Abstract
In monkeys, areas in the intraparietal sulcus (IPS) play a crucial role in visuospatial information processing. Despite many human neuroimaging studies, the location of the human functional homologs of some IPS areas is still a matter of debate. The aim of the present functional magnetic resonance imaging (fMRI) study was to identify the distinct locations of specific human IPS areas based on their functional properties using stimuli adapted from nonhuman primate experiments, in particular, surface orientation discrimination and memory guided saccadic eye movements (SEM). Intersubject anatomical variability likely accounts for much of the debate. By applying subject by subject analysis, we can demonstrate that sufficient intersubject anatomical and functional commonalities exist. Both the lateral bank of the anterior part of IPS, the putative human homolog of the area AIP, and the caudal part of the IPS (putative CIP) showed activation related to spatial discrimination of surface orientation. Eye tracking conducted during fMRI data acquisition allowed us to show that both areas were separated by an area related to SEM. This area was located in the middle region of the IPS (most probably including LIP), i.e., similar to the location observed in nonhuman primates. In 10 of 11 subjects our putative CIP activation was located in a medial side branch of the posterior part of the IPS, on the opposite side as described in nonhuman primates, making this landmark a useful anatomical marker for the location of CIP. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, AIP, CIP, LIP, human homolog
INTRODUCTION
On the basis of neurophysiologic evidence in monkeys, it was suggested that areas in the intraparietal sulcus (IPS) play a crucial role in tasks such as depth perception and visually guided hand movements [Sakata et al., 1997]. This is based on the fact that both the anterior and caudal regions of the monkey IPS, (AIP and CIP, respectively) encode visual three dimensional (3D) features of objects for grasping. Area AIP in the monkey houses neurons that are specifically related to visually guided hand movements [Taira et al., 1990; Sakata et al., 1995]. Many of these neurons are selectively activated not only during grasping a particular 3D object, but also during fixation upon these objects [Murata et al., 2000]. Indeed, in previous neuroimaging studies [Shikata et al., 2001, 2003], we observed signal changes in putative area AIP and area CIP elicited by a purely visual discrimination task, similar to the one used in nonhuman primates [Tsutsui et al., 2002].
In the monkey, area AIP is located in the antero‐lateral bank of the IPS, rostral to the lateral intraparietal area (LIP), which is functionally linked to saccadic eye‐movements [Andersen et al., 1990]. Area CIP is located in the lateral bank of the IPS caudal to area LIP and anterior to area V3a [Tsutsui et al., 2001]. Area AIP is functionally separated from LIP by the absence of saccadic eye movement responsive neurons [Murata et al., 2000], whereas the LIP border is defined by activation related to saccadic eye movements [Andersen et al., 1990; Gnadt and Andersen, 1988].
The location of putative human AIP has also been described as the rostral part of the lateral bank of the anterior IPS [Binkofski et al., 1998], and the crossing of the IPS with the postcentral sulcus [Faillenot et al., 2001; Frey et al., 2005]. In our previous study, we found activations in a visual discrimination task without hand‐manipulations, located posterior to the hand representation of S1/M1 [Shikata et al., 2001].
Although many studies have shown activation in putative human area AIP, there are only a few studies that investigated the topographical relationship between putative area AIP and CIP. Since the individual structure of the human IPS is known to be variable, it is crucial in neuroimaging studies to determine the location of these areas at a single subject level and to provide landmarks which may be helpful for further studies. Therefore, the aim of this study was to determine the location of putative human area AIP and area CIP and to differentiate it from an area related to performance of saccadic eye movements (probably including human area LIP), using a robust surface orientation discrimination task and memory guided saccadic eye‐movement task. The additional saccadic eye movement task was necessary to precisely localize both areas with respect to neighboring areas such as LIP. Recording of eye movements during scanning allowed us to ensure full compliance of subjects to the task requirements.
MATERIALS AND METHODS
Eleven right‐handed, healthy volunteers (mean, 25.0 ± 2.1 years; 5 females) with normal visual acuity gave their written informed consent to participate in this study. The protocol of the study was approved by the local ethics committee.
Experiment 1: Surface Orientation Discrimination with a Delayed Matching Task
The stimuli used in this experiment consisted of the texture elements evaluated already in our previous study [Shikata et al., 2003]. Figure 1a shows the task procedure of Experiment 1. In brief, texture element stimuli consisted of colored dots with a diameter of 0.5 degrees in the fronto‐parallel plane assembled into a perspective array. This plane was slanted 30 and 45 degrees with respect to the screen plane creating a pair of stimuli. The size of the dots was manipulated to generate the impression of a plane. This plane was then rotated about the saggital axis in 45 degree steps, resulting in 16 different orientations. The background of the texture was black. These texture stimuli were presented inside a 12.2 degree diameter aperture. The combination of 9 colors ranging from blue to yellow with 16 orientations resulted in 144 stimuli.
Figure 1.
