Abstract
Transcranial magnetic stimulation (TMS) can transiently modulate cortical excitability, with a net effect depending on the stimulation frequency (≤1 Hz inhibition vs. ≥5 Hz facilitation, at least for the motor cortex). This possibility has generated interest in experiments aiming to improve deficits in clinical settings, as well as deficits in the cognitive domain. The aim of the present study was to investigate the on‐line effects of low frequency (1 Hz) TMS on the EEG oscillatory activity in the healthy human brain, focusing particularly on the outcome of these modulatory effects in relation to the duration of the TMS stimulation. To this end, we used the event‐related desynchronization/synchronization (ERD/ERS) approach to determine the patterns of oscillatory activity during two consecutive trains of sham and real TMS. Each train of stimulation was delivered to the left primary motor cortex (MI) of healthy subjects over a period of 10 min, while EEG rhythms were simultaneously recorded. Results indicated that TMS induced an increase in the power of brain rhythms that was related to the period of the stimulation, i.e. the synchronization of the α band increased with the duration of the stimulation, and this increase was inversely correlated with motor‐evoked potentials (MEPs) amplitude. In conclusion, low frequency TMS over primary motor cortex induces a synchronization of the background oscillatory activity on the stimulated region. This induced modulation in brain oscillations seems to increase coherently with the duration of stimulation, suggesting that TMS effects may involve short‐term modification of the neural circuitry sustaining MEPs characteristics. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: rTMS, electroencephalography, event‐related desynchronization/synchronization, ERD/ERS, TMS/EEG coregistration, motor‐evoked potentials, MEP
INTRODUCTION
Transcranial magnetic stimulation (TMS) is an electrophysiological technique, which allows the investigation of the functional state of the human cerebral cortex (Heller and Van Hulsteyn, 1992). By means of a pulsed magnetic field created by a round or eight‐shaped coil positioned next to the scalp, electric currents are induced in the brain and these, in turn, produce transynaptic depolarization of neurons located in the superficial cortical layers (Heller and Van Hulsteyn, 1992). When delivered over the primary motor cortex (M1) with adequate intensity, magnetic stimuli induce neural efferent volleys along the corticospinal pathway and trigger electromyographic responses,—named motor‐evoked potentials (MEPs)—which can be recorded from the muscles contralateral to the site of stimulation (Barker et al., 1985). Amplitudes and latencies of MEPs are parameters, which allow the evaluation of the functional state of the corticospinal pathway, thus providing valuable information about the functioning of motor pathways in both physiological and pathological conditions (Barker et al., 1986; Rossini and Rossi, 1998). In general, motor responses induced by TMS are the result of a combination of excitatory/inhibitory events occurring at different neural levels along the motor pathway and the relative contribution of these events is far from being entirely clarified.
Technical advances in the early 1990s introduced a novel type of TMS able to deliver trains of repetitive stimuli (rTMS) opening new research directions. Since rTMS has been introduced, it has become evident that the effects of cortical stimulation may outlast the specific stimulation period, and this possibility has generated interest in experiments aiming to improve deficits in the cognitive domain (Cotelli et al., 2006) as well as in clinical applications in the field of neuropsychiatry (e.g. treatment of depression) and for treating movement disorders (George et al., 1999; Miniussi et al., 2005; Wassermann and Lisanby, 2001). Above all, the possibility of inducing long‐lasting changes in cortical excitability might explain the beneficial results obtained in depressed patients (Siebner and Rothwell, 2003), suggesting that TMS may induce modulations, or even a rearrangement, of synaptic efficiency within a given network. Nevertheless, the mechanisms underlying these changes in cortical function remain unclear.
It has been shown that several parameters, such as frequency, duration, and intensity of stimulation, influence the effects of TMS on cortical excitability. A low frequency stimulation (stimulus rates of 1 Hz or less) of the primary motor cortex is reported to lead to a transient decrease in corticospinal excitability (Chen et al., 1997), while higher frequencies (stimulus rates of more than 5 Hz) may promote a short‐term increase in cortical excitability (Berardelli et al., 1998; Di Lazzaro et al., 2002; Maeda et al., 2000; Pascual‐Leone et al., 1998; Peinemann et al., 2000).
With regard to the duration of suprathreshold TMS effects, Pascual‐Leone et al. (1994b) demonstrated a 3–4 min period of increased excitability after 10 pulses of 20 Hz rTMS. Berardelli et al. (1998) observed an increase in corticospinal excitability up to 900 ms after one train of 5 Hz rTMS and an increase in cerebral blood flow was observed at 10 min after 1 Hz stimulation to the motor cortex (Fox et al., 1997). All these studies suggest that the modulatory effects of rTMS on corticospinal excitability can vary from milliseconds to minutes, depending on frequency, stimulus intensity, intertrial interval, and duration of the rTMS. Nevertheless, stimulating the cerebral cortex has played an important role in therapeutic applications of rTMS. Therefore, the possibility to verify on line its inhibitory or facilitatory effects on bioelectrical activities of the stimulated cortex, as well as of cortical areas well outside the motor cortex, is of great interest to research and clinical application. Studying the modulations of ongoing oscillatory EEG activity by rTMS may be a key to verifying such effects. In general, voluntary movements are accompanied by a modulation in the α and β power bands, which is characterized by a decrement (event‐related desynchronization or ERD) starting about 1–3 s before the onset of a self‐paced finger or hand movement over contralateral sensorimotor areas and becoming bilateral when the movement begins; an increment (event‐related synchronization or ERS) occurring earlier for the β than for the α band can be observed after the movement execution (Derambure et al., 1993; Leocani et al., 1997; Manganotti et al., 1998; Pfurtscheller and Berghold, 1989; Pfurtscheller and Lopes da Silva, 1999; Stancak and Pfurtscheller, 1996). There is a general agreement that decreases in EEG power reflect oscillatory aspects of cortical activation (i.e. arousal) while increases of EEG power have been associated with predominantly inhibitory activities (Chen et al., 1998; Hummel et al., 2002; Pfurtscheller et al., 1996).
