Abstract
Variability in human behavior related to sex is supported by neuroimaging studies showing differences in brain activation patterns during cognitive task performance. An emerging field is examining the human connectome, including networks of brain regions that are not only temporally‐correlated during different task conditions, but also networks that show highly correlated spontaneous activity during a task‐free state. Both task‐related and task‐free network activity has been associated with individual task performance and behavior under certain conditions. Therefore, our aim was to determine whether sex differences exist during a task‐free resting state for two networks associated with cognitive task performance (executive control network (ECN), salience network (SN)) and the default mode network (DMN). Forty‐nine healthy subjects (26 females, 23 males) underwent a 5‐min task‐free fMRI scan in a 3T MRI. An independent components analysis (ICA) was performed to identify the best‐fit IC for each network based on specific spatial nodes defined in previous studies. To determine the consistency of these networks across subjects we performed self‐organizing group‐level ICA analyses. There were no significant differences between sexes in the functional connectivity of the brain areas within the ECN, SN, or the DMN. These important findings highlight the robustness of intrinsic connectivity of these resting state networks and their similarity between sexes. Furthermore, our findings suggest that resting state fMRI studies do not need to be controlled for sex. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, functional connectivity, cognition, default mode network, sex differences
INTRODUCTION
Sex differences exist for many types of social behavior, cognitive performance, emotional processes, and personality. For example, Crucian and Bernbaum [1998] found that men performed better than women in a mental rotation visuospatial task; whereas, another study found that women performed better than men in a verbal fluency task [Hyde and Linn, 1998]. Some studies suggest that women perform better in emotional tasks compared to men. For example, women identify facial emotional expressions more accurately [Rahman et al., 2004; Thayer and Johnsen, 2000], recall more emotional autobiographical events [Davis, 1999], and have better memory for emotional pictures compared to men [Canli et al., 2002]. It should be emphasized that such studies are based on relatively small group sizes and do not necessarily hold true for all individuals. However, the findings raise the possibility of sex as a potential confounder in brain imaging studies of human behavior. Indeed, some studies have found sex differences in functional cerebral activity during cognitive processes, even when controlling for performance differences [Jordan et al., 2002; Weiss et al., 2003]. Despite activating the same regions, the levels of activations varied between the sexes. Contradictory findings have been reported in some neuroimaging studies [Butler et al., 2007; Frings et al., 2006; Thomsen et al., 2000], in that no behavioral differences accompanied differences in brain activation, suggesting that different strategies may lead to the same behavioral outcome.
The execution of a cognitive task, and the strategy behind it, are likely the result of interaction and integration of connected brain networks that guide cognition, affect, and possibly interoceptive awareness, depending on the complexity and salience of the task. A number of groups have focused on sex‐based disparities in decision‐making or working memory tasks, specifically examining the integration of cognition and emotion in tasks requiring cognitive control of emotions [Cahill et al., 2001; Canli et al., 2000, 2002; Hamann and Canli, 2004; Hamann et al., 1999; Koch et al., 2007; Meriau et al., 2006]. These studies reported behavioral differences between men and women in emotional memories that were accompanied by distinct functional organization. Women showed activation in the brain areas associated with emotion in the right hemisphere during both the emotional experience and the memory encoding phases. In contrast, men activated the right hemisphere during the emotional experience and the left hemisphere for memory encoding. This may provide an advantage and contribute to the better memory capabilities for emotional events in women [Cahill, 2003; Cahill et al., 2001; Canli et al., 2000, 2002; Hamann and Canli, 2004].
Several studies have demonstrated sex differences in underlying mechanisms of cognitive control processes, specifically when resources are diverted from behavior to attend to emotional stimuli. For example, Koch et al. [2007] found that negative emotion induction impaired working memory performance in both sexes. However, women showed strong activation in areas associated with emotion (amygdala and orbitofrontal cortex; OFC), whereas men showed activation in regions considered important for attention and memory (superior parietal and middle temporal lobe). Therefore, there are sex differences in the interaction between cognition and emotion. The competing demands between emotion and cognition are expressed in a reciprocal relationship, balancing emotional and cognitive processing, between two adjacent brain regions: the ventral anterior cingulate cortex (vACC; ventral part of BA 32 and 24; equivalent to pregenual anterior cingulate, pgACC, based on Vogt's definition, see [Vogt, 2005]) and the dorsal ACC (dACC; dorsal part of BA 24 and 32; equivalent to anterior midcingulate cortex, aMCC, based on Vogt's definition, see [Bush et al., 2000; Vogt, 2005]). Specifically, when women, but not men, perform a cognitive task, there is vACC suppression and anti‐correlated functional connectivity with the dACC [Butler et al., 2007].
Furthermore, the execution of a cognitive task may depend on the degree of saliency and its relevance, whether emotional or homeostatic, which consequently influence brain function and behavior. For example, stronger functional connectivity between the dACC and prefrontal regions, especially the dorsolateral prefrontal cortex (DLPFC), has been associated with the ability to cognitively process emotions during perceptual decision‐making [Meriau et al., 2006]. What's more, emotional experiences have been correlated with interoceptive awareness [Critchley et al., 2004; Pollatos et al., 2007a] and with the level of activation in the dACC during an interoceptive awareness paradigm [Pollatos et al., 2007b]. Therefore, the dACC may play a role in the cognitive control of emotion, including the control of bodily responses elicited by emotional events.
