Abstract
According to recent neuroimaging studies, swallowing is processed within multiple regions of the human brain. In contrast to this, little is known about the cortical contribution and compensatory mechanisms produced by impaired swallowing. In the present study, we therefore investigated the cortical topography of volitional swallowing in patients with X‐linked bulbospinal neuronopathy (Kennedy disease, KD). Eight dysphagic patients with genetically proven KD and an age‐matched healthy control group were studied by means of whole‐head magnetoencephalography using a previously established swallowing paradigm. Analysis of data was carried out with synthetic aperture magnetometry (SAM). The group analysis of individual SAM results was performed using a permutation test. KD patients showed significantly larger swallow‐related activation of the bilateral primary sensorimotor cortex than healthy controls. In contrast to the control group, in KD patients the maximum activity was located in the right sensorimotor cortex. Furthermore, while in nondysphagic subjects a previously described time‐dependent shift from the left to the right hemisphere was found during the one second of most pronounced swallow‐related muscle activity, KD patients showed a strong right hemispheric activation in each time segment analyzed. Since the right hemisphere has an established role in the coordination of the pharyngeal phase of swallowing, the stronger right hemispheric activation observed in KD patients indicates cortical compensation of pharyngeal phase dysphagia. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: dysphagia, Kennedy‐disease, magnetoencephalography, cortical compensation, synthetic aperture magnetometry
INTRODUCTION
Although the brainstem has been the target of research related to the physiology of swallowing for decades [Jean, 2001], the cortical contribution to the coordination of this complex sensorimotor task is evolving only of late. By applying different methods such as functional magnetic resonance imaging (fMRI) [Hamdy et al., 1999a; Kern et al., 2001; Martin et al., 2001, 2004; Mosier et al., 1999; Mosier and Bereznaya, 2001], positron emission tomography (PET) [Hamdy et al., 1999b; Harris et al., 2005], transcranial magnetic stimulation (TMS) [Hamdy et al., 1996], and magnetoencephalography (MEG) [Dziewas et al., 2003, 2005; Furlong et al., 2004], and by using various paradigms, including volitional swallowing [Dziewas et al., 2003], autonomic swallowing [Martin et al., 2001], and reflexive swallowing [Kern et al., 2001], neuroimaging studies have suggested that swallowing is processed within multiple regions of the human brain. The most prominent swallow‐related activation foci involve—among others—the primary sensorimotor cortex, sensorimotor integration areas, the insula and frontal operculum, the anterior cingulate cortex, and the adjacent supplementary motor area.
Despite this considerable progress in understanding swallowing physiology, the cortical contribution to adaptation and to the compensation of impaired swallowing has been studied less systematically so far. Dysphagia, which is a prominent and disabling feature of a variety of neurovascular, neurodegenerative, and neuromuscular disorders, may be caused by lesions at any level of the central or peripheral nervous system affecting to different degrees motor output and sensory input [Hughes, 2003]. Because of its well‐defined and circumscribed pathology consisting of selective degeneration of spinal and, predominantly, bulbar motor neurons with intact first motor neuron and unimpaired oropharyngeal sensory afferents, X‐linked bulbospinal neuronopathy (Kennedy disease, KD) may be considered a candidate disease to study the cortical compensation of dysphagia. KD is a rare disorder with an incidence of 0.09/100,000/year and a prevalence of 1.6/100,000 [Guidetti et al., 2001]. A mutation characterized by an expansion of a polymorphic tandem CAG repeat in the first exon of the androgenreceptor gene was identified as its genetic cause [Amato et al., 1993]. Clinically, the middle‐aged patients present with bulbar and proximal weakness, muscular atrophy, fasciculations, and additional symptoms including gynecomastia and postural tremor. Dysphagia is commonly slowly progressive with the minority of patients suffering from severe complications like aspiration pneumonia [Sperfeld et al., 2002].
A major problem in investigating the cortical processing of swallowing with EEG or MEG is caused by the fact that—due to the intra‐ and interindividual variable duration of swallowing phases—swallowing does not result in evoked cortical potentials. Instead, swallowing like other voluntary movements results in circumscribed frequency changes of cortical rhythms. These types of changes are time‐locked to the event but not phase‐locked and thus cannot be extracted by simple linear methods, such as averaging, but may be detected by time–frequency analysis [Pfurtscheller and Lopes da Silva, 1999]. This might be due to a decrease or increase in synchrony of the underlying neuronal populations, otherwise known as event‐related desynchronization (ERD) [Pfurtscheller, 1977, 1992; Pfurtscheller and Aranibar, 1977] or synchronization (ERS) [Pfurtscheller, 1992]. Voluntary movement is known to result in desynchronizations in the upper alpha and lower beta band, localized close to sensorimotor areas [Derambure et al., 1993; Pfurtscheller and Aranibar, 1979; Pfurtscheller and Berghold, 1989; Stancak and Pfurtscheller, 1996]. These ERDs have also been reported in different swallowing studies [Dziewas et al., 2003; Furlong et al., 2004; Teismann et al., 2007].
