Abstract
Although preparation of voluntary movement has been extensively studied, very few human neuroimaging studies have examined preparation of an intentional reaction to a motor perturbation. This latter type of preparation is fundamental for adaptive motor capabilities in everyday life because it allows a desired motor output to be maintained despite changes in external forces. Using fMRI, we studied how the sensorimotor cortical network is implicated in preparing to react to a mechanical motor perturbation. While maintaining a given wrist angle against a small force, subjects were instructed to prepare a reaction to a subsequent wrist angle displacement. This reaction consisted of, either resisting the imposed movement, or remaining passive. During the preparation of both reactions we found an early implication of M1 and S1 but no implication at all of the higher order motor area preSMA. This is clearly different from what has been found for voluntary movement preparation. These results show that the sensorimotor network activation during preparation of voluntary motor acts depends on whether one expects a motor perturbation to occur: when external forces can interfere with ongoing motor acts, the primary sensorimotor areas must be ready to react as quickly as possible to perturbations that could prevent the goal of the ongoing motor act from being achieved. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: human, fMRI, anticipation, regions of interest, secondary motor areas, movement control
INTRODUCTION
Studying preparatory motor sets offers a privileged window for investigating the interaction between cognitive and sensorimotor functions in the brain [Evarts et al., 1984; Georgopoulos, 2000; Requin et al., 1991]. Optimal interaction with the natural environment involves preparation of voluntary motor acts as well as preparation of a reaction to possible perturbations of that motor act.1 Therefore, preparatory motor sets have been studied in two different ways. One approach consists of studying how the preparation of a voluntary movement in an environment influences sensorimotor cortex activation when there is no perturbation. In this case, mainly top‐down processes are devoted to organizing the motor command according to an a priori knowledge of the movement to come. The movement is then executed in response to an arbitrarily chosen exteroceptive (often visual) go‐signal. The cortical sensorimotor network involved in voluntary movement preparation has been extensively studied in human subjects by different means (e.g., regional blood flow with fMRI and PET [e.g., Ball et al., 1999; Cunnington et al., 2002; De Graaf et al., 2004; Lee et al., 1999; Wildgruber et al., 1997]; and the readiness potential using for instance EEG [e.g., Deecke et al., 1969; Leuthold and Jentzsch, 2001; Shibasaki and Hallet, 2006]), and is known to involve the rostral and caudal part of the supplementary motor area (preSMA and SMA proper, respectively), the ventral and dorsal premotor cortex (PMv, PMd), and, late during the preparation period, the primary motor cortex (M1), but not the primary somatosensory cortex (S1).
The second approach found in the literature for studying preparatory motor sets consists of studying neural activation related to anticipating a motor perturbation while performing some motor act [e.g., Evarts, 1973; Evarts and Tanji, 1974; Tanji and Evarts, 1976; for a recent review see Bonnard et al., 2004]. This kind of preparation involves integration of both top‐down and bottom‐up (proprioceptive) information, because the motor reaction following the mechanical perturbation is related to the somatosensory input: the stimulus initiating the limb response consists of the perturbation of that same limb [Evarts and Tanji, 1974]. This kind of preparation is, therefore, mainly based on readiness for selective use of proprioceptive input. It has, indeed, been shown that the reafferent sensory flow is processed differently according to the subject's intention about how to react to the perturbation. For instance, at the peripheral level, the amplitude of the long‐latency stretch response (LLSR) of the muscle stretched by the mechanical perturbation was modified depending on whether or not the subject was asked to resist the perturbation [e.g., Colebatch et al., 1979; Rothwell et al., 1980; Hammond, 1956].
In contrast to the brain mechanisms underlying voluntary movement preparation, those underlying preparation of a reaction to a motor perturbation are poorly understood. To investigate the involvement of the motor cortex in generating the LLSR, some human EEG experiments studied the short‐latency cortical potentials evoked by a mechanical perturbation, i.e., directly following the stretch but preceding the LLSR, as a function of the instruction of how to react [Abbruzzese et al., 1985; Crammond et al., 1986; MacKinnon et al., 2000]. In these studies, the classical “resist‐versus‐let‐go” paradigm [Bonnard et al., 1997; Feldman, 1966; Feldman and Levin, 1995; Latash, 1993] has been used in which subjects are instructed either to resist the perturbation (i.e., oppose to the imposed movement) or to let their hand go with the mechanical perturbation (i.e., be passive). Two of the studies [Abbruzzese et al., 1985; Crammond et al., 1986] reported different cortical evoked potentials when subjects reacted to the perturbation by opposing the imposed movement compared with when they remained passive. Although this latter result indeed suggests a different cortical neuronal state before the perturbation, no direct information concerning cortical activation during the preparation of the reaction (i.e., before the perturbation) was given. Only Crammond et al. [1986] shortly mentioned the existence of a readiness potential during the preparation, indeed suggesting cortical involvement in it. Some fMRI studies investigated the cerebral networks involved in the adaptation of motor responses to mechanical perturbations [e.g., Diedrichsen et al., 2005; Suminski et al., 2007]. However, they have not separated brain activity evoked by the perturbation (i.e., following it) from that during preparation of the intentional reaction to the perturbation (i.e., preceding it). So, very little information is available concerning the human brain network involved in the preparation of an intentional reaction to a mechanical limb perturbation.
The question of brain activity anticipating a motor perturbation has more often been addressed in single unit studies from nonhuman primates. Here, the standard protocol consists of pushing or pulling a cast attached to the forelimb in response to a sudden perturbation delivered via the cast [Evarts and Tanji, 1974; Tanji and Evarts, 1976; Tanji et al., 1980]. An instruction as to the direction of the monkey's movement is delivered some seconds prior to the occurrence of the perturbation. Correct performance, therefore, requires the animal to develop a preparatory state prior to the perturbation. In such experiments, anticipatory activity of primary sensorimotor cortex neurons with differential responses according to the instruction has been observed during preparation (without corresponding change of ongoing muscular activity). This suggests that by the time the perturbation occurred, the cerebral cortex was already in a different state depending on the instruction that had been given [Evarts and Tanji, 1974; Tanji and Evarts, 1976]. Indeed, the short latency motor cortex response evoked by the subsequent perturbation differed markedly depending upon the prior instruction [Evarts and Tanji, 1974]. It is, however, not clear which other cortical areas exert a modulatory influence over the sensorimotor cortex. In monkey, a possible implication of SMA has been investigated [e.g., Hummelsheim et al., 1986; Tanji et al., 1980], and the results indeed suggest that SMA plays a role in preparing to react to a motor perturbation. However, no distinction has been made between preSMA and SMA proper. Also, the involvement of the lateral premotor cortex has not been investigated, neither in monkey, nor in man.
In the present human study, we used the resist‐versus‐let‐go paradigm to study the cortical sensorimotor network involved in selective preparation of a reaction to a motor perturbation. We measured blood‐oxygen‐level‐dependent (BOLD) fMRI activity in an event‐related protocol with variable durations allowing us to isolate brain activity related to the preparation to react from other types of intervening brain activity (such as that related to nonselective attention or movement execution) with a time resolution of ∼1 s. To distinguish cerebral processes involved in “getting prepared” from those involved in “maintaining preparation,” we separately studied the first part of the preparation period during which no mechanical perturbation could occur but the subject had to prepare himself to be ready when the perturbation could occur, and the rest of the preparation period which was variable in duration and during which the subject had to maintain preparation because at any time the perturbation could occur.
