Abstract
The classic understanding of the role of the primary somatosensory cortex (SI) is to be a first major unimodal area processing somatosensory input and reflecting the physical location of peripheral stimulation in the form of the famous homunculus. Whereas in the past this functional topography was believed to be fixed, recent studies challenge this view. For example, in upper extremity amputees the cortical representation of the mouth was found to invade the region that formerly represented the amputated limb. Moreover, several studies demonstrated dynamic modulations of the body map in SI by tactile illusions. The present study aims to further explore the role of SI by creating an illusion of feeling a supernumerary artificial limb. Using an artificial hand and arm that were connected to their body, subjects were given the visual impression that they had a supernumerary third arm. The topography in SI was examined with neuromagnetic source localization. Results revealed that the participants not only viewed the artificial arm but felt to have three arms. Thus, a simple visuo‐tactile illusion evoked feelings of ownership of a supernumerary body part. Furthermore, during the illusion the cortical representation of the thumb shifted to a more medial and superior position. Because this modulation in SI could predict the strength of the feeling that the third arm was belonging to the own body, the results suggest that the somatosensory homunculus is reflecting the perceived shape of the body rather than physical aspects of peripheral stimulation even when feeling an artificial third arm. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: illusions, body image, multisensory, somatosensory cortex, touch, vision, magnetoencephalography
INTRODUCTION
It is long established that SI represents our body in the form of the somatosensory homunculus [Penfield and Boldrey, 1937]. However, the traditional view of a fixed body map representation in SI is questioned by the results of recent studies. For instance, in upper extremity amputees the cortical representation of the mouth region was found to invade the region that formerly represented the amputated limb [Yang et al., 1994]. Further studies demonstrated modulations in SI related to motor activity [Braun et al., 2001; Schaefer et al., 2005] or attention [Braun et al., 2002]. In addition, an increasing body of evidence suggests a role for SI in multisensory integration of visual and tactile information [e.g., Schaefer et al., 2006; Zhou and Fuster, 1997, 2000]. For example, it has been reported that viewing the body part enhances tactile performance at the stimulated side. Moreover, tactile performance was best when the body part was seen through a magnifying glass [Kennett et al., 2001]. This visuotactile enhancement has been linked to a reversible short‐term plasticity of tactile receptive fields in SI [Kennett et al., 2001; Taylor‐Clarke et al., 2002].
Recently, based on results that demonstrated dynamic modulations of the body map in SI by tactile illusions, several studies proposed that the functional topography in SI may reflect the perceived shape of the body rather than physical aspects of peripheral stimulation [Blankenburg et al., 2006; Chen et al., 2003; Schaefer et al., 2006]. For instance, Chen et al. [2003] demonstrated that simultaneous stimulation of two fingertips produced a single focal cortical activation located between the expected regions for single fingertip activation. Thus, they showed activity in SI in the absence of any real input.
Moreover, a recent study demonstrated that changes in the percepted shape of the body may affect functional topography in SI. Schaefer et al. [2007] created a simple illusion of feeling an elongated arm by using the dominance of the visual domain over the tactile sense. An artificial hand and arm, connected to the body of the subjects, were used to give the participants the visual impression that they had an extended arm. The results showed modulations in SI that could predict the strength of the illusory feeling of this extended arm, suggesting an involvement of SI during perceived changes in the size of body parts.
The current study aimed to extend the hypothesis that the somatosensory homunculus mirrors the phenomenally perceived shape of the body by investigating if even artificial supernumerary body parts may be represented in the somatosensory homunculus when illusory being felt. This would provide further support for the idea that SI is reflecting the perceived body map rather than simply mirroring physical aspects of peripheral stimulation. Moreover, it would show that the perceived body image in SI is not only prone to tactile illusions on the own body surface but also can be affected by broader and more general disturbances of the body scheme like feeling supernumerary limbs.