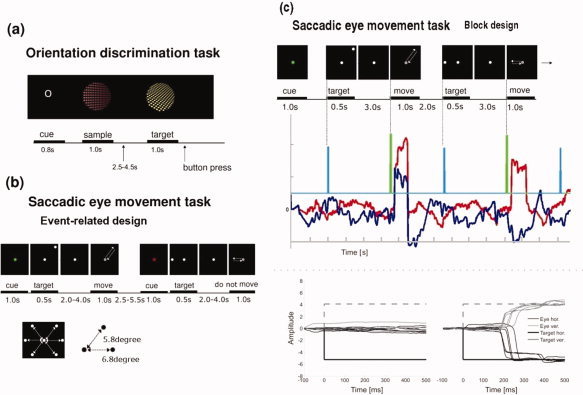
Paradigm as employed in the study. Experiment 1 (a) employed a surface orientation discrimination task in a delayed matching to sample paradigm. Stimuli were presented at the centre of the screen. The cue was followed by a sample stimulus. After the sample stimulus, a single matching stimulus was presented. Subjects were instructed to press the left button with their right index finger when the stimulus showed the same orientation (task O) or the same color (task C) as the sample and the right button with the middle finger when the matching stimulus was different. Subjects responses were recorded by index or forefinger button press. Stimuli were presented for 1,000 ms with a stimulus onset asynchrony randomised between 2,500 and 4,500 ms. Experiment 2 (b) was an event‐related ‘go – no go’ memory guided saccadic eye movement task. The event condition was cued to the subject by a red or green cue at trial onset, event structure was then identical. The inset shows the six possible target points at the points of a virtual hexagon. Distance from central fixation to target were 5.8 degree in diagonal and 6.8 degree in horizontal plane. (c) Saccadic eye movement data from one subject. Top panel shows two ‘go’ trials from the block designed ‘go – no go memory guided saccade’ experiment. In the middle row, eye movement recordings of the two trials are shown (red: horizontal eye movement, dark blue: vertical eye movement, light blue: time of ‘target onset’, green: onset of ‘move’ cue). The bottom panel shows all data for each eye movement to the upper left target (4 events) for one subject in one session. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Two different trial types, orientation judgment and color discrimination, were tested in a delayed matching to sample task. Visual stimuli were identical for both trial types. Each trial began with a cue letter, indicating the type of the next trial. The letter ‘O’ indicated an orientation trial; the letter ‘C’ indicated a color trial. Figure 1a shows the sequence of an ‘O’ trial. During the interstimulus interval, a white dot was presented as a fixation point (diameter 0.6°) at the centre of the screen. Subjects were instructed to fixate on this spot throughout all trials. The cue was followed by a sample stimulus. After the sample stimulus, a single matching stimulus was presented. Subjects were instructed to press the left button with their right index finger when the stimulus showed the same orientation (task O) or the same color (task C) as the sample and the right button with the middle finger when the matching stimulus was different. Subjects responses were recorded by index or forefinger button press. Stimuli were presented for 1,000 ms with a stimulus onset asynchrony randomized between 2,500 and 4,500 ms.
Experiment 2: Saccadic Eye Movement Task
All volunteers also participated in a second ‘go – no go’ memory guided saccadic eye movement experiment. This was composed of two runs, one blocked fMRI run to functionally localize the saccadic eye movement network for region of interest (ROI) analysis followed by an event related fMRI run to tease apart the differing components of the network. The two runs were measured and analyzed completely separately. By using colored cues subjects were informed before the onset of any event, or block of events whether they should actually perform a saccade (go) or not (no go). ROI's for the memory guided saccade network were determined by contrasting ‘go’ versus ‘no go’ blocks of stimuli from the first blocked run. The second event related experiment temporally separated the ‘salient visual stimulus identification’ and ‘memory guidance’ components from those of the actual ‘saccade generation’. As LIP is more heavily involved in these former components [Colby et al., 1996], we used this property of LIP as defined in the monkey to define LIP in humans. The separation was achieved by extending the delay between target presentation and saccade execution.
Figure 1b shows the procedure of the saccadic eye movement task in the event‐related session (Experiment 2b). The conditions ‘go’ and ‘no go’ were indicated to the subject, respectively, by a 1,000‐ms green or red color change of the white fixation dot. The following event sequence was then identical across conditions, although subjects were not allowed to make saccades during a ‘no go’ event. After the cue period, a white target point with the same size as the fixation point appeared in the peripheral visual field for 500 ms. Subjects were requested to maintain fixation until the central fixation point dimmed at a randomized interval between 2,000 and 4,000 ms after the disappearance of the peripheral target. This constituted our delay. The dimming of the fixation dot signaled the subject, in the case of a green cue, to execute a saccade towards the remembered target location and redirect their gaze back to the center point immediately afterwards. In the case of the red cue, subjects were to maintain fixation. Targets could appear in six possible locations, lying in the corners of a virtual hexagon, (see Figure 1b bottom). Twenty‐four ‘go’ events and 24 ‘no go’ events were presented in a pseudo‐random order during an 8‐min fMRI data acquisition session. The intertrial interval was jittered by ±1.5 s around a mean of 4 s.
In the block design experiment (Exp. 2a), blocks consisted of a stream of 12 ‘go’ or ‘no go’ events with the condition signaled at the onset of the block by a 1,000‐ms green or red color change of the white fixation dot. In contrast to the task in the event‐related experiment, the duration of the delay period and the period between tasks was fixed (delay = 3,000 ms, intertrial interval = 2,000 ms). Three blocks of 90‐s duration for each condition were presented interspersed with 20‐s rest periods in which only the fixation cross remained on the screen.
All visual stimuli were presented on a screen positioned within the scanner bore. The subjects viewed the screen through a mirror which was mounted directly above the eyes of the subject and slanted by 45 degrees. The effective field of view subtended 14 × 29° visual angle. The sequence of visual stimuli was controlled by a PC running the software “Presentation” (Neurobehavioral systems: http://www.neurobs.com, Albany, CA, USA). Before fMRI scanning, the volunteers were familiarized with the stimuli and practiced all tasks to ensure that these were performed correctly.
Image Acquisition
MR scanning was performed on a 3 T MRI scanner (Siemens Trio). The imaging sequence constituted, 36 slices of 3‐mm thickness with a 1‐mm gap between slices, TR = 2.1 s, TE = 25 ms, flip angle 80°, matrix 64*64, field of view 192 × 192 mm2, using a gradient echo echo‐planar (EPI) T2*‐sensitive sequence. The slices were centered on the parietal lobe but covered the whole brain. Echo‐planar images were collected over three sessions of 676 s, 558 s and 466 s for the surface discrimination, blocked saccades, and event related saccades experiments, respectively.
A high‐resolution (1 × 1 × 1 mm3 voxel size) structural MRI was also acquired for each subject using a standard 3D T1 weighted FLASH sequence in a separate session. Functional data were superimposed onto high‐resolution anatomical images.
Eye Position Recordings
Eye movement was measured during the performance of the experimental tasks inside the MRI scanner using an infrared limbus eyetracker (Cambridge Research Systems, UK; sampling rate: 500 Hz, spatial resolution ∼0.1°) during fMRI. Subject's eye movements were observed during scanning and recorded for later analysis. Because of technical reasons only eye recording data of six subjects could be used for further analysis. Analysis of the eye movement data was performed by using an in house semiautomatic computer program developed in Matlab (The MathWorks, Natick, MA). Blink artifacts could be identified automatically and were removed from the data prior to the behavioral analysis. The quality of the data was checked manually prior to, and after the automatic data analysis.
