Abstract
Background: Superior temporal lobe dysfunction is a robust finding in functional neuroimaging studies of schizophrenia and is thought to be related to a disruption of fronto‐temporal functional connectivity. However, the stage of the disorder at which these functional alterations occur is unclear. We addressed this issue by using functional MRI (fMRI) to study subjects in the prodromal and first episode phases of schizophrenia. Methods: Subjects with an at risk mental state (ARMS) for psychosis, a first psychotic episode (FEP), and controls were studied using fMRI while performing a working memory task. Activation in the superior temporal gyrus (STG) was assessed using statistical parametric mapping, and its relationship to frontal activation was examined using dynamic causal modeling. Results: The STG was differentially engaged across the three groups. There was deactivation of this region during the task in controls, whereas subjects with FEP showed activation and the response in subjects with ARMS was intermediately relative to the two other groups. There were corresponding differences in the effective connectivity between the STG and the middle frontal gyrus across the three groups, with a negative coupling between these areas in controls, a positive coupling in the FEP group, and an intermediate value in the ARMS group. Conclusions: A failure to deactivate the superior temporal lobe during tasks that engage prefrontal cortex is evident at the onset of schizophrenia and may reflect a disruption of fronto‐temporal connectivity. Qualitatively similar alterations are evident in people with prodromal symptoms of the disorder. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: schizophrenia, at risk mental state, functional neuroimaging, dynamic causal modeling, working memory
INTRODUCTION
Structural and functional neuroimaging studies have described abnormalities in a wide range of different cortical and subcortical areas in schizophrenia [McGuire and Matsumoto,2004]. One of the areas that is robustly implicated is the superior temporal gyrus (STG), which is a consistent site of gray matter volume reductions in structural neuroimaging studies of schizophrenia [Honea et al.,2005; Wright et al.,2000]. Functional neuroimaging studies in schizophrenia report abnormal activation of STG during performance of a range of cognitive tasks [Fletcher et al.,1996; Gur et al.,2007], and activity in these regions has been linked to the severity of auditory hallucinations [Allen et al.,2007; McGuire et al.,1995] and formal thought disorder [Kircher et al.,2001; McGuire et al.,1998].
Localized functional deficits do not appear to provide a satisfactory account of the range of clinical symptoms and cognitive impairments evident in schizophrenia, and it has been proposed that the disorder may be better understood in terms of faulty integration or connectivity between different brain areas [Friston and Frith,1995; McGuire and Frith,1996]. In particular, it has been suggested that functional connectivity between the frontal and superior temporal cortex may be particularly dysfunctional in patients with schizophrenia [Fletcher et al.,1996; Frith et al.,1995; Lawrie et al.,2002; Wolf et al.,2007]. However, there has been some inconsistency in the findings from studies of functional connectivity in schizophrenia, with two studies failing to find evidence of fronto‐temporal dysconnectivity [Dye et al.,1999; Spence et al.,2000]. An important factor in this variability of findings may be heterogeneity among the patient samples studied with respect to stage of illness and previous exposure to antipsychotic treatment [Fusar‐Poli et al.,2008]. The effect of these potentially confounding factors can be minimized by restricting studies to individuals in the early phase of psychosis, such that all the participants are at the same stage of illness and have received no or minimal previous treatment.
We adopted this approach in the present study, examining superior temporal lobe function and its functional connectivity in people who were experiencing prodromal symptoms but had not yet developed psychosis, and patients who just presented with a first episode of schizophrenia. As well as being medication‐naïve, the prodromal group was of particular interest, as it allowed us to examine whether temporal lobe dysfunction and abnormal functional connectivity are specific to schizophrenia or are also evident in people at high risk of the disorder. The prodromal phase, termed the at risk mental state (ARMS), is associated with neurobiological alterations qualitatively similar to those observed in schizophrenia [Fusar‐Poli et al.,2007], including cognitive deficits [Broome et al.,2007; Keefe et al.,2006; Wood et al.,2003], reductions in frontal and temporal gray matter volume [Borgwardt et al.,2007; Meisenzahl et al.,2008; Pantelis et al.,2003], and differential activation in frontal and temporal cortex during tasks of executive functions [Broome et al.,2009; Morey et al.,2005]. Although all subjects with an ARMS experience “prodromal” signs of psychosis, not all of them will subsequently develop a psychotic disorder. Large prospective studies following subjects with an ARMS have found that around 22–31% develop a psychotic disorder within the next year [Cannon et al.,2008; Yung et al.,2007]. The search for biomarkers, which might help predict which subjects will later develop psychosis, is thus of great clinical importance. Functional integration has not previously been studied in the ARMS. However, altered fronto‐parietal and fronto‐cerebellar connectivity have been reported in the relatives of patients with schizophrenia, who are at increased genetic risk for the disorder, and can experience psychotic symptoms similar to those in the ARMS [Whalley et al.,2005].