To this end a simple tactile illusion of a supernumerary artificial hand and arm was induced. Subjects had to wear a special shirt with an artificial third arm between both arms. This third arm was ending in front of the subjects with an artificial arm and a left hand made of rubber. Thus, the subjects viewed this artificial hand and arm connected to their body between the left arm and the middle of their body (see Fig. 1). In a control condition, the artificial hand and arm were put in front of the subjects. Hence, subjects could see the artificial hand and arm close to their body, but the artificial body parts were not connected to their body. In the rest condition subjects were told to rest without showing them any body parts. In each of the three conditions subjects were stimulated with a pneumatically stimulation device on the first (D1) and fifth digit (D5) of the left hand for mapping the cortical representations of those fingers with neuromagnetic source localization. On the basis of the known dominance of the visual modality over the tactile senses [Rock and Victor, 1964; Schaefer et al., 2007], we expected that the subjects would not only view the third arm but more or less would feel this third arm and transiently believe that this arm belongs to their own body. Because we hypothesized that even artificially body parts that were illusory felt might be represented in the somatosensory homunculus, we expected modulations in SI related to this illusory feeling.
Figure 1.
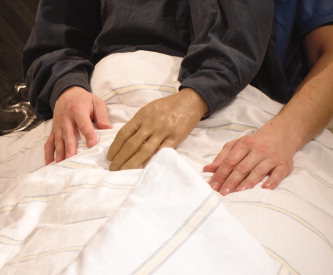
Attachment of the artificial third hand and arm to body of the subject in the illusion condition. The “third” arm was attached between mid of the bodyline and the left arm. During the control condition the artificial arm and hand were disconnected to the body and put in front of the participant. Thus, subjects were able to see this artificial arm close to their body but not connected to it. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
What type of change in SI may occur when subjects are feeling an additional hand and arm? We hypothesized that perceiving a supernumerary arm and hand would occupy additional space in the cortical representation of the real arm and hand. Thus, the original cortical representation of the hand and arm would be modulated when subjects were feeling the third arm and hand. However, the cortical representations of the upper or lower arm in SI are relatively small and difficult to measure. Thus, the current study focuses on modulations of cortical representations of D1 and D5 to examine a possible involvement of the somatosensory cortex when perceiving a change of the body shape.
MATERIALS AND METHODS
Participants
Eight right‐handed subjects (all females) with a mean age of 25 years (range 22–27) participated in the study. All subjects gave informed consent to the study, which adhered to the Declaration of Helsinki and was approved by the local human subjects' committee.
Procedure
The participants were seated on a comfortable chair with their head placed in the mould of the Dewar of a whole‐head magnetoencephalograph (MEG) system in a magnetically shielded room. A pneumatically driven stimulator was used for delivering tactile stimuli at the distal phalanges of D1 and D5 of the left hand. The stimulation device consisted of a membrane with a 10 mm diameter causing a distinct tactile sensation when inflated toward the skin. During each block D1 and D5 were stimulated. Each finger received 400 stimuli, resulting in 800 stimuli for each experimental block that lasted for ∼10 min. Stimuli were presented with an interstimulus interval of 650 ± 50 ms. Participants were instructed to ignore all tactile stimuli and not to move their head.
The study consisted out of three blocks (conditions). In the illusory third arm block (illusion condition) subjects wore a special shirt over their right arm, which left arm was ending in front of their body with an artificial arm and (left) hand made of rubber (filling consisted of wadding, weight of the artificial body part was 344 g). The participants viewed this artificial body part between their left arm and middle of the body as if it was a second left arm and hand (see Fig. 1). In the control condition, the artificial hand and arm were put in front of the subjects, enabling the subjects to view the artificial body part close to their body but not connected to it. In the rest condition subjects were told to rest without showing them the artificial body part. The blocks were presented in pseudorandomized order. Immediately after the blocks subjects had to complete a questionnaire where they had to indicate the occurrence of specific perceptual effects they had experienced. They were asked if they felt to “have three arms,” and if they felt that the “the artificial hand belongs to my body.” Subjects indicated their response on a six‐point scale ranging from “disagree completely” to “agree strongly.”
