Abstract
Recent brain imaging studies indicate that empathy for pain relies upon both the affective and/or the sensorimotor nodes of the pain matrix, and empathic neural responses are modulated by stimulus reality, personal experience, and affective link with others. The current work investigated whether and how empathic neural responses are modulated by emotional contexts in which painful stimulations are perceived. Using functional magnetic resonance imaging (fMRI), we first showed that perceiving a painful stimulation (needle penetration) applied to a face with neutral expression induced activation in the anterior cingulate cortex (ACC) relative to nonpainful stimulation (Q‐tip touch). However, when observation of the painful stimuli delivered to a neutral face was intermixed with observation of painful or happy faces, the ACC activity decreased while the activity in the face area of the secondary somatosensory cortex increased to the painful stimulation. Moreover, the secondary somatosensory activity associated with the painful stimulation decreased when the painful stimulation was applied to faces with happy and painful expressions. The findings suggest that observing painful stimuli in an emotional context weakens affective responses but increases sensory responses to perceived pain and implies possible interactions between the affective and sensory components of the pain matrix during empathy for pain. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: emotion, empathy, somatosensory cortex, anterior cingulate cortex, fMRI
INTRODUCTION
Regulation of appropriate social interactions and coordinated activity is crucially influenced by our ability to understand and share the feelings and intentions of other individuals [Decety and Lamm,2006; Lamm et al.,2007a, b; Preston and de Waal,2002; Singer,2006]. This empathic ability is called into play when we observe others suffering from either psychological (e.g. social rejection) or physical pain (e.g. being penetrated by a needle). Empathy for pain can occur at different phenomenological and neural levels; an onlooker's reactions to the pain of other individuals can be very different, depending on the degree of emotional sharing, evaluation of social bonds, and interpersonal relations between the onlooker and the observer, as demonstrated when viewing the pain of a loved one [Singer et al.,2004, 2006] and that of a stranger [Avenanti et al.,2005, 2006; Bufalari et al.,2007; Valeriani et al.,2008]. The empathic activation of the pain matrix may be triggered by symbols indicating others' pain [i.e., colorful shapes in Singer et al.,2004 and or words in Gu and Han,2007a], by direct observation of painful stimuli delivered to another's specific body part [Avenanti et al.,2005, 2006; Benuzzi et al.,2008; Bufalari et al.,2007; Gu and Han,2007b; Valeriani et al.,2008], or by perception of painful facial expressions [Botvinick et al.,2005, Lamm et al.,2007a; Saarela et al.,2007].
Neuroimaging and neurophysiological studies indicate that imagining or seeing the pain of others may generate activation of the affective nodes [e.g., the anterior cingulate cortex (ACC) and insula, Botvinick et al.,2005; Gu and Han,2007b; Jackson et al.,2005, 2006; Morrison et al.,2004; Singer et al.,2004, 2006], the sensorimotor nodes [e.g., the somatosensory cortex Avenanti et al.,2005, 2006; Benuzzi et al.,2008; Bufalari et al.,2007; Gu and Han,2007a; Valeriani et al.,2008], or both nodes of the pain matrix [Cheng et al.,2007; Moriguchi et al.,2007; Saarela et al.,2007]. In addition, neurophysiological indices of reactivity to others' pain such as the magnitude of blood oxygen level‐dependent (BOLD) signal recorded using functional magnetic resonance imaging (fMRI) [Jackson et al.,2005, 2006; Morrison et al.,2004; Saarela et al.,2007; Singer et al.,2004, 2006], the amplitudes of event‐related potentials (ERPs) [Fan and Han,2008; Han et al.,2008], the nonphase‐locked electroencephalogram (EEG) activity [Mu et al.,2008], and the amplitudes of motor and somatosensory‐evoked potentials [Avenanti et al.,2005, 2006; Bufalari et al.,2007] all correlate with subjective ratings of the affective or sensory qualities of the pain ascribed to the model. These findings indicate that both the affective and the sensory parts of the pain matrix are involved in empathy for pain.
However, empathic neural responses are strongly influenced by features of painful stimuli and contexts in which painful stimuli are perceived. For example, fMRI research showed evidence that the ACC activity associated with empathy for pain is stronger to pictures of hands in painful condition than to cartoons of hands in the same painful condition, suggesting that empathic responses depend on contextual reality of stimuli [Gu and Han,2007b]. Consistent with this, there is ERP evidence that the early neural activity linked to empathy for pain is reduced when the reality of painful stimuli is deteriorated by presenting painful stimulations in cartoon form [Fan and Han,2008]. Empathic neural responses also depend on subjective attitudes toward a target person who suffers from painful stimuli as activity in the insula related to empathy decreases when watching confederates who played unfairly receive pain compared with confederates who played fairly [Singer et al.,2006]. Observation of body parts being penetrated by needles induced increased activity in the ACC and insula in the control group but not in physicians who practice acupuncture [Cheng et al.,2007], suggesting a strong influence of personal experiences on empathic neural responses.
