Abstract
Functional neuroimaging studies have increasingly aimed at approximating neural substrates of human cognitive sex differences elicited by visuospatial challenge. It has been suggested that females and males use different behaviorally relevant neurocognitive strategies. In females, greater right prefrontal cortex activation has been found in several studies. The spatiotemporal dynamics of neural events associated with these sex differences is still unclear. We studied 22 female and 22 male participants matched for age, education, and nicotine with 29‐channel‐electroencephalogram recorded under a visual selective attention paradigm, the Attention Network Test. Visual event‐related potentials (ERP) were topographically analyzed and neuroelectric sources were estimated. In absence of behavioral differences, ERP analysis revealed a novel frontal‐occipital second peak of visual N100 that was significantly increased in females relative to males. Further, in females exclusively, a corresponding central ERP component at around 220 ms was found; here, a strong correlation between stimulus salience and sex difference of the central ERP component amplitude was observed. Subsequent source analysis revealed increased cortical current densities in right rostral prefrontal (BA 10) and occipital cortex (BA 19) in female subjects. This is the first study to report on a tripartite association between sex differences in ERPs, visual stimulus salience, and right prefrontal cortex activation during attentional processing. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: sex, gender, attention, attention network test, event‐related potentials
INTRODUCTION
Certain aspects of human cognition have consistently been demonstrated to differ between sexes. The majority of studies agree on sex‐related performance differences within the domains of visuospatial as well as semantic‐verbal capabilities: in semantic‐verbal tasks, female participants tend to outperform their male counterparts [e.g., Herlitz et al., 1997; Rossell et al., 2002; Wirth et al., 2007], whereas the opposite seems to be true for visuospatial paradigms [e.g., Astur et al., 1998, 2004; Linn and Peterson, 1985; see also Kimura, 2000 for a review].
Our knowledge of the biological mechanisms that may underlie these differences is greatly aided by rodent studies [see Jonasson, 2005 for a review]. Similar to humans, sex‐related visuospatial performance differences have been described using the Morris water maze [Isgor and Sengelaub, 1998; Roof and Havens, 1992]. Lesion studies demonstrated that frontal lesions induced significantly greater disruption of spatial performance in female than in male rats [Kolb and Cioe, 1996] whereas lesions of the entorhinal cortex affected male more than female rats [Roof et al., 1993]. Thus, it appears promising to investigate human sex‐related differences in visuospatial cognition by targeting these dimorphisms in functional neuroanatomy.
In the past few years, functional neuroimaging studies have increasingly aimed at approximating the neural substrates of human cognitive sex differences elicited by visuospatial challenge. Using mental rotation paradigms, several studies found significantly greater right prefrontal cortex activation in female participants relative to their male counterparts even in the absence of significant behavioral differences [Butler et al., 2006; Hugdahl et al., 2006; Weiss et al., 2003]; this activation pattern is thought to be indicative of stronger top–down processing during visuospatial challenge in females. Using a virtual maze navigation paradigm, Riepe and coworkers have demonstrated that male participants use both hippocampi during navigation whereas women tend to use the right hippocampus only while additionally activating right prefrontal cortex [Grön et al., 2000]. While divergent cortical activation patterns have consistently been identified by these studies, the spatiotemporal dynamics of underlying cortical events has not been assessed conclusively, given the relatively coarse temporal resolution of the functional neuroimaging methods used in those studies. Yet the chronology of neural events during visuospatial processing could provide useful insights into the neurobiology of human sex differences in this cognitive domain.
A few studies using event‐related potentials (ERPs) have tackled this issue so far, but the results—usually obtained applying the well‐studied visual oddball paradigm—remain inconsistent. A higher visual N100 has been attributed to females, at least at temporal electrodes [Vaquero et al., 2004]; on the other hand, posterior N100 was found to be higher in pre‐pubertal boys than in girls [Harter et al., 1989]. Similarly, higher amplitudes of visual P300 (P3b) have been found in females [Hoffman and Polich, 1999; Orozco and Ehlers 1998; Osterhout et al., 1997] as well as in males [Oliver‐Rodriguez et al., 1999; Vaquero et al., 2004]. Thus, no “classical” ERP component has yet been identified to clearly distinguish between sexes.
Targeting the mental rotation ERP, sex differences emerged at a latency of about 100‐300ms [Desrocher et al., 1995; Gootjes et al., 2008]. This is in line with other studies reporting on sex differences at this latency, although various measures were pursued, including visual evoked potentials [Emmerson‐Hanover et al., 1994], potential fields and global field power [Skrandies et al., 1999], face recognition‐related potentials [Proverbio et al., 2006], and event‐related oscillations [Güntekin and Basar, 2007]. Despite reporting partially divergent directions of obtained results, these studies indicate where in the temporal cascade of the visual processing stream sex differences might be sought. No electrophysiological study, however, has successfully linked sex differences during visuospatial processing with differential right prefrontal cortex activation that has been consistently identified in functional neuroimaging studies.
The right prefrontal cortex is—among other functions—involved in selective spatial attention [Desimone and Duncan, 1995; Yantis and Serences, 2003]. Due to the close relationship between selective spatial attention and visuospatial cognition, we applied the Attention Network Test (ANT). This paradigm allows for assessment of attentive functions of alerting, orienting, and executive control [Fan et al., 2002] as well as examination of ERPs during selective visual attention [Neuhaus et al., 2007]. We focused our analysis on ERP components N100 and P300 which have been—albeit inconsistently—associated with sex differences during selective visual attention in previous studies; special emphasis was put on components emerging at a latency of about 100–300 ms. Source analysis was applied to allow for estimation of underlying cortical generators of differential ERP components.
MATERIALS AND METHODS
Subjects
Forty‐four healthy subjects (22 f, 22 m) were included in this study. Participants were recruited via newspaper advertisements. Female and male participants were matched for age and education years; additionally, groups were matched for nicotine consumption since smoking status has been shown to affect cognitive ERP measures [Neuhaus et al., 2006]. None of the participants had a history of substance abuse other than tobacco smoking, of psychiatric axis I disorder according to DSM‐IV [American Psychiatric Association, 1994], or of severe medical or neurological disorder. All subjects were examined by a psychiatrist and were free of pharmacological treatment.
All participants were right‐handed, reported normal or corrected‐to‐normal vision, and were of Caucasian ethnicity. Demographic and basic neuropsychological data are provided in Table I. All subjects gave written, informed consent before participating. This study was approved by the ethics committee of the University Hospital Benjamin Franklin, Charité University Medicine Berlin, Germany, and was conducted in accordance with the Declaration of Helsinki.
