Abstract
INTRODUCTION:
Fibrinolysis was initially defined using rapid thrombelastography (rTEG). The cutoffs for the pathologic extremes of the fibrinolytic system, hyperfibrinolysis and shutdown, were both defined based on association with mortality. We propose to redefine these phenotypes for both TEG and for rotational thrombelastometry, the other commonly used viscoelastic assay.
METHODS:
Rotational thrombelastometry, rTEG, and clinical data were prospectively collected on trauma patients admitted to an urban Level I trauma center from 2010 to 2016. Hyperfibrinolysis was defined as the Youden index from EXTEM-clot lysis index 60 minutes after clotting time (CLI60) and rTEG-fibrinolysis 30 minutes after achieving MA (LY30) for predicting massive transfusion (>10 red blood cell units, or death per 6 hours after injury) as a surrogate for severe bleeding. Patients identified as having hyperfibrinolysis were then removed from the data set, and the cutoff for fibrinolysis shutdown was derived as the optimal cutoff for predicting mortality in the remaining patients.
RESULTS:
Overall, 216 patients (median age, 36 years (interquartile range, 27–49 years), 82% men, 58% blunt injury) were included. Of these, 16% required massive transfusion, and 12.5% died. Rapid thrombelastography phenotypes were redefined as hyperfibrinolysis: rTEG-LY30 greater than7.7%, physiologic rTEG-LY30 0.6% to7.6%, and shutdown rTEG-LY30 less than 0.6%. EXTEM-CLI60 fibrinolysis phenotypes were hyperfibrinolysis CLI60 less than 82%, physiologic (CLI60, 82–97.9%), and shutdown (CLI60 > 98%). Weighted kappa statistics revealed moderate agreement between rotational thrombelastometry– and rTEG-defined fibrinolysis (k = 0.51; 95% confidence interval, 0.39–0.63), with disagreement mostly in the shutdown and physiologic categories.
CONCLUSION:
We confirmed the U-shaped distribution of death related to fibrinolysis system abnormalities. Both rTEG LY30 and EXTEM CLI60 can identify the spectrum of fibrinolytic phenotypes, have moderate agreement, and can be used to guide hemostatic resuscitation.
LEVEL OF EVIDENCE:
Diagnostic study, level III.
Keywords: Rotational thrombelastometry, thrombelastography, hyperfibrinolysis, fibrinolysis shutdowns, fibrinolysis phenotypes
Using rapid thrombelastography (rTEG), we previously identified three fibrinolysis phenotypes.1,2 While hyperfibrinolysis (HYPER) is infrequent, it is the most lethal form of fibrinolysis, with a mortality greater than 40%.1,2 Conversely, fibrinolysis resistance or fibrinolysis shutdown (SD) has previously been identified as the most common abnormal fibrinolytic phenotype following injury and is associated with increased mortality compared to physiologic fibrinolysis (PHY).2–4 This is often due to macrothrombotic or microthrombotic complications such as pulmonary embolus, acute lung injury, or multiple organ failure (MOF).2–4 However, others have suggested that the SD phenotype is a misclassification related to insensitivity of TEG or ROTEM to detect lysis.5,6
The initial description of the abnormal lysis phenotypes1,2 was done using rapid thrombelastography, a whole blood viscoelastic assay that provides a comprehensive assessment of clot formation, clot remodeling, and degradation. The original TEG-based definition of the three fibrinolysis phenotypes, derived in a single trauma center,1 was subsequently confirmed in a large multicenter study.2 These studies focused on trauma patients with an Injury Severity Score (ISS) greater than 15,1,2 thus limiting the cutoffs application to more severely injured patients.
The other commonly used viscoelastic test is the rotational thromboelastometry (ROTEM)7,8 platform. Rotational thromboelastometry is also a viscoelastic assay that can perform multiple specialized assays, which include the EXTEM (activated with tissue factor) assay and APTEM assay, which is a modified EXTEM, in which aprotinin inhibits plasmin in vitro if systemic fibrinolysis is present.9 Thrombelastography has consistently defined fibrinolysis phenotypes by lysis 30 minutes after achieving maximum clot strength (LY30), and it has been the primary assay used to identify the postinjury spectrum of fibrinolysis phenotypes.2–4 Conversely, defining abnormal fibrinolysis using ROTEM is more complex. Inconsistent definitions of HYPER using ROTEM exist including the use of EXTEM clot lysis index 30 minutes after clotting time (CT) (CLI30), EXTEM CLI60, and EXTEM CT greater than APTEM CT or EXTEM maximum clot firmness (MCF) less than APTEM MCF as evidence for fibrinolysis.9–16 Only recently have ROTEM-derived definitions of fibrinolysis SD been evaluated6,15 and the best ROTEM measurement for detecting fibrinolysis SD has not been established.