Recently, fMRI has identified brain regions with similar functions to have highly correlated low frequency spontaneous fluctuations in neural activity during a task‐free state [Fox and Raichle, 2007]. Using independent component analysis (ICA), several studies [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006] have identified a network implicated in a wide range of cognitive processes and memory function. Seeley et al., [2007] further characterized this network and identified the existence of two functionally‐connected networks engaged during cognitive tasks that operate in the task‐free state. They defined one of these functionally‐connected networks as an executive control network (ECN), containing nodes in frontoparietal cortical areas: bilateral DLPFC, frontal eye fields (FEF), ventrolateral PFC, and dorsolateral parietal cortex. This ECN is thought to contribute to directed attention and working memory [Corbetta and Shulman, 2002; Curtis and D'Esposito, 2003; Miller and Cohen, 2001]. The second task‐free network is the salience network (SN) that operates when salient stimuli are perceived. The SN includes nodes in limbic and paralimbic structures (aMCC and orbital‐frontoinsula; OFI) as well as frontal, temporal, and parietal regions. These brain areas have also been found to be active while performing tasks that require interactions, depending on the task's characteristics and complexity, between cognitive and either emotional, sensory, or interoceptive stimuli [Corbetta and Shulman, 2002; Critchley, 2005; Critchley et al., 2004; Gray et al., 2002; Kerns et al., 2004; Miller and Cohen, 2001; Ochsner and Gross, 2005; Pessoa, 2008]. These two networks are distinct from the so‐called “default‐mode network” (DMN) that contains a different set of functionally‐connected regions, including the posterior cingulate cortex/precuneus (PCC/PCu) and the medial prefrontal cortex (mPFC), believed to be involved in self‐referential processing of both the internal and external environments [Fox et al., 2005; Greicius et al., 2003]. The DMN and the networks that are engaged during a cognitive task are anticorrelated: the former is deactivated during a cognitive task; whereas the latter are activated, suggesting that anticorrelated networks compete “between externally focused attention and processes subserving stimulus‐independent thought” [Fox et al., 2005]. Thus, the activity within these three functionally connected networks (DMN, ECN, and SN) during a task‐free condition can provide insight into the fundamental neural mechanisms of variability in human behavior.
The spontaneous intrinsic brain connectivity observable during the task‐free condition is also present during task performance and is thought to account for variability in human motor and cognitive behavior [Fox et al., 2006b; Hampson et al., 2006; Kelly et al., 2008] Therefore, the brain activity during a task‐free state might provide the basis for investigating individual differences in brain function [Buckner and Vincent, 2007]. Given the aforementioned sex differences in human behavior and brain activation during cognitive tasks, in addition to the association that was found between the spontaneous neural activity within networks of regions and human performance, it is possible that sex differences exist in cognitive networks during a task‐free state. Therefore, we hypothesized that in a task‐free state, men and women will have dissimilar sets of functionally connected brain areas implicated in cognitive task performance. These disparities might originate from the strength of connectivity between distinct brain areas that have a central role in cognition, such as the prefrontal and parietal cortices (i.e., ECN) with or without functional connectivity to other areas that are active during salience (aMCC and OFI) (i.e., SN). More specifically, based on cognitive behavioral studies, we expected that, in women, the ECN would demonstrate stronger functional connectivity to frontal brain areas and that the SN would show stronger functional connectivity to aMCC and OFI. Furthermore, we expected that these findings would be more apparent on the right side of the brain. To test this hypothesis, we acquired and analyzed task‐free fMRI data from healthy men and women and performed an ICA analysis. For each subject, we selected ICs related to the cognition networks (ECN and SN) and DMN based on specific spatial nodes that have been defined in previous studies [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; Seeley et al., 2007] and then performed a between‐group comparison in an ANOVA random effects analysis.
METHODS
Subjects
A total of forty‐nine right‐handed healthy subjects (26 women, 23 men) participated in the study and provided informed written consent to procedures approved by the University Health Network Research Ethics Board. There was no statistically significant difference in the mean age ± SD between the female (30 ± 8 years, range: 21–49) and male (30 ± 9 years, range 21–50) groups (P = 0.74).
MRI Data Acquisition and Preprocessing
Each subject underwent a T1‐weighted anatomical scan followed by a T2*‐weighted functional scan obtained on a 3T GE MRI system using an eight channel phased array head coil. A whole brain (124 sagittal slices, 24 × 24 cm2 FOV) high resolution (256 × 256 matrix, 1.5 mm × 0.94 mm × 0.94 mm voxels) anatomical scan was obtained using a 3D fast spoiled gradient‐echo (FSPGR) sequence (flip angle = 45°, TE = 5 ms, TR = 25 ms). T2* weighted fMRI scans were acquired with an echo‐planar pulse imaging (EPI) sequence (28 axial slices, 20 × 20 cm2 FOV, 64 × 64 matrix, 3.125 mm × 3.125 mm × 4 mm voxels, TE = 40 ms, TR = 2,000 ms). The 5 min and 8 s fMRI scan was acquired under a task‐free condition (i.e., resting state) during which subjects were instructed to relax, keep their eyes closed and to “not think about anything in particular” [Damoiseaux et al., 2006].
fMRI datasets were interpolated to 3 × 3 × 3 mm3 voxels and underwent preprocessing that included: head motion correction, slice timing correction, linear trend removal, and spatial smoothing with a 6 mm FWHM Gaussian kernel. fMRI data was aligned to the high‐resolution anatomical image, and normalized to standard Talairach space [Talairach and Tournoux, 1988] (voxels are reported as 1 mm × 1 mm × 1 mm). All data analysis was performed with BrainVoyager QX v1.8‐ v1.10 (Brain Innovation, Maastricht, Netherlands).
Functional Connectivity Network Analyses
We performed both individual spatial ICA (sICA) and self‐organizing group ICA (sog‐ICA). The ICA is a data‐driven method that models observed signals as a sum of statistically independent signals. By decomposing the time series into spatial components, ICA maximizes the spatial statistical independence of components each having a unique time course. Therefore, we used ICA to determine and exclude from further analysis components that reflect noise and to identify spatial maps that are related anatomically to the cognitive system (ECN, SN and DMN) in the task‐free state. In addition, we used the sog‐ICA to examine the consistency of these networks across subjects within each group.
