 | 0.012 | 0.001 | 1.475 | 0.042 | 1.420 | 0.021 | 0.235 | 0.685 | 0.022 | 0.578 | 0.018 | 0.001 |
Results of the two‐sample‐t‐tests, bold values indicate that results remained significant following Bonferroni correction (p <0.004).
Fractional Anisotropy
Significant main effects of group were observed for several regions of interests (ROIs). As can be seen in Table II, TBI patients showed reduced fractional anisotropy along the corticospinal tract [t(24) = −5.54, p < 0.001], cerebral peduncle [t(24) = −3.82, p < 0.001], anterior [t(24) = −3.25, p < 0.01] and posterior limb of the internal capsule [t(24) = −4.58, p < 0.001], cerebellum [t(24) = −7.29, p < 0.001], and posterior thalamic radiation [t(24) = −3.82, p < 0.001].
Axial and Radial Diffusivity
Investigating the changes in the underlying eigenvalues revealed that the TBI group showed significantly increased axial diffusivity (λ||) in the cerebellum [t(24) = 4.68, p < 0.001], and corticospinal tract [t(24) = 5.07, p < 0.001], relative to controls (Table II). Likewise, radial diffusivity (λ⊥) increased in the superior cerebellar peduncle [t(24) = 3.35, p < 0.01], posterior limb of the internal capsule [t(24) = 3.21, p < 0.01], cerebellum [t(24) = 5.10, p < 0.001], corticospinal tract [t(24) = 5.56, p < 0.001], and posterior thalamic radiation [t(24) = 3.75, p < 0.001]. Hence, a decrease of FA was mediated by the combined effects of λ|| and λ⊥ increase.
Relationship Between WM Integrity and Postural Control
To examine the relationship between WM integrity and postural control, correlations were determined for the entire group of TBI subjects in specific tracts (Table III). Significant correlations were obtained between the equilibrium score of condition 1 (eyes open, fixed surface and visual surround) and FA in the superior cerebellar peduncle (r = 0.83, p < 0.01), the anterior limb of the internal capsule (r = 0.78, p < 0.01), the cerebellum (r = 0.82, p < 0.01), and the medial lemniscus (r = 0.83, p < 0.01), with higher FA being related to higher scores on the SOT. Moreover, the equilibrium score of condition 3 (eyes open, fixed surface, sway referenced visual surround) was significantly related to mean FA in the fibers passing through the middle cerebellar peduncle (r = 0.78, p < 0.01; see Fig. 5). Hence, higher balance levels were associated with a higher WM anisotropy. Correlations that were significant at p < 0.05, but did not survive correction for multiple comparisons, are reported for descriptive purposes only in Table III.
Table III.
Results of the correlation analyses between FA and equilibrium scores
| ROI | Fractional anisotropy | ||||||
|---|---|---|---|---|---|---|---|
| EqC1 | EqC2 | EqC3 | EqC4 | EqC5 | EqC6 | Comp | |
| Middle cerebellar peduncle | 0.51 | 0.45 | 0.78 | 0.19 | 0.33 | 0.41 | 0.43 |
| p = 0.113 | p = 0.163 | p = 0.005 | p = 0.584 | p = 0.316 | p = 0.205 | p = 0.192 | |
| Inferior cerebellar peduncle | 0.30 | 0.39 | 0.24 | 0.24 | 0.11 | 0.21 | 0.22 |
| p = 0.369 | p = 0.236 | p = 0.469 | p = 0.485 | p = 0.757 | p = 0.545 | p = 0.506 | |
| Superior cerebellar peduncle | 0.83 | 0.48 | 0.65 | 0.27 | 0.61 | 0.35 | 0.51 |
| p = 0.002 | p = 0.139 | p = 0.030 | p = 0.416 | p = 0.046 | p = 0.287 | p = 0.113 | |
| Cerebral peduncle | 0.69 | 0.32 | 0.73 | 0.29 | 0.61 | 0.37 | 0.51 |
| p = 0.018 | p = 0.337 | p = 0.011 | p = 0.394 | p = 0.045 | p = 0.266 | p = 0.107 | |
| Anterior limb of the internal capsule | 0.78 | 0.65 | 0.64 | 0.40 | 0.44 | 0.43 | 0.52 |
| p = 0.004 | p = 0.029 | p = 0.033 | p = 0.220 | p = 0.179 | p = 0.184 | p = 0.099 | |
| Posterior limb of the internal capsule | 0.71 | 0.52 | 0.64 | 0.41 | 0.61 | 0.42 | 0.56 |
