Abstract
Previous studies on sex differences in neural responses to noxious stimuli yielded mixed results. Both increased and decreased brain activation in several brain areas in women as compared to men has been reported. The current event‐related functional magnetic resonance imaging study used a parametric design with different levels of the intensity of electrical stimulation in order to investigate sex differences in brain activation during pain processing. Four intensity levels, which were determined individually according to subjective ratings, ranging from stimulation below the stimulus detection threshold to moderately painful stimuli, were applied. Females experienced mild and moderate pain at lower stimulus intensity than males. Pronounced sex differences in brain activation were found in response to stimulation below the detection threshold and for the most intense pain stimuli in the medial prefrontal cortex (MPFC). Under both the conditions, women showed stronger activation in a region of the pregenual MPFC, which has been implicated in introspective, self‐focused informationprocessing. The results suggest that women, as compared to men, show increased self‐related attention during anticipation of pain and in response to intense pain. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: gender, brain, anticipation, insula, pain
INTRODUCTION
There is a considerable evidence for increased pain sensitivity in women compared to men [Berkley, 1997; Berkley and Holdcroft, 1999; Filingim, 2000]. Women show lower pain thresholds and less tolerance to somatic pain stimuli [Riley et al., 1998]. They report pain with greater frequency and are stronger affected by pain‐related diseases than men [Unruh, 1996]. Furthermore, women tend to pay more attention to pain and to respond to pain with stronger anxiety and catastrophizing [Berkeley, 1997; Edwards et al., 2004; Jones et al., 2003; Keefe et al., 2000; Koegh and Herdenfeldt, 2002; Rollman, 1995; Sullivan et al., 2000; but see Frot et al., 2004]. The latter findings suggest sex‐dependent cognitive and emotional responses to noxious stimuli. Several biological factors have been proposed to account for sex differences in pain responses, such as sex specificity in the opioid system [Filingim and Gear, 2004], in autonomic reactivity [Fillingim and Maixner, 1995; Lu et al., 2005; Tillisch et al., 2005], or in hormonal status [Aloisi, 2003; Craft et al., 2004]. However, the social, psychological, and biological factors for sex differences in pain processing are still poorly understood [Rollman et al., 2004].
A promising contribution to the understanding of factors underlying sex‐specific pain processing is the investigation of sex‐related differential neural responses to noxious stimuli [e.g. Derbyshire et al., 2002; Paulson et al., 1998]. Functional imaging studies with women and men showed that noxious stimuli are processed in several brain areas including thalamus, primary and secondary somatosensory cortex (S1 and S2), insula, anterior cingulate cortex (ACC), as well as medial prefrontal cortex (MPFC) [Apkarian et al., 2005; Baliki et al., 2006; Bornhoevd et al., 2002; Peyron et al., 2000; Tracey, 2005]. Regions such as the anterior insula, the ACC, or the MPFC seem to be more strongly involved in the subjective experience and appraisal of pain, whereas activation in other regions, such as somatosensory areas, correlates strongly with stimulus intensity [Albanese et al., 2007; Bornhovd et al., 2002; Büchel et al., 2002; Ochsner et al., 2005; Rainville et al., 1997]. However, also during pain anticipation, activation in somatosensory cortex and other pain‐related brain regions has been described suggesting a pronounced emotional and attentional modulation of brain activation [Chua et al. 1999; Ploghaus et al., 1999; Porro et al., 2002, 2003].
Previous studies on sex differences in brain activation to painful stimuli yielded mixed results. Both increased and decreased activation in several brain areas has been reported in females as compared to males. In a study by Paulson et al. [1998], participants received standardized painful or nonpainful heat stimuli. Women reported more pain and they showed greater activation to noxious stimuli in prefrontal areas, insula, and thalamus than men did. This study investigated brain responses to stimuli of equal objective intensity, which evoked, however, unequal pain experience in women and men. These behavioral differences seem to be related to the observed sex differences in brain activation. In a subsequent PET study [Derbyshire et al., 2002], pain experience was matched for the two sexes. Results of this study indicated pain‐related increased activation in perigenual ACC in women as compared to men. Remarkably, Derbyshire et al. [2002] found predominantly less activation to painful versus nonpainful stimuli in women than in men in several brain areas, including prefrontal, somatosensory, parietal, and insular cortex. A similar result of widely distributed decreased activation to painful stimuli in women compared to men has been reported very recently [Moulton et al., 2006]. Furthermore, attenuated insula activation has also been described in female patients with chronic visceral pain [Berman et al., 2000; Naliboff et al., 2003]. This effect seems partially to reflect sex differences in applied objective stimulus intensity [Berman et al., 2000]. In addition, relatively decreased activations to pain stimuli in females might also be due to increased anticipatory baseline activation in females as compared to males [Butler et al., 2005; Moulton et al., 2006; but see Berman et al., 2000].
There are no studies yet on sex differences in brain activation to noxious stimuli of varying intensity and during anticipation of these stimuli in healthy subjects. Such studies would allow investigating in detail sex‐specific stimulus–response curves. In the current event‐related functional magnetic resonance imaging (fMRI) study, we examined brain activation depending on the specific level of perceived stimulus intensity using four different intensity levels: (1) stimuli that could not be perceived (below detection threshold stimuli), (2) stimuli that were perceived but rated as nonpainful, (3) stimuli rated as mildly painful, and finally (4) stimuli rated as moderately painful [see also Bornhovd et al., 2002; Büchel et al., 2002]. By including stimulation below detection threshold, it was possible to assess brain activation during stimulus anticipation. Data analysis was based on subjective ratings. Thus, the main aim of this study was to investigate the sex‐related brain responses to not perceived but anticipated and to perceived noxious stimuli of varying intensity, whereby pain intensity was matched between sexes.