Although the technologies and reagents are similar between the two devices, the measurements reported differ and must not be generalized from one instrument to the other.10 Rapid TEG LY30 takes approximately 55 minutes to be finalized in severely injured trauma patients.11 This time frame roughly approximates the EXTEM lysis index 60 (LI60) derived using the ROTEM device, which quantifies the percent of clot strength remaining from maximum clot firmness 60 minutes from the initiation of the test. To our knowledge, the ROTEM-LI60 has not been used to define fibrinolysis abnormalities. Therefore, we hypothesize that abnormal fibrinolysis phenotypes (HYPER and fibrinolysis SD) can be defined by both ROTEM LI60 and rTEG, in an unselected population of trauma activation patients, and that both tests provide cutoffs that are associated with increased mortality.
METHODS
This is an analysis of prospectively collected data from our Trauma Activation Protocol study from 2010 to 2016 database, which includes patients who met criteria for the highest level of trauma team activation at the Ernest E. Moore Shock Trauma Center at Denver Health, an American College of Surgeons–verified and Colorado state–certified Level 1 trauma center affiliated with the University of Colorado Denver. Whole blood was collected from these patients under waiver of consent for minimal risk emergency research in the field or immediately upon arrival at the emergency department, and within less than 2 hours following injury.
Both ROTEM and rTEG were run immediately after sample collection, simultaneously, by onsite, full time, trained professional research assistants. This clinical study was approved by the Colorado multiple institutional review board.
Rotational Thromboelastometry
Rotational thromboelastometry (ROTEM) was performed on whole blood collected in vacuum tubes with citrate (3.2%) sample tubes (Vacutainer, Becton-Dickinson, Franklin Lakes, NJ) added to prevent clotting before analysis. The specific ROTEM assays used were EXTEM (activated with tissue factor). For each test, the reagents and the blood are pipetted semi-automatically into a single-use plastic cup that is then set onto a plastic pin on a rotating vertical axis guided by ball bearings. The increasing firmness of the clot gradually reduces the movement of the pin. This is continuously detected by using a light source, a reflecting mirror on the rotating axis, and a sensor chip. The reduction in movement is mathematically transformed into clot firmness (amplitude in millimeters) and plotted against time (in seconds), resulting in a thromboelastometric tracing. Rotational thromboelastometry tests were performed with 300 mL of citrated whole blood and 20 mL of 0.2-mol/L calcium chloride together with specific activators. In the standard global tests to measure clot strength, the activators used were 20 mL of rabbit brain thromboplastin (tissue factor) for EXTEM.12 Fibrinolysis was evaluated based on percentage of clot remaining compared to maximum clot firmness 60 minutes after CT (CLI60). This value was used, as it is temporally similar to LY30. LY30 occurs 30 minutes after maximum amplitude on TEG. While others have used maximum lysis to define fibrinolysis phenotypes in ROTEM,13 this parameter can be set to any period of observation based on institution and is not necessarily temporally related to the rTEG LY30 parameter. Given this, our analysis focused on defining fibrinolysis phenotypes using CLI60, which is temporally similar to rTEG LY30.
Rapid Thrombelastography
Rapid TEG (rTEG) was performed on whole blood collected in vacuum tubes with citrate (3.2%). Rapid TEG yields the following variables that were used to assess the dynamic process of clot formation and breakdown in this study: time to clot initiation (reaction time, minute), dynamics of clot formation (angle [α], degrees), clot strength (maximum amplitude [MA], mm), and fibrinolysis (lysis 30 minutes after MA is achieved [LY30, %]) run according to the manufacturer’s instructions on a TEG 5000 thrombelastograph hemostasis analyzer (Haemonetics Cooperation, Niles, IL).14
Data Analysis
Analyses of receiver operating characteristics (ROCs) curves were used to derive the cutoffs of rTEG LY30 and EXTEM CLI60 that defined HYPER, PHY, and SD. The ROC curve was drawn using logistic regression models with the rTEG LY30 and EXTEM LI60 as the independent variables.
The outcome for HYPER was life-threatening bleeding, as defined by the requirement for massive transfusion. To minimize survivor bias, we included death within 6 hours and massive transfusion (MT) as more than 10 red blood cell units.15 We derived the cutoff of rTEG LY30 and of EXTEM-CLI60 that best discriminated MT by the maximum Youden Index (sensitivity + specificity −1). Patients identified as having HYPER were then removed from the data set. The remaining patients formed the data set in which we derived the cutoff of rTEG LY30 and EXTEM CLI60 that best predicted mortality. The end point for this ROC curve was mortality instead of MT, as patients with SD typically die from organ failure, and not bleeding.1,2
SPSS version 23 (IBM, Armonk, NY, USA), SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), GraphPad Prism version 7.0a (GraphPad Software, Inc, La Jolla, CA), and Excel version 12.2.5 (Microsoft Corporation; Redmond, WA) were used for statistical analysis. All tests were two tailed with significance at p < 0.05. The Kruskal-Wallis test was used for continuous variables. The χ2 test was used for categorical variables. Dunn’s multiple comparisons were used for pairwise two-sided multiple comparison analysis. Agreement of rTEG LY30 and EXTEM CLI60 was compared using a test of symmetry and weighted kappa statistics. Kaplan-Meier survival curves were compared using the log-rank and Wilcoxon tests.