Image Processing and Statistical Analysis
Neuroimaging data analysis was performed using SPM2 (Welcome Department for Cognitive Neurology, London). Imaging series were realigned to the first volume [Friston et al., 1995a], slice‐time corrected within a volume, normalized to the MNI (Montreal Neurological Institute) [Evans et al., 1994] template using nonlinear basis functions [Friston et al., 1995b] and smoothed with a Gaussian kernel of 6 mm full‐width half‐maximum (FWHM). A high‐pass filter with a cut‐off period of 128 s was applied. The different tasks were modeled as delta functions convolved with a canonical hemodynamic response function (HRF). Specific effects were tested with appropriate linear contrasts of the parameter estimates, resulting in a t‐statistic for each voxel. A statistical parametric map of the t‐statistic for the parameter estimates was generated and subsequently transformed to a Z map. Because of the anatomical variability particularly prevalent in the IPS, data were only analyzed for each subject individually. Group analysis (with subjects considered as random effects) was conducted only upon the localizer scan, so as to provide a general functional localized region of interest (fROI) for saccade related activity.
T1 structural volumes were coregistered to the functional scans by normalizing them to a T1 template in the same space as the template used to normalize the functional data set.
Definition of Regions of Interest
In Experiment 1 (surface orientation discrimination task), data analysis was performed by modeling the visual stimuli presented during both trials (task O, C). Button presses were modeled as regressors of motor task. In individual analyses, we used a threshold of 0.05 corrected for multiple comparisons using a small volume correction for a region of interest defined as spheres of 20‐mm radius centered on the coordinate of putative AIP and CIP as reported in previous studies [Binkofski et al., 1999; Shikata et al., 2001] AIP; x = −40, y = −40, z = 40. x = 40, y = −40, z = 44. CIP; x = −15.7, y = −67.8, z = 56.8. x = 19.6, y = −66.8, z = 58.
In Experiment 2a (saccadic eye movements block design), to functionally localize the task related network we identified brain regions showing greater activity during ‘go’ blocks of executing saccades than ‘no go’ blocks of making no‐saccades at P < 0.001 (uncorrected). We used the results from this contrast at this explorative threshold to create functionally derived region of interest for the analysis of the event‐related data set for the comparison of the delay period of the ‘go’ versus ‘no go’ condition. This was done separately for each subject. The mean number of voxels activated in the contrasts, and therefore present in the mask, was 1222 ± 478 (mean ± standard error mean (SEM)).
RESULTS
Behavioral Performance
Task performance
The rate of correct responses was higher for color discrimination (97%) than for surface orientation discrimination (88%). The reaction times (RT) in discrimination trials were longer for orientation discrimination (923 ± 367 ms, mean ± SEM) than for color discrimination trials (760 ± 275 ms), although not significantly different (t(x) = n.s.). The average RT for both trial types was 843 ± 327 ms.
Eye movement monitoring
In the block design fMRI experiment, saccade ‘go’ trials and ‘no go’ trials were presented in alternating blocks. In fixation trials, the subjects maintained central fixation accurately with a standard deviation of eye position from 0.05 ± 0.08 to 0.42 ± 1.62 degrees of visual angle (mean ±SD). In the serial saccade trials, the subjects made a saccade in response to the shift of the target position, correct saccades accounted for 88.2% ± 6.6% of trials and the mean latency of saccadic eye movement onset was 252 ± 86 ms. Figure 1b shows an example of the saccadic eye movement recording from a representative volunteer. Time passes from left to right with the upper schema indicating onsets of each component of two sequential events. Below the schema we show the horizontal (red) and vertical (blue) eye movements measured during the trials. The final two graphs show the eye movements (horizontal and vertical) to the top right point of the hexagon during a single session (four events). In the event‐related fMRI experiment, the percentage of correct saccades was 85.7% ± 5.8% and the mean latency of saccade onset was 307 ± 95 ms.
In the orientation discrimination task, subjects maintained central fixation accurately with a standard deviation of eye movements of 0.09 ± 1.1 to 0.28 ± 2.7 degrees of visual angle in orientation trials and from 0.06 ± 1.2 to 0.85 ± 3.5 degrees in color discrimination trials. There was no significant difference between these two tasks.
FMRI Data
The topographical relation of intraparietal areas
The major aim of this study was to assess the topographical relationship between areas within the IPS which are involved in surface orientation discrimination and saccadic eye movements on an individual basis. Figure 2 shows the relative location of significant activation foci resulting from each individual obtained from orientation discrimination vs. color discrimination (AIP ‐ red and CIP ‐ green) and saccadic eye movements ‘go’ vs. ‘no go’ conditions (yellow in the middle part of the IPS).
Figure 2.
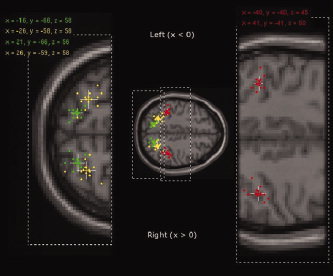
Peak point activation loci for each individual's analysis in Experiments 1 and 2. The cross shows the mean position of each data set. The colored part of the cross indicates standard error of mean and the white continuation of the cross indicates one standard deviation from the mean. All data is provided in the tables given as supplementary data. Red: loci for individual's significant activations in AIP elicited by orientation discrimination vs. color discrimination (P < 0.05 small volume corrected (20‐mm radius sphere centered at left AIP, x = −40, y = −40, z = 40; and right AIP, x = 40, y = −40, z = 44; (Binkofski et al., 1999)). Green: loci for individual's significant activations in CIP elicited by orientation discrimination vs. color discrimination (P < 0.05 small volume corrected (20 mm radius sphere centered at left CIP, x = −16, y = −68, z = 57; and right CIP, x = 20, y = −67, z = 58; (Shikata et al., 2001)). Yellow: loci for individual's significant activations in the middle part of IPS elicited by saccadic eye movements vs. fixation (P < 0.05 FDR small volume corrected using the functionally derived region of interest, (see Methods: Definition of Regions of Interest section)). Enlarged images are taken from MNI brain z = 58 (left) and z = 45 (right).
