Abstract
We designed a novel task, partially incongruent categorization (PIC), to examine the timing of cognitive control. In the PIC task, participants categorized the probe stimulus according to a specific concept, and the number of features corresponding to the concept was varied. When there was one feature (c1 condition), the probe would elicit only categorization, but when there was more than one feature (c2 and c3 conditions), the probe would also elicit cognitive control. Here, the high temporal resolution of event‐related potentials (ERPs) was utilized to investigate the temporal patterns of activity during conflict detection and control. Cognitive control elicited a N2 that was much larger in response to c2 and c3 than c1 in stimulus‐locked waveforms, and no difference was evident between c2 and c3. The N2 was followed by a P3 that was much less on c2 and c3 than c1 trials, with no difference between c2 and c3. A dipole source analysis for two difference waves, c2−c1 and c3−c1, further showed that the corresponding dipoles of the N2 and P3 in the cognitive control conditions were in the anterior cingulate cortex (ACC) and prefrontal cortex (PFC), respectively. Taken together, the present findings support that ERP components in response to the PIC task reflect the time course of cognitive control: the N2 responds to conflict information and subsequently activates the P3 to control this conflict. The connection between the ACC and PFC is supported by their sequential activation within trials. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: cognitive control, partially incongruent categorization, anterior cingulate cortex, prefrontal cortex, event‐related potentials, dipole source analysis
INTRODUCTION
Cognitive control, the ability to guide thought and action in accordance with internal intentions, lies at the heart of most higher mental faculties that make us human, such as planning, reasoning, problem solving, and language [Cohen et al.,2000]. Cognitive control involves dissociable brain structures, such as the anterior cingulate cortex (ACC) and prefrontal cortex (PFC). While the former is likely to be involved in evaluating the strength of control required, the latter may provide top‐down support of task‐appropriate behaviors [MacDonald et al.,2000]. Much research shows that the ACC detects and signals the occurrence of conflicts in information processing [e.g., Botvinick et al.,1999; van Veen and Carter,2002], and the PFC is involved in implementing control [e.g., Cohen et al.,2000; Gehring and Knight,2000; Kerns et al.,2004]. The conflict monitoring hypothesis [Botvinick et al.,2001] supposes that the conflict monitoring system first evaluates current levels of conflict, and then passes this information on to the centers responsible for control, triggering them to adjust the strength of their influence on processing.
Since the ACC responds to the occurrence of conflict and the PFC is involved in implementing control, it is reasonable to infer that activation of the ACC would trigger activation of the PFC [Botvinick et al.,2001]. However, there is little direct evidence of a connection between the detection of conflict in the ACC and subsequent greater control recruited in the PFC [Matsumoto and Tanaka,2004]. Kerns et al. [2004] demonstrated that conflict‐related activity in the ACC predicts both greater PFC activity and adjustments in behavior, supporting a role for the ACC in the engagement of cognitive control. These data provide strong support for the proposal that ACC activity is linked to subsequent implementation of control [Botvinick et al.,2004]. However, ACC activities on conflict and error trials were found to predict PFC activity on the following trial so that the connection between the ACC and PFC was obtained through analyzing different trial types [Kerns et al.,2004]. The same problem exists in other studies as well [e.g., Aron et al.,2004; Botvinick et al.,2004; Liston et al.,2006]. Activation of the ACC followed by PFC activation has not yet been recorded within one trial. It may be that only one of two processes, either conflict detection resulting in response selection [van Veen and Carter,2002] or conflict anticipation resulting in conflict control [Kerns et al.,2004] is needed when performing one trial. Alternatively, the limited temporal resolution of functional neuroimaging relative to the underlying neural events makes it difficult to discern whether increases in ACC activity are coincident with or produce increases in dorsolateral PFC activity [MacDonald et al.,2000].