MATERIALS AND METHODS
Subjects
Thirty‐six healthy, right‐handed volunteers (18 females), provided informed consent to participate in the study. The experimental procedures were approved by the Ethics Committee of the University of Jena. All participants were students of the University of Jena. Mean age of males was 23.5 years (range: 18–33) and of females 21.3 years (range: 18–26). All participants had normal or normal‐to‐corrected vision. For the analysis of fMRI‐data, six participants of each sex had to be excluded because of either insufficient number of ratings, strong movements during scanning, or significant signal artefacts. Mean age of the remaining group of 24 subjects (12 males) was 23.2 years for males (range: 18–33) and 21.8 years for females (range: 20–26).
Stimulation and Paradigm
Somatosensory electrical stimuli consisted of rectangular pulse of 20 ms duration generated by a constant current stimulator (DS7H; Digitimer, Welwyn Garden City, UK). Stimuli were applied subcutaneously to the tip of the index finger of the left hand through an isolated golden pin electrode with a diameter of 0.95 mm and a length of 1 mm [Bromm and Meier, 1984; Meissner et al., 2004; Miltner et al., 1989; Straube et al., 2007]. The pin was inserted into a small epidermal cavity of 1 mm diameter and about 1 mm depth and fixed with adhesive tape. The purpose of this preparation was to reduce skin resistance, and thus the current necessary to elicit a pain sensation. A flexible stainless‐steel electrode, fixed loosely around the first finger joint, served as a reference electrode. Subjects were grounded by using a broad, flexible, humid band electrode fixed around the wrist of the stimulated hand.
Individual stimulus intensities were determined prior to scanning by requesting participants to rate each stimulus on a Likert scale ranging from 1 to 5 (1, no sensation; 2, perceived but not painful; 3, mildly painful; 4, moderately painful; 5, strongly painful and not tolerable). Using a modified method of limits, three series of single electrical pulses with up and downgoing intensities were applied [Miltner et al., 1989]. Mean values for intensity 2, 3, and 4 (I‐2, I‐3, and I‐4) of the last two series were used in the experiment proper. For intensity 1 (I‐1), stimulus intensity was set 10% below the lowest perceived intensity, which allowed to investigate anticipatory effects but also to detect eventual sex‐dependent changes of perception thresholds during the experiment. During scanning, 25 electrical stimuli per stimulus intensity were applied in pseudo‐randomized order with an interstimulus interval of 11.2 s. Four seconds after the stimulus, subjects were requested to rate the intensity of each stimulus by lifting 1–4 fingers of the right hand, whereby the number of fingers corresponded to the perceived intensity level [see also Büchel et al., 2002]. To indicate the moment of rating, a cue was shown for 1,000 ms on an overhead mirror using a back‐projection screen. A fixation cross was presented for the rest of time. Subjects were familiarized with this procedure prior to the experiment proper. Behavioral data were analyzed using SPSS software (Version 13; SPSS, Chicago, IL). A P‐value of P < 0.05 was considered statistically significant. All data are expressed by means and standard error (M ± SE).
fMRI
In the 1.5 T magnetic resonance scanner (“Magnetom Vision plus”, Siemens, Medical Systems, Erlangen), one run of 404 volumes were measured using a T2*‐weighted echo‐planar sequence (TE = 50 ms, flip angle = 90°, matrix = 64 × 64, FOV = 192 mm, TR = 2,800 ms). Each volume comprised of 25 axial slices (thickness = 4 mm, gap = 1 mm, in plane resolution = 3 mm × 3 mm) being acquired parallel to the AC‐PC line so that they covered the whole brain. Additionally, a high‐resolution T1‐weighted anatomical volume was recorded. Before imaging, a shimming procedure to improve field homogeneity was performed. Visual inspection of the EPI‐data revealed signal loss due to susceptibility artefacts in the inferior parts of the frontal cortex. These regions were not included in the data analysis. The first four volumes of the functional run were discarded from analysis to ensure that steady state tissue magnetization was reached.
Preprocessing and analysis of functional data was performed using the software Brain Voyager QX (Version 1.9; Brain Innovation, Maastricht, The Netherlands). The volumes were realigned to the first volume in order to minimize the effects of head movements. Further, data preprocessing comprised a correction for slice time errors and spatial [8 mm full‐width half‐maximum isotropic Gaussian kernel (FWHMK)] as well as temporal (high pass filter: 8 cycles per run; low pass filter: 2,800 ms FWHMK) smoothing. Anatomical and functional images were coregistered and normalized to the Talairach space [Talairach and Tournoux, 1988]. Statistical analysis was performed by multiple linear regression of the signal time course at each voxel including a correction for serial correlations. The expected blood oxygen level‐dependent (BOLD) signal change for each event type (= predictor) was modeled by a canonical hemodynamic response function (modified gamma function; delta = 2.5, tau = 1.25). Events of interest were the subjectively rated stimulus intensities (1–4). Event of no interest was the motor response. Within‐group statistical comparisons were conducted using a mixed effect analysis, which considers intersubject variance and permits