Abstract
Neural correlates of driving and of decision making have been investigated separately, but little is known about the underlying neural mechanisms of decision making in driving. Previous research discusses two types of decision making: reward‐weighted decision making and cost‐weighted decision making. There are many reward‐weighted decision making neuroimaging studies but there are few cost‐weighted studies. Considering that driving involves serious risk, it is assumed that decision making in driving is cost weighted. Therefore, neural substrates of cost‐weighted decision making can be assessed by investigation of driver's decision making. In this study, neural correlates of resolving uncertainty in driver's decision making were investigated. Turning right in left‐hand traffic at a signalized intersection was simulated by computer graphic animation based videos. When the driver's view was occluded by a big truck, the uncertainty of the oncoming traffic was resolved by an in‐car video assist system that presented the driver's occluded view. Resolving the uncertainty reduced activity in a distributed area including the amygdala and anterior cingulate. These results implicate the amygdala and anterior cingulate as serving a role in cost‐weighted decision making. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: decision making, driving, cost, reward, amygdala, fMRI
INTRODUCTION
Driving is a daily activity for many people and an activity involved with life threatening risk. Lack of attention or wrong decision making during driving can cause fatal accidents. According to the US National Highway Traffic Safety Administration, motor vehicle traffic crashes were the leading cause of death for the age group 4 through 34 in 2003. Understanding of underlying neural mechanisms of decision making in driving is important for further development of automobile safety. There are several fMRI studies about driving [Calhoun et al., 2002; Horikawa et al., 2005; Spiers and Maguire, 2007; Uchiyama et al., 2003; Walter et al., 2001]. However, those studies are investigating overall aspects of driving including perception, attention, learning, memory, decision making, and action control. Therefore, there is no neural imaging study that exclusively investigates neural substrates for driver's decision making.
On the other hand, most previous research investigating the neural substrates involved in decision making is based on gambling tasks [e.g., Bechara et al., 1996, 1997, 1999; Cohen et al., 2005; Paulus et al., 2003; Paulus and Frank, 2006; Rogers et al., 1999]. Brain regions often reported for these studies are brain areas related to cognitive process (dorsal anterior cingulate, dorsolateral prefrontal cortex, and parietal cortex) and brain areas related to emotional process (ventromedial prefrontal cortex, amygdala, ventral anterior cingulate, and insula). Ernst and Paulus [2005] divided the decision making process in three stages [(1) assessment of options, (2) execution of an action, and (3) evaluation of an outcome] and proposed that both cognitive and affective brain circuits process these stages differentially. The most famous gambling task is called the Iowa Gambling Task that is developed by Bechara et al. [1994]. The task is designed to simulate real‐life decisions in terms of uncertainty, reward, and punishment. In the task, subjects are asked to choose between options that yield lower gain but smaller future loss (i.e., advantageous options) and those that yield high immediate monetary gain but larger future loss (i.e., disadvantageous options). During the task, normal subjects successfully adopt the advantageous options but individuals with ventromedial or amygdala lesions, who have difficulties in personal and social decision making even though their other intellectual abilities are preserved, often fail to adopt them [Bechara et al., 1994, 1997, 1999, 2003]. Bechara et al. [1998] reported that subjects with dorsolateral lesions performed defectively on a working memory task but not the gambling task and that subjects with ventromedial lesions were impaired on the gambling task but not the working memory task. This finding indicates the dissociation of working memory from decision making and the important role of emotion in decision making [Bechara et al., 2003]. In addition to them, enhanced activity in the ventromedial prefrontal cortex for risk taking decision‐making (i.e., choosing a low probability of winning a high reward) has been found in several neuroimaging studies with normal participants [Cohen et al., 2005; Critchley et al., 2001; Fukui et al., 2005; O'Doherty et al., 2003; Rogers et al., 1999]. In contrast, relatively few neuroimaging studies have reported enhanced activity in the amygdala for decision making in gambling tasks [Cohen et al., 2005; Hsu et al., 2005]. Differential contributions of the ventromedial prefrontal cortex and the amygdala to decision making have been suggested by a brain lesion study [Bechara et al., 1999]. Rushworth et al. [2007] discussed contrasting roles for the anterior cingulate and the ventromedial prefrontal cortex. They propose that the ventromedial prefrontal cortex is involved in reward expectations and the anterior cingulate is involved in cost‐benefit assessments. The role of ventromedial prefrontal cortex in reward anticipation or delay discounting based decision making has been reported by animal [Rudebeck et al., 2006] and neuroimaging [Cohen et al., 2005; Hampton and O'Doherty, 2007; O'Doherty et al., 2003; Rogers et al., 1999] studies. On the other hand, the role of the anterior cingulate in cost‐benefit assessment based decision making has been proposed by animal studies [Rudebeck et al., 2006; Rushworth et al., 2007; Walton et al., 2002, 2003]. Floresco and Ghods‐Sharifi [2007] suggest that the serial transfer of information between the amygdala and anterior cingulate guides response selection in effort‐based decision making. In their animal studies, the cost level was modulated by manipulating effort level: climbing a 30 cm barrier for the high reward or selecting an unoccupied arm for the low