RESULTS
There were 216 trauma activation patients from whom both ROTEM data and rTEG were obtained within 2 hours after injury. Patients’ demographics are shown in Table 1. These were severely injured patients with a median ISS of 16 (interquartile range, 5–29). Patients were 82% male, 58% suffered a blunt injury mechanism, 16% of patients had MT, and 12.5% died.
TABLE 1.
Demographic Characteristics of Trauma Activation Patients with ROTEM Data
| Variable | |
|---|---|
| Age, yrs | 36 (26–49) |
| ISS | 16 (5–29) |
| Male | 81% |
| Systolic blood pressure (SBP) | 108 (86–134) |
| Heart rate (HR) | 100 (80–118) |
| Glasgow Coma Scale (GCS) | 14 (6–15) |
| Temperature | 36.6 (36.3–36.8) |
| Calcium | 8 (7.5–8.5) |
| Lactate | 3.5 (2.4–5.6) |
| Base deficit | 7 (4–11) |
| Hgb | 13.7 (12.2–15.1) |
| Platelet count | 252 (205–308) |
| INR | 1.14 (1.05–1.31) |
| PTT (sec) | 28 (24.8–33.3) |
| Fibrinogen | 202.5 (136.3–263.8) |
| D-dimer | 2.74 (0.96–17.19) |
| EXTEM CT (sec) | 67 (57–78.3) |
| EXTEM angle (degrees) | 68 (63–72) |
| EXTEM MCF (mm) | 58 (51–62.3) |
| EXTEN CLI60 (%) | 94 (90.8–97) |
| rTEG ACT (sec) | 121 (113–136) |
| rTEG angle (degrees) | 70.5 (64.2–75.3) |
| rTEG MA (mm) | 61.5 (54.5–65.9) |
| rTEG LY30 (%) | 1.7 (0.7–2.9) |
| Blunt mechanism | 58% |
| Massive transfusion | 16% |
Data are presented as median and interquartile range.
ACT, activated clotting time; CLI60, clot lysis index 60 minutes after CT; CT, clotting time; Hgb, hemoglobin; INR, international normalized ratio; ISS, injury severity score; LY30, fibrinolysis 30 minutes after achieving MA; MA, maximum amplitude; MCF, maximum clot firmness; PTT, partial thromboplastin time.
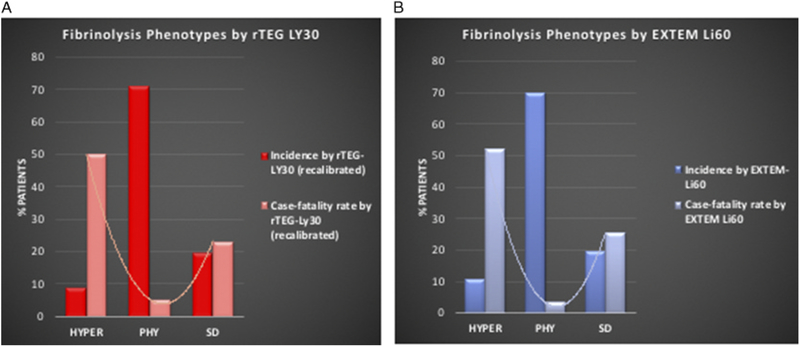
Rapid TEG defined HYPER as a Youden Index of rTEG-LY30 7.7%, the optimal cutoff to predict MT. Patients with systemic HYPER were removed, and the optimal cutoff of rTEG LY30 to predict death was used to define fibrinolysis SD. A Youden Index of 0.5% defined the cut point for SD. Therefore, fibrinolysis SD was defined as rTEG-LY30 less than 0.5%, PHYas 0.5% to 7.6%, and HYPER as greater than 7.7%. The incidence and case-fatality rate of lysis phenotypes, as defined by rTEG LY30, are shown in Figure 1A. Physiologic fibrinolysis was the most common phenotype (70.97%), followed by SD (19.82%) and HYPER (9.22%). Physiologic fibrinolysis had a significantly lower case-fatality rate compared to the two abnormal phenotypes (HYPER, 50%; and SD, 23.26%; p < 0.0001), confirming the previously described U-shaped association of abnormal lysis with mortality.
Figure 1.
Incidence of fibrinolysis phenotypes and mortality rate by fibrinolysis strata. A U-shaped distribution is illustrated using both (A) rTEG and (B) EXTEM assays.