population‐level inferences. First, voxel‐wise statistical maps were generated and the relevant, planned contrasts of predictor estimates (beta‐weights) were computed for each individual. Second, a random effect group analysis of these individual contrasts was performed. Analysis was conducted for each intensity as well as for two different stimulus–response functions (SRF). One SRF modeled a linear increase depending on perceived stimulus intensity (balanced contrast values for I‐1, I‐2, I‐3, I‐4: −3, −1, 1, 3) according to previous studies [Bornhovd et al., 2002; Büchel et al., 2002]. The other SRF modeled a nonlinear pain‐specific function irrespective of baseline activation (contrast values for I‐1 to I‐4: 0, −3, 1, 2). This contrast was masked with the contrasts: −1, 1, 0, 0 and 1, −1, 0, 0 (I‐1 to I‐4). Thus, only those voxels were considered as pain‐specific, which showed no significant activation difference while comparing I‐1 and I‐2 [see also Bornhovd et al., 2002]. Analysis was conducted in specific regions of interest (ROI), according to comparable earlier studies [Bornhovd et al., 2002; Büchel et al., 2002]. Coordinates of the ROIs were defined with the help of the Talairach daemon software (http://ric.uthscsa.edu/projects/talairachdaemon.html), which determines Brodmann areas (BA) or brain regions according to the stereotactic coordinate system of the Talairach atlas [Talairach and Tournoux, 1988]. The ROIs included the dorsal/ pregenual MPFC (medial BA 9 and 10 for z > 10; size: 10,395 mm3), anterior insula (insula for y ≥ 0; size: 10,773 mm3), ACC (for z > 10 and y < 0; size: 13,716 mm3), the whole thalamus (size: 12,771 mm3), SI (size: 12,690 mm3), and posterior insula/SII (insula for y < 0; size: 16,443 mm3). Figure 5 in supplementary materials indicates the ROIs graphically. To strike a balance between type I and type II errors, statistical parametric maps resulting from voxel‐wise analysis were considered statistically significant for clusters with voxel‐wise t‐values of P < 0.005 (uncorrected) and at least four contiguously activated 3 mm × 3 mm × 3 mm voxels [see also Straube et al., 2006a, b, 2007a, b]. However, we also tested whether the detected clusters survived a correction for multiple comparisons. We used the approach as implemented in Brain Voyager [Goebel et al., 2006] which is based on a 3D extension of the randomization procedure described by Forman et al. [1995]. First, voxel‐level threshold was set at P < 0.005 (uncorrected). Thresholded maps were then submitted to a correction for multiple comparisons based on the search space for each ROI. The correction criterion was based on the estimate of the map's spatial smoothness and on an iterative procedure (Monte Carlo simulation) for estimating cluster‐level false‐positive rates. After 1,000 iterations, the minimum cluster size threshold yielded a cluster‐level false‐positive rate of 5% was applied to the statistical maps that resulted from each of the different forms of data analysis (SRF vs. analysis of each intensity). All clusters reported in this article (see Results and Tables I, II, III) survived this ROI‐based control of multiple comparisons.
Table I.
Significant activation for each intensity
| Condition/ region | Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T‐value | Size | x | y | z | T‐value | Size | ||
| I‐1 | |||||||||||
| MPFC | R | 9 | 53 | 13 | 6.96 | 23 | |||||
| Insula | R | 48 | 5 | 4 | 5.02 | 17 | 45 | 11 | 13 | 5.32 | 12 |
| S1 | R | 54 | −16 | 40 | 5.02 | 13 | |||||
| Thalamus | R | 15 | −19 | 7 | 4.19 | 6 | |||||
| I‐2 | |||||||||||
| Insula | R | 45 | 8 | 10 | 6.65 | 111 | 42 | 5 | 16 | 5.68 | 83 |
| L | −47 | 8 | 10 | 5.54 | 93 | −32 | 7 | 16 | 6.69 | 76 | |
| ACC | −6 | 7 | 39 | 3.45 | 7 | −6 | 15 | 38 | 3.57 | 12 | |
| S1 | R | 52 | −11 | 43 | 6.80 | 11 | 57 | −14 | 29 | 5.52 | 14 |
| L | −54 | −13 | 43 | 6.25 | 12 | −43 | −30 | 53 | 5.13 | 16 | |
| p. insula/S2 | R | 30 | −22 | 14 | 6.06 | 14 | |||||
| L | −31 | −4 | 16 | 7.66 | 15 | ||||||
| Thalamus | R | 21 | −19 | 10 | 6.48 | 7 | |||||
| L | −15 | −19 | 7 | 4.89 | 5 | ||||||
| I‐3 | |||||||||||
| Insula | R | 43 | 17 | 4 | 7.15 | 117 | 40 | 8 | 7 | 9.18 | 134 |
| L | −39 | 14 | 7 | 7.35 | 83 | −39 | 11 | 10 | 10.02 | 148 | |
| ACC | 7 | 20 | 36 | 4.23 | 24 | −7 | 15 | 38 | 4.86 | 45 | |
| S1 | R | 54 | −10 | 43 | 6.28 | 37 | 57 | −16 | 33 | 6.45 | 25 |
| L | −42 | −28 | 34 | 7.68 | 26 | −51 | −11 | 43 | 7.68 | 27 | |
| p. insula/S2 | R | 36 | −1 | 17 | 5.25 | 19 | |||||
| L | −39 | −25 | 19 | 4.52 | 13 | −45 | −1 | 10 | 8.37 | 23 | |
| Thalamus | R | 6 | −22 | 7 | 4.60 | 14 | 6 | −19 | 4 | 6.27 | 31 |
| L | −6 | −19 | 4 | 6.06 | 23 | −9 | −19 | 1 | 6.40 | 29 | |
| I‐4 | |||||||||||
| Insula | R | 34 | 11 | 4 | 6.06 | 123 | 41 | 14 | 7 | 10.53 | 189 |
| L | −36 | 11 | 10 | 5.29 | 108 | −33 | 20 | 7 | 9.79 | 157 | |
| ACC | 11 | 3 | 41 | 4.25 | 43 | 6 | 7 | 40 | 5.43 | 53 | |
| S1 | R | 48 | −28 | 40 | 7.60 | 31 | 48 | −30 | 37 | 5.04 | 15 |
| L | −56 | −13 | 43 | 8.70 | 28 | −49 | −13 | 43 | 4.55 | 20 | |
| p. insula/S2 | R | 45 | −1 | 10 | 6.66 | 22 | |||||
| L | −45 | −7 | 10 | 8.41 | 28 | ||||||
| Thalamus | R | 12 | −19 | 7 | 4.28 | 5 | 6 | −19 | 1 | 8.85 | 41 |
| L | −18 | −19 | 4 | 6.37 | 6 | −12 | −19 | 10 | 7.17 | 36 | |
I, intensity; L, left; MPFC, medial prefrontal cortex; p., posterior; R, right; S, somatosensory cortex; size, number of voxels; (x, y, z), Talairach coordinates of peak voxel (activation threshold: P < 0.005, cluster ≥ 108 mm3).
