Abstract
Objective:
To apply and validate the use of electromyogram (EMG) recorded during functional magnetic resonance imaging (fMRI) in patients with movement disorders, to directly relate involuntary movements to brain activity.
Methods:
Eight “familial cortical myoclonic tremor with epilepsy” (FCMTE) patients, with tremor‐like cortical myoclonus and cerebellar Purkinje cell degeneration, and nine healthy controls performed hand posture and movement in an on/off fashion (block design). Superfluous movements were quantified as deviations in EMG power, positive and negative, with respect to the average EMG per session. This measure, “residual EMG” (r‐EMG), was derived by Gram–Schmidt orthogonalization. Activation maps resulting from conventional block regressors and novel r‐EMG regressors were compared.
Results:
In healthy participants, the block posture regressor identified mainly cerebellar activity and some activity in other areas belonging to motor circuitry. In FCMTE patients, no cerebellar activity was seen with the block posture regressor, compatible with cerebellar Purkinje cell changes in FCMTE. EMG power showed little variation during posture in healthy controls. Therefore, the r‐EMG regressor was almost constant and revealed no brain activity as expected. In contrast, in FCMTE patients the r‐EMG posture regressor was highly variable due to continuous myoclonic jerks. It identified sensorimotor cortical areas, compatible with cortical hyperexcitability in FCMTE patients.
Conclusion:
Conventional block regressors can be used to identify neuronal circuitry associated with a specific motor task, whereas r‐EMG regressors can help identify brain activation directly related to involuntary movements. Simultaneous EMG‐fMRI is complementary to conventional fMRI and will facilitate studies of hyperkinetic movement disorders. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: electromyogram, functional MRI, EMG‐fMRI, motor paradigm, cortical tremor, movement disorder, epilepsy, cerebellum
INTRODUCTION
The origin of hyperkinetic movements like tremor, myoclonus, dystonia, and tics is not fully understood. In this article, it will be argued that a measure derived from electromyogram (EMG), recorded during functional magnetic resonance imaging (fMRI), can be used to directly correlate superfluous movements and blood oxygen level dependent (BOLD) signal, thus providing insight into the origin of hyperkinetic movements.
It is well known that fMRI studies can provide information on brain activity during (in)voluntary movements. For the purpose of an event‐related design, isolated jerks such as tics and myoclonic jerks can be marked as events in the analysis. Until now, continuous hyperkinetic movements, like action tremor during posture, are commonly investigated with a conventional block (boxcar) design. In a block design, motor tasks—inducing the involuntary movements—are performed in an on/off fashion. In the analysis, task design is then correlated with BOLD signal. A limitation of a block design is that resulting brain activations are indistinguishably related to both the on/off involuntary movement and the simultaneously performed motor task. Different paradigms have been designed to discriminate between brain activation related to involuntary movements and task, for instance, comparing active and passive movements, which usually results in indirect measures of involuntary movements [Bucher et al., 1997].
To better quantify movement during a motor task, EMG can be recorded during hyperkinetic movements. The EMG amplitude can then be related to simultaneously acquired BOLD signal. However, in action‐induced hyperkinetic movements, such as in action tremor that is absent at rest, this measure still incorporates muscle activity related to task, and is not a sole reflection of hyperkinetic movements. However, after subtraction of the mean EMG per session from the raw EMG, the remaining signal (residual EMG, r‐EMG) theoretically represents the waxing and waning of hyperkinetic movements independent of the constant motor task [van Rootselaar et al., 2007].
Recently, we described the use of EMG‐fMRI in movement studies in healthy controls [van Rootselaar et al., 2007]. Participants performed voluntary semi‐irregular, flexion–extension movements of the wrist (blocks of slow (∼1 Hz) and fast (∼4 Hz) movements). EMG was recorded from wrist extensor muscles. First of all, it was shown that EMG amplitude information applied as a single regressor in an fMRI analysis identified motor circuitry during hand movements, similar to a block‐design based analysis. Second, r‐EMG was introduced, which quantifies movements as deviations in EMG power, positive or negative, with respect to the average EMG per session. r‐EMG correlated well with activity in brain areas known to be involved in modulation of movement. The same areas were also identified in a conventional analysis comparing fast and slow movements. It was concluded that r‐EMG is able to identify brain activation related to movement variability independent of a motor task [van Rootselaar et al., 2007].
The next step, and the purpose of this article, is to apply and validate the use of r‐EMG, theoretically representing the variation in hyperkinetic movement intensity independent of motor task, in patients with movement disorders. In previous studies investigating movement in normal and pathological conditions, EMG acquired during scanning has mainly been used to ascertain the onset of movement (timing information) [Liu et al., 2004; Oga et al., 2002; Richardson et al., 2006; Toma et al., 1999; van Rootselaar et al., 2007]. The use of amplitude information from EMG acquired during fMRI has been reported in a one‐patient study earlier [Richardson et al., 2006]. Richardson et al. recorded EMG‐fMRI in a single patient with cortical myoclonus and increased corticomuscular coherence and correlated the EMG power, in a band corresponding with the increased coherence, with BOLD signal. According to our knowledge, so far r‐EMG has not been used in fMRI patient studies.
To assess the value of r‐EMG in patients with hyperkinetic movements, in this study a homogenous group of patients with “familial cortical myoclonic tremor with epilepsy” (FCMTE) was included. This group of patients has been thoroughly investigated before, resulting in a good knowledge of the cerebral and cerebellar pathophysiology. This existing knowledge enables the validation of simultaneous EMG‐fMRI recordings and analysis, employing r‐EMG, in particular, in patients with a movement disorder. FCMTE is an autosomal dominantly inherited disorder, characterized by a continuous fine distal action myoclonus of known cortical origin [Ikeda et al., 1990; Striano et al., 2005; van Rootselaar et al., 2005]. Patients with FMCTE show increased cortical hyperexcitability as represented by features of cortical reflex myoclonus like a giant cortical sensory evoked potential (g‐SEP) induced by distal nerve stimulation [van Rootselaar et al., 2005]. Corticomuscular coherence studies in FCMTE patients point to a pathological cortical drive of the tremulous movements originating in the pre‐ or primary motor cortical areas [Grosse et al., 2003; van Rootselaar et al., 2006]. Postmortem investigations show typical cerebellar Purkinje cell degeneration in FCMTE patients [van Rootselaar et al., 2004]. It has been speculated that the cerebellar Purkinje cell degeneration leads to decreased cortical inhibition and thus to cortical hyperexcitability, as is described in other diseases characterized by cortical myoclonus of variable etiology [Hunt, 1921; Tijssen et al., 2000]. Based on our earlier studies, we hypothesize that r‐EMG can provide a measure of involuntary movements and will allow identification of brain areas involved in the generation of involuntary muscle activity, independent of the performed motor task, and will thus provide information complementary to the results of a conventional fMRI‐only recording and subsequent block design analysis.