Even though it has been previously demonstrated that TMS can modulate the ongoing oscillatory EEG activity, only a limited number of studies have investigated this topic. Recently, Strens et al. (2002) have evaluated the effects of rTMS in the α band after a train of 1,500 low frequency (1 Hz) stimuli delivered over the primary motor cortex at a subthreshold intensity. Recordings were taken prior to, immediately after, 25 min after, and 50 min after rTMS. Power decreased by 6% during the active compared to the rest state, but there was no apparent difference between the different active periods. Moreover, changes occurred on the hemisphere ipsilateral but not in the one contralateral to the stimulation.
In a coregistration EEG‐TMS study, Paus et al. (2001) reported that single‐pulse TMS induced a highly synchronous oscillation in the β range (15–30 Hz) that lasted for several hundred milliseconds. Moreover, they observed that the probability of potentiating such rhythmicity was linked to the intensity of stimulation: the only two subjects with a minimal oscillatory response were those with the lowest stimulation intensity. Fuggetta et al. (2005) showed that the magnetic stimulation applied to M1 produced a synchronization both in α and in β rhythms, which increased linearly with TMS intensity. In addition, this effect was clearly short‐lasting because it occurred within the first 500 ms after the magnetic stimulation. The TMS‐induced oscillations observed in Paus et al. (2001) and Fuggetta et al. (2005) have been more linked to the resetting of the ongoing oscillatory activity (produced by external magnetic stimulation of the brain) than to an idling state of the brain (Pfurtscheller et al., 1996). Resetting activity might be established in cortical networks or might be driven by a common thalamic pacemaker (Destexhe et al., 1999; Steriade and Amzica, 1996).
In the present study, we investigated for the first time the immediate effects of TMS on the ongoing EEG oscillatory activity in the healthy human brain, with particular focus on the relationship of such variations to the duration of the stimulating procedure. In practice, we divided 10 min of continuous real low frequency TMS into three consecutive periods and compared the cortical response from the first block of stimulation (from 0 to 3.33 min) to the second (from 3.34 to 6.66 min) and to the third (from 6.67 to 10 min) block of stimulation, using the ERD/ERS approach to determine the patterns of oscillatory activity during these three stimulation periods.
One of the basic features of ERD/ERS measurements is that the EEG power of an interval of interest (active period) is displayed relative to (i.e. as a percentage of) the power of the same EEG leads recorded during a reference period. In this study, the power in α and β frequency bands computed in the 480 ms following a low frequency rTMS (1 Hz) was compared with two different reference periods: a standard reference period 480 ms preceding each single pulse of the magnetic stimulation (standard reference) and a sham reference that was collected 480 ms following each single pulse of sham magnetic stimulation (sham reference) collected in a 10 min session just before real TMS. The standard reference was chosen according to the common procedure of using the few seconds before the event of interest (i.e. TMS pulse) as reference period, while the sham reference was chosen to better address the modulation of cortical oscillatory activity over time as well as the eventual effect of the acoustic event represented by the stimulator's noise. In fact, if modulations of cortical oscillatory activity induced by rTMS persist over time, this effect should affect both the interval preceding and following each magnetic stimulation pulse in a train of pulses. Thus, small changes should be detected by comparing the pre‐ and post‐TMS pulse periods, but larger changes should emerge when comparing the EEG power induced by real TMS with that induced by sham TMS.
MATERIALS AND METHODS
Procedure and Subjects
Six healthy right‐handed volunteers (three males and three females, mean age 34 years) were enrolled after giving written informed consent. None had history of neurological disorder or head injury. All experimental protocols had been approved by the local Ethics Committee. Real and sham TMS was applied over the left M1 simultaneously with EEG data collection. Each subject underwent an experiment consisting of two 10‐min sessions, a sham TMS and a real TMS session respectively, separated by some minutes stimulus‐free interval to allow replacement of the coil (from sham coil used in the first session to real coil used in the second session). For each session of stimulation, a train of 600 magnetic stimuli were delivered at 110% resting motor threshold with 1 Hz repetition rate. Subjects wore ear plugs and were seated in a comfortable armchair in an electrically‐insulated and sound‐proof room with their hands pronated in a relaxed position and eyes open.
Stimulation
TMS was carried out by a Magstim SuperRapid magnetic stimulator connected to four booster modules and a standard figure‐of‐eight shaped coil with an outer winding diameter of 70 mm (Magstim Company, Whitland, UK) that generates 2.2 T as a maximum output. In the present protocol individual biphasic stimuli were employed. The coil was placed tangentially to the scalp with the handle pointing backwards and laterally at about a 45° angle away from the midline. The current flow of the initial ringing phase of the biphasic pulse in the TMS coil induces a current flowing from posterior to anterior in the underlying motor cortex. To establish the motor “hot spot” and the resting motor threshold, the coil was moved in steps of 0.5 cm in the fronto‐central region of the scalp. The optimal position (“hot spot”) was functionally defined as the point where a specific TMS pulse induced a maximum evoked motor response from the abductor pollicis brevis (APB) muscle of the right hand. At this point, to assist in the position of the TMS over the subject's head, the coil was stabilized in the same position, with respect to the site of stimulation, by means of a mechanical support that consisted of a holding arm (Magic arm Manfrotto, with two large clamps) and a heavy duty tripod. Once the coil was immobilized, the resting motor threshold was determined as the lowest stimulus intensity, which produced in the APB muscle at least five MEPs of 50 μV out of 10 consecutive stimuli (Rossini et al., 1994).