To directly test the conflict monitoring hypothesis, both conflict detection and conflict control need to be elicited in one trial, and conflict detection should take place before conflict control. Therefore, we have designed the partially incongruent categorization (PIC) task. In general, judging an object's membership in a specific category requires categorization processing [Freedman et al.,2001; Thorpe et al.,1996]. According to this definition, the category is the basis of categorization. However, in the present study, the category is defined by features which present a concept. If some of the probe features are congruent with this concept, the probe is regarded as a member of the category. Besides the feature of the probe congruent with the concept, there is also one that is incongruent with the concept, and these two features are processed simultaneously. Although the congruent feature elicits matching and positive categorization, the incongruent feature elicits mismatching and negative categorization. However, only one congruent feature is required for a positive response. Therefore, in a condition where a positive response is required, the incongruent feature would elicit a task‐irrelevant negative categorization. To successfully complete task‐relevant positive categorization, participants are required to control the negative response. Thus, in the PIC task, the incongruent feature(s) elicit information conflict and subsequent response control.
Some researchers have suggested that the PFC evaluates the need for executive control whereas the ACC executes the control [e.g., Posner and DiGirolamo,1998; Turken and Swick,1999]. This has been termed the regulative hypothesis of cognitive control [Holroyd and Coles,2002; Johnston et al.,2007; Markela‐Lerenc et al.,2004; Roelofs,2003; Roelofs et al.,2006]. In our PIC task, we expect conflict detection and conflict control to be elicited successively within a trial. This allows the monitoring hypothesis to be directly examined, and this novel experimental paradigm is different from the paradigms typically used to test the regulative hypothesis.
We predicted that the interval between conflict onset and control engagement would be rather short. Therefore, in order to accurately record changes in brain activation, high temporal resolution event‐related potentials (ERPs) were recorded during the PIC process and dipole source analysis was used to localize the intracranial sources of relevant ERP components. The results of previous research indicate that early attention is reflected by the N1 component [e.g., Mangun,1995] and conflict detection is reflected by the N2 component [e.g., Lange et al.,1998; Liotti et al.,2000; Nieuwenhuis et al.,2003; van Veen and Carter,2002; Yeung and Cohen,2006; Yeung et al.,2004]. Liotti et al. [2000] and Lange et al. [1998] suggested that the N2 is related to activity in the ACC and van Veen et al. [2002] localized the source of the N2 to the ACC through dipole source localization analysis. The P3 is regarded as being related to inhibitory processing [Dimoska et al.,2003,2006; Kok et al.,2004) and it is also generally accepted that a distinction can be made between two P3 subcomponents, the P3a and P3b [Kok,2001]. The P3a has a fronto‐central scalp distribution [Linden,2005], which reflects activity of the frontal cortex [Polich,2004] and relates to the presentation of alarming stimuli [McCarthy et al.,1997]. According to the conflict monitoring hypothesis [Botvinick et al.,2001], conflict control is preceded by conflict detection; specifically, ACC activation is followed by PFC activation. In the present study, we expected to observe the N2 component followed by the P3 component within trials. We also aimed to separate the intracranial sources of the N2 and P3 through dipole source analysis. The source of the N2 was expected to be located in the ACC, whereas the source of the P3 was expected to be located in the PFC. These findings would provide direct evidence for the conflict monitoring hypothesis.
PARTICIPANTS AND METHODS
Participants
As paid volunteers, 15 adults (eight women, seven men) aged 21–26 years (mean age 23.2 years) participated in the experiment. All participants were healthy, right‐handed, and had normal or corrected to normal vision. The study was approved by the local Ethics Committee and participants received RMB40 for their participation, which provided sufficient motivation.