reward. Both bilateral inactivation of the amygdala and disconnection between the amygdala and anterior cingulate did not affect the preference for the high reward when the effort levels were the same but reduced the preference for the high reward when it required more effort than the low reward. Their study indicated that the reward anticipation was intact but the cost‐benefit assessment was impaired. These animal studies suggest that amygdala and anterior cingulate are implicated in effort‐based decision making. To our knowledge, there is no neuroimaging study with normal human subjects investigating effort‐based or cost‐based decision making. A recent study investigated neural substrates of decision making in avoiding loosing money [Kim et al., 2006], but the decisions were still based on reward anticipation because participants received $35 to start the task, had reward trials to win money, and did not have to worry about loosing their own money. In contrast, driving involves serious risks but not much rewards. Driving fast without causing accidents can be a psychological reward but the reward is very small considering the cost of causing accidents for most people. Therefore, it is reasonable to assume that the cost assessment is weighted more than reward anticipation for decision making in driving. Since the distinction between reward‐based and cost‐based decision making is not clear cut, we use terms “reward‐weighted” when the reward anticipation is weighted more than the cost assessment and “cost‐weighted” for the opposite.
In this study, neural substrates of driver's decision making were investigated. Specifically, we examined neural correlates of resolving uncertainty (i.e., ambiguity) in driver's decision making. We designed an fMRI experiment that simulates the intelligent system to assist drivers when they are turning right at a signalized intersection. Right‐turn road collision at an intersection is a common type of accident in left‐hand traffic and account for 10% of all traffic accidents in Japan [Traffic Accident Statistics of Japan]. Drivers who try to turn right often collide with oncoming vehicles because their view is occluded by cars at the center of the intersection that try to turn right from the opposite side. To simulate the system, a virtual space of a left‐hand traffic signalized intersection was constructed by 3D computer graphic animation. Driver's view videos and camera's view videos were made of the virtual space. The camera's view videos present perspectives from a traffic signal and provide drivers the view occluded by oncoming traffic. Then, the in‐car video assist system was simulated by presenting a small image of a camera's view video (i.e., auxiliary video) under a big image of a corresponding driver's view video (i.e., main video).
In decision making studies, two types of uncertainty are discussed and are often labeled as risky and ambiguous, respectively. A risky decision is a choice between a low probability of winning a high reward and a high probability of winning a low reward. On the other hand, the probabilities of winning are not informed for an ambiguous decision. Behavioral economics shows that many people prefer known probability choices to unknown probability choices, holding judged probability of outcomes constant [Einhorn and Hogarth, 1985; Ellsberg, 1961]. In a study of reward‐weighted ambiguous decision making, activity in the amygdala and orbitofrontal cortex was positively correlated with degree of ambiguity [Hsu et al., 2005]. The results suggest that neural substrates for risk and ambiguity share a general neural circuit and the corresponding activation levels are related to the degree of uncertainty (i.e., levels of information available to the decision maker). In this study, decision‐making when a driver's view is occluded corresponds to an ambiguity decision (i.e., no information to estimate probability of collision). The uncertainty level will be reduced if the driver can obtain information through the in‐car video assist system. Since driver's decision making is cost weighted, uncertainty level dependent activity is predicted to be present in the amygdala and anterior cingulate. The objective of this study is to explore brain regions that are activated more for the ambiguous condition than for the less ambiguous condition in the driving task. Regions involved with cost‐weighted decision making were investigated by this observation. Enhanced activation in the amygdala and anterior cingulate was expected.
MATERIALS AND METHODS
Participants
Fourteen adults (seven male; 21–46 years of age, mean 27.7) with no neurological or psychiatric history participated in this study. All participants had more than 3 years driving experience and gave written informed consent for experimental procedures approved by the ATR Human Subject Review Committee.
Stimuli and Procedure
Using the software package “DOGA‐LE3β” (DoGA Co., Ltd.), computer graphic (CG) animation sequences that simulate right‐turn across left‐hand traffic at a signalized intersection were designed. Half of the sequences had a truck at the center of the intersection to prevent views from a driver and the other half did not have any obstacles. Each sequence was composed of three parts; entering the intersection (2 s), waiting at the center of theintersection (1, 3, or 5 s), and starting to move forward (1.5 s). Three levels of waiting time were used to jitter response timing. The distances to the nearest vehicle in the on‐coming stream of traffic were also varied in five levels (2.5, 3, 3.5, 4, or 4.5 s in 60 km/h speed) so that participants can not tell how close the nearest vehicle in the on‐coming stream of traffic is (i.e., creating ambiguity when the driver's view is occluded by a big truck). By combining two levels of the obstacle situation, three levels of the waiting duration, and five levels of the distance, 30 CG animation sequences were constructed. Then, videos from two perspectives were generated from them. One perspective was from a driver trying to turn right. The other perspective was from a camera mounted on the traffic signal.