Table I.
Demographic and basic neuropsychological data
| Total | Female | Male | P | |
|---|---|---|---|---|
| N = 44 | N = 22 | N = 22 | — | |
| Age [years] | 30.50 ± 7.0 | 31.36 ± 8.2 | 29.64 ± 5.7 | 0.421a |
| Education [years] | 15.22 ± 2.1 | 15.11 ± 2.1 | 15.32 ± 2.2 | 0.755a |
| Nicotine [pack years] | 4.68 ± 6.3 | 3.66 ± 5.6 | 5.70 ± 7.0 | 0.289a |
| Video playing experienceb | 0/28/12/4 | 0/15/7/0 | 0/13/5/4 | 0.107c |
| LPS‐IQ | 114.75 ± 9.6 | 112.32 ± 10.1 | 117.18 ± 8.6 | 0.092a |
| MWT‐IQ | 114.57 ± 13.8 | 114.55 ± 9.8 | 114.59 ± 17.2 | 0.991a |
| DST | 63.02 ± 9.9 | 63.62 ± 10.9 | 62.43 ± 8.9 | 0.701a |
| TMT‐A | 27.00 ± 7.9 | 25.76 ± 5.8 | 28.24 ± 9.5 | 0.314a |
| TMT‐B | 54.29 ± 20.1 | 54.33 ± 22.0 | 54.23 ± 18.6 | 0.988a |
t‐test.
Factorized as: no computer experience/computer experience, but no video playing/occasional video playing (<2 h/week)/regular video playing (≥2 h/week)
χ2 test.
Abbreviations: LPS‐IQ, Leistungsprüfsystem (non‐verbal intelligence); MWT‐IQ, Mehrfachwortschatztest (verbal intelligence); DST, digit symbol test; TMT, trail making test.
Stimuli and Task
Subjects were seated in a slightly reclined chair with a head rest and viewed the 14‐inch cathode ray tube monitor from a distance of 60 cm. Behavioral responses were collected via two response keys on a keyboard resting on the subjects' lap. Visual stimuli were presented via Experimental Run Time System (ERTS; Berisoft Cooperation, Frankfurt/Main, Germany) on an IBM‐compatible personal computer running Windows 98.
A fixation cross (0.37° of visual angle) was visible in the center of the screen during the whole experiment. Cue stimuli (0.37°) appeared at 1.01° above or below the fixation cross (spatial cue), above and below the center (double cue), in the center (center cue), or were not displayed (no cue). Spatial cues always validly displayed the upcoming target's location. Target stimuli consisted of five horizontally arranged arrows or lines (3.2° for horizontal target stimulus contour) presented at 1.01° above or below the fixation cross. By left or right button press, subjects had to indicate the direction of the central arrow irrespective of flanking conditions. Flankers were either lines (neutral target condition) or arrows pointing to the same (congruent) or to the opposite direction (incongruent).
Saliency of presented stimuli was parametrized as the sum of visual angle of the respective stimulus plus vertical angle from fixation in the screen's center. Thus, visual stimuli were assigned the following values: no cue 0; center cue 0.37 (0.37° visual angle only); spatial cue 1.38 (0.37° plus 1.01° vertical angle); double cue 2.76 (2 × 1.38°); and each target 4.21 (3.2° plus 1.01°).
Each trial consisted of a variable fixation period (400–1,600 ms), an invariant cue presentation (100 ms) with subsequent fixation period (400 ms), and presentation of the target (maximum duration 1,700 ms) followed by a variable fixation period immediately after response. The duration of each trial summed up to 4,000 ms (Fig. 1). After a training block of 24 trials with full feedback, subjects had to perform three experimental blocks with a total of 288 pseudo‐randomized trials without feedback. Subjects were instructed to maintain focusing on the fixation cross throughout the experiment and to respond as fast and as accurately as possible.
Figure 1.
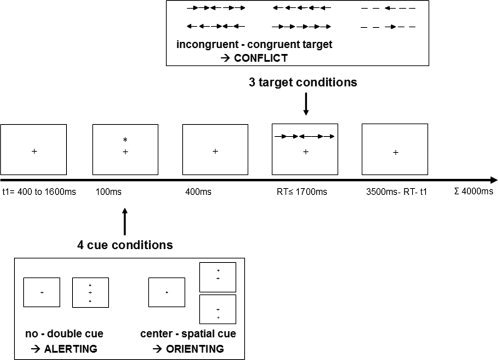
ANT design.
Attention network effects were calculated as reaction time (RT) differences of the following task conditions: alerting = RT targets (no previous cue) minus RT targets (previous double cue); orienting = RT targets (previous center cue) minus RT targets (previous spatial cue); conflict = RT incongruent targets minus RT congruent targets. Additionally, mean RT and mean accuracy were assessed. Only those trials that were correctly responded to within a time window of 100–1,000 ms were taken into further analysis.
ERP Acquisition and Analysis
EEG was recorded with 32 Ag/AgCl electrodes internally referenced to Cz using an electrode cap. The electrodes were positioned according to the International 10/20 system with the additional electrodes FC1, FC2, FC5, FC6, T1, T2, CP5, CP6, PO9, PO10, and Lo1. Electrode impedances were kept below 5 kΩ. EEG was assessed with a Neuroscan SynAmps (El Paso, TX, US) with a sampling rate of 250 Hz, gain 75,000, and an analogous 0.16 Hz high‐pass filter. EEG analysis was conducted with Brain Vision Analyzer 1.05 (Brain Products, Munich, Germany). Using EEG raw data, ocular artifact correction was performed using an independent component analysis approach [Jung et al., 2000]. Data were then re‐referenced to average reference and a digital low‐pass filter was applied at 45 Hz. After artifact rejection (≥80 μV at any electrode), data were segmented relative to stimulus onset (350 ms pre‐stimulus to 800 ms post‐stimulus) and submitted to baseline correction. At least 30 artifact‐free sweeps were averaged for each analyzed experimental condition; only correctly responded trials (100–1000 ms) were analyzed.
ERP components N100 and P300 were determined semi‐automatically with a visual control post hoc. N100 was identified at O1 and O2 as prominent negative deflections between 150 and 300 ms. N100 was then subdivided into two separate peaks: an earlier peak was identified between 150 and 210 ms and was computed for Fz, Pz, and Oz (interpolated: (O1+O2)/2); a later peak was identified between 200 and 300 ms and computed for Fz, Cz, Oz (interpolated). If no clear double peak was detectable, the largest deflection was accepted as first N100 peak if it occurred between 150 and 210 ms and as second N100 if it appeared between 210 and 300 ms. If only the first N100 peak was present, the amplitude of the second N100 peak was set at the corresponding latencies of the grand average. P300 was identified at Pz as a prominent positive deflection between 300 and 600 ms and was computed for Fz, Cz, and Pz.