Rotational thromboelastometry EXTEM CLI60 defined lysis phenotypes: the optimal cutoff for predicting MT was 82%. After excluding patients with a CLI60 less than 82%, another ROC curve for EXTEM CLI60 with the end point of mortality defined fibrinolysis SD as less than 98%. Therefore, we defined fibrinolysis SD as an EXTEM-LI60 98% or greater, PHY 82% to 97.9%, and HYPER less than 82%. The incidence and case-fatality rate of the EXTEM-CLI60 defined lysis phenotypes are shown in Figure 1B. These rates were remarkably similar to the rTEG lysis-derived rates as described in more detail below.
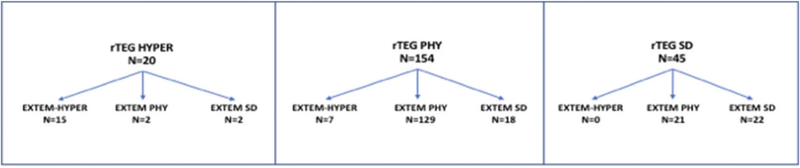
Rapid TEG and ROTEM show a large degree of lysis agreement. Overall, the two tests agreed in 76.8% of the patients, with most of the disagreement seen in distinguishing SD and PHY. Weighted kappa statistics yielded a value of 0.51 (95% confidence interval, 0.39–0.63) indicating moderate agreement between the two tests. The discordance between rTEG LY30 phenotype and EXTEM CLI phenotype is depicted in Figure 2.
Figure 2.
Discordance of rTEG LY30 and EXTEM CLI60. Patients stratified by rTEG-defined fibrinolysis phenotype as shown at the top. Patients were then stratified by EXTEM CLI60 phenotype within their rTEG-defined fibrinolysis phenotype.
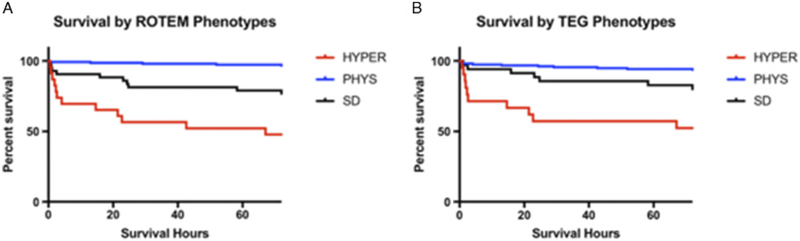
Patients’ characteristics by EXTEM CLI60 fibrinolysis phenotypes are depicted in Table 2. Significant differences were seen between groups in injury severity, systolic blood pressure, and laboratory measures of shock (base deficit and lactate). Furthermore, patients who presented with HYPER by ROTEM had an increased number of blood products transfused (Table 3) and more abnormal results of conventional coagulation assays. This same comparison was completed for redefined rTEG fibrinolysis phenotypes and showed similar findings (Supplemental Tables 1 and 2, Supplemental Digital Content 1, http://links.lww.com/TA/B257). Finally, survival time differed greatly between the groups. Survival based on groups stratified by either ROTEM or TEG phenotypes is shown in Figure 3. The HYPER group by TEG and ROTEM had an early drop in survival, while the SD cohort had a delayed mortality (log-rank p < 0.0001, Wilcoxon p < 0.0001). Physiologic fibrinolysis seems to impart a protective effect on the mortality by TEG and ROTEM.
TABLE 2.
Stratified ROTEM Fibrinolysis Phenotypes
| Variable | SD (CLI60 > 98%) | PHY (CLI60 > 82–97.9%) | HYPER (CLI60 < 82%) | Kruskal-Wallis p |
|---|---|---|---|---|
| Age, yrs | 32 (26–52) | 39 (27–48) | 35 (27–47) | 0.6332 |
| ISS | 25 (10–34)*,** | 13 (4–22) | 34 (28–43)* | <0.0001 |
| Male | 78% | 80.10% | 80.00% | 0.9057 |
| Systolic blood pressure | 106 (78–133)*,** | 117 (92–140) | 80 (0–100) | <0.0001 |
| Heart rate | 100 (81–116) | 101 (81–117) | 110 (66–132) | 0.6948 |
| Glasgow Coma Scale | 12 (3–15)* | 15 (10–15) | 3 (3–14)* | <0.0001 |
| Temperature | 36.5 (36.1–36.7) | 36.6 (36.3–36.8) | 36.4 (35.0–36.8) | 0.0967 |
| Calcium | 7.7 (7.1–8.3)* | 8 (7.6–8.5) | 7.9 (7.1–8.7) | 0.04 |
| Lactate | 3.9 (2.8–5.7)** | 3.2 (2.1–4.8) | 10.1 (5.8–14.6)* | <0.0001 |
| Base deficit | 8 (5–13)# | 6 (3–9) | 17 (11–22)* | <0.0001 |
| Hgb | 13.3 (11.6–14.6) | 14 (12.6–15.3) | 12.6 (9.9–15.1) | 0.0261 |
| Platelet count | 224 (191–280) | 258 (213–311) | 205 (124–284)* | 0.0093 |
| Blunt mechanism | 53.70% | 57.00% | 77.30% | 0.1545 |
p < 0.05 compared to PHYS,
p < 0.05 compared to HYPER.