The current work further investigated whether and how empathic neural responses to perception of painful stimulations are modulated by emotional contexts. In real social situations, people may perceive both the painful stimuli itself as well as emotional facial expressions induced by the painful stimuli. In addition, painful stimuli applied to a person may also induce emotional facial expressions in other observers. We know little about whether and how empathic neural responses to perceived painful stimulations are modulated by such emotional contexts. To investigate this, the present study first scanned one group of subjects, using fMRI, who observed painful stimuli (needle penetration) or nonpainful stimuli (Q‐tip touch) applied to faces with a neutral expression. Increases in neural activity to the painful compared with nonpainful stimuli were estimated to define empathic neural responses independent of facial emotional expressions. A second independent group of subjects was scanned while they observed the same neutral faces applied receiving the same painful and nonpainful stimuli. However, in this group, the neutral‐faced stimuli were intermixed with emotional (painful and happy) faces that also received painful and nonpainful stimuli. Compared with the results of the first subject group, the results of the second subject group may uncover whether neural activity in the affective and sensory parts of the pain matrix are modulated by contexts, in this case, the emotional expression of the faces of individuals receiving pain. One possibility is that, as empathic neural responses depend on top‐down attention to the painful contents of stimuli [Gu and Han,2007b], both the sensory and affective components of empathic responses to painful stimulation to neutral faces are reduced by facial emotional contexts that may distract attention away from the painful contents of the stimulation applied to the neutral faces. Alternatively, the sensory and affective components of empathic responses may be modulated by the emotional contexts in different ways if interactions exist between the affective and sensory components of the pain matrix during empathy for pain. If painful stimulation dominates empathic responses regardless of emotional contexts, we would expect similar empathic neural responses to painful stimuli applied to both neutral and emotional faces. If emotional facial expressions dominate empathic responses, however, the contrast between emotional faces that receive painful and nonpainful stimuli should decrease empathic responses to the painful stimulation.
MATERIALS AND METHODS
Subjects
Two independent groups of subjects were recruited in this study as paid volunteers. Twenty‐four healthy adults (12 males and 12 females, 18–25 years of age, mean ± SD = 21.0 ± 1.56) were scanned while observing only the face stimuli with neutral expressions (Group 1). Twenty‐two healthy adults (11 males and 11 females, 18–26 years of age, mean = 22.6 ± 2.30) were scanned while observing the face stimuli with both neutral and emotional (painful and happy) expressions (Group 2). All participants were right‐handed, had normal or corrected‐to‐normal vision, and reported no neurological or psychiatric history. Informed consent was obtained from all the participants before scanning. This study was approved by a local ethics committee.
Stimuli and Procedure
The visual stimuli consisted of 3‐second video clips showing human faces with either neutral, painful, or happy expressions. Examples of the different stimuli are illustrated in Figure 1. Six models (three males and three females) were employed to make the video clips. Each clip depicted a face that was penetrated by a needle (painful stimulation) or touched by a Q‐tip (nonpainful stimulus). The painful stimulation was applied to the left side of the faces in half video clips and to the right side of the faces in the other video clips. In each clip a model showed a neutral, painful, or happy facial expression that lasted throughout the clip. A total of 72 video clips were made for the present study. During the scanning procedure, participants were instructed to observe the video clips and to judge after each clip whether or not the model was feeling pain by pressing a button with the right index or middle finger. The video clips were presented through a projector onto a rear‐projection screen located at the subject's head. Each video clip subtended a visual angle of 21.4° × 17.1° at a viewing distance of 80 cm.
Figure 1.
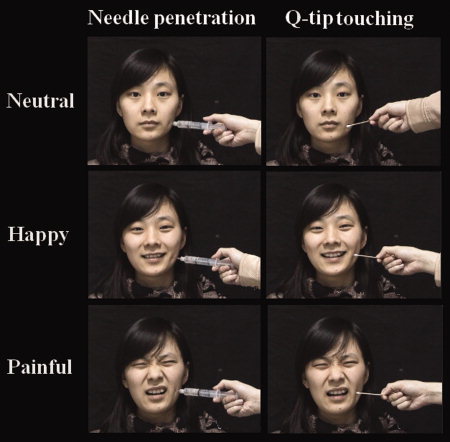
Illustration of the stimuli used in the current study. The first subject group was shown with only video clips of neutral faces with needle penetration or Q‐tip touch. The second subject group was shown video clips of neutral faces intermixed with painful and happy faces penetrated by the syringe or touched by the Q‐tip. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Two functional scans of 291 seconds were obtained from each subject of the first group. Six functional scans of 291 seconds were obtained from each subject of the second group. For each scan, 24 video clips were presented. The interstimulus interval between two successive clips lasted 9 seconds during which subjects fixated on a central cross. The last video clip in each scan was followed by a fixation of 12 seconds in order to record relevant BOLD signals.
For the first subject group, each scan consisted of 12 video clips showing only neutral faces with needle penetration (NF‐needle) and 12 video clips with the Q‐tip touching the face (NF‐Q‐tip). For the second subject group, each scan consisted of four clips in each stimulus conditions, i.e., NF‐needle, NF‐Q‐tip, painful faces with needle penetration (PF‐needle), painful faces with Q‐tip touch (PF‐Q‐tip), happy faces with needle penetration (HF‐needle), and happy faces with Q‐tip touch (HF‐Q‐tip). The video clips in different conditions were presented in a random order. The video clips showing painful or nonpainful stimulations to neutral faces were identical for the first and second subject groups. After the scanning procedure, each subject was shown the video clips again and was asked to answer specific questions related to the video clips. In particular, participants were asked to rate (1) the pain intensity felt by the model in the video clips (“How painful do you think the model feels?”) and (2) the unpleasantness felt by the onlooker during observation of the model undergoing needle penetration or Q‐tip touch (“How unpleasant do you feel when observing the video clip?”). The participants from the second group were also asked (3) to rate the intensity of the model's facial expression (“How strong is the model's emotional expression?”). Subjects answered the questions using a Likert‐type scale where 0 indicated no effect and 10 indicated maximal effect (e.g. extremely painful, extremely unpleasant, or extremely intense). The first and the second ratings assessed subjective feeling of anothers' pain and one's own emotional responses induced by painful stimuli. The third rating assessed subjective feeling of the models' facial emotions.
