Abstract
Recent theories of selective attention assume that the more attention is required by a task, the earlier are irrelevant stimuli filtered during perceptual processing. Previous functional MRI studies have demonstrated that primary visual cortex (V1) activation by peripheral distractors is reduced by higher task difficulty at fixation, but it remains unknown whether such changes affect initial processing in V1 or subsequent feedback. Here we manipulated attentional load at fixation while recording peripheral visual responses with high‐density EEG in 28 healthy volunteers, which allowed us to track the exact time course of attention‐related effects on V1. Our results show a modulation of the earliest component of the visual evoked potential (C1) as a function of attentional load. Additional topographic and source localization analyses corroborated this finding, with significant load‐related differences observed throughout the first 100 ms post‐stimulus. However, this effect was observed only when stimuli were presented in the upper visual field (VF), but not for symmetrical positions in the lower VF. Our findings demonstrate early filtering of irrelevant information under increased attentional demands, thus supporting models that assume a flexible mechanism of attentional selection, but reveal important functional asymmetries across the VF. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: attention, C1, EEG, V1, vision
INTRODUCTION
One of the most long‐standing discussions in cognitive psychology and cognitive neuroscience concerns the locus of attentional selection during perception [Broadbent, 1958; Mangun, 1995; Treisman, 1969]. Over the last decade, the load theory of selective attention, as proposed by Lavie [ 1995; Lavie et al., 2004; Lavie and Tsal, 1994], has received increasing research interest, as it integrates a range of disparate findings obtained with different experimental paradigms. According to this model, the locus of selection of perceptual information is not fixed at either early or late stages of perception, but varies depending on the amount of concurrently presented information and the cognitive demands associated with its processing. The bottleneck of attentional selection is thus thought of as an adaptive filtering mechanism which prevents cognitive resources from being overburdened, while at the same time ensuring a maximum intake of information under varying conditions.
Although Lavie's [ 1995] original concept of perceptual load was tested using either stimulus displays containing different amounts of information or the same stimulus displays with different amounts of cognitive processing, the latter type of manipulation is now commonly referred to as attentional load [Bahrami et al., 2007; Rees et al., 1997; Schwartz et al., 2005]. Results from previous fMRI studies [Bahrami et al., 2007; O'Connor et al., 2002; Pinsk et al., 2004; Schwartz et al., 2005] suggest that manipulations of attentional load may affect activity in several regions of the human visual cortex, including primary visual cortex (V1). Although these findings provide strong evidence for flexible mechanisms of attentional selection, it is still debated whether top‐down influences impact on information processing from the earliest stages in the cortex, as reported in a number of animal studies [Crist et al., 2001; for review, see Gilbert and Sigman, 2007], or whether modulations of early sensory cortex activity are the result of feedback influences from later stages of processing, which may operate on and reshape the still‐activated representations in lower‐level areas [Foxe and Simpson, 2002; Hupe et al., 1998; Lamme and Roelfsema, 2000; Martinez et al., 1999].
Evidence for the latter view comes from a large number of studies in which the earliest part of the visual evoked potential (VEP) was shown to be influenced only by physical stimulus characteristics, but not by manipulations of spatial attention [Handy et al., 2001; Heinze et al., 1994; Martinez et al., 1999; Noesselt et al. 2002]. Nevertheless, several recent studies have demonstrated that even the earliest cortical stages of visual processing as measured with EEG may be affected by factors other than simple visual features. For instance, the amplitude of the earliest component of the VEP, the C1 [Clark et al., 1995; Jeffreys and Axford, 1972], can be modified by emotional content [Halgren et al., 2000; Pourtois et al., 2004] and emotional associations [Stolarova et al., 2006] of visual stimuli, as well as following perceptual learning [Pourtois et al., 2008b]. In addition, one recent study suggested that the C1 component may be modulated by spatial attention [Kelly et al., 2008], unlike previously assumed [Martinez et al., 1999]. So far, this single study stands out as a striking exception to the lack of attentional effects typically reported for C1 responses.
In the present study, we sought to test the hypothesis that early visual cortex activity, as indexed by the retinotopic C1 component, may be affected by attentional load. This hypothesis was based on the combined evidence from previous fMRI studies in humans [Bahrami et al., 2007; O'Connor et al., 2002; Pinsk et al., 2004; Schwartz et al., 2005] demonstrating an influence of attentional load on V1 activity (but not whether attention affected early or late visual processing in V1), and animal electrophysiology [Crist et al., 2001; Gilbert et al., 2000; Ito and Gilbert, 1999] showing that attention can affect the earliest stages of visual information processing. Taking advantage of the high temporal resolution offered by EEG, we aimed to test whether early effects of attention on V1 activity predicted from the load theory of selective attention can also be observed in humans. We reasoned that previous failures to find such an effect may have been due to the high variability of visual cortex functional anatomy [Amunts et al., 2000; Dougherty et al., 2003] combined with stimulation protocols not optimized for eliciting clear V1 responses. We therefore adapted the paradigm employed by Schwartz et al. [ 2005] and recorded EEG responses to large‐scale, high‐contrast distractors presented at different locations in the peripheral visual field (VF) while subjects performed either an easy or a highly demanding task at fixation. Subjects were tested either in the upper or the lower VF. Our results reveal that attentional load modulates C1 amplitude for irrelevant visual distractors. However, these effects differed as a function of the part of the VF tested, suggesting asymmetries in attentional influences across the VF.
MATERIALS AND METHODS
Subjects
A total of 28 subjects (between 22 and 40 years old) were tested, 14 in the upper VF (11 male) and 14 in the lower VF (11 female). All of them had normal or corrected‐to‐normal vision and provided written informed consent. None of them reported any previous neurological or psychiatric disease. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee.
Stimuli
Stimuli were created using Cogent (http://www.vislab.ucl.ac.uk), a MATLAB toolbox allowing precise timing and synchronization with the EEG system, and presented on a 17″ CRT screen (viewing distance 40 cm, refresh cycle 60 Hz). A rapid serial visual presentation task consisting of differently colored (six colors) and differently oriented (two orientations) T‐shapes was presented at fixation (stimulus duration 250 ms; interstimulus interval 900–1243 ms), either at the bottom (upper VF group) or at the top of the screen (lower VF group). Task‐irrelevant arrays of white horizontal line elements were flashed in the periphery for 250 ms (8.7° × 37.8° of visual angle; Fig. 1A), either close to fixation or further away (vertical distance to the center of distractor 7.3° and 17.6°, respectively). These distractors followed targets after 250–493 ms. The screen background remained black throughout the experiment (Fig. 1B).