| p = 0.015 | p = 0.100 | p = 0.036 | p = 0.213 | p = 0.049 | p = 0.199 | p = 0.074 | |
| Cerebellum | 0.82 | 0.59 | 0.67 | 0.28 | 0.58 | 0.35 | 0.51 |
| p = 0.002 | p = 0.055 | p = 0.023 | p = 0.400 | p = 0.063 | p = 0.288 | p = 0.112 | |
| Brainstem | 0.57 | 0.51 | 0.73 | 0.19 | 0.47 | 0.43 | 0.47 |
| p = 0.065 | p = 0.112 | p = 0.011 | p = 0.585 | p = 0.145 | p = 0.191 | p = 0.144 | |
| Pons | 0.53 | 0.53 | 0.75 | 0.26 | 0.45 | 0.53 | 0.52 |
| p = 0.094 | p = 0.095 | p = 0.008 | p = 0.441 | p = 0.170 | p = 0.097 | p = 0.099 | |
| Corticospinal tract | 0.60 | 0.63 | 0.63 | 0.56 | 0.66 | 0.68 | 0.72 |
| p = 0.053 | p = 0.039 | p = 0.039 | p = 0.071 | p = 0.027 | p = 0.021 | p = 0.013 | |
| Medial lemniscus | 0.83 | 0.47 | 0.71 | 0.22 | 0.55 | 0.41 | 0.50 |
| p = 0.002 | p = 0.142 | p = 0.014 | p = 0.514 | p = 0.079 | p = 0.216 | p = 0.116 | |
| Posterior thalamic radiation | 0.67 | 0.70 | 0.56 | 0.66 | 0.65 | 0.63 | 0.71 |
| p = 0.023 | p = 0.018 | p = 0.076 | p = 0.027 | p = 0.029 | p = 0.038 | p = 0.013 | |
Bold values indicate scores remaining significant following Bonferroni correction (p < 0.007). Cursive values are significant at p < 0.05, but did not survive correction for multiple comparisons.
Figure 5.
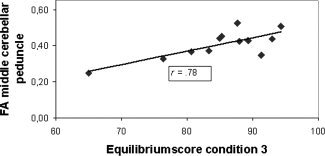
Scatter plot indicating the relationship between FA and equilibrium scores of the SOT within the TBI group.
Similar results were obtained using both axial and radial diffusivity indices: increased diffusivity (e.g., in the cerebellum, middle cerebellar peduncle, medial lemniscus, anterior limb of the internal capsule) was associated with lower performance on the SOT. However, we used FA as a damage marker without consideration of other DTI parameters, because FA has been suggested to be the most important DTI marker in patients with brain injury (Assaf and Pasternak,2008; Mori and van Zijl,2002).
DISCUSSION
In this study, we determined relations between brain WM structure and postural function in a young TBI group. First, we used DTI to assess WM differences between a TBI and matched control group in structures related to postural control. Second, the functional relevance of these microstructural abnormalities was underscored by statistically significant correlations between balance deficits and FA scores in the TBI group. To the best of our knowledge, this is the first time that such strong relationships between brain WM structure and postural control have been established.
Group Differences in Postural Control
Our behavioural results provide evidence of compromised sensorimotor processing as shown by disturbed postural control in TBI when compared with control subjects, especially in conditions where visual and vestibular inputs must be relied upon to produce stability. Balance deficits in TBI children have also been reported in studies using primarily clinical tests for field use (e.g. Gagnon et al.,1998,2001,2004; Geurts et al.,1996,1999). For example, children with mild TBI showed balance deficits at 12 weeks post injury on the balance subtest of the Bruininks‐Oseretsky Test of Motor Proficiency, the Postural Stress Test, and on the eyes‐closed conditions in the Pediatric Clinical Test of Sensory Interaction for Balance (Gagnon et al.,2004). Moreover, our results also paralleled those revealed with the SOT in mild TBI adults, showing increased postural instability as measured by the SOT composite score (Guskiewicz et al.,1997,2001; Kaufman et al.,2006; Riemann and Guskiewicz,2000). However, these findings cannot be compared directly with the results of this study due to the variation in patient populations (such as different age groups, severity and chronicity).