The aim of the present study was to use functional MRI to assess temporal lobe function and its connectivity in the ARMS and first episode schizophrenia in the context of a working memory task (the N‐back task). This paradigm was chosen for two reasons. First, performance on the N‐back is robustly impaired in both the ARMS and in schizophrenia [Wood et al.,2003]. Secondly, while functional imaging studies of the N‐back in schizophrenia indicate that there may be reduced engagement of prefrontal cortex in patients relative to controls, several have reported that patients show relatively increased activation of the STG [Meyer‐Lindenberg et al.,2001; Tan et al.,2006; Thermenos et al.,2005]. In healthy volunteers, performance of visually presented working memory tasks has been shown to be associated with deactivation of the superior temporal cortex [Crottaz‐Herbette et al.,2004], and relatively greater activation of this region in schizophrenia has been found to reflect a failure of superior temporal deactivation in patients [Menzies et al.,2007; Walter et al.,2007]. In the present study, we measured group differences in regional activation during a working memory task and then used dynamic causal modeling [DCM; Friston et al.,2003; Mechelli et al.,2003b] to examine effective connectivity within the network of regions it engaged. Effective connectivity refers to the influence that one neural system exerts over another and how this is affected by the experimental context.
Our first hypothesis was that the groups would show differential activation in the superior temporal cortex, with activation in the ARMS group intermediate to that in the FEP group and controls. The second hypothesis was that these differences in activation would be attributable to differences in effective connectivity between the STG and regions within frontal cortex.
METHODS
Subjects
A total of 39 subjects participated. All were right‐handed and native speakers of English. Subjects were excluded if there was a history of neurological disorder or if they met DSM‐IV criteria for a substance misuse disorder. ARMS group comprised 16 subjects who met PACE criteria [McGorry et al.,2003], recruited from OASIS, the local clinical service for people with ARMS [Broome et al.,2005]. The diagnosis was based on assessment by two experienced clinicians using the comprehensive assessment for the ARMS [CAARMS; Yung et al.,2003] and a consensus meeting with the clinical team. All these subjects were naïve to antipsychotic medication at the time of scanning. A first episode psychosis (FEP) group comprised ten patients who had recently presented with a first episode of psychosis to LEO (http://www.slam.nhs.uk/services/), the local clinical service for first episode patients. All met ICD‐10 criteria [World Health Organisation,1992] for a schizophreniform psychosis at the time of scanning and OPCRIT criteria [McGuffin et al.,1991] for schizophrenia when assessed 12 months after presentation. Three of the first episode patients were unmedicated at the time of scanning. The other seven had been treated with either oral Risperidone or Quetiapine for a mean of 10 days (95% CI 4.7–16.3) at mean doses of 1.7 and 63.75 mg, respectively. A control group comprised 13 healthy volunteers recruited via advertisements in the local media. The three groups were matched for age and gender, and there were no significant differences between the groups in IQ and sociodemographic variables.
N‐Back Task
Subjects were presented with a series of letters on a computer screen at 2‐s intervals in 30‐s blocks. During a baseline (0‐back) condition, they were required to move a joystick to the left when the letter “X” appeared. During 1‐back and 2‐back conditions, they were required to press a button on the joystick with their right index finger if the letter currently visible was the same as that presented one or two trials beforehand, respectively. The three conditions were presented in ten alternating blocks matched for the number of target letters per block (i.e., two or three), with each block preceded by an instruction slide. Reaction time and the accuracy of the responses were recorded on‐line, and afterwards compared between the three groups using one‐way ANOVA.