fMRI Image Acquisition and Analysis
Scanning was performed at Peking University First Hospital, on a GE 3‐T scanner with a standard head coil. Thirty‐two transverse slices of functional images covering the whole brain were acquired using a gradient‐echo echo‐planar pulse sequence (64 × 64 × 32 matrix with a spatial resolution of 3.75 × 3.75 × 4 mm, repetition time = 3,000 ms, echo time = 30 ms, FOV = 24 × 24 cm, flip angle = 90°). Anatomical images were obtained using a 3D FSPGR T1 sequence (256 × 256 × 128 matrix with a spatial resolution of 0.938 × 0.938 × 1.4 mm, TR = 7.4 ms, TI = 450 ms, TE = 3.0 ms, flip angle = 20°).
SPM2 (Statistical Parametric Mapping, the Wellcome Trust Centre for Neuroimaging, London, UK) was used for fMRI data analysis. The functional data were first time‐corrected to compensate for delays associated with acquisition time differences between slices during the sequential imaging. The functional images were then realigned to the first scan to correct for head motion between scans. All six movement parameters (translation; x, y, z and rotation; pitch, roll, yaw) were included in the statistical model. The anatomical image was coregistered with the mean functional image produced during the process of realignment. All images were normalized to a 2 × 2 × 2 mm3 Montreal Neurological Institute (MNI) template. Functional images were spatially smoothed using a Gaussian filter with the full‐width/half‐maximum parameter (FWHM) set to 8 mm. In addition, high pass temporal filtering with a cut‐off of 180 seconds was applied. The event‐related neural activity was modeled using a canonical hemodynamic response function (HRF). Effects at each voxel were estimated and regionally specific effects were compared using linear contrasts in individual participants using a fixed effect analysis.
For the first subject group, one contrast was calculated to define pain‐specific neural activations (NF‐needle vs. NF‐Q‐tip). Given the prior hypothesis of brain activation in association with empathy, significant activations were defined using threshold of P < 0.001 (uncorrected) and a spatial extent threshold of k = 50. The brain activations shown in the random effect analyses were then used to define regions‐of‐interest (ROIs), which were defined as spheres with 10‐mm diameter centered at the peak voxel in the activated clusters identified in the random effect analysis. To examine whether injected or touched neutral faces elicited different empathic neural responses when intermingled with emotional faces, parameter estimates of signal intensity in the ROIs to painful and nonpainful stimulations applied to the neutral faces were computed from the second subject group and compared using t‐tests. We further conducted repeated measures analyses of variance (ANOVAs) with stimulus (NF‐needle vs. NF‐Q‐tip) as within‐subjects independent variable and group (the first vs. second subject group) as between‐subjects variable to confirm the effect of emotional contexts produced by painful and happy faces on empathic responses to neutral faces. For the second subject group, the contrast of NF‐needle versus NF‐Q‐tip was calculated to define neural activation associated with the perceived pain with neutral faces. Then the parameter estimates of signal intensity in these ROIs to painful and happy faces receiving painful and nonpainful stimuli were calculated and compared using t‐tests. The signal intensities obtained from the second subject group were also subjected to two separate 2 × 2 ANOVAs with stimulus (needle vs. Q‐tip) and facial expression (neutral vs. painful and neutral vs. happy) as within‐subjects independent variables to confirm the effects of facial emotional contexts.
RESULTS
Behavioral Performance
Pain judgment during scanning
The number of trials in which subjects judged needle penetration as painful and Q‐tip as nonpainful during scanning are reported in Table I. There was no significant difference in judgment of Q‐tip as nonpainful between the two subject groups (t(44) = 0.117, P = 0.27). However, needle penetration applied to neutral faces was judged as painful less frequent by the second than by the first subject group (t(44) = 4.478, P < 0.001), suggesting the effect of emotional contexts that was specific to the subjective feelings of painful stimuli. Moreover, analyses of self‐reports in the second subject group indicate a strong contextual modulation of subjective reactivity to the same painful stimuli. Specifically, needle penetrations in painful faces were judged as painful much more often than in needle penetrations in neutral (t(21) = 3.596, P < 0.01) or happy face (t(21) = 4.506, P < 0.001. In a similar vein, Q‐tips were judged as nonpainful less frequent when touching a painful than a happy (t(21) = −4.146, P < 0.001) or a neutral face (t(21) = −4.190, P < 0.001).
Table I.
Percent of judgments about the needle or Q‐tip stimuli during the scanning procedure (mean ± SD)
| Group 1 (neutral face) | Group 2 (neutral face) | Group 2 (painful face) | Group 2 (happy face) | |
|---|---|---|---|---|
| Needle judged as painful (%) | 92.8 ± 11.9 | 55.3 ± 38.9 | 86.2 ± 13.3 | 50.6 ± 38.2 |
| Q‐tip judged as nonpainful (%) | 93.8 ± 7.7 | 90.2 ± 12.7 | 56.6 ± 37.2 | 90.0 ± 11.9 |
Rating of pain intensity and self‐unpleasantness after scanning
Table II shows the subjective rating scores along the Likert‐type scale for each video clip in the two subject groups outside scanner. The first subject group rated pain intensity and self‐unpleasantness significantly higher for observation of needle penetrations than Q‐tip touch (t(23) = 15.43 and 9.037, both P < 0.001). Consistent with the behavioral performances inside the scanner, the emotional context greatly influenced the subjective ratings of the video‐clip properties. Differential ratings (NF‐needle minus NF‐Q‐tip) of both pain intensity attributed to the observed actors with neutral expression and the related self‐ unpleasantness scores obtained after the scanning procedure were significantly higher in the first than the second subject groups (t(44) = 4.406 and 3.699, both P < 0.001).