Source Localization
Neuroelectric source imaging with LORETA [version 2005 March; Pascual‐Marqui et al., 1994, 1999] was used to compute the cortical distribution of electrical activity as recorded from scalp electrodes. This version of LORETA employs a three‐shell spherical head model registered to the Talairach atlas of the human brain [Talairach and Tournoux, 1988]. The solution space is restricted to the cortical grey matter and the hippocampus in the Talairach atlas, producing a total of 2,394 voxels. Without a priori assumptions on number and location of active sources, this solution to the inverse problem computes the current density at each voxel as the weighted sum of the scalp electric potentials. The unit of each voxel represents the electrical activity as squared magnitude (i.e., power [μV/Hz2]) of the computed current density [μA/mm3]. Current density maxima were regarded as spatially separate if the distance between the corresponding voxels was larger than 14 mm [Pascual‐Marqui et al., 1994].
Basic Neuropsychological Assessment
The German tests Mehrfachwortschatztest [MWT; Lehrl et al., 1995] and Leistungsprüfsystem [LPS; Horn, 1983] were employed to quantify non‐verbal and verbal intelligence, respectively. The Digit Symbol Test [DST; Wechsler, 1981] and Trail Making Tests A and B [TMT‐A/‐B; Reitan, 1959] were used to assess basic attentive and executive functions along with psychomotor function.
Statistical Analyses
Statistical calculations were carried out with SPSS for Windows 15.0 (Chicago, IL, US). Gaussian distribution of behavioral and ERP data was assessed with Kolmogorov‐Smirnov test; between‐group comparisons of demographic and behavioral data were then computed with t‐test or Pearson chi‐square. Examination of ERP data was performed with repeated measures analyses of variance entering stimuli and electrodes as within‐subject factors and sex and video playing experience as between‐subject factors. Although not significantly different between groups, video playing experience was introduced as a co‐factor since it has been shown to impact on sex differences in visuospatial cognition [Feng et al., 2007]. Video playing experience was factorized as no computer experience/computer experience, but no video playing/occasional video playing (<2 h/week)/regular video playing (≥2 h/week). Separate repeated measures analyses were computed for first N100 peak (6 cue and target stimuli × 3 electrodes: Fz, Pz, Oz), second N100 peak (6 stimuli × 3 electrodes: Fz, Cz, Oz), and P300 (3 target stimuli × 3 electrodes: Fz, Cz, Pz). Post hoc tests of significant within‐subjects factors were computed as post hoc ANOVAs. Correlation analysis between stimulus salience and mean sex differences of central ERP component amplitude was performed with Spearman rank order correlation, as only 7 separable stimulus conditions were available. Correlation analysis between ERP amplitude measures and RT was calculated with Pearson correlation.
Statistical imaging of current density differences was based on non‐parametric voxel‐by‐voxel t‐tests [Holmes et al., 1996]. This “maximum t‐statistic” is a non‐parametric analysis that offers, after a procedure of randomizations (5000 randomly created groups across conditions), a randomization distribution of the maximum statistic and will produce threshold values for single voxel Ps. This P value will be < 0.01 if the maximum of the observed statistical values is in the largest 1% of the randomization values, which is the case if it is greater than the 99th percentile of the randomization values. The time frame of interest for statistical imaging (150–300 ms) was selected on the basis of prior conventional ERP analysis.
For tests of demographic and basic neuropsychological differences, a conservative approach with P < 0.05 was chosen; for all other tests, a P < 0.01 was considered significant.
RESULTS
Demographic and Neuropsychological Data
Demographic and basic neuropsychological data of participants are shown in Table I. There were no significant differences between groups regarding distribution of age, education years, nicotine consumption, or video playing experience. Also, no significant differences were found for performance in the basic neuropsychological tests LPS, MWT, DST, and TMT.
Mean attention network effects are summarized in Table II, mean differential RTs are illustrated in Figure 2. There was no significant difference between groups for any of these behavioral measures. However, there was a statistical trend towards longer RT in female participants (P = 0.059). No significant differences between groups were found for performance accuracy.
Table II.
ANT behavioral data
| Total | Female | Male | P a | |
|---|---|---|---|---|
| Mean RT [ms] | 537.04 ± 69.5 | 556.76 ± 77.5 | 517.31 ± 55.4 | 0.059 |
| Mean accuracy [%] | 98.78 ± 1.1 | 98.90 ± 0.9 | 98.66 ± 1.3 | 0.468 |
| Alerting effect [ms] | 44.90 ± 25.9 | 43.60 ± 26.4 | 46.20 ± 25.9 | 0.742 |
| Orienting effect [ms] | 52.08 ± 22.7 | 49.77 ± 20.0 | 54.38 ± 26.4 | 0.507 |
| Conflict effect [ms] | 99.10 ± 34.1 | 104.78 ± 37.7 | 93.41 ± 29.9 | 0.274 |
t‐test.
Abbreviation: RT, reaction time.
Figure 2.
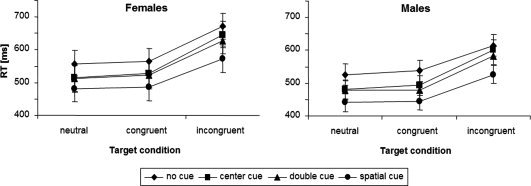
Differential reaction times of female and male participants across trials for different target and preceding cue conditions.
ERP Components: N100
Grand average ERP components at midline and occipital leads stratified by sex for cue and target conditions are illustrated in Figure 3. N100 displayed a double peak that was most prominent in female participants at electrodes Oz and Fz. Mean latencies for N100 (first peak, female vs. male) were 183.6 ms vs. 184.5 ms at Oz and 188.5 ms vs. 187.2 ms at Fz; for the second peak, mean latencies were 249.6 ms vs. 257.6 ms at Oz and 249.8 ms vs. 257.1 ms at Fz, respectively. This double peak configuration was present in 18 of 22 female and in 9 of 22 male participants as assessed by a ratio of second to first peak ≥0.3 for mean N100 across conditions. Moreover, exclusively in female subjects, a central positive component was found at a latency of 223.5 ms comparable to the second peak of N100.
Figure 3.