For the sham‐TMS condition, the Magstim Placebo Coil System was used. This is a device specially designed to replicate the standard figure‐of‐eight coil; it produces discharge noise without stimulating cortical tissue, since its magnetic field output is about ten‐times lower compared to that delivered by the standard coil. The experimental set‐up was therefore similar in both the sham and real TMS sessions.
EEG Recordings
TMS‐compatible EEG equipment (BrainAmp 32MRplus, BrainProducts GmbH, Munich, Germany) was used for recording TMS‐evoked potentials from the scalp. The EEG activity was continuously acquired from 19 scalp sites using electrodes mounted on an elastic cap, positioned according to the 10–20 International system. Additional electrodes were used as ground and reference. The ground electrode was placed in the midoccipital position (OZ). The left and right mastoid served as reference for all electrodes. A continuous recording mode without any sample and hold circuits was chosen. The design of new amplifiers allows appropriate selection of amplifier sensitivity and operational range that is adapted to the TMS stimulus magnitude (Bonato et al., 2006). This obviates the need to wait for the signal to recover after the TMS pulse.
The signal was digitized at a sampling rate of 2.5 kHz, using a 16 bit A/D‐Converter with 0.1 μV/bit sensitivity. Data were recorded with a band‐pass filter of 0.1–500 Hz. To minimize overheating of the electrodes located in the vicinity of the stimulating coil, magnetic field‐compatible Ag/AgCl‐coated electrodes were used. Skin/electrode impedance was measured with the dedicated BrainVision module and was confirmed to be ≤5 kΩ.
Horizontal and vertical eye movements were detected by recording the electrooculogram (EOG). The voltage difference between two electrodes located to the left and right of the external canthi recorded horizontal eye movements. The voltage difference between reference electrodes and electrodes located beneath the right eye recorded vertical eye movements and blinks.
EMG activity and MEPs from the right APB were recorded via surface electrodes in belly‐tendon montage; the signal was band‐pass filtered at 50–1,000 Hz with all the other parameters as for the EEG signal.
EEG Analysis
To characterize the cortical oscillatory activity, EEG data were analyzed offline with a commercial software (Scan 4.3, Compumedics Neuroscan). Since the first few milliseconds following the TMS pulse contained large and transient signals probably due to currents induced by the magnetic field, the EEG trace analyses began at 20 ms after magnetic stimulation. Epochs of 480 ms were obtained for the active period—from 20 to 500 ms after the real TMS—, for the standard reference—from −500 to −20 ms preceding the real TMS—, and for the sham reference—from 20 to 500 ms after the sham TMS pulse. For each type of period, the total 600 epochs were divided into three blocks of stimulation, each containing 200 trials (first: 1–200 magnetic stimuli; second: 201–400 magnetic stimuli; third: 401–600 magnetic stimuli). All the epochs were visually inspected and those with excessively noisy EEG (i.e. due to EMG contamination) or eye‐movement artifacts (blinks or saccades) were rejected from the analyses. Overall, the number of accepted epochs for each block ranged between 65 and 194. For each subject and for each epoch/sweep, the power spectra was estimated for the α (8–12 Hz) and β (12–30 Hz) frequency bands by means of the Fast Fourier transform (Hamming window; frequency resolution = 2,000 Hz). The mean band power was then obtained by averaging the power values of the sweeps for each block of stimulation. To quantify the EEG power changes induced by TMS, event‐related ERD/ERS were computed in accordance with the standard formula: [(band power in active period) − (band power in reference period)/(band power in reference period) × 100] (Pfurtscheller and Lopes da Silva, 1999). Two different ERD/ERS were computed depending on the 480 ms reference period used (standard reference, sham reference).
The ERD/ERS transformation is defined as the percentage decrease/increase of instant power density at the 'event' compared to a 'pre‐event' baseline. Therefore, event‐related power decreases (cortical activation state) are expressed as negative values, while event‐related power increases (cortical idling state) are expressed as positive values.
For each of the two frequency bands of interest (α 8–12 Hz; β 13–30 Hz), four factors were tested within subjects, using ANOVAs: reference period (standard reference vs. sham reference), stimulation block (first, second, third), region [frontal (F3, Fz, F4), central (C3, Cz, C4), parietal (P3, Pz, P4)], and side [right (F4, C4, P4), midline (Fz, Cz, Pz), left (F3, C3, P3)]. The Huynh–Feldt ε correction factor was applied where appropriate to compensate for possible effects of nonsphericity in the measurements compared. The correction factor reduces the degree of freedom of the usual F‐test; only the corrected probability values are reported. We used Statistica Data Analysis Software (Statsoft) to perform all the statistical analyses. In all conditions, the normal distribution was tested applying the Kolmogorov–Smirnov test (for all P ≫ 0.2). Post‐hoc tests were performed to investigate significant effects, by means of t tests, using the Bonferroni correction as appropriate in the case of multiple comparisons.
MEP Analysis
The MEPs recorded from the right APB were computed as the absolute amplitude between the two largest peaks of opposite polarity after 20 ms from the TMS pulse. MEPs amplitude was measured peak‐to‐peak from the initial down‐going deflection to the following up‐going one (Fig. 3). Mean MEP peak‐to‐peak amplitudes (mV) were normalized and calculated for each block of stimulation. To verify whether there was any correspondence between the modulatory effects of TMS on the amplitude of the MEPs and the modulatory effects of TMS on the event‐related synchronization, a Pearson's correlation (P < 0.05) coefficient was calculated between the changes in the MEPs and the changes in the event‐related synchronization over C3 and P3 through the three blocks of stimulation.
Figure 3.