The in‐car video assist system was simulated by presenting a small image of a camera's view video (i.e., auxiliary video) under a big image of a corresponding driver's view video (i.e., main video). The main video consisted of 9 by 12° visual angles and the auxiliary video consisted of 4.5 by 6° visual angles. Four types of experimental conditions were prepared: driver's perspective video when the view is occluded by a truck (DO), driver's perspective video when the view is not occluded (DN), driver's perspective video when the view is occluded with video from the perspective of the camera (DOC), and driver's perspective video when the view is not occluded with video from the perspective of the camera (DNC) (see Fig. 1 for examples). Samples of visual stimuli can be downloaded from our web site (http://www.cis.atr.jp/driving-mov/). The stimuli were presented through Victor's D‐ILA projector onto a rear‐projection screen located behind the subject's head. The screen was viewed with an angled mirror positioned on the head coil. Participants responded to each visual presentation by pressing one of the two buttons on a response box held in their left hand. They were instructed to press the left button if they decided to keep moving forward after the video disappeared and to press the right button if they decided to stop moving. The “keep moving forward” response means either “make a turn” or “inch forward into the intersection to get a better view.” Originally, during preliminary experiments, we were asking subjects to decide “make a turn” or “not make a turn.” However, with this instruction, certain people always chose “not make a turn” options for the DO and DOC condition. We assumed that they responded based on the condition without processing detail information of the visual stimuli. To facilitate active decision making process, we decided to use the “keep moving forward” response instead of the “make a turn” response and participants were instructed that “keep moving forward” did not mean exclusively “make a turn.” During the experiments, outcomes of participants' decisions were not provided. In other words, they never crashed during the experiment. Paulus et al. [2005] have reported that neural activation during assessment of a situation for decision making is critically dependent on previous outcomes. We did not choose to provide the outcomes in order to avoid this influence.
Figure 1.
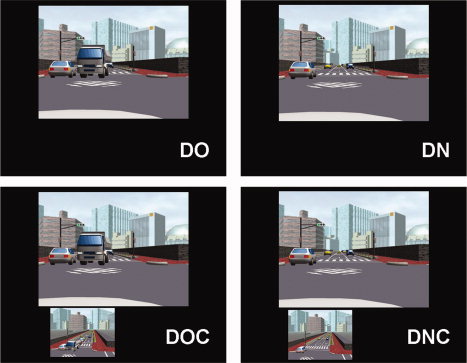
Examples of visual stimuli. DO, driver's perspective video when the view is occluded by a truck; DN, driver's perspective video when the view is not occluded by a truck; DOC, driver's perspective video when the view is occluded with a video from the perspective of the camera; DNC, driver's perspective video when the view is not occluded with a video from the perspective of the camera.
Trials were blocked by condition and each experimental condition block appeared five times in each run. Each experimental block (24 s) was alternated with a baseline block (6 s) in which just a fixation mark was presented. In each experimental block, video stimuli followed by a fixation mark for 1.5 s were presented three times and button responses were collected during these periods (Fig. 2A). For each run, all visual stimuli were pseudo randomly presented only once and there were 15 visual stimuli for each condition. Each participant performed two runs (i.e., 15 × 2 trials for each condition) and the order of experimental conditions was randomized across runs (Fig. 2B). Each run took about 10 min. Participants were asked to respond quickly to minimize differences in the hemodynamic response resulting from long response times and practiced outside of the scanner on a subset of the stimuli to familiarize themselves with the task.
Figure 2.
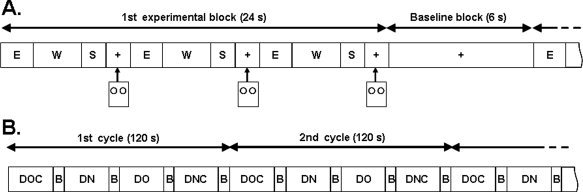
A: Block design for fMRI imaging. Each visual stimulus was composed of three parts; E, entering the intersection (2 s); W, waiting at the center of the intersection (1, 3, or 5 s); S, starting to move forward (1.5 s), and was followed by a 1.5 s fixation mark. Subjects responded by pressing a button during the fixation period. B: An example of an experimental condition order. The order of experimental blocks was randomized across runs. Each run took about 10 min. DO, DN, DOC, and DNC are experimental blocks (24 s); B is a baseline block (6 s).