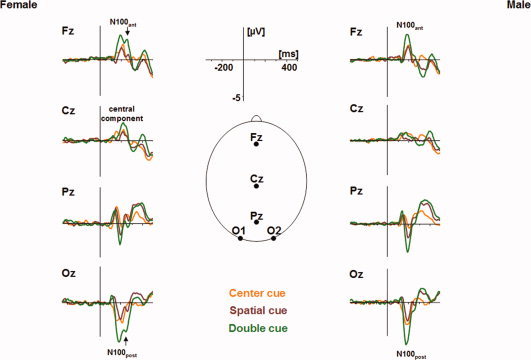
Grand average ERP components at midline and occipital leads following cue presentation stratified by sex. Oz is interpolated from O1 and O2. Note the polarity reversal of N100 over Cz as a consequence of reference system. A distinct N100 double peak is present at frontal (N100ant) and occipital (N100post) electrodes in female participants predominantly. At Cz, a positive shift at N100 latency is present in females exclusively. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For the first peak of N100, significant main effects of electrode (F = 97.396 (2, 38), P < 0.0001, partial η2 = 0.837) and of stimulus (F = 18.104 (5, 35), P < 0.0001, partial η2 = 0.720) were observed. The interaction of electrode × stimulus also yielded a significant effect (F = 15.984 (10, 30), P < 0.0001, partial η2 = 0.842). These results indicate that the first N100 peak varies according to scalp location and stimulus applied. There was, however, no significant main effect of interaction with sex on the dependent variable.
For the second peak of N100, several significant effects were observed. Similar to the first peak, significant main effects of electrode (F = 63.919 (2, 38), P < 0.0001, partial η2 = 0.771) and of interaction of electrode × stimulus (F = 5.577 (10, 30), P = 0.0001, partial η2 = 0.650) were observed. Additionally, significant main effects were observed for interactions of electrode × sex (F = 10.155 (2, 38), P = 0.0003, partial η2 = 0.348) as well as electrode × stimulus × sex (F = 3.289 (10, 30), P = 0.006, partial η2 = 0.523), thus indicating major modulating influences of sex. Post hoc analyses revealed significant sex differences of second N100 peak at all electrode sites analyzed (Fz: P = 0.001; Cz: P = 0.004; Oz: P = 0.003). When additionally stratifying for stimulus type at these electrodes, differential effects were observed, mainly pointing at significant effects of double cue and target conditions (Table III).
Table III.
Second N100 peak and central component amplitudes [μV] of visual ERP
| Total | Female | Male | P a | |
|---|---|---|---|---|
| Fzcenter cue | 1.16 ± 1.80 | 1.67 ± 1.81 | 0.58 ± 1.66 | 0.126 |
| Fzspatial cue | 1.46 ± 1.58 | 1.88 ± 1.51 | 1.04 ± 1.56 | 0.192 |
| Fzdouble cue | 2.43 ± 2.41 | 3.56 ± 2.26 | 1.30 ± 2.04 | 0.001 |
| Fzneutral target | 2.53 ± 2.02 | 3.06 ± 2.02 | 2.00 ± 1.92 | 0.006 |
| Fzcongruent target | 2.24 ± 2.10 | 2.80 ± 2.04 | 1.68 ± 2.06 | 0.021 |
| Fzincongruent target | 2.46 ± 2.15 | 2.98 ± 2.22 | 1.94 ± 1.98 | 0.006 |
| Czcenter cue | 2.52 ± 1.33 | 2.64 ± 1.34 | 2.40 ± 1.33 | 0.727 |
| Czspatial cue | 2.05 ± 1.69 | 2.25 ± 1.81 | 1.85 ± 1.58 | 0.291 |
| Czdouble cue | 3.13 ± 1.89 | 3.54 ± 1.96 | 2.73 ± 1.76 | 0.077 |
| Czneutral target | 1.52 ± 2.49 | 2.10 ± 2.15 | 0.94 ± 2.71 | 0.013 |
| Czcongruent target | 1.58 ± 2.52 | 2.34 ± 2.27 | 0.82 ± 2.58 | 0.009 |
| Czincongruent target | 1.39 ± 2.41 | 2.10 ± 2.10 | 0.70 ± 2.55 | 0.005 |
| Ozcenter cue | −1.75 ± 2.45 | −2.53 ± 2.67 | −0.67 ± 1.96 | 0.080 |
| Ozspatial cue | −1.55 ± 2.34 | −2.14 ± 2.75 | −0.97 ± 1.70 | 0.197 |
| Ozdouble cue | −3.54 ± 3.09 | −4.99 ± 3.44 | −2.10 ± 1.83 | 0.002 |
| Ozneutral target | −2.33 ± 2.00 | −2.79 ± 2.20 | −1.88 ± 1.71 | 0.042 |
| Ozcongruent target | −1.75 ± 2.10 | −2.16 ± 2.34 | −1.33 ± 1.76 | 0.109 |
| Ozincongruent target | −1.87 ± 1.94 | −2.49 ± 1.90 | −1.25 ± 1.82 | 0.016 |
Post hoc ANOVAS.
Significant differences at α = 0.01 are printed bold. Abbreviations: Fz, frontal midline electrode; Cz, central midline electrode; Oz, occipital midline electrode (interpolated).
The mean numbers of segments used for averaging were as follows (female vs. male): no cue 64.09 ± 7.2 vs. 66.09 ± 4.2; center cue 64.36 ± 6.2 vs. 67.3 ± 3.4; spatial cue 64.05 ± 7.1 vs. 67.45 ± 3.1; double cue 63.77 ± 7.7 vs. 67.27 ± 3.2; neutral target 69.82 ± 23.2 vs. 75.41 ± 21.0; congruent target 67.86 ± 25.1 vs. 76.27 ± 20.8; incongruent target 62.95 ± 21.9 vs. 69.50 ± 19.0. None of these differences were statistically significant.
ERP Components: P300
For P300, only a significant main effect of stimulus was observed (F = 22.382 (2, 38), P < 0.0001, partial η2 = 0.541), signifying P300 amplitude modulation according to preceding target type. There were also trends for interactions of stimulus × sex (F = 4.254 (2, 38), P = 0.022, partial η2 = 0.183) as well as electrode × stimulus (F = 3.169 (2, 38), P = 0.025, partial η2 = 0.260); however, no significant effects other than stimulus were detected in this model. Specifically, there were no significant main effects of interaction stimulus × electrode × sex on the dependent variable. Results of our P300 analysis are summarized in Table IV.
Table IV.