Table II.
Significant stimulus response functions
| Condition/ region | Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T‐value | Size | x | y | z | T‐value | Size | ||
| Intensity | |||||||||||
| Insula | R | 39 | 17 | −4 | 6.74 | 74 | 42 | 14 | 4 | 12.46 | 156 |
| L | −36 | 11 | −2 | 6.63 | 87 | −39 | 2 | 4 | 11.40 | 167 | |
| ACC | −1 | 26 | 34 | 5.33 | 47 | 6 | 11 | 42 | 10.32 | 54 | |
| S1 | R | 46 | −28 | 41 | 4.33 | 31 | 46 | −28 | 40 | 5.44 | 36 |
| L | −36 | −37 | 58 | 5.25 | 37 | −42 | −36 | 58 | 5.23 | 18 | |
| p. insula/S2 | R | 39 | −4 | 13 | 7.26 | 15 | |||||
| L | −39 | −1 | 4 | 10.42 | 23 | ||||||
| Thalamus | R | 4 | −21 | 10 | 4.45 | 8 | |||||
| L | −3 | −19 | 13 | 5.19 | 11 | ||||||
| Pain | |||||||||||
| Insula | R | 47 | 9 | 10 | 4.56 | 14 | 42 | 11 | 5 | 10.72 | 128 |
| L | −36 | 14 | −5 | 4.20 | 9 | −39 | 8 | 7 | 12.30 | 145 | |
| ACC | 0 | 31 | 33 | 3.43 | 7 | 6 | 8 | 42 | 6.41 | 43 | |
| S1 | R | 40 | −36 | 50 | 4.90 | 25 | 45 | −27 | 40 | 7.89 | 15 |
| L | −29 | −37 | 59 | 6.65 | 38 | ||||||
| p. insula/ S2 | R | 44 | −1 | 7 | 5.89 | 7 | |||||
| L | −36 | −1 | −5 | 4.59 | 9 | −37 | −1 | 7 | 6.28 | 11 | |
| Thalamus | R | 6 | −16 | 1 | 4.55 | 7 | |||||
| L | −12 | −16 | 4 | 3.26 | 6 | ||||||
L, left; p., posterior; R, right; S, somatosensory cortex; size, number of voxels; (x, y, z), Talairach coordinates of peak voxel (activation threshold: P < 0.005, cluster ≥ 108 mm3).
Table III.
Significant sex differences in brain activation
| Condition/ region | Women > men | Men > women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T‐value | Size | x | y | z | T‐value | Size | ||
| Pain‐SRF | |||||||||||
| MPFC | R | 7 | 54 | 19 | 3.43 | 18 | |||||
| I‐1 | |||||||||||
| MPFC | R | 7 | 53 | 21 | 3.77 | 27 | |||||
| I‐3 | |||||||||||
| Insula | L | −33 | 20 | 8 | 3.40 | 23 | |||||
| I‐4 | |||||||||||
| MPFC | R | 4 | 55 | 21 | 3.33 | 10 | |||||
I, intensity; L, left; MPFC, medial prefrontal cortex; R, right; size, number of voxels; SRF, stimulus response function; (x, y, z), Talairach coordinates of peak voxel (activation threshold: P < 0.005, cluster ≥ 108 mm3).
RESULTS
Behavioral Data
Analysis of differences in objective stimulus intensities associated with individual ratings was conducted using a 4 × 2 factorial design with the within‐subject factor Intensity (I‐1, I‐2, I‐3, I‐4) and the between‐subject factor Sex (male, female). ANOVA showed a main effect of Intensity [F(3,102) = 21.74, P < 0.0001] and an interaction of Intensity by Sex [F(3,102) = 3.11, P < 0.05]. Planned t‐tests revealed that intensity did not differ between sexes for I‐1 (1.36 ± 0.29 mA vs. 1.07 ± 0.19 mA; t = 0.81, P > 0.05) and I‐2 (4.16 ± 1.23 mA vs. 2.56 ± 0.39 mA; t = 1.24, P > 0.05) but for I‐3 (11.53 ± 3.60 mA vs. 4.73 ± 1.04 mA; t = 1.81, P < 0.05) and I‐4 (18.87 ± 4.46 mA vs. 9.38 ± 2.75 mA; t = 1.82, P < 0.05). This means that less absolute stimulus intensity was required to induce mild and moderate pain in women compared to men (see Fig. 1).
Figure 1.
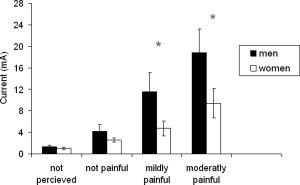
Sex differences in intensity ratings. Intensity did not differ between sexes for I‐1 (not perceived) and I‐2 (not painful) but for I‐3 (mildly painful) and I‐4 (moderately painful). Thus, less absolute stimulus intensity was required to induce mild and moderate pain in women compared to men. The asterisk indicates significant differences between sexes.
There was no significant interaction between Sex and Intensity regarding the number of ratings during the scanning session (P > 0.05). Across all subjects, the following mean numbers of ratings per intensity were provided: 28.33 ± 6.62 (I‐1), 27.13 ± 7.10 (I‐2), 27.38 ± 7.51 (I‐3), and 17.17 ± 7.11 (I‐4).
fMRI‐Data
Analysis for each sex
First, we analyzed activation to each level of stimulus intensity separately for men and women. Results are given in Table I. Overall, both sexes showed similar coordinates and t‐values for activation maxima within most ROIs. Women‐specific activation was found in the MPFC and S1 during anticipation (I‐1), while men but not women displayed activation in posterior insula/S2 during I‐4. Second, we analyzed the SRFs for each sex. Women and men showed voxels correlating with stimulus intensity in anterior insula, ACC, and S1 (see Table II). Coordinates of peak voxels were comparable between both sexes (see Table II). Additionally, men showed a significant correlation of activation with stimulus intensity in posterior insula/S2 and thalamus. For the pain SRF, women and men showed clusters correlating with pain intensity in anterior insula, ACC, posterior insula/S2, and S1 (Table II). Except for the ACC, coordinates of peak voxels were similar in both sexes. Men, but not women, showed a significant activation for the pain SRF in the thalamus. According to this SRF, Figure 2 displays significant activation maxima for each sex, or in case of the thalamus (where activation in women did not survive the cluster threshold) the peak voxel of a nonsignificant cluster in women.