Data Acquisition
Images were acquired in a 1.5 T MRI scanner (Signa LX‐GE system) at the Maudsley Hospital in London, using a TR of 2000 ms and TE 40 ms, 38 × 3 mm2 slices, with an interslice gap of 0.3 mm gap in 14 axial planes.
fMRI Data Analysis
All analyses were performed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) running in Matlab 7.0 (Mathworks, Sherborn, MA). Preprocessing included realignment of all volumes in each subject using the first as reference, normalization to a standard MNI template using nonlinear‐basis functions, and spatial smoothing with a 6 mm full width at half maximum isotropic Gaussian kernel. An event‐related analysis was performed on the block‐design‐acquired data. This type of analysis has shown to yield a more accurate model than an epoch‐analysis [Mechelli et al.,2003a] and furthermore allowed us to model error trials (either missed targets or wrong nontargets) separately. Eight experimental conditions comprising the target and nontarget events in each of the three task conditions (0‐back, 1‐back, and 2‐back) plus instructions and error trials were modeled by convolving their respective onset times with a canonical hemodynamic response function. A general linear model was used to calculate the parameter estimates for all brain voxels, and contrasts were created for each subject comparing nontarget events while performing 1‐back and 2‐back tasks, respectively, versus 0‐back condition (baseline). Nontarget (rather than target) events were chosen as their greater number made them more appropriate for an event‐related analysis, and because they were not associated with motor responses that might contribute to the BOLD response. A second level analysis was performed using the pooled 1‐ and 2‐back contrasts to identify areas activated in association with working memory consistently in the three groups, independent of mnemonic load (threshold of P < 0.05, corrected with FWE with clusters >10, masked with contrasts of activated regions for each independent group at P < 0.001). Likewise, differences in activation between the three groups were investigated using a statistical threshold of P < 0.05, corrected with FWE and an extent threshold of 10 voxels. Within our a priori region of interest, the superior temporal cortex, group‐related differences were identified using a statistical threshold of P < 0.001 and an extent threshold of 10 voxels.
DCM Analysis
The aim of DCM is to estimate and make inferences about the influence that one neural system exerts over another and how this is affected by the experimental context. In this study, DCM was used to find whether the differences found in the activation in the temporal lobe between the three groups were explained or modulated by abnormal connectivity from another brain region. No direct anatomical connections need to be assumed in DCM, since it could happen that two remote areas are functionally connected through another relay region. Three distinct sets of connectivity parameters are estimated. A first set scales the direct and extrinsic influence of inputs on brain states in any particular region. These parameters are generally of little interest in the context of DCM but are the primary focus in classical analyses of regionally specific effects. A second set of parameters (which is the primary focus in this study) refers to the “endogenous connections” that couple neuronal states in different regions and allow one to estimate the rate of change of neuronal activity in one area induced by activity in another. As such, this characterization does not depend on the units of activity per se, but on the “speed” or the rate of interregional influences. A third set of parameters or “bilinear terms” reflects changes in the intrinsic coupling between regions that are induced by experimental manipulation and were not included in this study. The reader is referred to Friston et al. [2003] and Mechelli et al. [2003b] for further information about DCM.
In the present study, the DCM analysis was implemented in SPM5. As previously mentioned, we used a lower statistical threshold (P < 0.001 uncorrected) to identify areas to be included in the DCM analysis in our a priori region of interest, namely the superior temporal cortex. Other regions entered in the analysis were identified according to two criteria: Firstly, regions activated in all three groups in association with the task; secondly, regions where there was differential activation between the FEP and control groups, with activation in the ARMS group at an intermediate level.
We limited the analysis to a single hemisphere to minimize the number of statistical comparisons and to avoid excessive computational demands. The left hemisphere was selected, because the group differences in activation during this particular task were more marked than in the right (see results below). Individual subject maxima for each subject and for each region were identified allowing for intersubject variation of the coordinates of ±6 units in each of the coronal, sagittal, and axial planes. Their first principal component (eigenvariate) was extracted using a contrast for nontarget events only. We built a model in which all the regions identified (using the functional criteria above) were unidirectionally connected to the temporal lobe. External inputs (all nontargets events) were allowed to enter the model affecting each of the areas included, except the temporal lobe, to study the effect they had on the latter. Individual connectivity parameters between the different areas to the temporal lobe were compared between the three groups using ANOVA on ranks, since it was not normally distributed. Finally, subjects within the ARMS group who later made a transition to psychosis were compared to those who did not in relation to its connectivity profile.
RESULTS
Behavioral Results
There were no significant group differences in number of missed targets in the three groups, with controls missing 1, ARMS 2.9, and FEP 3.1 (P = 0.19). There were no significant group differences in number of misidentified nontargets either, with FEP making 5.6 errors, ARMS 1.6, and control 2.2 (P = 0.15).
fMRI Results
Areas activated in all three groups
During the N‐back task (1‐back and 2‐back combined versus baseline), all three groups showed activation in a bilateral network of areas comprising the prefrontal, insular, cingulate, supplementary motor, posterior parietal, and cerebellar cortex, plus the right caudate nucleus (Fig. 1 and Table I).