Magnetic Source Imaging
Recording of somatosensory evoked magnetic fields (SEFs) were carried out with a whole head MEG‐system with 148 first‐order gradiometers (4D‐Neuroimaging, Irvine, CA). The MEG data were acquired with a sampling rate of 2034 Hz and high‐pass filtered at 0.1 Hz. Each trial was epoched into 400 ms windows and averaged across trials for D1 and D5. Somatotopic representations of the stimulated fingers were determined by source modeling of the earliest prominent activity peak of the magnetic brain response ranging in a time window from 35 to 85 s (M60 component) [Braun et al., 2001; Elbert et al., 1995; Schaefer et al., 2006]. The generator of the M60 component has been related to neural sources in cortical area 3b of SI by previous work (e.g., Hari et al., 1993]. Ipsilateral activity was modeled by an additional source when it improved the explained variance [Zhu et al., 2007]. To determine the neuromagnetic sources dipole localizations were overlaid onto individual magnetic resonance images using CURRY multi‐modal neuroimaging software (Neuroscan, El Paso, TX).
Changes in the topographical localization of D1 and D5 were assessed using a distance measure between the equivalent dipole locations of D1 and D5. To observe modulations of the cortical representations of the fingers separately, D1 (or D5, respectively) in rest state served as a reference for potential modulations of D5 (or D1, respectively). The dipole locations were specified in polar coordinates [Braun et al., 2001; Schaefer et al., 2006]. Cortical shifts along the postcentral gyrus were expressed by polar angle Δϑ, which describes shifts in the medio–lateral direction. Representational changes in anterior–posterior direction were expressed by differences in polar angle Δφ or by differences in eccentricity (Δr) and can be related to a change in source extent. Changes in the amount of cortical activity were examined by comparing the dipole strengths.
A repeated measurements analysis of variance (ANOVA) with the factor “condition” (rest, control, illusion) was performed for statistical comparisons of differences of the cortical distances. Significance levels were adjusted with the Greenhouse‐Geisser epsilon coefficient [Jennings and Wood, 1976]. Dipole parameters were then subjected to t‐tests for paired samples.
RESULTS
Behavioral Results
All subjects felt the sensory stimulation on their real hand and not on the artificial limb. Behavioral results revealed that during the illusion condition six of eight subjects felt three arms. Two subjects did not feel any illusion at all (mean 3.00, ±1.69, on a six‐point scale ranging from “disagree completely” to “agree strongly”). Further, five of the eight subjects felt the third arm as belonging to their own body, whereas three subjects refused this completely (mean 2.88, ±1.81). Thus, although the extent of the perceptual illusion differed across the participants, most of our subjects felt the artificial third arm and hand as belonging to their own body. This illusion disappeared in the control condition, where the third arm was no longer connected to the body of the participants. Only one subject mildly claimed to feel three arms (1.10, ±0.32; t(7) = 3.63, P < 0.01). None of the participants felt the third arm as belonging to their own body (t(7) = 2.93, P < 0.05).
Neuromagnetic Source Localization
The neuromagnetic data revealed a clear dipolar neuromagnetic response in the contralateral hemisphere of each subject. An example of the time course of the evoked magnetic activity and the corresponding scalp topography for one representative subject is shown in Figure 2.
Figure 2.