MRI Data Acquisition and Preprocessing
For structural and functional brain imaging, Shimadzu‐Marconi's Magnex Eclipse 1.5T PD250 was used at the ATR Brain Activity Imaging Center. Functional T2*‐weighted images were acquired using a gradient echo planar imaging sequence (TR = 3000 ms, TE = 49 ms, flip angle = 90°, field of view = 192 mm × 192 mm, matrix size = 64 × 64 pixels, 30 slices, slice thickness = 4 mm, slice gap = 1 mm, 204 scans per run). The first four scans from each run were discarded to allow for T1 equilibration effects. Images were preprocessed using programs within SPM2 (Wellcome Department of Cognitive Neurology, London). Images were realigned, slice time corrected, spatially normalized (voxel size 2 mm × 2 mm × 4 mm) by using a template defined by the Montreal Neurological Institute (MNI), and were smoothed using a twice voxel size (6 mm × 6 mm × 10 mm) FWHM Gaussian kernel. Before the acquisition of functional images, T2‐weighted anatomical images were acquired in the same plane as the functional images (voxel size = 0.75 mm × 0.75 mm × 5mm). T1‐weighted anatomical images (voxel size 1 mm × 1 mm × 1 mm) were also acquired.
fMRI Data Analysis
Preprocessed MRI data were analyzed statistically on a voxel‐by‐voxel basis using SPM2 [240 s high pass filter, serial correlations corrected by an autoregressive AR (1) model]. The task‐related neural activity was modeled with a series of events convolved with a canonical hemodynamic response function. The following contrast images were calculated for every subject: two simple contrasts DO> DOC, DN > DNC, DOC > DO, DNC > DC and one interaction (DO > DOC) > (DN > DNC). Participant specific contrast images were used as inputs for the second level analysis. At the second level, one‐sample t tests were conducted. Two contrast images [(DO > DOC) and (DO > DOC) − (DN > DNC)] for each participant were used as inputs for the one way within subjects analysis of variance (ANOVA). In the ANOVA test, areas activated for the DO > DOC inclusively masked by the interaction were obtained. The DO > DOC contrast captures the effect of uncertainty when the driver's view is occluded but itself can not rule out the effect of the auxiliary video. The interaction was used as an inclusive mask to control for this confound. The DOC > DO and DNC > DN contrasts were assessed to investigate the enhanced neural activity caused by the auxiliary video. For all tests, a height threshold of P < 0.05 (FDR corrected) and an extent threshold of P < 0.05 uncorrected were employed. The percent signal change was the relative change from the mean MR signal. The MNI coordinates were converted to Talairach coordinates [Talairach and Tournoux, 1988] using a nonlinear transform method (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
RESULTS
Behavioral Performance
Participants, debriefed after scanning, reported that visual stimuli were made well and realistic. Button press responses were analyzed using a two factor [view occlusion (occluded or not) and system availability (available or not)] within subject ANOVA. The dependent variable was mean reaction time. A significant main effect was found only for the system availability [i.e., (DOC > DO) and (DNC > DN)] [F(1,13) = 5.233, P < 0.05]. The main effect of view occlusion [i.e., (DO > DN) and (DOC > DNC)] and the interaction were not significant. Participants responded slower when the system was available for both occluded and nonoccluded view conditions (mean ± SEM in ms: DO = 512.4 ± 46.7, DOC = 0.562.8 ± 59.9, DN = 472.2 ± 71.0, DNC = 530.4 ± 67.3).
Brain Imaging
To investigate neural effects of resolving uncertainty when the driver's view is occluded by a big truck, a simple effect of the system availability when the driver's view was occluded (i.e., DO > DOC) was assessed. Moreover, an interaction between the view occlusion and the system availability [i.e., (DO > DOC) > (DN > DNC)] was assessed to make sure that the effects found in the DO > DOC contrast were not because of different number of videos presented on the screen. Then, the brain areas that were active in both contrasts were obtained by inclusively masking the DO > DOC contrast with the interaction (Table I, Fig. 3A). Greater brain activity for the uncertainty (i.e., DO) condition than the resolved uncertainty (i.e., DOC) condition was observed in a distributed area including anterior cingulate, amygdala, cuneus, inferior parietal lobule, insula, hippocampus, and caudate. In contrast, there was no significant difference in the system availability when the driver's view was not occluded (i.e., DN > DNC).
Table I.
