Abstract
One important aspect of self‐control is refraining voluntarily from already planned behavior, by a final intervention before commitment to action. Despite its crucial role in human existence, and clear social implications, this aspect of self‐control has proved hard to study experimentally. One recent study used a perceptual timing paradigm to identify specific activations in the dorsal fronto‐median cortex (dFMC) associated with voluntary inhibition of action (Brass and Haggard 2007: J Neurosci 27:9141–9145). Here, we extend this work in two important new directions. First, we developed a more naturalistic task that gives participants a strong reason to inhibit or to execute actions, and therefore involves self‐control in the sense of voluntary inhibition of prepotent impulsive responses. Second, we investigated the relation between dFMC and other cognitive‐motor areas using effective connectivity analysis. We show that dFMC is activated when inhibiting prepared responses to external events. Moreover, its effective connectivity suggests that it allows intentional inhibition of action through top‐down inhibition of premotor areas. This view of dFMC is consistent with a new view of self‐control as a key stage in a cognitive‐motor interface. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: fMRI, inhibition, volition, motor intention, prefrontal cortex, intentional inhibition, self‐control
INTRODUCTION
Imagine you are a heavy smoker trying to quit. Your thoughts are drawn to the fact that there is another pack of cigarettes in the drawer. Do you go and get it or not? Although the environment is full of tempting motivations for action, humans also possess mechanisms to resist them. Moreover humans can postpone immediate gratification of a desire to act, often to achieve a delayed, alternative or more abstract benefit. A person addicted to cigarettes might manage to bear symptoms like irritability, weight gain, and periods of strong craving in order to reach the long‐term goal of overcoming the nicotine dependence.
Indeed, human society relies precisely on balancing an individual's reasons to act against the interests of others. The capacity to refrain from actions in such circumstances is an important aspect of “self‐control.” Self‐control breaks the normal flow from intention to action. An individual with self‐control may form an intention to perform an action, but will not necessarily implement the intention. They may refrain from translating the impulse to act into a physical motor action. The inability to voluntarily control one's intentions occurs in several clinical disorders like addiction, attention‐deficit/hyperactivity disorder (ADHD) and certain personality disorders. Given the importance of self‐control in social settings, such disorders often have a very high social cost.
Previous research in cognitive neuroscience has focused on externally triggered inhibition rather than the voluntary inhibition of action characteristic of self‐control. For example, the classic NoGo paradigm and stop‐signal paradigm involve frequent stimulus‐driven responses which participants sometimes inhibit when instructed by an external signal [Band and van Boxtel, 1999; Logan, 1995; Logan and Cowan, 1984]. Externally triggered inhibition is associated with lateral brain regions, in particular right inferior frontal gyrus [Aron and Poldrack, 2005; Rubia et al., 2001]. But self‐control in daily life cannot rely on external signals. There is no red‐light inside the drawer warning us to ignore the cigarettes: inhibition of action must then be internally generated. However, such endogenous inhibition is difficult to study experimentally, because, by definition, it is not associated with any stimulus or any response.
In one recent study, a subjective temporal judgment was used to identify endogenous inhibition in the absence of overt action [Brass and Haggard, 2007]. Participants were asked to perform spontaneous key presses while watching a rotating clock hand. They were further asked to cancel the impending action at the last possible moment on some trials that they themselves freely selected. After each trial they reported the clock position at which they experienced the intention to act [Libet et al., 1983], both on trials where they performed the action, and also on trials where they voluntarily “vetoed” it. Activation of the dorsal fronto‐median cortex (dFMC) was associated with vetoing the neural processes translating intentions into actions.
However, this study could not conclusively identify when intentional inhibition took place. Participants were instructed to decide “at the last possible moment.” Nevertheless, they could, in principle, have decided at the start of some trials that they would not act on that trial. In that case, they might not even prepare any action, and no veto process would be required. If such predecision occurred, then dFMC activation would not reflect endogenous inhibition, but some other process. Second, the “free‐choice” task used by Brass and Haggard, like others in the voluntary action literature, lacked the context of reasons for performing and inhibiting action that generally exists outside the laboratory. Participants simply pressed a button or did not. Their choice had neither antecedent reasons nor consequent effects. However, from an evolutionary point of view a self‐control mechanism should have evolved especially for regulation of impulsive responses, as in the cases of delayed gratification and social compromise described earlier.
In addition, we wanted to relate “veto” activity to a more general theoretical model of action generation. One recent model views voluntary action as a product of three specific kinds of action decisions [Brass and Haggard, 2008]: what‐, when‐ and whether‐ decisions. The rostral cingulate zone (RCZ) has been associated with “what‐decisions” involving selection between different response alternatives [Cunnington et al., 2006; Debaere et al., 2003; Lau et al., 2004; Müller et al., 2007; van Eimeren et al., 2006]. We wondered if “whether‐decisions,” for example between executing an action or vetoing an action, might also involve the RCZ. We distinguished between a decision process regarding whether to execute or veto, and the decision outcome, in favor of one of these alternatives. In neuroimaging terms, the decision process would be present irrespective of outcome, and so would not appear in a contrast of veto‐action activations. In contrast, dFMC activation reported by Brass and Haggard [2007] was specific to veto trials, and absent in action trials, suggesting it reflects a decision outcome. We therefore compared the activity in both areas to each participant's frequency of inhibited actions. Finally, we reasoned that both veto decisions and action decisions must be expressed by regulating levels of activity in action execution areas. We therefore investigated the specific role of veto decisions in action generation using effective connectivity analyses.