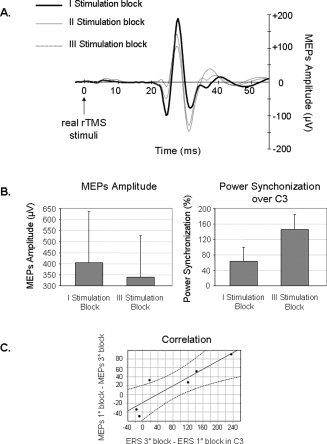
Panel A: Motor‐evoked potential recorded from the right APB; grand averaged data elicited during real TMS of the left MI in three successive stimulation blocks: the first: 1–200 magnetic stimuli (solid line), the second: 201–400 magnetic stimuli (thin line), and the third: 401–600 magnetic stimuli (dashed line). Panel B: Mean amplitude of the motor‐evoked potential (on the left) and of the power synchronization recorded over the electrode C3 (on the right) elicited during the first and the third blocks of stimulation. Bars correspond to the standard error of mean. Panel C: The scatterplot shows the significant correlation between the changes in the amplitude of the MEPs, i.e. a decrease, on the y‐axis and the changes in the power synchronization recorded over the electrode C3, i.e. an increase, on the x‐axis, between the first and the third stimulation blocks. The dotted lines represent 95% confidence limits.
RESULTS
Subjects did not report any adverse side effects during the course of the experiment. Mean motor threshold was 62%, ranging from 58 to 65%, therefore the mean stimulation intensity was 68% of the maximum output of the stimulator.
In both the frequency bands, rTMS induced a general increase in EEG power oscillations (ERS), which reached larger amplitudes in the α compared to the β band independently of the baseline.
α Band
The statistical analysis performed on the α frequency band revealed a significant effect of region [F(2, 10) = 4.40, P = 0.042]. Planned t tests proved that the synchronization over the central electrodes (mean = 52.69%; ±SD = 24.90) was larger compared to that recorded over the frontal electrodes (mean = 17.97%; ±SD = 13.02) (P = 0.042), while no difference emerged between central and parietal electrodes (P = 0.44). A significant effect of side [F(2, 10) = 10.55, P = 0.01] also emerged, indicating that the left hemisphere (ipsilateral to the TMS stimulation) showed a larger synchronization amplitude (mean = 56.48%; ±SD = 24.48) compared to the right hemisphere (mean = 21.37%; ±SD = 13.80) (P = 0.004) and to the midline (mean = 29.09%; ±SD = 16.23) (P = 0.02). There was no difference between the right side and the midline (P = 1.0). Finally, a four‐way interaction [reference period × stimulation block × region × side: F(8, 40) = 2.76, P = 0.01] showed a difference in the synchronization amplitude power between the first and the third stimulation blocks on C3 (mean = 64.62%; ±SD = 43.95 vs. mean = 146.10%; ±SD = 46.19) and P3 (mean = 26.78%; ±SD = 16.11 vs. mean = 92.79%; ±SD = 23.09) electrodes particularly when the sham TMS reference was used (Figs. 1A and 2). As a matter of fact, the post hoc analysis revealed significant differences between the two blocks directly for C3 (P < 0.001) and P3 (P < 0.001) electrodes with respect to the sham TMS reference. The same comparison using the standard reference showed a significant difference between the first and the third stimulation blocks on P3 (P = 0.048), but not on C3 (P = 1.0) electrodes. This result was indicative of an increasing modulatory effect related to the duration of the stimulation that was also partly reference‐specific.
Figure 1.
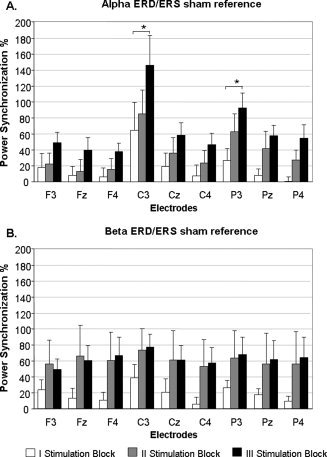
Average data of the event‐related power modulations induced by 1 Hz rTMS for the α frequency band (Panel A) and for the β frequency band (Panel B) using the sham TMS reference. The data are shown as a function of three successive stimulation blocks: first in white—from trial 1 to 200; second in gray—from trial 201 to 400; third in black—from trial 401 to 600. On the x‐axis the analyzed recording electrodes are reported. Bars correspond to the standard error of mean.
Figure 2.
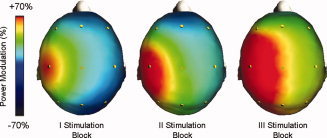
Scalp distribution maps of the average ERD/ERS induced by the real rTMS for the α frequency band, with sham as reference, represented separately for the three stimulation blocks. Red color represents maximum relative synchronization.
β Band
The statistical analysis performed on the β frequency band showed a significant two‐way interaction [region × side: F(4, 20) = 4.17, P = 0.01] indicating that the synchronization power was larger over the C3 electrode (ipsilateral to the TMS stimulation) compared to all the frontal electrodes (all P < 0.013), to the contralateral C4 (P < 0.001) and to the parietal Pz and P4 electrodes (all P < 0.005). There was a trend for this difference to be larger when the sham TMS reference was considered with respect to the standard reference [reference period × region × side: F(4, 20) = 2.72, P = 0.058] (Fig. 1B). Moreover, the main factor of reference period approached significance [F(1, 5) = 6.13, P = 0.056], revealing an interesting trend whereby real TMS induced a larger synchronization power relative to the sham TMS reference (mean = 47.01%; ±SD = 26.93) than to the standard (i.e., pre real TMS pulse) reference (mean = 8.79%; ±SD = 10.75).