Partially Incongruent Categorization Paradigm
In this PIC task, geometric stimuli are presented varying along three attributes (color, stripe orientation, and shape) and having four possible features for each attribute (see Fig. 1). The PIC task consists of two phases: forming a concept and categorization. When forming a concept, participants are presented two stimuli simultaneously and are asked to infer the common feature or features [Bigman and Pratt,2004]. During categorization, they must decide whether the probe stimulus shares a feature with the two former stimuli. They respond by pushing one button if the probe shares a feature with the previously presented stimuli (positive response), and another button if it does not (negative response). The critical element in the design of the PIC task is that no matter how many features are involved in the concept, only one feature of the probe is congruent with the two previous stimuli during a positive response. Features pertaining to the concept decide which attributes of the forthcoming probe will be attended, and features not relevant to the concept decide which attributes will be omitted from attentional processing. For example, if the congruent feature is “red”, participants will attend to the color of the probe, but not to the stripe orientation or shape. Information that is irrelevant to a task elicits cognitive control processing [Barcelo et al.,2006; Kerns et al.,2004], but this depends on whether the information is attended by participants. When irrelevant information is omitted from attentional processing, it does not interrupt processing of relevant information.
Figure 1.
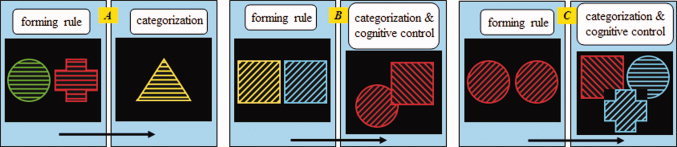
The main cognitive processes in the PIC task. In condition c1 (A), if the feature relevant to the concept were 0° (A, left), then the participants would form anticipation to the “stripe orientation” attribute. After the presentation of the probe, this attribute would be attended, but the “color” and “shape” attributes would be omitted from attention. For trials requiring a positive response (when the probe stimulus has a stripe orientation of 0°; A, right), the stimulus would not elicit conflict and control. In condition c2 (B), if the features relevant to the concept were square and 45° (B, left), then the participants would form anticipation to “shape” and “stripe orientation.” After presentation of the probe, both of these attributes would be attended, but “color” would be omitted from attention. Trials requiring a positive response could have either a square probe or a probe with 45° stripe orientation. For example, it may be a circle with 45° stripe orientation or a square with 135° orientation (B, right). However, only one feature would be congruent with participants' anticipation and the other feature would become an attended incongruent feature, eliciting conflict and control during categorization. Similarly to c2, in condition c3 (C), if the features in the concept were red, circle, and 45° (C, left), but positive response trials only had one congruent feature (C, right), both conflict and control would be elicited, but the number of conflicting features attended to in c3 is more than in c2.
For positive responses, only one feature is anticipated to be congruent with the probe; therefore, the number of features in the concept decides whether conflict and control are involved in categorization. With only one feature in the concept (c1 condition; Fig. 1A), cognitive control is not elicited because only one feature is attended to, and that is the congruent feature. However, cognitive control is elicited when more than one feature pertains to the concept. In this study, we designed two conditions with more than one feature related to the concept. The c2 condition (Fig. 1B) involves two features and the c3 condition (Fig. 1C) involves three features. In these two conditions, concept‐forming results in attention toward two and three features, respectively, but there is only one congruent feature and the others are incongruent. Therefore, these incongruent features become attended irrelevant information and elicit cognitive control. It is worth noting that the congruent feature is assigned randomly so that the incongruent feature cannot be predicted.
Stimuli
The stimuli were all familiar geometric figures and each stimulus had a specific feature in each of three attributes: color (yellow, blue, green, red), shape (triangle, square, circle, cross), and stripe orientation (0°, 45°, 90°, 135°). The combination of the four feature levels and three attributes resulted in 4 × 4 × 4 = 64 different stimuli. The stimuli were all drawn in CorelDRAW 11 (Corel Corporation, Ottawa, Canada), and were individually exported and saved as bitmap files. The sizes of the figures were: 4.56 cm base and 5.92 cm high for triangles, 4.28 cm edge for squares, 4.28 cm diameter for circles, and 4.24 cm edge for crosses (see Fig. 1). During the experiment, the distance between participants' eyes and the screen was about 1.5 m; therefore horizontal and vertical angles were both less than 3.5°.