Figure 1.
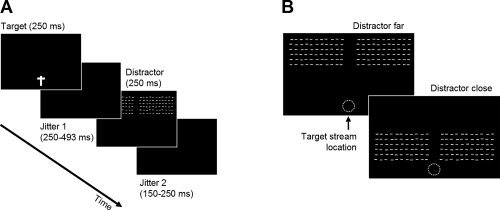
Experimental stimuli. A: Time‐course of a single trial. On each trial, subjects had to detect either a single feature (color) or a feature conjunction (color and orientation of the T‐shape) at fixation while distractors were presented unpredictably. A jitter was introduced between onsets of the central task stimuli and peripheral distractors in order to distinguish between neural responses elicited by each type of event. B: Distractors were presented in the periphery, either close to or far from fixation (denoted by a white dotted circle which was not shown in the experiment). Distractors were always irrelevant to the task and subjects were instructed to ignore them. Distractors for upper VF are shown; for subjects tested in lower VF, the display was inverted.
The manipulation of distractor location was introduced to replicate the findings of Schwartz et al. [ 2005], who observed suppression of activity elicited by a distracting stimulus close to fixation, but less suppression in more eccentric areas of the VF. However, ERPs elicited by more eccentric distractors turned out to be difficult to interpret, with only two subjects in the upper and six subjects in the lower VF group showing a clear C1. We therefore limited our analyses of distractor‐related activity to stimuli presented close to fixation, which elicited a clear C1 in all subjects and conditions.
Procedure
Subjects were placed in a quiet, dimly lit, and electrically shielded recording booth. Four blocks of 410 trials each were presented. At the beginning of alternating blocks, participants were instructed to press the space‐bar of a standard computer keyboard only if they saw either (i) an upright or upside‐down red T‐shape (pop‐out detection, low attentional load) or (ii) an upright yellow or an upside‐down green T‐shape (conjunction discrimination, high attentional load). The two tasks alternated between blocks, with the starting condition counterbalanced across participants. Subjects were instructed to respond as correctly and as rapidly as possible. Pseudo‐random trains of stimuli were created for each block of 410 trials; about 32 of these trials were target trials requiring a motor response. In each block, 62 distractors were presented in each eccentricity condition, 46 of which were uncontaminated by target‐related motor activity. The large number of nondistractor trials was required to generate a strong and stable attentional set from to the central load task, ensuring a valid measurement of attentional load, and also to avoid visual adaptation/habituation to the distractors. Instructions stressed that randomly occurring distractors in the periphery were task‐irrelevant and to be ignored. Each block lasted approximately 10 min, including a short break after half of the trials had been completed. Figure 1B depicts the sequence of visual events forming a single trial.
Data Recording and Analysis
Scalp‐EEG was recorded from 62 Ag/AgCl electrodes (Neuroscan, Synamps, El Paso, TX) positioned according to the extended international 10–20 EEG system [Oostenveld and Praamstra, 2001]. Signals were amplified at 30 K and band‐pass filtered between 0.01 and 100 Hz; a 50 Hz notch‐filter was applied to filter line noise. Horizontal and vertical electro‐oculograms (EOG) were monitored using four bipolar electrodes. Both EEG and EOG were acquired continuously at 500 Hz.
Using Brain Vision Analyzer 1.05 (Brain Products, Munich, Germany), eye‐blink artifacts were semiautomatically corrected using the procedure described by Gratton et al. [ 1983] and a 0.5 Hz high‐pass filter was applied. Epochs from −100 ms to +600 ms around stimulus‐onset were extracted and baseline‐corrected for the 100 ms preceding stimulus‐onset. Epochs with EEG or residual EOG exceeding ±80 μV were rejected. Single‐trial VEPs were then averaged and low‐pass filtered at 30 Hz and the C1, P1, and N1 components were semiautomatically identified based on their distinctive polarities, latencies, and topographic properties. Their peak amplitudes and latencies were measured in each participant at electrode sites determined from the grand averages.
We then tested for topographical differences between load conditions during these components, using a microstate segmentation analysis [Pasqual‐Marqui et al., 1995] as implemented in the software Cartool (http://brainmapping.unige.ch). This analysis is based on the assumption that while a given distribution of voltage values across the scalp may reflect any combination of distributed neural generators, different distributions necessarily imply different neural generators [Lehmann and Skrandies, 1980]. It has been demonstrated that voltage distributions (or voltage maps) do not change randomly over the course of an ERP but remain stable for several milliseconds, reflecting so‐called EEG microstates which in turn are assumed to reflect different stages of information processing [Michel et al., 2001; Pourtois et al., 2005, 2008a]. To detect such microstates, voltage maps corresponding to each time‐frame of a grand‐average ERP are subjected to a K‐means spatio‐temporal cluster analysis which segments the data into periods of stable topographical patterns, varying only in intensity over time [Pasqual‐Marqui et al., 1995]. Voltage maps obtained from the grand averages are then fitted back to the data of individual subjects, to allow for statistical comparison between conditions based on several fit indices, such as the duration and onset time of a dominant map as well as the global explained variance (GEV), the latter being an estimate of the goodness of fit. We used the following standard settings [cf. Michel et al., 2001; Pasqual‐Marqui et al., 1995]: A K‐Means algorithm was run on the first 300 ms poststimulus of grand averages from both load conditions, separately for upper and lower VF groups. Individual microstates were considered as reliable if they persisted for at least three time‐frames (i.e., 6 ms). Analyses were calculated using 5–25 initial clusters and the optimal number of clusters was determined objectively using both cross‐validation [Pasqual‐Marqui et al., 1995] and Krzanowski‐Lai [Tibshirani and Walther, 2005] criteria. Fitting onto individual subject data was then performed for periods showing significant effects of task conditions.
Finally, a local auto‐regressive average (LAURA) procedure was employed to estimate electrical sources in the brain volume corresponding to the scalp topographies identified by the segmentation procedure [Grave de Peralta Menendez et al., 2004]. This distributed source localization analysis does not use any a priori assumption on the number and position of neural generators, but determines the most likely configuration of activity simultaneously in a large number of solution points (4,024 in our case) placed throughout the cortical grey matter. We opted for this method because it allows for a flexible spatial distribution of activations, as elicited by the large‐scale peripheral distractors used.