Because a reaction to a perturbation is sensorimotor‐driven by proprioceptive inflow from the perturbed limb whereas voluntary movements are arbitrarily cued by an exteroceptive go‐signal, one could expect differences between the brain mechanisms underlying the preparation of a reaction to a perturbation from those observed in the preparation of a voluntary movement, particularly in the primary sensorimotor areas (M1 and S1). Moreover, given the results in the literature concerning voluntary movement preparation and the results obtained in nonhuman primates on selective anticipation of a motor perturbation, we were also interested in some specific brain areas, such as preSMA, SMA proper, PMv, and PMd. Because of the high intersubject variability for some of these anatomical areas, we defined regions of interest (ROIs) on the basis of subjects' individual brain anatomy and based the analysis on the mean BOLD signal measured in each of these ROIs.
METHODS
Subjects
Eleven normal right‐handed volunteers (3 females and 8 males, aged 24–42 years) participated in the study. They were screened for MRI compatibility during a medical visit and their right‐handedness was confirmed by systematic questioning about daily manipulations. All subjects gave written informed consent and were paid for their participation. The experiment was approved by the local ethic committee (CCPPRB Marseille 1, ref. 01/14).
Protocol and Experimental Design
During scanning, the subjects laid on the MRI bed, slightly turned on their right side with their right forearm and hand fixed in a semi‐prone position to a custom‐designed and carefully calibrated pneumatic manipulandum (see Fig. 1) at the right side of their body. The manipulandum allowed only flexion/extension movements of the wrist and was used to generate the perturbation of the wrist angle. A standard linear potentiometer was fixed on the rotation axis of the manipulandum to record the subjects' wrist angle. With the help of mirror glasses, the subjects were looking at a computer screen on which the instruction appeared.
Figure 1.
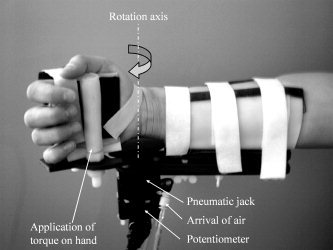
Photograph of the custom‐made pneumatically controlled manipulandum. The manipulandum is attached to the forearm and hand of the subject and controls the torque at the wrist joint. Under the wrist joint, the potentiometer and air tubes can be seen.
The trial sequence was as follows (see Fig. 2). The hand of the subject was passively positioned by the manipulandum to an initial flexed position (individually chosen for each subject in order to be comfortable and then the same throughout the whole experiment, Fig. 2A). The trial did not start until this position was reached. The subjects had to maintain this position against a very small force2 (0.5 N, in the direction of wrist extension) while waiting for the instruction to be presented on the computer screen 2–12 s later (pre‐instruction period, Fig. 2B). The instruction was a red or green filled circle presented in the middle of the computer screen for 500 ms. The red circle instructed the subject to resist the upcoming mechanical perturbation; the green circle to let the hand go during the perturbation. The perturbation was delivered after an unpredictable time period of 2–12 s following the instruction (Fig. 2C). During this preparation period the subjects could prepare their reaction to the upcoming motor perturbation according to the instruction. The pre‐instruction and preparation time windows were chosen to isolate the BOLD response related to the preparation from other intervening brain activity. Indeed, they make the pre‐instruction and preparation‐related activity temporally independent from preceding transient events (such as the turning‐on of the small torque, and the visual instruction signal). These time windows are compatible with those used in the literature for similar studies [Toni et al., 1999, 2002]. In addition, a preparation period with variable durations ensures that the subjects prepare a reaction to the perturbation and do not anticipate an action starting at the same time as (or even before) the perturbation. The perturbation, a torque generating a force of about 40.5 N at the hand in the direction of wrist extension, was applied for 150 ms. The duration of the motor response evoked by the perturbation was on average 550 ms and on the most 880 ms (Fig. 2D). All torque, including the small one, was turned off immediately after the perturbation and subjects waited passively for their hand to be returned to the initial position (Fig. 2E), allowing the subject to rest. The duration of the rest period varied between 1 and 4 s.
Figure 2.
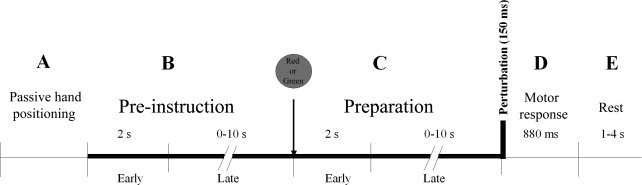
Time course of trials. (A) At the start of each trial, the subject's hand was passively returned by the manipulandum to an initial individually chosen flexed position. (B) Pre‐instruction period: The subjects had to maintain this position against a very small force (0.5 N, in the direction of wrist extension, represented by the thick bold line) while waiting for the instruction to be presented on the computer screen 2–12 s later. The instruction was a red‐ or green‐filled circle presented in the middle of the computer screen for 500 ms. The red circle instructed the subject to resist the upcoming mechanical perturbation; the green circle to let the hand go during the perturbation. (C) Preparation period: The subject prepared for the upcoming motor perturbation according to the instruction while maintaining the initial hand position against the small torque (thick bold line). The perturbation was then delivered 2–12 s following the instruction. It consisted of a torque generating a force of about 40.5 N at the hand in the direction of wrist extension applied for 150 ms. The torque was completely turned off immediately after the perturbation (end of thick bold line). (D) Motor response evoked by the perturbation with a duration of maximum 880 ms. (E) Rest period (1–4 s) during which the subjects waited passively for their hand to be returned to the initial position. Early pre‐instruction and early preparation represent the first 2 s of these respective periods; late pre‐instruction and late preparation represent the remaining part of the periods.
Subjects performed 128 trials, 64 trials with the instruction to resist and 64 trials with the instruction to let the hand go. Trials were pseudo‐randomized before the experiment according to the type of instruction and the duration of each period of the trial (pre‐instruction, preparation, rest) and distributed among four sessions in such a way that the mean duration of the pre‐instruction and preparation periods were the same within each session. The duration of each session was ∼9 min (because a trial did not start as long as the hand did not reach the initial position, the total duration of the sessions could not be precisely determined beforehand).
Prior to the fMRI experiment subjects underwent a training session. Subjects sat in a comfortable slightly upright position to see a computer screen, with their forearm horizontal as it would be during scanning. Surface electromyographical (EMG) measures of a subset of relevant wrist muscles [flexor carpi radialis (FCR) and extensor carpi radialis (ECR)], were used to train the subjects to keep a constant EMG level during pre‐instruction and preparation periods throughout the whole experiment. In particular, they learned to anticipate the mechanical perturbation without co‐contracting the antagonistic muscles. This latter strategy, which would indeed be efficient for resisting the perturbation [Abbs and Gracco, 1984], had to be avoided to ensure that eventual BOLD signal increase in sensorimotor areas during the preparation period relative to the pre‐instruction period, or during the preparation to resist relative to preparation to let‐go, could not be related to higher muscle force production. At the end of the training session, for each of the recorded muscles and both instructions, we calculated the mean rectified EMG level during the preparation period. A repeated measures ANOVA for the within‐subjects factors of muscle (FCR vs. ECR) and type of instruction (resist vs. let‐go) showed that, indeed, the EMG level was similar for the two types of instructions (main effect of instruction: F(1,10) = 0.31, P > 0.05).