Group Differences in Diffusion Tensor Imaging
We found that mean FA in several fiber systems was lower in a young group suffering from moderate‐to‐severe TBI when compared with a typically developing group that was matched on demographic variables. These findings extend previous adult DTI studies showing long‐term WM disruption following TBI (Benson et al.,2007; Kraus et al.,2007; Mathias et al.,2004; Salmond et al.,2006), as well as case reports suggesting decreased FA in children with TBI (Ewing‐Cobbs et al.,2006). However, in contrast to previous DTI studies, which used a priori ROI analyses, making them vulnerable to intra‐ and inter‐subject variability, we applied an automated observer‐independent approach for assessing groupwise microstructural differences in several WM pathways/regions of the brain, as discussed next.
Firstly, we demonstrated significantly lower FA in the internal capsule of our TBI patients compared with controls. This region is a site with a predilection to shearing injuries (DAIs) (Gentry et al.,1988). More specifically, we found FA to be decreased in several motor tracts and regions, including the corticospinal tract and the cerebral peduncle. Recent studies using predefined ROIs for analysis of diffusion characteristics along WM tracts also demonstrated FA reduction in these two regions (Kraus et al.,2007; Sidaros et al.,2008). Because our field of view was not confined to these ROIs, we discovered WM disintegration at different levels of the motor system, including the cerebellum, i.e. a decrease in anisotropy and increase in diffusivity. The pattern of WM cerebellar impairment corresponds well with previous studies. MRI findings in children confirm that the cerebellum is often affected even when the initial injury does not involve the cerebellum (Spanos et al.,2007; Soto‐Ares et al.,2001). The indirect damage can be obtained via several mechanisms, such as mechanical forces, metabolic changes, and presynaptic hyperexcitability (Potts et al.,2009). This is further supported by studies using animal models of indirect and direct TBI trauma (Igarashi et al.,2007; Park et al.,2006,2007). Following either direct or indirect cerebellar injury, several pathophysiologic mechanisms mediating TBI‐induced cerebellar damage can be identified. These include Purkinje cell injury or loss due to excitotoxic injury, activation of an inflammatory response, and traumatic axonal injury. Moreover, functional (compensatory) changes in the cerebellum have been reported in our previous fMRI study (Caeyenberghs et al.,2009), suggesting that brain injury may lead to cerebellar disorganisation. This study complements previous work, emphasizing the vulnerability of the cerebellum to TBI.
The posterior thalamic radiation was also affected in the TBI group, impairing peripheral sensory input to the cortex and reciprocal corticothalamic projections (Kamali et al.,2009). This suggest that WM projections to or from sensory cortex, besides classical pyramidal motor tracts, may play an important role in the pathophysiology of motor disabilities in TBI. Contribution of sensory pathways to motor outcome has also been demonstrated in children with cerebral palsy (Hoon et al.,2002; Thomas et al.,2005).
Although global FA is commonly reported in pediatric TBI studies (Ewing‐Cobbs et al.,2008; Wilde et al.,2006), the underlying eigenvalues are also valuable because these may show more specific associations with certain pathological processes (Pierpaoli et al.,2001; Song et al.,2002). Human (Arfanakis et al.,2002; Concha et al.,2006) and animal studies (Boska et al.,2007; Mac Donald et al.,2007) suggest that the second and third eigenvalues (radial diffusivity) assess myelin changes. The first eigenvalue (axial diffusivity) is more related to axonal morphology and degradation. Here, we found increased radial and axial diffusivity in the TBI group for specific ROIs, suggesting damage to both myelin and axons. Kraus et al. (2007) found similar changes in patients with moderate‐to‐severe TBI, whereas only axial diffusivity was elevated in patients with mild TBI. This suggests a continuum of widespread neural changes in moderate to severe TBI affecting tissue organization, myelin, and axonal integrity. Regardless of the exact proportionate contribution of these pathological processes, the observed DTI pattern reflects the consequences of DAI and secondary insults.