RESULTS
Behavioral Performance
Accuracy data were analyzed using nonparametric Friedman Analysis of Variance [Friedman, 1937], as absolute numbers of errors were low, with none of the participants committing more than five misses or more than seven false alarms per block of 410 trials (∼32 of which were true targets requiring a motor response). Results demonstrated a significant effect of Attentional Load. Both misses [χ2(3) = 10.4, P = 0.014] and false alarms [χ2(3) = 48.2, P < 0.001] were more frequent under high load, underlining the increased difficulty of this condition. On the other hand, Bonferroni‐corrected Mann‐Whitney tests did not uncover any significant differences on either of the two accuracy measures between the upper (0.93 misses/1.20 false alarms on average) and lower VF (1.14/2.21) groups.
Reaction times (RTs) for correctly detected targets were analyzed using repeated‐measures ANOVAs with Attentional Load (high/low) and Block (first/second) as within‐subjects factors and VF (upper/lower) as between‐subjects factor. Again, significant main effects were found for Attentional Load [low (mean ± SE): 478 ± 10 ms, high: 623 ± 11 ms; F(1, 26) = 367.6, P < 0.001, partial η2 = 0.934] and Block [first: 546 ± 11, second: 556 ± 10 ms; F(1, 26) = 4.6, P = 0.042, partial η2 = 0.150]. The effect of Block may reflect fatigue, but note that this effect is based on a very small difference in RTs (∼10 ms) compared with the effect of Attentional Load (∼150 ms).
Although the error rate was low (as required by the task instructions), it is remarkable that both error rates and RT measurements showed significant effects of attentional load, thus confirming that task instructions successfully modulated demands on attentional resources.
Central Target VEPs
To characterize activity induced by the task at fixation, we first analyzed trials where the central task did not require a motor response and was not followed by a peripheral distractor. There were approximately 390 such trials in each load condition, 25–33% of which were excluded during data preprocessing. (Note that ERPs to imperative target stimuli were not computed due to the small number of trials).
Grand‐averaged data for the central stimulus did not show a C1 component, as would be expected following central presentation in the VF [Clark et al., 1995; Jeffreys and Axford, 1972]. Based on the grand‐average topographies, we selected electrodes P3‐P8 and P05‐PO8 for analysis of the P1 component. Peak amplitude data were entered into a repeated‐measures ANOVA with Attentional Load (low/high) as within‐subjects factor and VF (upper/lower) as between‐subjects factor. In all peak analyses, data were collapsed across the two hemispheres (because hemispheric asymmetries were not relevant for our study) as well as across electrodes (because changes in voltage topographies were examined separately, see Topographic Analyses). Results indicated no differences between peak amplitudes of P1 under low versus high load (P = 0.19) or for lower versus upper VF groups (F < 1). P1 peak latencies were also unaffected by these experimental factors (both F < 1).
Analyses of the N1 component were conducted on the same electrodes as for P1, that is, P3‐P8 and PO5‐PO8, and with the same factors. Peak amplitudes were modulated by Attentional Load, as expected due to changes in visual discrimination demands [Hopfinger and West, 2006], with more negative voltages observed under high load [−3.5 ± 0.7] than under low load [−3.0 ± 0.68 μV; F(1, 26) = 5.94, P = 0.022, partial η2 = 0.186]. There was no effect of VF group on peak amplitudes and no effect of Attentional Load or VF on N1 latencies (all F < 1).
Figure 2 illustrates the grand averages under low‐ and high‐load conditions for upper VF subjects. The main effect of Attentional Load on peak amplitudes was qualitatively similar in the lower VF group. Additional analysis using peak‐to‐peak measurements (N1 minus P1 amplitudes [cf. Picton et al., 2000]) as the dependent variable confirmed these results, with the main effect of Attentional Load even more significant (P = 0.001) than when simple peak amplitude measurements were used, and again no interaction between load and VF group.
Figure 2.
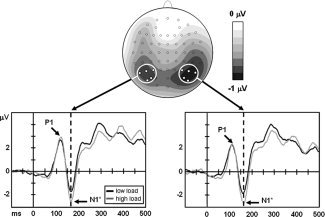
Grand averages elicited by central stimuli (task‐relevant but nontargets), for subjects tested in upper VF. Top: Difference topography (high minus low load) at the time of the N1 peak (∼165 ms). Electrode sites included in statistical analyses of P1 and N1 components are highlighted. Bottom: Grand averages across the indicated electrodes over left and right hemispheres, respectively. *P < 0.05.
Peripheral Distractor VEPs
As the C1 reverses polarity with upper versus lower VF stimulation [Clark et al., 1995; Jeffreys and Axford, 1972], a difference score was computed between the two load conditions and then used for combined analyses in the two groups of subjects (with scores from lower VF subjects sign‐inverted). Based on the grand‐average topographies, we selected a 3 × 2 electrode grid for C1 peak analyses: CP1, CPz, CP2, P1, Pz, and P2. Although topographies for upper and lower VF stimulation were not perfectly equivalent, the maximum of the C1 component was captured well with these leads in both groups (see Fig. 3).
Figure 3.
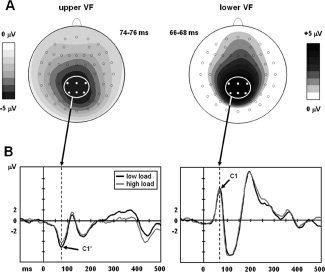
C1 grand average topographies (A) and ERPs (B) in response to distractors close to fixation. Data from subjects tested in upper VF are shown on the left, and those tested in lower VF on the right. Topographies are shown for low attentional load. ERPs in (B) are averages across the electrodes indicated in (A). *P < 0.05.
A clear C1 was evoked in all 28 subjects by distractors close to fixation (see Materials and Methods). Again, we performed an ANOVA with Attentional Load (low/high) as within‐subjects and VF (upper/lower) as between‐subjects factor. Results showed a significant Attentional Load × VF interaction [mean differences: 0.95 ± 0.40 (upper VF) and −0.26 ± 0.40 μV (lower VF); F(1, 26) = 4.72, P = 0.039, partial η2 = 0.154]. Additional analyses conducted separately for each group showed that C1 peak amplitudes were significantly reduced under high attentional load following distractors in upper VF [F(1, 13) = 6.17, P = 0.027, partial η2 = 0.322], but not for distractors in lower VF (F < 1). Peak latencies of the C1 were not affected either by Attentional Load (P = 0.20) or by its interaction with VF (F < 1). The VF factor itself was also nonsignificant (F < 1).
