Abstract
Purpose:
Primary Ciliary Dyskinesia (PCD) is a rare disorder of mucociliary clearance leading to recurrent upper and lower respiratory tract infections. PCD is difficult to clinically distinguish from other entities leading to recurrent oto-sino-pulmonary infections, including primary immunodeficiency (PID). Nasal nitric oxide (nNO) is a sensitive and specific diagnostic test for PCD, but it has not been thoroughly examined in PID. Past publications have suggested an overlap in nNO levels among subjects with PCD and PID. We sought to determine if nNO measurements among patients diagnosed with PID would fall significantly above the established PCD diagnostic cutoff value of 77 nL/min.
Methods:
Children >5 years old and adults with definitive PID or PCD diagnoses were recruited from outpatient subspecialty clinics. Participants underwent nNO testing by standardized protocol using a chemiluminiescence analyzer and completed a questionnaire concerning their chronic oto-sino-pulmonary symptoms, including key clinical criteria specific to diagnosed PCD (neonatal respiratory distress at term birth, year-round cough or nasal congestion starting before 6 months of age, any organ laterality defect).
Results:
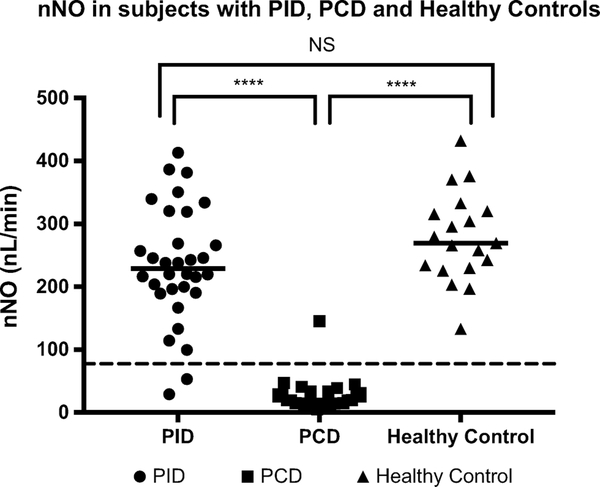
Participants included 32 patients with PID, 27 patients with PCD and 19 healthy controls. Median nNO was 228.9.1 nL/min in the PID group, 19.7 nL/min in the PCD group and 269.4 in the healthy controls (p<0.0001). Subjects with PCD were significantly more likely to report key clinical criteria specific to PCD, but approximately 25% of PID subjects also reported at least 1 of these key clinical criteria (mainly year-round cough or nasal congestion).
Conclusions:
While key clinical criteria associated with PCD often overlap with the symptoms reported in PID, nNO measurement by chemiluminescence technology allows for effective discrimination between PID and PCD.
Keywords: Primary ciliary dyskinesia, primary immunodeficiency, nasal nitric oxide
Introduction:
Primary ciliary dyskinesia (PCD) is a rare, genetic disease characterized by abnormal motile ciliary structure, function and biogenesis [1]. The resulting impairment of mucociliary clearance from the lungs, paranasal sinuses, and middle ear leads to chronic respiratory symptoms from the early postnatal period, with recurrent oto-sino-pulmonary infections and significant pulmonary morbidity over time [1, 2]. Recognizing PCD is difficult, as the symptoms seen in PCD often overlap with those in other chronic respiratory diseases, including cystic fibrosis, asthma, recurrent viral infections and primary immunodeficiency (PID). Through deep phenotyping of PCD populations, researchers have discovered four key clinical features that are sensitive and specific for PCD diagnosis, including 1) neonatal respiratory distress requiring at least 24 hours of supplemental oxygen or positive pressure support, despite term birth, 2) year-round, productive (often wet) cough starting before 6 months of age, 3) year-round, non-seasonal nasal congestion starting before 6 months of age and 4) any organ laterality defect (including situs inversus totalis or situs ambiguus) [3].
Despite improved clinical phenotyping, there is still no single diagnostic test that detects all cases of PCD. The “gold standard” for PCD diagnosis has traditionally been nasal or endobronchial biopsy for ciliary ultrastructural analysis by transmission electron microscopy (TEM), but this is estimated to capture only 70% of affected individuals [4], as forms of PCD exist with normal TEM ultrastructure. More than 40 different genes are known to cause PCD. Although new genes are being discovered at an accelerated pace through whole exome sequencing, genetic testing identifies only 70% of PCD patients [5]. High speed video microscopy (HSVM) is another PCD diagnostic tool, but this too fails to detect all patients with PCD. Moreover, each of these tests can give non-diagnostic results, while TEM and HSVM require specialized expertise to perform correctly.