Data Acquisition
Behavioral data
The hand movements were recorded by sampling the signal from the potentiometer at a frequency of 100 Hz by a Labview program (LabVIEW 6.1). This signal was analyzed in real time to control the experiment (initial position), and saved on a hard disk for later off‐line analysis together with the time points of the main tasks events.
fMRI data
Imaging was performed using the 3T whole‐body imager MEDSPEC 30/80 AVANCE (Brüker) in the Marseille fMRI research centre. A quadrature head coil was used for excitation and reception. For all participants, the experiment began with the acquisition of high‐resolution structural T1‐weighted images (MPRAGE sequence; axial volume; TE 5 ms, TR 25 ms; voxel size 1 × 0.75 × 1.22 mm3; total acquisition time of 15 min). The functional images were acquired using a standard T2*‐weighted echo‐planar sequence (TE 35 ms; TR 1.66 s; 20 axial slices; thickness 3 mm; inter‐slice gap 1 mm; interleaved acquisition; matrix 64 × 64; voxel size 3 × 3 mm). The slices covered the whole upper part of the brain and were parallel to the Anterior Commissure–Posterior Commissure (AC–PC) line. A total of 340 volumes was acquired for each session, taking 9 min and 24 s.
To allow a characterization of the hemodynamic evoked response at a finer temporal resolution than the actual TR [Josephs et al., 1997], the time course of trials was not synchronized with the MRI acquisition. Therefore, to temporally relate the different experimental events with the recorded BOLD signal, the slice number of the functional MRI acquisition was saved along with the time instants of the other experimental variables (hand position, stimulus presentation, and other events).
Analysis
Behavioral data
For each trial, the potentiometer signal was visually inspected to check whether the subject performed the task correctly; this required maintaining the hand in a steady‐state position during the pre‐instruction and preparation periods and to let‐go or resist as instructed directly following the perturbation onset. A visual inspection was sufficient because a clear distinction in the angular displacement of the wrist at the end of the motor response between the two types of trials could be seen, and the trajectory was highly repeatable for each type of trial (see Fig. 5). The trials in which the subjects did not perform correctly were processed differently (see further below). To determine the mean wrist displacement for the correct trials, we calculated the difference between the wrist angle at the start of the trial and the maximum wrist angle obtained after perturbation onset.
Figure 5.
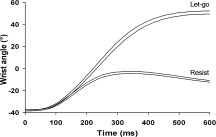
Wrist angle (interval of the mean ± SE), measured with the potentiometer, as a function of time and for the two instructions, from one subject. The motor perturbation was delivered at time 0. Note the small interval for each of the two types of instructions, as well as the clear difference between them.
We also ensured that the subjects produced a similar level of muscle co‐contraction in the resist trials as in the let‐go trials during the preparation period. This analysis was based on the fact that a higher level of co‐contraction in the agonist and antagonist muscles would result in an increased stiffness at the wrist. The stiffness of an effector system can be derived from its reactions to an external perturbing force [Hogan, 1984; Houk and Rymer, 1981]. Indeed, an increased stiffness would in turn increase the resistance of the hand to the applied perturbation, and consequently, decrease the speed of the hand movement in the very early part of the perturbation. Therefore, we compared the wrist displacement during the first part of the motor response where the LLSR component did not yet influence the trajectory, that is, the first 90 ms following the perturbation onset [e.g., MacKinnon et al., 2000], for the let‐go and resist trials. A similar wrist displacement indicates that a similar level of muscle co‐contraction was produced before the occurrence of the perturbation.
fMRI data
Statistical parametric mapping software (spm99) was used for image processing and analysis (http://www.fil.ion.ucl.ac.uk/spm/). Figure 3 shows a flow diagram of the image processing and statistical analysis. The functional images were interpolated in time to correct phase advance during volume acquisition (slice timing), and then realigned to the first image of each session. The motion parameters were later taken as covariates of noninterest regressors in the statistical analysis (to avoid an influence of a possible correlation between head motion and the time course of the trials on the results). The anatomical reference images and the realigned functional images of all subjects were transformed to a common standard stereotactic MNI space in the following way. For each subject, the normalization parameters were calculated for the anatomical image with the help of a standard MNI T1 template, resulting in a normalized anatomical image with a voxel size of 1 × 1 × 1 mm3. The normalization parameters were then applied to the functional images of the subject, giving normalized functional images with a voxel size of 3 × 3 × 3 mm3. The functional data were then temporally filtered using a 120 ms period high‐pass filter and a Gaussian low‐pass filter with a 4 s of full width at half maximum (FWHM).
Figure 3.
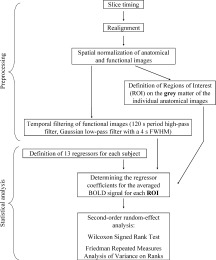
Flow diagram of the pre‐processing and the statistical analysis of the fMRI images. For each of the 11 subjects, after slice timing and realignment of the images, the anatomical and functional images were normalized. Then, the functional images were temporally filtered. ROIs were defined on the gray matter of the individual anatomical images (see Fig. 4). Then, for each subject, the regressors were defined and the regressor coefficients calculated with Marsbar. Finally, a nonparametric second‐order random‐effect analysis was applied to find significant group differences between the regressors of interest for each ROI.
For each subject, 12 regions of ROIs were manually defined on the gray matter using anatomical landmarks from the individual normalized MRI data. These ROIs, represented in Figure 4, are considered to be functionally homogeneous areas. We identified the segment of the central sulcus with a characteristic knob shape on axial slices [Bonnard et al., 2007; Yousry et al., 1997]. This segment, known as the “hand area,” is considered to be a reliable anatomical landmark for the localization of the functional projection area of the hand in the central sulcus [Boling et al., 1999; Rumeau et al., 1994; Sastre‐Janer et al., 1998; Yousry et al., 1997]. Within this hand area, we defined two ROIs along the central sulcus. Firstly, for both left and right hemispheres, the hand area of M1 (M1HaL and M1HaR, respectively) on the anterior bank of the central sulcus, consistent with the anatomical data from Picard and Strick [2001]. Secondly, the hand area of S1 (S1HaL and S1HaR) between the posterior bank of the central sulcus and the border of the post central sulcus (Fig. 4A). Brodmann's area (BA) 6 is usually subdivided into a medial part (supplementary motor area or SMA, Fig. 4B) and a dorsolateral one (premotor area, Fig. 4A). Although the exact border between these regions is not clear (because SMA can slightly overlap the superior margin of the hemisphere [Rizzolatti et al., 1996]), this superior margin provides a very distinct landmark that we used to define the superior border of SMA. Inferiorly, SMA is bounded by the cingulate sulcus [Roland and Zilles, 1996]. There is agreement that the vertical planes intersecting the anterior commissure (VCA) and the posterior commissure (VCP) constitute approximately the anterior and posterior borders of SMA proper or SMAp (SMApL and SMApR) [Geyer et al., 2000]. At the anterior edge of SMA proper, we defined preSMA (PreSMAL and PreSMAR). The anterior border of preSMA was taken as the vertical plane parallel and 15 mm anterior to the VCA plane. Such a border approximately coincides with the anterior border of BA6 on the medial surface of the hemisphere given in the Talairach and Tournoux atlas [Talairach and Tournoux, 1988], and is very close to the border taken by Crespo‐Facorro et al. [1999] in the commonly used frontal parcellation scheme. The premotor cortex (PM), within precentral gyrus was subdivided into a ventral part (PMvL, PMvR) and a dorsal part (PMdL, PMdR). The anterior border corresponded to the fundus of the precentral sulcus. The dorsal border of PMd corresponded to the superior border of SMAp. The exact border between PMd and PMv is not known in human brain [Picard and Strick, 2001; Rizzolatti et al., 2002]. Therefore, the border between PMd and PMv was precisely aligned with the dorsal border of M1HaL and M1HaR, such that the so‐called hand area of PM [Picard and Strick, 2001] was included in PMv. The ventral border of PMv was aligned with the most ventral part of the central sulcus. Note that neither BA 44 nor pre‐PMd [Rizzolatti et al., 2002] were included in our definition of PM.
Figure 4.