Figure 2.
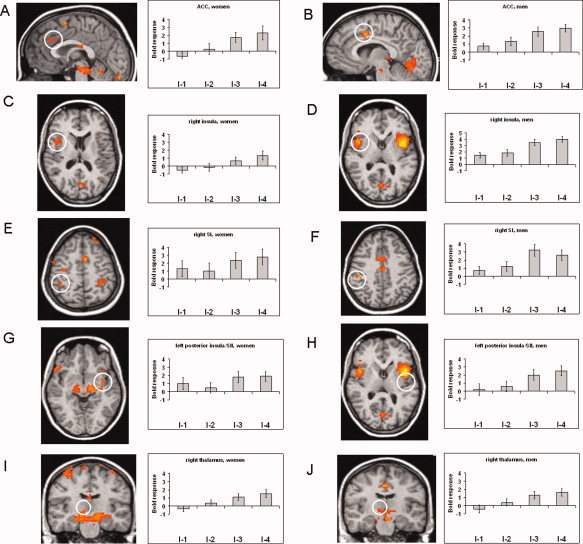
Pain‐related activation (based on the pain SRF) in woman and men within ROIs. Activation is shown for the ACC (A, women, x = 1; B, men, x = 1), right insula (C, women, z = 10; D, men, z = 5), right SI (E, women, z = 50; F, men, z = 40), left posterior insula/SII (G, women, z = −5; H, men, z = 7), right thalamus (I, women, y = −16, not significant at cluster level; J, men, y = −16). Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right). The plots show parameter estimates for each rating (mean ± standard error for maximally activated voxel).
Comparisons between sexes
In a further step, we analyzed sex differences in brain activation by means of between‐group comparisons with sex as group factor. There was no main effect of sex on brain responses independently of stimulus intensity. The analysis of sex differences for each intensity revealed differential activation for I‐1 and I‐4 in the right pregenual MPFC. For both intensities, there was an increased activation in woman as compared to men (Table III; Fig. 3). Talairach coordinates (x, y, z) for the maximal activated voxel were almost identical: 7, 53, 21 (I‐1) and 4, 55, 21 (I‐4). There was no difference between sexes when comparing the SRFs related to stimulus intensity. However, when comparing the pain‐related SRFs, we found increased activation in the MPFC in women compared to men (Table III; Fig. 3). The activation profiles across intensities of this region, as depicted in Figure 3, indicate a u‐shaped SRF in women, while men displayed no clear modulation of activation by stimulus intensity or rather an inverted u‐function, respectively. These differential types of SRFs explain why sex differences in activation of the MPFC were detected with the pain‐related SRF (which excludes intensity 1) but not with the intensity‐related SRF (which assumes a linear increase).
Figure 3.
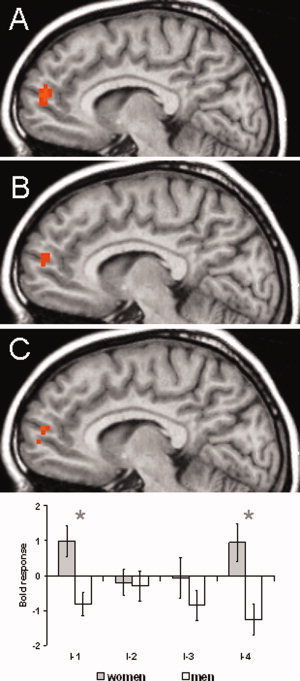
Differences in brain activation between women and men in the MPFC. Increased activation in women was found for (A) I‐1 (not perceived; y = 7), (B) I‐4 (moderately painful, y = 7), and (C) the pain SRF (y = 7). Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right). The plots show parameter estimates for each rating (mean ± standard error for maximally activated voxel at I‐1. The asterisk indicates significant differences between sexes.
Besides the effects found in the MPFC, there was a significant influence of sex on brain activation in the left insular cortex for the intensity I‐3. Here, men showed greater activation compared to women. Figure 4 displays the parameter estimates of the maximally activated voxel for the cluster in this anterior part of the left insula. This figure indicates also a tendency for increased activation in men compared to women during moderate pain (I‐4; P < 0.01). One has to consider that there is confound between sex and stimulus intensity. Men received higher stimulation than women for I‐3 and I‐4. To estimate whether the difference between women and men in brain activation might simply result from the difference between subjects with high and low pain thresholds, we investigated activation differences between low and high pain sensitive men (high threshold: 30.50 mA; low threshold: 5.17 mA; separated by median split). We have to acknowledge that this analysis of high/low threshold men is limited by very small sample size. However, even at a very lenient threshold of P < 0.05 (uncorrected), there was no activation difference in the insula for I‐3 or I‐4 between both groups. Thus, the difference in applied intensity does not seem to be the main reason for the observed sex differences in brain activation during the painful intensities.
Figure 4.
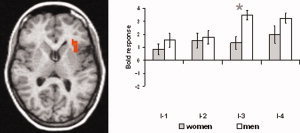
Differences in brain activation between women and men in left anterior insula. Increased activation in men was found for I‐3 (mildly painful). Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right, z = 8). The plots show parameter estimates for each rating (mean ± standard error for maximally activated voxel), indicating also a tendency of increased activation in men compared to women for I‐4 (moderately painful). The asterisk indicates significant differences between sexes.