Table II.
Results of subjective rating (mean ± SD)
| Question 1 | Question 2 | Question 3 | |
|---|---|---|---|
| Group 1 | |||
| NF‐needle | 7.37 ± 1.57 | 6.32 ± 2.50 | |
| NF‐Q‐tip | 0.75 ± 0.80 | 1.20 ± 1.28 | |
| Group 2 | |||
| NF‐needle | 5.32 ± 2.67 | 5.29 ± 2.26 | 0.15 ± 0.64 |
| NF‐Q‐tip | 2.10 ± 1.87 | 3.01 ± 1.92 | 0.05 ± 0.19 |
| PF‐needle | 7.61 ± 2.01 | 4.87 ± 2.52 | 6.13 ± 1.47 |
| PF‐Q‐tip | 5.06 ± 2.75 | 2.17 ± 1.65 | 5.72 ± 1.64 |
| HF‐needle | 4.76 ± 2.96 | 5.54 ± 2.35 | 4.39 ± 2.74 |
| HF‐Q‐tip | 1.86 ± 1.51 | 4.78 ± 2.29 | 5.18 ± 2.31 |
Question 1: How intensely do you think the model is feeling pain in the video clip? Question 2: How unpleasant do you find the video clip? Question 3 (only for the second subject group): How intense do you think the model's emotional facial expression is?
The second subject group rated pain intensity significantly higher for needle penetration than Q‐tip touch regardless of the model's facial expression (neutral face: t(21) = 4.886, P < 0.001; happy expression: t(21) = 4.237, P < 0.001; painful expression: t(21) = 4.225, P < 0.001). Q‐tip touch was rated as more painful when the model displayed painful rather than neutral facial expression (t(21) = 5.505, P < 0.001). Rating of pain intensity of the Q‐tip touch did not differ between happy and neutral faces (t(21) = 1.060, P > 0.1). Self‐unpleasantness ratings were higher when perceiving needle penetration relative to Q‐tip touching for neutral (t(21) = 4.486, P < 0.001) and happy (t(21) = 4.328, P < 0.001) faces but not for painful faces (t(21) = 1.656, P > 0.1), suggesting that the painful facial emotion induced comparable self‐unpleasantness regardless of perceived painful stimulation. When observing the model touched by the Q‐tip, self‐unpleasantness was rated significantly higher for neutral faces than painful faces (t(21) = 5.032, P < 0.001) and happy faces than neutral face (t(21) = −2.811, P < 0.05).
Rating of facial expression
Ratings of facial expression intensity did not differ significantly between faces bearing needle penetration and Q‐tip touching regardless of facial expression (neutral face: t(21) = 0.863, P > 0.1; happy face: t(21) = 1.511, P > 0.1; painful face: t(21) = 1.807, P > 0.05), suggesting comparable subjective feelings of facial expression regardless of painful or nonpainful stimulations. Relative to the neutral faces, ratings of emotional expressions were significantly higher for painful (Q‐tip touching: t(21) = 15.78, P < 0.001; needle penetration: t(21) = 14.73) and for happy faces (Q‐tip touching: t(21) = −10.53, P < 0.001; needle penetration: t(21) = 8.421, P < 0.001), confirming subjective feeling of the models' painful and happy expressions.
fMRI Results
Empathic neural responses to neutral faces presented alone
To identify the neural substrates underlying empathy for pain induced by painful stimulation in the first subject group, the contrast of NF‐needle versus NF‐Q‐tip was calculated using a whole‐brain statistical parametric mapping analysis and showed increased activations in the ACC (MNI coordinates x/y/z = 4/40/38, Z = 4.31, voxel number = 327) and bilateral prefrontal cortex (−52/16/16, Z = 4.31, voxel number = 217; 52/22/20, Z = 4.51, voxel number = 349; see Fig. 2a).
Figure 2.
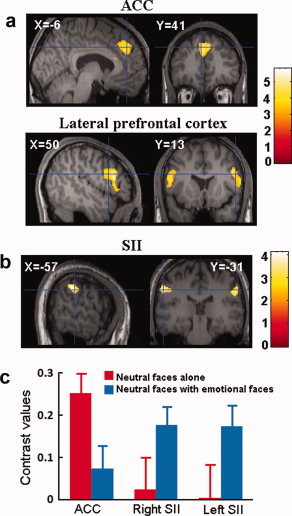
(a) Needle penetration to neutral faces induced increased activation in the ACC and bilateral prefrontal cortex in the first subject group; (b) needle penetration to neutral faces induced increased activation in bilateral SII in the second subject group; (c) contrast values in the ACC and the left and right SII regions differentiating needle penetration, and Q‐tip touch applied only to neutral faces when they were presented alone to the first subject group or when they were intermixed with emotional (painful and happy) faces and presented to the second subject group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Modulation of empathy‐related ACC activity by emotional contexts
To investigate if observation of painful stimulation delivered to neutral faces also induced increased empathic responses in the ACC when the neutral faces were intermixed with emotional faces, we first calculated the parameter estimates of signal intensity from the second subject group in the ROIs defined based on the ACC activation observed in the first subject group. Paired t‐tests did not show significant difference in signal intensity in the ACC related to NF‐needle and NF‐Q‐tip in the second subject group. To further confirm the differential activations between the first and second subject groups, a mixed model ANOVA of signal intensity in the ACC was conducted with stimuli (needle vs. Q‐tip) as a within‐subjects variable and group (the first vs. second subject group) as a between‐subjects variable. There was a significant interaction of stimulus × group (F(1,44) = 6.225, P = 0.016), confirming that the ACC activation indexed by the contrast value of needle vs. Q‐tip conditions was weaker in the second than in the first subject group (Fig. 2c).