Greater activity for DO relative to DOC
| Brain region | BA | Coordinates (mm) | Peak t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Cuneus | L | 17 | −14 | −80 | 4 | 8.64 |
| Inferior parietal lobule | L | 40 | −65 | −32 | 31 | 7.50 |
| Insula | L | −40 | −11 | 4 | 7.38 | |
| R | 38 | 5 | −14 | 5.15 | ||
| Inferior frontal gyrus | L | 45 | −24 | 33 | 6 | 7.43 |
| Postcentral gyrus | R | 43 | 48 | −6 | 19 | 7.00 |
| L | −50 | −9 | 19 | 5.47 | ||
| Anterior cingulate gyrus | R | 24 | 12 | −8 | 41 | 6.88 |
| L | −12 | 17 | 29 | 4.31 | ||
| Cerebellum | R | 26 | −70 | −30 | 6.84 | |
| Middle temporal gyrus | L | 21 | −53 | −16 | −6 | 6.25 |
| Medial frontal gyrus | L | 10 | −10 | 51 | 5 | 6.17 |
| Hippocampus | L | −30 | −10 | −13 | 6.16 | |
| R | 38 | −16 | −13 | 5.47 | ||
| Thalamus | L | −26 | −21 | 12 | 5.78 | |
| Amygdala | L | −24 | 1 | −19 | 5.56 | |
| Caudate nucleus | R | 24 | 27 | −1 | 5.60 | |
| L | −22 | 11 | 22 | 4.82 | ||
Figure 3.
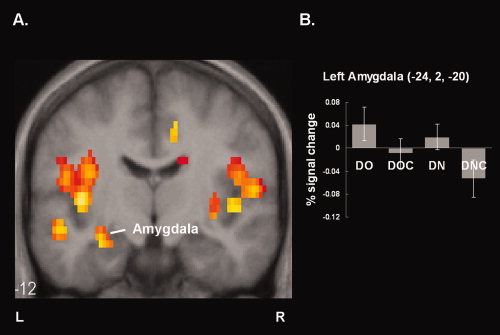
Neural correlates of resolving uncertainty when the driver's view is occluded by a big truck. A: Reduced activations (P < 0.05, FDR corrected) are plotted on coronal slices of the spatially normalized mean T1 weighted image. B: Mean percent signal changes in the left amygdala.
Then, enhanced neural activity caused by the auxiliary video was examined. Greater brain activity for the DOC than the DO condition (i.e., DOC > DO contrast) was observed in occipital cortex and left middle frontal gyrus (Table II, Fig. 4A). Greater brain activity for the DNC condition than the DN condition (i.e., DNC > DN contrast) were observed in occipital cortex (Table III, Fig. 4B).
Table II.
Greater activity for DOC relative to DO
| Brain region | BA | Coordinates (mm) | Peak t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Fusiform gyrus | L | 37 | −28 | −61 | −16 | 13.15 |
| R | 30 | −53 | −7 | 8.12 | ||
| Middle frontal gyrus | L | 6 | −32 | −1 | 55 | 8.52 |
| Occipitotemporal cortex | R | 19 | 36 | −79 | 22 | 8.43 |
| L | −48 | −73 | 7 | 6.13 | ||
| Superior parietal lobule | R | 7 | 32 | −54 | 54 | 8.38 |
| L | −31 | 58 | 51 | 8.24 | ||
| Precuneus | L | 7 | −16 | −65 | 51 | 7.61 |
| R | 14 | −71 | 51 | 5.68 | ||
| Superior occipital gyrus | L | 19 | −28 | −78 | 33 | 7.70 |
| R | 19 | 33 | −74 | 33 | 5.53 | |
| Cuneus | L | 17 | 8 | −93 | 8 | 6.70 |
| R | −10 | −72 | 4 | 6.69 | ||
| Lingual gyrus | R | 18 | 10 | −62 | 7 | 6.17 |
| Inferior frontal gyrus | R | 44 | 44 | 11 | 25 | 5.14 |
| Cerebellum | R | 12 | −71 | −13 | 4.99 | |
| L | 0 | −73 | −17 | 4.32 | ||
| Middle occipital gyrus | R | 19 | 44 | −74 | −3 | 4.60 |
Figure 4.
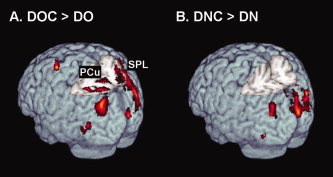
Enhanced neural activations (P < 0.05, FDR corrected) caused by the auxiliary video are plotted on a rendered brain. For both contrasts, significant differences were found in occipital cortex. Different activity in bilateral precuneus and superior parietal lobule was only found for the DOC > DO condition. Region abbreviations: PCu, precuneus; SPL, superior parietal lobule.
Table III.