METHODS
Participants
Eight FCMTE patients and nine healthy controls were investigated. The FCMTE patients belonged to a Dutch pedigree, described in detail previously [van Rootselaar et al., 2002, 2004, 2006]. All patients were affected according to research criteria; they suffered from distal action myoclonus (clinically resembling essential tremor) and had a history of generalized tonic‐clonic seizures (GTCS) or features of cortical reflex myoclonus [van Rootselaar et al., 2002]. At rest the tremulous movements were absent. The clinical characteristics are summarized in Table I. Median age was 44 years (range, 19–59 years) and median disease duration was 12.5 years (range, 7–34 years). All patients, except the three youngest, used antiepileptic drugs (AEDs; Table I). The nine healthy participants, five of which were included in our earlier EMG‐fMRI validation study [van Rootselaar et al., 2007], with no known neurological history, were gender‐ and age‐matched with the FCMTE patients (median age, 42 years; range, 31–63 years). All participants gave their informed consent. The study protocol was approved by the medical‐ethical board of the University Medical Centre Groningen, The Netherlands. Recordings were performed at the BCN‐NeuroImaging Center, Groningen.
Table I.
Familial cortical myoclonic tremor with epilepsy patients
| Patient | Age (in years) | Gender | Symptoms | AED | Clinical electrophysiology | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tremora | GTCSa | seiz fra | EEG changes | g‐SEP | C‐reflex | EEG‐EMG coherence | ||||
| FCMTE 1b | 19 | F | 12 | − | − | − | − | − | + | nd |
| FCMTE 2 | 31 | F | 20 | − | − | − | − | + | + | + |
| FCMTE 3 | 33 | M | 22 | − | − | − | − | + | + | + |
| FCMTE 4 | 43 | M | 19 | 20 | + | PhB, VPA, CBZ | nd | nd | nd | + |
| FCMTE 5 | 45 | F | 38 | 42 | ± | VPA, CZP | not specific | + | + | + |
| FCMTE 6 | 46 | M | 12 | 31 | ± | VPA, CZP | nd | nd | nd | + |
| FCMTE 7 | 56 | M | 30 | 43 | + | OCB, CZP | spike‐wave | − | + | + |
| FCMTE 8 | 59 | M | 45 | 52 | ± | VPA, CBZ | spike‐wave | + | − | + |
AED: anti‐epileptic drug; C‐reflex: cortical reflex, long latency reflex; CBZ: clobazam; CZP: clonazepam; EEG: electroencephalogram; EMG: electromyogram; F: female; FCMTE: familial cortical myoclonic tremor with epilepsy; g‐SEP: giant sensory evoked potential; GTCS: generalized tonic clonic seizure; M: male; nd: not done; OCB: oxcarbamazepine; PhB: phenobarbital; seiz fr: seizure frequency; VPA: valproic acid; −: negative/none; ±: good control with AEDs; +: present/few seizures per year on AEDs.
Age at onset.
Left handed.
Motor Tasks
The test setting and motor tasks are comparable to our validation study in healthy subjects [van Rootselaar et al., 2007]. Participants laid supine on the scanner bed and were instructed to only move their right arm as visually instructed using slides. The slides were projected on a screen at the head end of the MRI scanner. Participants saw the screen via a mirror attached to the head coil, preventing a vision of their own hand or arm. Three tasks were performed: (1) “rest,” when tremor was clinically absent; (2) “posture,” maintained extension and pronation of right arm, hand, and fingers, inducing an action tremor in the patient group; and (3) “movement,” a self‐paced flexion–extension movement of the right wrist evenly divided in “fast” movement and “slow” movement tasks to introduce movement frequency variability, keeping the arm extended with the hand in the vertical plane. In the “fast” movement task, subjects were instructed to move their hand as fast as possible while keeping the rest of their body as still as possible. For the “slow” movement task, subjects were instructed to complete one flexion–extension wrist movement within ∼1 s. All tasks were demonstrated and practiced outside the scanner. Each task block lasted for 30 s and was repeated either five times (“rest”) or 10 times (“posture” and “movement”) in a semirandom fashion. Sessions were performed twice by each participant. A scan is defined as the time required to image the brain once (3 s, see fMRI analysis below). At the end of each session, two additional scans were added to better capture the slow return to baseline of the BOLD response.
EMG Recordings and Analysis
EMG recordings and analysis have been described in detail before [van Rootselaar et al., 2007] but this is repeated and summarized here for clarity. Commercially available MR compatible equipment and software was used (Brain Products GmbH, Munich, Germany). Pairs of sintered silver/silver‐chloride EMG electrodes with current‐limiting resistors (5 kΩ [Lemieux et al., 1997]) were attached, one pair above the right wrist extensor (RE) muscles, ∼5 and 9 cm distal to the right elbow joint, and another pair above the right first dorsal interosseus (RFDI) muscle and the metacarpophalangeal joint. The electrode wires were twisted per pair to minimize the differential effect of the magnetic field on the EMG leads. A reference electrode was positioned on the right elbow joint, and a ground electrode was placed on the left elbow joint [Lemieux et al., 1997]. The signals were digitized, transmitted via an optical cable to a PC outside the MR room, and stored at a sampling rate of 5,000 Hz. Offline, the data were corrected for fMRI artifacts [Allen et al., 2000; van Duinen et al., 2005]. To reduce movement artifacts, bipolar derivations for each muscle were high‐pass filtered at 10 Hz, and rectified to enhance the information on EMG burst‐frequency (tremor) of the signal, thereby recovering the low frequency EMG content [Myers et al., 2003]. The artifact induced in the EMG due to echo‐planar imaging (EPI) has a very clear signature in frequency space: its spectrum has peaks at multiples of the slice frequency. For the present study, EPI induces spectral peaks at ∼16 Hz and its harmonics (i.e., 32, 48, 64 Hz, etc.). Before artifact correction, these peaks dominate the spectrum. Successful correction should result in a typical EMG spectrum, without any visible peaks due to EPI. Therefore, the quality of the corrected EMG signal was established not only by visual inspection but also by assessment of the power spectrum per task block (30 s) for remaining fMRI artifact power peaks. Per participant, the EMG channel with the best artifact correction was selected for further analysis. Then, frequency extraction from 1 to 250 Hz was applied to this channel, calculating the average power (square of spectral amplitude) in this range for each data point in mV2. This yields a signal representing the variation over time of the EMG power between 1 and 250 Hz. The upper limit was set at 250 Hz, because there is generally no significant EMG power above this value [Basmajian and Luca, 1985; Lutzenberger et al., 1985]. A smaller frequency band (e.g., around tremor frequency) was considered, but the relative power variation in such a band did not show any differences from the 1‐ to 250‐Hz band in a representative sample of participants. The data were subsequently imported into Matlab (version 6.5, The Mathworks, Natick, MA) and the EMG power was averaged over segments of 3 s, i.e., the time required to acquire one scan. This yielded an EMG power vector of length 252, with one entry for every scan, suitable to use as a regressor in an fMRI analysis.