Empathic neural responses to neutral faces intermixed with emotional faces
To examine the neural activity linked to painful stimulation in the neutral faces intermixed with the emotional faces, a whole‐brain analysis was conducted to contrast NF‐needle versus NF‐Q‐tip in the second subject group. This revealed increased activations in the face area of the secondary somatic sensory cortex (SII) in both the left and right hemispheres (−56/−34/38, Z = 3.44, voxel number = 138; 58/−28/34, Z = 3.49, voxel number = 127; Fig. 2b). Signal intensity was also computed in both subject groups from the ROIs defined based on the SII activations observed in the second subject group. The contrast values of needle versus Q‐tip conditions were larger in the second than in the first subject group, resulting in a marginally significant interaction of stimulus × group (F(1,44) = 3.905, P = 0.054, combined SII signals in the left and right hemispheres, Fig. 2c).
Modulation of empathy‐related SII activity by emotional contexts
To assess whether facial expressions modulate the SII empathic responses to perceived painful stimulation, signal intensity in the ROIs of the left and right SII related to NF‐needle was obtained from the video clips showing painful and happy faces. The two‐way ANOVAs showed a significant interaction of stimulus and facial expression (neutral vs. happy), confirming the difference in SII activity linked to painful stimulation between neutral and happy expressions (left SII: F(1,21) = 6.398, P < 0.05; right SII: F(1,21) = 0.807, P < 0.01), indicating that happy expression decreased the empathy‐related SII activity. The interaction of stimulus and facial expression (neutral vs. painful) was not significant (left SII: F(1,21) = 1.333, P > 0.1; right SII: F(1,21) = 1.797, P > 0.1), suggesting that modulation of SII activity by painful expression was not as strong as that by happy expression. Post hoc t‐tests showed that the SII signal intensity did not differ significantly between painful and neutral stimulation when models in the video clips showed painful or happy expressions (PF‐needle vs. PF‐Q‐tip: t = 0.968 and 0.709 for the left and right SII respectively, both P > 0.1; HF‐needle vs. HF‐Q‐tip: t = −0.219 and −1.784 for the left and right SII respectively, both P > 0.05, see Fig. 3).
Figure 3.
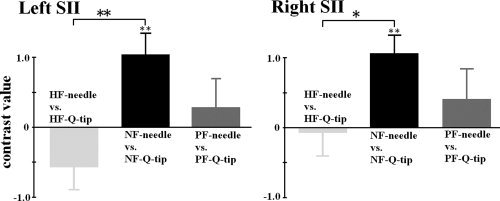
Contrast values in the left and right SII regions differentiating needle penetration and Q‐tip touch applied to neutral, painful, and happy faces.
Empathic neural responses to painful expression
To identify neural substrates linked to perceived dynamic painful facial expressions, we calculated the contrast of PF‐Q‐tip versus NF‐Q‐tip in the second subject group using the whole‐brain analysis. Relative to neutral faces with Q‐tip touch, faces with painful expressions and Q‐tip touch elicited increased activations in the ACC (6/26/44, Z = 3.65, voxel number = 311), bilateral prefrontal cortex (−44/18/34, Z = 3.61, voxel number = 172; 48/20/30, Z = 3.53, voxel number = 275), and the left insula (−36/24/2, Z = 3.10, voxel number = 85, see Fig. 4a–c). The contrast between happy faces with Q‐tip touch versus neutral faces with Q‐tip touch did not show any significant brain activation.
Figure 4.
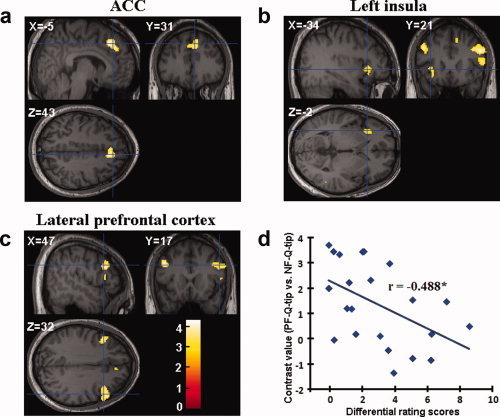
Observation of painful faces touched by the Q‐tip induced increased activation relative to observation of neutral faces touched by the Q‐tip in (a) the ACC, (b) the left insula, and (c) the bilateral prefrontal cortex; (d) correlation between the contrast values in the left prefrontal cortex differentiating painful and neutral faces touched by the Q‐tip and differential subjective rating scores of others' pain. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Correlation between subjective ratings and empathy‐related neural activity
Finally, to estimate the relation between subjective feeling and empathy‐related neural activity, we calculated correlations between the signal intensity in the brain areas activated by perceived pain and subjective rating scores of the pain attributed to the model and self‐unpleasantness derived from seeing the video clip from both subject groups. We found a significant correlation between the magnitude of the left prefrontal activity differing between painful and neutral faces with Q‐tip touch and subjective rating scores of the intensity of pain in others in the second subject group (r = −0.488, P < 0.05, Fig. 4d). No other significant correlation was observed in the first and second subject groups.