ERP Recording
Electroencephalography (EEG) was conducted with the 64‐channel (Neuroscan, El Paso, TX) EEG recording system, with references on the left and right mastoids [average mastoid reference, Luck,2005]. The electrooculogram (EOG) was recorded with electrodes placed above and below the left eye. All interelectrode impedance was maintained below 5 kΩ during recording. The EEG and EOG were continuously sampled at 500 Hz with DC‐100 Hz bandpass and 50 Hz notch on. Trials contaminated with EOG artifacts (mean EOG voltage exceeding ±80 μV) or those with artifacts due to amplifier clipping, bursts of electromyographic (EMG) activity, or peak‐to‐peak deflection exceeding ±80 μV were excluded from averaging.
Procedure and Tasks
The experimental procedure is illustrated in Figure 2. At first, a fixation cross was presented in the center of the screen for 500 ms. Next, two stimuli (s1 and s2) were presented for 1500 ms and participants were instructed to infer common features between the two stimuli for the categorization concept. Finally, after a 500–1500 ms interval, a probe stimulus was presented and participants judged whether the probe belonged to the same category as the previous stimuli. The probe was terminated by key press or after 2,000 ms.
Figure 2.
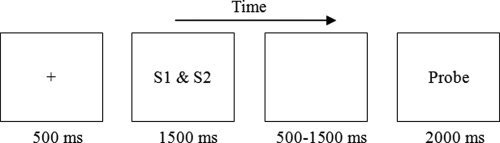
The experimental procedure for one trial in the present study.
If a feature in the probe was congruent with the anticipated feature, participants were instructed to make a positive response by pressing a key; if no feature in the probe was congruent with the anticipated feature, participants made a negative response by pressing another key. Sufficient practice was provided before the formal experiment, and only participants with over 90% correct practice trials were allowed to proceed to the experiment. All 15 participants met this requirement after a block of 80 practice trials was completed.
Experimental Design
Three experimental conditions were designed, corresponding to different numbers of features involved in the categorization concept. There was one feature in the concept for c1, two features in c2 and three features in c3. There were two types of responses made by the participants: the positive and the negative, and thus there were six treatments (three conditions * two response types). There were 80 experimental trials in each treatment and therefore 480 trials total were completed in eight blocks. Within each block, 10 trials were assigned to each treatment. When positive responses were required, only feature‐matching took place in c1 trials, whereas both feature‐matching and feature conflict took place in c2 and c3. However, when negative responses were required, only feature conflict took place in all three conditions. When none of the probe features were congruent with the concept, the information in the probe was all task‐relevant (incongruent features) and elicited no cognitive control. In the present study, only data from correct positive responses were analyzed.
ERP Data Analysis and Statistics
ERP waveforms were time‐locked to the onset of the probe. The averaged epoch for ERPs was 700 ms including a 100 ms baseline. The following 12 sites were chosen for statistical analysis: F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, and CP4 (see Fig. 3).
Figure 3.
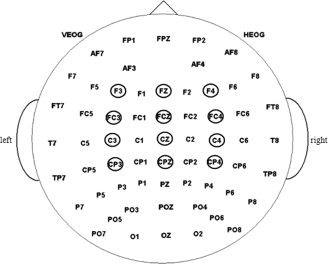
The 64‐channel Neuroscan electrode montage. The statistical analysis is based on data recorded by electrodes located within the small circles.
The amplitude (from baseline to peak) and latency of the N1 component were measured in a 80–140 ms time window. The amplitude and latency of the P2 were measured in a 180–230 ms time window. For the N2, the amplitude and latency were measured in a 240–300 ms time window. The amplitude and latency of the P3 were measured in a 340–400 ms time window. The amplitudes and latencies of the above four components were analyzed using two‐way repeated measures analysis of variance (ANOVA) with factors of condition (c1, c2, and c3) and electrode site (12 sites). The P values of all main and interaction effects were corrected using the Sphericity method for repeated‐measures effects.