For unclear reasons, nasal nitric oxide (nNO) levels are diminished in PCD, which led to nasal measurement of this exhaled gas as a screening test for PCD [6–8]. Past publications have validated nNO measurement as 98% sensitive and > 99% specific for PCD, when using a diagnostic cutoff of 77nL/min [9]. Additionally, nNO testing is rapid, non-invasive, inexpensive for patients, easy to perform and results are immediately available. As a result, recent clinical practice guidelines suggest nNO measurement, performed by a standard operating protocol, with chemiluminescence technology, as a first line diagnostic test for PCD [10, 2]. However, nNO measurement has not been extensively evaluated in diseases that present with symptoms similar to PCD, such as those seen in certain PIDs. PIDs are inborn errors of immunity, some of which compromise host defenses in the respiratory tract. Consequently, patients with these PIDs can phenocopy some aspects of patients with PCD and past publications have suggested an overlap in nNO levels among subjects with PCD and PID [11, 12]. We sought to prospectively examine the ability of nNO measurement, using an established and validated diagnostic methodology in accordance with clinical guidelines [13, 10], to discriminate between the clinically similar entities of PCD and a well-phenotyped group of patients with PID. We hypothesized that nNO levels in patients with PID would fall above the PCD diagnostic cutoff of 77 nL/min, particularly when repeated on multiple occasions.
Methods
Study Population
Participants were recruited from adult and pediatric Immunodeficiency and Pulmonology clinics at the McGill University Health Center (MUHC). Recruitment was limited to subjects ≥5 years old, who were able to cooperate with the technique required to obtain reliable nNO values. Recruited participants had a clear basis (either clinical or genetic) for their diagnosis of PID as well as histories of chronic ear, sinus or pulmonary disease in their medical records (table 1) [14]. Diagnoses were reviewed with a board-certified immunologist (RA), when necessary. Subjects were clinically stable and free of upper or lower respiratory tract infections in the 2 weeks prior to nNO measurement. The participants were in clinic for a minimum of 2 hours prior to nNO testing. During this period, there was no exercise, smoking, nasal instrumentation, nasal medication administration, nor known food or drink consumption.
Table 1:
Subject Characteristics
| PCD (n=27) | PID (n=32) | |
|---|---|---|
| Median age in years (IQR) | 18.6 (9.7–38.6) | 16.5 (9.8–45.3) |
| Male sex (%) | 12 (44) | 17 (53) |
| White race (%) | 18 (67) | 26 (81) |
| Median FEV1 % predicted (IQR) | 81 (59–91) | 94.1 (87–101)* |
| Median FVC % predicted (IQR) | 92 (80–99) | 97 (87–106) |
| Median FEV1/FVC % predicted (IQR) | 88 (83–89) | 87 (83–90) |
| Treatment nasal CS (%) | 17 (63) | 17 (53) |
| Treatment nasal saline lavage (%) | 13 (48) | 0 (0)* |
| Previous FESS (%) | 12 (44) | 4 (13)* |
| Past nasal polyps (%) | 6 (22) | 0* |
| Prophylactic oral antibiotics (%)† | 8 (30) | 12 (38) |
| Prophylactic inhaled antibiotics (%)‡ | 2 (7) | 0 |
| IVIG replacement (%) | 0 | 23 (72)* |
| Current smoker (%) | 0 | 6 (19)* |
| Smoker in the home (%) | 0 | 7 (22)* |
PID = primary immunodeficiency disease; PCD = primary ciliary dyskinesia; FEV1 = Forced expiratory volume in 1 second, FVC= forced vital capacity; CS = corticosteroid; IVIG = intravenous immunoglobulin
Statistically significant values using Fisher Exact Test
includes azithromycin, moxifloxacin, ciprofloxacin, TMP-SMX, amoxicillin
includes inhaled tobramycin
Nasal nitric oxide values and clinical research data from all definitively diagnosed PCD subjects, followed in the PCD clinics of the MUHC, were included in this study. All PCD subjects were diagnosed through either a classic, disease causing ultrastructural defect on TEM or two pathogenic variants in one of the known PCD genes. High speed videomicroscopy analysis was not performed on this population.
A standardized clinical questionnaire was administered to all PID subjects on the day of enrollment (see supplemental materials). In addition, medical records were reviewed for pertinent clinical information. Spirometry was also performed on the day of study enrollment. If spirometry was not feasible at the study visit, clinical spirometry testing within 6 months was abstracted from the medical record, if available. The study design (14–019-PED) was reviewed by the Research Ethics Board of the MUHC. Informed consent was obtained from participants included in the study.