In this study, we investigated the cortical topography of volitional swallowing in patients with KD using a previously established swallowing paradigm [Dziewas et al., 2003, 2005; Teismann et al., 2007]. Capitalizing on the high temporal resolution of MEG, we paid special attention to rapid shifts of hemispheric lateralization during the act of swallowing [Teismann et al., in press].
PATIENTS AND METHODS
Patients and Healthy Control Subjects
Eight patients with genetically proven KD recruited from our neuromuscular outpatient clinic (mean age 53.1 years, range 43–71 years) and eight age‐matched healthy control subjects (mean age 44.6 years, range 33–60 years, P = 0.1415 for the comparison of mean age between both groups) participated in this study. KD patients had a history of dysphagia for several years (see Results section for details). Control subjects were free of stroke, neuromuscular or neurodegenerative disorders, or other conditions potentially being associated with dysphagia. None of them reported swallowing difficulties. When systematically questioned, all control subjects denied coughing during eating, slow eating, and avoidance of certain food consistencies, fatigable swallowing, or weight loss.
The local ethics committee approved the protocol of the study. Informed consent was obtained from each subject after the nature of the study was explained in accordance to the principles of the Declaration of Helsinki.
Fiberoptic Endoscopic Evaluation of Swallowing
The basic fiberoptic endoscopic evaluation of swallowing (FEES) protocol as published by Langmore and previously applied in our department [Dziewas et al., 2007] was performed with the patient sitting upright. FEES allows visualization of the entire pharyngeal swallow, except for a very brief period when the contracting pharyngeal walls obstruct the optical tip of the endoscope. Penetration, aspiration, leakage, and residues as signs for dysphagia can be evaluated [Langmore, 2001].
Dysphagia Limit Test
Before each MEG recording, a dysphagia limit was quantified according to the protocol of Ertekin et al. [ 1996]. Here, the subjects of both groups were instructed to swallow increasing bolus sizes of water (1, 3, 5, 10, 15, 20 ml). The maximum bolus size swallowed without piecemeal deglutition was specified.
Swallowing Screening Test
Before MEG recording was started, a dysphagia screening test was performed according to the protocol by Hughes and Wiles [ 1996]. Control subjects and patients were asked to drink 150 ml of water from a plastic beaker. They were instructed to drink “as quickly as is comfortably possible.” Subjects were observed from the side, and the number of swallows counted by observing the movements of the thyroid cartilage. A stopwatch was started when the water first touched the bottom lip, and stopped when the larynx came to rest for the last time.
Intraoral Infusion
To facilitate volitional swallowing during MEG recording, water was infused into the oral cavity via a flexible plastic tube 4.7 mm in diameter attached to a fluid reservoir. The reservoir bag was positioned about 1‐m above the mouth of each subject when seated. The tip of the tube was placed in the corner of the mouth between the buccal part of the teeth and the cheek. The tube was gently fixed to the skin with tape. The side chosen for tube placement was alternated between subjects. The infusion flow was individually adjusted to the subject's request and ranged between 8 and 12 ml/min. The aim was to establish a swallowing frequency of four to six times per minute.
MEG Recording
During 15 min of MEG recording, the subject swallowed in a self‐paced manner, without external cueing. Swallowing acts were recorded and identified by electromyographic recording. MEG data were collected using a whole head 275‐channel SQUID sensor array (Omega 275, CTF Systems, Canada) housed in a magnetically shielded room. The recorded magnetic fields were sampled with a frequency of 600 Hz. The data were filtered during acquisition using a 150 Hz low‐pass filter. Recordings were performed while subjects were seated in a comfortable upright position and watching a silent movie of their choice.
EMG Recording
Surface EMG was measured with two pairs of bipolar skin electrodes (Ag‐AgCl) placed on the submental muscle groups [Ding et al., 2002; Vaiman et al., 2004]. The electrodes were connected to a bipolar amplifier (DSQ 2017E EOG/EMG system, CTF Systems), and the nominal gain was set at 1. EMG data was high‐pass filtered with 0.1 Hz before markers were manually set.
Data Analysis
Each individual's EMG signal was used to mark the beginning of main muscle activation (M1) and the end of the task‐specific muscle activity (M2) for every single swallow.