Orientation discrimination
Strong activations (P < 0.05 FDR & Small Volume Corrected with a spherical ROI) were shown by all subjects but as expected were spread across a limited spatial range of (<20 mm for AIP and left CIP and <30 mm across right CIP) building well separated clusters which represent the IPS areas in question. Mean peak point loci from this analysis are given in Figure 2 which provides an overview of the mean location of each of these areas.
Two different IPS activation sites were observed in each hemisphere: the first included the anterior part of the lateral bank and bottom of the IPS, and the second included a caudal region in a side branch of the IPS. The side branch lay anterior to the parieto‐occipital sulcus. Bilateral activations within the AIP‐ROI were observed in 9 of 11 subjects and in two subjects in the right hemisphere only at the level of P < 0.05 FDR and small volume corrected (S7, S11). Examples of activation foci in individual subjects best representing the data are shown in Figure 3 (S1, 2, 3, 9). Views of the rendered brain viewed from the left and from above are shown. Significant activations for surface orientation vs. color discrimination are shown in red. In the enlarged views (Fig. 3), activations located at the crossing of the IPS with the postcentral sulcus are shown (letter a). The location and Z‐scores of the peak activations in each individual subject are listed in supplemental data Table I.
Figure 3.
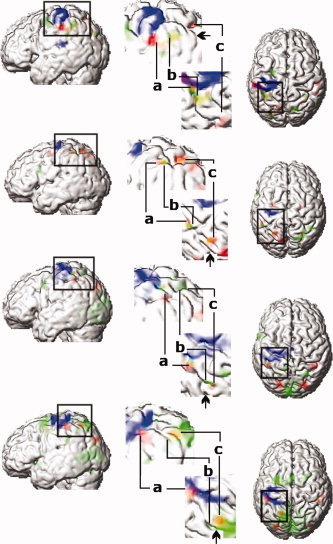
Activated regions in four individual subjects, (top to bottom). Significant activations for surface orientation vs. color discrimination (red) (P < 0.001 uncorrected), for the motor response of the left index and middle finger (blue) (P < 0.05 corrected) and for delayed period for saccadic eye movements (green) (P < 0.05 corrected within fROI). Orange color indicates overlap between the areas of surface orientation discrimination and delay period prior to saccadic eye movements (Sub 9). First column, rendered brain viewed from left. Central column, an enlarged view of the areas around the IPS viewed from the right and from above. Right column, rendered brain viewed from above. In the enlarged view, activations during orientation discrimination (a, AIP; c, CIP) are located in anterior and posterior part of IPS. Activations related to the delay period prior to saccadic eye movement (b, middle part of IPS) are located between AIP and CIP. Arrows in enlarged view indicate border of parietal and occipital lobe in IPS.]
Table I.
Coordinates of the anterior part of the IPS with neuroimaging experiments mentioned in discussion
| Study | L | R | ||||||
|---|---|---|---|---|---|---|---|---|
| Coordination | Coordination | |||||||
| X | Y | Z | X | Y | Z | |||
| PET study | ||||||||
| Faillenot et al., 1997 | 34 | −52 | 44 | |||||
| Taira et al., 1998 | −40 | −42 | 40 | 37 | −46 | 43 | ||
| Faillenot et al., 1999 | 46 | −44 | 48 | |||||
| MRI study | ||||||||
| Binkofski et al., 1998 | −45 | −35 | 43 | |||||
| Binkofski et al., 1999 | −40 | −40 | 40 | 40 | −40 | 44 | ||
| Chao and Martin, 2000 | −32 | −47 | 42 | |||||
| −30 | −39 | 47 | ||||||
| Faillenot et al., 2001 | −40 | −40 | 56 | 36 | −40 | 52 | ||
| Jäncke et al., 2001 | −40 | −44 | 40 | 32 | −30 | 40 | ||
| Buccino et al., 2001 | −36 | −40 | 52 | 40 | −40 | 52 | ||
| Shikata et al., 2001 | −37 | −40 | 47 | 45 | −32 | 51 | ||
| Grefkes et al., 2002 | −40 | −42 | 36 | |||||
| Mecklinger et al., 2002 | −44 | −46 | 42 | |||||
| Shikata et al., 2003 | −36 | −39 | 39 | 39 | −39 | 39 | ||
| Frey et al., 2005 | −40 | −33 | 43 | |||||
| Tanabe et al., 2005 | 38 | −40 | 44 | |||||
| Mean | −38.5 | −40.5 | 43.6 | 38.7 | −40.3 | 45.7 | ||
| ±SD | 4.2 | 3.9 | 5.6 | 4.4 | 6.3 | 4.8 | ||
Significant activation of the caudal part of the IPS was found in 10 of 11 subjects bilaterally, and in one subject only in the right hemisphere (S11). As presented in Figures 2 and 3, activations of the caudal part of the IPS were located in a side branch of the posterior IPS. This side branch of the posterior IPS lies rostral to the parieto‐occipital sulcus. As shown in Figures 2 and 3, such activation located in the side branch of the posterior IPS (colored green) was also clearly shown in most of our subjects. In fact, in all subjects who had this side branch of the IPS, the activation sites in the caudal IPS were located between the crossing and the end of the side branch of the posterior IPS. In one subject, in whom this side branch could not be detected in the right hemisphere, the activation of the caudal part of IPS was located at the parietal end of the horizontal part of the IPS (S3). The locations and Z‐scores of the peak activations in each individual subjectare provided as supplemental data (Table II). The mean location of the activations were x = −39.6 ± 4.9, y = −40.1 ± 2.6, z = 44.7 ± 4.9 and x = 41.2 ± 5.0, y = −40.6 ± 5.3, z = 50.2 ± 4.8 mm in AIP (red in Figure 2), x = −15.5 ± 3.4, y = −66.3 ± 4.1, z = 58.4 ± 4.3 and x = 21.0 ± 6.0, y = −65.7 ± 5.0, z = 55.9 ± 6.6 in CIP (green in Fig. 2) and x = −25.6 ± 6.3, y = −57 ± 6.1, z = 58.4 ± 6.7 on the left and x = 26.1 ± 6.0, y = −59.4 ± 6.7, z = 57.6 ± 5.2 in the middle part of IPS (yellow in Fig. 2). Further clusters of activation (all bilateral) were observed in the inferior frontal lobes and the posterior end of the superior frontal sulci in the frontal lobe. In occipital lobes, bilateral clusters lay within the middle occipital gyri and interoccipital sulci (at the boundary with the IPS).