“Residual EMG” Regressors
In our earlier study, we showed that a conventional block design analysis and an EMG‐based analysis can be merged, allowing the identification of brain activity specifically related to movement variation [van Rootselaar et al., 2007]. For this type of integrated analysis, it is necessary to mathematically preprocess the EMG regressor according to a procedure known as “Gram–Schmidt orthogonalization” [van Rootselaar et al., 2007]. This procedure has also been used to study correlations between EEG and brain activity [Feige et al., 2005]. For one specific task the r‐EMG vector r‐EMG task with respect to the corresponding block design vector block task was calculated as follows:
Here, (·) denotes the inner product of two vectors and EMG task is a vector of length 252 with entries equal to the mean EMG power per scan during task execution and equal to 0 elsewhere. The entries in block task, which is also a vector of length 252, are equal to 1 for every scan during task execution and equal to 0 elsewhere (this describes a boxcar design). The procedure is illustrated in Figure 1. In this manner, the information that is already present in the block regressor is subtracted from the EMG regressor, allowing simultaneous inclusion of the two into one design. The preprocessed EMG vector is, for use in the analysis, referred to as r‐EMG regressor. In nonmathematical terms, the r‐EMG regressor is equal to the additional EMG (positive or negative) relative to the mean EMG value across the task. During the posture task it represents the waxing and waning of tremulous movement intensity, and during movement it represents the variations in EMG power due to changes in the frequency of movement [van Rootselaar et al., 2007].
Figure 1.
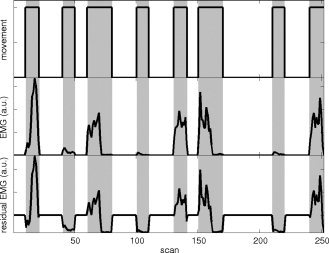
Gram–Schmidt orthogonalization. Block and EMG‐derived regressors for the movement task across one complete session (12.5 min) in a healthy participant. Horizontal axis: scannumber (1 scan is 3s) time in minutes; vertical axis: regressor (EMG in arbitrary units, a.u.). Gray: movement task intervals. Top: block design (boxcar type) regressor for movement; the task is performed in an on/off fashion. Middle: Electromyogram (EMG) regressor for movement. Note that the movement task was evenly divided in fast (higher EMG power) and slow movement (lower EMG power). Bottom: Gram–Schmidt orthogonalization of the EMG regressor with respect to the block design regressor results in the residual EMG regressor. This is the additional EMG (positive or negative) relative to the mean EMG power (shown here before convolution with the canonical haemodynamical response function and scaling with the standard deviation).
Subsequently, the derived r‐EMG regressors were convolved with the canonical haemodynamical response function (HRF, identical to the HRF in Statistical Parametric Mapping 2, SPM2; http://www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Cognitive Neurology, London [Friston et al., 1995a]) and then scaled by their respective standard deviations. Finally, the two original block design regressors (block posture and block movement) and the two derived r‐EMG regressors (r‐EMG posture and r‐EMG movement) were used together in one fMRI design (see below).
fMRI Recordings and EMG‐fMRI Analysis
Functional images were acquired using a 3T Philips Intera MRI scanner (Best, The Netherlands), using a standard transmit/receive head coil. The following pulse sequence parameters were used: Fast Field Echo (FFE) single shot EPI; 46 contiguous slices; slice thickness, 3.5 mm; field of view, 224 × 224 mm2; scanning matrix, 64 × 64; transverse slice orientation; repetition time (TR) = 3 s; echo time (TE) = 35 ms; flip angle, 90°; total acquisition time per session = 12:30 min. In addition, T1‐weighted 3D FFE anatomical images of the entire brain were obtained with the following pulse sequence parameters: field of view 256 × 256 mm2; scanning matrix, 256 × 256; 120 slices; slice thickness, 1 mm; transverse slice orientation; TE = 4.6 ms; TR = 25 ms; flip angle, 30°.
Single‐subject first‐level analysis was performed in SPM2. The functional images were realigned, normalized to standard brain coordinates (Montreal Neurological Institute (MNI) standard space, http://www.bic.mni.mcgill.ca/cgi/icbm_view), and spatially smoothed with an isotropic 8‐mm full‐width at half maximum (FWHM) Gaussian kernel. The actual on‐ and offsets of movement or posture, as detected by EMG, were used to temporally correct the block regressors. In our analysis, we applied a well‐established method: statistical parametric mapping (SPM) based on a general linear model (GLM; [Friston et al., 1995a, b]). The GLM for a time‐series can be written in matrix notation as
Here, in the context of fMRI analysis, X is a column vector of mean corrected values from a single voxel. The columns of G, the so‐called design matrix, model the effects of interest and the effects of no interest [Friston et al., 1995b]. In our case the effects of interest are modeled by both block and r‐EMG regressors, whereas the effects of no interest, or confounds, are modeled by motion parameters [Friston et al., 1996]. The column vector β consists of parameters for the effects modeled by each column of the design matrix [Friston et al., 1995b].