The beginning of the main muscle activation was defined as an enduring larger than 50% increase in amplitude or frequency of the EMG signal after an initial increase of more than 50% of EMG activity defining the onset of swallowing preparation. The end of the task‐specific muscle activity was defined as a decrease in amplitude or frequency of the EMG signal greater than 50%. To estimate the maximal null distribution (see later), a third marker was set to distinguish background activity from the onset of swallowing preparation (M0). For analysis of the whole swallowing execution, phase time intervals were defined as follows (see Fig. 1):
-
1
1 s Execution stage: −0.4 to 0.6 s in reference to M1.
-
2
1 s Resting stage: 0 to 1 s in reference to M2.
-
3
1 s Background active: −1 to 0 s in reference to M0.
-
4
1 s Background control: −2 to −1 s in reference to M0.
Figure 1.
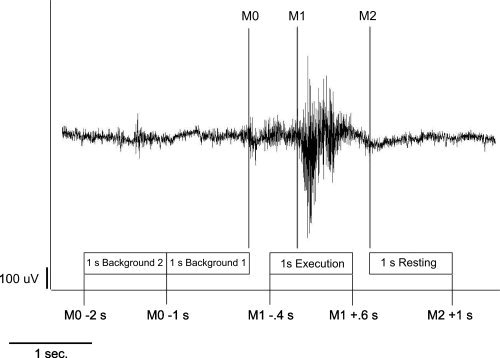
Definition of the swallowing execution and resting stage of swallowing‐related muscle activity. The EMG recording of one swallowing act is shown (surface electrodes, recording from the submental muscles). For the 1‐s analysis of the whole swallowing phase with SAM, the beginning (M1) and the end (M2) of main muscle activation were marked. The swallowing execution phase and the corresponding resting phase were defined. To estimate the maximal null distribution a third marker (M0) at the beginning of task‐specific muscle activity was set and two background phases were defined (Patients and Methods section).
To examine the chronological sequence of brain activation, the execution stage was divided into five parts, each lasting 200 ms. Time intervals including the according resting stages for the subsequent analysis were defined as follows (see Fig. 2):
-
1
200 ms Execution stage 1 (E1): −0.4 to −0.2 s in reference to M1.
-
2
200 ms Execution stage 2 (E2): −0.2 to 0.0 s in reference to M1.
-
3
200 ms Execution stage 3 (E3): 0.0 to 0.2 s in reference to M1.
-
4
200 ms Execution stage 4 (E4): 0.2 to 0.4 s in reference to M1.
-
5
200 ms Execution stage 5 (E5): 0.4 to 0.6 s in reference to M1.
-
6
200 ms Resting stage (R): 0 to 0.2 s in reference to M2.
-
7
200 ms Background active (B1): −0.2 to 0 s in reference to M0.
-
8
200 ms Background control (B2): −0.4 to −0.2 s in reference to M0.
Figure 2.
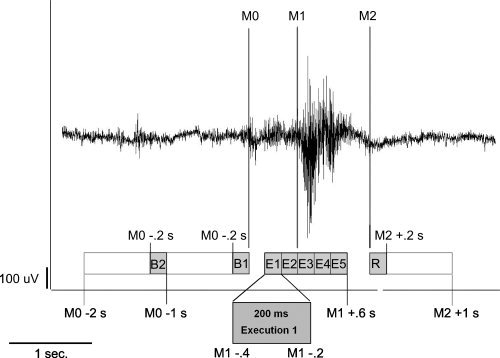
To analyze the cortical activation within the early and later stages of the execution phase, this1‐s interval is divided into five successive 200‐ms time intervals (E1–E5). The corresponding resting stage (R) and two background stages (B1 and B2) are also shortened to 200 ms (Patients and Methods section).
In this study, the recorded MEG data were filtered within three different frequency bands: alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–80 Hz). From the filtered MEG data, SAM was used to generate a 20 × 20 × 14 cm3 volumetric pseudo‐t‐images [Vrba and Robinson, 2001], with 3‐mm voxel resolution for all the three frequency bands. A pseudo‐t‐value cancels the common‐mode brain activity by subtracting the source power found in a defined control stage from the source power in the active stage. To account for uncorrelated sensor noise, this difference was normalized by the mapped noise power [Sekihara et al., 2005; Vrba and Robinson, 2001]. For analyzing cortical activity during the movement stage (1) the corresponding resting stage (2) served as control. The required similarity between the resting stage and the two background stages in patients as well as in controls was proven before by a direct comparison of these three stages.