P300 peak amplitudes [μV] of visual ERP
| Total | Female | Male | P a | |
|---|---|---|---|---|
| Fzneutral target | 2.29 ± 2.08 | 2.62 ± 2.03 | 1.96 ± 2.12 | 0.195 |
| Czneutral target | 4.58 ± 2.37 | 4.28 ± 2.43 | 4.89 ± 2.33 | 0.239 |
| Pzneutral target | 4.91 ± 2.03 | 4.57 ± 2.29 | 5.26 ± 1.73 | 0.153 |
| Fzcongruent target | 2.43 ± 2.14 | 2.61 ± 2.02 | 2.24 ± 2.28 | 0.329 |
| Czcongruent target | 4.44 ± 2.50 | 4.04 ± 2.78 | 4.83 ± 2.18 | 0.138 |
| Pzcongruent target | 4.56 ± 2.00 | 4.33 ± 2.18 | 4.79 ± 1.83 | 0.278 |
| Fzincongruent target | 2.73 ± 2.21 | 2.99 ± 2.35 | 2.48 ± 2.09 | 0.289 |
| Czincongruent target | 4.61 ± 2.50 | 4.17 ± 2.49 | 5.04 ± 2.49 | 0.267 |
| Pzincongruent target | 3.67 ± 1.89 | 3.39 ± 2.06 | 3.95 ± 1.70 | 0.177 |
Post hoc ANOVAS.
Abbreviations: Fz, frontal midline electrode; Cz, central midline electrode; Pz, parietal midline electrode.
Correlation Analyses
A non‐parametric correlation analysis revealed a strong association between stimulus salience and peak differences between sexes at Cz (Spearman's ρ = 0.964; P = 0.0005; Fig. 4). To further validate this correlation, another repeated measures ANOVA was computed entering stimulus type with 2 levels (cues, targets). Again, significant main effects of electrode, electrode × sex, and electrode × stimulus (F = 9.358 (2, 38), P = 0.0005, partial η2 = 0.330) were observed; however, there was no significant main effect of electrode × stimulus × sex (F = 1.448 (2, 38), P = 0.248, partial η2 = 0.071), thus ruling out a major influence of behavioral stimulus relevance, i.e., cue vs. target, on the observed sex differences.
Figure 4.
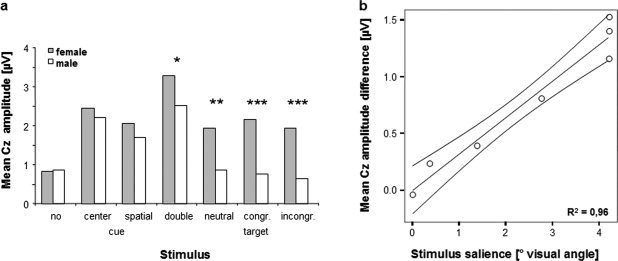
(a) Mean central ERP component amplitude differences between sexes for different stimuli; * < 0.1; ** < 0.05; *** < 0.01 (b) Stimulus salience (visual angle plus vertical angle from fixation) is plotted against mean central component amplitude difference (4 cues, 3 targets; female minus male); regression line with 95% mean prediction interval.
A parametric correlation analysis was performed between RTs and second N100 peak amplitude measures at Fz, Cz, and Oz (interpolated). There was no correlation between mean RT and mean amplitudes of the second N100 peak neither were there any significant (α = 0.01) correlations for single conditions.
Source Localization
To estimate neural sources of sex differences in visual ERP, a between‐group contrast of current densities was calculated at 170–270 ms post target stimuli, corresponding to the central component observed in female participants. t statistics showed significant current density increases in right rostral prefrontal cortex (Brodmann area (BA) 10; Talairach x, y, z: 18, 59, 15; t = 4.32) and in right occipital cortex (BA 19; x, y, z: 11, −81, 36; t = 4.22) in female compared to male participants; t‐values ≥ 3.69 were significant at a level of P = 0.01.
When computing corresponding cortical responses to cue stimuli, a comparable pattern emerged for female relative to male subjects with current density increases in right rostral prefrontal cortex (BA 10; x, y, z: 11, 59, 29; t = 5.93) and right occipital cortex (BA 19; x, y, z: 4, −74, 29; t = 5.28); additionally, increases were observed in right temporal cortex (BA 22; x, y, z: 46, −11, 1; t = 4.58) and in the precentral region (BA 6; x, y, z: 18, −11, 71; t = 4.53); t‐values ≥ 3.71 were significant at a level of p = 0.01 (Fig. 5).
Figure 5.
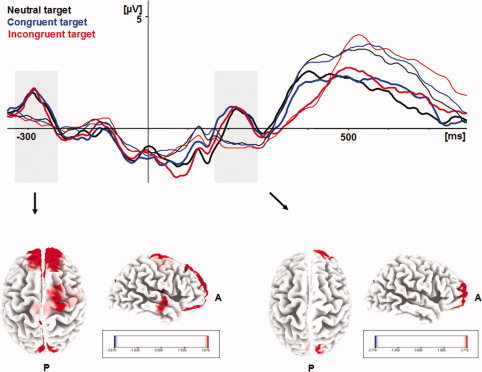
Grand average target ERP components at Cz by sex (female, bold lines; male, thin lines) and target conditions. Time frame of interest for source localization is 170–270 ms post cue (left) and post target (right), respectively. For both contrasts between sexes, significant current density increases are observed in right ventral frontal cortex and right occipital cortex in female relative to male participants. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
An additional source analysis was calculated for the P300 responses within a time frame of 300–600 ms post target stimuli, although only a statistical trend for a differential interaction of stimulus × sex was observed at the electrode level. t statistics revealed statistical trends towards higher occipital current densities in male participants for each P300 condition, i.e., neutral targets (BA 17; x, y, z: 11, −81, 8; t = 3.10, threshold t = 3.76); congruent targets (BA 18; x, y, z: 11, −88, 8; t = 3.37, threshold t = 3.81); and incongruent targets (BA 18; x, y, z: 4, −88, 22; t = 3.23, threshold t = 3.98).
DISCUSSION
The main results of this study are (1) a double peak of N100 and a corresponding prominent central ERP component at 170–270 ms exclusively in female participants, (2) a strong correlation between stimulus salience and sex difference of the central ERP component amplitude, and (3) increased current density in rostral prefrontal (BA 10) and occipital (BA 19) cortices in female participants at 170–270 ms post stimulus during attentional processing. These latencies indicate that sex differences occur at a relatively early time point within the temporal cascade of the attentional processing stream. Our results are strongly supported by previous electrophysiological studies on different functional sexual dimorphisms occurring up to 300 ms [Desrocher et al., 1995; Emmerson‐Hanover et al., 1994; Gootjes et al., 2008; Güntekin and Basar 2007; Proverbio et al., 2006; Skrandies et al., 1999]. Our findings were observed in the absence of significant behavioral differences and are unlikely to be confounded by group disparities other than sex as distribution of demographic variables including video playing experience and performance on basic neuropsychological tasks were not significantly different between groups in multivariate analysis. This is the first study to report on a tripartite association of sex differences in visual ERPs, stimulus salience, and right prefrontal cortex activation during attentional processing.