Figure 1.
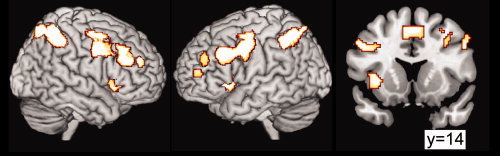
Areas activated when subjects from the three groups were performing the working memory tasks. Regions showed correspond to the combined 1‐ and 2‐back conditions against 0‐back condition, with a threshold of P < 0.05, corrected with FWE with clusters >10 and masked with contrasts of activated regions for each independent group at P < 0.001. Coordinates and Z‐scores are reported in Table I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Areas activated during the working memory task in the three groups
| Region | Cluster size | Co‐ordinates | Z‐score |
|---|---|---|---|
| Right middle frontal gyrus | 1,568 | 32, 0, 56 | Inf |
| 46, 4, 38 | Inf | ||
| 40, 32, 40 | 7.25 | ||
| 45 | 38, 50, 28 | 6.8 | |
| Left parietal lobe | 940 | −36, −50, 50 | Inf |
| −14, −72, 56 | 7.35 | ||
| Right parietal lobe | 1,313 | 38, −42, 44 | Inf |
| 28, −64, 58 | 7.21 | ||
| 36, −56, 54 | 7.21 | ||
| Left middle frontal gyrus | 1,734 | −26, 0, 56 | Inf |
| −46, 26, 38 | 7.75 | ||
| −40, 2, 34 | 7.57 | ||
| 1,568 | −44, 44, 26 | 5.73 | |
| Supplementary motor area/cingulate gyrus | 324 | 0, 14, 52 | 7.33 |
| 8, 20, 42 | 6.77 | ||
| 6, 4, 56 | 6.06 | ||
| Left insula | 380 | −34, 18, 2 | 7.32 |
| Right insula | 308 | 38, 24, −4 | 7.1 |
| Left cerebellum | 18 | −32, −60, −32 | 6.6 |
| Right caudate | 125 | 16, −4, 20 | 6.53 |
| Right cerebellum | 16 | 30, −66, −30 | 6.4 |
| Left superior frontal gyrus | 42 | −36, 56, 14 | 6.07 |
| 89 | −36, 46, 30 | 6.01 |
Areas differentially activated between groups
In the whole brain analysis, no significant differences between groups were detected at a threshold of P < 0.05 (corrected for multiple comparisons across the whole brain). However, in the a priori region of interest, the superior temporal cortex, the FEP group showed greater activation than controls bilaterally, with differences more marked in the left hemisphere (Z‐score 3.9, cluster size 126; P < 0.001) than the right (Z‐score 3.27, cluster size 13; P < 0.001). This reflected activation of this region relative to baseline in the FEP group, in contrast to the deactivation seen in controls (see Fig. 2). The level of activation in the ARMS group was intermediate to that in the other two groups.
Figure 2.
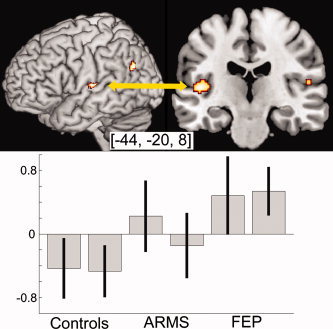
Comparison of FEP and controls when performing the working memory task. Only areas significant at P < 0.001 uncorrected and with cluster size >10 are shown. Bold signal from the highlighted area in the STG is shown in the lower graph. The two columns in each group represent the 1‐ and 2‐back conditions. Notice that in both conditions the FEP group showed activation of the STG, in contrast to the deactivation evident in controls. The ARMS group showed an intermediate pattern of activation in this region. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DCM Results
We then used DCM to examine whether the group difference in superior temporal gyrus activation could be explained by an abnormal interaction between this region and frontal areas. The three frontal regions (the supplementary motor cortex, insula, and middle frontal gyrus) activated by the task in all three groups were selected to enter the DCM analysis, along with the posterior parietal cortex, which was also activated in all three groups and was included as an internal control (representing a nonfrontal region engaged by the task) (see Fig. 1). Table II shows the coordinates of the five regions in the model and their Z‐scores for each group for the [1‐back + 2‐back] > baseline contrast.