Dipole Source Analyses
The Brain Electrical Source Analysis toolkit (BESA 2003, v.5.1.2600.1106, MEGIS Software GmbH, Munich, Germany) was used to perform dipole source analysis. The head model is a four‐shell ellipsoidal head. To focus on the scalp electrical activity related to the processing of pure cognitive control, the averaged ERPs evoked by c1 (no cognitive control) were subtracted from the ERPs evoked by c2 and c3 (cognitive control), and two difference waves were obtained accordingly (c2−c1 and c3−c1). Principal Component Analysis (PCA) was employed in the interval of 240–300 ms for the N2 and 340–400 ms for the P3 in order to estimate the number of dipoles needed to explain the difference waves. When the number of dipoles was determined with PCA, software automatically determined the dipoles' location, and the relevant residual variance criterion was used.
RESULTS
Behavioral Performance
The reaction times for correct responses and accuracy were analyzed using repeated‐measures ANOVAs with the three conditions as independent variables. Specific effects were tested using paired‐sample t tests. Reaction time (Mean ± SE) was 649 ± 31.7 ms on c1, 824 ± 36.4 ms on c2, and 846 ± 36.6 ms on c3 trials (Fig. 4, left). The response times exhibited significant effects of condition, F (2,28) = 48.777, P < 0.001. Participants responded more slowly to the probe on c2 than on c1 trials, t (14) = 8.274, P < 0.001 and on c3 than on c1 trials, t (14) = 9.074, P < 0.001, but response times on c2 and c3 trials did not differ, t (14) = 0.952, P = 0.357. Accuracy (Mean ± SE) was 0.97 ± 0.0074 on c1, 0.96 ± 0.0077 on c2 and 0.94 ± 0.009 on c3 trials (Fig. 4, right). There was a significant effect of condition, F (2,28) = 10.876, P = 0.002 such that more errors were made on c2 than on c1 trials, t (14) = 2.892, P = 0.012, on c3 than on c1 trials, t (14) = 3.62, P = 0.003, and on c3 than on c2 trials, t (14) = 2.803, P = 0.014.
Figure 4.
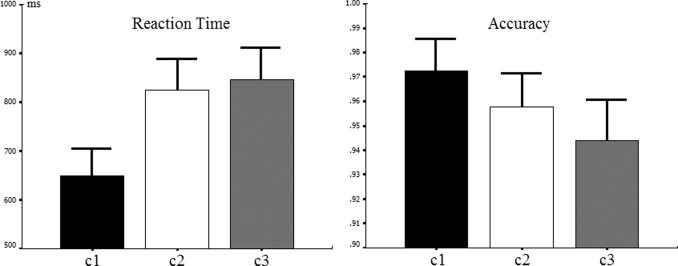
Mean response times for the c1, c2, and c3 conditions in the PIC task (left). Mean accuracies for the same three conditions (right). Error bars indicate the SE.
ERP Data Analysis and Statistics
Early components N1 and P2
The ERP waveforms for the three conditions at the electrode sites selected for analysis are shown in Figure 5. The early components N1 and P2 were elicited by all three conditions. There was a significant main effect of condition for N1 amplitude (F (2,28) = 3.684, P = 0.038) with means of −2.25 ± 0.42 μV for c1, −2.27 ± 0.33 μV for c2, and −3.09 ± 0.45 μV for c3. The results of Pairwise Comparisons of Means showed that N1 amplitude was higher for c3 than c1 (F (1,14) = 6.904, P = 0.02), whereas c1 and c2 and c2 and c3 did not differ (F (1,14) = 2.67, P = 0.125 and F (1,14) = 1.487, P = 0.243, respectively). There was no significant main effect of condition for N1 latency (F (2,28) = 0.794, P = 0.462; 100 ± 3.7 ms for c1, 101 ± 3.2 ms for c2, and 99 ± 2.5 ms for c3).
Figure 5.