The effects of interest “block posture” and “block movement” were modeled by a boxcar function and convolved with the canonical HRF in SPM2. These two block regressors are the first two columns in the design matrix G and are similar to the regressors in a conventional block design. We added the two r‐EMG regressors, for posture and movement, to the design matrix G. Estimates for β were derived in single‐subject fixed‐effects analyses. For the second level within group and between group comparisons, nonparametric permutation tests were performed since sample size was limited (i.e., the nonparametric equivalent of a t‐test; Statistical non‐Parametric Mapping toolbox, http://www.sph.umich.edu/ni-stat/SnPM/ [Nichols and Holmes, 2002]). Tests were performed for the different regressors, i.e., block posture, block movement, r‐EMGposture, and r‐EMGmovement, (1) within groups (512 permutations in controls, 256 permutations in FCMTE patients), and (2) between groups per task (10,000 permutations). Additionally, since wrist extension–flexion in controls can be considered a simulation of tremulous activity in FCMTE patients [Bucher et al., 1997], we compared brain activation resulting from the r‐EMGposture analysis in FCMTE patients to brain activation resulting from the r‐EMGfast movement analysis in controls. Permutations were performed using the pseudo‐T maps (10 mm FWHM isotropic Gaussian kernel applied to the standard deviation map). Within groups, we reported activations for voxels detected at P < 0.05 (familywise error corrected) when part of a cluster ≥ 15 voxels. For between group comparisons, we reported activations for voxels detected at P < 0.001 (uncorrected) when part of a cluster ≥ 45 voxels.
RESULTS
EMG Accuracy and Artifact Removal
Both the changing magnetic field gradients and the movement of EMG leads in the magnetic field introduced major artifacts in the EMG signal. After fMRI artifact correction [Allen et al., 2000], the movement artifacts were removed by a 10‐Hz high‐pass filter. We ensured that artifact correction of the EMG signal (Fig. 2) was sufficient by visual inspection and by verification of the power spectrum per 30 s epoch, as explained earlier. A typical example of EMG spectra during posture before and after artifact correction is given in Figure 3. Overall, artifacts were removed satisfactorily from the recordings of at least one muscle in all participants.
Figure 2.
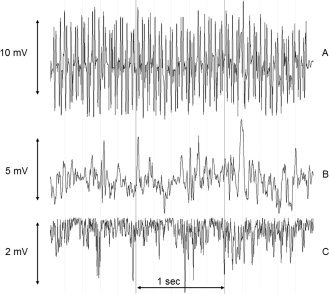
Artifact removal from EMG signal. Horizontal axis: time in seconds, vertical axis: potential in millivolts (mV). Sequential fMRI artifact correction (top → middle) and movement artifact correction (middle → bottom) on 3 s of EMG acquired during posture in an FCMTE patient. A: Bipolar extensor EMG before artifact correction. The high frequency artifacts due to fMRI are clearly visible. The irregularity in the baseline is due to movement. B: The same bipolar EMG signal after fMRI artifact correction [Allen et al., 2000]. Note the reduction in amplitude. The remaining sinusoidal signal is the result of movement of the EMG leads in the magnetic field. The EMG itself is superimposed on this signal, but of lower amplitude. C: After application of a 10‐Hz high‐pass filter and rectification of the signal, the EMG bursts related to the tremulous limb movements during posture are clearly visible.
Figure 3.
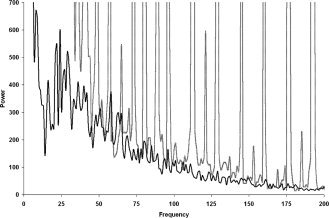
Power spectrum during posture in an FCMTE patient. Gray: before artifact correction, black: after artifact correction. Frequency in Hz; power in μV2. Notice the peaks at multiples of the slice frequency (16 Hz) in the spectrum before correction.
Task Execution
Spectral analysis (results not shown) showed that “fast” movement resulted in a frequency of movement of ∼4 Hz and “slow” movement in a frequency of movement of ∼1 Hz. EMG power variations reflected that task execution was variable within and between tasks, sessions, and participants. Deviations in task on‐ and offsets were identified with EMG and corrected for in the analysis. Per session, the mean number of task blocks in which the task onset was delayed by one scan (3 s) was 4.4 in FCMTE patients and 3.3 in controls. In two FCMTE patients, delay in onset was two scans (6 s) on four occasions in total. Differences between groups were not significant (P = 0.233, two‐sample t‐test, equal variances assumed).
EMG‐fMRI Analysis, Group Results
Results within groups are presented in Tables II and III. Differences between patients and controls are presented in Table IV. Main results are summarized below and illustrated in Figure 4. MNI coordinates, pseudo‐T, and P‐values are given in the tables.
Table II.
Posture, results for blockposture and r‐EMGposture, regressor within groups
| Regressor posture | Group | Voxels, n | Area | Side | Pseudo‐T | x a | y a | z a |
|---|---|---|---|---|---|---|---|---|
| Block | Controls | 816 | Vermis VI | M | 8.48 | 4 | −64 | −22 |
| —b | Cerebellum IV,V | R | 8.46 | 10 | −56 | −20 | ||
| —b | Cerebellum VI | R | 6.53 | 23 | −46 | −28 | ||
| 436 | Precentral gyrus | L | 7.17 | −34 | −26 | 58 | ||
| 63 | SMA | M | 5.50 | 2 | 10 | 46 | ||
| 27 | Frontal sup | L | 5.84 | −28 | −8 | 68 | ||
| 23 | Frontal inf, oper | R | 5.59 | 58 | 14 | 4 | ||
| 17 | Temporal pole sup | R | 5.46 | 54 | 16 | −8 | ||
| FCMTE | 196 | Frontal inf, oper | R | 7.30 | 56 | 10 | 10 | |
| —b | Temporal pole sup | R | 6.75 | 56 | 12 | −6 | ||
| 22 | Frontal mid | R | 6.08 | 42 | 50 | 20 | ||
| 104 | Insula | R | 6.90 | 38 | 20 | 0 | ||
| 151 | Insula | L | 7.02 | −44 | 10 | −8 | ||
| —b | Temporal pole sup | L | 6.62 | −56 | 8 | −6 | ||
| 33 | Supramarginal | L | 6.32 | −60 | −48 | 26 | ||
| r‐EMG | Controls | 0 | — | |||||
| FCMTE | 135 | Parietal inf | R | 6.71 | 38 | −46 | 40 | |
| 115 | Parietal inf | L | 6.06 | −42 | −44 | 46 | ||
| —b | Parietal sup | L | 5.97 | −46 | −46 | 54 | ||
| 15 | Insula | L | 6.19 | −40 | 14 | −4 |
MNI: Montreal Neurological Institute standard space; CO: controls; FCMTE: familial cortical myoclonic tremor with epilepsy; block: block regressor; r‐EMG: residual EMG regressor; L: left, contralateral; R: right, ipsilateral; M: midline, not confined to one side; cerebellum x: cerebellar hemispheral lobule x; inf: inferior gyrus; mid: middle gyrus; oper: pars opercularis; SMA: supplementary motor area; sup: superior gyrus; tri: pars triangularis; vermis x: vermal lobule x.