Activations related to saccadic eye movements
Comparison of ‘go target onset’ versus ‘no go target onset’ in Experiment 2 revealed activations related to saccadic eye movements. Strong, (P < 0.05, FDR & Small Volume Corrected with fROI) activations within the middle part of the IPS are shown in yellow in Figure 2. Mean peak point loci from this analysis are given in Figure 2 which provides an overview of the spread of data in this region. Activations within the middle part of the IPS were bilaterally located between the anterior and caudal parts of the IPS and were adjacent to the activation of the caudal part of the IPS. Ten of eleven subjects showed bilateral focal activations in the middle part of the IPS. One subject showed distinct activations along the IPS but only on the left side (n = 1/11). Seven of 11 subjects, however, showed more than two activations or peaks in the middle part of the IPS (see S2, S3 in Figure 3). Besides the middle part of the IPS, ‘go’ versus ‘no go’ target onset revealed increased signal around the posterior end of the superior frontal sulcus, (SFS), in the SMA, in the middle part of the IPS, in the occipital lobe (calcarine) and in the cerebellum, (P < 0.05, FDR & Small Volume Corrected with fROI).
The topographical relation of intraparietal areas
Figure 3 shows the topographical map of the relative localization of activations related to orientation discrimination (red), ‘go’ vs. ‘no go’ (green) and finger movement execution (blue) in individual subjects (S1,2,3,9). The activation related to orientation discrimination vs. color only; represents putative AIP and CIP (Fig. 3, red). Area AIP is located at the intersection of the postcentral sulcus and the IPS (Fig. 3, letter a) anterior‐lateral and inferior to the area activated by saccadic eye movements (green). The activation of the caudal part of the IPS, suggested as putative site of CIP (Fig. 2, green) is located in the side branch of the IPS and postero‐medial to the area related to saccadic eye movements (green activations in Fig. 3) or overlapped with activation in the caudal part of the IPS (orange activation in S9). In all subjects saccade related activation was located medial and posterior to the anterior part of the IPS.
One of 11 subjects showed bilateral overlapping activation of the caudal part of the IPS by orientation and saccade related activation (S9, see Fig. 3). Three subjects showed such an overlap of activation in the left hemisphere only (S6, S10, S11).
The location of the hand/finger representation of sensori‐motor cortex in relation to AIP
The location of the hand/finger representation within the sensori‐motor cortex was assessed by modeling individual button presses with the right hand in an event‐related fashion. Subjects were required to respond with their right index or middle finger during Experiment 1, which resulted in activation centered on the left central sulcus, including the pre‐ and postcentral gyrus. The mean coordinates of this activation calculated from all subjects was x = −47.1 ± 7.1, y = −30 ± 6.4, z = 60 ± 6.7 mm.
We investigated the relative distribution of the hand/finger representation in the sensory‐motor cortex to the surface orientation related area in the anterior part of the IPS. Figure 3 shows the locations of the hand/finger movements (blue) in relation to AIP (red). AIP is located postero‐inferior to the hand representation area in every subject. On the medio‐lateral aspect, 9 of 11 subjects show that the activation in the anterior part of the IPS (AIP) is medial to the hand representation within the sensori‐motor cortex. In one subject the x‐coordinate for the anterior intraparietal and hand/finger activation was identical. In the remaining subjects, the activation of the anterior part of the IPS was localized lateral to the hand representation in the sensori‐motor cortex. The antero‐posterior (y) coordinate of the anterior IPS activation (AIP) was found to be posterior to the hand representation of M1/S1 in all subjects. Along the inferior‐superior (z) axis, in all subjects the anterior IPS activation (AIP) was localized inferior to M1/S1. The mean distance between anterior IPS activation and M1/S1 was x = 10.1 ± 6.3, y = 12.4 ± 6.3, z = 14.6 ± 8. The corresponding Euclidean distance was 23.3 ± 6.8 mm (range 15–38.5 mm).
DISCUSSION
We investigated the topographical layout of the putative functional equivalents of human areas AIP, CIP, and a saccade related area (including a human equivalent of area LIP) using a surface orientation discrimination task with a small motor component and a memory guided saccadic eye movement task.
The orientation discrimination related activation in the anterior part of the IPS was located on the antero‐lateral bank of the IPS and at the crossing of the IPS with the postcentral sulcus and represented the human homolog of AIP. AIP was also located caudal to the finger and hand representation area. This activation was adjacent to an area that was related to saccadic‐eye‐movements (putative equivalent of LIP).
Orientation discrimination related activations in the caudal part of the IPS (CIP) was located in the medial side branch of the IPS in those subjects in whom such a side branch was present. The activation related to saccadic‐eye‐movements (including putative area LIP) was located both medial and posterior to the AIP area.