To address these points, we devised a task in which participants had to intentionally stop an externally triggered impulsive response. Although the task bears some conceptual resemblance with the task of Brass and Haggard [2007], it is much closer to real‐life situations. Participants saw a white marble on a platform of a steep ramp which they could set in motion by means of a button press. In 50% of the trials, the marble turned green and participants had to stop the marble by pressing the button a second time as fast as possible. Since slow responding was punished by a subtraction of monetary reward and the shattering of the marble accompanied by a glass breaking sound, this keypress was the natural response. If the marble did not change color, its progress was noticeably slower, and the task was to choose between performing the prepared action to stop the marble (decide‐go) and voluntarily refraining from it (decide‐nogo). This decision could be made only when the marble's continued white color was registered. Crucially, then, the keypress action was triggered by the marble's motion, but the inhibition of the action, when it occurred, was uncued and endogenous. Moreover the decision was made against a context favoring action provided by the color‐change trials where not acting was punished by subtraction of monetary reward. To encourage our participants to consciously decide how to behave we asked them to report the position of the marble when they made their choice. We compared trials in which participants voluntarily chose not to respond with trials in which they chose to respond. This contrast should reveal brain areas involved in voluntarily inhibiting a strong impulsive response tendency. Furthermore, we set out to explore brain areas functionally associated with dFMC to assess how dFMC modulates areas involved in forming motor intentions.
MATERIALS AND METHODS
Participants
Sixteen healthy volunteers participated on the basis of informed consent and with ethical committee approval and according to the Declaration of Helsinki. All subjects had normal or corrected‐to‐normal vision. No subject had a history of neurological, major medical, or psychiatric disorder. The participants (11 women and 5 men) had a mean age of 21.2 (ranging from 19 to 31) were all right‐handed as assessed by a handedness questionnaire [van Strien, 1992 mean score = 9.8].
Behavioral Task
Participants viewed a white ramp displayed on a black background. At the beginning of each trial a white marble appeared on a platform at the top of the ramp (Fig. 1). Participants pressed a button with their right index finger to set the marble in motion down. They viewed the marble rolling down the ramp (in fact 16 rapidly presented static images showing the marble at successive locations on the ramp), and finally a shattered marble beneath the ramp accompanied by a sound of breaking (Visuastim MR‐compatible headphones). The task involved three different randomized conditions: the (1) green, (2) white, and (3) red marble condition.
Figure 1.
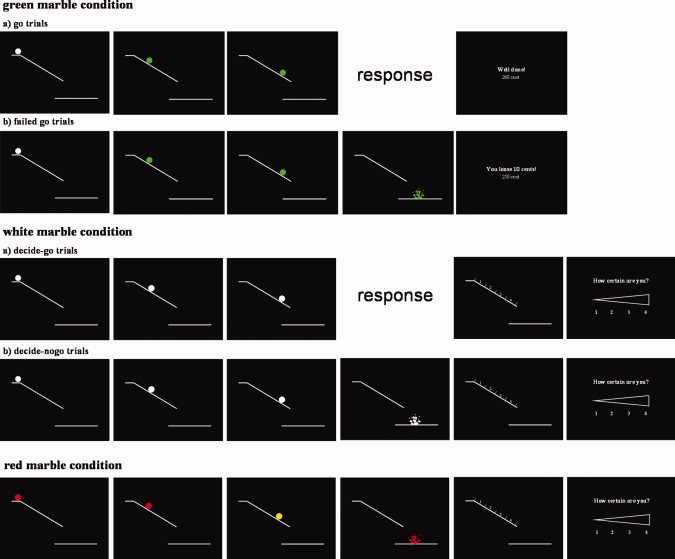
Schematic drawing of the experimental conditions.
In the green marble condition, the marble changed color to green as soon as it began to move, and all the successive static images showed a green marble. Participants were instructed to respond to the green marble by pressing the key to stop the marble from falling off the end of the ramp. If subjects succeeded, they avoided the unpleasant breaking‐glass sound and received positive feedback as follows. Participants received virtual 300 cents at the start of each block and lost 10 cents every time they failed to respond in time to a green marble. Feedback after each trial reported “you loose 10 cents” for a failed response, “well done!” for a successful response, and the current balance in cents. This reinforcement aimed to ensure participants were maximally prepared to respond when the marble began to move. The speed of the marble was adjusted by a staircase procedure. The experiment started with a marble duration of 30 ms each. Afterward, the speed was continuously modified during the experiment. If the subject succeeded in giving a response in time, the presentation duration of each marble was decreased by 10 ms, making the task more difficult; conversely, if participants failed to respond in time, the presentation duration increased by 10 ms, making the task easier. The staircase variable was allowed to fluctuate between 20 and 80 ms allowing a response window between 320 and 1280 ms. The green marble condition served to ensure high response preparation throughout the experiment. In the analysis, green marble trials were divided into go trials in which subjects responded before the marble shattered and failed‐go trials in which subjects responded after the marble shattered.