MNI.
Same activated region.
Table III.
Movement, blockmovement and r‐EMGmovement, within groups
| Regressor | Group | Voxels, n | Area | Side | Pseudo‐T | x a | y a | z a |
|---|---|---|---|---|---|---|---|---|
| Block | Controls | 4,969 | Cerebellum VI | R | 13.35 | 15 | −55 | −24 |
| —b | Cerebellum VI | L | 7.71 | −28 | −58 | −26 | ||
| 1,709 | Postcentral gyrus | L | 8.89 | −40 | −20 | 48 | ||
| —b | Precentral gyrus | L | 8.77 | −30 | −22 | 70 | ||
| 1,155 | Cingulum mid | M | 8.55 | 3 | 7 | 41 | ||
| —b | SMA | M | 7.32 | −2 | −2 | 50 | ||
| 523 | Temporal pole sup | R | 6.97 | 48 | 8 | −10 | ||
| 895 | Pallidum | L | 9.63 | −28 | −8 | −4 | ||
| —b | Thalamus | L | 7.44 | −16 | −20 | 4 | ||
| 359 | Thalamus | R | 8.70 | 16 | −14 | 18 | ||
| FCMTE | 981 | Cerebellum IV, V | R | 10.66 | 16 | −50 | −22 | |
| —b | Cerebellum VI | R | 6.36 | 34 | −54 | −30 | ||
| —b | Vermis VII | M | 6.18 | 2 | −64 | −24 | ||
| 916 | Parietal sup | L | 9.25 | −42 | −42 | 60 | ||
| —b | Postcentral gyrus | L | 7.89 | −32 | −32 | 56 | ||
| 270 | Temporal pole sup | L | 8.31 | −48 | 10 | −10 | ||
| 732 | SMA | M | 8.17 | −4 | −6 | 50 | ||
| —b | Cingulum mid | M | 6.87 | 2 | 16 | 36 | ||
| 65 | Postcentral gyrus | R | 6.68 | 54 | 54 | 22 | ||
| 720 | Insula | R | 7.76 | 38 | 12 | 0 | ||
| —b | Temporal pole sup | R | 7.63 | 54 | 12 | −8 | ||
| —b | Frontal inf oper | R | 7.16 | 56 | 10 | 12 | ||
| 151 | Frontal mid | R | 6.52 | 32 | 54 | 22 | ||
| —b | Frontal inf, tri | R | 6.47 | 32 | 40 | 16 | ||
| 47 | Thalamus | L | 6.84 | −14 | −10 | 16 | ||
| r‐EMG | Controls | 293 | Vermis VII | M | 6.81 | 2 | −64 | −24 |
| —b | Cerebellum IV, V | R | 6.54 | 10 | −54 | −18 | ||
| FCMTE | 0 | — |
MNI: Montreal Neurological Institute standard space; CO: controls; FCMTE: familial cortical myoclonic tremor with epilepsy; block: block regressor; r‐EMG: residual EMG regressor; L: left, contralateral; R: right, ipsilateral; M: midline, not confined to one side; cerebellum x: cerebellar hemispheral lobule x; inf: inferior gyrus; mid: middle gyrus; oper: pars opercularis; SMA: supplementary motor area; sup: superior gyrus; tri: pars triangularis; vermis x: vermal lobule x.
MNI.
Same activated region.
Table IV.
Group comparisons
| Task | Regressor | Comparison | Voxels, n | Area | Side | P Uncorrecteda | x b | y b | z b |
|---|---|---|---|---|---|---|---|---|---|
| Posture | Block | — | |||||||
| r‐EMG | FC‐CO | 191 | Frontal mid | L | 0.0003 | −40 | 40 | 18 | |
| —c | Frontal mid | L | 0.0007 | −40 | 40 | 30 | |||
| —c | Frontal inf tri | L | 0.0006 | −36 | 40 | 10 | |||
| 84 | Frontal inf tri | R | 0.0002 | 50 | 40 | 18 | |||
| 37 | Supramarginal | L | 0.0009 | −54 | −38 | 32 | |||
| Movement | Block | — | |||||||
| r‐EMG | FC‐CO | 209 | Precentral | L | 0.0007 | −42 | 4 | 40 | |
| Posture (FC)/fast movement (CO) | r‐EMG | FC‐CO | 367 | Parietal inf | R | 0.0001 | −38 | −42 | 36 |
| 122 | Frontal mid | R | 0.0000 | 48 | 42 | 20 | |||
| 221 | Precentral | L | 0.0001 | −40 | 6 | 60 | |||
| —c | Frontal mid | L | 0.0007 | −30 | 0 | 66 | |||
| —c | Precentral | L | 0.0009 | −36 | −16 | 68 | |||
| 224 | Parietal inf | L | 0.0009 | −46 | −46 | 52 | |||
| —c | Angular | L | 0.0009 | −40 | −50 | 36 | |||
| 207 | Precentral | L | 0.0003 | −42 | 6 | 42 | |||
| —c | Frontal inf oper | L | 0.0010 | −40 | 4 | 24 | |||
| 222 | SMA | L | 0.0002 | 0 | 0 | 62 | |||
| 94 | Frontal mid | L | 0.0007 | −38 | 40 | 18 | |||
| —c | Frontal inf tri | L | 0.0008 | −36 | 40 | 10 | |||
| —c | Frontal inf orb | L | 0.0003 | −32 | 38 | −4 | |||
| CO‐FC | — |
MNI: Montreal Neurological Institute standard space; CO: controls; FCMTE: familial cortical myoclonic tremor with epilepsy; block: block regressor; r‐EMG: residual EMG regressor; L: left, contralateral; R: right, ipsilateral; M: midline, not confined to one side; cerebellum x: cerebellar hemispheral lobule x; inf: inferior gyrus; mid: middle gyrus; oper: pars opercularis; SMA: supplementary motor area; sup: superior gyrus; tri: pars triangularis; vermis x: vermal lobule x.