MEPs
As can be seen in Figure 3A, the mean amplitude of the MEPs decreased in the second and third blocks of stimulation in comparison to the first block. Nevertheless this difference did not approach a significant value in statistical analysis (P = n.s.; first block 404.14 μV vs. third block 338.57 μV). Since a difference between the first and the third stimulation blocks emerged in the analysis of α band synchronization over C3 and P3 electrodes when the ERS was computed using the sham TMS reference (reference period × stimulation block × region × side), a correlation analysis was performed between the changes of the MEPs and of the ERS in the three intervals (α band; sham TMS reference). The decrease in the MEPs amplitude correlated with the increase of the power synchronization over the C3 electrode (Fig. 3B). As a matter of fact, a significant correlation emerged between the difference in the amplitude of the MEPs between the first and the third stimulation blocks and the difference in the power synchronization recorded over the electrode C3 between the two blocks (r = 0.88, P = 0.02) (Fig. 3C). A correlation approaching significance was also observed between the first and the second stimulation blocks (r = 0.79, P = 0.063). No correlation was found between the decrease of the MEPs and the increase of the synchronization amplitude over the P3 electrode.
DISCUSSION
The present study was designed to explore EEG power modulations induced by low frequency rTMS in the α and β frequency bands. According to previous studies (Fuggetta et al., 2005; Paus et al., 2001), a widespread synchronization of α and β activity has been observed after magnetic stimulation. In self‐paced movements, the power synchronization typically emerges after the onset of the movement and it has been linked to an idling (Pfurtscheller et al., 1996) or “nil‐working” state (Mulholland, 1995) or to an inhibitory control of neuronal activity (Hummel et al., 2002; Pfurtscheller and Andrew, 1999; Suffczynski et al., 1999), while desynchronization is present during self‐paced movement and is correlated with the activation of motor areas (Pfurtscheller, 1992). Since in the present study subjects were in a relaxed state and had no process to control, it is most likely that the synchronization observed after the cortical stimulation reflects resetting of the oscillators, as previously suggested (Fuggetta et al., 2005; Paus et al., 2001). Nevertheless, it has recently been suggested that α ERS may stem principally from rhythmic fluctuations of inhibitory neurons (Klimesch et al., 2007), and therefore it may play an active role in the inhibitory control of cortical processing as evidence against the idling hypothesis.
There was a slight difference in the power modulation patterns between α and β bands. In general, a larger synchronization was reached in the α rhythm than in the β rhythm. Furthermore, in the α band, larger amplitude was observed over the stimulated hemisphere than to the contralateral one, while in the β band only the central parietal region showed a focal difference. This result is in line with findings, which state that the effect of TMS is strongest where the induced electric field is strongest (Rothwell, 1991), in this case in the left motor area. The larger involvement of the posterior regions relative to the central stimulated area in β, and partly also in the α band, could be explained by the close connections between motor and somatosensory regions, the so called sensory‐motor area. The use of suprathreshold intensity induced muscle twitches that could modulate central processing via sensory afferents. It has been shown that increase of β rhythm, contralateral to the stimulated hand, can be found in sensorimotor areas following peripheral somatosensory stimulation (for a review, see Neuper et al., 2006); this observation would support our results, accounting for the β rhythm synchronization. However, these studies also suggest an associated desynchronization of the α rhythm (Neuper et al., 2006) and this is inconsistent with our results. Therefore, the effect due to the afferent input (muscle twitches) could account for synchronization of the β, but not of the α rhythm. α rhythm includes what is called the μ rhythm (10 Hz), that tends to be associated with motor activity, and several experiments suggest that the synchronization of the μ rhythm is associated with cortical inhibition of the motor cortex (Pfurtscheller et al., 1996). Finally, the coil position or its orientation could also account for these differences in the patterns of α and β bands (Amassian et al., 1989; Pascual‐Leone et al., 1994a; Ruohonen et al., 1996).
In contrast with our results a previous study by Strens et al. (2002) found a decrease of α power immediately after subthreshold 1 Hz rTMS. This study differs from ours since we did use an on‐line recording and the stimulation intensity was 110% of the individual motor threshold. Such differences in the experimental setting can account for different results. In fact other studies (Fuggetta et al., 2005; Paus et al., 2001) suggest that the intensity of stimulation determines the increase of power induced by TMS and that these effects are short lasting.
To characterize a possible strengthening of the effects induced by the TMS on cortical activity, we compared EEG power modulations, as well as MEPs amplitude, in three consecutive periods during a train of stimulation. An increase in the power synchronization from the first to the third block of stimulation appeared only in the α band and inversely correlated with the MEPs amplitude. This result suggests that the increasing modulatory effect of rTMS on EEG activity over the course of the stimulation may relate to the amount of energy transferred to the brain. Changes in the α band may represent activities related to source generators in motor areas as measured by movement‐related EEG signal power and coherence modulation (Gerloff et al., 1998; Leocani et al., 1997; Manganotti et al., 1998; Pfurtscheller et al., 1994; Salmelin and Hari, 1994; Toro et al., 1994). In addition, the α band has been documented to be more reactive than broad β band to movement programming and execution (Manganotti et al., 1998) and to single pulse TMS (Fuggetta et al., 2005). More significant differences were observed between the first, second and third blocks of stimulation when the real TMS condition was compared with the sham baseline than when it was compared with the pre‐TMS baseline. The TMS likely affected cortical activity over the entire session of stimulation by increasing the band power synchronization both in the active (post‐TMS pulse) and in the reference period (pre‐TMS pulse). Consequently, the ratio between them remained the same and did not reveal evident power modulations related to TMS duration. On the contrary, the sham magnetic stimulation did not produce any power alterations, therefore representing the ideal reference period. This interpretation is supported by the study of Fuggetta and colleagues (2005) in which they demonstrated that the sham condition did not produce any effect on the oscillatory EEG activity.