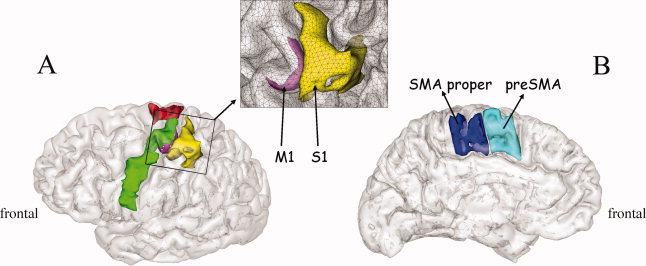
ROIs superimposed on the gray matter of the reconstructed brain from one subject. (A) Lateral view on which can be seen S1Ha (yellow), M1Ha (magenta), PMd (red), PMv (green). Note that the ventral part of M1 in the depth of the Rolandic fissure is not visible. (B) Medial view with SMA proper (blue) and preSMA (turquoise). Inset: Detailed view of M1Ha and S1Ha superimposed on the triangular mesh of the gray/white matter interface. For description of the anatomical delimitations of the different ROIs see the Methods section.
For each subject, a general linear model was applied to the time course of the signal averaged over all voxels for each ROI (see Fig. 3). Each regressor was formed by a box‐car convolved with a canonical hemodynamic response function. In the present study, we were particularly interested in the preparation period before the mechanical perturbation. For the analysis of the fMRI data, we divided the preparation period in two parts. The early preparation period was the first 2 s of the preparation period during which no mechanical perturbation could occur but the subject had to get prepared for the upcoming perturbation. The late preparation period was the rest of the preparation period, which was variable in duration, during which the subject had to maintain preparation because at any time the perturbation could occur (Fig. 2C). This distinction was made to distinguish cerebral processes involved in preparation from those involved in maintaining the prepared state. For technical reasons we made the same subdivision for the pre‐instruction period (Fig. 2B). Statistical analysis with spm99 is based on comparison of the regression coefficients. One needs, therefore, to have regressors with comparable amplitudes. Because events with variable durations give regressors with highly variable amplitudes, we had to model the phase during which the subjects waited for the instruction with regressors of the same length as the preparation phases to be able to have valid contrasts between these two experimental phases.
Besides the above‐mentioned six motion parameter regressors, data were analyzed by modeling a total of 13 experimental events: the first 2 s of the pre‐instruction period (EarlyPreInstr), the rest of the pre‐instruction period (2 s up to appearance of instruction; LatePreInstr), the first 2 s of the preparation period to resist (EarlyPrepRES) or to let‐go the hand (EarlyPrepLG), the late preparation periods (2 s up to the perturbation; LatePrepRES, LatePrepLG), the motor response to the perturbation when the instruction had been to let‐go or to resist, the rest period, and the return period. Finally, for every incorrect trial we added three regressors (early preparation, late preparation, motor response). So, the statistical model took into account all (known) cognitive and motor components of the protocol whether they were of interest or not for the present study. By doing this, the regressors explained much of the variance due to cognitive and motor processes. Therefore, the residues are independent and identically distributed in time and space, and the estimation of the regressor coefficients of interest is improved.
The following contrasts were of particular interest for the present study. Firstly, to study early preparation‐related BOLD activity, we contrasted the BOLD signal obtained during the early preparation period with that obtained during the early pre‐instruction period [i.e., (EarlyPrepLG + EarlyPrepRes) − 2*EarlyPreInstr, hereafter termed EarlyPrep − EarlyPreInstr]. Then, to study how the prepared state is maintained, the same contrast was calculated for the rest of the (variable) duration of the pre‐instruction and preparation periods [i.e., (LatePrepLG + LatePrepRes)‐2*LatePreInstr, hereafter termed LatePrep‐LatePreInstr]. Finally, to elucidate eventual differences in BOLD signal between the two types of preparation, we contrasted EarlyPrepLG with EarlyPrepRes and LatePrepRes with LatePrepLG.
We used the freely available SPM toolbox Marsbar (http://www.marsbar.sourceforge.net) [Brett et al., 2002] to perform the region‐of‐interest analysis. The analysis is basically the same as that conducted in SPM99, but is based on the BOLD signal averaged over all voxels contained within the ROI of the non‐smoothed functional images. For each ROI and each contrast, we performed a Wilcoxon signed rank test on the difference between the regressor coefficients to decide whether an ROI was involved in early and/or late preparation and whether a difference between the two types of preparation existed. Then, to take into account the intersubject homogeneity with respect to preparation‐related activity found for the ROIs, for each contrast and each hemisphere, a Friedman Repeated Measures Analysis of Variance on Ranks was performed on the difference between the regressor coefficients.
RESULTS
Behavioral Data
The initial wrist angle ranged from 25 to 55° of flexion over all subjects (mean ± SD: 42° ± 7.3°) and was consistent within subjects (indeed, a trial did not start before the hand had reached the initial self‐chosen position). Motor responses to the perturbation were clearly differentiated between the two types of instructions, with a small movement amplitude for the resist trials and a large movement amplitude for the let‐go trials (see Fig. 5). It was therefore easy to identify error trials (i.e., let‐go their hand whereas instructed to resist or vice versa) by visual inspection. The mean percentage of correct trials was 83% (53 trials) for resist and 78% (50 trials) for let‐go. For correct trials, the mean wrist movement amplitude was 93° ± 7.4° (mean ± SD) following the let‐go instruction and 28° ± 6.1° following the resist instruction (Student's t test, t(10) = 20.9, p < 0.001). To show the stability of the movement amplitudes within each type of trial (resist and let‐go), we grand averaged the individual standard errors (SE). The mean standard error was 1.1° for the let‐go trials and 0.9° for the resist trials. Figure 5 shows an example of the responses for the two types of trials for a typical subject. The, indeed, high reproducibility of the trajectories can be seen from the width of the curves representing the interval between the mean ± SE.
To verify that the subjects had not modified their level of co‐contraction in the preparation period, we analyzed the wrist displacement obtained after the first 90 ms following the perturbation onset. The results show that for all subjects the hand displacements for the first 90 ms (mean ± SE: 3.0° ± 0.1°) were similar for the resist and let‐go conditions (p > 0.05), implying that the subjects had the same level of global wrist stiffness, and hence the same global muscle co‐activation level, for the two types of preparation.
fMRI Data
For each separate ROI, we performed a Wilcoxon signed rank test across subjects on the individual differences in regressor coefficients between the preparation period and the pre‐instruction period. Table I gives the results for the two main contrasts (EarlyPrep‐EarlyPreInstr and LatePrep‐LatePreInstr). It can be seen that some areas showed preparation‐related activity throughout the preparation period (such as M1HaL, S1HaL, SMApL), others were activated only at the start (SMApR and PMdR, bilateral PMv), and some areas did not show any preparation‐related activity (such as M1HaR, SMApR, PMdR, and bilateral preSMA).
Table I.