For analysis of single conditions, the significance of activated brain regions was assessed by the permutation test method described by Chau et al. [ 2004]. The maximal null distribution was estimated by comparing Background stages 1 (active) and 2 (control) [Chau et al., 2004; Nichols and Holmes, 2002]. For the comparison of the different groups, a standard permutation test for unpaired samples was performed [Nichols and Holmes, 2002].
Hemispheric lateralization concerning the five different time intervals of swallowing related activation was quantified using a lateralization index (LI), which was calculated as (L − R)/(L + R), where L and R are the cumulative pseudo‐t‐activation in the sensorimotor cortex (BA 3, 1, 2, and 4, according to the Talairach atlas) of the left and right hemisphere, respectively. A positive LI indicates left hemispheric lateralization, whereas a negative LI indicates stronger right hemispheric activation. A LI of about 0 represents indeterminate dominance, 1, respectively, −1 are indicating unilateral activation [Dziewas et al., 2003; Yetkin et al., 1995].
RESULTS
All KD patients reported of having noticed swallowing difficulties for several years prompting them to eat more slowly and carefully without, however, necessitating special adaptation of food and fluids in any of them. None of the patients suffered from recurrent bronchial infections or was hospitalized due to aspiration pneumonia. FEES revealed moderate pharyngeal phase dysphagia in all eight patients. As most prominent finding, incomplete bolus clearance during the swallow with residues in the valleculae and pyriform sinus was identified. No signs of sensory deficits were found.
The swallowing screening test revealed no differences in swallowing speed, volume per swallow, and swallowing capacity between KD patients and healthy control subjects. A tendency for a reduced swallowing capacity in KD patients is found (see Table I).
Table I.
Results of the DYSPHAGIA limit and the swallowing screening test in Kennedy patients and healthy controls
| No. | Dysphagia limit (ml) | Volume per swallow (ml) | Duration per swallow (s) | Capacity (ml/s) |
|---|---|---|---|---|
| Kennedy patients | ||||
| K1 | 20 | 25.00 | 0.95 | 26.41 |
| K2 | 20 | 15.00 | 2.70 | 5.56 |
| K3 | 20 | 16.67 | 1.44 | 11.58 |
| K4 | 20 | 50.00 | 4.30 | 11.63 |
| K5 | 20 | 21.43 | 0.79 | 27.13 |
| K6 | 20 | 30.00 | 1.26 | 23.81 |
| K7 | 20 | 25.00 | 1.48 | 16.89 |
| K8 | 15 | 13.64 | 2.15 | 6.34 |
| Mean | 19.38 | 24.59 | 1.88 | 13.06 |
| Healthy controls | ||||
| C1 | 20 | 21.43 | 1.14 | 18.79 |
| C2 | 20 | 15.00 | 0.98 | 15.31 |
| C3 | 20 | 25.00 | 0.78 | 31.65 |
| C4 | 20 | 18.75 | 0.98 | 19.13 |
| C5 | 20 | 16.67 | 0.79 | 21.10 |
| C6 | 20 | 50.00 | 1.90 | 26.32 |
| C7 | 20 | 25.00 | 1.25 | 20.00 |
| C8 | 20 | 25.00 | 0.92 | 27.17 |
| Mean | 20 | 24.61 | 1.09 | 22.50 |
All participants tolerated the MEG measurements without any complications, in particular no aspiration with coughing was observed. A sufficient number of swallows was recorded by submental EMG.
During the execution of swallowing, single subject SAM analysis of MEG data showed in all subjects ERD in the beta frequency band localized within the primary sensorimotor cortex. In other frequency bands and other cortical areas no systematic activation was observed. Because the muscular activation during deglutition cortical activation changes in lower brain areas are difficult to interpret. Group analysis resulted in significant ERD in the beta frequency band both in the patient and in the control group. The extent of brain activation was broader in patients than in control subjects (5,555 active voxels vs. 4,327 active voxels). Also, the maximum ERD (amplitude) was slightly stronger in KD patients when compared with the control group (−0.482 vs. −0.447). In the control group, maximum activation was localized within the left primary sensorimotor cortex (minimum pseudo‐t‐value in the left hemisphere: −0.447, in the right hemisphere: −0.406), whereas the patient group showed a right hemispheric dominance in this respect (minimum pseudo‐t‐value in the left hemisphere: −0.362, in the right hemisphere: −0.482; see Fig. 3). In contrast to control subjects, brain activation extended into the prefrontal cortex and the posterior parietal cortex in KD patients (see Table II).
Figure 3.