Nasal nitric oxide measurements
Nasal nitric oxide measurements were performed using a CLD88 chemiluminescence analyzer (EcoPhysics, AG, Duernten, Switzerland) per the standard operating protocol of the Genetic Diseases of Mucociliary Clearance Consortium (GDMCC) and the PCD Foundation Clinical and Research Center Network [13]. To perform this test, a plastic catheter with a surrounding soft nasal sponge was placed into one nare to ensure an airtight seal. Participants performed manoeuvres to close their velum, which prevents dilution of nasal gas (containing much higher nitric oxide levels) with gas from the lower airways. To accomplish this, participants inhale to total lung capacity and exhale into a resistor or a child’s party favour, which is partially occluded at one end, for at least 10 seconds. This technique produces an initial washout phase followed by a nitric oxide concentration plateau phase, which signifies steady state nitric oxide sampling from the nasal cavity. The same procedure is repeated in the contralateral nare. The mean of two separate plateau measurements (at least 3 seconds duration each, with <5% variation from the horizontal axis) were calculated for each nare, followed by a calculation of the mean for both nares together to yield an average test result in parts per billion. Final values are expressed in nanoliters per minute, which are a product of the concentration (in parts per billion) and the flow rate of transnasal airflow in the sampling catheter (in liters/minute) yielding nasal nitric oxide production values (in nL/minute). A standard transnasal airflow sampling rate of 0.33 liters per minute was verified before and after each subject tested. Ambient nitric oxide levels were recorded and were consistently less than 50 parts per billion. Measurements were performed by one of three operators, who were extensively trained and experienced with the standardized nNO measurement protocol. All nNO raw data curves were reviewed and verified by the lead device operator (AJS).
Statistics
All statistical analyses were performed using Prism GraphPad (Version 7.0c, 2007, California). Demographic data is reported as the median with interquartile range. Differences in clinical symptoms and demographic features were computed using Fisher exact tests at a 5% level of significance. Nasal nitric oxide values between subjects with PCD, PID and healthy controls were compared using a Kruskal-Wallis test, given the non-parametric data distribution. Post-hoc pairwise comparisons were performed by Mann-Whitney U tests.
Results
Demographic data for the PCD and PID subjects are shown in table 1. Clinical characteristics for both groups are described in table 2. Among the 32 participants with PID, diagnoses included: polysaccharide antibody deficiency (n=2), common variable immunodeficiency (n=14), hypogammaglobulinemia (n=8); X-linked agammaglobulinemia (n=2), combined immunodeficiency (n=2), X-linked neutropenia (n=1), complement deficiency (n=1), autosomal recessive RAG1 deficiency (n=1) and autosomal recessive STAT1 deficiency (n=1).
Table 2:
Clinical Features of Patients with PCD and PID
| PCD (n=27) n (%) |
PID (n=32) n (%) |
p-value | Sens (%) |
Spec (%) |
|
|---|---|---|---|---|---|
| 4 key clinical features of PCD [3] | |||||
| Neonatal respiratory distress despite term birth† | 19 (70) | 1 (3) | p <0.0001* | 70.4 | 96.9 |
| Daily, year-round wet cough starting before 6 months old | 26 (96) | 3 (9) | p <0.0001* | 96.2 | 90.6 |
| Daily, year-round nasal congestion starting before 6 months old | 25 (93) | 3 (9) | p < 0.0001* | 92.6 | 90.6 |
| Any organ laterality defect | 18 (67) | 1 (3) | p< 0.0001* | 66.6 | 96.1 |
| Daily, year-round wet cough AND daily, year round nasal congestion | 24 | 3 | p < 0.0001* | 88.9 | 90.6 |
| Neonatal respiratory distress AND any organ laterality defect | 13 | 0 | p< 0.0001* | 48.1 | 100 |
| 1 key PCD clinical feature | 27 (100) | 8 (25) | p< 0.0001* | ||
| ≥ 2 key PCD clinical features | 27 (100) | 5 (16) | p < 0.0001* | ||
| Other Clinical Features in Adults With PCD [40] | |||||
| Bronchiectasis on CT chest | 14 (88) (n=16) |
8 (38) (n=21) |
p = 0.003 * | ||
| Pansinusitis on CT sinus | 8 (57) (n=14) |
5 (42) (n=12) |
p = 0.7 | ||
Neonatal respiratory distress defined as need for supplemental oxygen or positive pressure support for more than 24 hours, despite term birth
Statistically significant value using Fisher exact test
Abbreviations- PCD: Primary Ciliary Dyskinesia; PID: primary immunodeficiency; sens: sensitivity; spec: specificity; CT: computed tomography
Subjects with PCD were significantly more likely to have key clinical features of PCD, including a history of neonatal respiratory distress, daily year-round wet cough before 6 months of age, daily year-round nasal congestion before 6 months of age or organ laterality defects than subjects with PID. Sensitivities and specificities were calculated for each symptom individually (table 2). Twenty-five percent of PID patients had ≥1 key clinical feature of PCD, while 16% had ≥2 key PCD clinical features on history. Sensitivity and specificity for the combination of daily year-round wet cough and daily year-round nasal congestion starting before 6 months of age was 88.9% and 90.6% respectively for the diagnosis of PCD in this study. Sensitivity and specificity for the combination of neonatal respiratory distress and organ laterality defects was 98.1% and 100% respectively (table 2). Sinusitis verified on CT scan was not significantly more prevalent in the PCD group (57%) versus the PID group (42%, p=0.7) and there was no significant difference in historically reported sinusitis episodes between groups. Despite similarities in median age and sex distribution between groups, 14/16 (87%) of PCD patients had bronchiectasis on CT scan compared to 8/21 (38%) PID patients who underwent CT imaging (p< 0.003) before study enrollment.