DISCUSSION
To assess whether and how facial emotional contexts modulate empathic neural responses induced by physical painful stimulations applied to neutral faces, we compared behavioral and neural responses with painful stimulation (needle penetration) and nonpainful stimulation (Q‐tip touch) applied to the neutral faces which were presented alone or were presented in facial emotional contexts (i.e., intermixed with painful and happy faces). Our behavioral data showed that the perception of emotional faces greatly influenced participants' subjective reactivity to observation of painful stimulation applied to neutral faces. The participants judged that the actors receiving needle penetration were feeling pain over 90% of the trials when the neutral faces were presented alone. However, this value dropped greatly when the neutral faces were presented in a context of other emotional faces. In addition, subjective ratings of others' pain and self‐unpleasantness associated with the painful stimulation applied to the neutral faces also decreased in the facial emotional context. The behavioral measurements indicated that affective consequences of perceived painful stimulation to the neutral faces were weakened by the presence of facial emotional contexts.
In line with the behavioral results, we found that, when the neutral faces were presented alone, perceived painful stimulation led to increased activation in an important part of the affective pain matrix, namely the ACC, which resulted active in previous fMRI studies of empathy for pain [Botvinick et al.,2005; Gu and Han,2007b; Jackson et al.,2005, 2006; Lamm et al.,2007a; Morrison et al.,2004; Singer et al.,2004]. One novel finding of the present study is that the facial emotional context linked to the presence of painful and happy faces produced two opposite effects on the neural activity associated with the perceived painful stimulation delivered to the neutral faces. The first effect consisted in a suppression of the ACC activation related to the painful stimulation to the neutral faces. The second effect consisted in increased activation in the face areas of bilateral SII during observation of syringes penetrating neutral faces that were intermixed with painful and happy faces. The bilateral SII activations are consistent with the fact that the painful stimulation was applied to the left side of the neutral faces in half of the videos and to the right side of the faces in the other videos.
It is unlikely that the absence of ACC activation in the second subject group reflected these subjects' lack of empathy for pain of others because they showed increased affective responses in the ACC and the anterior insula to painful faces relative to neutral faces, which is consistent with the results reported in the previous work [Botvinick et al.,2005; Lamm et al.,2007a]. Our results suggest that the absence of the somatosensory activity in association with empathy for pain in previous fMRI studies [Benuzzi et al.,2008; Cheng et al.,2007; Jackson et al.,2006; Lamm et al.,2007b; Moriguchi et al.,2007] is not due to a lack of sensitivity of technique in detecting somatosensory activity. Together with the studies that recorded motor‐evoked potentials [Avenanti et al.,2005, 2006], somatosensory‐evoked potentials [Bufalari et al.,2007; Valeriani et al.,2008], and magnetoencephalography [Cheng et al.,2008], our fMRI results confirmed that the somatosensory cortex also plays a pivotal role in the perception of pain in other individuals. Most importantly, our findings indicate that the neural activities in the affective and the sensory parts of the pain matrix are modulated by the emotional contexts in different ways. This implies the existence of the interaction between affective and sensory components of empathic responses.
It has been well documented that first‐hand pain experience engages somatosensory activity in SII [e.g., Raij et al.,2005; Singer et al.,2004]. Studies in humans [Godinho et al.,2006] and mice [Langford et al.,2006] also showed evidence that sensitivity to painful stimulation is enhanced by perceived pain in others. In addition, the amplitudes of somatosensory‐evoked potentials elicited by painful stimulation were increased when human subjects perceived others in painful rather than nonpainful conditions [Bufalari et al.,2007; Godinho et al.,2006]. These results indicate that perception of others in pain increases pain sensitivity by enhancing somatosensory responses to one's own first‐hand painful stimulation. Such modulation of somatosensory activity by perceived pain occurred quickly after sensory stimulation (e.g., within a few hundred milliseconds). Our fMRI results, however, imply long‐latency inhibition of SII activity to perceived pain by emotional contexts of facial expressions given the long latency of blood oxygen level‐dependent signal. Similar interactions between the affective and sensory nodes of the pain matrix during empathy were observed in a previous fMRI study where directing subjects' attention to sensory rather than affective consequences of painful stimulation resulted in decreased ACC activity but increased sensorimotor activity [Lamm et al.,2007b]. While the ACC supports the affective component of empathy for pain [Singer et al.,2004], it is also involved in regulation of subjective feelings of pain‐related unpleasantness [Bush et al.,2000] and in shifting perspective from others to the self during empathy for pain [Jackson et al.,2006]. As we found decreased ACC activity that was accompanied by increased activity in SII to neutral faces when presented in a facial emotional context, it may be speculated that the ACC possibly both supports the affective component of empathy (i.e., sharing others' feelings and producing emotional responses) and underlies the modulation of sensory component of empathy mediated by SII. The interactions between the sensory and affective components of empathic responses may help to understand human social behaviors. For instance, evolutionary psychological research suggests that empathy is a candidate mechanism to underlie altruistic behaviors in response to another's pain, need, or distress [de Waal,2008]. Apparently, enhancement of pain sensitivity is a great disadvantage to one's own action (e.g., mild stimulation may produce strong subjective feeling of pain) and thus does not help to conduct efficient altruistic behaviors. While affective empathic response may serve to generate the motivation to help others [de Waal,2008], inhibition of the somatosensory activity induced by affective empathic responses, as suggested by our fMRI results, may help to implement altruistic behaviors efficiently.