DISCUSSION
This study investigated sex differences in ratings and neural responses during anticipation and perception of nonpainful and painful electrical stimuli. The behavioral data are in accordance with previous findings showing lowered pain thresholds and greater pain sensitivity in women as compared to men [Berkley, 1997; Berkley and Holdcroft, 1999; Filingim, 2000]. The fMRI data indicated overall similar brain activation in men and women to stimuli matched for perceived stimulus intensity. However, there were specific sex differences. Most importantly, we detected increased brain activation in the pregenual MPFC in woman as compared to men during anticipation of stimulation and in response to the strongest pain stimulus. In contrast, activation in the anterior insula was increased to mildly and, as a tendency, also to moderately painful stimuli in men.
The observation that men and woman showed activation in regions consistently activated in pain studies is in accordance with previous work [Derbyshire et al., 2002; Paulson et al., 1998]. The areas of the so‐called pain matrix such as ACC, insula, thalamus, and somatosensory cortex have been demonstrated to be increasingly activated with increasing pain intensity [Bornhovd et al., 2002]. As far as perceived pain intensity is not comparable for both sexes, differences in brain activation to similar physical stimuli might reflect differences in pain perception. For example, Paulson et al. [1998] investigated brain responses to standardized painful or nonpainful heat stimuli, which were administered with equal intensity for both sexes. In this study, females showed greater pain ratings along with stronger brain activation in the insula and thalamus. In a subsequent study of Derbyshire et al. [2002], pain experience was equalized for the two sexes instead to deliver stimuli of equal objective intensity. Differences between males and females were investigated for the voxels that correlated positively with subjective pain ratings. The authors found decreased activation in somatosensory, parietal, and insular cortex in woman compared to men, while women exhibited stronger responses in the perigenual ACC. Significantly, decreased activation in women compared to men in several brain areas during painful laser stimulation has been also reported very recently [Moulton et al., 2006].
In this study, only in the insula a statistically significant increase of activation in men as compared to women was detected. Stronger insula activation in men than in women, though comparable intensity ratings in both sexes, has been also described in patients suffering from chronic pain [Berman et al., 2003; Naliboff et al., 2003]. Besides its role in pain processing, the insular cortex has been shown to be involved in aversive emotions [e.g., Phan et al., 2002; Straube et al., 2004, 2006a, b] and generally interoceptive awareness [e.g., Craig, 2002; Critchley, 2004]. However, although the insula is involved in pain and generally in emotional processes, previous studies found that affective reports of pain correlated with activation in rostral ACC and MPFC rather than with insula activation [Berman et al., 2000; Mertz et al., 2000; Rainville et al., 1997]. It has been suggested that the insula responses might be more strongly coupled to objective stimulus intensities during painful stimulation [Berman et al., 2000; Naliboff et al., 2003], which were increased in men compared to women. However, we found no activation difference in the insula to painful stimulation when comparing high and low painsensitive men. This result does not suggest that the sex‐difference in insula activation during the painful stimulation is simply a result of sex‐differences in objective stimulus intensities. Noteworthy, the insula is involved in the representation of sympathetic arousal [e.g., Critchley, 2004]. Thus, increased insula responses might also correlate with sympathetic arousal reactions during painful stimulation that seem to be increased in men, as compared to women [Tousignant‐Laflamme and Marchand, 2006].
In the anterior pregenual MPFC, women showed a u‐shaped SRF, while men displayed no clear modulation of activation or rather an inverse u‐function, respectively. In a study by Bornhovd et al. [2002], a u‐shaped SRF in medial prefrontal areas was also found in response to anticipated, nonpainful, and painful laser stimuli. Since this study did not investigate sex differences, it remained unclear whether eventual sex‐specific activation patterns within the MPFC might exist. Bornhovd et al. suggested that activation in this region most likely reflects negative affect, which is especially pronounced during anticipation of pain and during the exposure to stronger pain stimuli. In line with this proposal, there is considerable evidence that anticipatory anxiety correlates with activation in the MPFC [Simpson et al., 2001; Straube et al., 2007a]. Recently, Ochsner et al. [2006] reported a positive correlation between individual differences in anxiety sensitivity and activation to painful heat in pregenual MPFC. In addition, activation in the pregenual and dorsomedial prefrontal cortex has been generally implicated in evaluative, emotional, self‐referential, and self‐regulative processing [Kalisch et al., 2006; Ochsner et al., 2006; Phan et al., 2002]. In an actual review, Amodio and Frith [2006] suggested that the anterior pregenual MPFC might be especially engaged in metacognitive processes such as thinking about unpleasantness of stimuli, for example the perceived unpleasantness of pain.
Self‐focusing and rumination, which correlate with medial prefrontal activation, are more pronounced in women compared to men during coping behavior [Tamres et al., 2002]. Furthermore, there is some evidence that females show increased anxiety sensitivity compared to males, which seems to have an effect on sex differences in pain processing [Jones and Zachariae, 2003; Koegh et al., 2004; Robin et al., 1987; Rollman, 1995; but see Frot et al., 2004]. Increased anxiety and worry are also related to pain disorders [Rollman et al., 2004]. Women are significantly stronger affected by chronic pain diseases than men [Unruh, 1996]. Remarkably, only activation in dorsal and pregenual MPFC has been reported to correlate with pain intensity during spontaneous pain in chronic back pain patients [Baliki et al., 2006]. Taken together, increased medial prefrontal activation and self‐focused attention in woman compared to men might be a key mechanism to understand sex‐differences in pain processing.
Some limitations of this study should be mentioned. One limitation is a relatively small final sample for the analysis of imaging data. With a larger sample size, more significant results might be found. For example, when inspecting the t‐values, men showed a tendency of higher activation in several areas during the painful stimulation. Furthermore, additional measures of behavioral, subjective, and physiological responses would be helpful in order to investigate potential relations between different sex‐related factors and brain activation during expectation and experience of painful stimulation.