Clusters ≥ 45 voxels.
MNI.
Same activated region.
Figure 4.
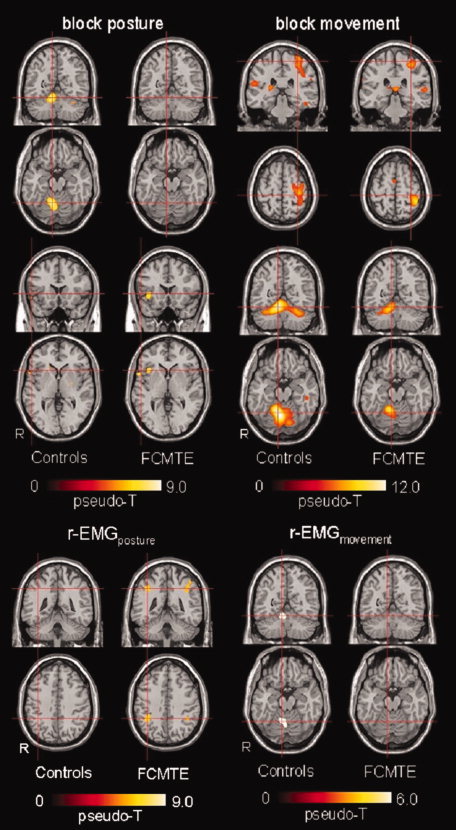
Block versus EMG‐based fMRI analysis, group results. Activation maps per group projected on a normalized single‐participant T1 image. Controls: n = 9; FCMTE: n = 8; n = number of participants. Only main significant activations are illustrated. Upper panel: activations maps resulting from block design regressors. Left: posture. Top: coronal and transverse sections focused at MNI (9,−56,−18), cerebellar hemispheral lobule IV–V; bottom: coronal and transverse sections focused at MNI (52,18,2), frontal inferior gyrus, pars triangularis. Right: movement. Top: coronal and transverse sections focused at MNI (−32,−30,62), postcentral gyrus; bottom: coronal and transverse sections at MNI (9,−56,−18), cerebellar hemispheral lobule IV–V. Only significant activations have been plotted (posture: pseudo‐T > 5.17 for controls and pseudo‐T > 5.69 for FCMTE; movement: pseudo‐T > 5.39 for controls and pseudo‐T > 5.42 for FCMTE). Note the different color scales. Lower panel: Activations maps resulting from r‐EMG regressors. Left: posture. Coronal and transverse sections focused at MNI (38,−44, −41), parietal inferior gyrus. Right: movement. Coronal and transverse sections focused at MNI (9, −56, −18), cerebellar hemispheral lobule IV–V. Only significant activations have been plotted (posture: pseudo‐T > 5.34 for controls and pseudo‐T > 5.61 for FCMTE; movement: pseudo‐T > 5.48 for controls and pseudo‐T > 5.52 for FCMTE). Note the different color scales. See Tables II and III for a complete overview of all significant activations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Block regressors
In healthy controls, the blockposture regressor mainly identified the ipsilateral cerebellum and the contralateral precentral gyrus. Additional smaller areas of activation were the supplementary motor area (SMA) and bilateral frontal areas. The blockmovement regressor was primarily associated with activation in areas specifically belonging to motor circuitry known to be involved in hand movement, i.e., contralateral primary sensorimotor cortex and thalamus, ipsilateral cerebellum and SMA. Additionally, the right superior temporal pole and left pallidum were activated.
In FCMTE patients, the blockposture regressor mainly correlated with brain activity in bilateral frontolateral areas (inferior frontal gyrus pars opercularis, superior temporal pole, middle frontal gyrus, and insula). Additional minor activity was observed in the left supramarginal cortex. The blockmovement regressor was primarily associated with activation in areas specifically belonging to hand motor circuitry, i.e., contralateral primary sensorimotor cortex and thalamus, ipsilateral cerebellum and SMA. Additional activation was seen in ipsilateral frontolateral areas.
r‐EMG regressors
In healthy controls the r‐EMGposture regressor yielded no significant results, while the r‐EMGmovement regressor identified ipsilateral cerebellar activity. In FCMTE patients, the r‐EMGposture regressor identified bilateral activity of bilateral inferior and right superior parietal cortex. Some additional activation was seen in left insula. The r‐EMGmovement regressor identified no significant brain activity in FCMTE patients.
Between group differences
Areas of increased brain activity were detected in FCMTE patients when compared with controls: (mainly) contralateral frontolateral areas for the r‐EMGposture regressor and contralateral precentral areas for the r‐EMGmovement regressor. For the block‐regressors, no significant group differences were found. When comparing r‐EMGposture in FCMTE patients to r‐EMGfast movement in controls at P < 0.001 (uncorrected), the right parietal inferior cortex was more active in patients (Fig. 5). When the same comparison was considered at a lower threshold (P < 0.01 (uncorrected) for clusters ≥ 90 voxels), the corresponding parietal area on the left side, SMA, left precentral cortex, and bilateral frontolateral areas were additionally revealed. The inverse comparison showed no additional activity in controls compared with FCMTE patients, not even at the lower threshold. To better understand these results, we also considered the within‐group analysis of r‐EMGfast movement in controls at a lower threshold. This indicated, besides right cerebellar and vermis activity, additional activity in left precentral and bilateral postcentral areas but none in SMA, bilateral inferior parietal, or frontolateral areas.
Figure 5.