The interaction between different subunits of the pain matrix during the first‐hand pain experience has been reported in previous research. For instance, Valet et al., [2004] found that the ACC exerts top‐down influences on the periaqueductal gray and posterior thalamus to gate pain modulation during distraction. The descending influences from the ACC may elicit inhibition of nocioceptive transmission via brainstem structures [Tracey and Mantyh,2007]. How does the ACC interact with the somatosensory cortex during empathy for pain? Anatomical connections of the ACC include most of the sensory cortex including the somatosensory cortex [see Öngür and Price,2000 for review]. The functional connectivity between these brain areas also increased during the first‐hand pain experience and psychologically induced pain [Raij et al.,2005]. In view of this, it may be proposed that the ACC could modulate the somatosensory activity via direct connectivity. Alternatively, since the ACC plays a key role in descending pain modulation, ACC activation induced by perceived pain may first modulate subcortical structures [e.g. lateral thalamic nuclei, Stevens et al.,1993], which in turn modulate the SII activity. Although we tried to conduct functional connectivity analyses to assess these possibilities, we failed to find reliable signal changes in any brain areas that index functional connectivity changes as a function of stimulus valence (painful vs. nonpainful). Thus, these possible mechanisms need to be investigated in future work.
The present study also showed that the somatosensory activity induced by perceived pain decreased when painful stimulation was applied to emotional faces, particularly when faces showed emotions (e.g. happiness) conflicting with those supposedly generated by painful stimulation (i.e., painful expression). This result suggests that the pain matrix integrates information from both the valence of stimulation and the facial expression. However, facial expressions seem to dominate our understanding of others' emotional states. The happy facial expression weakened empathic neural responses by either withdrawing attention away from the painful stimulation (i.e., needle penetration) or deteriorating the reality of painful stimulation, as both mechanisms modulate empathy for pain [Gu and Han,2007b]. Similarly, the needle penetration applied to a painful face failed to induce increased activation of the pain matrix relative to Q‐tip touching to the painful face, possibly because either the painful expressions dominate the observed painful stimulation that cannot thus further modulate the activity of the pain matrix or because the painful expression may result in the interpretation of the Q‐tip touch as painful. Previous studies showed that perception of others in pain activates not only the ACC but also the lateral prefrontal cortex [Gu and Han,2007b; Jackson et al.,2005, 2006; Lamm et al.,2007a; Ochsner et al., 2008]. Similarly, we found increased activation in these brain areas associated with painful expressions. However, subjective feelings concerning others pain correlated positively with ACC activity [Jackson et al.,2005; Saarela et al.,2007], but negatively with lateral prefrontal activity in the current work. Such differential correlation patterns suggest that the lateral prefrontal cortex is likely to engage in emotional regulation, which plays an important role in empathy [Decety,2006], more than in the inhibition of somatosensory activity.
Finally, we found that the prefrontal activity differentiating between painful and neutral faces with Q‐tip touching correlated negatively with the subjective scores of the intensity of pain in others in the second subject group. Similarly, a prior fMRI study reported that (ventral) prefrontal cortex negatively correlate with activity in the ACC and personal distress felt during social exclusion [Eisenberger et al.,2003]. Taken together, these brain imaging results suggest that the prefrontal cortex may be involved in emotional regulation when suffering from physical pain and social exclusion.
CONCLUSION
Our findings provide the first piece of neuroimaging evidence that empathic neural responses to others' pain are modulated by emotional contexts. The pattern of activity modulation in the ACC and SII by the facial emotional context of the stimuli implies that there is an interaction between the affective and sensory parts of the pain matrix during empathy for pain. Our fMRI findings suggest that the ACC not only functions to support the affective responses during empathy but may work to inhibit the somatosensory activity during empathy as well. Such interactions between the sensory and affective components of the pain matrix during empathy may serve to support efficient altruistic actions. It should be noted that our proposed model of the affective‐sensory relationship is speculative. Future research may investigate how such relationship may be realized by examining mutual interactions between different parts of the pain matrix.
Acknowledgements
The authors thank Sook‐Lei Liew for helpful comments on an early draft of this article.