Structural Integrity of the Brain (DTI) and its Relation to Postural Control
Our ultimate goal was to investigate whether damage in motor and sensory WM tracts/regions in the TBI group was associated with impairments in balance, as assessed by the SOT protocol of the EquiTest. Lower balance levels were associated with a lower WM anisotropy. More specifically, significant correlations were obtained between the SOT equilibrium score of condition 1 and FA in the superior cerebellar peduncle, the anterior limb of the internal capsule, the cerebellum, and the medial lemniscus. Also, the equilibrium score of condition 3 was significantly related to mean FA of the middle cerebellar peduncle. Interestingly, in most of the regions where FA values in TBI patients were significantly lower than controls (e.g. anterior limb of the internal capsule, cerebellum, and medial lemniscus), high correlations with the equilibrium scores were observed (Tables II and III). Even FA values for the cerebellar peduncles, which did not differ significantly between groups, were correlated with equilibrium. This in agreement with a previous study of Hong et al. (2009), showing that the junction and peri‐junctional areas between the brainstem and cerebellum are most vulnerable to DAI. Evaluation of the cerebellar peduncles using DTI could be helpful in patients with ataxia following TBI.
Moreover, the importance of the changes in diffusivity in this study is also highlighted by the significant correlations between radial/axial diffusivity and the equilibrium scores. Increases in diffusivity (both axial and radial) were associated with poor scores on condition 1 and 3 of the SOT.
Against our expectations, significant correlations were primarily observed for the less challenging postural control conditions and only for a specific set of WM tracts. However, it is important to note that high correlations were observed across the postural conditions even though these did not survive Bonferroni correction (see Table III). For example, performance on condition 5 was positively related to the mean FA in the posterior thalamic radiation and the superior cerebellar peduncle.
Limitations of this study pertain to the small sample size and the cross sectional design. Despite the clear findings, their replication in a larger sample is mandatory. In addition, further histopathology studies are required to investigate the processes underlying the DTI findings. Such research should also be explored for broader age ranges because the effects of TBI on FA and diffusivity indices may be age‐dependent. Finally, longitudinal studies are needed to determine how changes in DTI metrics are related to recovery and objective measures of postural control.
Taking into account these limitations, this study is the first report illustrating associations between microstructure and postural control scores in a young TBI group. Correlations between indices derived from diffusion‐weighted imaging during acute scanning and injury severity have previously been noted (Huisman et al.,2004), as well as with neurocognitive measures in chronic TBI patients (Kraus et al.,2007; Salmond et al.,2006). Motor indices, though not investigated in the head injury literature, have previously been reported to be associated with DTI measures in other disorders. For example, DTI metrics are correlated with tests of upper‐limb function in patients with congenital hemiplegia and chronic stroke patients (Bleyenheuft et al.,2007; Schaechter et al.,2009). Unfortunately, DTI studies that use quantitative measures of kinematics are scarce.
CONCLUSIONS
This is the first report demonstrating a strong relation between reduced FA and balance deficits in a young TBI group using quantitative force‐platform recordings. DTI may complement prognosis and may serve as a biomarker for TBI severity and potential functional motor impairment. Such work may clarify the brain structure‐function relations that mediate the development of postural control and provide a basis for early prognosis and effective treatment of balance deficits.
Acknowledgements
Support for this study was provided through a grant from the Research Programme of the Research Foundation ‐ Flanders (FWO) (Levenslijn # 7.0004.05), as well as Grant P6/29 from the Interuniversity Attraction Poles program of the Belgian federal government. Caeyenberghs K. is funded by a PhD fellowship of the Research Foundation ‐ Flanders (FWO).