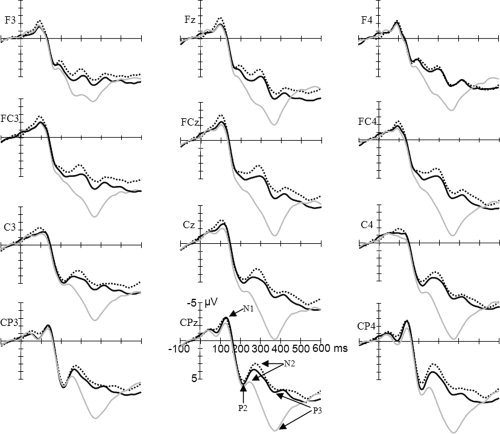
Grand average (n = 15) of ERPs in response to c1 (gray line), c2 (black line), and c3 (dotted line) at the 12 electrode sites chosen for statistical analysis. Time = 0 ms corresponds to the onset of target stimulus presentation. N1, P2, N2, and P3 are indicated on the waveform plots. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
There was a significant main effect of condition for P2 amplitude (F (2,28) = 6.231, P = 0.011), with means of 7.3 ± 1.27 μV for c1, 5.7 ± 1.28 μV for c2, and 5.614 ± 1.28 μV for c3. The results of Pairwise Comparisons of Means showed that P2 amplitude was higher for c1 than c3 (F (1,14) = 12.517, P = 0.003) and c2 (F (1,14) = 12.517, P = 0.003), whereas c2 and c3 did not differ (F (1,14) = 0.075, P = 0.789). There was no significant main effect of condition for P2 latency (F (1,14) = 2.556, P = 0.131; 225 ± 4.3 ms for c1, 191 ± 20.3 ms for c2, and 218 ± 4.1 ms for c3).
Late components N2 and P3
As shown in Figure 5, the late N2 and P3 components were elicited by all three conditions. There was a significant main effect of condition for N2 amplitude (F (2,28) = 17.768, P < 0.001), with means of 5.7 ± 1.24 μV for c1, 3.3 ± 1.12 μV for c2, and 2.6 ± 1.12 μV for c3. The results of Pairwise Comparisons of Means showed that N2 amplitude was higher for c2 than c1 (F (1,14) = 18.924, P = 0.001), and for c3 than c1 (F (1,14) = 50.713, P < 0.001), but c2 and c3 did not differ (F (1,14) = 1.219, P = 0.288). There was no significant hemisphere effect of N2 amplitude (F (1,14) = 0.166, P = 0.69; 3.76 ± 1.06 μV in the left hemisphere and 3.9 ± 1.12 μV in the right hemisphere). There was a significant main effect of condition on N2 latency (F (2,28) = 15.429, P < 0.001; 258 ± 4.2 ms for c1, 276 ± 6.2 ms for c2, and 278 ± 5.1 ms for c3). The results of Pairwise Comparisons of Means showed that N2 latency was shorter for c1 than c2 (F (1,14) = 15.686, P = 0.001) and for c1 than c3 (F (1,14) = 32.124, P < 0.001), but c2 and c3 did not differ (F (1,14) = 0.377, P = 0.549).
There was a significant main effect of condition for P3 amplitude (F (2,28) = 51.185, P < 0.001). The means of the conditions were 11.8 ± 1.06 μV for c1, 7.6 ± 1.22 μV for c2, and 7.06 ± 1.18 μV for c3. The results of Pairwise Comparisons of Means showed that P3 amplitude was higher for c1 than c2 (F (1,14) = 74.588, P < 0.001) and c3 (F (1,14) = 110847, P < 0.001), but c2 and c3 did not differ (F (1,14) = 0.783, P = 0.391). There was a significant hemisphere effect on P3 amplitude (F (1,14) = 10.121, P = 0.007; 7.8 ± 1.04 μV in the left hemisphere and 9.2 ± 1.13 μV in the right hemisphere). There was no significant main effect of condition on P3 latency (F (2,28) = 1.107, P = 0.345; 367 ± 5.2 ms for c1, 371 ± 5.7 ms for c2, and 374 ± 6.1 ms for c3).