Table II.
Co‐ordinates and Z‐scores of the five regions entered in the DCM model
| Area (all in left hemisphere) | Co‐ordinates | Z‐scores ([1‐back + 2‐back] > 0‐back) | ||||
|---|---|---|---|---|---|---|
| x | y | z | Controls | ARMS | FEP | |
| Superior temporal gyrus | −44 | −20 | 8 | −2.87 | 0.21 | 2.81 |
| Supplementary motor area | 0 | 14 | 52 | 6.17 | 4.14 | 4.10 |
| Middle frontal gyrus | −46 | 26 | 38 | 5.09 | 5.98 | 4.17 |
| Insula | −34 | 18 | 2 | 4.54 | 4.89 | 4.82 |
| Posterior parietal cortex | −36 | −50 | 50 | 7.09 | 5.16 | 4.50 |
A model connecting these four regions to superior temporal lobe was created for entering DCM analysis as shown in Figure 3.
Figure 3.
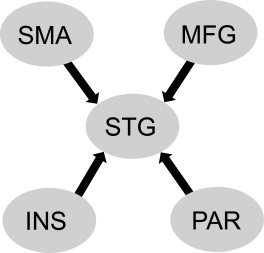
Model connecting selected regions to superior temporal gyrus (STG). Note that the directions of the connections are unidirectional to STG. SMA = supplementary motor area; MFG = middle frontal gyrus; INS = insula; PAR = posterior parietal.
Individual coupling parameters between the four selected regions and the STG as modeled were compared using ANOVA on ranks as the data were found to be non‐normally distributed. The connection between the middle frontal gyrus and the STG was the only one that was significantly different between the three groups (H = 6.659, two degrees of freedom, P = 0.036).
Post‐hoc analysis corrected for multiple comparisons using a Bonferroni‐based method showed a significant difference between FEP and controls, with FEP having a positive coupling between the middle frontal gyrus and superior temporal regions (median 0.00830; 25% 0.00670, 75% 0.0195), while controls showed a negative coupling (median −0.0076; 25% −0.0157, 75% 0.0078). In the ARMS group, there was an intermediate coupling between that in the other two groups (median 0.00180; 25% −0.00380, 75% 0.0160). The coupling parameters in each subject are displayed in the scatter plot in Figure 4.
Figure 4.
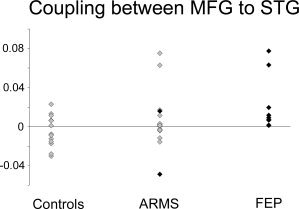
Scatter plot of coupling parameters (rate of change in activation per unit of time) for each subject in the three groups. Coupling between prefrontal and superior temporal cortex was positive in the FEP group, negative in controls, and around neutral in the ARMS group. Highlighted with black diamonds are the two subjects in the ARMS group who later made the transition to psychosis (see text).
Clinical Correlates
During the 24‐month follow‐up period, two of the ARMS group made a transition to a psychotic disorder. Their coupling parameters were 4th and 16th (of n = 16) when ranked from highest to lowest within the cohort (subjects highlighted in Fig. 4 with black).
DISCUSSION
The aims of the present study were to investigate (i) whether there was differential activation of superior temporal cortex in ARMS, FEP, and control subjects during a working memory task analyzed with an event‐related analysis, and if so, (ii) whether this could be explained by the altered connectivity between this region and frontal cortex. We found that the FEP group and to a lesser extent the ARMS group expressed increased activation in the superior temporal cortex relative to the control group in the working memory task. We then constructed a DCM model comprising areas that were consistently activated in all groups (prefrontal, insula, supplementary motor areas, and posterior parietal). The three selected frontal regions have been previously reported as sites of abnormalities in structural and functional neuroimaging studies of schizophrenia [supplementary motor area—Ortuño et al.,2005; insula—O'Daly et al.,2007; prefrontal cortex—Fu et al.,2005], raising the possibility that their connections to STG could be altered in FEP and ARMS. Examination of the individual connections of each area with the STG revealed a differential coupling between middle frontal gyrus and STG between the three groups, with controls displaying a negative coupling, FEP a positive coupling, and the coupling in the ARMS intermediate and close to neutral.