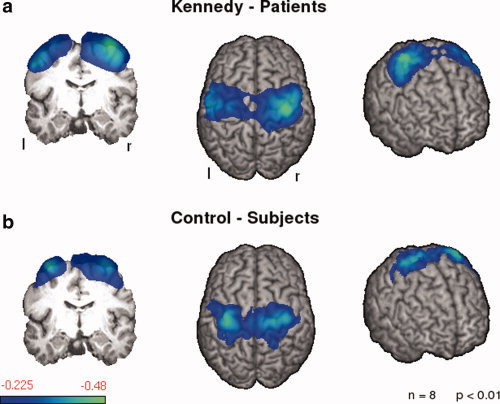
Event‐related desynchronizations in the beta frequency band (13–30 Hz) during the 1‐s execution phase of volitional swallowing. Significant activation in the group analysis is shown for patients and controls (P < 0.05). The color bar represents the t‐value. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
Localization of significant swallowing‐related cortical activation in both groups of subjects (P < 0.05)
| Hz | Coordinates of peaks | Pseudo‐t‐value | Cortical region | Brodman‐area |
|---|---|---|---|---|
| KD patients | ||||
| Beta (13–30 Hz) | [l.: −56 −9 47] | −0.362011 | GPrC, GPoC, GFs/m (r.) | 1, 2, 3, 4, 6 (l. & r.) |
| [r.: 36 −11 70] | −0.482141 | GPrC, GPoC, GFm (l.); | ||
| Control subjects | ||||
| Beta (13–30 Hz) | [l.: −36 −14 73] | −0.425359 | GPrC, GPoC, GFs/m (l. & r.) | 1, 2, 3, 4, 6 (l. & r.) |
| [r.: 24 −17 73] | −0.370315 | |||
GFm/s, Gyrus frontalis medialis/superior; GPoC, Gyrus postcentralis; GPrC, Gyrus precentralis; l = left hemisphere; r = right hemisphere.
Separate calculation of the SAM images for each 200‐ms interval resulted in ERD of rhythmic brain activity within sensorimotor cortex in each individual subject and interval. In healthy control subjects, group analysis showed a small left hemispheric activation during the first 400 ms without any right hemispheric involvement (LI: 1), a bilateral but slightly left lateralized activation within the third time interval (LI: 0.12), a bilateral and slightly right lateralized activation within the fourth time interval (LI: −0.1) and, finally, a strongly right lateralized activation within the last time frame (LI: −0.76) (see Fig. 4b). In contrast, group analysis revealed an early, large, and persistent right hemispheric activation in the patient group. Thus, in the first 400‐ms, cortical activation was observed bilaterally and was slightly lateralized to the right (LI: −0.63 and −0.57), within the third (LI 0.46) and fourth (LI: 0.21) time interval, bilateral activation showed a minor left lateralization, while during the last 200 ms, activation was clearly more pronounced within the right hemisphere (LI: −0.53) (see Fig. 4a).
Figure 4.
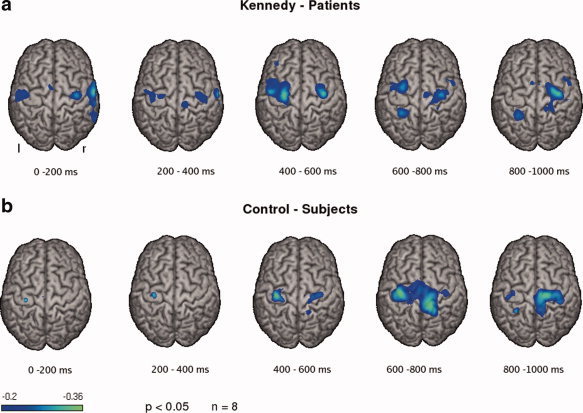
Event‐related desynchronizations in the beta frequency band during the five successive 200‐ms time intervals of the swallowing execution phase is shown for both groups. Significant activation in the group analysis is shown (P < 0.05). The color bar represents the t‐value. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This MEG study examined the cortical representation of volitional swallowing in patients with KD. The first main finding was that KD patients showed a stronger and broader swallow‐related activation of the primary sensorimotor cortex than healthy control subjects that even extended into the premotor cortex and into the somatosenory integration area. The notion that these changes in brain activation reflect functional reorganization of the primary sensorimotor cortex is corroborated by two other recent imaging studies on dysphagic patients. Mosier et al. [ 2005] studied four patients treated with partial glossectomy due to carcinoma of the tongue by means of fMRI. In these patients, the authors found a significantly increased activation of the primary sensory cortex, the parietal cortex, and the cerebellum. Using TMS, Hamdy et al. [ 1997] observed that dysphagia in patients with hemispheric stroke was associated with smaller pharyngeal responses to stimulation of the unaffected hemisphere than in nondysphagic stroke patients. Interestingly, with recovery from dysphagia this pharyngeal representation increased in size thereby suggesting a compensatory reorganization of the unaffected hemisphere [Hamdy et al., 1996]. Furthermore, although unrelated to dysphagia, the study by Reddy et al. [ 2002] is remarkably in keeping with our own findings. In patients with limb weakness because of a motor neuropathy, these authors found an increased activation of the bilateral primary sensorimotor cortex and the supplementary motor cortex during a simple finger movement task.