Results of the Wilcoxon signed rank test (second order group level) of the individual differences in beta‐values found with Marsbar for all ROIs and the two main contrasts
| EarlyPrep‐EarlyPreInstr | LatePrep‐LatePreInstr | |||||
|---|---|---|---|---|---|---|
| Median | Quartile deviation | z value | Median | Quartile deviation | z value | |
| S1HaL | 0.0933 | 0.0478 | 2.9333** | 0.0094 | 0.0042 | 2.6667** |
| S1HaR | 0.0535 | 0.0433 | 1.6889* | −0.0058 | 0.0089 | 0.7111 ns |
| M1HaL | 0.0591 | 0.0386 | 2.7556** | 0.0101 | 0.0057 | 2.8444** |
| M1HaR | −0.0024 | 0.0328 | 0 ns | −1.0064 | 0.0105 | 1.0667 ns |
| SMApL | 0.1234 | 0.0671 | 2.2222* | 0.0169 | 0.0115 | 2.4889** |
| SMApR | 0.0458 | 0.0485 | 1.5111 ns | 0.0093 | 0.009 | 1.5111 ns |
| PMvL | 0.0720 | 0.0535 | 2.3111* | 0.0075 | 0.0101 | 1.5111 ns |
| PMvR | 0.0533 | 0.0543 | 1.8667* | 0.0024 | 0.0054 | 0.5333 ns |
| PMdL | 0.0295 | 0.0351 | 1.5111 ns | 0.0083 | 0.0079 | 2.6667** |
| PMdR | 0.0336 | 0.0421 | 1.6 ns | 0.0014 | 0.0052 | 0.4444 ns |
| PreSMAL | 0.0329 | 0.042 | 1.0667 ns | −0.0075 | 0.0128 | 0.3556 ns |
| PreSMAR | 0.0043 | 0.0546 | 1.1556 ns | 0.0016 | 0.006 | 0.7111 ns |
For the anatomical description of ROIs see the text and Figure 4. L, left; R, right.
P < 0.01;
P < 0.05;
ns = nonsignificant.
Because the Wilcoxon signed rank test was carried out for each ROI separately, it is difficult to directly compare ROIs (indeed, it might be that, for a given ROI, only some of the subjects show high preparation‐related activity, whereas others show it for another ROI). Therefore, to take into account the intersubject homogeneity with respect to preparation‐related activity found for the ROIs (which will make inter‐ROI comparisons valid), for each hemisphere and for the two main contrasts, we performed a Friedman Repeated Measures Analysis of Variance on Ranks (11 subjects × 6 ROI for each hemisphere) on the individual differences in regressor coefficients between the preparation period and the pre‐instruction period. First, concerning the contrast EarlyPrep‐EarlyPreInstr, the left‐sided ROIs showed differences in median values greater than would be expected by chance (Chi‐square = 28.299, df = 5, P < 0.001). The post hoc comparisons (Tukey‐like test) showed significant differences (p < 0.05) between S1HaL and PreSMAL, between S1HaL and PMdL, between SMApL and PMdL, and, of particular interest for the present study, between SMApL and PreSMAL. For the right‐sided ROIs, no significant difference in median values was found (Chi‐square = 5.078, df = 5, p = 0.406). So, although for the early preparation period we found a significant increase in BOLD signal for some individual right‐sided ROIs (i.e., PMvR and S1HaR), they were not significantly different from the other right‐sided ROIs. Concerning the contrast LatePrep‐LatePreInstr, the left‐sided ROIs, again, showed differences in median values greater than would be expected by chance (Chi‐square = 14.896 df = 5, p = 0.011). The post hoc Tukey‐like test, however, only showed a significant difference between SMApL and preSMAL (p < 0.05). For the right‐sided ROIs, although none of these ROIs individually showed a significant increase in BOLD signal during the late preparation phase (see Table I), we found a significant difference in median values (Chi‐square = 14.532 df = 5, p = 0.013). Post hoc testing revealed differences between SMApR and S1HaR and between SMApR and M1HaR (p < 0.05), which is not surprising given the global decrease in BOLD signal for S1HaR and M1HaR and the (nonsignificant) increase for SMApR during the late preparation phase (see median values in Table I).
Finally, the direct contrast between the two types of preparation (resist and let‐go) did not reveal any significant difference in BOLD signal.
DISCUSSION
The main goal of the present work was to investigate the sensorimotor cortical network involved in the preparation of a reaction to a motor perturbation (sensorimotor‐driven by proprioceptive input), and to compare the results to what is known from the literature concerning preparation of voluntary movement (arbitrarily cued by exteroceptive go‐signals). Globally, in contrast to voluntary movement preparation, the results show a significant activation of the contralateral hand area of M1 and S1 throughout the preparation period but no activation of the higher order motor area preSMA.
Before discussing these results in detail, we would like to emphasize some important methodological points. Firstly, the present experiment was designed to ensure that the observed changes in BOLD signal were specifically related to the preparation of an intentional reaction to an upcoming perturbation. In our analysis, the preparation period was always contrasted with the pre‐instruction period, not with a “rest” condition. A similar level of global attention was required during these two periods of the task in which the subject was waiting either for the instruction cue (pre‐instruction period) or for the perturbation (preparation period). Therefore, although some attention differences between the pre‐instruction and the preparation period do remain (due to the fact that the subjects were waiting for different types of events), the difference in the level of activity between these two periods revealed by our contrasts is unlikely to reflect a general state of alertness or attention.
Secondly, we found a similar wrist displacement at 90 ms after perturbation onset for both types of instructions, showing similar stiffness at the wrist joint. These results indicate that during the preparation period the subjects had produced similar levels of EMG co‐contraction following the two instructions. Very importantly, this observation was corroborated by the lack of BOLD level difference between the two types of preparation. Consequently, the changes in the BOLD signal revealed by our contrasts can be interpreted as modulation of the cortical activity specifically related to the preparation to react to a perturbation, and cannot be attributed to differences in on‐going muscle force production.
Implication of the Primary Sensorimotor Areas in Anticipating a Motor Perturbation
The present results show that both M1HaL and S1HaL are active throughout the entire preparation period prior to the perturbation. For two reasons, the combined activation of these adjacent areas is unlikely to be due to the spreading of the BOLD signal from one area into the other due to the smoothing procedure or averaging across subjects. Firstly, for the ROI analysis we did not spatially smooth the images. Secondly, we defined the ROI for both areas on the basis of the individual anatomy of each subject before combining the results for each ROI over the whole group. Therefore, although some spreading cannot be completely excluded, it is likely that the significant BOLD signal over the hand area of the sensorimotor cortex reflects activation of both M1 and S1 during the preparation period.
Concerning M1, our results are in line with those obtained from perturbation experiments in monkeys rather than voluntary preparation studies in humans. Using the push–pull protocol, it has been demonstrated that single units recorded in M1 from macaque monkeys show changes in activity as early as 200 ms following an instruction to either push or pull a lever in response to a perturbation [Evarts and Tanji, 1974], whereas in several fMRI studies in humans investigating the preparation of voluntary movements, activation of M1 is either not significant [Lee et al., 1999; Toni et al., 1999] or occurs late during the preparation period [Ball et al., 1999; Cunnington et al., 2003; Wildgruber et al., 1997]. MEG/EEG and intracranial electrical recording studies also showed activity in M1 just before movement onset [Ball et al., 1999; Rektor et al., 1994; Shibasaki and Ikeda, 1996; Toro et al., 1993]. So, it appears that M1 is differently involved during the preparation of a reaction to a motor perturbation compared to the preparation of voluntary movement.
Regarding S1, there is strong evidence that the activation seen here reflects anticipatory tuning of S1 to receive proprioceptive input from the perturbed limb. In a combined EEG/fMRI study on voluntary movement, Ball et al. [1999] did not find any activity in this area before self‐paced movement onset, nor did Cunnington et al. [2002] in an event‐related fMRI study using an auditory go‐signal. In another voluntary movement reaction time study, in which the instruction (serving as the go‐signal at the same time) was either auditory, visual, or tactile, the primary sensory cortex corresponding to the modality of the signal has been found to be activated [Weeks et al., 2001]. Although this latter study did not allow isolation of the preparation period from other task‐related activity, the authors suggested that this primary sensory cortex activation might be related to higher attention for the cue. This might explain part of our S1 activation during the preparation period. However, in the present study, the go‐signal is not an arbitrary somatosensory signal; the information triggering the muscle contraction consists of the proprioceptive inflow caused by perturbation of the very same muscle that is involved in the ongoing motor task. In other words, the perturbation gives the trigger for action via the muscle that must react, and is thus directly entering the sensorimotor loop. This is never the case in arbitrarily cued voluntary movement preparation. Tanji and Evarts [1976], using the push–pull protocol in monkeys, found selective anticipatory tuning of neurons in S1 during preparation for a motor perturbation. This strongly suggests that the significant increase in S1 BOLD signal during the preparation period is not only due to directed attention to the somatosensory inflow, but also reflects the selective anticipatory tuning of neural circuits in S1 to receive the reaction‐relevant upcoming information.