In the key white marble condition, the marble did not change color and was considerably slower. Participants were instructed to choose between responding or inhibiting the response, and to approximately balance the frequency of the two choices. If subjects responded they stopped the marble from breaking; if they decided not to respond the marble fell from the ramp and the breaking glass sound was presented. Accordingly, white marble trials were divided into decide‐go and decide‐nogo trials depending on the choice of the participants. The speed of the white marbles was set to that currently reached in green trials plus 30 ms, to allow sufficient time to choose between responding and inhibiting.
After each white marble trial, participants indicated the marble location on the ramp where they decided to respond or inhibit (location judgment). For this purpose, the ramp was displayed on the screen with the numbers 1–4 depicted above the ramp indicating descending locations on the ramp (Fig. 1). Additionally, participants had to judge how certain they were about this location (certainty judgment). A certainty judgment scale was presented on the screen depicting a gradient from low certainty to high certainty labeled with the numbers 1–4, on which 1 indicates the lowest and 4 the highest certainty (Fig. 1). Participants responded with their index or middle finger of their left (for 1 and 2) or right (for 3 and 4) hand. The written instruction stated that “1” was to be pressed in response to the location and certainty judgment screen in the case that the response was given impulsively, because a green trial was expected. These judgments allowed us to distinguish prepotent responses associated with initial preparation from those initiated after a specific decision triggered by detecting a white marble. That is, a rapid response on a white marble trial might in fact be a false positive or error of commission. Since participants were highly prepared to respond rapidly to marble motion in green marble trials, they might respond to the marble's initial motion, without registering that the marble remained white. We reasoned that participants would report early decisions, and/or uncertainty of decision on any such trials.
In the analysis, we therefore only analyzed white marble trials which appeared to involve deliberate decision, as opposed to prepotent responses. That is, if participants indicated that the marble was at the top of the ramp when they decided (response of “1” in the location judgment), and/or reported uncertainty about their decision (certainty reply of “1”), the trial was discarded. Thus, we ensure only to include trials in which subjects did not decide in advance and were able to report the decision time. The aim was to subtract the decision process out of the decide‐nogo brain activity when calculating the contrast decide‐nogo versus decide‐go condition.
In the red marble condition, a red marble appeared at the starting position. Participants were instructed to initiate the trial by pressing the button under their right index finger and simply observe the marble rolling down and falling off the ramp without responding. At a random position on the ramp the marble briefly flashed yellow, and participants later judged where on the ramp this change occurred, and reported their certainty level. This served as a control for the precision of the location judgments on the ramp. The marble motion was identical to the white marble condition. Red marble trials therefore contained similar stimuli, and a similar absence of response to white marble trials on which participants inhibited actions. Furthermore, we could use these trials to determine the brain areas that are involved in the decision to act or not.
Finally, we included null events in which the screen remained black for the average length of the white marble condition.
To summarize, the green marble condition created a prepotent response tendency to press the button to stop the marble's motion. Since it contained a feedback manipulation and did not involve the two judgments employed after the white and red marble condition those trials were included for psychological purposes only. It provided a background context for the white marble condition, in which participants themselves decided whether to make this response or to inhibit it. The red marble condition served as a control for the stimulus and judgment aspects of the task.
All in all the experiment consisted of four blocks of 84 trials each (36 green, 24 white, 12 red trials, and 12 null events per block). Before scanning, subjects were trained with one practice block of 84 trials comparable with the blocks used in the scanner. The experiment in the scanner lasted about 40 min.
Scanning Procedure
Subjects were positioned head first and supine in the magnet bore. Images were collected with a 3T Magnetom Trio MRI scanner system (Siemens Medical Systems, Erlangen, Germany), using an 8‐channel radiofrequency head coil. First, 176 high‐resolution anatomical images were acquired using a T1‐weighted 3D MPRAGE sequence [TR = 2530 ms, TE = 2.58 ms, image matrix = 256 × 256, FOV = 220 mm, flip angle = 7°, slice thickness = 0.90 mm, voxel size = 0.9 mm × 0.86 mm × 0.86 mm (resized to 1 mm × 1 mm ×1 mm)]. Whole brain functional images were collected using a T2*‐weighted EPI sequence, sensitive to BOLD contrast (TR = 2000 ms, TE = 35 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 17%, voxel size 3.5 mm × 3.5 mm × 3 mm, 30 axial slices). A varying number of images were acquired per run due to the self‐paced initiation of trials.