Localization of Human Area AIP
The location of area AIP in humans was first described by Binkofski et al. [1998] using a combination of lesion studies and fMRI. They located area AIP in the anterior and horizontal part of the IPS and determined the coordinates of AIP in the left hemisphere with functional neuroimaging in five healthy subjects. Despite the differences in experimental design and data analysis, the activation of area AIP was identified in a number of studies, including the present results, at/around the intersection of the IPS and the postcentral sulcus. In a PET study using axis orientation and object matching, the location of area AIP was described within the anterior part of the IPS in the right hemisphere [Faillenot et al., 1997, 1999; Taira et al., 1998]. In contrast, during axis orientation discrimination matched with hand movements, the location of area AIP was activated only in the left hemisphere [Taira et al., 1998]. In an fMRI study during orientation discrimination of objects and gratings, the activation was located bilaterally in the anterior part of IPS, at the intersection of the IPS and the postcentral sulcus [Faillenot et al., 2001]. In many fMRI studies, activation of the AIP homolog in humans has been proposed [Binkofski et al., 1999; Buccino et al., 2001; Frey et al., 2005; Grefkes et al., 2002; Jäncke et al., 2001; Mecklinger et al., 2002; Tanabe et al., 2005]. Even without actual grasping movement, tasks concerned with 3D features of objects seem to elicit activation in putative human area AIP even in the absence of any overt movements as seen in monkey studies [Chao and Martin, 2000; Mecklinger et al., 2002; Murata et al., 1997], (see Table I for coordinates).
In primate experiments, the caudal border between AIP and LIP was identified by mapping the change in functional properties of neurons from being visual fixation neurons to those showing activity related to saccadic eye movements [Murata et al., 2000]. LIP neurons were originally recognized by their consistent visual, delay‐period, and saccade‐related response in the delayed saccade task [Andersen et al., 1990; Gnadt and Andersen, 1988]. LIP may house numerous complex functions other than those captured by the memory guided saccade task, such as processing of spatial selectivity, of the presaccadic period and of spatial remapping [Colby et al., 1996; Duhamel et al., 1992].
Our main goal was to delineate the border of area AIP and area CIP with respect to neighboring areas. A saccadic paradigm was chosen for localization of the human functional homolog of LIP, because it has provided very consistent results in the original physiological functional mapping experiments in monkeys.
It should, however, be mentioned that it is still a matter of debate which human area constitutes the exact homolog of area LIP in the monkey. Silver et al. [2005] suggested that human LIP might have more subdivisions than monkey LIP. In the monkey, area LIP is located along the lateral bank of the intraparietal sulcus, posterior to AIP and anterior to area CIP. However, several studies have proposed the human homolog of LIP as being part of the IPS [Heide et al., 2001; Merriam et al., 2003; Sereno et al., 2001].
Of these reports, some have localized putative human LIP using topographic organization of the activation in the delay period prior to saccades [Sereno et al., 2001; Schluppeck et al., 2005] and/or directional attention [Silver et al., 2005; Vandenberghe et al., 2005]. In another approach, Medendorp et al. [2003] and Merriam et al. [2003] also reported significant activation in contralateral PPC using a saccadic remapping task. On the basis of the properties of macaque LIP neurons which include preparatory activation for saccades, Astafiev et al. [2003] showed two activation foci within the IPS which were described as human functional homologs to monkey LIP and VIP. LIP activation foci as described in these reports ranged from y = −48 [Heide et al., 2001] to y = −76 [Schluppeck et al., 2005] along the posterior part of the IPS. Human subdivisions of LIP (not seen in monkeys) may play a role in precise localization of LIP in imaging experiments [Silver et al., 2005].
The present findings show that area AIP activated during orientation discrimination is discretely located anterior and lateral to the saccade related activation sites of the IPS. There are only a few neuroimaging studies addressing the issue of the border between AIP and the area for saccadic eye movements. In each of these studies, activation within the IPS due to hand movement, including hand preshaping and grasping, always lay anterior to activations related to saccades [Kawashima et al., 1996; Simon et al., 2002]. Even so, activations indicating AIP were still anterior to the IPS activation elicited by saccadic eye movements. Using an attentional saccadic eye movement task and a pointing task, Astafiev et al. [2003] mapped activation foci to the VIP/LIP complex. They reported this VIP/LIP complex activation as lying posterior to AIP. Not only during saccadic preparation, but also during preparation of the pointing task, there was no activation in AIP.
Based on these studies, it is has also been suggested that the area in the anterior part of the IPS, which is related to hand manipulation, may be spatially separated from the area related to saccadic eye movements.
We also investigated the spatial relationship between area AIP and the area activated by simple finger movements, i.e., sensori‐motor cortex, involved in making the button press in Experiment 1. This analysis was performed in analogy to previous monkey studies demonstrating that area AIP is located in the anterior IPS caudal to the finger and hand representation in S1 [Gallese et al., 1994; Murata et al., 2000]. We also found activation for surface orientation discrimination in the anterior part of the IPS (AIP) posterior to the hand representation in the sensori‐motor cortex, at the intersection of the IPS and the postcentral sulcus. Despite the interindividual variation of sulcal anatomy, the relationship of the location was almost identical to that reported by Frey et al. [2005].
Localization of Human Area CIP
Area CIP in the monkey is located on the lateral bank of the intraparietal sulcus, caudal to area LIP, and rostral to area V3a [Shikata et al., 1996; Taira et al., 2000; Tsutsui et al., 2001]. The border between area CIP and LIP was defined by the lack of eye movement related activity in CIP. The border of CIP to area V3a could be identified by the larger receptive fields in CIP compared to V3a. However, even in the monkey, anatomical boundaries of this area are still vague and have not been standardized yet. Area CIP might correspond to anatomical area LOP (lateral occipital parietal) identified by Van Essen [Lewis and Van Essen 2000a; Van Essen, 2003]. Area LOP contains visual neurons and is located between LIP and V3a [Lewis and Van Essen, 2000b]. The posterior part of LOP is partially included in the oculomotor network [Schall et al., 1995].
Only few neuroimaging studies in humans showed activations at the putative site of area CIP using discrimination of surface features. In our previous study, we reported the location of area CIP using surface orientation discrimination by texture gradient [Shikata et al., 2001]. In other studies, perception of surface orientation as studied by using shading and rotated objects in depth, activated either the right [Taira et al., 2001] or bilateral caudal part of IPS [James et al., 2002]. However, it is unclear whether these activations which are some 20 mm apart comprise the same functional area. One reason for the observed differences may be the diversity of the tasks used. Another explanation might be related to interindividual anatomical differences. In this present study, 10 out of 11 subjects showed a side branch of the IPS and the activation of area CIP was located at the intersection of that branch and the IPS. The other subject (S4) showed activation of area CIP at the end of the horizontal part of the IPS. Therefore, we propose that the location of CIP is variable across subjects and should be assessed individually.