In conclusion, this study suggests that processing of anticipated and experienced painful stimuli is closely associated with differential sex‐related activation in anterior pregenual MPFC. In this area, women showed an increased activation compared to men. There was no evidence of increased activation in women in other brain areas, at least when perceived intensities and pain responses were equalized between sexes. In contrast, we found some evidence for decreased activation in women in the anterior insula during painful conditions. On the basis of the putative role of the anterior MPFC, increased activation in this area suggests stronger self‐related processing of anticipated and experienced, clearly painful stimuli in women, as compared to men. Furthermore, this differential processing modus might contribute to increased pain sensitivity in women.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Figure 5
REFERENCES
- Albanese MC,Duerden EG,Rainville P,Duncan GH ( 2007): Memory traces of pain in human cortex. J Neurosci 27: 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM ( 2003): Gonadal hormones and sex differences in pain reactivity. Clin J Pain 19: 168–174. [DOI] [PubMed] [Google Scholar]
- Aloisi AM,Pari G,Ceccarelli I,Vecchi I,Ietta F,Lodi L,Paulesu L ( 2005): Gender‐related effects of chronic non‐malignant pain and opioid therapy on plasma levels of macrophage migration inhibitory factor (MIF). Pain 115: 142–151. [DOI] [PubMed] [Google Scholar]
- Amodio DM,Frith CD ( 2006): Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci 7: 268–277. [DOI] [PubMed] [Google Scholar]
- Apkarian AV,Bushnell MC,Treede RD,Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Baliki MN,Chialvo DR,Geha PY,Levy RM,Harden RN,Parrish TB,Apkarian AV ( 2006): Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ ( 1997): Sex differences in pain. Behav Brain Sci 20: 371–380. [DOI] [PubMed] [Google Scholar]
- Berkley KJ,Holdcroft A ( 1999): Sex and gender differences in pain In: Wall PD,Melzack R, editors. Textbook of Pain, 4th ed. London: Churchill Livingstone; pp 951–965. [Google Scholar]
- Berman S,Munakata J,Naliboff BD,Chang L,Mandelkern M,Silverman D,Kovalik E,Mayer EA ( 2003): Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain (London, England) 4: 157–172. [DOI] [PubMed] [Google Scholar]
- Bornhovd K,Quante M,Glauche V,Bromm B,Weiller C,Buchel C ( 2002): Painful stimuli evoke different stimulus‐response functions in the amygdala, prefrontal, insula and somatosensory cortex: A single‐trial fMRI study. Brain 125: 1326–1336. [DOI] [PubMed] [Google Scholar]
- Bromm B,Meier W ( 1984): The intracutaneous stimulus: A new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol 6: 405–410. [PubMed] [Google Scholar]
- Büchel C,Bornhovd K,Quante M,Glauche V,Bromm B,Weiller C ( 2002): Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single‐trial laser functional magnetic resonance imaging study. J Neurosci 22: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T,Pan H,Epstein J,Protopopescu X,Tuescher O,Goldstein M,Cloitre M,Yang Y,Phelps E,Gorman J,Ledoux J,Stern E,Silbersweig D ( 2005): Fear‐related activity in subgenual anterior cingulate differs between men and women. Neuroreport 16: 1233–1236. [DOI] [PubMed] [Google Scholar]
- Chua P,Krams M,Toni I,Passingham R,Dolan R ( 1999): A functional anatomy of anticipatory anxiety. Neuroimage 9: 563–571. [DOI] [PubMed] [Google Scholar]
- Craft RM,Mogil JS,Aloisi AM ( 2004): Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain 8: 397–411. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2004): The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101: 6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW,Nichols TE,Firestone L,Townsend DW,Jones AK ( 2002): Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain 3: 401–411. [DOI] [PubMed] [Google Scholar]
- Edwards RR,Haythornthwaite JA,Sullivan MJ,Fillingim RB ( 2004): Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory‐induced pain. Pain 111: 335–341. [DOI] [PubMed] [Google Scholar]
- Fillingim R ( 2000): Sex gender and pain: a biopsychosocial framework In: Fillingim R,editor. Sex, Gender, and Pain. Seattle, WA: IASP Press; pp 1–6. [Google Scholar]
- Fillingim RB,Maixner W ( 1995): Gender differences in the responses to noxious stimuli. Pain Forum 4: 209–221. [Google Scholar]
- Fillingim RB,Gear RW ( 2004): Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain 8: 413–425. [DOI] [PubMed] [Google Scholar]
- Forman SD,Cohen JD,Fitzgerald M,Eddy WF,Mintun MA,Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a clustersize threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Frot M,Feine JS,Bushnell MC ( 2004): Sex differences in pain perception and anxiety. A psychophysical study with topical capsaicin. Pain 108: 230–236. [DOI] [PubMed] [Google Scholar]
- Goebel R,Esposito F,Formisano E ( 2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A,Zachariae R,Arendt‐Nielsen L ( 2003): Dispositional anxiety and the experience of pain: gender‐specific effects. Eur J Pain 7: 387–395. [DOI] [PubMed] [Google Scholar]
- Kalisch R,Wiech K,Critchley HD,Dolan RJ ( 2006): Levels of appraisal: a medial prefrontal role in high‐level appraisal of emotional material. Neuroimage 30: 1458–1466. [DOI] [PubMed] [Google Scholar]
- Keefe FJ,Lefebvre JC,Egert JR,Affleck G,Sullivan MJ,Caldwell DS ( 2000): The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain 87: 325–334. [DOI] [PubMed] [Google Scholar]
- Keogh E,Herdenfeldt M ( 2002): Gender, coping and the perception of pain. Pain 97: 195–201. [DOI] [PubMed] [Google Scholar]