fMRI Data Preprocessing and Main Analysis
The fMRI data were analyzed with statistical parametric mapping, using the SPM5 software (Wellcome Department of Cognitive Neurology, London, UK). The first four scans of all EPI series were excluded from the analysis to minimize T1 relaxation artefacts. A mean image for all scan volumes was created, to which individual volumes were spatially realigned by rigid body transformation. The high resolution structural image was coregistered with the mean image of the EPI series. The structural image was normalized to the Montreal Neurological Institute template. The normalization parameters were then applied to the EPI images to ensure an anatomically informed normalization. A commonly applied filter of 8 mm FWHM (full‐width at half maximum) was used. The time series data at each voxel were processed using a high‐pass filter with a cut‐off of 256 s to remove low‐frequency drifts. The subject‐level statistical analyses were performed using the general linear model. The main events of interest were the periods after the onset of the white marble. Vectors containing the event onsets were convolved with the canonical hemodynamic response function (HRF) to form the main regressors in the design matrix (the regression model). The vectors were also convolved with the temporal derivatives and the resulting vectors were entered into the model. The statistical parameter estimates were computed separately for each voxel for all columns in the design matrix. Contrast images were constructed from each individual to compare the relevant parameter estimates for the regressors containing the canonical HRF. The group‐level random effects analysis was then performed. One‐sample t‐test was performed for each voxel of the contrast images. The main comparison of interest to explore voluntary inhibition was a contrast of trials in the white marble condition in which participants freely chose not to respond (decide‐nogo) with trials in which they chose to respond (decide‐go) time‐locked to the start of the marble's motion. The two comparisons of interest to explore brain areas associated with the decision to act or not were (1) a contrast of white marble trials in which participants chose to respond (decide‐go) with red marble trials in which participants did not have to make a decision and were not allowed to respond (red‐nogo) and (2) a contrast of white marble trials in which participants chose not to respond (decide‐nogo) with red marble trials (red‐nogo). Resulting statistical values were thresholded at P < 0.05 (FDR‐corrected) with a volume greater than 350 mm3 (10 adjacent voxels). They were overlaid onto a normalized structural image of a single subject.
Percent Signal Change Analysis
With the percent signal change analysis, we aimed at comparing RCZ and dFMC activity, to distinguish between the decision process and the decision outcome. Therefore, we took a sphere with 6 mm radius around the peak voxel of the contrast decide‐go versus red‐nogo for the RCZ ROI (0, 32, 35) and the same sphere around the peak voxel of the contrast decide‐nogo versus decide‐go for the dFMC ROI (−7, 42, 21).
For the correlational percent signal change analysis, we defined a ROI consisting of the functional cluster in dFMC resulting from the whole‐brain contrast of the decide‐nogo versus decide‐go condition. We correlated those percent signal changes with each participant's behavioral probability of inhibiting an action: #decide‐nogo trials/(#decide‐nogo trials + #decide‐go trials).
For each subject, region and condition separately the mean percent signal change over a time window of 4–6 s after stimulus onset was calculated and compared by use of paired t‐tests.
Effective Connectivity Analysis
The effective connectivity, also called psychophysiological interaction [PPI, Friston et al., 1997] analysis explores whether connections between brain areas are modulated by psychological factors. It assesses whether the effective connectivity between a seed region and all other voxels in the brain is changed by an experimental condition. We explored PPI for a volume of interest (VOI) in the dFMC. Individual VOIs were defined as 6 mm radius spheres, with the center being the local maximum in the contrast of decide‐go and decide‐nogo versus null events closest to the peak voxel of the dFMC activation found of the main contrast decide‐nogo versus decide‐go (−7, 42, 21). The significance for the VOI extraction was set to P = 0.001, k = 5 (uncorrected). The time‐series data of the first eigenvariate of the VOI was extracted. Then one vector containing the main effect of the contrasts of interest (P regressor, psychological variable) and a second vector representing the VOI time‐course (Y regressor, physiological variable) and a third vector was generated contrasting the time‐series of the estimated neural response for the conditions of interest (PPI regressor, interaction of the psychological and physiological variable). Then the PPI analysis convolves those regressors with the canonical hemodynamic response function to estimate the effects of the regressors. Brain sites receiving contextual influences of dFMC that were stronger during the decide‐nogo compared with the decide‐go condition were determined by a t‐test. This second‐level random effects analysis was thresholded at P = 0.001, k = 5 (uncorrected).
RESULTS
Behavioral Data
Mean reaction times (RTs) for the different response conditions are displayed in Table I. Paired t‐tests reveal that white‐marble decide‐go trials, in which participants intentionally decided to act, were significantly longer than RTs in purely reactive green‐marble go trials (t(15) = −8.34, P < 0.001). Table I shows that the additional processes of decision in the former case add an average 873 ms to the RT.
Table I.
Mean RT (ms) and standard deviation (SD)
| Condition | Mean RT | SD |
|---|---|---|
| Go trials | 259 | 28 |
| Failed go trials | 278 | 32 |
| Successful decide‐go trials | 1132 | 418 |
| Unsuccessful decide‐go trials | 353 | 179 |
Our participants succeeded in responding in time on 52.5% of green marble trials, and received on average 440 cent in addition to the basic payment at the end of the experiment. The distribution of successful (meaning that the location and certainty judgment were greater than 1) decide‐go and decide‐nogo trials in the white marble condition was nearly equal (48.2% vs. 51.8%). To exclude that participants use simple alternation strategies to achieve an equal distribution we calculated the mean random number generation (RNG) measure proposed by Evans [1978]. This measure considers the randomness of the sequence, namely the dependency between one choice and the next. The score has a range between 0 (perfect equality of all possible transitions, no predictability) and 1 (paired sequences are completely predictable). Our results show low RNG of 0.226 with slightly less response repetitions then expected according to an equal distribution of response transition probabilities. In the random generation literature, this is a phenomenon called repetition avoidance.
On average, we excluded 13.2% of the decide‐go trials and 9.6% of the decide‐nogo trials due to either a location and/or a certainty judgment of 1.