Several considerations should be made in relation to the use of the sham condition and possible potential confounds. The first is related to the order of the sham condition in the present experiment (i.e. sham always preceded to real stimulation) and the second is that indeed sham stimulation by itself is not an ideal control condition in the sense that it does not reproduce the “skin sensation” that one gets with real TMS. As regards the first point, the choice of this order raises the possibility that the increase in α power throughout the 10‐min session could be a result of changes in subjects' arousal during the real stimulation. Nevertheless, the choice of this experimental procedure was based on the fact that it was not possible to counterbalance the sham and real sessions since the possibility of a lasting effect after TMS may have influenced a subsequent sham recording. Moreover, the specificity of the results, that were localized and lateralized, cannot account for the explanation of a general arousal effect. In the same vein, an increase of synchronization should also have been present over the first 10 min in the sham condition, since subjects were in a relaxed state and had no process to control skin sensation or twitches induced by TMS; nevertheless, no differences were present between the first and the last block in the sham condition.
As regards the second point, a different control condition, like real TMS on occipital cortex, might have been a superior approach instead of sham. Nevertheless, to try to ensure that changes in “performance” are specifically attributable to the effects that TMS induces upon the brain, it was necessary to have a control condition free from influences of specific TMS effects, since the stimulation of other areas as control condition could have produced an unbalanced baseline condition inducing modifications of the general brain response.
The main finding of the present experiment is the correlation between the progressive decrease of MEPs amplitude and the simultaneous increase in EEG synchronization, which in itself provides additional information on plasticity in the human brain. It has been known for some time that MEPs amplitude tends to decrease progressively during recurrent TMS at a slow repetition‐rate; this phenomenon has been ascribed to self‐defending mechanisms against the impact of stimulation delivered from the outside within the intra‐cortical circuitry (Rossini et al., 1991). However this explanation is unlikely from a phylogenic point of view since there are no reasons that such a mechanism could be useful. A more probable explanation is that such modulation can be ascribed to the activation of a form of neuronal gain‐control, suggesting an active inhibitory mechanism for the control of cortical processing, congruent also with the idea that α ERS may stem principally from rhythmic fluctuations of inhibitory neurons.
Recently it has been documented that low frequency TMS (≤1 Hz) given at subthreshold (≤95% resting threshold) or suprathreshold (≥110% resting threshold) intensity produce a transient decrease in corticomotor excitability that lasts seconds to minutes (Chen et al., 1997; Maeda et al., 2000; Muellbacher et al., 2000; Touge et al., 2001). In addition, low frequency (1 Hz) rTMS over the motor cortex produced an increase in ipsilateral cortico‐cortical coherence immediately after the rTMS (Strens et al., 2002). It has been suggested that rTMS is able to excite cortical interneurones, thereby acting transynaptically on pyramidal cells. Subthreshold low frequency rTMS has also been shown to decrease regional cerebral blood flow, consistent with the idea of TMS‐induced activation of local inhibitory mechanisms (Paus et al., 1998; Speer et al., 2000). Moreover, using evoked potentials, it has been shown that 1 Hz rTMS of human primary motor cortex changes cortical excitability at the site of stimulation as well as in ipsilateral somatosensory cortex, probably via cortico‐cortical pathways between motor and sensory cortex (Enomoto et al., 2001). Thus, in agreement with previous publications, we can hypothesize that rTMS produces changes in cortical inhibitory mechanisms responsible for the development of cortical oscillations and increased connectivity (Contreras et al., 1997; Paulus et al., 1999; Rubin and Terman, 2000). The decrease in MEPs amplitude during 1 Hz TMS stimulation is consistent with the potentiation of inhibitory mechanisms related to this kind of stimulation. On the other hand, the reduction in MEPs amplitudes can be related to a reduction in synaptic efficacy under the stimulated site; in this case, there is less postsynaptic efficacy for a fixed excitatory input driven by the magnetic pulse and therefore less pre‐motor neuron (i.e. pyramidal cells) activity, resulting in a reduced motor response (Lee et al., 2003).
Although the magnitude of this effect was clear, so far we can only say that rTMS produces changes in cortical excitability at the site of stimulation as well as in correlated areas. Some data indicate that the effects of cortical stimulation may not only induce a modification of cortical excitability at the site of stimulation, but there is also the possibility of a subcortical contribution to its effects. A few studies have analyzed possible activation of subcortical areas or changes of neuroactive substances after TMS in humans, showing modulation at distant levels (Strafella et al., 2001, 2003; but see Shaul et al., 2003 in human neuroblastoma cells; Szuba et al., 2001 in thyroid hormone; Zangen and Hyodo, 2002 in neurotransmitter). Indeed, the possibility to disentangle focal from distant effects induced by TMS upon different structures of the central nervous system may have valuable implications, and combining EEG recording with TMS is a fascinating way to study these aspects.
In summary, slow rTMS over primary motor cortex induces a synchronization of α and β bands that preferentially affects the stimulated hemisphere. This power modulation seems to increase over time in relation to the duration of real stimulation and correlates with MEPs reduction, suggesting that TMS may affect the mechanisms regulating short‐term synaptic efficacy of the intracortical circuitries, inducing decrease of cortical excitability or increase of inhibition expressed as increase in cerebral synchronization. Future studies on the healthy brain during and after different motor tasks, as well as in pathophysiological conditions dealing with brain excitability, will add new information to these interesting aspects of brain neurophysiology.
Acknowledgements
The authors thank Dr Harris for useful suggestions.