- Keogh E,Hamid R,Hamid S,Ellery D ( 2004): Investigating the effect of anxiety sensitivity, gender and negative interpretative bias on the perception of chest pain. Pain 111: 209–217. [DOI] [PubMed] [Google Scholar]
- Lu Q,Zeltzer LK,Tsao JC,Kim SC,Turk N,Naliboff BD ( 2005): Heart rate mediation of sex differences in pain tolerance in children. Pain 118: 185–193. [DOI] [PubMed] [Google Scholar]
- Meissner W,Weiss T,Trippe RH,Hecht H,Krapp C,Miltner WHR ( 2004): Acupuncture decreases somatosensory evoked potential amplitudes to noxious stimuli in anesthetized volunteers. Anesth Analg 98: 141–147. [DOI] [PubMed] [Google Scholar]
- Mertz H,Morgan V,Tanner G,Pickens D,Price R,Shyr Y,Kessler R ( 2000): Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 118: 842–848. [DOI] [PubMed] [Google Scholar]
- Miltner W,Johnson R E. Jr,Braun C,Larbig W ( 1989): Somatosensory event‐related potentials to painful and non‐painful stimuli: Effects of attention. Pain 38: 303–312. [DOI] [PubMed] [Google Scholar]
- Moulton EA,Keaser ML,Gullapalli RP,Maitra R,Greenspan JD ( 2006): Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol 291: R257–R267. [DOI] [PubMed] [Google Scholar]
- Naliboff BD,Berman S,Chang L,Derbyshire SWG,Suyenobu B,Vogt BA,Mandelkern M,Mayer EA ( 2003): Sex‐related differences in IBSpatients: Central processing of visceral stimuli. Gastroenterology 124: 1738–1747. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Beer JS,Robertson ER,Cooper JC,Gabrieli JD,Kihsltrom JF,D'Esposito M ( 2005): The neural correlates of direct and reflected self‐knowledge. Neuroimage 28: 797–814. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Ludlow DH,Knierim K,Hanelin J,Ramachandran T,Glover GC,Mackey SC ( 2006): Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE,Minoshima S,Morrow TJ,Casey KL ( 1998): Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R,Laurent B,Garcia‐Larrea L ( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager T,Taylor SF,Liberzon I ( 2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Ploghaus A,Tracey I,Gati JS,Clare S,Menon RS,Matthews PM,Rawlins JN ( 1999): Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981. [DOI] [PubMed] [Google Scholar]
- Rainville P,Duncan GH,Price DD,Carrier B,Bushnell MC ( 1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–971. [DOI] [PubMed] [Google Scholar]
- Porro CA,Baraldi P,Pagnoni G,Serafini M,Facchin P,Maieron M,Nichelli P ( 2002): Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22: 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA,Cettolo V,Francescato MP,Baraldi P ( 2003): Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage 19: 1738–1747. [DOI] [PubMed] [Google Scholar]
- Riley JL,Robinson ME,Wise EA,Myers CD,Fillingim RB ( 1998): Sex differences in the perception of noxious experimental stimuli: a metaanalysis. Pain 74: 181–187. [DOI] [PubMed] [Google Scholar]
- Robin O,Vinard H,Vernet‐Maury E,Saumet JL ( 1987): Influence of sex and anxiety on pain threshold and tolerance. Funct Neurol 2: 173–179. [PubMed] [Google Scholar]
- Rollman GB ( 1995): Gender differences in pain: Role of anxiety. Pain Forum 4: 231–234. [Google Scholar]
- Rollman GB,Abdel‐Shaheed J,Gillespie JM,Jones KS ( 2004): Does past pain influence current pain: Biological and psychosocial models of sex differences. Eur J Pain 8: 427–433. [DOI] [PubMed] [Google Scholar]
- Simpson JR Jr,Drevets WC,Snyder AZ,Gusnard DA,Raichle ME ( 2001): Emotion‐induced changes in human medial prefrontal cortex. II. During anticipatory anxiety. Proc Natl Acad Sci USA 98: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T,Kolassa IT,Glauer M,Mentzel HJ,Miltner WHR ( 2004): Effect of task conditions on brain responses to threatening faces in social phobics, an event‐related functional magnetic resonance imaging study. Biol Psychiatry 56: 921–930. [DOI] [PubMed] [Google Scholar]
- Straube T,Glauer M,Dilger S,Mentzel HJ,Miltner WHR ( 2006a): Effects of cognitive‐behavioral therapy on brain activation in specific phobia. Neuroimage 29: 125–135. [DOI] [PubMed] [Google Scholar]
- Straube T,Mentzel HJ,Miltner WHR ( 2006b): Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 59: 162–170. [DOI] [PubMed] [Google Scholar]
- Straube T,Mentzel HJ,Miltner WHR ( 2007a): Neural correlates of anticipatory anxiety in specific phobia. Neuroimage 37: 1427–1436. [DOI] [PubMed] [Google Scholar]
- Straube T,Weiss T,Mentzel HJ,Miltner WHR ( 2007b): Time course of amygdala activation during aversive conditioning depends on attention. Neuroimage 34: 462–469. [DOI] [PubMed] [Google Scholar]
- Sullivan M,Tripp D,Santor D ( 2000): Gender differences in pain and pain behavior: The role of catastrophizing. Cognitive Ther Res 24: 121–134. [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotactic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Tamres L,Janicki D,Helgeson V ( 2002): Sex differences in coping behavior: A meta‐analytic review and an examination of relative coping. Pers Soc Psychol Rev 6: 2–30. [Google Scholar]
- Tillisch K,Mayer EA,Labus JS,Stains J,Chang L,Naliboff BD ( 2005): Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut 54: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant‐Laflamme Y,Marchand S ( 2006): Sex differences in cardiac and autonomic response to clinical and experimental pain in LBP patients. Eur J Pain 10: 603–614. [DOI] [PubMed] [Google Scholar]
- Tracey I ( 2005): Nociceptive processing in the human brain. Curr Opin Neurobiol 15: 478–487. [DOI] [PubMed] [Google Scholar]
- Unruh A ( 1996): Gender variations in clinical pain experience. Pain 65: 123–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Figure 5