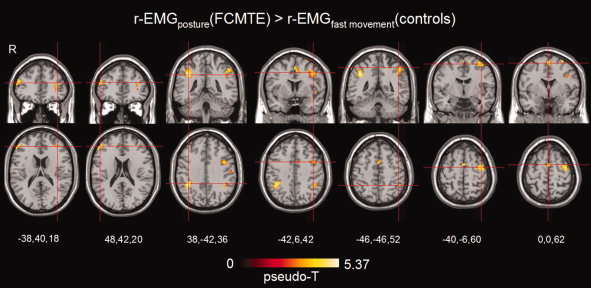
Group results for r‐EMG posture (FCMTE) > r‐EMG fast movement (controls). This contrast compares the tremulous movements in FCMTE patients with simulated tremor in healthy controls applying r‐EMG regressors. From left to right slices go from lower to higher positions in the brain. Coronal and transverse sections are focused at (from left to right): frontal mid (L), frontal mid (R), parietal inferior (R), precentral (L), parietal inferior (L), precentral (L), and SMA activations. Only significant activations have been plotted at P < 0.001 (uncorrected). Activation maps are projected on a normalized single‐participant T1 image. See Table IV and the text for a detailed description of these activations. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The results, in a homogenous group of patients with tremor of known cortical origin and cerebellar pathology, indicate that adding regressors derived from EMG acquired simultaneously with MRI to the fMRI analysis gives additional information on pathophysiology of hyperkinetic movements compared with a solely block‐design based fMRI analysis. r‐EMG is, after orthogonalization of the EMG with respect to the block‐design, the deviation in amplitude over time with respect to the average EMG over one condition, in one individual. The block‐ and r‐EMG‐regressors are (mathematically) independent and can thus be incorporated in the same design matrix. The block‐ and EMG‐derived regressors provide complementary information. As will be argued below, the conventional block regressors mainly point to neuronal circuitry associated with the motor task, while the r‐EMG regressors help to identify brain activation which is known to be related to involuntary movements in this group of patients.
Block‐Regressors
In healthy controls, both the blockposture and the blockmovement regressors correlated with activity of the contralateral primary motor cortex and ipsilateral cerebellum, as expected for motor tasks. This confirms that block design regressors can identify task‐related brain activity and indicates that recording and processing of EMG does not disturb the fMRI data acquisition and block analysis.
In FCMTE patients, “posture” induces the distal myoclonic movements, which makes the posture task the most interesting to discuss from a clinical perspective. Group results for blockposture did not reveal cerebellar activity in patients. This is in line with the known cerebellar Purkinje cell degeneration in FCMTE [van Rootselaar et al., 2004]. Not only pathological studies but also functional studies, such as eye movements abnormalities, point toward cerebellar dysfunction in six of the eight FCMTE patients included in the current study (unpublished results). Group‐comparison showed no significant differences for cerebellar activity in patients and controls, probably due to the small group sizes and large interindividual variability, especially in the patient group.
The blockmovement regressor identified motor circuitry in FCMTE patients, similar to healthy controls. There were no significant differences between groups for this regressor, although the cluster size of the cerebellar activity in the FCMTE patients was about a fifth of that found in the healthy controls which could again be in line with the cerebellar pathology in these patients. There are several explanations for remaining cerebellar activity in these patients with cerebellar pathology. First, postmortem studies revealed remaining normal Purkinje cells [van Rootselaar et al., 2004]. Second, the cerebellum is highly differentiated with respect to several functions and has a somatotopical differentiation [Manni and Petrosini, 2004]. “Movement” is likely to involve more activation or different activation of diverse cerebellar areas compared with posture, which would then result in suprathreshold cerebellar fMRI activity in the group analysis for movement. Previous studies showed that changes in the frequency of wrist flexion–extension movements is a cerebellar function [Thach et al., 1992; van Rootselaar et al., 2007]. Therefore, even in patients with cerebellar Purkinje cell degeneration, remaining cerebellar activity is likely to be found.
These results support the idea that a block regressor identifies brain areas involved in the performance of a specific motor task.
r‐EMG Regressors
In healthy controls, no significant activations were identified for r‐EMGposture (Fig. 4, Table II). This is not surprising because, in case posture was performed constantly and well‐timed, r‐EMGposture is virtually constant. In healthy subjects, who are able to perform posture in a steady fashion, indeed only minor deviations were seen in the EMG with respect to the block‐design and the r‐EMG regressor showed little variation and did not correlate significantly with BOLD signal variations anywhere in the brain.
In FCMTE patients with continuous hyperkinetic movements, r‐EMGposture represents the presence and severity of the tremor during posture. It needs to be stressed that the residual EMG regressor is not the EMG signal as recorded from the muscle. This signal is preprocessed first, i.e., filtered, rectified, averaged over scans, and orthogonalized. It is thus a reflection of the waxing and waning EMG signal with respect to the task, i.e., the involuntary movements. In FCMTE patients the r‐EMGposture regressor correlated mainly with bilateral activity in the (secondary) sensory cortex (Fig. 4, Table II). In FCMTE, it has been speculated that the cortex is the origin of the tremor. This speculation is supported by electrophysiological findings like a giant cortical somatosensory evoked potential and increased corticomuscular coherence [van Rootselaar et al., 2006]. In cortical myoclonus, hyperexcitability of the sensorimotor cortex has been hypothesized to lead to myoclonic jerks [Hallett et al., 1979]. It can be questioned whether this activity of the sensory areas is due to increased afferent input. However, bilateral activity of the secondary sensory areas was seen, instead of (mainly) contralateral activity in the primary sensory cortex—as would be expected from afferent input.
To further investigate whether hand movements by themselves would lead to activity of the sensory areas, we compared r‐EMG activation maps of healthy controls performing fast wrist flexion–extension movements with r‐EMG activation maps of FCMTE patients performing posture. Fast hand movements in controls have previously been compared with action tremor in an fMRI study of essential tremor [Bucher et al., 1997]. The cortical tremor (FCMTE) patients in the present study, however, showed activity in the parietal inferior gyrus (the secondary sensory cortex) which was absent in controls during fast hand movement, indicating that this activity is unlikely to be normal afferent input (Table IV, Fig. 5).
For r‐EMGposture, significantly more activity was seen in the middle frontal gyrus in FCMTE patients compared with healthy controls (Table IV). This may be in accordance with the known cortical hyperexcitability in this patient group. Alternatively, this may indicate compensatory activity. Therefore, in patients with continuous tremulous movements, it can be concluded that the r‐EMG regressor correlates with brain areas that, based on our knowledge of FCMTE and cortical myoclonus in general, are very likely to be involved in the generation of the hyperkinetic movements.
These results show that r‐EMG regressors can help to identify brain activation related to involuntary movements, complementary to brain areas identified by conventional block design regressors.
CONCLUSIONS
Application of EMG during fMRI and an integrated EMG‐fMRI analysis technique have additional value over fMRI alone. Recording of muscle activity during scanning allows the use of an EMG‐derived measure of hyperkinetic movements as a regressor in the fMRI analysis. These methods, allowing to identify brain activity related to superfluous movements and changes in task performance, will facilitate future studies of movement disorders.
Acknowledgements
The authors wish to thank Anita Kuiper for operating the MR scanner. RR is a Hazewinkel‐Beringer fellow.