RT analysis showed a characteristic pattern of attention network effects that are comparable to the results published originally [Fan et al., 2002] and by our group [Neuhaus et al., 2007]. Attention network effects were comparable between sexes and no significant differences were found. However, there was a trend towards different mean RTs between sexes indicating a slightly slower overall performance in female participants. Speculatively, the effect size elicited by flanker‐type paradigms might be too small to reliably evoke significant differential RTs between females and males in this study. Parametric post hoc correlation analysis indicated that RT differences are independent from differences in ERP amplitudes; thus, the present results from ERP analysis do rather not reflect behavioral differences between sexes but may be interpreted in terms of more fundamental differences.
ERP components revealed the classic componentry of posterior N100‐P200 for non‐target stimuli and additional P300 for target stimuli in both sexes. With the present analysis, earlier findings on parietal P300 modulation according to target complexity and on central P300 latency increase as a function of flanker conflict effect are confirmed [Neuhaus et al., 2007]; however, only statistical trends for effects of sex were obtained for the P300 component. Likewise, source analysis revealed statistical trends towards higher occipital activation in males that correspond to higher posterior P300 relative to female participants. Perhaps these results reflect the heterogeneity of findings obtained for this ERP component so far [Hoffman and Polich, 1999; Oliver‐Rodriguez et al., 1999; Orozco and Ehlers 1998; Osterhout et al., 1997; Vaquero et al., 2004]. Hypothetically, there might by a sex difference in visual P300, but with the current design this question cannot be conclusively answered.
Sex differences were found for visual N100 that is considered an index of perceptual discrimination processes [Vogel and Luck, 2000]. In female participants only, a second N100 peak and a corresponding central component were present. N100 double peak was detectable in posterior as well as anterior leads for both cue and target conditions; polarity reversal at the frontal lead is attributable to the reference system with Cz used as internal reference during EEG recording. The finding of a double‐peaked N100 following visual stimuli has not been described yet. Studies using auditory stimulation, however, have already reported this distinct N100 morphology at frontal electrodes and suggested that the second peak may indicate the amount of attention allocated to stimulus processing [Mulert et al., 2001]. The impact of selective attention on N100 is well known as “N1‐effect” [Hillyard et al., 1973], “negative difference” [Hansen and Hillyard, 1980], or “processing negativity” [Näätänen and Picton, 1987] which refers to an augmented negativity of N100 during attentional stimulus processing. Accordingly, the second cognitive N100 component has been shown to be modulated by effort and task difficulty [Mulert et al., 2005, 2007].
In females exclusively, a central ERP component emerged at a latency that was comparable to the second N100 peak. Speculatively, this central component reflects a summation effect of higher frontal and occipital N100 activity in females as a consequence of the second N100 peak. A strong positive correlation was found between differential central ERP component amplitude as a function of sex and visual angle of presented stimuli. In other words, as visual stimulus salience increases, this central ERP component escalates in females whereas no comparable ERP modulation is observed in males. This central ERP component may thus be reflective of increasingly effortful visual stimulus discrimination in females as stimulus salience increases.
This assumption is consistent with the conceptualization of N100 as an index of visual stimulus discrimination in general [Hopf et al., 2002; Vogel and Luck, 2000]. Our hypothesis also corroborates earlier findings on the second N100 component peak as a function of effort and task difficulty in the auditory modality described by Mulert and coworkers [ 2005, 2007]. Further, the present findings seem to extend their observations to the visual modality, suggesting either comparable cognitive processes for these modalities or a fundamental cognitive mechanism operating at a supramodal level.
For the respective time frame (170–270 ms), source localization analyses consistently indicated greater cortical current density in right rostral prefrontal cortex (BA 10) and in extrastriate visual cortex (BA 19) in females. Due to the very similar frontal‐occipital pattern, this current density configuration is particularly suitable to explain the observed voltage differences on the scalp. Moreover, a recent structural neuroimaging study found that orbitofrontal gray matter is larger in females when controlling for total intracranial volume [Gur et al., 2002]. This is also consistent with the present results: larger cortical volumes can principally account for increased scalp potentials that are reflected in the topographic distribution of the second N100 peak in our data.
Functionally, the role of BA 19 can be clearly attributed to visual stimulus processing; however, the question of functional significance of BA 10 has been addressed only recently and remains a matter of debate. A recent meta‐analysis on functional neuroimaging studies reporting activation peaks in rostral prefrontal cortex described segregation between emotional and cognitive tasks along a medial–lateral axis with lateral BA 10 being more associated with behavioral guidance during cognitive tasks [Gilbert et al., 2006]. This view is further substantiated by an increasing number of studies on top–down influences in sensory processing, especially top–down modulation of visual processing by prefrontal cortex [e.g., Gazzaley et al., 2007; Kastner and Ungerleider, 2001; Sehatpour et al., 2008; see also Gilbert and Sigman, 2007 for a review]. Top–down influences dynamically set sensory cortices in a specific working mode as behaviorally required. Although former studies did not identify BA 10 as a source of prefrontal top–down control, the results obtained here suggest a considerable role of this cortical area in top–down processes, at least in females. Specifically, the prefrontal‐occipital pattern together with the correlation between scalp potentials and visual stimulus salience strongly suggests an amplification of sensory stimulus representation in BA 19 by prefrontal BA 10. It is proposed that BA 10 harbors a top–down calibration mechanism for representation and discrimination of complex visual stimuli in females. In sum, we interpret our findings as evidence for stronger top–down control in females during attentional processing, especially when stimuli are salient.