REFERENCES
- Adams JH, Graham DI, Scott G, Parker LS, Doyle D ( 1980): Brain damage in fatal non‐missile head injury. J Clin Pathol 33: 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Carew JD, Meyerand ME ( 2002): Independent component analysis applied to diffusion tensor MRI. Magn Reson Med 47: 354–363. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O ( 2008): Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: a review. J Mol Neurosci 34: 51–61. Review. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A ( 2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44: 625–632. [DOI] [PubMed] [Google Scholar]
- Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, Millis SR, Makki M, Latif Z, Coplin W, Meythaler J, Haacke EM ( 2007): Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma 24: 446–459. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Grandin CB, Cosnard G, Olivier E, Thonnard JL ( 2007): Corticospinal dysgenesis and upper‐limb deficits in congenital hemiplegia: a diffusion tensor imaging study. Pediatrics 120: e1502–e1511. [DOI] [PubMed] [Google Scholar]
- Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, Hahn T, Gendelman HE, Mosley RL ( 2007): Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson's disease. Neurobiol Dis 26: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Wenderoth N, Smits‐Engelsman BC, Sunaert S, Swinnen SP ( 2009): Neural correlates of motor dysfunction in children with traumatic brain injury: exploration of compensatory recruitment patterns. Brain 132: 684–694. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C ( 2006): Diffusion tensor imaging of time‐dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 32: 1090–1099. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Ewing‐Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J ( 2006): Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol 27: 879–881. [PMC free article] [PubMed] [Google Scholar]
- Ewing‐Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM ( 2008): Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon I, Forget R, Sullivan SJ, Friedman D ( 1998): Motor performance following a mild traumatic brain injury in children: an exploratory study. Brain Inj 12: 843–853. [DOI] [PubMed] [Google Scholar]
- Gagnon I, Friedman D, Swaine B, Forget RJ ( 2001): Balance findings in a child before and after a mild head injury. Head Trauma Rehabil 16: 595–602. [DOI] [PubMed] [Google Scholar]
- Gagnon I, Swaine B, Friedman D, Forget R ( 2004): Children show decreased dynamic balance after mild traumatic brain injury. Arch Phys Med Rehabil 85: 444–452. [DOI] [PubMed] [Google Scholar]
- Ge Y, Law M, Grossman RI ( 2005): Applications of diffusion tensor MR imaging in multiple sclerosis. Ann N Y Acad Sci 1064: 202–219. [DOI] [PubMed] [Google Scholar]
- Gentry LR ( 1994): Imaging of closed head injury. [Review]. Radiology 191: 1–17. [DOI] [PubMed] [Google Scholar]
- Gentry LR, Godersky JC, Thompson B ( 1988): MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 150: 663–672. [DOI] [PubMed] [Google Scholar]
- Geurts AC, Knoop JA, van Limbeek J ( 1999): Is postural control associated with mental functioning in the persistent postconcussion syndrome? Arch Phys Med Rehabil 80: 144–149. [DOI] [PubMed] [Google Scholar]
- Geurts AC, Ribbers GM, Knoop JA, van Limbeek J ( 1996): Identification of static and dynamic postural instability following traumatic brain injury. Arch Phys Med Rehabil 77: 639–644. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM ( 2001): Postural stability assessment following concussion: one piece of the puzzle. [Review]. Clin J Sport Med 11: 182–189. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM ( 1997): Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exer 29: S213–S221. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA ( 1992): Movement Assessment Battery for Children: Manual. London: Psychological Corporation. [Google Scholar]
- Hong JH, Kim OL, Kim SH, Lee MY, Jang SH ( 2009): Cerebellar peduncle injury in patients with ataxia following diffuse axonal injury. Brain Res Bull 80: 30–35. [DOI] [PubMed] [Google Scholar]
- Hoon AH Jr, Lawrie WT Jr, Melhem ER, Reinhardt EM, Van Zijl PC, Solaiyappan M, Jiang H, Johnston MV, Mori S ( 2002): Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology 59: 752–756. [DOI] [PubMed] [Google Scholar]
- Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty‐Alva N, Ozsunar Y, Wu O, Sorensen AG ( 2004): Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 25: 370–376. [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Potts MB, Noble‐Haeusslein LJ ( 2007): Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp Neurol 203: 258–268. [DOI] [PubMed] [Google Scholar]
- Kamali A, Kramer LA, Butler IJ, Hasan KM ( 2009): Diffusion tensor tractography of the somatosensory system in the human brainstem: initial findings using high isotropic spatial resolution at 3.0 T. Eur Radiol 19: 1480–1488. [DOI] [PubMed] [Google Scholar]
- Kaufman KR, Brey RH, Chou LS, Rabatin A, Brown AW, Basford JR ( 2006): Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med Eng Phys 28: 234–239. [DOI] [PubMed] [Google Scholar]
- Kennedy MR, Wozniak JR, Muetzel RL, Mueller BA, Chiou HH, Pantekoek K, Lim KO ( 2009): 3hite matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc 15: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JF, McArthur DL ( 1996): Epidemiologic aspects of brain injury. Neurol Clin 14: 435–450. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM ( 2007): White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130: 2508–2519. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H ( 2001): Diffusion tensor imaging: concepts and applications. [Review]. J Magn Reson Imaging 13: 534–546. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, Jones, DK ( 2009): ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: 17th Annual Meeting of Intl Soc Mag Reson Med, Hawaii, USA, p. 3537.