To control for the accuracy of the timing judgment and for the consistency of the certainty judgment, we evaluated the responses in the red marble condition. The timing judgment of the yellow color change was correct in 96.3% of the trials. Comparing the certainty judgment for correct versus incorrect trials regarding the timing, we find a highly significant difference (certainty judgment for correct timing: 3.1, certainty judgment for incorrect timing: 1.8; t(15) = 26.59, P < 0.001). This difference confirms that participants' confidence in their timing judgments reflected their actual performance. Therefore, we felt that timing and certainty judgments could reliably be used to classify white marble trials into those with a prepotent response, and those resulting from a deliberate decision process.
fMRI Data
Whole‐brain analysis
The whole‐brain contrast decide‐nogo versus decide‐go revealed three activated clusters: left dFMC (BA 9), right primary, and secondary auditory cortex namely Heschl's gyrus (HG) and planum temporale (PT) (BA 41, 42) and right inferior parietal lobe (IPL, BA 40) (Fig. 2A, Table II). The dFMC activation (−7, 42, 21, MNI coordinate) is close to that reported previously [Brass and Haggard, 2007: −2, 41, 37, MNI coordinate], though slightly more ventral.
Figure 2.
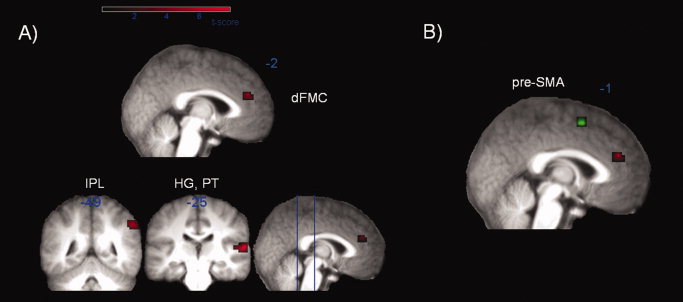
A) Main contrast of decide‐nogo versus decide‐go. Activation map averaged over 16 subjects (FDR‐corrected P < 0.05, k 5 10 voxel) mapped onto an anatomical mean image of all subjects. Colored labels indicate positive T values. Displayed are activities in dorsal fronto‐median cortex (dFMC), inferior parietal lobe (IPL), Heschl's gyrus and planum temporale (HG, PT). B) Significant changes in effective connectivity (decide‐nogo vs. decide‐go) between the cluster of the seed region in dorsal fronto‐median cortex (dFMC) depicted in red and the presupplementary motor area (pre‐SMA) (P < 0.001, k 5 5) in green mapped onto an anatomical mean image of all subjects.
Table II.
Areas showing significant activation in whole brain contrast decide‐nogo versus decide‐go
| Area | BA | Peak coordinates (MNI) | Z‐score | Extent | P (FDR‐corrected) |
|---|---|---|---|---|---|
| Heschl's gyrus and planum temporale (HG, PT) | 41, 42 | 64, −25, 11 | 4.99 | 49 | 0.005 |
| Dorsal fronto‐median cortex (dFMC) | 9 | −7, 42, 21 | 4.16 | 12 | 0.031 |
| Inferior parietal lobe (IPL) | 40 | 56, −49, 42 | 4.06 | 19 | 0.034 |
When taking into account all decide‐go and decide‐nogo trials without excluding those with low location and certainty judgments and therefore loosening the criterion to exclude trials with impulsive responding or preplanning we find a similar left dFMC and auditory cortex but not the IPL activation.
The fact that dFMC is more active in decide‐nogo trials compared with decide‐go trials implies that dFMC cannot be the locus of deciding between acting and stopping because the decision process should be involved in both conditions. To identify the brain region that is involved in the decision to act and the decision to stop, we looked at the contrasts of decide‐go versus red‐nogo (Tables I and II in Supp. Info. Material) and decide‐nogo versus red‐nogo. As predicted in both contrasts RCZ is activated.
Percent signal change analysis
To compare the signal strength in the decide‐go and decide‐nogo condition, we performed a percent signal change analysis in RCZ and dFMC. Paired t‐tests revealed a significant difference between the percent signal change in the decide‐go and the decide‐nogo condition in dFMC (t(15) = −5.569; P < 0.001), whereas no significant difference was observed between the percent signal changes in the decide‐go and the decide‐nogo condition in RCZ (t(15) = −0.515; P = 0.614) (Fig. 3). In a repeated measures ANOVA on the percent signal change values the interaction of the factor ROI (dFMC vs. RCZ) and condition (decide‐go vs. decide‐nogo) is significant (F(1,15) = 29.637, P < 0.001).
Figure 3.
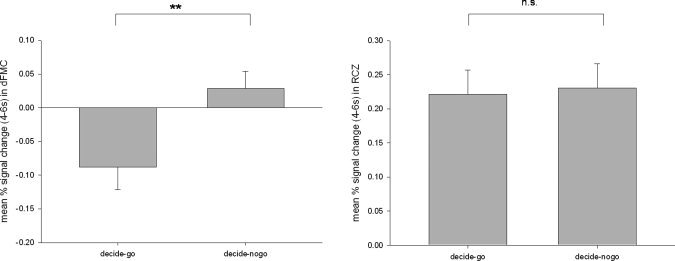
Percent signal changes for a ROI in RCZ (sphere with radius 6 mm around peak voxel 0, 32, 35 of the contrast decide‐go vs. red‐nogo) and dFMC (sphere with radius 6 mm around peak voxel −7, 42, 21 of the contrast decide‐nogo vs. decide‐go). **P < 0.001; n.s., not significant.