Subsequent VEP components were analyzed separately for upper and lower VF groups. No clear P1 was observed following lower VF stimulation [see also Clark et al., 1995], in line with previous findings with similar stimulus parameters [Pourtois et al., 2008b] and probably due to the overlap between C1 and P1. Instead, in lower VF subjects, we observed a centrally distributed negative component reminiscent of what Clark et al. [ 1995] termed the N90op. Neither peak amplitudes nor latencies of this component (as measured at electrodes CP1, CPz, CP3, P1, Pz, P2, PO3, POz, and PO4) were influenced by Attentional Load (all F < 1). Likewise, no significant effects were obtained for the P1 component in upper VF subjects (measured at electrodes P1, Pz, P2, PO3, POz, PO4, O1, Oz, and O2, in keeping with a more posterior distribution than N90op; all F < 1).
The N1 response to peripheral stimuli was observed bilaterally, in both lower and upper VF subjects, with its peak situated over lateral centro‐parietal electrode sites. For the upper VF group, electrodes CP1‐CP6 and P1‐P6 were selected to analyze the N1 voltage and latency, with Attentional Load as within‐subjects factor, but this showed no significant modulation for either measure of this component (both P > 0.37). Likewise, for the lower VF group, no effects of Attentional Load on N1 amplitude or latency (as measured at electrodes CP3‐CP6 and P3‐P6) were observed (all F < 1). In the latter group, however, evaluation of the N1 was complicated by its partial overlap in time and space with the aforementioned N90op, and we therefore confirmed these results using a microstate segmentation analyses (see later) that allowed us to better disentangle these two negative‐going components.
Topographic Analyses
To complement the peak analyses described above, we investigated scalp voltage distributions in the different experimental conditions across time using a microstate segmentation analysis (see Materials and Methods) as implemented in Cartool (http://brainmapping.unige.ch). Spatio‐temporal K‐means cluster analyses [Pasqual‐Marqui et al., 1995] were first conducted on the grand averages of ERPs to central target stimuli in both load conditions, with the lower and upper VF groups analyzed separately. Results demonstrated a high degree of similarity for the successive microstates between low‐ and high‐load conditions in both groups; with the earliest indication of topographic differences between load conditions arising at ∼250 ms poststimulus. Combined with the peak measures, this topographic analysis suggests that the significant differences in N1 amplitude (peak ∼150 ms) evoked by the central stimuli (see above) was the result of differing strength of activity within the same set of neural generators.
We then tested for differences in voltage topography in response to the peripheral distractors. Based on the results of the peak analyses reported in the preceding section, we conducted separate analyses for upper and lower VF subjects. The segmentations showed a high degree of topographic similarity between low‐ and high‐load conditions, particularly in the lower VF group, where the first indication of topographical differences were present at ∼240 ms poststimulus. In the lower VF group, however, different maps were already observed during the first 100 ms following distractor presentation, in addition to differences at later stages of processing (see Fig. 4). The first difference was seen during the initial period after distractor onset (Maps 1 vs. 2, 0–50 ms), while another difference was also present later (Maps 4 vs. 5, 70–100 ms).
Figure 4.
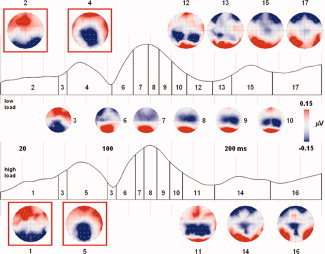
Microstate segmentation (first 300 ms poststimulus) of grand averages elicited by distractors in upper VF. Map numbers are superimposed on global field power traces of low‐ and high‐load conditions. Topographic maps differing between load conditions are displayed at the top and bottom, respectively. For the highlighted maps, a significant Load × Map interaction (P < 0.05) was observed after fitting onto single‐subject ERPs (backfitting was done only for the first 100 ms poststimulus). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We tested these differences by fitting the respective maps obtained by segmentation of the grand‐average data onto individual subject ERPs from each load condition, and then compared the number of time‐frames during which each map was present (TF criterion), as well as the amount of topographical variance explained by each map (GEV criterion). When examining the early succession of topographical maps in the first 100 ms poststimulus onset, we found a significant Load×Map interaction for the TF criterion [F(1, 13) = 9.39, P = 0.009], indicating that Map 1 was present significantly longer under high than low load (P = 0.02, paired t‐test) and vice versa for Map 2 (P = 0.03). The same pattern of results was observed for the GEV criterion, although the interaction term did not quite reach significance (P = 0.057). Analysis of Maps 4 and 5 yielded similar results, with a significant Load × Map interaction for the TF criterion [F(1, 13) = 5.73, P = 0.032] and a marginally significant effect for the GEV criterion (P = 0.09). However, post‐hoc t‐tests indicated that differences between load conditions were significant only for Map 5 (TF criterion, P = 0.02; GEV criterion, P = 0.03).
Taken together, these results suggest very early differences in the configuration of neural generators implicated in the processing of task‐irrelevant distractors as a function of attentional load. Importantly, these differences were most pronounced before C1 or during its rising phase, suggesting that these changes may reflect a possible source of the attentional influences on C1 peak amplitude as reported earlier. Again, no such difference between low‐ and high‐load conditions was observed in lower VF subjects.
Source Localization
Finally, we applied a Local Autoregressive Average [LAURA; cf. Grave de Peralta Menendez et al., 2004] distributed source localization algorithm on the VEPs elicited by distractors in upper VF. Using the approximate time‐windows for which significant topographic differences were observed (0–60 and 60–100 ms poststimulus), we calculated inverse solutions for each subject and condition and subsequently averaged them. As expected, distractor‐related neural activity common to both load‐conditions was primarily observed in early visual areas, with a clear maximum located near the occipital pole (Fig. 5A), although weaker source activity was also found in higher areas along the temporal visual stream. This was the case for both time‐windows of interest, although overall activity was stronger during the 60–100 ms interval than during the first 60 ms (data not shown).
Figure 5.