Activation of M1 and S1 may be required for preparing a motor act in an environment with nonstable external forces, allowing one to react as soon as possible whenever a correct execution of the motor act is in danger following an unexpected modification of external forces (such as wind, or somebody bumping against us). At the peripheral level, it has been shown that the reafferent sensory flow following the perturbation is processed differently according to the subject's intention about how to react to a perturbation. Indeed, the amplitude of the LLSR of the muscle stretched by the mechanical perturbation is modified as a function of the instruction to be active or passive relative to the perturbation [e.g., Colebatch et al., 1979; Hammond, 1956; Rothwell et al., 1980]. Studies in monkeys [Strick and Preston, 1982; Tanji and Wise, 1981; Wiesendanger and Miles, 1982] and humans [Balzamo et al., 2004; Geyer et al., 1996; Moore et al., 2000] have demonstrated direct tactile and proprioceptive input to the primary motor cortex. Therefore, the highly significant increase of BOLD signal in M1 and S1 during early preparation in the present study, is consistent with the idea of a presetting of primary motor and somatosensory cortex neurons to be able to react quickly and accurately to the proprioceptive inflow caused by the external perturbation of the ongoing motor act.
Implication of the Higher Order Motor Areas in Anticipating a Motor Perturbation
Our results show that contralateral SMA proper is implicated from the beginning of the preparation phase. This is in line with results obtained with single‐cell recordings in monkey SMA, showing a very early instruction‐induced modulation in the discharge of SMA neurons during the period between the instruction and the mechanical perturbation [Tanji and Taniguchi, 1978; Tanji et al., 1980]. Moreover, SMA activity seems to affect the LLSR. Hummelsheim et al. [1986] reported that, in monkeys, microstimulation of the SMA could decrease the response of motor cortical cells to peripheral afferent input, suggesting that the SMA could, indeed, exert a modulatory influence on the size of the LLSR. It has also been reported that a patient with an infarction in right SMA showed abnormalities in the long‐latency stretch reflex evoked by a mechanical perturbation in the arm contralateral to the lesion [Dick et al., 1987]. So, these studies argue in favor of an early involvement for SMA in modulating responses of the sensorimotor cortex to sensory inputs. This hypothesis is further reinforced by our data showing combined activation of contralateral SMAp, S1 and M1 from the early preparation period onwards.
During the preparation of a voluntary movement, SMAp seems to be activated later during the preparation period. For instance, Lee et al. [1999], in a reaction time fMRI study in which an instruction indicated which kind of voluntary movement was to be produced 6 s later, found SMAp to be activated about 2 s after cue presentation. This is in contrast to our findings, but in their experiment the go‐signal never arrived earlier then 6 s after the instruction, whereas here the go‐signal (i.e., the perturbation) could potentially arrive 2 s after the instruction. So, the differential timing of SMAp activation in the preparation of voluntary movements and in the preparation to a motor perturbation could be due to differences in the experimental protocols or could reflect a distinct functional implication of this area in these tasks.
We clearly found an implication of PM in the preparation for a mechanical perturbation. The ROI analysis revealed early PMv and late PMd preparation‐related activity. The pattern of PMv and PMd activation presented here differs from the pattern of activation observed during the preparation of a voluntary movement. Indeed, for voluntary movement, contralateral PMd is usually involved early in the preparation phase [Churchland et al., 2006; Mathews et al., 2006]. It has been suggested that PMd is involved in the preparation of an action, whereas PMv seems to be implicated in the specification of the target to be reached [e.g., Hoshi and Tanji, 2002, 2004]. However, in most of the studies on the role of PM in voluntary movement preparation, the task of the subject is to prepare a movement toward a visually perceived spatial target and then to execute it in response to a go‐signal. In the present study, although we do have a visual stimulus, it does not contain any relevant spatial information for the execution of the task, whereas our “go‐signal” (the muscle stretch) is highly relevant for the execution of the task. So, our results seem to emphasize the functional difference between preparing to act and preparing to react.
Finally, we found early bilateral PMv activity (Table I), but the post hoc analysis did not show any significant difference between the right‐sided ROIs. It remains to be seen, therefore, whether ipsilateral PMv activity can be confirmed. However, the lack of significant early preparation‐related PMd activity was confirmed with the post hoc analysis. Indeed, left PMd behaved significantly differently from the other left‐sided significantly active ROIs. To the best of our knowledge, the present study is the first to report an implication of lateral premotor cortex in the preparation of an intentional reaction to a mechanical perturbation.
In the present study we did not find a preparation‐related BOLD signal increase in preSMA, not even at the start of the preparation phase. Indeed, the ROI analysis showed significant differences between SMAp (found to be active during preparation) and preSMA activity, both for the early and late preparation periods.
Neurophysiological data showed that preparatory activity related to voluntary movement was more frequent in preSMA than in SMAp of monkey brains [Matsuzaka et al., 1992]. Lee et al. [1999], in their fMRI study on voluntary movement preparation, found clear bilateral preSMA BOLD signal increase immediately after the presentation of the cue. Also, Sakai et al. [2000] suggested a role of preSMA in response selection for voluntary movement. So, our results for preSMA clearly differ from those obtained for voluntary movement preparation. It appears that preSMA is not specifically involved in the preparation of a reaction to a motor perturbation. This is supported by neurophysiological finding from Matsuzaka et al. [1992] showing that, although preSMA is responsive to visual input, it does not appear to be responsive to somatosensory inputs.
It makes sense that we did not find preSMA to be similarly implicated in the preparation of a reaction to a possible perturbation. Indeed, once the goal of a motor act is determined and its execution has started, the network has to be ready to handle eventual deviations of the ongoing motor act caused by external forces, information that is not entering via a higher order motor area such as preSMA but rather via primary sensorimotor structures.
Same Sensorimotor Network for Prepare to Resist and Prepare to Let‐Go
We observed a very significant percentage of BOLD signal change in M1HaL and S1HaL during the preparation period with respect to the pre‐instruction period, but no difference between the two types of preparation. This observation is intriguing because the behavioral responses for the two types of instruction were clearly differentiated and well‐adapted to the instruction. In their push–pull protocol, Evarts and Tanji [1974] and Tanji and Evarts [1976] detected anticipatory activity of neurons in the monkey primary motor and somatosensory cortex which was differentiated according to the instruction, some of them increasing their discharge and some of them decreasing it. The lack of significant difference between the two types of preparation in our fMRI data might indicate that they involve populations of neurons that are equally distributed within the network and thus cannot be distinguished with fMRI. However, firstly, it has to be noted that there is no simple relation between unitary neuron discharge and global BOLD signal, in particular when some neurons increase and others decrease their discharge rate, neural processes that both influence BOLD signal. Secondly, the difference in the behavioral response between the two types of instruction could also be the consequence of a presetting of structures downstream from the primary sensorimotor cortex.