A major limitation of this study is constituted by the lack of control for hormone status in female participants. It has been shown that cognitive skills are influenced by sex hormone status across the menstrual cycle [Hampson, 1990]. Therefore, studies on cognitive sex differences are thought to require statistical control for sex hormone levels. On the other hand, our sample might be large enough to assume normal distribution of sex hormones; nevertheless, a replication study with control of sex hormone levels is needed to validate our findings. A minor limitation arises from potential lack of generalization of our results as a relatively homogenous sample in terms of age and education has been investigated. Next, our paradigm was not designed to explicitly allow for assessment of stimulus salience; thus our results warrant replication in studies employing more specific paradigms. Finally, given that processing demands for cue and target conditions are qualitatively different with respect to behavioral relevance, the integration of both stimulus types into the same correlation analysis of the central ERP component may be questionable; however, a parametric analysis indicated that stimulus type did not interact with sex differences of the central ERP component. Further, our approach might be justified by its explorative nature and may stimulate further research with more appropriate study paradigms.
In conclusion, however, we identified a distinct ERP feature that occurs during attentional processing of increasingly complex visual stimuli and that distinguishes between healthy female and male participants. ERPs were generated in right ventral prefrontal as well as right occipital cortex suggesting a top–down influence of prefrontal on occipital cortex in females. Functionally, our findings may indicate augmentation of sensory stimulus representation and discrimination by a prefrontal mechanism. Our results indicate that we detected a complex association of sex differences in visual ERPs, stimulus salience, and right prefrontal cortex activation that point to relative differences in visual attentional processing between sexes.
Acknowledgements
This work was part of the doctoral thesis of Melanie Gross. We thank all participants of this study. There are no conflicts of interest relating to this manuscript for any of the authors.
REFERENCES
- American Psychiatric Association ( 1994): Diagnostic and Statistic Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Press. [Google Scholar]
- Astur RS,Ortiz ML,Sutherland RJA ( 1998): Characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav Brain Res 93: 185–190. [DOI] [PubMed] [Google Scholar]
- Astur RS,Tropp J,Sava S,Constable RT,Markus EJ ( 2004): Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res 151: 103–115. [DOI] [PubMed] [Google Scholar]
- Butler T,Imperato‐McGinley J,Pan H,Voyer D,Cordero J,Zhu Y‐S,Stern E,Silbersweig D ( 2006): Sex differences in mental rotation: Top–down versus bottom‐up processing. Neuroimage 32: 445–456. [DOI] [PubMed] [Google Scholar]
- Desimone R,Duncan J ( 1995): Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222. [DOI] [PubMed] [Google Scholar]
- Desrocher ME,Smith ML,Taylor MJ ( 1995): Task and sex differences in performance of mental rotation: Evidence from event‐related potentials. Brain Cogn 28: 14–38. [DOI] [PubMed] [Google Scholar]
- Emmerson‐Hanover R,Shearer DE,Creel DJ,Dustman RE ( 1994): Pattern reversal evoked potentials: Gender differences and age‐related changes in amplitude and latency. Electroencephalogr Clin Neurophysiol 92: 93–101. [DOI] [PubMed] [Google Scholar]
- Fan J,McCandliss BD,Sommer T,Raz A,Posner MI ( 2002): Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14: 340–347. [DOI] [PubMed] [Google Scholar]
- Feng J,Spence I,Pratt J ( 2007): Playing an action video game reduces gender differences in spatial cognition. Psychol Sci 18: 850–855. [DOI] [PubMed] [Google Scholar]
- Gazzaley A,Rissman J,Cooney J,Rutman A,Seibert T,Clapp W,D'Esposito M ( 2007): Functional interactions between prefrontal and visual association cortex contribute to top–down modulation of visual processing. Cereb Cortex 17: i125–i135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SA,Sigman M ( 2007): Brain states: Top–down influences in sensory processing. Neuron 54: 677–696. [DOI] [PubMed] [Google Scholar]
- Gilbert SA,Spengler S,Simons JS,Frith CD,Burgess PW ( 2006): Differential functions of lateral and medial rostral prefrontal cortex (Area 10) revealed by brain–behavior associations. Cereb Cortex 16: 1783–1789. [DOI] [PubMed] [Google Scholar]
- Gootjes L,Bruggeling EC,Magnée T,Van Strien JW ( 2008): Sex differences in the latency of the late event‐related potential mental rotation effect. Neuroreport 19: 349–353. [DOI] [PubMed] [Google Scholar]
- Grön G,Wunderlich AP,Spitzer M,Tomczak R,Riepe MW ( 2000): Brain activation during human navigation: Gender‐different neural networks as substrates of performance. Nat Neurosci 3: 404–408. [DOI] [PubMed] [Google Scholar]
- Güntekin B,Basar E ( 2007): Brain oscillations are highly influenced by gender differences. Int J Psychophysiol 65: 294–299. [DOI] [PubMed] [Google Scholar]
- Gur RC,Gunning‐Dixon F,Bilker WB,Gur RE ( 2002): Sex differences in temporo‐limbic and frontal brain volumes of healthy adults. Cereb Cortex 12: 998–1003. [DOI] [PubMed] [Google Scholar]
- Hampson E ( 1990): Variations in sex‐related cognitive abilities across the menstrual cycle. Brain Cogn 14: 26–43. [DOI] [PubMed] [Google Scholar]
- Hansen JC,Hillyard SA ( 1980): Endogenous brain potentials associated with selective auditory attention. Electroencephalogr Clin Neurophysiol 49: 277–290. [DOI] [PubMed] [Google Scholar]
- Harter M,Miller S,Price N,Lalonde M,Keyes A ( 1989): Neural processes involved in directing attention. J Cogn Neurosci 1: 223–237. [DOI] [PubMed] [Google Scholar]
- Herlitz A,Nilsson LG,Backman L ( 1997): Gender differences in episodic memory. Membr Cognit 25: 801–811. [DOI] [PubMed] [Google Scholar]
- Hillyard SA,Hink RF,Schwent VL,Picton TW ( 1973): Electrical signs of selective attention in the human brain. Science 182: 177–180. [DOI] [PubMed] [Google Scholar]
- Hoffman L,Polich J ( 1999): P300, handedness, and corpus callosal size: Gender, modality, and task. Int J Psychophysiol 31: 163–174. [DOI] [PubMed] [Google Scholar]
- Holmes AP,Blair RC,Watson JD,Ford I ( 1996): Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab 16: 7–22. [DOI] [PubMed] [Google Scholar]