Pulmonary function was assessed using Global Lung Initiative predictive equations [15]. The FEV1 values were significantly different between PCD and PID groups, with a median FEV1 of 81% and 94% predicted, respectively (p=0.004). The FVC and FEV1/FVC values did not vary significantly between disease groups. Some therapeutic regimens also significantly differed between the PCD and PID groups. 48% of PCD subjects versus 0% of PID subjects were on daily nasal saline lavage (p<0.0001). Subjects with PCD were more likely to have been diagnosed with past nasal polyps (p=0.07) and to have undergone past functional endoscopic sinus surgery (FESS) (p=0.008). Other treatment modalities were similar between groups. For example, 30% of PCD subjects versus 38% of PID subjects received prophylactic oral antibiotics (p= 0.59) and 63% of PCD subjects versus 53% of PID subjects (p=0.63) were on daily nasal corticosteroids, (table 1).
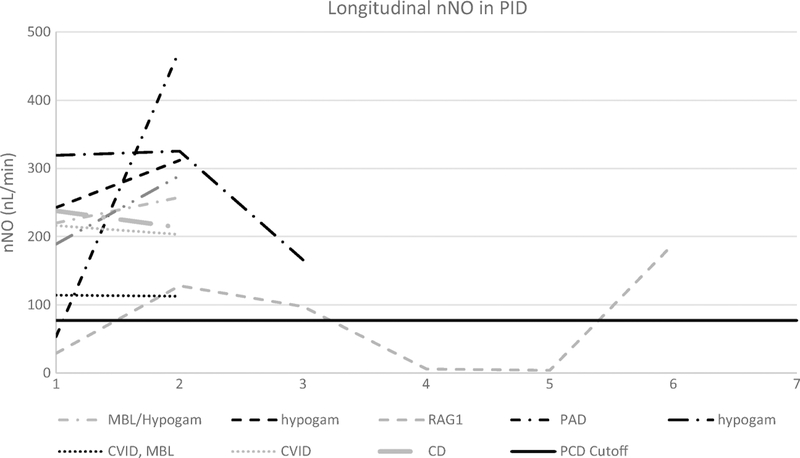
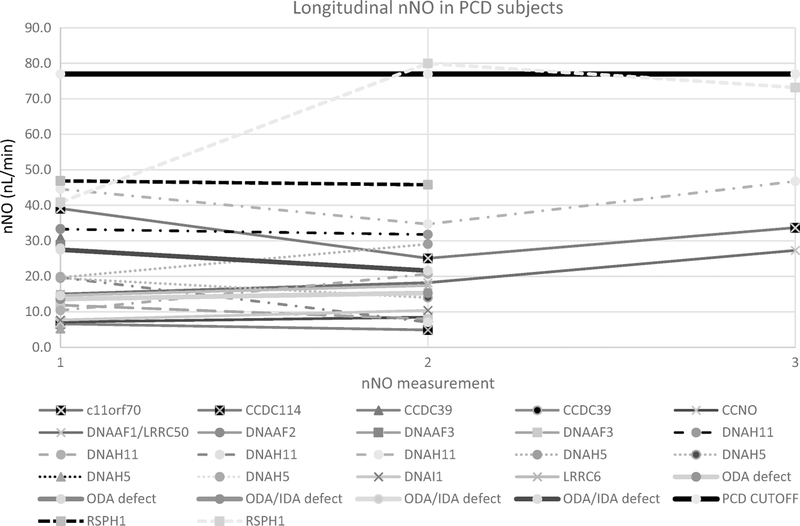
Initial nNO testing was performed in 32 PID and 27 PCD subjects, while repeat nNO testing was performed in 9 PID and 17 PCD cases. In addition, 19 healthy controls underwent nNO testing. Median initial nNO values in the PCD, PID and healthy control groups were 19.7, 228.9 and 269.4 nL/min, respectively (p<0.0001) (Fig1). Two PID subjects had initial nNO levels below the diagnostic cutoff of 77 nL/min, but both increased above 77 nL/min on repeat testing (Fig 2). However, one PID subject with normal nNO values on repeat testing subsequently redeveloped nNO values below 77 nL/min. Of the PCD subjects, all but one had initial nNO levels below the diagnostic cutoff of 77 nL/min. On repeat nNO testing, only 1 PCD participant had nNO levels rise slightly above 77 nL/min (Fig3). Thus, the sensitivity and specificity of repeat nNO measurements in distinguishing subjects with PCD from subjects with PID in this population was 97% and 91% respectively, using the cutoff of 77 nL/min.
Fig 1.