Moreover, we performed correlation analyses to relate the dFMC activation to voluntary inhibition of action. We correlated each participant's behavioral probability of inhibiting an action with their inhibition‐related activation, namely the difference between dFMC activity for inhibition and action trials. Participants who expressed less inhibition also showed smaller inhibition‐specific dFMC activation (r = 0.51, P < 0.05), replicating an association reported previously [Brass and Haggard, 2007]. The result is consistent with the view that dFMC is involved in the voluntary inhibition of actions.
Effective connectivity analysis
To explore the effective connectivity pattern of dFMC, we applied PPI to the contrast decide‐nogo–decide‐go. If dFMC truly inhibits actions that are already prepared and intended, we hypothesized that it should influence motor preparation areas such as the pre‐SMA more strongly in decide‐nogo than in decide‐go trials. This hypothesis was confirmed by a significant difference in effective connectivity with pre‐SMA (Fig. 2B, Table III), consistent with modulation of motor intentions.
Table III.
Areas showing significant activation in effective connectivity with dorsal fronto‐median cortex
| Area | BA | Peak coordinates (MNI) | Z‐score | Extent |
|---|---|---|---|---|
| Pre‐SMA | 6 | −7, 14, 56 | 3.24 | 11 |
DISCUSSION
The present study provides further evidence for the assumption that the dFMC is involved in voluntary inhibition of action [Brass and Haggard, 2007]. However, in contrast to previous work the present paradigm investigates the voluntary inhibition of a prepotent, impulsive response tendency. It is therefore much closer to real‐life situations requiring self‐control than the “spontaneous free choice” tasks used previously. Since our task clearly involved both advance preparation of a prepotent response, and a decision to voluntarily inhibit this response on certain trials, we ruled out possible confounds of earlier studies, notably predecision. Thus, we can more confidently identify dFMC activation with a specific process of self‐initiated inhibition of preplanned actions. The conclusion that the dFMC is involved in self‐control is further supported by the positive correlation between the percentage of inhibition trials with dFMC activation. We suggest that dFMC is not involved in deciding between acting and inhibiting because this would require that dFMC is active in both conditions. Rather, we suggest that RCZ is the location of this decision process, since it is involved in the decision to act as well as in the decision not to act [Cunnington et al., 2006; Debaere et al., 2003; Lau et al., 2004; Müller et al., 2007; van Eimeren et al., 2006]. Most importantly, we report the first evidence regarding how dFMC may exert control over brain areas involved in the intentional execution of action. We found an increased effective connectivity of dFMC and pre‐SMA in the intentional inhibition condition compared with the action condition. This finding is consistent with the view that dFMC modulates brain areas involved in motor preparation. It confirms a broadly hierarchical view of action control, with voluntary inhibition modulating premotor processing.
Remarkably, the dFMC activation is very specific in the current task. Only bilateral auditory cortex and inferior parietal activity were found in addition to dFMC. The activation in the auditory cortex is clearly elicited by the sound of glass breaking when participants voluntarily inhibit the action that stops the marble. Activity in the inferior parietal lobe (IPL) has often been reported in tasks involving self‐initiated versus triggered action [Jahanshahi et al., 1995; Jenkins et al., 2000]. This might imply that the selection to inhibit voluntarily involves more selection effort or more attention than responding.
The Role of dFMC in Self‐Control
As outlined above, contrasting voluntary inhibition of an impulsive action with execution of the same action results in a single, isolated prefrontal cortex activation. This activation is located close to those in two recent studies on intentional inhibition [Brass and Haggard, 2007; Campbell‐Meiklejohn et al., 2008]. In the study by Brass and Haggard [2007] participants were asked to voluntarily stop a self‐initiated action at the last possible moment. In the study by Campbell‐Meiklejohn et al. [2008] participants had to stop loss chasing in a more complex gambling task. From a phenomenological perspective all three tasks are extremely different. Nevertheless, they result in a common fronto‐median activation. Taken together evidence is accumulating that a specific region in the fronto‐median wall is responsible for the intentional inhibition of action [see also Brass and Haggard, 2008].
Functional Connections of dFMC
Although there is accumulating evidence for the role of dFMC in intentional inhibition of action, it is still completely unknown how this region implements such control. The PPI analysis revealed effective connectivity of dFMC with an area involved in intentional action, namely pre‐SMA. These data suggest that dFMC operates by influencing brain areas involved in intentional action. DTI studies provide anatomical support for this connectivity between dFMC and pre‐SMA [Johansen‐Berg et al., 2004].
The posterior part of the fronto‐median cortex including SMA and pre‐SMA has been associated with the initiation and selection of movements [Thaler et al., 1995], especially in the context of internally guided actions [Deiber et al., 1999; Lau et al., 2006]. Deecke [1996] proposed that SMA is involved in the channeling of motivation into execution of movement. Moreover, several studies suggest the SMA or pre‐SMA to be a generator of the Bereitschaftspotential (BP, readiness potential) occurring prior to movement onset [Deecke, 1996; Pedersen et al., 1998; Rektor, 2002]. Electrical stimulation of the pre‐SMA can produce an “urge” to move [Fried et al., 1991].