Continuous variables are presented as median and interquartile range, while categorical variables are presented as percent. Differences between groups were identified with the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. Dunn’s multiple comparisons were used for pairwise two-sided multiple comparison analysis.
Hgb, hemoglobin; HYPER, hyperfibrinolysis; INR, international normalized ratio; PTT, partial thromboplastin time; HYPER, hyperfibrinolysis.
TABLE 3.
Conventional Coagulation and Blood Product Transfusion Characteristics of ROTEM Fibrinolysis Phenotypes
| Variable | SD (CLI60 > 98%) | PHY (CLI60 > 82–97.9%) | HYPER (CLI60 < 82%) | Kruskal-Wallis p |
|---|---|---|---|---|
| INR | 1.26(1.08–1.39)*,** | 1.08(1.02–1.2) | 1.55 (1.34–1.94)* | <0.0001 |
| PTT | 30 (26.38–36.63)*,** | 27.15 (23.78–30.35) | 44.25 (39.83–63.23)* | <0.0001 |
| Platelet count | 224 (191–280) | 258 (213–311) | 205 (124–284)* | 0.0093 |
| Fibrinogen | 173 (114–204)* | 234 (190–294) | 123 (100–158)* | 0.0018 |
| D-dimer | 2.96 (2.12–9.55) | 2.28 (0.52–11.88) | 19.86 (7.33–20.01) | 0.0968 |
| 6-hr RBCs | 0 (0–6)*,** | 0 (0–1) | 13 (7–23)* | <0.0001 |
| 6-hr plasma | 2 (0–4)*,** | 0 (0–1) | 7 (3–12)* | <0.0001 |
| 6-hr platelets | 0 (0–1)*,** | 0 (0–0) | 1 (1–2)* | <0.0001 |
| 6-hr cryo | 0 (0–0)** | 0 (0–0) | 0 (0–2)* | <0.0001 |
p < 0.05 compared to PHYS,
p < 0.05 compared to HYPER.
Patients who had hyperfibrinolysis received increased blood product transfusions and also had more abnormal conventional coagulation assay values. Differences between groups were identified with the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. Dunn’s multiple comparisons were used for pairwise two-sided multiple comparison analysis.
Cryo, cryoprecipiate.
Figure 3.
Kaplan-Meier cruves for survaival in the first 72 hours based on fibirnolysis phenotype by (A) ROTEM and (B) TEG. Hyperfibrinolysis (HYPER) shows early mortality, while the shutdown fibirnolysis (SD) phenotype has a more delayed mortality. The physiologic fibrinolysis group (PHYS) seems to have a protective effect on both ROTEM and TEG. Log-rank p < 0.0001,Wilcoxon p < 0.0001 for ROTEM and TEG comparisons.
DISCUSSION
Using ROTEM and rTEG, we have identified three distinct phenotypes of fibrinolysis in injured patients. Furthermore, we redefined rTEG fibrinolysis phenotypes in an unselected population of trauma activation patients. We described moderate agreement and symmetry between recalibrated rTEG LY30 and EXTEM CLI60 as tests for defining fibrinolysis phenotypes. A U-shaped distribution of mortality similar to what has been shown with TEG was generated for EXTEM-defined fibrinolysis phenotypes, with the greatest mortality on the extremes of fibrinolysis. Finally, these two technically different assays share almost 77% agreement for defining fibrinolysis phenotypes.
Our data confirm a recently published study from Toronto that ROTEM can effectively determine fibrinolysis phenotypes.13 While our study and the study by Gomez-Builes et al. both use ROTEM to identify fibrinolysis phenotypes, different assays were used. This suggests that there are multiple values within the ROTEM platform that can discriminate fibrinolysis phenotypes. Furthermore, our data are consistent with our previous studies that used TEG to define and stratify fibrinolytic phenotypes.1,2 We defined three distinct phenotypes based on EXTEM CLI60 that are able to discriminate mortality. Similar to the work by Gomez-Builes et al., the distribution of mortality was similar as two previously published studies evaluating fibrinolysis phenotypes using rTEG.1,2 The highest mortality was seen in the HYPER cohort, followed by SD fibrinolysis, and what seemed to be a protective degree of fibrinolysis in the PHY group (Fig. 1). This was further illustrated on both ROTEM and TEG with increased early mortality in the HYPER group (Fig. 3A and B). While in the study by Gomez-Builes et al., SD was no longer associated with mortality after multivariate analysis could be attributed to overadjustment.17 Since fibrinolysis abnormalities are mediators of the effects of injury severity and shock on mortality, adjusting for these effects obliterates its association with mortality owing to overadjustment.