CONCLUSION
To prepare to act and to prepare to react to motor perturbations are both fundamental aspects of motor control underlying the adaptive capabilities of motor function, and in daily life they are constantly and intimately intermingled. In this study, we investigated the sensorimotor network involved in the preparation of a reaction to a motor perturbation. We found an early activation of M1 and S1 during the preparation of the reaction to a perturbation, but no activation of the higher order motor area preSMA, which is clearly different from the sensorimotor cortical network involved in preparing an action. Firstly, this confirms that the primary sensorimotor cortex functions as an integrative centre for both top‐down cognitive information and bottom‐up sensory information and not as a simple executing relay. Secondly, these results suggest that the activation of the sensorimotor network during the preparation of a voluntary motor act is highly dependent on whether one expects a motor perturbation to occur: when external forces can interfere with ongoing motor acts, the primary sensorimotor areas must be ready to react as quickly as possible to perturbations that could prevent the goal of the ongoing motor act from being achieved.
Acknowledgements
The authors gratefully thank Prof. J.M. Viton for assuming the medical responsibility for the experiment and T. Brochier for his useful comments on the manuscript.
Footnotes
Well‐known examples of motor perturbations are those caused by external forces such as wind, people bumping‐in to you, or sideways accelerations of a car while driving.
The rational for having the subjects maintain the tonic 0.5 N force prior to the perturbation is that when a muscle is slightly active, the information inflow from the muscle following the mechanical perturbation is more important and, thus, the perturbation is better perceived. This makes it easier for the subject to prepare the required reaction.
REFERENCES
- Abbruzzese G,Berardelli A,Rothwell JC,Day BL,Marsden CD ( 1985): Cerebral potentials and electromyographic responses evoked by stretch of wrist muscles in man. Exp Brain Res 58: 544–551. [DOI] [PubMed] [Google Scholar]
- Abbs JH,Gracco VLI ( 1984): Control of complex motor gestures: Orofacial muscle responses to load perturbations of lip during speech. J Neurophysiol 51: 705–723. [DOI] [PubMed] [Google Scholar]
- Ball T,Schreiber A,Feige B,Wagner M,Lücking CH,Kristeva‐Feige R ( 1999): The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. Neuroimage 10: 682–694. [DOI] [PubMed] [Google Scholar]
- Balzamo E,Marquis P,Chauvel P,Régis J ( 2004): Short‐latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre‐ and postcentral areas. Clin Neurophysiol 115: 1616–1623. [DOI] [PubMed] [Google Scholar]
- Boling W,Olivier A,Bittar RG,Reutens D ( 1999): Localization of hand motor activation in Broca's pli de passage moyen. J Neurosurg 91: 903–910. [DOI] [PubMed] [Google Scholar]
- Bonnard M,Pailhous J,Danion F ( 1997): Intentional on‐line adaptation of rhythmic movements during a hyper‐ to microgravity change. Motor Control 1: 247–262. [Google Scholar]
- Bonnard M,De Graaf JB,Pailhous J ( 2004): Interactions between cognitive and sensorimotor functions in the motor cortex: Evidence from the preparatory motor sets anticipating a perturbation. Rev Neurosci 15: 371–382. [DOI] [PubMed] [Google Scholar]
- Bonnard M,Galléa C,De Graaf JB,Pailhous J ( 2007): Corticospinal control of the thumb‐index grip depends on precision of force control: A transcranial magnetic stimulation and functional magnetic resonance imagery study in humans. Eur J Neurosci 25: 872–880. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton J‐L,Valabregue R,Poline J‐B ( 2002): Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan.
- Churchland MM,Yu BM,Ryu SI,Santhanam G,Shenoy KV ( 2006): Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci 26: 3697–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG,Gandevia SC,McCloskey DI,Potter EK ( 1979): Subject instruction and long‐latency reflex responses to muscle stretch. J Physiol Lond 292: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crammond DJ,MacKay WA,Mruphy JT ( 1986): Evoked potentials from passive elbow movements. II. Modification by motor intent. Electroencephal Clin Neurophysiol 64: 144–158. [DOI] [PubMed] [Google Scholar]
- Crespo‐Facorro B,Kim J‐J,Andreasen NC,O'Leary DS,Wiser AK,Bailey JM,Harris G,Magnotta VA ( 1999): Human frontal cortex: An MRI‐based parcellation method. Neuroimage 10: 500–519. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Windischberger C,Deecke L,Moser E ( 2002): The preparation and execution of self‐initiated and externally‐triggered movement: A study of event‐related fMRI. Neuroimage 15: 373–385. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Windischberger C,Deecke L,Moser E( 2003): The preparation and readiness for voluntary movement: a high‐field event‐related fMRI study of the Bereitschafts‐BOLD response. Neuroimage 20: 404–412. [DOI] [PubMed] [Google Scholar]
- Deecke L,Scheid P,Kornhumer HH ( 1969): Distribution of readiness potential, pre‐motion positivity and motor potential of the human cerebral cortex preceding voluntary finger movement. Exp Brain Res 7: 158–168. [DOI] [PubMed] [Google Scholar]
- De Graaf JB,Galléa C,Pailhous J,Anton J‐L,Roth M,Bonnard M ( 2004): Awareness of muscle force during movement production: An fMRI study. NeuroImage 21: 1357–1367. [DOI] [PubMed] [Google Scholar]
- Dick JP,Rothwell JC,Day BL,Wise RJ,Benecke R,Marsden CD ( 1987): Modulation of the long‐latency reflex to stretch by the supplementary motor area in humans. Neurosci Lett 75: 349–54. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J,Hashambhoy Y,Rane T,Shadmehr R ( 2005): Neural correlates of reach errors. J Neurosci 25: 9919–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV ( 1973): Motor cortex reflexes associated with learned movement. Science 179: 501–503. [DOI] [PubMed] [Google Scholar]
- Evarts EV,Tanji J ( 1974): Gating of motor cortex reflexes by prior instruction. Brain Res 71: 479–494. [DOI] [PubMed] [Google Scholar]
- Evarts EV,Shinoda Y,Wise SP. ( 1984). Neurophysiological approaches to higher brain functions. NewYork: Wiley. [Google Scholar]
- Feldman AG ( 1966): Functional tuning of the nervous system with control of movement or maintenance of a steady posture. II. Controllable parameters of the muscle Biofizika 11: 565–578. [Google Scholar]
- Feldman AG,Levin MF ( 1995): The origin and use of positional frames of reference in motor control. Behav Brain Sci 18: 723–806. [Google Scholar]
- Georgopoulos AP ( 2000): Neural aspects of cognitive motor control. Curr Opin Neurobiol 10: 238–241. [DOI] [PubMed] [Google Scholar]
- Geyer S,Ledberg A,Schleicher A,Kinomura S,Schormann T,Burgel U,Klinberg T,Larsson J,Zilles K,Roland PE( 1996): Two different areas within the primary motor cortex of man. Nature 382: 805–807. [DOI] [PubMed] [Google Scholar]
- Geyer S,Matelli M,Luppino G,Zilles K ( 2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol 202: 443–474. [DOI] [PubMed] [Google Scholar]
- Hammond PH ( 1956): The influence of prior instruction to the subject on an apparently involuntary neuromuscular response. J Physiol Lond 132: 17P–18P. [PubMed] [Google Scholar]