Individual‐level ICA was applied to the preprocessed functional time series using a “plugin” extension of BrainVoager QX v1.10 [Goebel et al., 2006]. The best‐fit components for the DMN, ECN, and SN were selected by visual inspection of 30 components from each individual ICA with each component thresholded for a z‐score of 2 assigned to every voxel in the brain. Identification of the individual best‐fit ICs for the DMN, ECN, and SN was based on a three‐step spatial and temporal pattern‐matching process in each individual using specific spatial nodes from previous studies as well as spatial maps and time‐course activity extracted from seed region analyses that we ran for each individual (see Fig. 1).
Figure 1.
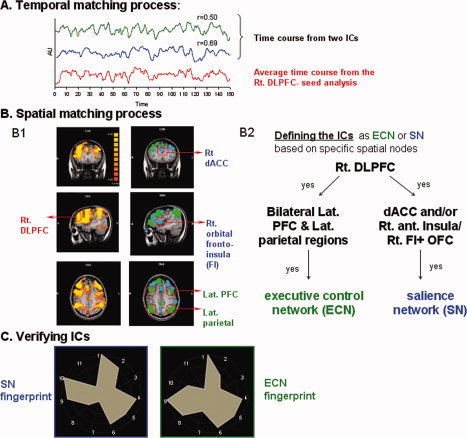
Three‐step spatial and temporal‐matching process used for selection of individual best‐fit independent components. To select the executive control network (ECN) and salience network (SN) components for each individual, the time course of 30 independent components (ICs) was correlated with the average time course from right dorsolateral prefrontal cortex (right DLPFC) [region of interest (ROI) in our seed region analysis]. A: Two components from one representative subject are shown. The most highly correlated ICs based on the Pearson correlation coefficient was transferred to the second step in which the spatial maps of these ICs were inspected for spatial matching with specific nodes from previous studies and with the individual right DLPFC‐seed region analysis map (B1, B2). B: The left column in B1 shows the seed‐region analysis map from a representative subject. In the right column two IC‐spatial maps (green and blue) from the same representative subject are superimposed. The two most matched ICs from each subject were identified as ECN (green) and SN (blue) based on specific spatial nodes (B2). C: A fingerprint analysis was used to characterize the chosen ICs for each network in space and time domain and to verify the signal as arising from a neural BOLD response. The 11 fingerprint parameters are: (1) Degree of clustering; (2) Skewness; (3) Kurtosis; (4) Spatial entropy; (5) One lag autocorrelation; (6) Temporal entropy; (7) Power in the band: 0–0.008 Hz; (8) Power in the band: 0.008–0.02 Hz; (9) Power in the band: 0.02–0.05 Hz; (10) Power in the band: 0.05–0.1 Hz; (11) Power in the band: 0.1–0.25 Hz. The identical process was done for the default mode network except that the ROI for temporal correction and spatial matching was the posterior cingulate cortex/precuneus and its functional connectivity map.
A seed‐region analysis was performed using two regions of interest (ROIs) identified as major nodes of functional maps of previously reported cognitive tasks, task‐free networks, and anatomical landmarks: The DMN seed was centered on the bilateral posterior cingulate/precuneus (PCC/PCu) (BA23/31 for PCC and BA7 for PCu: x = ±7, y = −55, z = 26; size: 12 × 12 × 27 mm3, bilaterally) [De Luca et al., 2006; Fox et al., 2005; Fransson, 2005; Greicius et al., 2003; Harrison et al., 2008; Seminowicz and Davis, 2007]. The seed for the ECN and SN was the right DLPFC (BA9 in the middle frontal gyrus; x = 42, y = 34, z = 27; size: 5 × 5 × 5 mm3) [Bush et al., 2003; Kumari et al., 2004; Seminowicz and Davis, 2007]. The ROI in the DLPFC was chosen for three reasons: (1) it is commonly viewed as a cognitive area where cognition, emotion, and interoceptive awareness integrate [Gray et al., 2002; Indovina and Macaluso, 2007; Miller and Cohen, 2001; Perlstein et al., 2002; Pessoa, 2008], (2) it is part of the functionally connected ECN and SN under task‐free condition, and (3) it has been proposed to mediate the functional interaction between ECN and SN [Fox et al., 2006a; Seeley et al., 2007]. For each subject, the averaged BOLD signal time‐course from each ROI was used as a regressor to identify brain regions whose BOLD signal fluctuations were highly correlated with the ROI. The individual correlation maps were thresholded at a corrected value of P < 0.05 (derived from an uncorrected P < 0.0001 and cluster threshold of 150 mm3 contiguous voxels, based on a Monte Carlo simulation implemented in the AFNI software with the AlphaSim application) and used as a template for selecting the individual ICs corresponding to the ECN, SN, and DMN. In addition, the individual correlation maps from each group were entered into a second‐level, group random‐effect analysis thresholded at a corrected value of P < 0.05 (corrected for multiple comparisons using Bonferroni correction).
In the first step, the components were sorted by their degree of temporal correlation with the average time course from all voxels in the PCC/PCu and Rt. DLPFC ROIs (Fig. 1a). In the second step, a spatial matching process was implemented to identify ICs that had the same spatial pattern as the individual corrected seed region analysis correlation map. This seed region analysis was done for the PCC/PCu (for the DMN) and right DLPFC (for the ECN/SN). We also examined the spatial similarity of each ICs with spatial nodes related to the ECN/SN and DMN reported in previous studies [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; Seeley et al., 2007]. The chosen ICs from the second step were classified as ECN and SN based on previous literature examining resting‐state networks using ICA. For the ECN, the specific spatial nodes included the dorsal frontoparietal regions (bilateral DLPFC, BA9/46 and lateral parietal cortex, BA40). For the SN network, the specific spatial nodes included the right anterior insula (AI) or right OFI (BA13 and BA47) and/or the aMCC (dorsal part of BA24 and 32). In view of the fact that we looked for the functional connectivity of these brain areas with “cognitive” areas, the DLPFC (BA 9/46) was also included as part of the SN's spatial nodes (Fig. 1b). In the third step, we verified the best‐fit component for each network using its IC‐fingerprint. An IC‐fingerprint is a polar plot describing power in various frequency bands, skewedness, kurtosis, clustering, spatial entropy, temporal entropy, and one lag autocorrelation [De Martino et al., 2007] (Fig. 1c). ICs reflecting similar process types have similar fingerprints, and so this tool is useful to distinguish a BOLD response from various types of artifact (motion, EPI susceptibility) or physiological “noise” (e.g., vascular modulation). Specifically, the BOLD response is characterized by a distinctive fingerprint with a high degree of clustering, high one lag autocorrelation, and high power in the 0.01–0.1 Hz band [see De Martino et al., 2007]. Group analyses were performed on the subjects' best‐fit IC for each network, generating random‐effects group t‐maps corrected for multiple comparisons using false discovery rate (FDR). Comparison between groups was based on a random‐effects ANOVA (corrected P < 0.05).