Previous studies identified fibrinolysis SD as the most common fibrinolytic phenotype; however, with this recalibration in an unselected population of trauma activation patients, which included patients ultimately found to have ISS less than 16, we found that the PHY phenotype was the most common phenotype by more than twofold in all patients that had both rTEG and ROTEM measured. However, there were too few patients to effectively differentiate the cause of death between ROTEM fibrinolysis phenotypes.
Using the previous definitions of rTEG fibrinolysis phenotypes, the PHY fibrinolysis window is relatively narrow (0.9–2.9%), while the range using ROTEM is much wider (82–97.9%). Similarly, the recalibrated rTEG definitions of fibrinolysis phenotypes showed a wider range (LY30, 0.5–7.6%). This underscores a key point that in an unselected group of injured patients, injury patterns and severity are not consistent, and periodically redefining fibrinolysis phenotypes may better capture appropriate thresholds for different patient populations or institutions over time.
Sample timing is critical, since phenotypes change rapidly during the first few hours.16 Additionally, phenotype association with mortality would be expected to change as therapy changes and, consequently, would also be dependent on institution resuscitation protocol. Large randomized controlled trials and initial Cochrane analyses of viscoelastic assays in trauma resuscitation suffer from a “one size fits all” approach to very sick patients. The benefit of viscoelastic assays to guide resuscitation in the very severely injured patients may not be uncovered with large randomized controlled trials or with Cochrane analyses, which cut a wide swath and, by doing so, may miss the significant contribution to the understanding of trauma induced coagulopathy (TIC) for patients in which a model of precision-based medicine may better serve to improve outcomes in critically injured patients.17
Fibrinolysis using ROTEM in our study was evaluated based on the EXTEM CLI60, the percentage of clot remaining compared to MCF 60 minutes after CT. In contrast, LY30 used by rTEG occurs 30 minutes after maximum amplitude and gives the percentage decrease in amplitude at 30 minutes after MA.18 Previous studies have shown that the median time to MA using rapid TEG is approximately 22 minutes,19 with LY30 resulting 30 minutes from this time point. The median CT in our study was 67 seconds, with CLI60 occurring 60 minutes after this point. Therefore, the LY30 and CLI60 should represent a similar time frame and measures of fibrinolysis can be determined in less than 10 minutes from one another. Importantly, moderate agreement was seen between rTEG and EXTEM definitions of fibrinolysis phenotypes. Thrombelastography is used at our institution and more commonly in the United States, while ROTEM is prominently used in European centers.20 While TEG LY30 and EXTEM CLI60 are temporally similar, they are not defined at the exact same time, which may also explain why there is no complete agreement between assays. Although the technologies and reagents are similar, the measurements reported are not the same and cannot be generalized from one instrument to the other.10 The fact that there is disagreement could be multifactorial. Many of the patients with disagreement are at the cut point of fibrinolysis phenotypes. A patient being on opposite sides of a cut point when comparing the two tests may simply be normal instrumentation error. It could also be secondary to differences in the assay, specifically timing of measurement and that citrated rTEG is measuring amount of clot breakdown and EXTEM is measuring amount of clot remaining. Finally, after manual review of the TEG and ROTEM tracings, there were five discordant patients, which could be explained by clot retraction that resulted in a questionable lysis profile. However, the fact that we have now defined three distinct fibrinolysis phenotypes with different incidence and mortality rates (Fig. 1) indicates that these phenotypes are not just a byproduct of the TEG platform.
A critical aspect of optimizing early trauma resuscitation is understanding the mechanisms that drive derangements in the coagulation system and approaches to treating these derangements. Hyperfibrinolysis has been associated with increased levels of tissue plasminogen activator (tPA).21–23 While hemorrhagic shock does not uniformly provoke systemic HYPER in trauma patients, examples from nonsurgical patients suggest that inadequate tissue perfusion is a common feature.2,24 In our cohort, hyperfibrinolytic patients had more severe physiologic and laboratory findings of shock illustrated by a low blood pressure, elevated lactate, and more pronounced base deficit (Table 2 and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/TA/B257). This is in contrast to fibrinolysis SD, which is thought to be driven primarily by inhibition of plasminogen activation through plasminogen activator inhibitor-1 (PAI-1) inhibition of tPA.4 Furthermore, there are exogenous factors that can affect the release of PAI-1 and ultimately influence fibrinolytic phenotype. For instance, others have established a link between acute alcohol intoxication and increased risk of thrombotic complications through elevated levels of PAI-1.25,26 There is a greater increase in PAI-1 release compared to tPA in those who consume a larger amount of alcohol, thereby increasing the PAI-1:tPA ratio,27 which would presumably shift the fibrinolytic pathway toward an SD phenotype.