Interestingly, other studies [Nachev et al., 2005, 2007; Sumner et al., 2007] show that pre‐SMA plays a critical role in preventing actions, especially in situations of response conflict. Our finding of connectivity between dFMC and pre‐SMA sheds light on the apparent paradox of action‐promoting and action‐preventing functions being colocated in the pre‐SMA. We suggest that dFMC provides an intentional mechanism for stopping an ongoing action in a top‐down fashion. Inputs from dFMC to pre‐SMA therefore potentially control whether actions occur or not. Pre‐SMA BOLD responses correlating with action inhibition might reflect the braking input from dFMC to pre‐SMA [Nachev et al., 2005]. Lesions of the pre‐SMA might prevent this input from being effective, producing the patterns of prepotent responding characteristic of patients with pre‐SMA lesions [Nachev et al., 2007]. Interestingly, patients with anarchic hand syndrome, which generally involves unilateral pre‐SMA lesion, appear to retain the intention to inhibit stimulus‐driven actions, but cannot actually inhibit them. Thus, though they may decide not to perform an action, and are fully mindful of the need not to perform it, they are unable to prevent the action occurring. They may resort to physical inhibition by restraining the affected hand with the intact hand, rather than the intentional inhibition provided by an intact dFMC‐pre‐SMA pathway [Boccardi et al., 2002; Della Sala, 1991]. We speculate that the pre‐SMA involvement in many situations of suppressing competing plans may reflect not only processing of conflict within the pre‐SMA, but also top‐down inputs from dFMC.
Individual Differences and Clinical Aspects
We found differences between individuals in dFMC activation that correlated with the frequency of inhibiting actions. This suggests a trait‐like predisposition for self‐control. Individual differences in impulsivity are well‐known. In everyday life they are expressed as inability to inhibit appropriate behaviors, the inability to wait, and the tendency to act without forethought [Logan et al., 1997; Reynolds et al., 2006]. Further research has to clarify whether such personality characteristics are related to interindividual differences in dFMC function. Moreover, dysfunctionality of self‐control plays a crucial role in so‐called impulse‐control disorders as e.g., ADHD, obsessive‐compulsive disorder, or pathological gambling [Campbell‐Meiklejohn et al., 2008]. The present experimental paradigm would be well suited to test differences in self‐control related brain activity between clinical and healthy populations.
CONCLUSION
To conclude, the current findings demonstrate the role of dFMC in intentional inhibition of action, and its effective connectivity with areas involved in intention and preparation of action. We used a naturalistic task involving clear response affordances and impulsive actions, close to real‐life experiences of action decision. dFMC was involved in intentional inhibition of such responses, in addition to intentional inhibition of self‐generated actions reported previously. In accordance with a functional fractionation of intentional action by Brass and Haggard [2007], we could dissociate the area that is involved in the implementation of intentional inhibition (dFMC) from the area involved in the decision whether to act or not (RCZ). Our results represent a further step in addressing the question of self‐control. Future research may benefit from taking a cognitive‐motor approach to study clinical disorders of self‐control.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. Areas showing significant activation in whole brain contrast decide‐go vs. red‐nogo. Supporting Information Table 2. Areas showing significant activation in whole brain contrast decide‐nogo vs. red‐nogo.
Acknowledgements
The authors would like to thank Dorit Wenke for helpful discussion of the experimental design.
REFERENCES
- Aron AR,Poldrack RA ( 2005): The cognitive neuroscience of response inhibition: Relevance for genetic research in attention‐deficit/hyperactivity disorder. Biol Psychiatry 57: 1285–1292. [DOI] [PubMed] [Google Scholar]
- Band GPH,van Boxtel GJM ( 1999): Inhibitory motor control in stop paradigms: Review and reinterpretation of neural mechanisms. Acta Psychologica 101: 179–211. [DOI] [PubMed] [Google Scholar]
- Brass M,Haggard P ( 2007): To do or not to do: The neural signature of self‐control. J Neurosci 27: 9141–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M,Haggard P ( 2008): The what, when, whether model of intentional action. Neuroscientist 14: 319–325. [DOI] [PubMed] [Google Scholar]
- Boccardi E,Della Sala S,Motto C,Spinnler H ( 2002): Utilisation behaviour consequent to bilateral SMA softening. Cortex 38: 289–308. [DOI] [PubMed] [Google Scholar]
- Campbell‐Meiklejohn DK,Woolrich MW,Passingham RE,Rogers RD ( 2008): Knowing when to stop: The brain mechanisms of chasing losses. Biol Psychiatry 63: 293–300. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Windischberger C,Robinson S,Moser E ( 2006): The selection of intended actions and the observation of others' actions: A time‐resolved fMRI study. Neuroimage 29: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Debaere F,Wenderoth N,Sunaert S,van Hecke P,Swinnen SP ( 2003): Internal vs external generation of movements: Differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19: 764–776. [DOI] [PubMed] [Google Scholar]