REFERENCES
- Amassian VE,Cracco RQ,Maccabee PJ ( 1989): Focal stimulation of human cerebral cortex with the magnetic coil: A comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol 74: 401–416. [DOI] [PubMed] [Google Scholar]
- Barker AT,Jalinous R,Freeston IL ( 1985): Non‐invasive magnetic stimulation of human motor cortex. Lancet 1: 1106–1107. [DOI] [PubMed] [Google Scholar]
- Barker AT,Freeston IL,Jabinous R,Jarratt JA ( 1986): Clinical evaluation of conduction time measurements in central motor pathways using magnetic stimulation of human brain. Lancet 1: 1325–1326. [DOI] [PubMed] [Google Scholar]
- Berardelli A,Inghilleri M,Rothwell JC,Romeo S,Curra A,Gilio F,Modugno N,Manfredi M ( 1998): Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res 122: 79–84. [DOI] [PubMed] [Google Scholar]
- Bonato C,Miniussi C,Rossini PM ( 2006): Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co‐registration study. Clin Neurophysiol 117: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Chen R,Classen J,Gerloff C,Celnik P,Wassermann EM,Hallett M,Cohen LG ( 1997): Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology 48: 1398–1403. [DOI] [PubMed] [Google Scholar]
- Chen R,Yaseen Z,Cohen LG,Hallett M ( 1998): Time course of corticospinal excitability in reaction time and self‐paced movements. Ann Neurol 44: 317–325. [DOI] [PubMed] [Google Scholar]
- Contreras D,Destexhe A,Steriade M ( 1997): Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J Neurophysiol 78: 335–350. [DOI] [PubMed] [Google Scholar]
- Cotelli M,Manenti R,Cappa SF,Geroldi C,Zanetti O,Rossini PM,Miniussi C ( 2006): Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol 63: 1602–1604. [DOI] [PubMed] [Google Scholar]
- Derambure P,Dujardin K,Defebvre L,Bourriez JL,Jacquesson JM,Guieu JD ( 1993): [Spatiotemporal study of event‐related desynchronization during self‐paced movement]. Neurophysiol Clin 23: 337–351. [DOI] [PubMed] [Google Scholar]
- Destexhe A,Contreras D,Steriade M ( 1999): Cortically‐induced coherence of a thalamic‐generated oscillation. Neuroscience 92: 427–443. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V,Oliviero A,Mazzone P,Pilato F,Saturno E,Dileone M,Insola A,Tonali PA,Rothwell JC ( 2002): Short‐term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res 147: 108–113. [DOI] [PubMed] [Google Scholar]
- Enomoto H,Ugawa Y,Hanajima R,Yuasa K,Mochizuki H,Terao Y,Shiio Y,Furubayashi T,Iwata NK,Kanazawa I ( 2001): Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol 112: 2154–2158. [DOI] [PubMed] [Google Scholar]
- Fox P,Ingham R,George MS,Mayberg H,Ingham J,Roby J,Martin C,Jerabek P ( 1997): Imaging human intra‐cerebral connectivity by PET during TMS. Neuroreport 8: 2787–2791. [DOI] [PubMed] [Google Scholar]
- Fuggetta G,Fiaschi A,Manganotti P ( 2005): Modulation of cortical oscillatory activities induced by varying single‐pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. Neuroimage 27: 896–908. [DOI] [PubMed] [Google Scholar]
- George MS,Lisanby SH,Sackeim HA ( 1999): Transcranial magnetic stimulation: Applications in neuropsychiatry. Arch Gen Psychiatry 56: 300–311. [DOI] [PubMed] [Google Scholar]
- Gerloff C,Richard J,Hadley J,Schulman AE,Honda M,Hallett M ( 1998): Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531. [DOI] [PubMed] [Google Scholar]
- Heller L,Van Hulsteyn DB ( 1992): Brain stimulation using electromagnetic sources: Theoretical aspects. Biophys J 63: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F,Andres F,Altenmuller E,Dichgans J,Gerloff C ( 2002): Inhibitory control of acquired motor programmes in the human brain. Brain 125: 404–420. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Sauseng P,Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Lee L,Siebner HR,Rowe JB,Rizzo V,Rothwell JC,Frackowiak RS,Friston KJ ( 2003): Acute remapping within the motor system induced by low‐frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 5308–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L,Toro C,Manganotti P,Zhuang P,Hallett M ( 1997): Event‐related coherence and event‐related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self‐paced movements. Electroencephalogr Clin Neurophysiol 104: 199–206. [DOI] [PubMed] [Google Scholar]
- Maeda F,Keenan JP,Tormos JM,Topka H,Pascual‐Leone A ( 2000): Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 800–805. [DOI] [PubMed] [Google Scholar]
- Manganotti P,Gerloff C,Toro C,Katsuta H,Sadato N,Zhuang P,Leocani L,Hallett M ( 1998): Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62. [DOI] [PubMed] [Google Scholar]
- Miniussi C,Bonato C,Bignotti S,Gazzoli A,Gennarelli M,Pasqualetti P,Tura GB,Ventriglia M,Rossini PM ( 2005): Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: An efficacious therapy for major drug‐resistant depression? Clin Neurophysiol 116: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Muellbacher W,Ziemann U,Boroojerdi B,Hallett M ( 2000): Effects of low‐frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111: 1002–1007. [DOI] [PubMed] [Google Scholar]
- Mulholland T ( 1995): Human EEG, behavioral stillness and biofeedback. Int J Psychophysiol 19: 263–279. [DOI] [PubMed] [Google Scholar]
- Neuper C,Wortz M,Pfurtscheller G ( 2006): ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159: 211–222. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A,Cohen LG,Brasil‐Neto JP,Hallett M ( 1994a): Non‐invasive differentiation of motor cortical representation of hand muscles by mapping of optimal current directions. Electroencephalogr Clin Neurophysiol 93(1): 42–48. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A,Valls‐Sole J,Wassermann EM,Hallett M ( 1994b): Responses to rapid‐rate transcranial magnetic stimulation of the human motor cortex. Brain 117: 847–858. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A,Tormos JM,Keenan J,Tarazona F,Canete C,Catala MD ( 1998): Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15: 333–343. [DOI] [PubMed] [Google Scholar]