Activation related to orientation discrimination in some volunteers overlapped with saccade related activation. With respect to the overlap of activation in area CIP, the limitation of spatial resolution of fMRI should be taken into account. This is because some parts of area CIP/LOP in the monkey are located inferior to area LIP and superior to area V3a at the lateral bank of the IPS in the same coronal plane [Lewis and Van Essen, 2000b]. If human topography is similar to that of the monkey, it is possible that fMRI fails to detect spatial differences between area CIP and saccade related areas in humans.
Another important issue is the functional difference between humans and monkeys. Human area V3a is providing direct projections to area CIP [Adams, 1997] and may have greater functional significance than in monkeys [Orban et al., 2003]. It has also been suggested that the correspondence between the macaque IPS and the human IPS is not complete and the regional differences to the human parietal cortex are much greater than to the occipital cortex [Orban et al., 2004].
The present findings suggest that putative area AIP in humans has a similar topographical relationship to S1 and to putative area LIP as in the monkey parietal cortex. Furthermore, putative area AIP has clearly distinct functions from its neighboring areas and shows much less individual variability than putative human area CIP or LIP. Putative human area CIP is located upon the medial side branch of IPS prior to the junction with the parieto‐occipital sulcus, and therefore differs from the monkey area CIP. When interpreting activations within the posterior parietal cortex, these anatomical differences amongst individuals should be taken into account.
Supporting information
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .
Acknowledgements
We thank the physics group and the computing staff at Neuroimage Nord for their assistance. F.H. was supported by the German Research Council (DFG). C.B. and F.B. were supported by the Volkswagenstiftung. NeuroImage Nord is supported by the BMBF (01GO0207 and 01GW0571) and the DFG.
REFERENCES
- Adams DL ( 1997): Functional organization of the monkey visual cortex for stereoscopic depth. PhD. Thesis. London: University College London.
- Anderson RA,Bracewell RM,Barash S,Gnade JW,Fogassi L ( 1990): Eye position effects on visual, memory, and saccade‐related activity in areas LIP and 7a of macaque. J Neurosci 10: 1176–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV,Shulman GL,Stanley CM,Snyder AZ,Van Essen DV,Corbetta M ( 2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F,Dohle C,Posse S,Stephan KM,Hefter H,Seitz RJ,Freund H‐J ( 1998): Human anterior intraparietal area subserves prehension. Neurology 50: 1253–1259. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Buccino G,Posse S,Seitz RJ,Rizzolatti G,Freund H‐J ( 1999): A fronto‐parietal circuit for object manipulation in man: Evidence from an fMRI study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Buccino G,Binkofski F,Fink GR,Fadiga L,Fogassi L,Gallese V,Seitz RJ,Rizzolatti G,Freund H‐J ( 2001): Action observation activated premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Chao LL,Martin A ( 2000): Representation of manipulable man‐made objects in the dorsal stream. NeuroImage 12: 478–484. [DOI] [PubMed] [Google Scholar]
- Colby CL,Duhamel JR,Goldberg ME ( 1996): Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysi 76: 2841–2852. [DOI] [PubMed] [Google Scholar]
- Duhamel JR,Colby CL,Goldberg ME ( 1992): The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92. [DOI] [PubMed] [Google Scholar]
- Evans AC,Kambar M,Collins DL,MacDonald D ( 1994): An MRI‐based probabilistic atlas of neuroanatomy. In magnetic resonance scanning and epilepsy. New York: Plenum; pp 263–274. [Google Scholar]
- Faillenot I,Toni I,Decety J,Grégoire MC,Jeannerod M ( 1997): Visual pathways for object‐oriented action and object recognition. Functional anatomy with PET. Cereb Cortex 7: 77–85. [DOI] [PubMed] [Google Scholar]
- Faillenot I,Decety J,Jeannerod M ( 1999): Human brain activity related to the perception of spatial features of objects. Neuroimage 10: 114–124. [DOI] [PubMed] [Google Scholar]
- Faillenot I,Sunaert S,Van Hecke P,Orban GA ( 2001): Orientation discrimination of objects and gratings compared: An fMRI study. Eur J Neurosci 13: 585–596. [DOI] [PubMed] [Google Scholar]
- Frey SH,Vinton D,Norlund R,Grafton ST ( 2005): Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res 23: 397–405. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Ashburner J,Frith CD,Poline J‐B,Heather JD,Frackowiak RSJ ( 1995a): Spatial registration and normalization of images. Hum Brain Map 2: 1–25. [Google Scholar]
- Friston KJ,Holmes AP,Worsley KP,Poline J‐B,Frith CD,Frackowiak RSJ ( 1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Map 2: 189–210. [Google Scholar]
- Gallese V,Murata A,Kaseda M,Niki N,Sakata H ( 1994): Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport 5: 1525–1529. [DOI] [PubMed] [Google Scholar]
- Gnadt JW,Andersen RA ( 1988): Memory related motor planning activity in posterior parietal cortex of macaque Exp Brain Res 70: 216–220. [DOI] [PubMed] [Google Scholar]
- Grefkes C,Weiss PH,Zilles K,Fink GR ( 2002): Crossmodal processing of object features in human anterior intraparietal cortex: An fMRI study implies equivalencies between humans and monkeys. Neuron 35: 173–184. [DOI] [PubMed] [Google Scholar]
- Heide W,Binkofski F,Seitz RJ,Posse S,Nitschke MF,Freund H‐J,Kömpf D ( 2001): Activation of frontoparietal cortices during memorized triple‐step sequences of saccadic eye movements: An fMRI study. Eur J Neurosci 13: 1177–1189. [DOI] [PubMed] [Google Scholar]