Initial nNO in primary immunodeficiency, PCD and healthy controls were compared using a Kruskal-Wallis test with post-hoc pairwise Mann-Whitney U tests. Horizontal lines represent the group median. Dashed line represents the PCD diagnostic cut-off of 77 nL/min. ****p < 0.0001. Only two PCD subjects (both with pathogenic variants in RSPH1) had initial or repeat nNO values above the PCD diagnostic cutoff of 77 nL/min (solid black horizontal line). This is consistent with previous observations that pathogenic variants in RSPH1 can sometimes result in nNO measurements above the diagnostic cutoff [29]. Two PID subjects initially had nNO measurements below the diagnostic cutoff of 77nL/min. Both of these subjects eventually had nNO measurements that rose above 77 nL/min, though one subject with RAG1 immunodeficiency had repeat nNO values fall below this cutoff.
Fig 2.
CVID – Common variable immunodeficiency, hypogam – hypogammaglobulinemia, PAD-polysaccharide antibody deficiency, MBL – Mannose binding lectin deficiency, XLA – X-linked agammaglobulinemia, CD – complement deficiency
Fig 3.
One PCD subject with RSPH1 and longitudinal measurements mutation had one of three values above the diagnostic cutoff of 77 nL/min. As noted, this is consistent with observations that RSPH1 mutations can occasionally generate nNO measurements above 77 nL/min.
Discussion
PCD and certain PIDs can both manifest with recurrent respiratory tract infections. Accurately distinguishing these two entities has important clinical implications. This study confirms that nNO testing accurately discriminates between PCD and PID in most cases. Most importantly, repeat nNO testing in PID patients with initially low nNO values normalized on subsequent measurements (above 77 nL/min) in all subjects, while nNO remains persistently low in most subjects with PCD. As nNO values can be transiently decreased by nasal bleeding, acute viral respiratory infection and other disease states, repeat nNO testing is vital in patients with key PCD clinical symptoms [16, 17]. Only a few conditions, including allergic rhinitis or acute bacterial sinusitis [18, 19], have been reported to artificially raise nNO values. However, these conditions have also been reported to decrease nNO values in certain settings [20, 21]. PID subjects with transiently low nNO values in this study likely had occult viral infections or other secondary causes of their low nNO levels. Normalization of nNO values over time argues strongly against PCD and should direct clinicians to pursue further investigations for PID and/or other respiratory diseases [22].
In addition to nNO values, clinical symptom history by standardized questionnaire appears to provide good diagnostic discrimination between PCD and PID. For each of the four key PCD clinical criteria, subjects with PCD were significantly more likely to possess the symptom than subjects with PID. Sensitivities and specificities in PCD were highest for daily year-round wet cough and daily year-round nasal congestion beginning before 6 months of age. Although the presence of an organ laterality defect had a sensitivity of only 67%, the specificity was quite high, suggesting that a laterality defect, when present, should point the clinician toward the diagnosis of primary ciliary dyskinesia. A substantial percentage of PID subjects (25%) displayed one or more of these key clinical criteria, emphasizing the significant overlap in symptoms between these two entities, and the value of a rapid, non-invasive and inexpensive test (like nNO measurement) to delineate between them. Clinical immunologists may benefit from this quick diagnostic test when key PCD clinical features are present, as it may direct towards nNO testing (a test not always considered in the evaluation of such patients) and avoid extensive and costly immunological testing.
Even though the clinical features of PCD and PID closely overlap, nNO testing in PID has not been widely investigated to distinguish between these diseases. Early publications on nNO values in PID included limited numbers of subjects, yet clinical information on the type of immune dysfunction are lacking, and the actual number of studied PID patients is quite small [11, 23]. Boon and colleagues explored nNO testing in 48 subjects with humoral PID, yet they did not collect detailed information on chronic respiratory symptoms and they enrolled many subjects with more debatable forms of immune dysfunction, including isolated IgA deficiency and IgG subclass deficiency [12] – abnormalities that are also reported in subjects without symptoms of immunodeficiency. To our knowledge, our study is the first to examine nNO testing in a well-phenotyped population with clinically significant forms of PID. Furthermore, while past studies report nNO values in humoral PID [12, 23], we report nNO values in a broader array of PID, including humoral, innate and combined immunodeficiencies. Although our numbers are small, these PID patients display chronic respiratory symptoms overlapping with PCD, emphasizing the real-world similarities between these rare diseases.
This is also the first report on diagnostic accuracy of nNO testing in PID patients since the current PCD diagnostic cutoff of 77 nL/min was rigorously validated through multi-center study [13]. Past studies of nNO in PID used different nNO diagnostic cutoff values (62.5 and 90 nL/min) and measurement protocols (breath hold or exhalation against resistance), which may have affected diagnostic accuracy conclusions [11, 12]. This study employs the standard operating protocol used by the Genetic Diseases of Mucociliary Clearance Consortium and the PCD Foundation Clinical and Research Center Network: these networks are comprised of 17 accredited PCD specialty centers across North America, and this identical nNO protocol was used to establish the current PCD diagnostic nNO cutoff of 77 nL/min [13]. This protocol also falls within the recommendations of the American Thoracic Society/European Respiratory Society for nNO measurement [24].