Greater activity for DNC relative to DN
| Brain region | BA | Coordinates (mm) | Peak t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Occipitotemporal cortex | R | 19 | 36 | −80 | 26 | 7.97 |
| L | −30 | −79 | 19 | 6.78 | ||
| Cuneus | R | 17 | 8 | −92 | 16 | 7.80 |
| L | −12 | −70 | 3 | 6.98 | ||
| Fusiform gyrus | R | 19 | 30 | −59 | −7 | 7.16 |
| L | −20 | −66 | −7 | 5.03 | ||
| Superior occipital gyrus | R | 19 | 20 | −74 | 41 | 6.38 |
| Middle occipital gyrus | R | 19 | 40 | −74 | −3 | 5.87 |
| Lingual gyrus | R | 19 | 14 | −47 | −1 | 5.31 |
| Superior parietal lobule | R | 7 | 34 | −52 | 50 | 5.31 |
| Precuneus | L | 7 | −2 | −68 | 48 | 4.85 |
DISCUSSION
In the present study, neural substrates of resolving uncertainty in driver's decision making were investigated. This investigation yielded that resolving uncertainty in driver's decision making reduced activity in a distributed area including amygdala and anterior cingulate. Unlike many reward‐weighted decision making studies, neural change in ventromedial prefrontal cortex was not found. This finding supports the role of the amygdala and anterior cingulate in cost‐weighted decision making.
Most of the areas that are activated more for the uncertainty condition relative to the resolved uncertainty condition (i.e., DO > DOC) were consistent with previous studies. The neural activity change was not due to a different number of videos because they were not found when the driver's view was clear (i.e., DN > DNC). In contrast with previous studies, in this study, greater activity in the ventromedial prefrontal cortex was not found but greater activity in the amygdala and anterior cingulate was found. We believe that these discrepancies are due to the different nature of the decision‐making tasks between studies. In other words, the neural substrates of decision making in the gambling task (i.e., reward‐weighted decision) are different from ones of decision making in the driving task (i.e., cost‐weighted decision). The activation in the amygdala and anterior cingulate in this study is in consensus with results of effort‐based decision making in rodents. In those studies, cost levels are controlled by increasing the effort required to obtain a larger reward. The lesions in the anterior cingulate [Rudebeck et al., 2006; Walton et al., 2002, 2003] and amygdala [Floresco and Ghods‐Sharifi, 2007; Floresco and Tse, 2007] did not impair reward anticipation but cost‐reward assessment. These results indicate the involvement of these regions in cost‐weighted decision making.
In this study, the level of uncertainty was varied by presenting missing information (i.e., driver's occluded view) through the auxiliary video. To speculate the uncertainty levels for each condition, additional behavioral tests were performed with another set of participants. They performed the same task outside the MRI scanner and were asked to rate each condition on a 1–9 anxiety scale where 1 = least, 9 = most, anxiety. The mean rating and standard error of mean for each condition were DO, 8.09 ± 0.29; DOC, 5.45 ± 0.43; DN, 2.73 ± 0.5; DNC, 2.09 ± 0.42 (mean ± SEM). The significant differences were found for the main effects of view occlusion [F(1,10) = 90.888, P < 0.001] and of system availability [F(1,10) = 32.563, P < 0.001] and the interaction [F(1,10) = 31.429, P < 0.001]. The significant main effect of view occlusion indicates that participants felt more anxiety when their view was occluded. The significant main effect of system availability indicates that the auxiliary video reduced their anxiety. The significant interaction indicates that the auxiliary video reduced their anxiety more when their view was occluded than when their view was not occluded. Because people feel more anxiety for unpredictable aversive events than the same events when they are anticipated [Karim and Balleine, 2007], these results can be interpreted as evidence that participants felt more uncertain for the DO condition than the DOC condition.
In addition to its involvement in decision making, the amygdala is reported to be implicated in anxiety. The amygdala plays a very important role in emotional processes and is considered to be crucial for learning conditioned fear and for experiencing anxiety [Dalton et al., 2005; Davidson, 2002; Davis and Whalen, 2001]. Decreased activity in the amygdala may imply that providing the occluded view information reduces partcipants' anxiety level when they have to turn right at the intersection where their view is occluded. To estimate anxiety level for each condition, we obtained percentage signal changes in the left amygdala. Positive correlation between amygdala activity and stimulus intensity [Anderson et al., 2003; Small et al., 2003] or subjective arousal level [Phan et al., 2003, 2004] has been reported. Percent signal changes are plotted in Figure 3B. Less activity in the left amygdala was found for the DOC condition than for the DO condition indicating that participants felt less anxiety when uncertainty was resolved. Moreover, the same tendency was seen when driver's view was not occluded (i.e., DN and DNC comparison). This may imply that a shifted view from the camera on the traffic signal provides additional information for judging a distance to oncoming traffic and reduced participants' anxiety level even when their view is not occluded. In addition to the role of the amygdala in anxiety, involvement of the amygdala in other functions such as reward processing and self‐relatedness has been reported. There are possibilities that the activity in the amygdala may account for those functions.