Self‐organizing group ICA (sog‐ICA) was performed using the BrainVoyager QX v1.10 sog‐ICA plugin (Brain Innovation, Maastricht, Netherlands) [Esposito et al., 2005; Goebel et al., 2006]. In general, the sog‐ICA plugin identifies spatial patterns that are common across subjects, while addressing as much as possible the intersubject variability and “clustering” the components in a subject space. We analyzed 20 subjects randomly selected from each group, while controlling for age [mean age for females: 27 ± 6 years old (range: 21–42) and for males: 29 ± 7 years old (range: 21–43, P = 0.45] and set the number of components to 30 ICs per subject. We did not use all subjects in the sog‐ICA analysis due to a software limitation; however, other sog‐ICA studies could detect significant group differences with an n of 20 subjects/group [Esposito et al., 2008].
We performed sog‐ICA, separately for the females and males groups, and this revealed 30 within‐group spatial patterns. (“group clusters”). We fed the within‐group spatial patterns into another sog‐ICA (“mega” sog‐ICA) to objectively select the homologous group clusters between the two groups (“super clusters”) (see Fig. 2). Next, we visually inspected the 30 super clusters and selected the networks based on previous studies; the DMN was defined based on activity in the PCC/PCu, medial prefrontal and lateral parietal and we also identified the network that includes the prefrontal and parietal regions that are functionally integrated during a wide range of cognitive processes [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006; Fox et al., 2005; Seeley et al., 2007]. Finally, we generated two random‐effects group t‐maps from the individual ICs that composed the selected super clusters and used a random effects ANOVA for between‐group comparison. Correction for multiple comparisons was done with FDR (P < 0.05).
Figure 2.
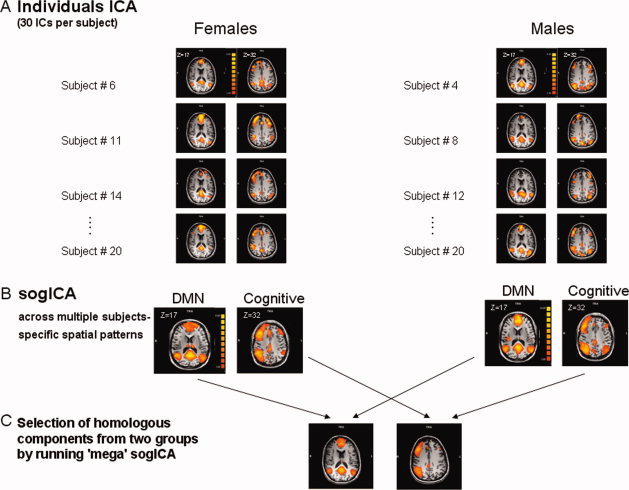
Self organizing independent component analysis (sog‐ICA). A: Thirty ICs from each individual (one from each subject, four components shown) were entered into the sog‐ICA that clustered them together to generate 30 common group clusters (in the spatial domain) across subjects for each group. B: The 30 group clusters from each group were entered into a mega‐sog‐ICA to identify 30 homologous clusters (“super clusters”) between the females and males groups (C).
RESULTS
The ICA process formed an independent spatial component for the DMN that was easily identified in all subjects, and was composed of the PCC/PCu, medial prefrontal, and lateral parietal cortices (see Fig. 3). The ECN and SN were identified by ICA as two different spatial components, each having a unique time‐course. The ECN and the SN could be identified in the majority of subjects (21 females and 19 males for the ECN, 18 females and 16 males for the SN). Random‐effects group spatial maps, for females and males for the ECN (see Fig. 4 and Table I (Supporting Information)), and for the SN (see Fig. 5 and Table II (Supporting Information)), illustrate that these two networks are composed of the middle and inferior frontal gyri (BA9/46, and BA10) and the inferior parietal gyrus (BA40). However, the SN included additional areas that were not part of the ECN, such as AI/FI, aMCC (BA24, 32), and pgACC, bilaterally. Additionally, areas in the superior and middle frontal gyrus (BA6 and BA9), showed stronger functional connectivity to nodes of the SN compared to nodes of the ECN (see Fig. 6). In contrast, the inferior lateral parietal gyrus (BA39,40) was more functionally connected to other nodes in the ECN (see Fig. 6). No sex differences were found in the group‐level random effects maps for the DMN, ECN, and SN. However, thresholding these random effects ANOVA maps at an uncorrected P < 0.001 revealed a difference between sexes in only one region of the ECN; women showed higher functional connectivity in the medial prefrontal cortex (−3, 56, 29; BA 9, 2012 voxels) to other brain areas within the network.
Figure 3.
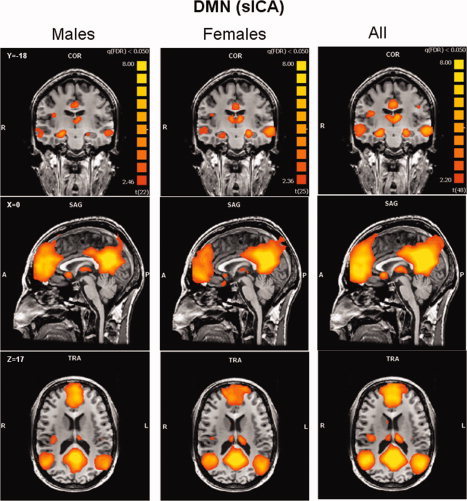
Default mode network. Group‐level random‐effects maps for the default mode network identified by spatial ICA for males, females and all subjects. Maps are projected onto a single subject anatomical scan. Statistical threshold was set at a corrected P = 0.05 (false discovery rate).