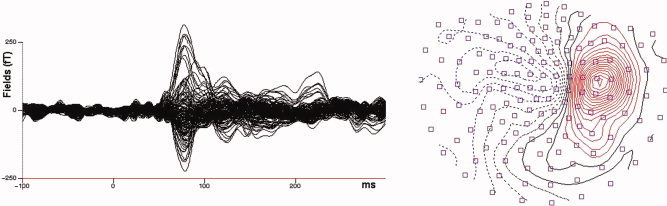
Topographic map and waveform of the evoked magnetic response of D1 (rest state, data of one representative subject). Time courses of single MEG channels are superimposed from 148 sensors. Topographic map shows the magnetic potential pattern at the first prominent peak after stimulus onset (nasion is up, right side displays the right hemisphere, left side the left hemisphere). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
An ANOVA testing for changes of the cortical representations of D1 revealed a main effect for condition (polar angle Δϑ; F(2;14) = 3.92; P < 0.05). Post‐hoc t‐tests showed that the polar angle Δϑ between the cortical representations of D1 (various conditions) and D5 (rest state) was significantly smaller in the illusion condition compared to rest (t(7) = 2.60, P < 0.05). The polar angle Δϑ during the illusion condition was also significantly smaller compared to the control condition (t(7) = −2.17, P < 0.05). This modulation corresponded to a shift of the cortical representation along the central sulcus to a more medial and superior position (see Figures 3 and 4 and Table I). Furthermore, this shift in SI was significantly positively correlated with the magnitude of the subjects' feeling that the artificial third arm belongs to their own body (Spearman's Rho, r = 0.74, P < 0.05, n = 8). No significant effects were found in radial eccentricity (Δr) or in the anterior–posterior direction (polar angle Δφ) of the dipole sources of D1 and D5. Further, there were no significant differences for modulations of the cortical representation of D5. Statistical analysis of the dipole moments yielded no significant effects.
Figure 3.
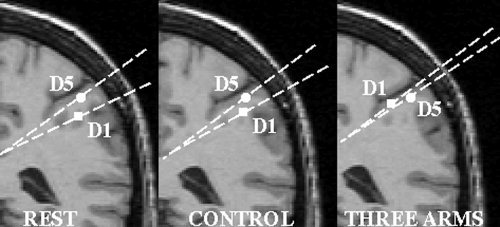
Dipole sources of the SEFs of D1 (squares) and D5 (circles, here always rest state for picture purposes) for one representative subject overlaid onto a coronal MRI slice. The positions of the cortical representations are specified in polar coordinates. Differences of the distances for the cortical representations between rest or control condition and illusion condition (three arms) are visible.
Figure 4.
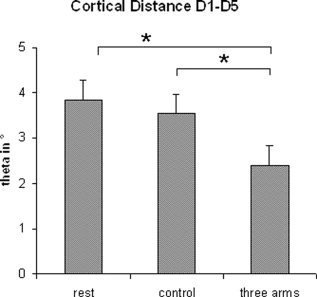
Mean differences and standard errors for the cortical distances between the cortical representations of D1 (various conditions) and D5 (rest state) in polar angle Δϑ (group data). Note the differences between the illusion condition (three arms) and rest state or control, pointing to a shift of the cortical representation of D1 to a more medial and superior position.
Table I.
Results of the neuromagentic source imaging and ratings of the subjects (coordinates in x—(medial–lateral), y—(anterior–posterior), z—(inferior–superior) dimensions; dipole strength; goodness of fit (Gof))
| Subj. | D1rest | D5rest | D1‐control | D5‐control | D1‐three arms | D5‐three arms | Feeling three arms | Feeling the artifical arm |
|---|---|---|---|---|---|---|---|---|
| 1 | −45,−48, 119; 12.2 uAmm; Gof 0.87 | −43,−44, 124; 14.6 uAmm; Gof: 0.95 | −44,−46,118; 19.2 uAmm; Gof 0.90 | −33,−42,127; 10.2 uAmm; Gof 0.86 | −42,−44,120; 22.4 uAmm; Gof 0.93 | −43,−48,122; 11.0 uAmm; Gof 0.89 | 1 | 1 |
| 2 | −20,12, 128; 15.7 uAmm; Gof 0.93 | −37, −3,130; 24.2uAmm; Gof 0.97 | −28, −6,131; 27.0uAmm; Gof 0.93 | −39, −11,136; 26.6uAmm; Gof 0.95 | −28,4,122; 23.3 uAmm; Gof 0.91 | −38, −13,135; 20.9uAmm; Gof 0.91 | 3 | 1 |