One PID subject had several low nNO values (3/6 measurements <77 nL/min) during this study. This subject was investigated for PCD and had normal electron microscopy and a panel of 21 PCD genes that did not reveal any pathogenic variants, yet neither of these negative tests effectively rules out PCD. Eventually, this subject was discovered to have homozygous, pathogenic variants in RAG1 on targeted genetic analysis following abnormal V-ß repertoire testing. The variable nNO values in this case may be due to the difficulty in clearing nasal viral infections, known to decrease nNO values, associated with combined immunodeficiency [25–28]. Alternatively, an oligogenic syndrome, consisting of RAG1 deficiency with a PCD defect due to one of the newly discovered genes not accounted for in the panel, is possible, especially as the subject’s parents were consanguineous, thereby increasing the risk of recessive diseases. Among the PCD subjects, two patients had nNO measurements above the diagnostic cutoff of 77 nL/min. Both of these subjects carried two pathogenic variants in RSPH1, resulting in a form of PCD affecting radial spoke heads and sometimes producing nNO levels above 77 nL/min [29]. RSPH1 pathogenic variants are a relatively rare cause of PCD, which affect only 2% of diagnosed individuals and do not cause organ laterality defects [30]. In the future, there may be more forms of genetically diagnosed PCD with nNO levels above the accepted cutoff of 77 nL/min, but RSPH1 is the only currently known PCD gene that regularly results in non-diagnostic nNO levels. This finding should be kept in mind when clinically evaluating patients with a PCD phenotype, but for which nNO and immunologic investigations are unrevealing. With increased availability of commercial genetic testing, it may be possible to run combined PID and PCD panels, further aiding in diagnosis.
Approximately one-third of PID subjects (31%) had nNO levels that were above 77nL/min, yet still fell below nNO values commonly seen in healthy subjects (generally >200 nL/min) [31]. For many of these PID subjects, repeat nNO measurements were not available, so it is unclear how these values might have progressed over time. Of the subjects with intermediate nNO values, 4 of them underwent repeat testing, and 3 of them had at least one test greater than 200 nL/min. Intermediate nNO values have been described in cystic fibrosis [32] and heterotaxy syndrome [33] and therefore, may also be present among patients with certain subtypes of PID. Multi-center, cross sectional analysis of large numbers of PID patients, with systematic PCD and immunologic evaluation, would be necessary to uncover such nNO trends and clinical correlations in various subtypes of PID. In our study, 55% of PCD patients had at least partial, normal clinical immune testing, yet only 34% of our PID cohort had clinical PCD testing, reflecting the real-world clinical practice of individual subspecialties. Although presumably rare, reports of oligogenic syndromes, with co-occurrence of PCD and humoral PID, are published on a few patients [34, 35].
Treatment with nasal saline lavage (p<0.0001) or FESS (p=0.008) was significantly more common in subjects with PCD versus those with PID. Nasal corticosteroid use did not differ significantly between groups. Although some small studies suggest that nNO increases post-FESS [21, 36, 37] others contradict this, and a meta-analysis did not find convincing support for the notion that nNO is a useful marker of clinical improvement post-FESS [38]. Similarly, there is conflicting evidence from a handful of small studies about whether the use of nasal corticosteroids influences nNO levels [37–39].
This study does have certain limitations. Our sample sizes are relatively small, although this is not surprising given that PCD and PID are both rare diseases. Additionally, approximately one third of PID participants underwent repeat nNO testing, so the ability to extrapolate patterns from this longitudinal data is limited. At the time of study, many PID participants were receiving immunoglobulin replacement with control of their clinical symptoms but were asked to recall symptoms at the time of their PID diagnosis, lending itself to potential recall bias. However, this bias is reduced by our meticulous review of past clinical symptoms in participants’ medical records. Lastly, this is not a study of prospectively referred symptomatic subjects with possible PID or PCD, so the diagnostic accuracy of nNO testing is possibly overestimated. Nonetheless, a previous multi-site, prospective study of nNO in children with chronic oto-sino-pulmonary disease has confirmed the excellent diagnostic accuracy (sensitivity 98%; specificity >99%) of nNO for PCD versus other respiratory diseases [12].
Conclusions
This study confirms that nNO measurement discriminates between patients with primary ciliary dyskinesia and various forms of primary immunodeficiency. This suggests that nNO can be employed as an early, non-invasive and relatively inexpensive test in the investigation of pediatric and adult patients with chronic oto-sino-pulmonary symptoms and assist in proper referral of patients to immunology versus pulmonology subspecialists. Standardized operating protocols and chemiluminescence analyzers are essential for accurate nNO measurements.