This study provides further evidence for a perturbation of fronto‐temporal connectivity in schizophrenia and the first evidence that alterations in connectivity may also be a feature, albeit to a lesser degree, in nonpsychotic people experiencing prodromal symptoms of the disorder. Most previous evidence of fronto‐temporal dysconnectivity in schizophrenia has been based on studies of chronically ill patients. As a result, it was not possible to know whether the findings were related to effects of several years of illness or its treatment with antipsychotic medication, both of which are associated with functional and volumetric changes in the brain [Cahn et al.,2002; Chakos et al.,1994; Dazzan et al.,2005]. Here, we were able to minimize these potential effects by studying patients who had only recently developed psychosis and had relatively little previous exposure to antipsychotic medication. Our data indicate that an alteration in fronto‐temporal coupling is already present in the early stages of schizophrenia and thus unlikely to be attributable to effects of illness progression or treatment subsequent to the first episode of psychosis, as it could apply to data from patients with chronic psychosis. However, there still could be a progressive deterioration of fronto‐temporal coupling in the period between the ARMS and the transition to frank psychosis, as there is evidence that there are longitudinal volumetric changes in cortical gray matter at this stage [Borgwardt et al.,2008; Pantelis et al.,2003]. To address this issue, the present study would need to be repeated in a larger sample with a larger number of transitions. We cannot exclude the possibility that the small amount of antipsychotic medication given to some participants in the FEP group may have influenced the results. Previous studies have shown that BOLD signal [Jones et al.,2004; Snitz et al.,2005] as well as functional connectivity [Stephan et al.,2001] was affected by atypical antipsychotics. The small number of subjects taking antipsychotic medication did not allow us to test for these effects statistically; however, the fact that qualitatively similar abnormalities were evident in the medication naïve ARMS group indicates that the contribution of antipsychotic medication to our results was not significant. Our findings of altered connectivity in the ARMS group are consistent with data from a recent study of another group at increased risk of psychosis, the relatives of patients with schizophrenia, although that study involved a different task that engaged different set of regions, and it examined inter‐regional correlations rather than effective connectivity [Whalley et al.,2005].
Abnormal frontal lobe function has been consistently implicated in schizophrenia. As we found that the abnormal temporal activation was related to a reversed coupling with a frontal region, the temporal dysfunction could be interpreted as an epiphenomenon of a macrocircuit alteration stemming from abnormal frontal activity. This mechanism was originally suggested by Friston and Frith [1995], who explained hyperactivation of the temporal lobe in schizophrenia during a verbal fluency task as a “second‐order effect” of perturbed frontal lobe function.
The presence of qualitatively similar changes in the ARMS to those in first episode schizophrenia suggests that this may be a correlate of the increased vulnerability of this group to the disorder. There was some heterogeneity in the strength of fronto‐temporal coupling population within the ARMS sample: some subjects had positive coupling parameters similar to those in patients with FEP, while others displayed a negative coupling, as in most of the controls (Fig. 4). However, the subjects within the ARMS group who later made a transition to psychosis did not appear to be outliers and their coupling parameters were not as positive as the FEP sample (highlighted in Fig. 4), although the small number of subjects involved precludes concluding anything from this observation about the predictive value of these changes. Whether this coupling parameter could inform prediction of subsequent conversion to psychosis will require investigation in samples large enough to yield a larger subgroup of subjects who make a transition.
There are a number of strengths to the present investigation. First, we used an event‐related analysis that modeled errors independently. Controlling for performance may be critical to identifying the involvement of the superior temporal gyrus in visually presented working memory tasks; in one study the failure to deactivate the STG in schizophrenia was only evident when patients and controls were adequately matched according to response rates (Thermenos 05). Second, we chose a hypothesis‐driven approach using DCM to look at the effective connectivity, constraining the initial areas studied that could affect STG on the basis of the results found in the classical fMRI data instead of using an analysis of functional connectivity, which is based on simple correlations.
As explained in the Methods section, we restricted our analysis to the left hemisphere. Although the right hemisphere showed similar activation during the task, we cannot necessarily conclude that similar connectivity alterations would be found. Finally, we note that the difference in effective connectivity between prefrontal and superior temporal cortex was detected without performing a correction for multiple comparisons to account for the fact that a total of four pathways were investigated within our network of interest. However, a typical correction for multiple independent comparisons, such as Bonferroni, would have been inappropriate (i.e., too conservative) because the four pathways investigated were not independent but part of the same functional network. Further studies are required to replicate our finding in independent samples of individuals with FEP and ARMS.
CONCLUSIONS
In conclusion, the present investigation suggests that the failure to deactivate the superior temporal cortex previously reported in patients with chronic schizophrenia is also evident at the onset of psychosis and, to a lesser extent, in subjects at high risk of the disorder. Furthermore, the present investigation provides support to the idea that this dysfunction can be explained in terms of fronto‐temporal dysconnectivity.
Acknowledgements
OASIS is supported by the Guy's and St Thomas' Charitable Foundation and the South London and Maudsley NHS Trust. The authors would like to thank all the clients, staff, and referrers of both OASIS and Lambeth Early Onset Services.
REFERENCES
- Allen P, Amaro E, Fu CH, Williams SC, Brammer MJ, Johns LC, McGuire PK ( 2007): Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry 190: 162–169. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher‐Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pfluger M, Rechsteiner E, D'souza M, Stieglitz RD, Radu EW, McGuire PK ( 2007): Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry 61: 1148–1156. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pflüger MO, Stieglitz RD, Radue EW, Riecher‐Rössler A ( 2008): Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res 106( 2/3): 108–114. [DOI] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Johns LC, Valmaggia LR, Tabraham P, Gafoor R, Bramon E, McGuire PK ( 2005): Outreach and support in south London (OASIS): Implementation of a clinical service for prodromal psychosis and the at risk mental state. Eur Psychiatry 20( 5/6): 372–378. [DOI] [PubMed] [Google Scholar]
- Broome MR, Johns LC, Valli I, Woolley JB, Tabraham P, Brett C, Valmaggia L, Peters E, Garety PA, McGuire PK ( 2007): Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br J Psychiatry 191 ( Suppl 51): s38–s42. [DOI] [PubMed] [Google Scholar]
- Broome MR, Matthiasson P, Fusar‐Poli P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SCR, Brammer MJ, Chitnis X, McGuire PK ( 2009): Neural correlates of executive function and working memory in the ‘at‐risk mental state’. Br J Psychiatry 194: 25–33. [DOI] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS ( 2002): Brain volume changes in first‐episode schizophrenia: A 1‐year follow‐up study. Arch Gen Psychiatry 59: 1002–1010. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R ( 2008): Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Arch Gen Psychiatry 65: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M ( 1994): Increase in caudate nuclei volumes of first‐episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 151: 1430–1436. [DOI] [PubMed] [Google Scholar]
- Crottaz‐Herbette S, Anagnoson RT, Menon V ( 2004): Modality effects in verbal working memory: Differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage 21: 340–351. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, Fearon P, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM ( 2005): Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: The AESOP study. Neuropsychopharmacology 30: 765–774. [DOI] [PubMed] [Google Scholar]
- Dye SM, Spence SA, Bench CJ, Hirsch SR, Stefan MD, Sharma T, Grasby PM ( 1999): No evidence for left superior temporal dysfunction in asymptomatic schizophrenia and bipolar disorder. PET study of verbal fluency. Br J Psychiatry 175: 367–374. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ ( 1996): Local and distributed effects of apomorphine on fronto‐temporal function in acute unmedicated schizophrenia. J Neurosci 16: 7055–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD ( 1995): Schizophrenia: A disconnection syndrome? Clin Neurosci 3: 89–97. [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF ( 1995): Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 167: 343–349. [DOI] [PubMed] [Google Scholar]
- Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK ( 2005): Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. Am J Psychiatry 162: 485–494. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P ( 2007): Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta‐analysis. Neurosci Biobehav Rev 31: 465–484. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Allen P, McGuire P ( 2008): Neuroimaging studies of the early stages of psychosis: A critical review. Eur Psychiatry 23: 237–244. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, Kanes SJ, Arnold SE, Gur RC ( 2007): Visual attention circuitry in schizophrenia investigated with oddball event‐related functional magnetic resonance imaging. Am J Psychiatry 164: 442–449. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE ( 2005): Regional deficits in brain volume in schizophrenia: A meta‐analysis of voxel‐based morphometry studies. Am J Psychiatry 162: 2233–2245. [DOI] [PubMed] [Google Scholar]
- Jones HM, Brammer MJ, O'Toole M, Taylor T, Ohlsen RI, Brown RG, Purvis R, Williams S, Pilowsky LS ( 2004): Cortical effects of quetiapine in first‐episode schizophrenia: A preliminary functional magnetic resonance imaging study. Biol Psychiatry 56: 938–942. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA ( 2006): A longitudinal study of neurocognitive function in individuals at‐risk for psychosis. Schizophr Res 88( 1–3): 26–35. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK ( 2001): Neural correlates of formal thought disorder in schizophrenia: Preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry 58: 769–774. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC ( 2002): Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51: 1008–1011. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ ( 2003): The “close‐in” or ultra high‐risk model: A safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull 29: 771–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I ( 1991): A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 48: 764–770. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Frith CD ( 1996): Disordered functional connectivity in schizophrenia. Psychol Med 26: 663–667. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Matsumoto K ( 2004): Functional neuroimaging in mental disorders. World Psychiatry 3: 6–11. [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, Murray RM, David AS, Frackowiak RS, Frith CD ( 1995): Abnormal monitoring of inner speech: A physiological basis for auditory hallucinations. Lancet 346: 596–600. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF ( 1998): Pathophysiology of ‘positive’ thought disorder in schizophrenia. Br J Psychiatry 173: 231–235. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Henson RN, Price CJ, Friston KJ ( 2003a) Comparing event‐related and epoch analysis in blocked design fMRI. Neuroimage 18: 806–810. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ ( 2003b): A dynamic causal modeling study on category effects: Bottom–up or top–down mediation? J Cogn Neurosci 15: 925–934. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Gaser C, Bottlender R, Schmitt GJ, McGuire P, Decker P, Burgermeister B, Born C, Reiser M, Möller HJ ( 2008): Structural brain alterations in subjects at high‐risk of psychosis: A voxel‐based morphometric study. Schizophr Res 102( 1–3): 150–162. [DOI] [PubMed] [Google Scholar]
- Menzies L, Ooi C, Kamath S, Suckling J, McKenna P, Fletcher P, Bullmore E, Stephenson C ( 2007): Effects of gamma‐aminobutyric acid‐modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry 64: 156–167. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF ( 2001): Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 158: 1809–1817. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A ( 2005): Imaging frontostriatal function in ultra‐high‐risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry 62: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daly OG, Frangou S, Chitnis X, Shergill SS ( 2007): Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res 156: 15–21. [DOI] [PubMed] [Google Scholar]
- Ortuño FM, Lopez P, Ojeda N, Cervera S ( 2005): Dysfunctional supplementary motor area implication during attention and time estimation tasks in schizophrenia: A PET‐O15 water study. Neuroimage 24: 575–579. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK ( 2003): Neuroanatomical abnormalities before and after onset of psychosis: A cross‐sectional and longitudinal MRI comparison. Lancet 361: 281–288. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A 3rd, Cohen JD, Cho RY, Becker T, Carter CS ( 2005): Lateral and medial hypofrontality in first‐episode schizophrenia: Functional activity in a medication‐naive state and effects of short‐term atypical antipsychotic treatment. Am J Psychiatry 162: 2322–2329. [DOI] [PubMed] [Google Scholar]
- Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JF, Grasby PM ( 2000): Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 176: 52–60. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O'Leary DS, Andreasen NC ( 2001): Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol Med 31: 1065–1078. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer‐Lindenberg A, Egan MF, Weinberger DR, Callicott JH ( 2006): Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry 163: 1969–1977. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Buka SL, Poldrack RA, Koch JK, Tsuang MT, Seidman LJ ( 2005): The effect of working memory performance on functional MRI in schizophrenia. Schizophr Res 74( 2/3): 179–194. [DOI] [PubMed] [Google Scholar]
- Walter H, Vasic N, Hose A, Spitzer M, Wolf RC ( 2007): Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: Evidence from event‐related fMRI. Neuroimage 35: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM ( 2005): Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain 128 ( Pt 9): 2097–2108. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) . 1992. ICD‐10: The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: WHO. [Google Scholar]
- Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, Ragland JD ( 2007): Alterations of fronto‐temporal connectivity during word encoding in schizophrenia. Psychiatry Res 154: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, Mahony K, Brewer W, Smith DJ, McGorry PD ( 2003): Spatial working memory ability is a marker of risk‐for‐psychosis. Psychol Med 33: 1239–1247. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe‐Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET ( 2000): Meta‐analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16–25. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD ( 2003): Psychosis prediction: 12‐month follow up of a high‐risk (“prodromal”) group. Schizophr Res 60: 21–32. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, Berger G, Francey S, Hung TC, Nelson B, Phillips L, McGorry P ( 2007). Declining transition rate in ultra high risk (prodromal) services: Dilution or reduction of risk? Schizophr Bull 33: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]