- James TW,Humphrey GK,Gati JS,Menon RS,Goodale MA ( 2002): Differential effects of viewpoint on object‐driven activation in dorsal and ventral streams. Neuron 35: 793–801. [DOI] [PubMed] [Google Scholar]
- Jäncke L,Kleinschmidt A,Mirzazade S,Shah NJ,Freund H‐J ( 2001): The rule of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cerebral Cortex 11: 114–121. [DOI] [PubMed] [Google Scholar]
- Kawashima R,Naitoh E,Matsumura M,Itoh H,Ono S,Satoh K,Gotoh R,Koyama M,Inoue K,Yoshioka S,Fukuda H ( 1996): Topographic representation in human intraparietal sulcus of reaching and saccade. Neuroreport 7: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Lewis JW,Van Essen DD ( 2000a): Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto‐occipital cortex. J Comp Neurol 428: 79–111. [DOI] [PubMed] [Google Scholar]
- Lewis JW,Van Essen DD ( 2000b): Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137. [DOI] [PubMed] [Google Scholar]
- Mecklinger A,Grunenewals C,Besson M,Magnie MN,Von Cremon Y ( 2002): Separable neuronal circuitries for manipulable and non‐manipulable objects in working memory. Cerebral Cortex 12: 1115–1123. [DOI] [PubMed] [Google Scholar]
- Medendorp WP,Goltz HC,Vilis T,Crawford JD ( 2003): Gaze‐centered updating of visual space in human parietal cortex. J Neurosci 23: 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EP,Genovese CR,Colby CL ( 2003): Spatial updating in human parietal cortex. Neuron 39: 361–373. [DOI] [PubMed] [Google Scholar]
- Murata A,Fadiga L,Fogassi L,Gallese V,Raos V,Rizzolatti G ( 1997): Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78: 2226–2230. [DOI] [PubMed] [Google Scholar]
- Murata A,Gallese V,Luppino G,Kaseda M,Sakata H ( 2000): Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601. [DOI] [PubMed] [Google Scholar]
- Orban GA,Fize D,Peuskens H,Denys K,Nelissen K,Sunaert D,Todd J,Vanduffel W ( 2003): Similarities and differences in motion processing between the human and macaque brain: Evidence from fMRI. Neuropsychologia 41: 1757–1768. [DOI] [PubMed] [Google Scholar]
- Orban GA,Van Essen D,Vanduffel W ( 2004): Comparative mapping of higher visual area in monkeys and humans. Trends Cogn Sci 8: 315–323. [DOI] [PubMed] [Google Scholar]
- Sakata H,Taira M,Murata A,Mine S ( 1995): Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex 5: 429–438. [DOI] [PubMed] [Google Scholar]
- Sakata H,Taira M,Kusunoki M,Murata A,Tanaka Y ( 1997): The TINS lecture; The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci 20: 350–357. [DOI] [PubMed] [Google Scholar]
- Schall JD,Morel A,King DJ,Bullier J ( 1995): Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. J Neurosci 15: 4464–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D,Glimcher PW,Heeger DJ ( 2005): Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol 94: 1372–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI,Pitzalis S,Martinez A ( 2001): Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294: 1350–1354. [DOI] [PubMed] [Google Scholar]
- Shikata E,Tanaka Y,Nakamura H,Taira M,Sakata H ( 1996): Selectivity of the parietal visual neurones in 3D orientation of surface of stereoscopic stimuli. Neuroreport 7: 2389–2394. [DOI] [PubMed] [Google Scholar]
- Shikata E,Hamzei F,Glauche V,Knab R,Dettmers C,Weiller C,Büchel C ( 2001): Surface orientation discrimination activated caudal and anterior intraparietal sulcus in humans: An event‐related fMRI study. J Neurophysiol 85: 1309–1314. [DOI] [PubMed] [Google Scholar]
- Shikata E,Hamzei F,Glauche V,Koch M,Weiller C,Binkofski F,Büchel C ( 2003): Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur J Neurosci 17: 1105–1110. [DOI] [PubMed] [Google Scholar]
- Silver MA,Ress D,Heeger DJ ( 2005): Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol 94: 1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O,Mangin J‐F,Cohen L,Le Bihan D,Dehaene S ( 2002): Topographical layout of hand, eye, calculation and language‐related areas in the human parietal lobe. Neuron 33: 475–487. [DOI] [PubMed] [Google Scholar]
- Taira M,Mine S,Georgopoulos AP,Murata A,Sakata H ( 1990) Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res 83: 29–36. [DOI] [PubMed] [Google Scholar]
- Taira M,Kawashima R,Inoue K,Fukuda H ( 1998): A PET study for axis orientation discrimination. Neuroreport 9: 283–288. [DOI] [PubMed] [Google Scholar]
- Taira M,Tsutsui KI,Jiang M,Yara K,Sakata H ( 2000): Parietal neurons represent surface orientation from the gradient of binocular disparity. J Neurophysiol 83: 3140–3146. [DOI] [PubMed] [Google Scholar]
- Taira M,Nose I,Inoue K,Tsutsui K ( 2001): Cortical areas related to attention to 3D surface structures based on shading: an fMRI study. Neuroimage 14: 959–966. [DOI] [PubMed] [Google Scholar]
- Tanabe HC,Kato M,Miyauchi S,Hayashi S,Yanagida T ( 2005): The sensorimotor transformation of cross‐modal spatial information in the anterior intraparietal sulcus as revealed by functional MRI. Brain Res Cog Brain Res 22: 385–396. [DOI] [PubMed] [Google Scholar]
- Tsutsui KI,Jiang M,Yara K,Sakata H,Taira M ( 2001): Integration of perspective and disparity cues in surface‐orientation‐selective neurons of area CIP. J Neurophysiol 86: 2856–2867. [DOI] [PubMed] [Google Scholar]
- Tsutsui KI,Sakata H,Naganuma T,Taira M ( 2002): Neural correlates for perception of 3D surface orientation from texture gradient. Science 298: 409–412. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R,Geeraerts S,Molenberghs P,Lafosse C,Vandenbulcke M,Peeters K,Peeters R,Van Hecke P,Orban GA ( 2005): Attentional responses to unattended stimuli in human parietal cortex. Brain 128: 2843–2857. [DOI] [PubMed] [Google Scholar]
- Van Essen DC ( 2003): Organization of visual areas in macaque and human cerebral cortex In: Chalupa L,Werner JS, editors. The Visual Neurosciences. MA: MIT Press; pp 507–521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .