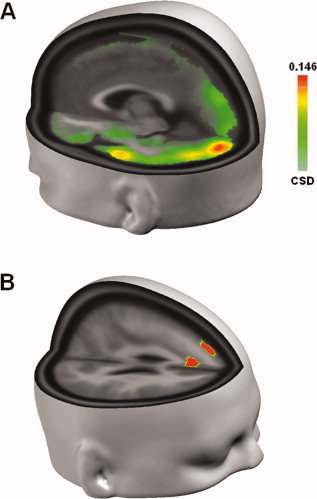
A: Distributed inverse solution results for distractors in upper VF across subjects and conditions. Maximum activity was observed near the occipital pole. Data are for the time‐window during which C1 topography persisted (60–100 ms, see Fig. 4). A similar pattern of activity was observed for the first 60 ms poststimulus, although at lower levels of overall activity. B: Results of paired t‐test on distributed inverse solutions. Activity was compared between load conditions across all cortical generators, and those exceeding a significance criterion of 0.005 and an extent threshold of ≥3 are shown. The same time‐window as in Figure 5 is displayed (no significant differences were observed during the first 60 ms poststimulus). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To pinpoint the neural correlates of load‐induced differences observed in both waveform and microstate analyses, we then compared the activity of each of the 4,024 cortical generators between the two load‐conditions and across subjects, using a paired t‐test with a significance criterion of α = 0.005 and an extent threshold of ≥3 contiguous generators.
As shown in Figure 5B, significant differences were observed for the second time‐window from 60 to 100 ms, where activity in medial and dorsal prefrontal cortex in the left hemisphere was reduced under high attentional load. Considering the extent of these areas as well as their distance from the electrodes used for C1 measurements, it seems unlikely that the observed differences in source activity can explain the amplitude and topographic effects reported earlier. We therefore assume that higher attentional load did not induce differences in the configuration of neural activity in occipital cortex (but amplitude differences only) or that the head model used did not offer sufficient spatial resolution to detect any subtle load‐related differences in early visual cortex. By contrast, differential activity in prefrontal cortex might indicate the recruitment of cognitive resources under high attentional load and a concomitant reduction of activity in default‐ or resting‐state‐networks.
DISCUSSION
Using a well‐established experimental paradigm that induces different degrees of attentional load at fixation [Bahrami et al., 2007; Lavie, 2006; Schwartz et al., 2005], we demonstrate a modulation of visual responses to peripheral distractors involving the very early stages of visual cortex activity in humans. To our knowledge, only a single ERP study [Kelly et al., 2008] recently described an effect of spatial attention on the C1, which is the earliest component of the VEP and is considered to reflect the first volley of sensory information reaching V1 [Foxe and Simpson, 2002; Jeffreys and Axford, 1972]. Here, we show for the first time that C1 can be modulated by attentional load, providing new support for the view that even initial inputs associated with C1 are sensitive to attentional influences. In addition, in our study, topographical analyses suggest that load‐induced changes in C1 amplitude are related to subtle shifts in neural generators even before the component's peak. We note that feedback effects have not been reported on the rising phase of the C1 [Foxe and Simpson, 2002; Vanni et al., 2004], arguing against the notion that our observations are linked to recurrent processing in V1. Finally, distributed source localization results indicate disengagement of medial and dorsal prefrontal cortex with increasing task demands, pointing to possible sources of top‐down effects modulating the processing of task‐irrelevant distractors due to changes in activity in executive frontal networks.
These data go beyond many previous EEG studies suggesting that attention does not affect primary visual cortex activity as indexed by the C1 [Handy et al., 2001; Heinze et al., 1994; Martinez et al., 1999; Noesselt et al., 2002], but are in agreement with a number of animal studies showing early attentional effects on V1 activity that may be unrelated to feedback influences from later stages of processing [Gilbert and Sigman, 2007]. Our findings also converge with previous behavioral [McAnany and Levine, 2007; Rubin et al., 1996; Yeshurun and Carrasco, 1998], electrophysiological [Pourtois et al., 2008b], and fMRI [Liu et al., 2006] evidence suggesting major functional asymmetries across the upper and lower VF, since a significant effect of attentional load was detectable only following peripheral stimulation above the horizontal meridian.
Modulation of Early Visual Processing by Attention
Top‐down attentional modulations of early visual cortex activity, including V1, have been consistently observed in animal studies [Crist et al., 2001; Li et al., 2004] before feedback from later stages of visual processing. Our results provide important evidence that similar effects of attention may be observed in human primary visual cortex. By contrast, previous studies examining primary visual cortex activity in the context of manipulations of attention in humans have usually emphasized effects on P1 and N1, but reported an absence of effects on the C1 [Fu et al., 2008; Handy et al., 2001; Heinze et al., 1994; Martinez et al., 1999; Noesselt et al., 2002]. Possible reasons for these negative findings include the use of relatively small‐scale stimuli [e.g., Handy et al., 2001], ineffective stimulus presentation on the horizontal midline [Martinez et al., 1999], or confounding stimulus differences between attentional conditions [e.g., Fu et al., 2008].
On the other hand, several recent studies reported that early visual cortex activity may be influenced by factors not directly related to attention or physical characteristics of the stimuli. For example, Halgren et al. [ 2000] as well as Pourtois et al. [ 2004] observed C1 modulations as a function of the emotional content of rapidly presented faces. Similarly, using an emotional conditioning procedure, Stolarova et al. [ 2006] found that C1 amplitude was increased for grating patterns previously associated with threat‐related cues. Moreover, we [Pourtois et al., 2008b] previously showed that perceptual learning can also influence C1 amplitude and even more recently, Kelly et al. [ 2008] elegantly demonstrated an effect of spatial attention on C1 amplitudes using an individualized mapping procedure to account for large individual differences in the component's topography. Considering the high variability of human visual cortex functional anatomy [Amunts et al., 2000; Dougherty et al., 2003], these studies suggest that stimulation protocols tuned to the receptive field characteristics of V1 [Pourtois et al., 2004, 2008b; Stolarova et al., 2006] and/or individual mapping procedures such as employed by Kelly et al. [ 2008] are necessary to uncover subtle effects of higher cognitive processes on initial processing in V1.
In the present study, we used large‐scale, high‐contrast stimuli in the peripheral VF to demonstrate that increased attentional load at fixation leads to stronger filtering of distractors and an associated reduction of C1 amplitudes. Our results thus support the load theory of attentional selection [Lavie et al., 2004], according to which increased attentional demands for the central task may lead to a diversion of resources away from peripheral distractors and reduce their processing at early cortical stages. By contrast, previous ERP studies on attentional demands [Heinze et al., 1994; Martinez et al., 1999; Noesselt et al., 2002] often used tasks with a comparatively low impact on processing resources, which could in turn explain the comparatively late stages at which attentional filtering was observed.
Interestingly, Kelly et al. [ 2008] observed increases in C1 amplitude with spatial attention, presumably linked to enhanced contrast perception [cf. Talgar and Carrasco, 2002; Yeshurun and Carrasco, 1998]. Whether C1 amplitude increases or decreases might thus depend on the task‐relevance of the stimuli used to trigger the C1. In the present experiment, as well as in our previous study of perceptual learning [Pourtois et al., 2008b], peripheral stimuli were task‐irrelevant and thus suppressing neural processing of these distractors at early stages—as reflected in reduced C1 amplitudes—would benefit task performance on stimuli presented at fixation. By contrast, in the study of Kelly et al. [ 2008], subtle changes in contrast had to be detected in the peripheral stimuli used to elicit C1 responses. An enhanced representation of these stimuli would aid task performance, in accordance with their finding of higher C1 amplitudes under increased spatial attention. It thus seems that the interaction of visual cortex functional anatomy, experimental stimulus characteristics, and attentional task demands determines whether or not modulations of early primary visual cortex activity can be detected, and whether such modulations are reflected in increased or decreased EEG signals.
From a more general viewpoint, it seems unlikely that attentional effects should be observed across the whole visual cortex except V1. In natural situations, where stimulation does not occur within short and clearly separated time‐windows, information extracted in higher‐order visual cortex is presumably crucial to shape or refine the processing of subsequent stimuli from the earliest cortical stages onwards [Hupe et al., 2001]. The demonstration by Hupe et al. [ 2001] of transient MT/V5 inactivation leading to changes in firing frequency of V1 neurons from the very first time‐bin of activation underlines the functional importance of ongoing top‐down input to early visual cortex [see also Foxe and Simpson, 2002]. The fact that neuronal activity in V1 elicited by the same visual stimuli may change as a function of task demands [Crist et al., 2001] indicates that top‐down influences can affect V1 excitability [see also Bestmann et al., 2007; Ruff et al., 2006]. Although both animal [Mehta et al., 2000] and human studies [Schwartz et al., 2005] suggest that attentional effects are less pronounced at lower levels of the visual cortex hierarchy, it is thus highly plausible that top‐down control plays an important role in shaping sensory processing within early visual areas [Hupe et al., 1998], and our results add an important piece of evidence to the emerging view of primary visual cortex as an adaptive processor [Gilbert and Sigman, 2007; Gilbert et al., 2001] rather than a specialized and inflexible module for the treatment of low‐level visual information.
Recent studies have demonstrated modulations of prestimulus α oscillations by spatial attention, linking them to an active, retinotopically specific process of distractor suppression [Kelly et al., 2006; Rihs et al., 2007]. Future research will have to test whether a similar mechanism may explain distractor suppression in paradigms where spatial attention is fixed but other attentional parameters are manipulated, as in the present study.
Differences in Attentional Effects Across the Visual Field
Manipulation of attentional load in the task performed at fixation elicited the expected pattern of behavioral and electrophysiological effects. Subjects were slower to react and committed more errors under high load, in accordance with earlier reports [Bahrami et al., 2007; Lavie, 1995]; and VEPs elicited by central target stimuli (see Fig. 2) showed enhanced occipito‐parietal N1 amplitudes in this condition, in agreement with numerous studies demonstrating effects of endogenous attention on this component [Doallo et al., 2006; Eimer, 1998; Hillyard and Anllo‐Vento, 1998; Hopfinger and West, 2006; Mangun, 1995; Vogel and Luck, 2000]. Importantly, this attentional modulation of VEPs to central stimuli was similar in upper and lower VF groups, whereas attentional effects on VEPs to the peripheral distractors revealed a clear asymmetry between upper and lower VF, with significant reductions of C1 amplitude only in the former group.
We have previously reported a similar asymmetry in C1 modulation [Pourtois et al., 2008b] and discussed possible sources of this effect. In particular, physiological differences along the upper versus lower hemiretina systems [Lehmann and Skrandies, 1979; Previc, 1990; for review, see Skrandies, 1987] may interact with attentional states in such a way as to produce seemingly contradictory results if the same stimuli are presented in different parts of the VF. This has been elegantly demonstrated by Carrasco and coworkers [Talgar and Carrasco, 2002; Yeshurun and Carrasco, 1998], who found that the same attentional manipulation may lead to performance increases or decreases depending on the eccentricity of stimulation. They interpreted this effect as a consequence of differences in spatial resolution and contrast sensitivity across the retina, with spatial attention being applied to areas of high spatial resolution resulting in reduced perception of low‐resolution stimuli. However, this hypothesis cannot readily explain the asymmetries observed in the present paradigm, where peripheral stimuli were completely irrelevant and thus ignored by the subjects.
Nevertheless, psychophysically relevant differences between upper and lower VF seem a likely explanation for the differential effects observed. We surmise that differences in several physiological properties such as contrast sensitivity, spatial resolution, and conduction velocity may give rise to different degrees of load‐sensitivity in upper and lower VF. This hypothesis is in accordance with the proposal that in humans, ecological constraints should favor higher spatial resolution in lower VF [Previc, 1990; Skrandies, 1987]. Furthermore, given the predominant projections of the upper VF to the ventral temporal stream, and the major role of attention for gating visual processing along object recognition pathways [Chelazzi, 1995], it is possible that attentional filtering might have a stronger impact on the upper than lower VF. Conversely, therefore, stimuli presented in the lower VF may be more resistant to modulation by attentional load, as found in the present study. The fact that Kelly et al. [ 2008] found effects of spatial attention on C1 amplitudes in both upper and lower VF is probably linked to stimulus differences: As noted by Previc [ 1990], asymmetries between upper and lower VF are most pronounced at low spatial frequencies and when large stimuli are used (as in the present study), whereas such asymmetries are much reduced at high spatial frequencies and for smaller stimuli [as employed by Kelly et al., 2008].
CONCLUSION
We have demonstrated a modulation of the first component of the VEP in response to peripheral distractors as a function of attentional load of a task at fixation. The effect was selectively observed in the upper VF. This is the first demonstration of attentional load effects on the very early stages of visual processing in humans, corresponding to the initial inputs into V1. We suspect that previous results showing the C1 to be unaffected by attentional manipulations are related to insufficient load being imposed on the attentional domain under study, differences in physical stimulation between experimental conditions, or individual differences in functional anatomy precluding reliable assessment of C1.
Acknowledgements
The authors would like to thank Christoph Michel and Gregor Thut of the Functional Brain Mapping Lab for helpful discussions; and Rolando Grave de Peralta and Sara Gonzalez of the Electrical Neuroimaging Group for the realistic head model and the inverse solutions used in this work.
REFERENCES
- Amunts K,Malikovic A,Mohlberg H,Schormann T,Zilles K ( 2000): Brodmann's areas 17 and 18 brought into stereotaxic space—Where and how variable? Neuroimage 11: 66–84. [DOI] [PubMed] [Google Scholar]
- Bahrami B,Lavie N,Rees G ( 2007): Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol 17: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S,Ruff CC,Blakemore C,Driver J,Thilo KV ( 2007): Spatial attention changes excitability of human visual cortex to direct stimulation. Curr Biol 17: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE ( 1958): Perception and Communication. London: Pergamon Press. [Google Scholar]
- Chelazzi L ( 1995): Neural mechanisms for stimulus selection in cortical areas of the macaque subserving object vision. Behav Brain Res 71: 125–134. [DOI] [PubMed] [Google Scholar]
- Clark VP,Fan S,Hillyard SA ( 1995): Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp 2: 170–187. [Google Scholar]
- Crist RE,Li W,Gilbert CD ( 2001): Learning to see: Experience and attention in primary visual cortex. Nat Neurosci 4: 519–525. [DOI] [PubMed] [Google Scholar]
- Doallo S,Holguin SR,Cadaveira F ( 2006): Attentional load affects automatic emotional processing: Evidence from event‐related potentials. Neuroreport 17: 1797–1801. [DOI] [PubMed] [Google Scholar]
- Dougherty RF,Koch VM,Brewer AA,Fischer B,Modersitzki J,Wandell BA ( 2003): Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3: 586–598. [DOI] [PubMed] [Google Scholar]
- Eimer M ( 1998): Mechanisms of visuospatial attention: Evidence from event‐related brain potentials. Vis cogn 5: 257–286. [Google Scholar]
- Foxe JJ,Simpson GV ( 2002): Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res 142: 139–150. [DOI] [PubMed] [Google Scholar]
- Friedman M ( 1937): The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Stat Assoc 32: 675–701. [Google Scholar]
- Fu S,Zinni M,Squire PN,Kumar R,Caggiano DM,Parasuraman R ( 2008): When and where perceptual load interacts with voluntary visuospatial attention: An event‐related potential and dipole modeling study. Neuroimage 39: 1345–1355. [DOI] [PubMed] [Google Scholar]
- Gilbert C,Ito M,Kapadia M,Westheimer G ( 2000): Interactions between attention, context and learning in primary visual cortex. Vision Res 40: 1217–1226. [DOI] [PubMed] [Google Scholar]
- Gilbert CD,Sigman M ( 2007): Brain states: Top‐down influences in sensory processing. Neuron 54: 677–696. [DOI] [PubMed] [Google Scholar]
- Gilbert CD,Sigman M,Crist RE ( 2001): The neural basis of perceptual learning. Neuron 31: 681–697. [DOI] [PubMed] [Google Scholar]
- Gratton G,Coles MG,Donchin E ( 1983): A new method for off‐line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55: 468–484. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R,Murray MM,Michel CM,Martuzzi R,Gonzalez Andino SL ( 2004): Electrical neuroimaging based on biophysical constraints. Neuroimage 21: 527–539. [DOI] [PubMed] [Google Scholar]
- Halgren E,Raij T,Marinkovic K,Jousmaki V,Hari R ( 2000): Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex 10: 69–81. [DOI] [PubMed] [Google Scholar]
- Handy TC,Soltani M,Mangun GR ( 2001): Perceptual load and visuocortical processing: Event‐related potentials reveal sensory‐level selection. Psychol Sci 12: 213–218. [DOI] [PubMed] [Google Scholar]
- Heinze HJ,Mangun GR,Burchert W,Hinrichs H,Scholz M,Munte TF,Gos A,Scherg M,Johannes S,Hundeshagen H,Gazzaniga MS,Hillyard SA( 1994): Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Hillyard SA,Anllo‐Vento L ( 1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB,West VM ( 2006): Interactions between endogenous and exogenous attention on cortical visual processing. NeuroImage 31: 774–789. [DOI] [PubMed] [Google Scholar]
- Hupe JM,James AC,Payne BR,Lomber SG,Girard P,Bullier J ( 1998): Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394: 784–787. [DOI] [PubMed] [Google Scholar]
- Hupe JM,James AC,Girard P,Lomber SG,Payne BR,Bullier J ( 2001): Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol 85: 134–145. [DOI] [PubMed] [Google Scholar]
- Ito M,Gilbert CD ( 1999): Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22: 593–604. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA,Axford JG ( 1972): Source locations of pattern‐specific components of human visual evoked potentials. I. Component of striate cortical origin. Exp Brain Res 16: 1–21. [DOI] [PubMed] [Google Scholar]
- Kelly SP,Lalor EC,Reilly RB,Foxe JJ ( 2006): Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95: 3844–3851. [DOI] [PubMed] [Google Scholar]
- Kelly SP,Gomez‐Ramirez M,Foxe JJ ( 2008): Spatial attention modulates initial afferent activity in human primary visual cortex. Cereb Cortex (doi: 10.1093/cercor/bhn022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA,Roelfsema PR ( 2000): The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci 23: 571–579. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 1995): Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform 21: 451–468. [DOI] [PubMed] [Google Scholar]
- Lavie N ( 2006): The role of perceptual load in visual awareness. Brain Res 1080: 91–100. [DOI] [PubMed] [Google Scholar]
- Lavie N,Tsal Y ( 1994): Perceptual load as a major determinant of the locus of selection in visual attention. Percept Psychophys 56: 183–197. [DOI] [PubMed] [Google Scholar]
- Lavie N,Hirst A,de Fockert JW,Viding E ( 2004): Load theory of selective attention and cognitive control. J Exp Psychol Gen 133: 339–354. [DOI] [PubMed] [Google Scholar]
- Lehmann D,Skrandies W ( 1979): Multichannel evoked potential fields show different properties of human upper and lower hemiretina systems. Exp Brain Res 35: 151–159. [DOI] [PubMed] [Google Scholar]
- Lehmann D,Skrandies W ( 1980): Reference‐free identification of components of checkerboard‐evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48: 609–621. [DOI] [PubMed] [Google Scholar]