Although CG animation sequences used in this study consisted of three stages (entering the intersection, waiting at the center of the intersection and starting to move forward), the neural responses for each stage can not be distinguished in this study. It is because we employed a block design. Considering that a big truck that occluded the drivers' view was already at the intersection when the drivers enter the intersection for the DO conditions, information about on‐coming traffic was already missing from the first stage. An uncertainty level in participants may have been modulated by time, by the difference in stages for decision making, or by something else. To investigate differential neural processes for each stage, further research using an event related design has to be done.
Increased activity by providing a view from the traffic signal (i.e., auxiliary video) was observed mainly in occipital cortex for both occluded view and clear view conditions (DOC > DO; DNC > DC). This increased activity is considered to be due to increased load on visual processing in order to watch two videos simultaneously. This increased load can be speculated to account for the slower reaction times for the system available conditions than the system not available conditions. The cuneus corresponds to the primary visual area, V1. The lateral occipitotemporal region observed in this study corresponds to the V5/MT that is involved in the processing of motion [Tootell et al., 1995] and medial fusiform regions correspond to the V4 that is involved in the processing of color and luminance constancy [Bartles and Zeki, 2000]. Enhanced activation in these regions was reported for the natural movement scenes (e.g., riding through a street) [Stiers et al., 2006]. Left middle frontal gyrus (BA 6), bilateral precuneus and superior parietal lobule were only found for the occluded view condition (see Fig. 4). It is very unlikely that the left BA 6 activity is caused by the button responses because subjects were using their left thumb to press buttons. It may reflect timing‐related functions such as synchronization of the two videos [Dreher et al., 2002; Rubia et al., 1998]. The bilateral activation of the precuneus and superior parietal lobule is reported for shifting attention for visual stimuli [Le et al., 1998]. These results may indicate that subjects pay more attention to the video from the perspective of the traffic signal in the occluded view condition than in the clear view condition.
CONCLUSION
This study shows brain regions that involve driver's decision making. Considering that driving is involved with high cost but not much rewards, we assume that the cost‐weighted decision is weighted more than reward‐weighted decision in driving. In contrast with the role of ventromedial prefrontal cortex in reward‐weighted decision making, the results implicate the amygdala and anterior cingulate as serving a role in cost‐weighted decision making.
Acknowledgements
This research was supported in part by the SCOPE, Ministry of Internal Affairs and Communications.
REFERENCES
- Anderson AK,Christoff K,Stappen I,Panitz D,Ghahremani DG,Glover G,Gabrieli JDE,Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Bartles A,Zeki S ( 2000): The architecture of the colour centre in the human visual brain, new results and a review. Eur J Neurosci 12: 172–193. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio AR,Damasio H,Anderson SW ( 1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio H,Damasio AR ( 2003): Risky business: Emotion, decision‐making, and addiction. J Gambl Stud 19: 23–51. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio H,Damasio AR ( 1999): Different contributions of the human amygdala and ventromedial prefrontal cortex to decision‐making. J Neurosci 19: 5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A,Damasio H,Tranel D,Damasio AR ( 1997): Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–1295. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio H,Tranel D,Anderson SW ( 1998): Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 18: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callhoun VD,Pekar JJ,McGinty VB,Adali T,Watson TD,Pearlson GD ( 2002): Different activation dynamics in multiple neural systems during simulated driving. Hum Brain Mapp 16: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX,Heller AS,Ranganath C ( 2005): Functional connectivity with anterior cingulate and orbitofrontal cortices during decision‐making. Cogn Brain Res 23: 61–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD,Christopher J,Mathias J,Dolan RJ ( 2001): Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29: 537–545. [DOI] [PubMed] [Google Scholar]
- Dalton KM,Kalin NH,Grist TM,Davidson R ( 2005): Neural‐cardiac coupling in threat‐evoked anxiety. J Cogn Neurosci 17: 969–980. [DOI] [PubMed] [Google Scholar]
- Davidson RJ ( 2002): Anxiety and affective style: Role of Prefrontal cortex and amygdala. Biol Psychiatry 51: 68–80. [DOI] [PubMed] [Google Scholar]
- Davis M,Whalen PJ ( 2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6: 13–34. [DOI] [PubMed] [Google Scholar]