Physiologically, there are several mechanisms at different levels of the nervous system that contribute to a task‐specific increase of cortical activation under the condition of motor denervation: (i) Latent intracortical connections may be unmasked by local disinhibition [Jacobs and Gonoghue, 1991]; (ii) modulation of synaptic efficacy may lead to long‐term potentiation or long‐term depression [Hess and Donoghue, 1994]; (iii) axonal sprouting with alterations in synapse shape, number, size and type may be involved [Kaas, 1991]; (iv) adaptive changes in subcortical nuclei may drive altered patterns of cortical recruitment [Kaas et al., 1999]; and (v) reinnervation of muscles by surviving horn motor neurons may form new motor units, the recruitment of which would involve usually not activated cortical areas [Wu and Kaas, 1999]. Given the chronic and slowly progressive nature of KD, it is quite possible that apart from the first mechanism, which is assumed to play a major role in short‐term plasticity after an acute injury, all the other mechanisms of motor system reorganization are involved to different degrees in our patient collective.
The second main finding of this study points to a stronger swallow‐related involvement of the right hemisphere in patients with KD as compared to healthy controls. As has been established previously in different studies, our control group showed left hemisphere dominance for volitional swallowing. In contrast to this finding, in KD patients the maximum activity was located in the right sensorimotor cortex. Furthermore, while in nondysphagic subjects the previously described time‐dependent shift from the left to the right hemisphere was found [Teismann et al., in press], KD patients showed right hemispheric activation in each time segment. In particular, in the first 400‐ms bilateral activation was seen, whereas control subjects only presented with left hemispheric activation. The interpretation of these findings draws upon previous research suggesting that different components of swallowing are differently lateralized. First insights into this topic were generated by lesion studies. Thus, Robbins et al. [ 1993] identified oral stage dysfunction to be associated with left hemispheric infarction, whereas pharyngeal stage dysfunction was related to right hemispheric infarction. Similarly, another study showed that right hemispheric stroke led to dysfunction and dysmotility of the pharyngeal stage of deglutition [Daniels et al., 1996]. These results were corroborated by a recent study employing a dual‐task interference paradigm to investigate swallowing lateralization [Daniels et al., 2006]. Based on their findings, Daniels et al. suggested a left hemisphere control for volitional aspects of swallowing and a right hemisphere control for reflexive swallowing behavior. Interestingly, the aforementioned time‐dependent shift of cortical activation from an early involvement of the left to a late involvement of the right hemisphere is also well in line with this notion of a left hemispheric dominance for the oral stage and a right hemispheric dominance for the pharyngeal stage of swallowing [Teismann et al., in press]. Interpreting the pattern of cortical activation observed in KD patients in the light of these findings one may suggest that the stronger right hemispheric activation, both in size and time, is indicative of a cortical compensation of pharyngeal phase dysphagia, which in turn was demonstrated by FEES.
Finally, it seems unlikely that the measured differences between the patient and the control group were due to nonspecific effects of muscle weakness. In spite of pathological findings in FEES, KD patients did not fare worse than healthy control subjects in the water swallowing screening task. Taking into account that the swallowing paradigm used during MEG measurement was even less demanding and, in contrast to most fMRI studies, enabled swallowing in the physiological upright sitting position, there should not have been a confounding effect of task difficulty.
In conclusion, this is one of the very first studies showing adaptive cortical changes in patients with neurogenic dysphagia. In future trials, we will aim at investigating diseases causing discrete lesions of other parts of the complex sensorimotor network regulating the act of swallowing.