- Li W,Piech V,Gilbert CD ( 2004): Perceptual learning and top‐down influences in primary visual cortex. Nat Neurosci 7: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T,Heeger DJ,Carrasco M ( 2006): Neural correlates of the visual vertical meridian asymmetry. J Vis 6: 1294–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR ( 1995): Neural mechanisms of visual selective attention. Psychophysiology 32: 4–18. [DOI] [PubMed] [Google Scholar]
- Martinez A,Anllo‐Vento L,Sereno MI,Frank LR,Buxton RB,Dubowitz DJ,Wong EC,Hinrichs H,Heinze HJ,Hillyard SA ( 1999): Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369. [DOI] [PubMed] [Google Scholar]
- McAnany JJ,Levine MW ( 2007): Magnocellular and parvocellular visual pathway contributions to visual field anisotropies. Vision Res 47: 2327–2336. [DOI] [PubMed] [Google Scholar]
- Mehta AD,Ulbert I,Schroeder CE ( 2000): Intermodal selective attention in monkeys. I. Distribution and timing of effects across visual areas. Cereb Cortex 10: 343–358. [DOI] [PubMed] [Google Scholar]
- Michel CM,Thut G,Morand S,Khateb A,Pegna AJ,Grave de Peralta R,Gonzalez S,Seeck M,Landis T ( 2001): Electric source imaging of human brain functions. Brain Res Brain Res Rev 36: 108–118. [DOI] [PubMed] [Google Scholar]
- Noesselt T,Hillyard SA,Woldorff MG,Schoenfeld A,Hagner T,Jancke L,Tempelmann C,Hinrichs H,Heinze HJ ( 2002): Delayed striate cortical activation during spatial attention. Neuron 35: 575–587. [DOI] [PubMed] [Google Scholar]
- O'Connor DH,Fukui MM,Pinsk MA,Kastner S ( 2002): Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5: 1203–1209. [DOI] [PubMed] [Google Scholar]
- Oostenveld R,Praamstra P ( 2001): The five percent electrode system for high‐resolution EEG and ERP measurements. Clin Neurophysiol 112: 713–719. [DOI] [PubMed] [Google Scholar]
- Pasqual‐Marqui RD,Michel CM,Lehmann D ( 1995): Segmentation of brain electrical activity into microstates: Model estimation and validation. IEEE Trans Biomed Eng 42: 658–665. [DOI] [PubMed] [Google Scholar]
- Picton TW,Bentin S,Berg P,Donchin E,Hillyard SA,Johnson R,Miller GA,Ritter W,Ruchkin DS,Rugg MD,Taylor MJ( 2000): Guidelines for using human event‐related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 37: 127–152. [PubMed] [Google Scholar]
- Pinsk MA,Doniger GM,Kastner S ( 2004): Push‐pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol 92: 622–629. [DOI] [PubMed] [Google Scholar]
- Pourtois G,Grandjean D,Sander D,Vuilleumier P ( 2004): Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex 14: 619–633. [DOI] [PubMed] [Google Scholar]
- Pourtois G,Thut G,Grave de Peralta R,Michel C,Vuilleumier P ( 2005): Two electrophysiological stages of spatial orienting towards fearful faces: Early temporo‐parietal activation preceding gain control in extrastriate visual cortex. Neuroimage 26: 149–163. [DOI] [PubMed] [Google Scholar]
- Pourtois G,Delplanque S,Michel C,Vuilleumier P ( 2008a): Beyond conventional event‐related brain potential (ERP): Exploring the time‐course of visual emotion processing using topographic and principal component analyses. Brain Topogr 20: 265–277. [DOI] [PubMed] [Google Scholar]
- Pourtois G,Rauss KS,Vuilleumier P,Schwartz S ( 2008b): Effects of perceptual learning on primary visual cortex activity in humans. Vision Res 48: 55–62. [DOI] [PubMed] [Google Scholar]
- Previc FH ( 1990): Functional specialization in the lower and upper visual‐fields in humans—Its ecological origins and neurophysiological implications. Behav Brain Sci 13: 519–541. [Google Scholar]
- Rees G,Frith CD,Lavie N ( 1997): Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science 278: 1616–1619. [DOI] [PubMed] [Google Scholar]
- Rihs TA,Michel CM,Thut G ( 2007): Mechanisms of selective inhibition in visual spatial attention are indexed by α‐band EEG synchronization. Eur J Neurosci 25: 603–610. [DOI] [PubMed] [Google Scholar]
- Rubin N,Nakayama K,Shapley R ( 1996): Enhanced perception of illusory contours in the lower versus upper visual hemifields. Science 271: 651–653. [DOI] [PubMed] [Google Scholar]
- Ruff CC,Blankenburg F,Bjoertomt O,Bestmann S,Freeman E,Haynes JD,Rees G,Josephs O,Deichmann R,Driver J ( 2006): Concurrent TMS‐fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol 16: 1479–1488. [DOI] [PubMed] [Google Scholar]
- Schwartz S,Vuilleumier P,Hutton C,Maravita A,Dolan RJ,Driver J ( 2005): Attentional load and sensory competition in human vision: Modulation of fMRI responses by load at fixation during task‐irrelevant stimulation in the peripheral visual field. Cereb Cortex 15: 770–786. [DOI] [PubMed] [Google Scholar]
- Skrandies W ( 1987): The upper and lower visual field of man: Electrophysiological and functional differences In: Ottoson D, editor.Progress in Sensory Physiology, Vol. 8 Berlin: Springer; 2–93. [Google Scholar]
- Stolarova M,Keil A,Moratti S ( 2006): Modulation of the C1 visual event‐related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cereb Cortex 16: 876–887. [DOI] [PubMed] [Google Scholar]
- Talgar CP,Carrasco M ( 2002): Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychon Bull Rev 9: 714–722. [DOI] [PubMed] [Google Scholar]
- Tibshirani R,Walther G ( 2005): Cluster validation by prediction strength. J Comput Graph Stat 14: 511–528. [Google Scholar]
- Treisman AM ( 1969): Strategies and models of selective attention. Psychol Rev 76: 282–299. [DOI] [PubMed] [Google Scholar]
- Vanni S,Warnking J,Dojat M,Delon‐Martin C,Bullier J,Segebarth C ( 2004): Sequence of pattern onset responses in the human visual areas: An fMRI constrained VEP source analysis. Neuroimage 21: 801–817. [DOI] [PubMed] [Google Scholar]
- Vogel EK,Luck SJ ( 2000): The visual N1 component as an index of a discrimination process. Psychophysiology 37: 190–203. [PubMed] [Google Scholar]
- Yeshurun Y,Carrasco M ( 1998): Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