- Della Sala S,Marchetti C,Spinnler H ( 1991): Right‐sided anarchic (alien) hand: A longitudinal study. Neuropsychologia 29: 1113–1127. [DOI] [PubMed] [Google Scholar]
- Deecke L ( 1996): Planning, preparation, execution, and imagery of volitional action. Cogn Brain Res 3: 59–64. [DOI] [PubMed] [Google Scholar]
- Deiber MP,Honda M,Ibanez V,Sadato N,Hallett M ( 1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J Neurophysiol 81: 3065–3077. [DOI] [PubMed] [Google Scholar]
- Evans FJ ( 1978): Monitoring attention deployment by random number generation: An index to measure subjective randomness. Bull Psychon Soc 12: 35–38. [Google Scholar]
- Fried I,Katz A,McCarthy G,Sass KJ,Williamson P,Spencer SS,Spencer DD ( 1991): Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci 11: 3656–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ,Buechel C,Fink GR,Morris J,Rolls E,Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M,Jenkins IH,Brown RG,Marsden CD,Passingham RE,Brooks DJ ( 1995): Self‐initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement‐related potentials in normal and Parkinson's disease subjects. Brain 118: 913–933. [DOI] [PubMed] [Google Scholar]
- Jenkins IH,Jahanshahi M,Jueptner M,Passingham RE,Brooks DJ ( 2000): Self‐initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123: 1216–1228. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H,Behrens TEJ,Robson MD,Drobnjak I,Rushworth MFS,Brady JM,Smith SM,Higham DJ,Matthews PM ( 2004): Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101: 13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HC,Rogers RD,Ramnani N,Passingham RE ( 2004): Willed action and attention to the selection of action. Neuroimage 21: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Lau HC,Rogers RD,Passingham RE ( 2006): Dissociating response selection and conflict in the medial frontal surface. Neuroimage 29: 446–451. [DOI] [PubMed] [Google Scholar]
- Libet B,Gleason CA,Wright EW,Pearl DK ( 1983): The time of conscious intention to act in relation to onset of cerebral activity (readiness‐potential): The unconscious initiation of a freely voluntary act. Brain 106: 623–642. [DOI] [PubMed] [Google Scholar]
- Logan GD ( 1995): On the ability to inhibit thought and action: A user's guide to the Stop Signal Paradigm In: Dagenbach D,Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. San Diego: Academic Press. [Google Scholar]
- Logan GD,Cowan WB ( 1984): On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev 91: 295–327. [DOI] [PubMed] [Google Scholar]
- Logan GD,Schachar RJ,Tannock R ( 1997): Impulsivity and inhibitory control. Psychol Sci 8: 60–64. [Google Scholar]
- Müller VA,Brass M,Waszak F,Prinz W ( 2007): The role of the preSMA and the rostral cingulate zone in internally selected actions. Neuroimage 37: 1354–1361. [DOI] [PubMed] [Google Scholar]
- Nachev P,Rees G,Parton A,Kennard C,Husain M ( 2005): Volition and conflict in human medial frontal cortex. Curr Biol 15: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P,Wydell H,O'Neill K,Husain M,Kennard C ( 2007): The role of the presupplementary motor area in the control of action. Neuroimage 36 ( Suppl 2): T155–T163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JR,Johannsen P,Bak CK,Kofoed B,Saermark K,Gjedde A ( 1998): Origin of human motor readiness field linked to left middle frontal gyrus by MEG and PET. Neuroimage 8: 214–220. [DOI] [PubMed] [Google Scholar]
- Rektor I ( 2002): Scalp‐recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators—A hypothesis. Clin Neurophysiol 113: 1998–2005. [DOI] [PubMed] [Google Scholar]
- Reynolds B,Ortengren A,Richards JB,de Wit H ( 2006): Dimensions of impulsive behaviour: Personality and behavioural measures. Pers Individ Diff 40: 305–315. [Google Scholar]
- Rubia K,Russel T,Overmeyer S,Brammer MJ,Bullmore ET,Sharma T,Simmons A,Williams SCR,Giampietro V,Andrew CM,Taylor E ( 2001): Mapping motor inhibition: Conjunctive brain activations across different versions of Go/No‐Go and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Sumner P,Nachev P,Morris P,Peters AM,Jackson SR,Kennard C,Husain M ( 2007): Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54: 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler D,Chen YC,Nixon PD,Stern CE,Passingham RE ( 1995): The functions of the medial premotor cortex. I. Simple learned movements. Exp Brain Res 102: 445–460. [DOI] [PubMed] [Google Scholar]
- van Eimeren T,Wolbers T,Munchau A,Buchel C,Weiller C,Siebner HR ( 2006): Implementation of visuospatial cues in response selection. Neuroimage 29: 286–294. [DOI] [PubMed] [Google Scholar]
- van Strien JW ( 1992): Classificatie van links‐ en rechtshandige proefpersonen (Classification of left‐ and right‐handed subjects.). Nederlandes Tijschrift voor de Psychologie en Haar Grensgebieden 47: 88–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. Areas showing significant activation in whole brain contrast decide‐go vs. red‐nogo. Supporting Information Table 2. Areas showing significant activation in whole brain contrast decide‐nogo vs. red‐nogo.