- Paulus W,Korinth S,Wischer S,Tergau F ( 1999): Differential inhibition of chromatic and achromatic perception by transcranial magnetic stimulation of the human visual cortex. Neuroreport 10: 1245–1248. [DOI] [PubMed] [Google Scholar]
- Paus T,Jech R,Thompson CJ,Comeau R,Peters T,Evans AC ( 1998): Dose‐dependent reduction of cerebral blood flow during rapid‐rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol 79: 1102–1107. [DOI] [PubMed] [Google Scholar]
- Paus T,Sipila PK,Strafella AP ( 2001): Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J Neurophysiol 86: 1983–1990. [DOI] [PubMed] [Google Scholar]
- Peinemann A,Lehner C,Mentschel C,Munchau A,Conrad B,Siebner HR ( 2000): Subthreshold 5‐Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired‐pulse inhibition. Neurosci Lett 296: 21–24. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G ( 1992): Event‐related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol 83: 62–69. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Andrew C ( 1999): Event‐Related changes of band power and coherence: Methodology and interpretation. J Clin Neurophysiol 16: 512–519. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Berghold A ( 1989): Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 72: 250–258. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Neuper C,Berger J ( 1994): Source localization using event‐related desynchronization (ERD) within the alpha band. Brain Topogr 6: 269–275. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A,Jr ,Neuper C ( 1996): Event‐related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int J Psychophysiol 24: 39–46. [DOI] [PubMed] [Google Scholar]
- Rossini PM,Rossi S ( 1998): Clinical applications of motor evoked potentials. Electroencephalogr Clin Neurophysiol 106: 180–194. [DOI] [PubMed] [Google Scholar]
- Rossini PM,Desiato MT,Lavaroni F,Caramia MD ( 1991): Brain excitability and electroencephalographic activation: Non‐invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res 567: 111–119. [DOI] [PubMed] [Google Scholar]
- Rossini PM,Barker AT,Berardelli A,Caramia MD,Caruso G,Cracco RQ,Dimitrijevic MR,Hallett M,Katayama Y,Lucking CH,Maertens de Noordhout AL,Marsden CD,Murray NMF,Rothwell JC ( 1994): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92. [DOI] [PubMed] [Google Scholar]
- Rothwell JC ( 1991): Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalogr Clin Neurophysiol Suppl 43: 29–35. [PubMed] [Google Scholar]
- Rubin J,Terman D ( 2000): Geometric analysis of population rhythms in synaptically coupled neuronal networks. Neural Comput 12: 597–645. [DOI] [PubMed] [Google Scholar]
- Ruohonen J,Panizza M,Nilsson J,Ravazzani P,Grandori F,Tognola G ( 1996): Transverse‐field activation mechanism in magnetic stimulation of peripheral nerves. Electroencephalogr Clin Neurophysiol 101: 167–174. [DOI] [PubMed] [Google Scholar]
- Salmelin R,Hari R ( 1994): Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550. [DOI] [PubMed] [Google Scholar]
- Shaul U,Ben‐Shachar D,Karry R,Klein E ( 2003): Modulation of frequency and duration of repetitive magnetic stimulation affects catecholamine levels and tyrosine hydroxylase activity in human neuroblastoma cells: Implication for the antidepressant effect of rTMS. Int J Neuropsychopharmacol 6: 233–241. [DOI] [PubMed] [Google Scholar]
- Siebner HR,Rothwell J ( 2003): Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp Brain Res 148: 1–16. [DOI] [PubMed] [Google Scholar]
- Speer AM,Kimbrell TA,Wassermann EM,J DR,Willis MW,Herscovitch P,Post RM ( 2000): Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 48: 1133–1141. [DOI] [PubMed] [Google Scholar]
- Stancak A,Jr ,Pfurtscheller G ( 1996): Event‐related desynchronisation of central beta‐rhythms during brisk and slow self‐paced finger movements of dominant and nondominant hand. Brain Res Cogn Brain Res 4: 171–183. [DOI] [PubMed] [Google Scholar]
- Steriade M,Amzica F ( 1996): Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci USA 93: 2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP,Paus T,Barrett J,Dagher A ( 2001): Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21: RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP,Paus T,Fraraccio M,Dagher A ( 2003): Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126 (Part 12): 2609–2615. [DOI] [PubMed] [Google Scholar]
- Strens LH,Oliviero A,Bloem BR,Gerschlager W,Rothwell JC,Brown P ( 2002): The effects of subthreshold 1 Hz repetitive TMS on cortico‐cortical and interhemispheric coherence. Clin Neurophysiol 113: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Suffczynski P,Pjin JP,Pfurtscheller G,Lopes da Silva F. 1999. Event‐related dynamics of alpha band rhythm: A neuronal network model of focal ERD/sorround ERS In: Pfurtscheller G,Lopes da Silva F, editors. Event‐Related Desynchronization. Handbook of Electroencephalography and Clinical Neurophysiology. Amsterdam: Elsevier; pp 67–85. [Google Scholar]
- Szuba MP,O'Reardon JP,Rai AS,Snyder‐Kastenberg J,Amsterdam JD,Gettes DR,Wassermann E,Evans DL ( 2001): Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry 50: 22–27. [DOI] [PubMed] [Google Scholar]
- Toro C,Deuschl G,Thatcher R,Sato S,Kufta C,Hallett M ( 1994): Event‐related desynchronization and movement‐related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol 93: 380–389. [DOI] [PubMed] [Google Scholar]
- Touge T,Gerschlager W,Brown P,Rothwell JC ( 2001): Are the after‐effects of low‐frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol 112: 2138–2145. [DOI] [PubMed] [Google Scholar]
- Wassermann EM,Lisanby SH ( 2001): Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clin Neurophysiol 112: 1367–1377. [DOI] [PubMed] [Google Scholar]
- Zangen A,Hyodo K ( 2002): Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 13: 2401–2405. [DOI] [PubMed] [Google Scholar]