REFERENCES
- Amato AA,Prior TW,Barohn RJ,Snyder P,Papp A,Mendell JR ( 1993): Kennedy's disease: A clinicopathologic correlation with mutations in the androgen receptor gene. Neurology 43: 791–794. [DOI] [PubMed] [Google Scholar]
- Chau W,McIntosh AR,Robinson SE,Schulz M,Pantev C ( 2004): Improving permutation test power for group analysis of spatially filtered MEG data. Neuroimage 23: 983–996. [DOI] [PubMed] [Google Scholar]
- Daniels S,Foundas A,Iglesia G,Sullivan M ( 1996): Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis 6: 30–34. [DOI] [PubMed] [Google Scholar]
- Daniels SK,Corey DM,Fraychinaud A,DePolo A,Foundas AL ( 2006): Swallowing lateralization: The effect of modified dual‐task interference. Dysphagia 21: 21–27. [DOI] [PubMed] [Google Scholar]
- Derambure P,Defebvre L,Dujardin K,Bourriez JL,Jacquesson JM,Destee A,Guieu JD ( 1993): Effect of aging on the spatio‐temporal pattern of event‐related desynchronization during a voluntary movement. Electroencephalogr Clin Neurophysiol 89: 197–203. [DOI] [PubMed] [Google Scholar]
- Ding R,Larson C,Logemann J,Rademaker A ( 2002): Surface electromyographic and electroglottographic studies in normal subjects under two swallow conditions: Normal and during the Mendelsohn manuever. Dysphagia 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Dziewas R,Sörös P,Ishii R,Chau W,Henningsen H,Ringelstein EB,Knecht S,Pantev C. ( 2003): Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144. [DOI] [PubMed] [Google Scholar]
- Dziewas R,Sörös P,Ishii R,Chau W,Henningsen H,Ringelstein EB,Knecht S,Pantev C ( 2005): Cortical processing of esophageal sensation is related to the representation of swallowing. Neuroreport 16: 439–443. [DOI] [PubMed] [Google Scholar]
- Dziewas R,Warnecke T,Schnabel M,Ritter M,Nabavi DG,Schilling M,Ringelstein EB,Reker T ( 2007): Neuroleptic‐induced dysphagia: Case report and literature review. Dysphagia 22: 63–67. [DOI] [PubMed] [Google Scholar]
- Ertekin C,Aydogdu I,Yüceyar N ( 1996): Piecemeal deglutition and dysphagia limit in normal subjects and in patients with swallowing disorders. J Neurol Neurosurg Psychiatry 61: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong PL,Hobson AR,Aziz Q,Barnes GR,Singh KD,Hillebrand A,Thompson DG,Hamdy S ( 2004): Dissociating the spatio‐temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage 22: 1447–55. [DOI] [PubMed] [Google Scholar]
- Guidetti D,Sabadini R,Ferlinie A,Torrente I ( 2001): Epidemiological survey of X‐linked bulbar and spinal muscular atrophy, or Kennedy disease, in the province of Reggio Emilia, Italy. Eur J Epidemiol 17: 587–591. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Aziz Q,Rothwell JC,Crone R,Hughes DG,Tallis RC,Thompson DG ( 1997): Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 350: 686–692. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Aziz Q,Rothwell JC,Singh KD,Barlow J,Hughes DG,Tallis RC,Thompson DG ( 1996): The cortical topography of human swallowing in health and disease. Nat Med 2: 1217–1224. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Mikulis D,Crawley A,Xue S,Lau H,Henry S,Diamant N. ( 1999a): Cortical activation during human volitional swallowing: An event‐related fMRI study. Am J Physiol Gastrointest Liver Physiol 277: G219–G225. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Rothwell J,Brooks D,Bailey D,Aziz Q,Thompson D. ( 1999b): Identification of the cerebral loci processing human swallowing with H2 150 PET activation. J Neurophysiol 81: 1917–1926. [DOI] [PubMed] [Google Scholar]
- Harris ML,Julyan P,Kulkarni B,Gow D,Hobson A,Hastings D,Zweit J,Hamdy S ( 2005): Mapping metabolic brain activation during human volitional swallowing: A positron emission tomography study using [18F]fluorodeoxyglucose. J Cerebr Blood Flow Metab 25: 520–526. [DOI] [PubMed] [Google Scholar]
- Hess G,Donoghue JP ( 1994): Long‐term potentiation of horizontal connections provides a mechanism to reorganize cortical maps. J Neurophysiol 71: 2543–2547. [DOI] [PubMed] [Google Scholar]
- Hughes TAT ( 2003): Neurology of swallowing and oral feeding disorders: Assessment and management. J Neurol Neurosurg Psychiatry 74: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TAT,Wiles CM ( 1996): Clinical measurement of swallowing in health and in neurogenic dysphagia. Q J Med 89: 109–116. [DOI] [PubMed] [Google Scholar]
- Jacobs KM,Gonoghue JP ( 1991): Reshaping the cortical motor map by unmasking latent intracortical connections. Science 22: 944–947. [DOI] [PubMed] [Google Scholar]
- Jean A ( 2001): Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev 81: 929–969. [DOI] [PubMed] [Google Scholar]
- Kaas JH ( 1991): Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci 14: 137–167. [DOI] [PubMed] [Google Scholar]