- Hopf JM,Vogel E,Woodman G,Heinze HJ,Luck SJ ( 2002): Localizing visual discrimination processes in time and space. J Neurophysiol 88: 2088–2095. [DOI] [PubMed] [Google Scholar]
- Horn W ( 1983): Leistungsprüfsystem—andanweisung, 2nd ed Göttingen: Hogrefe. [Google Scholar]
- Hugdahl K,Thomsen T,Ersland L ( 2006): Sex differences in visuo‐spatial processing: An fMRI study of mental rotation. Neuropsychologia 44: 1575–1583. [DOI] [PubMed] [Google Scholar]
- Isgor C,Sengelaub DR ( 1998): Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats Horm Behav 34: 183–198. [DOI] [PubMed] [Google Scholar]
- Jonasson Z ( 2005): Meta‐analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci Biobehav Rev 28: 811–825. [DOI] [PubMed] [Google Scholar]
- Jung TP,Makeig S,Westerfield M,Townsend J,Courchesne E,Sejnowski TJ ( 2000): Removal of eye activity artifacts from visual event‐related potentials in normal and clinical subjects. Clin Neurophysiol 111: 1745–1758. [DOI] [PubMed] [Google Scholar]
- Kastner S,Ungerleider SG ( 2001): The neural basis of biased competition in human visual cortex. Neuropsychologia 39: 1263–1276. [DOI] [PubMed] [Google Scholar]
- Kimura D ( 2000): Sex and Cognition. Cambridge, MA: MIT Press. [Google Scholar]
- Kolb B,Cioe J ( 1996): Sex‐related differences in cortical function after medial frontal lesions in rats. Behav Neurosci 110: 1271–1281. [DOI] [PubMed] [Google Scholar]
- Lehrl S,Triebig G,Fischer B ( 1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335–345. [DOI] [PubMed] [Google Scholar]
- Linn MC,Peterson AC ( 1985): Emergence and characterization of sex differences in spatial ability: A meta‐analysis. Child Dev 56: 1479–1498. [PubMed] [Google Scholar]
- Mulert C,Gallinat J,Pascual‐Marqui R,Dorn H,Frick K,Schlattmann P,Mientus S,Herrmann WM,Winterer G ( 2001): Reduced event‐related current density in the anterior cingulate cortex in schizophrenia. Neuroimage 13: 589–600. [DOI] [PubMed] [Google Scholar]
- Mulert C,Menzinger E,Leicht G,Pogarell O,Hegerl U ( 2005): Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56: 65–80. [DOI] [PubMed] [Google Scholar]
- Mulert C,Leicht G,Pogarell O,Mergl R,Karch S,Juckel G,Möller HJ,Hegerl U ( 2007): Auditory cortex and anterior cingulate cortex sources of the early evoked gamma‐band response: Relationship to task difficulty and mental effort. Neuropsychologia 45: 2294–2306. [DOI] [PubMed] [Google Scholar]
- Näätänen R,Picton T ( 1987): The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 24: 375–425. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH,Bajbouj M,Kienast T,Kalus P,von Haebler D,Winterer G,Gallinat J ( 2006): Persistent dysfunctional frontal lobe activation in former smokers. Psychopharmacology 186: 191–200. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH,Koehler S,Opgen‐Rhein C,Urbanek C,Hahn E,Dettling M ( 2007): Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: An event‐related potential study. J Psychiatr Res 41: 635–644. [DOI] [PubMed] [Google Scholar]
- Oliver‐Rodríguez JC,Guan Z,Johnston VS ( 1999): Gender differences in late positive components evoked by human faces. Psychophysiology 36: 176–185. [PubMed] [Google Scholar]
- Orozco S,Ehlers CL ( 1998): Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry 44: 281–289. [DOI] [PubMed] [Google Scholar]
- Osterhout L,Bersick M,McLaughlin J ( 1997): Brain potentials reflect violations of gender stereotypes. Membr Cognit 25: 273–285. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Michel CM,Lehmann D ( 1994): Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int J Psychophysiol 18: 49–65. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Lehmann D,Koenig T,Kochi K,Merlo MC,Hell D,Koukkou M ( 1999): Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic‐naive, first‐episode, productive schizophrenia. Psychiatry Res 90: 169–179. [DOI] [PubMed] [Google Scholar]
- Proverbio AM,Brignone V,Matarazzo S,Zotto MD,Zani A ( 2006): Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia 44: 2987–2999. [DOI] [PubMed] [Google Scholar]
- Reitan RM ( 1955): The relation of the trail making test to organic brain damage. J Consult Psychol 19: 393–394. [DOI] [PubMed] [Google Scholar]
- Roof RL,Havens MD ( 1992): Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res 572: 310–313. [DOI] [PubMed] [Google Scholar]
- Roof RL,Zhang Q,Glasier MM,Stein DG ( 1993): Gender‐specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res 57: 47–51. [DOI] [PubMed] [Google Scholar]
- Rossell SL,Bullmore ET,Williams SCR,David AS ( 2002): Sex differences in functional brain activation during a lexical visual field task. Brain Lang 80: 97–105. [DOI] [PubMed] [Google Scholar]
- Sehatpour P,Molholm S,Schwartz TH,Mahoney JR,Mehta AD,Javitt DC,Stanton PK,Foxe JJ ( 2008): A human intracranial study of long‐range oscillatory coherence across a frontal‐hippocampal‐occipital brain network during visual object processing. Proc Natl Acad Sci USA 105: 4399–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrandies W,Reik P,Kunze C ( 1999): Topography of evoked brain activity during mental arithmetic and language tasks: Sex differences. Neuropsychologia 37: 421–430. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Vaquero E,Cardoso MJ,Vazquez M,Gomez CM ( 2004): Gender differences in event‐related potentials during visual‐spatial processing. Int J Neurosci 114: 541–557. [DOI] [PubMed] [Google Scholar]
- Vogel EK,Luck SJ ( 2000): The visual N1 component as an index of a discrimination process. Psychophysiology 37: 190–203. [PubMed] [Google Scholar]
- Wechsler D ( 1981): Wechsler Adult Intelligence Scale‐Revised (WAIS‐R). New York, NY: Psychological Corporation. [Google Scholar]
- Weiss E,Siedentopf CM,Hofer A,Deisenhammer EA,Hoptman MJ,Kremser C,Golaszewski S,Felber S,Fleischhacker WW,Delazer M ( 2003): Sex differences in brain activation pattern during a visuospatial cognitive task: A functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett 344: 169–172. [DOI] [PubMed] [Google Scholar]
- Wirth M,Horn H,Koenig T,Stein M,Federspiel A,Meier B,Michel CM,Strik W ( 2007): Sex differences in semantic processing: Event‐related brain potentials distinguish between lower and higher order semantic analysis during word reading. Cereb Cortex 17: 1987–1997. [DOI] [PubMed] [Google Scholar]
- Yantis S,Serences JT ( 2003): Cortical mechanisms of space‐based and object‐based attentional control. Curr Opin Neurobiol 13: 187–193. [DOI] [PubMed] [Google Scholar]