Supplementary Material
Acknowledgments:
We would like to acknowledge Whitney Wolf (research technician) for specimen and DNA handling and processing and Invitae (San Francisco, California) for performing next generation sequencing of known PCD genes. We also appreciate Shrikant Mane, Francesc Lopez-Giraldez and Weilai Dong (Yale Center for Mendelian Genomics, [UM1 HG006504]) for providing whole exome sequencing and bioinformatics support.
Funding: Funds to support this project were received from the Montreal Children’s Hospital Foundation and the National Institute of Health (NIH) research grants U54HL096458, 5R01HL071798. The Genetic Disorders of Mucociliary Clearance (U54HL096458) is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Disease Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR). NCATS funded through a collaboration between NCATS and NHLBI. The content of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Ethical Approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare no conflicts of interest with regards to the publication of this paper.
References
- 1.Leigh MW, O’Callaghan C, Knowles MR. The Challenges of Diagnosing Primary Ciliary Dyskinesia. Proceedings of the American Thoracic Society. 2011;8(5):434–7. doi: 10.1513/pats.201103-028SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatric Pulmonology. 2016;51(2):115–32. doi: 10.1002/ppul.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann Am Thorac Soc 2016;13(8):1305–13. doi: 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouis P, Yiallouros PK, Middleton N, Evans JS, Kyriacou K, Papatheodorou SI. Prevalence of primary ciliary dyskinesia in consecutive referrals of suspect cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis. Pediatr Res 2017;81(3):398–405. doi: 10.1038/pr.2016.263. [DOI] [PubMed] [Google Scholar]
- 5.Zariwala MA, Knowles MR, Leigh MW. Primary Ciliary Dyskinesia In: MP A HH A RA P, editors. GeneReviews. Seattle: University of Wahington; 2007. [updated 2015 Sept 3]. [Google Scholar]
- 6.Lundberg J, Weitzberg E, Nordvall S, Kuylenstierna R, Lundberg J, Alving K. Primarily nasal origin of exhaled nitric oxide and absence in Kartagener’s syndrome. European Respiratory Journal. 1994;7(8):1501–4. [DOI] [PubMed] [Google Scholar]
- 7.Collins SA, Gove K, Walker W, Lucas JS. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta-analysis. European Respiratory Journal. 2014;44(6):1589–99. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AJ, Josephson M, Rosenfeld M, Yilmaz O, Davis SD, Polineni D et al. Accuracy of Nasal Nitric Oxide Measurement as a Diagnostic Test for Primary Ciliary Dyskinesia. A Systematic Review and Meta-analysis. Annals of the American Thoracic Society. 2017;14(7):1184–96. doi: 10.1513/AnnalsATS.201701-062SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2018;197(12):e24–e39. doi: 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvàth I, Loukides S, Wodenhouse T, Csiszér E, Cole P, Kharitonov S et al. Comparison of exhaled and nasal nitric oxide and exhaled carbon monoxide in patients with and without primary ciliary dyskinesia Thorax. 2003;58:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boon M, Meyts I, Proesmans M, Vermeulen FL, Jorissen M, De Boeck K. Diagnostic accuracy of nitric oxide measurements to detect primary ciliary dyskinesia. Eur J Clin Invest 2014;44(5):477–85. doi: 10.1111/eci.12254. [DOI] [PubMed] [Google Scholar]
- 13.Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc 2013;10(6):574–81. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley ME, Notarangelo LD, Etzioni A. Diagnostic Criteria for Primary Immunodeficiencies. Clinical Immunology. 1999;93(3):190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. European Respiratory Journal. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marthin JK, Philipsen MC, Rosthoj S, Nielsen KG. Infant nasal nitric oxide over time: natural evolution and impact of respiratory tract infection. European Respiratory Journal. 2018;51(6):1702503. doi: 10.1183/13993003.02503-2017. [DOI] [PubMed] [Google Scholar]
- 17.NAKANO H, IDE H, IMADA M, OSANAI S, TAKAHASHI T, KIKUCHI K et al. Reduced Nasal Nitric Oxide in Diffuse Panbronchiolitis. American Journal of Respiratory and Critical Care Medicine. 2000;162(6):2218–20. doi: 10.1164/ajrccm.162.6.2003051. [DOI] [PubMed] [Google Scholar]
- 18.Suojalehto H, Vehmas T, Lindström I, Kennedy DW, Kilpeläinen M, Plosila T et al. Nasal nitric oxide is dependent on sinus obstruction in allergic rhinitis. The Laryngoscope. 2014;124(6). [DOI] [PubMed] [Google Scholar]