- Kaas JH,Florence SL,Jain N ( 1999): Subcortical contributions to massive cortical reorganizations. Neuron 22: 657–660. [DOI] [PubMed] [Google Scholar]
- Kern MK,Jaradeh S,Arndorfer RC,Shaker R ( 2001): Cerebral cortical represenatation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360. [DOI] [PubMed] [Google Scholar]
- Langmore SE. 2001. Scoring a FEES examination In: Langmore SE, editor. Endoscopic Evaluation and Treatment of Swallowing Disorders. New York, Stuttgart: Thieme; pp 101–143. [Google Scholar]
- Martin RE,Goodyear BG,Gati JS,Menon RS ( 2001): Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950. [DOI] [PubMed] [Google Scholar]
- Martin RE,MacIntosh BJ,Smith RC,Barr AM,Stevens TK,Gati JS,Menon RS ( 2004): Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443. [DOI] [PubMed] [Google Scholar]
- Mosier K,Bereznaya I ( 2001): Parallel cortical networks for volitional control of swallowing. Exp Brain Res 140: 280–289. [DOI] [PubMed] [Google Scholar]
- Mosier K,Liu WC,Behin B,Lee C,Baredes S ( 2005): Cortical adaptation following partial glossectomy with primary closure: Implications for reconstruction of the oral tongue. Ann Otol Rhinol Laryngol 114: 681–687. [DOI] [PubMed] [Google Scholar]
- Mosier KM,Liu WC,Maldjian JA,Shah R,Modi B ( 1999): Lateralization of cortical function in swallowing: A functional MR imaging study. Am J Neuroradiol 20: 1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Nichols TE,Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G ( 1977): Graphical display and statistical evaluation of event‐related desynchronization (ERD). Electroencephalogr Clin Neurophysiol 43: 757–760. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G ( 1992): Event‐related synchronization (ERS): An electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol 83: 62–69. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Aranibar A ( 1977): Event‐related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 42: 817–826. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Aranibar A ( 1979): Evaluation of event‐related desynchronization (ERD) preceding and following voluntary self‐paced movement. Electroencephalogr Clin Neurophysiol 46: 138–146. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Berghold A ( 1989): Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 72: 250–258. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Reddy H,Bendahan D,Lee MA,Johansen‐Berg H,Donaghy M,Hilton‐Jones D,Matthews PM ( 2002): An expanded cortical representation for hand movement after peripheral motor denervation. J Neurol Neurosurg Psychiatry 72: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J,Levine RL,Maser A,Rosenbeck JC,Kempster GB ( 1993): Swallowing after unilateral stroke of the cerebral hemisphere. Arch Phys Med Rehab 74: 1295–1300. [DOI] [PubMed] [Google Scholar]
- Sekihara K,Sahani M,Nagarajan SS ( 2005): Localization bias and spatial resolution of adaptive and non‐adaptive spatial filters for MEG source reconstruction. Neuroimage 25: 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperfeld AD,Karitzky J,Brummer D,Schreiber H,Häussler J,Ludolph AC,Hanemann O ( 2002): X‐linked bulbospinal neuronopathy—Kennedy disease. Arch Neurol 59: 1921–1926. [DOI] [PubMed] [Google Scholar]
- Stancak A,Jr .,Pfurtscheller G ( 1996): Event‐related desynchronisation of central beta‐rhythms during brisk and slow self‐paced finger movements of dominant and nondominant hand. Brain Res Cogn Brain Res 4: 171–183. [DOI] [PubMed] [Google Scholar]
- Teismann IK,Dziewas R,Steinstraeter O,Pantev C ( 2007): Time‐dependent hemispheric shift of the cortical control of volitional swallowing. Hum Brain Mapp. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann IK,Steinstraeter O,Stoeckigt K,Suntrup S,Wollbrink A,Pantev C,Dziewas R ( 2007): Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiman M,Eviatar E,Segal S ( 2004): Surface electromyographic studies of swallowing in normal subjects: A review of 440 adults. Report 1. Quantitative data: Timing measures. Otolaryngol Head Neck Surg 131: 548–555. [DOI] [PubMed] [Google Scholar]
- Vrba J,Robinson SE ( 2001): Signal processing in magentoencephalography. Methods 25: 249–271. [DOI] [PubMed] [Google Scholar]
- Wu CW,Kaas JH ( 1999): Reorganization in primary motor cortex of primates with long‐standing therapeutic amputations. J Neurosci 19: 679–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin FZ,Hammeke TA,Swanson SJ,Morris GL,Mueller WM,McAuliffe TL,Haughton VM ( 1995): A comparison of functional MR activation patterns during silent and audible language tasks. AJNR Am J Neuroradiol 16: 1087–1092. [PMC free article] [PubMed] [Google Scholar]