REFERENCES
- Avenanti A,Bueti D,Galati G,Aglioti SM( 2005): Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8: 955–960. [DOI] [PubMed] [Google Scholar]
- Avenanti A,Paluello IM,Bufalari I,Aglioti SM( 2006): Stimulus‐driven modulation of motor‐evoked potentials during observation of others' pain. Neuroimage 32: 316–324. [DOI] [PubMed] [Google Scholar]
- Benuzzi F,Lui F,Duzzi D,Nichelli PF,Porro CA( 2008): Does it look painful or disgusting? Ask your parietal and cingulate cortex. J Neurosci 28: 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M,Jha AP,Bylsma LM,Fabian SA,Solomon PE,Prkachin KM ( 2005): Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 25: 312–319. [DOI] [PubMed] [Google Scholar]
- Bufalari I,Aprile T,Avenanti A,Di Russo F,Aglioti SM ( 2007): Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Cheng Y,Lin CP,Liu HL,Hsu YY,Lim KE,Hung D,Decety J ( 2007): Expertise modulates the perception of pain in others. Curr Biol 17: 1708–1713. [DOI] [PubMed] [Google Scholar]
- Cheng Y,Yang CY,Lin CP,Lee PR,Decety J ( 2008): The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage 40: 1833–1840. [DOI] [PubMed] [Google Scholar]
- Decety J ( 2006): Human empathy. Jpn J Neuropsychol 22: 11–33. [Google Scholar]
- Decety J,Lamm C ( 2006): Human empathy through the lens of social neuroscience. Scientific WorldJournal 6: 1146–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM ( 2008): Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol 59: 279–300. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI,Lieberman MD,Williams KD ( 2003): Does rejection hurt? An fMRI study of social exclusion. Science 302: 290–292. [DOI] [PubMed] [Google Scholar]
- Fan Y,Han S ( 2008): Temporal dynamic of neural mechanisms involved in empathy for pain: An event‐related brain potential study. Neuropsychologia 46: 160–173. [DOI] [PubMed] [Google Scholar]
- Godinho F,Magnin M,Frot M,Perchet C,Garcia‐Larrea L ( 2006): Emotional modulation of pain: Is it the sensation or what we recall? J Neurosci 26: 11454–11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X,Han S ( 2007a): Neural substrates underlying evaluation of pain in actions depicted in words. Behav Brain Res 181: 218–223. [DOI] [PubMed] [Google Scholar]
- Gu X,Han S ( 2007b): Attention and reality constraints on the neutral processes of empathy for pain. Neuroimage 36: 256–267. [DOI] [PubMed] [Google Scholar]
- Han S,Fan Y,Mao L ( 2008): Gender difference in empathy for pain: An electrophysiological investigation. Brain Res 1196: 85–93. [DOI] [PubMed] [Google Scholar]
- Jackson PL,Brunet E,Meltzoff AN,Decety J ( 2006): Empathy examined through the neural mechanisms involved in imaging how I feel versus how you feel pain. Neuropsychologia 44: 752–761. [DOI] [PubMed] [Google Scholar]
- Jackson PL,Meltzoff AN,Decety J ( 2005): How do we perceive the pain of others? A window into the neural processes involved inempathy. NeuroImage 24: 771–779. [DOI] [PubMed] [Google Scholar]
- Lamm C,Batson CD,Decety J ( 2007a): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19: 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C,Nusbaum HC,Meltzoff AN,Decety J ( 2007b): What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE 2: e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ,Crager SE,Shehzad Z,Smith SB,Sotocinal SG ( 2006): Social modulation of pain as evidence for empathy in mice. Science 312: 1967–1970. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y,Decety J,Ohnishi T,Maeda M,Mori T,Nemoto K,Matsuda H,Komaki G ( 2007): Empathy and judging other's pain: An fMRI study of alexithymia. Cereb Cortex 9: 2223–2234. [DOI] [PubMed] [Google Scholar]
- Morrison I,Lloyd D,di Pellegrino G,Roberts N ( 2004): Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cogn Affect Behav Neurosci 4: 270–278. [DOI] [PubMed] [Google Scholar]
- Mu Y,Fan Y,Mao L,Han S ( 2008): Event‐related theta and alpha oscillations mediate empathy for pain. Brain Res 1234: 128– 136. [DOI] [PubMed] [Google Scholar]
- Öngür D,Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Preston SD,de Waal FBM ( 2002): Empathy: Its ultimate and proximate bases. Behav Brain Sci 25: 1–72. [DOI] [PubMed] [Google Scholar]
- Raij TT,Numminen J,Närvänen S,Hiltunen J,Hari R ( 2005): Brain correlates of subjective reality of physically and psychologically induced pain. Proc Natl Acad Sci USA 102: 2147– 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela MV,Hlushchuk Y,Williams AC,Schurmann M,Kalso E,Hari R ( 2007): The compassionate brain: Humans detect intensity of pain from another's face. Cereb Cortex 17: 230–237. [DOI] [PubMed] [Google Scholar]
- Singer T ( 2006): The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev 30: 855–863. [DOI] [PubMed] [Google Scholar]
- Singer T,Seymour B,O'Doherty J,Kaube H,Dolan RJ,Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Singer T,Seymour B,O'Doherty JP,Stephan KE,Dolan RJ,Frith CD ( 2006): Empathic neural responses are modulated by the perceived fairness of others. Nature 439: 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RT,London SM,Apkarian AV ( 1993): Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res 631: 241–246. [DOI] [PubMed] [Google Scholar]
- Tracey I,Mantyh PW ( 2007): The cerebral signature for pain perception and its modulation. Neuron 55: 377–391. [DOI] [PubMed] [Google Scholar]
- Valeriani M,Betti V,Le Pera D,De Armas L,Miliucci R,Restuccia D,Avenanti A,Aglioti SM ( 2008): Seeing the pain of others while being in pain: A laser‐evoked potentials study. Neuroimage 40: 1419–1428. [DOI] [PubMed] [Google Scholar]
- Valet M,Sprenger T,Boecker H,Willoch F,Rummeny E,Conrad B,Erhard P,Tolle TR ( 2004): Distraction modulates connectivity of the cingulo‐frontal cortex and the midbrain during pain—An fMRI analysis. Pain 109: 399–408. [DOI] [PubMed] [Google Scholar]