Fibrinolysis SD is not as well understood as systemic HYPER, partially because it manifests late following injury and seems to be a significant factor in delayed mortality.1,2 Elevated levels of PAI-1 activity, from platelets or endothelium, are associated with increased fibrinolysis SD.28 However, others have suggested that the SD phenotype is a misclassification related to insensitivity of TEG or ROTEM to detect lysis.5,6 In these patient’s, biomarkers were indicative of massive fibrinolytic activation potentially driven by endothelial bound plasminogen receptors (S100A10), which may indicate a subtype of fibrinolysis phenotypes.6 Similarly, the spectrum of fibrinolysis phenotypes may also be affected by tPA-sensitive and tPA-nonsensitive patients,28 also identifying subtypes of the classically described SD, PHY, and HYPER phenotypes. Regardless of these potential subtypes, SD is associated with an increased mortality due to macrothrombotic and microthrombotic complications such as venous thrombotic events and MOF.2 The CRASH-2 trial suggested that the early empiric use of tranexamic acid (TXA) reduces the rate of death in injured patients but identified increased mortality when this therapy was delivered more than 3 hours after injury.29 Our group has suggested that there should be a selective use of TXA in the injured patient, as administration to those with an SD phenotype may be critical in the pathogenesis of postinjury organ failure and thrombotic complications30 in TEG. Furthermore, the use of TXA in those with PHY fibrinolysis has been shown to increase mortality in that patient population.31
This study has several limitations. It was limited to one time point before a trauma patient’s resuscitation in the hospital. Recent evidence suggests a patient’s initial fibrinolysis phenotype will change during the course of his or her resuscitation.2 This change in phenotype has been illustrated by what is referred to as reperfusion fibrinolysis SD, which occurs 2 hours after injury.16 There was a relatively small sample size of patients in each group (SD, PHYS, and HYPER), yet the mortality difference was significant; thus, statistical power was not an issue. Multivariate studies are urgently needed to validate these findings, as well as those by Gomez-Builes et al. using multicenter designs, despite the difficulties in standardizing the resuscitations between centers. Furthermore, the current sample was not large enough to examine the effect of fibrinolysis phenotypes with specific injury patterns.
The addition of tissue factor to TEG or EXTEM has been thought to reduce the sensitivity of both tests by masking coagulation abnormalities.32,33 For this reason, the use of native assays (without activators), for example, remains relevant for use in research owing to its ability to dampen false signals to determine fibrinolysis and therefore the cutoff points, which predict transfusion needs in TIC.32 However, a recent comparison of native citrated kaolin, and rapid TEG suggested that transfusion thresholds can be used with confidence without concern for masking coagulation abnormalities by using an activated TEG, as there was significant overlap in the performance of the these three TEG assays.32
In conclusion, severely injured trauma patients present with a spectrum of postinjury fibrinolysis previously identified by TEG and now identified using ROTEM. The TEG and ROTEM platforms have moderate agreement in their ability to derive fibrinolysis phenotypes and can help stratify patients into different groups with different mortality. Appreciation of fibrinolysis SD as a common presenting phenotype in injured patients should warrant considerations for the judicious use of antifibrinolytics and, furthermore, stimulate thought toward treatments that are aimed at minimizing microthrombotic and macrothrombotic events and ultimately MOF.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart Lung and Blood Institute UM1-HL120877, in addition to the Department of Defense USAMRAA and W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood Institute, or the Department of Defense. Additional research support was provided by Haemonetics and Instrumentation Laboratories with shared intellectual property.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg 2014;77(6):811–817; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J. Am. Coll. Surg 2016;222(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent Fibrinolysis Shutdown Is Associated with Increased Mortality in Severely Injured Trauma Patients. J. Am. Coll. Surg 2017;224(4):575–582. [DOI] [PubMed] [Google Scholar]
- 4.Moore EE, Moore HB, Gonzalez E, Chapman MP, Hansen KC, Sauaia A, Silliman CC, Banerjee A. Postinjury fibrinolysis shutdown: Rationale for selective tranexamic acid. J Trauma Acute Care Surg 2015;78(6 Suppl 1): S65–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas JC, Wade CE, Cotton BA, George MJ, Holcomb JB, Schreiber MA, White NJ. PROPPR Study Group. Teg Lysis Shutdown Represents Coagulopathy in Bleeding Trauma Patients: Analysis of the Proppr Cohort. Shock. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gall LS, Vulliamy P, Gillespie S, Jones TF, Pierre RSJ, Breukers SE, Gaarder C, Juffermans NP, Maegele M, Stensballe J, et al. The S100A10 Pathway Mediates an Occult Hyperfibrinolytic Subtype in Trauma Patients. Ann. Surg 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma Acute Care Surg 2009;67(1):125–131. [DOI] [PubMed] [Google Scholar]