- Leemans A, Jones DK ( 2009): The B‐matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61: 1336–1349. [DOI] [PubMed] [Google Scholar]
- Leemans A, Sijbers J, De Backer S, Vandervliet E, Parizel PM ( 2005): Affine coregistration of diffusion tensor magnetic resonance images using mutual information. Lecture Notes Comput Sci 3708: 523–530. [Google Scholar]
- Levin HS ( 2003): Neuroplasticity following non‐penetrating traumatic brain injury. Brain Inj 17: 665–674. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Yeakley J, Song J, Scheibel RS, Harward H, Fletcher JM, Kufera JA, Davidson KC, Bruce D ( 1997): Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: a test of the Ommaya‐Gennarelli model. Neurosurgery 40: 432–440. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D ( 2007): Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 27: 11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Bigler ED, Jones NR, Bowden SC, Barrett‐Woodbridge M, Brown GC, Taylor DJ ( 2004): Neuropsychological and information processing performance and its relationship to white matter changes following moderate and severe traumatic brain injury: a preliminary study. Appl Neuropsychol 11: 134–152. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J ( 2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40: 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, van Zijl PC ( 2002): Fiber tracking: principles and strategies—a technical review. [Review]. NMR Biomed 15: 468–480. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae‐Poetscher LM ( 2005): MRI Atlas of the Human White Matter. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Nashner LM, Peters JF ( 1990): Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin 8: 331–349. [PubMed] [Google Scholar]
- Park E, Liu E, Shek M, Park A, Baker AJ ( 2007): Heavy neurofilament accumulation and alpha‐spectrin degradation accompany cerebellar white matter functional deficits following forebrain fluid percussion injury. Exp Neurol 204: 49–57. [DOI] [PubMed] [Google Scholar]
- Park E, McKnight S, Ai J, Baker AJ ( 2006): Purkinje cell vulnerability to mild and severe forebrain head trauma. J Neuropathol Exp Neurol 65: 226–234. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P ( 2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Potts MB, Adwanikar H, Noble‐Haeusslein LJ ( 2009): Models of Traumatic Cerebellar Injury. Cerebellum 8: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann BL, Guskiewicz KM ( 2000): Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train 35: 19–25. [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Sullivan SJ ( 1996): Motor fitness in children and adolescents with traumatic brain injury. Arch Phys Med Rehabil 77: 1062–1065. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD ( 2006): Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 29: 117–124. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N ( 2009): Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients (in press). [DOI] [PMC free article] [PubMed]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E ( 2008): Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131: 559–572. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Smits‐Engelsman BCM ( 1998): Movement Assessment Battery for Children (handleiding) [Dutch Manual]. Lisse: Swets & Zeitlinger. [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Sosin DM, Sniezik JE, Thurman DJ ( 1996): Incidence of mild and moderate brain injury in the United States. Brain Inj 10: 47–54. [DOI] [PubMed] [Google Scholar]
- Soto‐Ares G, Vinchon M, Delmaire C, Abecidan E, Dhellemes P, Pruvo JP ( 2001): Cerebellar atrophy after severe traumatic head injury in children. Childs Nerv Syst 17: 263–269. [DOI] [PubMed] [Google Scholar]
- Spanos GK, Wilde EA, Bigler ED, Cleavinger HB, Fearing MA, Levin HS, Li X, Hunter JV ( 2007): Cerebellar atrophy after moderate‐to‐severe pediatric traumatic brain injury. Am J Neuroradiol 28: 537–542. [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S ( 2005): Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 128: 2562–2577. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Leemans A, D'Agostino E, De Backer S, Vandervliet E, Parizel PM, Sijbers J ( 2007): Nonrigid coregistration of diffusion tensor images using a viscous fluid model and mutual information. IEEE Trans Med Imaging 26: 1598–1612. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Sijbers J, D'Agostino E, Maes F, De Backer S, Vandervliet E, Parizel PM, Leemans A ( 2008): On the construction of an inter‐subject diffusion tensor magnetic resonance atlas of the healthy human brain. Neuroimage 43, 69–80. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS ( 2006): Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma 23: 1412–1426. [DOI] [PubMed] [Google Scholar]