- Dreher J‐C,Koechlin E,Ali SO,Grafman J ( 2002): The role of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Einhorn HJ,Hogarth RM ( 1985): Ambiguity and uncertainty in probabilistic inference. Psychol Rev 92: 433–461. [Google Scholar]
- Ellsberg D ( 1961): Risk, ambiguity, and the Savage axioms. Q J Econ 75: 643–669. [Google Scholar]
- Ernst M,Paulus MP ( 2005): Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biol Psychiatry 58: 597–604. [DOI] [PubMed] [Google Scholar]
- Floresco SB,Ghods‐Sharifi S ( 2007): Amygdala‐prefrontal cortical circuitry regulates effort‐based decision making. Cereb Cortex 17: 251–260. [DOI] [PubMed] [Google Scholar]
- Floresco SB,Tse MT ( 2007): Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdale‐prefrontal cortical pathway. J Neurosci 27: 2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H,Murai T,Fukuyama H,Hayashi T,Hanakawa T ( 2005): Functional activity related to risk anticipation during performance of the Iowa gambling task. Neuroimage 24: 253–259. [DOI] [PubMed] [Google Scholar]
- Hampton AN,O'Doherty JP ( 2007): Decoding the neural substrates of reward‐weighted decision making with fMRI. Proc Natl Acad Sci USA 104: 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa E,Okamura N,Tashiro M,Sakurada Y,Maruyama M,Arai H,Yamaguchi K,Sasaki H,Yanai K,Itoh M ( 2005): The neural correlates of driving performance identified using positron emission tomography. Brain Cogn 58: 166–171. [DOI] [PubMed] [Google Scholar]
- Hsu M,Bhatt M,Adolphs R,Tranel D,Camerer CF ( 2005): Neural systems resoponding to degrees of uncertainty in human decision‐making. Science 310: 1680–1683. [DOI] [PubMed] [Google Scholar]
- Karim N,Balleine B ( 2007): Ambiguity and anxiety: When a glass half full is empty. Nat Neurosci 10: 807–808. [DOI] [PubMed] [Google Scholar]
- Kim H,Shimojo S,O'Doherty JP ( 2006): Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol 4: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TH,Pardo JV,Hu X ( 1998): 4T‐fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- O'Doherty J,Critchley H,Deichmann R,Dolan RJ ( 2003): Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci 23: 7931–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP,Feinstein JS,Leland D,Simmons AN ( 2005): Superior temporal gyrus and insula provide response and outcome‐dependent information during assessment and action selection in a decision‐making situation. Neuroimage 25: 607–615. [DOI] [PubMed] [Google Scholar]
- Paulus MP,Frank LR ( 2006): Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage 20: 668–677. [DOI] [PubMed] [Google Scholar]
- Paulus MP,Rogalsky C,Simmons A,Feinstein JS,Stein MB ( 2003): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. Neuroimage 19: 1439–1448. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Decker LR,Noll DC,Nichols TE,Britton JC,Liberzon I ( 2003): Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: A fMRI study. Biol Psychiatry 533: 211–215. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Ho SH,Britton JC,Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Rogers RD,Owen AM,Middleton HC,Williams EJ,Pickard JD,Sahakian BJ,Robbins TW ( 1999): Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 20: 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K,Overmeyer S,Taylor E,Brammer M,Williams S,Simmons A,Andrew C,Bullmore E ( 1998): Prefrontal involvement in temporal bridging and timing movement. Neuropsychologia 36: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH,Walton ME,Smyth AN,Bannerman DM,Rushworth MF ( 2006): Separate neural pathways process different decision costs. Nat Neurosci 9: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS,Behrens TEJ,Rudebeck PH,Walton ME ( 2007): Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 11: 168–176. [DOI] [PubMed] [Google Scholar]
- Small DM,Gregory MD,Mak YE,Gitelman D,Musulam MM,Parrish T ( 2003): Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39: 701–711. [DOI] [PubMed] [Google Scholar]
- Spiers HJ,Maguire EA ( 2007): Neural substrates of driving behaviour. Neuroimage 36: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiers P,Peeters R,Lagae L,Van Hecke P,Sunaert S ( 2006): Mapping multiple visual areas in the human brain with a short fMRI sequence. Neuroimage 29: 74–89. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Plannar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Tootell RB,Reppas JB,Kwong KK,Malach R,Born RT,Brady TJ,Rosen BR,Belliveau JW ( 1995): Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traffic Accident Statistics of Japan ( 2006): National Police Agency, Tokyo.
- Uchiyama Y,Ebe K,Kozato A,Okada T,Sadato N ( 2003): The neural substrates of driving at a safe distance: A functional MRI study. Neurosci Lett 352: 199–202. [DOI] [PubMed] [Google Scholar]
- Walter H,Vetter SC,Grothe J,Wunderlich AP,Hahn S,Spitzer M ( 2001): The neural correlates of driving. Neuroreport 12: 1763–1767. [DOI] [PubMed] [Google Scholar]
- Walton ME,Bannerman DM,Rushworth MF ( 2002): The role of rat medial frontal cortex in effort‐based decision making. J Neurosci 22: 10996–11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME,Bannerman DM,Alterescu K,Rushworth MF ( 2003): Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort‐related decisions. J Neurosci 23: 6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]