- 8.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J ThrombHaemost 2013;11(2):307–314. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J CardiothoracVascAnesth 2012;26(6):1083–1093. [DOI] [PubMed] [Google Scholar]
- 10.Saraçoğlu AYA, Tetik S. The role of viscoelastic tests in trauma: “TEG and ROTEM”. J Pharmacol Med Chem 2017;1(1):1–5. [Google Scholar]
- 11.Moore HB, Moore EE, Chapman MP, Huebner BR, Einersen PM, Oushy S, Silliman CC, Banerjee A, Sauaia A. Viscoelastic Tissue Plasminogen Activator Challenge Predicts Massive Transfusion in 15 Minutes. J. Am. Coll. Surg 2017;225(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, Pichlmaier M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. T. J Thorac Cardiovasc Surg 2009;138(3):694–702. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB. Harmful or Physiologic: Diagnosing Fibrinolysis Shutdown in a Trauma Cohort With Rotational Thromboelastometry. AnesthAnalg 2018;127(4):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan KL, Summerhayes RG, Ignjatovic V, Horton SB, Monagle PT. Reference values for kaolin-activated thromboelastography in healthy children. AnesthAnalg 2007;105(6):1610–1613. [DOI] [PubMed] [Google Scholar]
- 15.Nunns GR, Moore EE, Stettler GR, Moore HB, Ghasabyan A, Cohen M, Huebner BR, Silliman CC, Banerjee A, Sauaia A. Empiric transfusion strategies during life-threatening hemorrhage. Surgery. 2018;164(2):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore HBM, Moore EE, Gonzalez E, Huebner BJ, Sheppard F, Banerjee A, Sauaia A, Silliman CC. Reperfusion Shutdown: Delayed Onset of Fibrinolysis Resistance after Resuscitation from Hemorrhagic Shock Is Associated with Increased Circulating Levels of Plasminogen Activator Inhibitor-1 and Postinjury Complications. Blood. 2016;128:206. [Google Scholar]
- 17.Maslove DM, Lamontagne F, Marshall JC, Heyland DK. A path to precision in the ICU. Crit Care. 2017;21(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Luz LT, Nascimento B, Rizoli S. Thrombelastography (TEG): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma ResuscEmerg Med 2013;21:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman MP, Moore EE, Moore HB, Gonzalez E, Morton AP, Chandler J, Fleming CD, Ghasabyan A, Silliman CC, Banerjee A, et al. The “Death Diamond”: Rapid thrombelastography identifies lethal hyperfibrinolysis. J Trauma Acute Care Surg 2015;79(6):925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli S. TEG(R) and ROTEM(R) in trauma: similar test but different results? World J Emerg Surg 2012;7: Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma Acute Care Surg 2008;64(5): 1211–1217; discussion 7. [DOI] [PubMed] [Google Scholar]
- 22.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6): 514–521. [DOI] [PubMed] [Google Scholar]
- 23.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg 2016;80(1):16–23; discussion −5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schochl H, Cadamuro J, Seidl S, Franz A, Solomon C, Schlimp CJ, Ziegler B. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013; 84(4):454–459. [DOI] [PubMed] [Google Scholar]
- 25.Spoerke N, Underwood S, Differding J, Van P, Sambasivan C, Shapiro D, Schreiber M. Effects of ethanol intoxication and gender on blood coagulation. The J Trauma Acute Care Surg 2010;68(5):1106–1111. [DOI] [PubMed] [Google Scholar]
- 26.Djousse L, Pankow JS, Arnett DK, Zhang Y, Hong Y, Province MA, Ellison RC. Alcohol consumption and plasminogen activator inhibitor type 1: the National Heart, Lung, and Blood Institute Family Heart Study. J Am Heart Assoc 2000;139(4):704–709. [DOI] [PubMed] [Google Scholar]
- 27.Dimmitt SB, Rakic V, Puddey IB, Baker R, Oostryck R, Adams MJ, Chesterman CN, Burke V, Beilin LJ. The effects of alcohol on coagulation and fibrinolytic factors: a controlled trial. Blood Coagul Fibrinolysis 1998; 9(1):39–45. [DOI] [PubMed] [Google Scholar]
- 28.Moore HB, Moore EE, Huebner BR, Dzieciatkowska M, Stettler GR, Nunns GR, Lawson PJ, Ghasabyan A, Chandler J, Banerjee A, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg 2017;83(6):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013; 17(10):1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016;56(Suppl 2):S110–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore HB, Moore EE, Huebner BR, Stettler GR, Nunns GR, Einersen PM, Silliman CC, Sauaia A. Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. J Surg Res 2017; 220:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman JR, Moore EE, Chapman MP, Banerjee A, Silliman CC, Ghasabyan A, Chandler J, Samuels JM, Sauaia A. Rapid TEG efficiently guides hemostatic resuscitation in trauma patients. Surgery. 2018;164(3):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stettler GR, Moore EE, Moore HB, Lawson PJ, Fragoso M, Nunns GR, Silliman CC, Banerjee A. Thrombelastography indicates limitations of animal models of trauma-induced coagulopathy. J Surg Res 2017;217: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.