| 3 | −43,4,129; 17.7 uAmm; Gof 0.93 | −45,11,138; 11.7 uAmm; Gof 0.85 | −46,2,136; 17.6 uAmm; Gof 0.95 | −44,6,144; 15.0 uAmm; Gof 0.94 | −38,19,134; 12.9 uAmm; Gof 0.90 | −44,8,142; 10.2 uAmm; Gof 0.85 | 3 | 4 |
| 4 | −56, −34,113; 8.1uAmm; Gof 0.93 | −47, −29,121; 9.7uAmm; Gof 0.93 | −48, −30,109; 13.4uAmm; Gof 0.95 | −45, −28,117; 16.0uAmm; Gof 0.96 | −46, −28,111; 13.6uAmm; Gof 0.87 | −36, −22,116; 19.3 uAmm; Gof 0.90 | 4 | 3 |
| 5 | −32, −20,122, 13.7uAmm; Gof 0.94 | −14, −3,131; 4.9uAmm; Gof 0.85 | −37, −23,119; 14.9uAmm; Gof 0.95 | −38, −25,106; 11.3uAmm; Gof 0.85 | −32, −16,127; 13.1uAmm; Gof 0.93 | −34, −20,99; 14.4uAmm; Gof 0.88 | 2 | 3 |
| 6 | −35,−31,125; 21.1uAmm; Gof 0.97 | −37,−31,133; 9.7uAmm; Gof 0.87 | −27,−45,137; 7.2uAmm; Gof 0.85 | −46,−27,151; 3.7uAmm; Gof 0.85 | −33,−41,138; 6.0uAmm; Gof 0.87 | −34,−34,149; 4.1uAmm; Gof 0.89 | 4 | 4 |
| 7 | −44,−8,140; 9.2uAmm; Gof 0.92 | −34,15,142; 14.1 uAmm; Gof 0.98 | −37,−19,131; 17.0uAmm; Gof 0.92 | −36,−13,143; 20.0uAmm; Gof 0.91 | −33,−20,136; 17.4uAmm; Gof 0.86 | −34,−11,138; 20.4uAmm; Gof 0.92 | 1 | 1 |
| 8 | −38,−33,126; 25.0uAmm; Gof 0.88 | −40,−29,139; 18.0uAmm; Gof 0.94 | −43,−30,134; 17.6uAmm; Gof 0.91 | −44,−28,145; 8.0uAmm; Gof 0.97 | −35,−33,125; 26.1uAmm; Gof 0.91 | −53,−29,142; 14.6uAmm; Gof 0.89 | 6 | 6 |
To investigate if other variables might have systematically influenced the neuromagnetic source localizations, we examined the signal‐to‐noise‐ratios of the dipole solutions. An ANOVA with the factor “condition” (rest, control, illusion) did not show any significant effects. Further, we examined the goodness of fit of the dipole solutions in an analogous way. The results failed to show any significant effects.
The experimental conditions might also have affected the latency of the peaks of the SEFs. However, an ANOVA with the factor “condition” (rest, control, illusion) revealed no significant effects on the latencies.
DISCUSSION
The present study aimed to examine the body map in SI when subjects were feeling the illusion of having a supernumerary arm. Results revealed that most of the participants were not only seeing a third arm and hand connected to their body but felt this artificial third arm as belonging to their own body. Moreover, this feeling was significantly positively correlated with modulations in the somatosensory homunculus: the more the subjects felt this third arm and hand as their own arm and hand, the more the cortical representation of D1 was shifting to a more medial and superior position on the somatosensory cortex. Thus, a simple visual illusion seems to dynamically affect the topography of the body map in SI.
In recent years, several studies have shown that the body map in SI is not fixed but can be altered. Changes in the maps of the body surface following intensive and long‐lasting experience of altered sensory input have been demonstrated in both animals and humans [Elbert et al., 1995; Flor et al., 1995; Jenkins et al., 1990]. Further, recent studies report that the functional organization of SI may also be modulated dynamically depending on specific tasks and contexts [Braun et al., 2001, 2002; Schaefer et al., 2005]. Moreover, it has been demonstrated that tactile illusions affect the topography of the somatosensory homunculus, thus showing that SI reflects the perceived rather than physical aspects of peripheral stimulation [Blankenburg et al., 2006; Chen et al., 2003; Schaefer et al., 2006].
A recent study further suggested that the topography of SI reflects the perceived rather than actual afferent input simply by changing the visual appearance of the own body [Schaefer et al., 2007]. Using an artificial hand and arm, subjects were given the visual impression that they had an extended arm. This “morphed” body part was not only seen but also felt by many subjects. Schaefer et al. [2007] reported modulations in SI that were significantly positively correlated with the illusory feeling of this extended arm and suggested an involvement of SI during perceived changes in the size of body parts.
The results of the present study further support these results. Early studies have shown that vision often dominates the tactile sense (e.g., Rock and Victor, 1964]. We tried to make use of this by eliciting a tactile illusion merely by viewing an artificial third arm and hand that were connected to the subject's body. Most of the participants reported the sensation to feel the third arm more or less. This feeling was associated with modulations in SI. The changes in SI could predict the strength of the illusion. Thus, together with the results of the other studies, the present study suggest that the topography in SI mirrors the phenomenally perceived body image rather than physically stimulated locations of tactile stimuli on the body surface. Moreover, the present results extend previous studies by demonstrating that even artificially new body parts that are transiently believed to belong to the own body seem to be reflected within SI.
It seems remarkable that simply viewing the own body “disguised” elicits feelings of a morphed body, associated with modulations in SI topography. Several studies demonstrated that vision can dominate the tactile sense to an extent that subject's body feelings are modulated or distorted (e.g., Rock and Victor, 1964; Schaefer et al., 2007]. Whereas these studies manipulated the visual sense to evoke illusory feelings, other studies changed proprioception [Goodwin et al., 1972] or the tactile sense [Botvinick and Cohen, 1994] to induce similar illusions. However, although the strength of the illusion in the current study seems to be weaker compared with, for example, the rubber hand illusion [Botvinick and Cohen, 1994], the present study (along with Rock and Victor, 1964 and Schaefer et al., 2007] demonstrates that just viewing the own body morphed or disguised is sufficient to influence the perception of the body for many subjects. Thus, simply seeing the own body as looking different can change the perception and feeling of our own body. This may also be supported by the work of contemporary artists, for example, the work of Cindy Sherman, whose pictures of puppets in different situations often offend our feelings, although we know and easily can see that these images depict puppets and not humans.
How may vision of the new third arm have affected the somatosensory homunculus? We suggest that higher cortical areas, containing bimodal neurons sensitive for both, visual and tactile senses, may have influenced SI. Areas 3a and 2 of the somatosensory cortices have dense reciprocal connections with the motor system of the frontal lobe and areas in the posterior parietal cortex. Previously, activation of the prefrontal/premotor region has been linked to several tactile related tasks, including multisensory integration [Fink et al., 1999] or illusions of the body image [Ehrsson et al., 2004]. Thus, those areas might have had altered the map in SI via top‐down feedback. Similar top–down projections from multimodal to unimodal areas have been shown in the visual cortex of monkeys [Rockland and Ojima, 2003].
It has recently been shown that in upper extremity amputees the cortical representation of the mouth region invades the region that formerly represented the amputated limb, whereby this cortical shift is associated with the magnitude of phantom limb pain [Flor et al., 1995]. What may be the functional explanation for the shift we report here? The present study reports a modulation of the cortical representation of D1 when feeling a third arm, but no modulations of D5. Because the representation of D1 is shifted to a more medial and superior position, we suggest that the “new” third hand might be located directly below the cortical representation of D1. Additional space for the new body part is needed and this new space seems to be close to the original cortical representations of the hand and arm. Because there is more anatomical coherence in the representations above the hand (hand, arm, shoulder) compared with the functional topography below the hand (hand, face, nose), we speculate that the new hand and arm will be represented between the representations of the face and the original hand (instead of a position superior to the original hand). The results of the study provide support for this hypothesis by showing that the cortical representation of D1 was moving to a more medial and superior position during the illusion. Nevertheless, since we do not have information about modulations of other cortical representations (e.g., the arm, other fingers, or the mouth), this argument remains speculative. Furthermore, the current study cannot give any information on the topography of the ipsilateral SI, which might have been used as a reference to modulations on the contralateral side. However, pilot data revealed that the illusory effects we wanted to elicit disappeared when the experiment lasted too long. Thus, future studies are needed to support the results of dynamic modulations in the somatosensory cortex associated with the feeling of a supernumerary limb.
Whereas many studies describe and examine patients who lost a limb, so far there are only few reports of patients who feel supernumerary phantom limbs. Hari et al., [1998] and McGonigle et al. [2002] report a case study of patient E.P. with a right frontomesial lesion who sporadically experiences a supernumerary “ghost” arm. Neuromagnetic recordings revealed that activity of the left secondary somatosensory cortex was strongly suppressed during the perception of the ghost arm [Hari et al., 1998]. FMRI results showed activation of the supplementary motor area (SMA) when the ghost arm was present [McGonigle et al., 2002]. Another case study is reported by Halligan et al. [1993], who describe a patient after a hematoma within the right basal ganglia. This patient reported to feel a third arm that consisted over several months. Although this third arm caused distress for the patient and confused him, he had normal cognition and was fully oriented. Similar case studies are reported by Sellal et al. [1996] and Weinstein et al. [1954]. However, because the underlying neural mechanisms for feeling supernumerary phantom limbs after brain lesions are still seem to be only little understood, it remains unclear if illusions of having a third arm induced by visual manipulations in healthy subjects as reported in the current study are based on similar neural circuits.
REFERENCES
- Blankenburg F,Ruff CC,Deichmann R,Rees G,Driver J ( 2006): The cutaneous rabbit illusion affects human primary sensory cortex somatotopically. PloS Biol 4: e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M,Cohen J ( 1998): Rubber hand ‘feel’ touch that eyes can see. Nature 391: 756. [DOI] [PubMed] [Google Scholar]
- Braun C,Heinz U,Schweizer R,Wiech K,Birbaumer N,Topka H ( 2001): Dynamic organization of the somatosensory cortex induced by motor activity. Brain 124: 2259–2267. [DOI] [PubMed] [Google Scholar]
- Braun C,Haug M,Wiech K,Birbaumer N,Elbert T,Roberts L ( 2002): Functional organization of primay somatosensory cortex depends on the focus of attention. Neuroimage 17: 1451–1458. [DOI] [PubMed] [Google Scholar]
- Chen LM,Friedman RM,Roe AW ( 2003): Optical imaging of a tactile illusion in area 3b of the primary somatosensry cortex. Science 302: 881–885. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH,Spence C,Passingham RE ( 2004): That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305: 875–877. [DOI] [PubMed] [Google Scholar]
- Elbert T,Pantev C,Wienbruch C,Rockstroh B,Taub E ( 1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Fink G,Marshall JC,Halligan PW,Frith CD,Driver J,Frackowiak RS,Dolan RJ ( 1999): The neural consequences of conflict between intention and the senses. Brain 122: 497–512. [DOI] [PubMed] [Google Scholar]
- Flor H,Elbert T,Knecht S,Wienbruch C,Pantev C,Birbaumer N,Larbig W,Taub E ( 1995): Phantom limb pain as a perceptual correlate of massive reorganization in upper limp amputees. Nature 375: 482–484. [DOI] [PubMed] [Google Scholar]
- Goodwin GM,McCloskey DI,Mathews PBC ( 1972): The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95: 705–748. [DOI] [PubMed] [Google Scholar]
- Halligan PW,Marshall JC,Wade DT ( 1993): Three arms: a case study of supernumerary phantom limb after right hemisphere stroke. J Neurol Neurosurg Psychiatry 56: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R,Karhu J,Hämläinen M,Knuutila J,Salonen O,Sams M,Vilkman V ( 1993): Functional organization of the human first and second somatosensory cortices. A neuromagnetic study. JNeurosci 5: 724–734. [DOI] [PubMed] [Google Scholar]