REFERENCES
- Allen PJ,Josephs O,Turner R ( 2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12: 230–239. [DOI] [PubMed] [Google Scholar]
- Basmajian JV,Luca CJ ( 1985): Description and analysis of the EMG signal In: Muscles Alive: Their Functions Revealed by Electromyography. Baltimore MD: Williams and Wilkins; pp 65–100. [Google Scholar]
- Bucher SF,Seelos KC,Dodel RC,Reiser M,Oertel WH (1997): Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol 41: 32–40. [DOI] [PubMed] [Google Scholar]
- Feige B,Scheffler K,Esposito F,Di Salle F,Hennig J,Seifritz E ( 2005): Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol 93: 2864–2872. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JB,Frith CD,Frackowiak RSJ ( 1995a): Statistical Parametric Maps in functional imaging: A general linear approach. Human Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ,Holmes AP,Poline JB,Grasby PJ,Williams SCR,Frackowiak RSJ,Turner R ( 1995b): Analysis of fMRI time series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Williams S,Howard R,Frackowiak RS,Turner R ( 1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Grosse P,Guerrini R,Parmeggiani L,Bonanni P,Pogosyan A,Brown P ( 2003): Abnormal corticomuscular and intermuscular coupling in high‐frequency rhythmic myoclonus. Brain 126: 326–342. [DOI] [PubMed] [Google Scholar]
- Hallett M,Chadwick D,Marsden CD ( 1979): Cortical reflex myoclonus. Neurology 29: 1107–1125. [DOI] [PubMed] [Google Scholar]
- Hunt JR ( 1921): Dyssynergica cerebellaris myoclonica—Primary atrophy of the dentate system. Brain 44: 490–538. [Google Scholar]
- Ikeda A,Kakigi R,Funai N,Neshige R,Kuroda Y,Shibasaki H ( 1990): Cortical tremor: A variant of cortical reflex myoclonus. Neurology 40: 1561–1565. [DOI] [PubMed] [Google Scholar]
- Lemieux L,Allen PJ,Franconi F,Symms MR,Fish DR ( 1997): Recording of EEG during fMRI experiments: Patient safety. Magn Reson Med 38: 943–952. [DOI] [PubMed] [Google Scholar]
- Liu JZ,Zhang L,Brown RW,Yue GH ( 2004): Reproducibility of fMRI at 1.5 T in a strictly controlled motor task. Magn Reson Med 52: 751–760. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W,Elbert T,Rockstroh B,Birbaumer N ( 1985): Das EEG. Psychophysiologie und Methodik von Spontan‐EEG und ereigniskorrelierten Potentialen. [Google Scholar]
- Manni E,Petrosini L ( 2004): A century of cerebellar somatotopy: A debated representation. Nat Rev Neurosci 5: 241–249. [DOI] [PubMed] [Google Scholar]
- Myers LJ,Lowery M,O'Malley M,Vaughan CL,Heneghan C,St. Clair GA,Harley YX,Sreenivasan R ( 2003): Rectification and non‐linear pre‐processing of EMG signals for cortico‐muscular analysis. J Neurosci Methods 124: 157–165. [DOI] [PubMed] [Google Scholar]
- Nichols TE,Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oga T,Honda M,Toma K,Murase N,Okada T,Hanakawa T,Sawamoto N,Nagamine T,Konishi J,Fukuyama H,Kaji R,Shibasaki H ( 2002): Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer's cramp: An fMRI study. Brain 125: 895–903. [DOI] [PubMed] [Google Scholar]
- Richardson MP,Grosse P,Allen PJ,Turner R,Brown P ( 2006): BOLD correlates of EMG spectral density in cortical myoclonus: Description of method and case report. Neuroimage 32: 558–565. [DOI] [PubMed] [Google Scholar]
- Striano P,Zara F,Striano S ( 2005): Autosomal dominant cortical tremor, myoclonus and epilepsy: Many syndromes, one phenotype. Acta Neurol Scand 111: 211–217. [DOI] [PubMed] [Google Scholar]
- Thach WT,Goodkin HP,Keating JG ( 1992): The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15: 403–442. [DOI] [PubMed] [Google Scholar]
- Tijssen MA,Thom M,Ellison DW,Wilkins P,Barnes D,Thompson PD,Brown P ( 2000): Cortical myoclonus and cerebellar pathology. Neurology 54: 1350–1356. [DOI] [PubMed] [Google Scholar]
- Toma K,Honda M,Hanakawa T,Okada T,Fukuyama H,Ikeda A,Nishizawa S,Konishi J,Shibasaki H ( 1999): Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: An event‐related fMRI study. J Neurosci 19: 3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen H,Zijdewind I,Hoogduin H,Maurits N ( 2005): Surface EMG measurements during fMRI at 3T: Accurate EMG recordings after artifact correction. Neuroimage 27: 240–246. [DOI] [PubMed] [Google Scholar]
- van Rootselaar F,Callenbach PM,Hottenga JJ,Vermeulen FL,Speelman HD,Brouwer OF,Tijssen MA ( 2002): A Dutch family with ‘familial cortical tremor with epilepsy’. Clinical characteristics and exclusion of linkage to chromosome 8q23.3–q24.1. J Neurol 249: 829–834. [DOI] [PubMed] [Google Scholar]
- van Rootselaar AF,Aronica E,Jansen Steur EN,Rozemuller‐Kwakkel JM,de Vos RA,Tijssen MA ( 2004): Familial cortical tremor with epilepsy and cerebellar pathological findings. Mov Disord 19: 213–217. [DOI] [PubMed] [Google Scholar]
- van Rootselaar AF,van Schaik IN,van den Maagdenberg AM,Koelman JH,Callenbach PM,Tijssen MA ( 2005): Familial cortical myoclonic tremor with epilepsy: A single syndromic classification for a group of pedigrees bearing common features. Mov Disord 20: 665–673. [DOI] [PubMed] [Google Scholar]
- van Rootselaar AF,Maurits NM,Koelman JH,van der Hoeven JH,Bour LJ,Leenders KL,Brown P,Tijssen MA ( 2006): Coherence analysis differentiates between cortical myoclonic tremor and essential tremor. Mov Disord 21: 215–222. [DOI] [PubMed] [Google Scholar]
- van Rootselaar AF,Renken R,de Jong BM,Hoogduin JM,Tijssen MA,Maurits NM: fMRI analysis for motor paradigms using EMG‐based designs: A validation study. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