- Hogan N ( 1984): Adaptive control of mechanical impedance by coactivation of antagonistic muscle. IEEE Trans Automat Control 29: 681–690. [Google Scholar]
- Hoshi E,Tanji J ( 2002): Contrasting neuronal activity in the dorsal and ventral premotor areas during preparation to reach. J Neurophysiol 87: 1123–1128. [DOI] [PubMed] [Google Scholar]
- Hoshi E,Tanji J ( 2004): Functional specialization in dorsal and ventral premotor areas. Prog Brain Res 143: 507–511. [DOI] [PubMed] [Google Scholar]
- Houk JC,Rymer WZ ( 1981): Neural control of muscle length and tension In: Brooks V,editor.Handbook of Physiology, Section 1 Vol. 2 Bethesda, MD: American Physiological Society; pp 257–323. [Google Scholar]
- Hummelsheim H,Wiesenganger M,Bianchetti M ( 1986): The supplementary motor area modulates perturbation‐evoked discharges of neurons in the precentral motor cortex. Neurosci Lett 67: 119–122. [DOI] [PubMed] [Google Scholar]
- Josephs O,Turner R,Friston KJ ( 1997): Event‐related fMRI. Hum Brain Mapping 5: 243–248. [DOI] [PubMed] [Google Scholar]
- Latash ML. ( 1993): Control of Human Movement. Champaign, IL: Human Kinetic. [Google Scholar]
- Lee K‐M,Chang K‐H,Roh J‐K ( 1999): Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 9: 117–123. [DOI] [PubMed] [Google Scholar]
- Leuthold H,Jentzsch I ( 2001): Neural correlates of advance movement preparation: A dipole source analysis approach. Cogn Brain Res 12: 207–224. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD,Verrier MC,Tatton WG ( 2000): Motor cortical potentials precede long‐latency EMG activity evoked by imposed displacement of the human wrist. Exp Brain Res 131: 477–490. [DOI] [PubMed] [Google Scholar]
- Mathews S,Ainsley Dean PJ,Sterr A( 2006): EEG dipole analysis of motor‐priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol 117: 2675–2683. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y,Aizawa H,Tanji J ( 1992): A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: Neuronal activity during a learned motor task. J Neurophysiol 68: 653–662. [DOI] [PubMed] [Google Scholar]
- Moore CI,Stern CE,Corkin S,Fischl B,Gray AC,Rosen BR,Dale AM ( 2000): Segregation of somatosensory activation in the human Rolandic cortex using fMRI. J Neurophysiol 84: 558–569. [DOI] [PubMed] [Google Scholar]
- Picard N,Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Rektor I,Feve A,Buser P,Bathien N,Lamarche M ( 1994): Intracerebral recording of movement related readiness potentials: An exploration in epileptic patients. Electroencephalogr Clin Neurophysiol 90: 273–283. [DOI] [PubMed] [Google Scholar]
- Requin J,Brener J,Ring C ( 1991): Preparation for action In: Jennings JR,Coles MG, editors. Handbook of Cognitive Psychophysiology: Central and autonomic nervous system approaches. New York: Wiley; pp 357–448. [Google Scholar]
- Rizzolatti G,Luppino G,Matelli M ( 1996): The classic supplementary motor area is formed by two independent areas In: Luders HO,editor.Advances in Neurology: Supplementary Sensorimotor Area, Vol. 70 Philadelphia, PA: Lippincott Raven; pp 45–56. [PubMed] [Google Scholar]
- Rizzolatti G,Fogassi L,Gallese V ( 2002): Motor and cognitive functions in the ventral premotor cortex. Curr Opin Neurobiol 12: 149–154. [DOI] [PubMed] [Google Scholar]
- Roland PE,Zilles K ( 1996): Functions and structures of the motor cortices in humans. Curr Opin Neurobiol 6: 773–781. [DOI] [PubMed] [Google Scholar]
- Rothwell JC,Traub MM,Marsden CD ( 1980): Influence of voluntary intent on the human long‐latency stretch reflex. Nature 286: 496–499. [DOI] [PubMed] [Google Scholar]
- Rumeau C,Tzourio N,Murayama N,Peretti‐Viton P,Levrier O,Joliot M,Mazoyer B,Salamon G ( 1994): Location of hand function in the sensorimotor cortex: MR and functional correlation. Am J Neuroradiol 15: 567–572. [PMC free article] [PubMed] [Google Scholar]
- Sakai K,Hikosaka O,Takino R,Miyauchi S,Nielsen M,Tamada T ( 2000): What and when: Parallel and convergent processing in motor control. J Neurosci 20: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre‐Janer FA,Regis J,Belin P,Mangin J‐F,Dormont D,Masure M‐C,Remy P,Froulin V,Samson Y ( 1998): Three‐dimensional reconstruction of the human central sulcus reveals a morphological correlate of the hand area. Cereb Cortex 8: 641–647. [DOI] [PubMed] [Google Scholar]
- Shibasaki H,Hallet M ( 2006): What is the Bereitschaftspotential? Clin Neurophysiol 117: 2341–2356. [DOI] [PubMed] [Google Scholar]
- Shibasaki H,Ikeda A ( 1996): Generation of movement‐related potentials in the supplementary sensorimotor area. Adv Neurol 70: 117–125. [PubMed] [Google Scholar]
- Strick PL,Preston JB ( 1982): Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol 48: 150–159. [DOI] [PubMed] [Google Scholar]
- Suminski AJ,Rao SM,Mosier KM,Scheidt RA ( 2007): Neural and electromyographic correlates of wrist posture control. J Neurophysiol 97: 1527–1545. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P. ( 1988): Co‐planar stereotaxic atlas of the human brain. 3‐dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical. [Google Scholar]
- Tanji J,Evarts EV ( 1976): Anticipatory activity in motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 39: 1062–1068. [DOI] [PubMed] [Google Scholar]
- Tanji J,Taniguchi K ( 1978): Does the supplementary motor area play a part in modifying motor cortex reflexes? J Physiol Paris 74: 317–318. [PubMed] [Google Scholar]
- Tanji J,Wise SP ( 1981): Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 45: 467–481. [DOI] [PubMed] [Google Scholar]
- Tanji J,Taniguchi K,Saga T ( 1980): Supplementary motor area: Neuronal response to motor instruction. J Neurophysiol 43: 60–68. [DOI] [PubMed] [Google Scholar]
- Toni I,Schluter ND,Josephs O,Friston K,Passingham RE ( 1999): Signal‐, set‐ and movement‐related activity in the human brain: An event‐related fMRI study. Cereb Cortex 9: 35–49. [DOI] [PubMed] [Google Scholar]
- Toni I,Shah NJ,Fink GR,Thoenissen D,Passingham RE,Zilles K ( 2002): Multiple movement representations in the human brain: An event‐related fMRI study. J Cogn Neurosci 14: 769–784. [DOI] [PubMed] [Google Scholar]
- Toro C,Matsumoto J,Deuschl G,Roth BJ,Hallett M ( 1993): Source analysis of scalp‐recorded movement‐related electrical potentials. Electroencephalogr Clin Neurophysiol 86: 167–175. [DOI] [PubMed] [Google Scholar]
- Weeks RA,Honda M,Catalan M‐J,Hallett M ( 2001): Comparison of auditory, somatosensory, and visually instructed and internally generated finger movements: A PET study. Neuroimage 14: 219–230. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M,Miles TS ( 1982): Ascending pathway of low‐threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiol Rev 62: 1234–1270. [DOI] [PubMed] [Google Scholar]
- Wildgruber D,Erb M,Klose U,Grodd W ( 1997): Sequential activation of supplementary motor area and primary motor cortex during self‐paced finger movement in human evaluated by functional MRI. Neurosci Lett 227: 161–164. [DOI] [PubMed] [Google Scholar]
- Yousry TA,Schmid UD,Alkadhi H,Schmidt D,Peraud A,Buettner A,Winkler P ( 1997): Localization of the motor hand area to a knob on the precentral gyrus. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]