- 19.Struben VMD, Wieringa MH, Feenstra L, De Jongste JC. Nasal nitric oxide and nasal allergy. Allergy.2006;61(6):665–70. doi:doi: 10.1111/j.1398-9995.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Takabayashi T, Imoto Y, Sakashita M, Narita N, Fujieda S. Reduced nasal nitric oxide levels inpatients with eosinophilic chronic rhinosinusitis. Allergology International. 2018. doi: 10.1016/j.alit.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, McKnight CL, Aves T, Yip J, Grewal AS, Gupta S. Nasal nitric oxide as a marker of sinus mucosal health in patients with nasal polyposis. International Forum of Allergy & Rhinology. 2015;5(10):894–9. doi:doi: 10.1002/alr.21598. [DOI] [PubMed] [Google Scholar]
- 22.Chawla K, Hazucha M, Dell SD, Ferkol T, Sagel SD, Rosenfeld M et al. A Multi-Center, Longitudinal Study of Nasal Nitric Oxide in Children with Primary Ciliary Dyskinesia. American journal of respiratory and critical care medicine. 2010;181. doi: 10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A6726. [DOI] [Google Scholar]
- 23.Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax. 2002;57(7):586–9. doi: 10.1136/thorax.57.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deschamp AR, Schornick L, Clem C, Hazucha M, Shapiro AJ, Davis SD. A comparison of nasal nitric oxide measurement modes. Pediatr Pulmonol 2017;52(11):1381–2. doi: 10.1002/ppul.23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Österback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. Journal of Allergy and Clinical Immunology. 2010;126(1):120–6. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC et al. Respiratory Syncytial Viral Infection in Children with Compromised Immune Function. New England Journal of Medicine. 1986;315(2):7781. doi: 10.1056/nejm198607103150201. [DOI] [PubMed] [Google Scholar]
- 27.Klimov AI, Rocha E, Hayden FG, Shult PA, Roumillat LF, Cox NJ. Prolonged Shedding of Amantadine-Resistant Influenza A Viruses by Immunodeficient Patients: Detection by Polymerase Chain Reaction-Restriction Analysis. The Journal of Infectious Diseases. 1995;172(5):1352–5. [DOI] [PubMed] [Google Scholar]
- 28.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged Shedding of Multidrug-Resistant Influenza A Virus in an Immunocompromised Patient. New England Journal of Medicine. 2003;348(9):867–8. doi: 10.1056/nejm200302273480923. [DOI] [PubMed] [Google Scholar]
- 29.Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE et al. Mutations in RSPH1 Cause Primary Ciliary Dyskinesia with a Unique Clinical and Ciliary Phenotype. American Journal of Respiratory and Critical Care Medicine. 2014;189(6):707–17. doi: 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro AJ, Leigh MW. Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure. Ultrastructural Pathology. 2017;41(6):373–85. doi: 10.1080/01913123.2017.1362088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leigh MW, Ferkol TW, Davis SD, Lee H-S, Rosenfeld M, Dell SD et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Annals of the American Thoracic Society. 2016;13(8):1305–13. doi: 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas SR, Kharitonov SA, Scott SF, Hodson ME, Barnes PJ. Nasal and Exhaled Nitric Oxide is Reduced in Adult Patients With Cystic Fibrosis and Does Not Correlate With Cystic Fibrosis Genotype. CHEST Journal. 2000;117:1085–9. [DOI] [PubMed] [Google Scholar]
- 33.Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA et al. High prevalence of respiratory ciliarydysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125(18):2232–42. doi: 10.1161/CIRCULATIONAHA.111.079780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skorpinski EW, Kung SJ, Yousef E, McGeady SJ. Diagnosis of common variable immunodeficiency in apatient with primary ciliary dyskinesia. Pediatrics. 2007;119(5):e1203–5. doi: 10.1542/peds.2006-2396. [DOI] [PubMed] [Google Scholar]
- 35.Boon M, De Boeck K, Jorissen M, Meyts I. Primary ciliary dyskinesia and humoral immunodeficiency—is there a missing link? Respir Med 2014;108(6):931–4. doi: 10.1016/j.rmed.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Colantonio D, Brouillette L, Parikh A, Scadding GK. Paradoxical low nasal nitric oxide in nasal polyposis. Clinical & Experimental Allergy. 2002;32(5):698–701. doi:doi: 10.1046/j.1365-2222.2002.01379.x. [DOI] [PubMed] [Google Scholar]
- 37.Ragab SM, Lund VJ, Saleh HA, Scadding G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy. 2006;61(6):717–24. doi: 10.1111/j.1398-9995.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- 38.Phillips PS, Sacks R, Marcells GN, Cohen NA, Harvey RJ. Nasal nitric oxide and sinonasal disease: a systematic review of published evidence. Otolaryngology--Head and Neck Surgery. 2011;144(2):159–69. [DOI] [PubMed] [Google Scholar]
- 39.Baraldi E, Azzolin NM, Carra S, Dario C, Marchesini L, Zacchello F. Effect of topical steroids on nasal nitric oxide production in children with perennial allergic rhinitis: a pilot study. Respiratory Medicine. 1998;92(3):558–61. doi: 10.1016/S0954-6111(98)90308-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.