Figure 4.
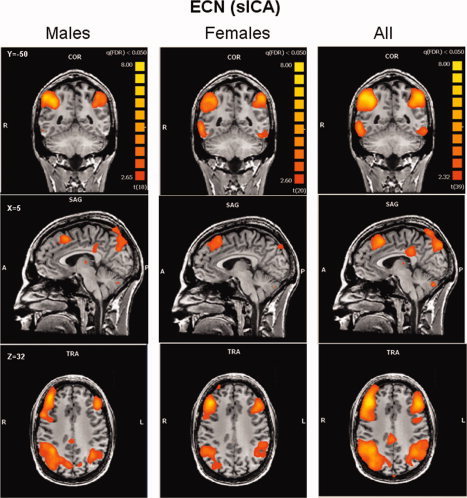
Executive control network. Group‐level random‐effects maps for the executive control network identified by spatial ICA for males, females and all subjects. Maps are projected onto a single subject anatomical scan. Statistical threshold was set at a corrected P = 0.05 (false discovery rate).
Figure 5.
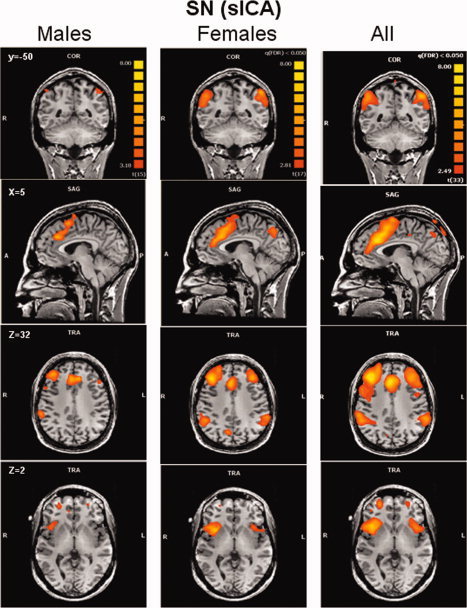
Salience network. Group‐level random‐effects maps for the salience network identified by spatial ICA for males, females and all subjects. Maps are projected onto a single subject anatomical scan. Statistical threshold was set at a corrected P = 0.05 (false discovery rate).
Figure 6.
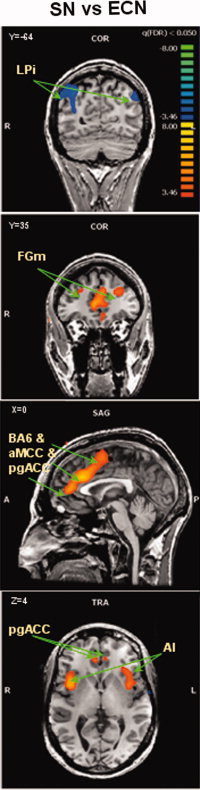
Salience network versus executive control network random‐effects contrast map for all subjects, between the ICs for the salience network and the ICs for the executive control network (SN minus ECN). LPi, Inferior lateral parietal; FGm, Middle frontal gyrus (BA 9); aMCC, anterior middle cingulated cortex; pgACC, pregenual anterior cingulate cortex; IA, anterior insula.
For both sexes the right DLPFC connectivity involved dorsal frontoparietal areas (BA9/46, 40, FEF), SMA, inferior frontal cortex (BA10, 47), the temporoparietal junction, as well as cingulate and paracingulate cortices (aMCC; BA24, 32), bilateral insula, and subcortical regions (basal ganglia, thalamus). Thus, the right DLPFC showed connectivity to regions that we specified as part of the ECN and SN based on previous studies, and is therefore a common node of the two networks. No sex differences were found in the temporal correlation (i.e., strength of the whole component functional connectivity) of the ECN and SN with the right DLPFC time course. The correlation coefficient for the ECN network was r = 0.6 for females and r = 0.58 for males (P = 0.8) and the correlation coefficient for SN was r = 0.54 for females and r = 0.48 for males (P = 0.48).
The IC‐fingerprint associated with the ICs of the DMN, ECN, and SN showed a high degree of similarity to each other, with a high value of degree of clustering, temporal autocorrelation, and peak in frequency power between 0.02 and 0.1 Hz (see Fig. 7). The fingerprint is depicted by a polar plot with 11 axes, each corresponding to the median value of the parameter normalized to 0–1. The IC‐fingerprints for the ECN and SN show remarkable similarity to previously reported IC‐fingerprints associated with task and nontask BOLD networks [De Martino et al., 2007] that were shown to be distinct from fingerprints related to artifacts (e.g., motion, scanner noise, etc.). There were no sex differences in any of the 11 parameters' values for three networks (P > 0.22; Bonferroni corrected for multiple comparisons).
Figure 7.
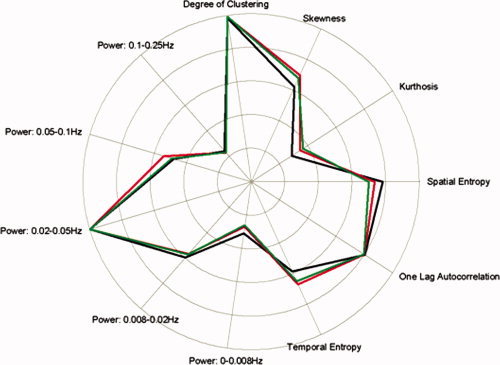
IC‐fingerprints of the executive control, salience, and default mode networks. Each fingerprint is a polar plot chart of the IC representation in multidimensional space, characterized by 11 measures of the normalized spatial, temporal and spectral parameters. The median value from all subjects (females and males) was calculated for each parameter and plotted separately for each network: executive control (black), salience (red) and default mode (green). Note that the fingerprints of the three networks are remarkably similar and typical for BOLD signals associated with neural activity.
The mega sog‐ICA procedure successfully clustered the DMN from females and males group clusters to a super cluster, with high spatial similarity distances (r = 0.83). The DMN group spatial map for each sex formed by sog‐ICA (see Fig. 1 and Table III both in the Supporting Information) was similar to the group spatial map produced by sICA (see Fig. 3). There were no sex differences in the DMN cluster based on random effects ANOVA between groups. Another cluster that was extracted from the mega sog‐ICA with high spatial similarity distances (r = 0.85) included mainly the dorsal frontoparietal areas (BA9/46, 40, FEF), inferior frontal cortex (BA10, 47), PCC, paracingulate (on the border between BA32 and 9), right AI, thalamus and basal ganglia (Fig. 2 and Table IV both in the Supporting Information), that are functionally connected in a wide range of cognitive processes. Chi‐square analysis revealed that 55% of the females but only 25% of the males showed activation in right aMCC and/or right AI (P = 0.053). Specifically, 45% of the females demonstrated activation in the aMCC versus 10% of the males (P = 0.013) whereas for the AI, 40% of the females versus 15% of the males showed activation (P = 0.077). Similar to the finding for the DMN, no sex differences were found in the group‐level cluster after running random‐effects ANOVA comparing between groups. However, the number of subjects within each group that demonstrated activation in the right aMCC and/or right AI, areas that distinguish the ECN and the SN, showed a trend toward differences between females and males.
DISCUSSION
In this study, we sought to determine whether inherent differences in intrinsic task‐free brain networks have the potential to contribute to sex‐differences in human behavior. Therefore, we specifically tested whether there were sex differences in the intrinsic brain connectivity of the DMN and two cognitive networks (ECN and SN) during a task‐free resting state. The DMN, ECN, and SN were clearly identified using sICA in both men and women with no significant sex differences. The data highlight that, in both men and women, the ECN and SN contain a distinct constellation of nodes anchored by a common node in the right DLPFC. This common right DLPFC node may act to direct processing streams to one network or the other depending on the task/stimulus or salience. The similarity between sexes in these task‐free networks support previous studies that propose that sex differences in patterns of brain activation evoked during cognitive‐task performance arise from individual strategies rather then differences in the composition of task‐free networks.
Previous fMRI studies have identified some sex differences in the strength of activation in brain areas that show some task responsivity in both sexes. For example, during visuospatial cognitive tasks, men tend to have stronger parietal activation; whereas women tend to have stronger inferior frontal and temporal activation [Jordan et al., 2002; Kucian et al., 2005; Thomsen et al., 2000; Weiss et al., 2003]. This disparity might be related to different strategies employed by each sex while solving a cognitive task. However, everyday activities are assembled from complex cognitive‐emotional behaviors and there is evidence that the emotional content of a stimulus and the emotional state of the subject can influence cognitive function [Dolan, 2002]. What's more, fMRI studies evaluating sex differences in neural responses to emotional stimuli have found that some of the same regions are activated. However, there are some notable incongruities, primarily located within the limbic system that can be characterized as differences in levels or laterality of activations [for review article see Hamann and Canli, 2004]. Interestingly, a meta‐analysis by Wager and Ochsner [2005] that examined sex differences in responses to emotional stimuli identified differences in lateralization of activation, but not levels of activation.
Complex cognitive‐emotional behaviors require a coalition of activity in affective and cognitive networks to regulate and integrate information between regions [Pessoa, 2008]. It has been suggested that this integration occurs in the DLPFC (BA 9/46) [Fales et al., 2008; Gray et al., 2002; Miller and Cohen, 2001; Pessoa, 2008], an area implicated in top‐down regulation of emotional processes, and in the aMCC, an area implicated in conflict monitoring that could interrupt performance and engage the DLPFC to alter attention resources [Botvinick et al., 2001; Davis et al., 2005; Meriau et al., 2006]. It is noteworthy that these two brain regions were also found to integrate interoceptive information [Critchley et al., 2004]. We found that the DLPFC is functionally connected to the dorsal frontoparietal cortex, SMA, TPJ, inferior frontal cortex, ACC, AI, and subcortical areas. This pattern of brain activation involves regions that were found to be active during different cognitive tasks. The dorsal frontoparietal cortex is engaged in goal‐directed attention and memory tasks, and can be modulated by brain areas activated by salient stimuli, such as the TPJ, inferior frontal gyrus, AI, and ACC/SMA [Corbetta and Shulman, 2002]. Seeley et al. [2007] found that the ECN and SN share a common node in the right DLPFC. Our study supports this finding because we found that these two networks are strongly temporally correlated with the right DLPFC, compared to other resting state‐networks identified by ICA. Our finding that both networks were temporally correlated with a mutual node in the right DLPFC supports the role of the DLPFC as a potential brain area where interaction could occur between the two networks in both sexes. However, the medium strength temporal correlation (r = 0.5) between ECN and SN to the right DLPFC suggests that there may be more common nodes where there is either integration or interaction between these separate neural networks.
It is not yet understood whether salient processes, including emotional processes, interact with cognition differently in men and women. To examine sex differences in interactions between salient stimuli with a cognitive task, Koch et al. [2007] gave a negative olfactory stimulation to subjects in the MRI while they were performing a memory task. They found that men demonstrated an extensive fronto‐parietal‐cingulate network; whereas in women, there was no difference in the interaction paradigm, compared to the control paradigm (neutral olfactory stimulus). Furthermore, women showed greater activation in emotional brain areas, namely the orbitofrontal cortex and the amygdala, and men showed more parietal and occipital activations compared to females. Thus, men may integrate cognition and emotion information within the cognitive control network, whereas women may engage parallel processing of cognition and emotion information with relative hyperactivity in emotional areas.
The above mentioned studies highlight the sex differences in brain areas evoked by cognitive tasks (i.e., task‐positive networks). However, previous studies have not evaluated sex differences in the DMN deactivation evoked by a cognitive task. Given that patterns of spontaneous activity predict the way in which the brain responds across a wide variety of task conditions [for review, see Fox and Raichle, 2007] we expected that differences in patterns of activation between sexes would be reflected in the functional connectivity during a task‐free condition. However, the findings from the data‐driven analyses performed with the random effects analysis, do not support rejecting the null hypothesis, and therefore indicate that there are no significant sex differences in these functionally connected networks. Our findings indicate that controlling for sex may not be necessary in fMRI studies of resting state networks. However, non sex‐based individual differences in task‐free activity have been reported. For example, functional connectivity of the dACC with other nodes within the SN was positively correlated with anxiety [Seeley et al., 2007], resting state brain metabolism in the prefrontal and striatal regions varied with personality traits [Kim et al., 2008], connectivity between nodes in the DMN positively correlated with memory performance [Hampson et al., 2006], and DLPFC connectivity with other brain areas was positively correlated with intelligence [Song et al., 2008]. These types of studies typically use equal number of female and male subjects to control for potential sex differences, which our data suggest is unnecessary. Based on previous studies showing that both men and women are capable of activating these networks during cognitive task, and our findings that those networks continue to spontaneously fluctuate in both sexes, we provide support that the networks are primed to be prompted depending on the salience and complexity of a task. These networks might be modified based on individual differences whether they are related to intelligence, personality traits, state or strategies based on knowledge derived from previous experience. More recently there has been interest in the way the task‐free networks are modulated during cognitive task. Calhoun et al. [2008], using ICA of fMRI data, found that the task‐free networks are temporally and spatially modulated during cognitive task conditions. This raises the possibility that these task‐free networks may be modulated differently in both sexes and these modulations might even correlate with behavior in specific brain areas.
One limitation of our study arises from the issue of the estimated number of ICs for fMRI data analysis. Typically there are a large number of ICs in sICA, up to the number of time samples in the scan, and so there are a variety of methods to estimate the optimal number of components. In this study, based on our experience and in accordance with Brainvoyager recommendations, we used 30 components, equivalent to 20% of the 150 time points in our task‐free scan that generate stable results in ICA. Other studies used principle component analysis prior to ICA decomposition [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006], which determines how much noise is left in the data but does not take into account the actual structure of interesting signal in the data. Furthermore, we did not use variance‐based automated methods for choosing the number of components [Beckmann et al., 2005; Damoiseaux et al., 2006; De Luca et al., 2006, Li et al., 2007], because we were not concerned with the variance of the signals. Rather, we were concerned with optimizing the signals' spatial patterns relative to our hypotheses. Moreover, Li et al. [2007] proposed a new method to obtain a set of effectively independent and identically distributed data samples for order selection. Although the networks that we found during task‐free condition are identical to those that were found in previous studies, they are highly dependent on our choice of the number of ICs in ICA.
Another potential limitation in our study is that our study participants spanned a wide age range from 21 to 42 years old. This range was based on the commonly used range of ages in imaging studies. Although age‐related changes in brain activity under task‐free condition have been reported, they were found for subjects older than ours [Damoiseaux et al., 2008; Sambataro et al., in press]. Furthermore, we age‐matched our subjects between the two groups. Therefore, although we cannot totally rule out age effects, their contribution to the findings are likely minimal.
In conclusion, we have found a remarkable similarity between sexes in spatial topography of activation of the DMN, ECN, and SN. This finding can be used for future studies as an a priori hypothesis that brains of both sexes will potentially respond in the same way during cognitive tasks. Furthermore, our results advocate that resting state fMRI studies can be done without controlling for sex.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Default Mode Network. Group‐level random‐effects maps for the default mode network identified by sog‐ICA for males, females and all subjects. Maps are projected onto a single subject anatomical scan. Statistical threshold set to a corrected p=0.05 (false discovery rate).
Supporting Information Figure 2. A network activated in cognitive processes (CN). Group‐level random‐effects maps for a network implicated in cognitive tasks identified by sog‐ICA. Although the sog‐ICA did not extract separated clusters for the executive control and salience networks, the network identified here is typically active during a wide range of goal‐directed cognitive tasks and working memory function. Maps are projected onto a single subject anatomical scan. Statistical threshold set to a corrected p=0.05 (false discovery rate).
Supporting Information Table 1.
Supporting Information Table 2.
Supporting Information Table 3.
Supporting Information Table 4.
Acknowledgements
The authors thank Fabrizio Esposito (Brain Innovation B.V.) for guidance in the use and interpretation of group ICA in BrainVoyager. Karen D. Davis is a Canada Research Chair in Brain and Behaviour. This study was supported from funds from the CRC program and a CIHR grant to KDD.
REFERENCES
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL ( 2007): Unrest at rest: The importance of the default activity and spontaneous network corellations. Neuroimage 37: 1091–1099. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA ( 2003): The multi‐source interference task: Validation study with fMRI in individual subjects. Mol Psychiatry 8: 60–70. [DOI] [PubMed] [Google Scholar]
- Butler T, Imperato‐McGinley J, Pan H, Voyer D, Cunningham‐Bussel AC, Chang L, Zhu YS, Cordero JJ, Stern E, Silbersweig D ( 2007): Sex specificity of ventral anterior cingulate cortex suppression during a cognitive task. Hum Brain Mapp 28: 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L ( 2003): Sex‐related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci 985: 163–173. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT ( 2001): Sex‐related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 75: 1–9. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD ( 2008): Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive task. Hum Brain Mapp 29: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L ( 2000): Event‐related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20: RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD ( 2002): Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA 99: 10789–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2005): Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493: 154–166. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ ( 2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Crucian GP, Berenbaum SA ( 1998): Sex Differences in Right Hemisphere Tasks. Brain Cogn 36: 377–389. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA ( 2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM ( 2005): Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci 25: 8402–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ ( 1999): Gender differences in autobiographical memory for childhood emotional experiences. J Pers Soc Psychol 76: 498–510. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM ( 2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29: 1359–1367. [DOI] [PubMed] [Google Scholar]
- De Martino F, Gentile F, Esposito F, Balsi M, Di Salle F, Goebel R, Formisano E ( 2007): Classification of fMRI independent components using IC‐fingerprints and support vector machine classifiers. Neuroimage 34: 177–194. [DOI] [PubMed] [Google Scholar]
- Dolan RJ ( 2002): Emotion, cognition, and behavior. Science 298: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G, Goebel R, Seifritz E, Di Salle F ( 2005): Independent component analysis of fMRI group studies by self‐organizing clustering. Neuroimage 25: 193–205. [DOI] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Goebel R, Di Salle F ( 2008): Independent component model of the default‐mode brain function: Combining individual‐level and population‐level analyses in resting‐state fMRI. Magn Reson Imaging 26: 905–913. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI ( 2008): Altered emotional interference processing in affective and cognitive‐control brain circuitry in major depression. Biol Psychiatry 63: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME ( 2006a): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME ( 2006b): Coherent spontaneous activity accounts for the trail‐to‐trail variability in human evoked brain responses. Nat Neurosci 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze‐Bonhage A ( 2006): Gender‐related differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport 17: 417–421. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E ( 2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME ( 2002): Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA 99: 4115–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Canli T ( 2004): Individual differences in emotion processing. Curr Opin Neurobiol 14: 233–238. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD ( 1999): Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 2: 289–293. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT ( 2006): Brain connectivity related to working memory performance. J Neurosci 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Ortiz H, Soriano‐Mas C, Yucel M, Pantelis C, Cardoner N ( 2008): Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA 105: 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Linn MC ( 1998): Gender differences in verbal ability: A meta analysis. Psychol Bull 104: 53–69. [Google Scholar]
- Indovina I, Macaluso E ( 2007): Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb Cortex 17: 1701–1711. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wustenberg T, Heinze HJ, Peters M, Jancke L ( 2002): Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia 40: 2397–23408. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP ( 2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39: 527–537. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kim S, Hwang J, Park H, Kim S ( 2008): Resting brain metabolic correlates of neuroticism and extraversion in young men. Neuroreport 19: 883–886. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Stocker T, Shah NJ, Amunts K, Kircher T, Schneider F, Habel U ( 2007): Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia 45: 2744–2754. [DOI] [PubMed] [Google Scholar]
- Kucian K, Loenneker T, Dietrich T, Martin E, von Aster M ( 2005): Genser differences in brainactivation patterns during mental rotation and number related cognitive task. Psychol Sci 47: 112–131. [Google Scholar]
- Kumari V, ffytche DH, Williams SC, Gray JA ( 2004): Personality predicts brain responses to cognitive demands. J Neurosci 24: 10636–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD ( 2007): Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp 11: 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriau K, Wartenburger I, Kazzer P, Prehn K, Lammers CH, van der Meer E, Villringer A, Heekeren HR ( 2006): A neural network reflecting individual differences in cognitive processing of emotions during perceptual decision making. Neuroimage 33: 1016–1027. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA ( 2002): Dissociation in human prefrontal cortex of affective influences on working memory‐related activity. Proc Natl Acad Sci USA 99: 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L ( 2008): On the relationship between emotion and cognition. Nat Rev Neurosci 9: 148–158. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R ( 2007a): Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp 28: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C ( 2007b): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141: 178–87. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD, Abrahams S ( 2004): Sex, sexual orientation, and identification of positive and negative facial affect. Brain Cogn 54: 179–185. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS: Age‐related alterations in default mode network: Impact on working memory performance. Neurobiol Aging ( 2008) Jul 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD ( 2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD ( 2007): Interactions of pain intensity and cognitive load: The brain stays on task. Cereb Cortex 17: 1412–1422. [DOI] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T ( 2008): Brain spontaneous functional connectivity and intelligence. Neuroimage 41: 1168–1176. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux, P ( 1988): Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers. [Google Scholar]
- Thayer JF, Johnsen BH ( 2000): Sex differences in judgement of facial affect: A multivariate analysis of recognition errors. Scand J Psychol 41: 243–246. [DOI] [PubMed] [Google Scholar]
- Thomsen T, Hugdahl K, Ersland L, Barndon R, Lundervold A, Smievoll AI, Roscher BE, Sundberg H ( 2000): Functional magnetic resonance imaging (fMRI) study of sex differences in a mental rotation task. Med Sci Monit 6: 1186–1196. [PubMed] [Google Scholar]
- Vogt BA ( 2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Ochsner KN ( 2005): Sex differences in the emotional brain. Neuroreport 16: 85–87. [DOI] [PubMed] [Google Scholar]
- Weiss E, Siedentopf CM, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M ( 2003): Sex differences in brain activation pattern during a visuospatial cognitive task: A functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett 344: 169–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1. Default Mode Network. Group‐level random‐effects maps for the default mode network identified by sog‐ICA for males, females and all subjects. Maps are projected onto a single subject anatomical scan. Statistical threshold set to a corrected p=0.05 (false discovery rate).
Supporting Information Figure 2. A network activated in cognitive processes (CN). Group‐level random‐effects maps for a network implicated in cognitive tasks identified by sog‐ICA. Although the sog‐ICA did not extract separated clusters for the executive control and salience networks, the network identified here is typically active during a wide range of goal‐directed cognitive tasks and working memory function. Maps are projected onto a single subject anatomical scan. Statistical threshold set to a corrected p=0.05 (false discovery rate).
Supporting Information Table 1.
Supporting Information Table 2.
Supporting Information Table 3.
Supporting Information Table 4.