- Hari R,Hänninen R,Mäkinen T,Jousmäki V,Forss N,Seppä M,Salonen O ( 1998): Three hands: fragmentation of human bodily awareness. Neurosci Lett 240: 131–134. [DOI] [PubMed] [Google Scholar]
- Jenkins WM,Merzenich MM,Ochs MT,Allard T,Guic‐Robles E ( 1990): Functional reorganization of SI in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104. [DOI] [PubMed] [Google Scholar]
- Jennings JR, and Wood CC ( 1976): The e‐adjustment procedure for repeated‐measures analyses of variance. Psychophysiol 13: 277–278. [DOI] [PubMed] [Google Scholar]
- Kennett S,Taylor‐Clarke M,Haggard P ( 2001): Noninformative vision improves the spatial resolution of touch in humans. Curr Biol 11: 1188–1191. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ,Hänninen R,Salenius S,Hari R,Frackowiak RS,Frith CD ( 2002): Whose arm is it anyway? An fMRI case study of supernumerary phantom limb. Brain 125: 1265–1274. [DOI] [PubMed] [Google Scholar]
- Penfield W,Boldrey E ( 1937): Somatic motor and sensory representation in cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Rock I,Victor J ( 1964): Vision and touch: An experimentally created conflict between the two senses. Science 143: 594–596. [DOI] [PubMed] [Google Scholar]
- Rockland KS,Ojima H ( 2003): Multisensory convergence in calcarine viusal areas in macaque monkey. Intern J Psychophysiol 50: 19–26. [DOI] [PubMed] [Google Scholar]
- Schaefer M,Flor H,Heinze H‐J,Rotte M ( 2005): Dynamic shifts in the organization of primary somatosensory cortex induced by bimanual spatial coupling of motor activity. NeuroImage 25: 395–400. [DOI] [PubMed] [Google Scholar]
- Schaefer M,Noennig N,Heinze H‐J,Rotte M ( 2006): Fooling your feelings: Artificially induced referred sensations are linked to a modulation of the primary somatosensory cortex. Neuroimage 29: 67–73. [DOI] [PubMed] [Google Scholar]
- Schaefer M,Flor H,Heinze H‐J,Rotte M ( 2007): Morphing the body: illusory feeling of an elongated arm affects somatosensory homunculus. NeuroImage 36: 700–705. [DOI] [PubMed] [Google Scholar]
- Sellal F,Renaseau‐Leclerc C,Labrecque R ( 1996): The man with 6 arms. An analysis of supernumerary phantom limbs after right hemisphere stroke. Rev Neurol (Paris) 152: 190–195. [French]. [PubMed] [Google Scholar]
- Taylor‐Clarke M,Kennett S,Haggard P ( 2002): Vision modulates somatosensory cortical processing. Curr Biol 12: 233–236. [DOI] [PubMed] [Google Scholar]
- Weinstein EA,Kahn RL,Malitz S,Rozanski J ( 1954): Delusional reduplication of parts of the body. Brain 77: 45–60. [DOI] [PubMed] [Google Scholar]
- Yang TT,Gallen C,Schwartz B,Bloom FE,Ramachandran VS,Cobb S ( 1994): Sensory maps in the human brain. Nature 368: 592–593. [DOI] [PubMed] [Google Scholar]
- Zhou YD, and Fuster JM ( 1997): Neuronal activity of somatosensory cortex in a cross‐modal (visuo‐haptic) memory task. Exp Brain Res 116: 551–555. [DOI] [PubMed] [Google Scholar]
- Zhou YD,Fuster JM ( 2000): Visuo‐tactile cross‐modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA 97: 9777–9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z,Nagarajan SS,Zumer JM,McGonigle DJ,Disbrow EA ( 2007): Spatiotemporal integration of tactile information in human somatosensory cortex. BMC Neurosci 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]