Topographical Maps and Dipole Analyses of Difference Waves
According to Figure 6, the activity of the two difference waves at 280 ms was mainly in the midline frontal scalp and activity at 370 ms was in the right frontal scalp. In order to localize the encephalic source of the difference waves at these timepoints, BESA was applied to segments of the grand‐average waveforms that had produced significant contrasts (c1 vs. c2 and c1 vs. c3) in the preceding statistical analyses. The results were accepted when the software showed an acceptable fit (residual variance <15%).
Figure 6.
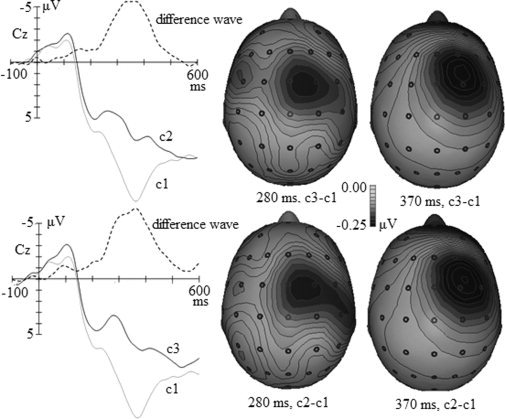
Grand average of ERPs in response to c1, c2, c3, and the difference waves (c2−c1 and c3−c1) at Cz and topographic maps of the difference waves at 280 ms and 370 ms. The two grand averages and c2−c1 difference waves are presented in the upper left panel, and the two grand averages and c3−c1 difference waves are in the lower left panel. The topographic maps of the c2−c1 difference wave at 280 ms and 370 ms are presented in the upper right panel, and those of the c3−c1 difference wave at 280 and 370 ms are presented in the lower right panel.
For the c2−c1 difference wave, we fitted the waves within a 240–300 ms window for the N2 component and a 340–400 ms window for the P3 component in accordance with the latencies of the N2 and P3 indicated in Figure 4. First, PCA was employed in these intervals to estimate the number of dipoles needed for fitting. The first component in the 240–300 ms window accounted for 96.5% of variance, and that in the 340–400 ms window accounted for 99.4%. Therefore, one dipole was used to fit the waves in each window. The dipole for the 240–300 ms window was located approximately in the ACC and accounted for the variance with a residual variance of 8.87%. The dipole for the 340–400 ms window was in the right inferior PFC and accounted for the variance with a residual of 11.17%. The locations of these two dipoles are shown in Figure 7.
Figure 7.
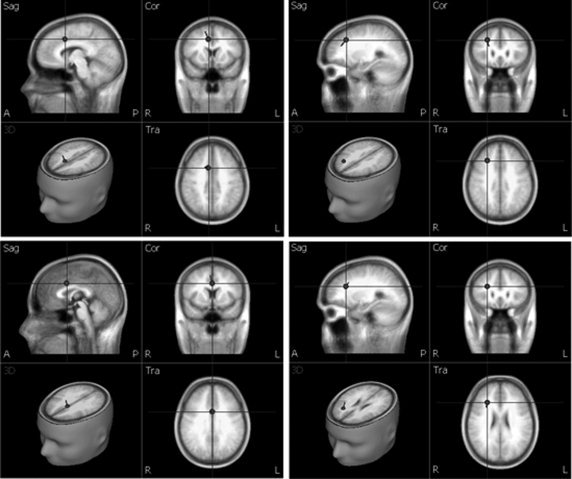
Dipole source localizations for the difference wave c2−c1 and c3−c1 at peak latencies of the N2 and P3. The top left panel is the fitted dipole within the 240–300 ms window presented in the sagittal, coronal, and transverse sections (Talairach coordinates x = 8.3, y = 11.2, z = 38.7). The top right panel is the fitted dipole within the 340–400 ms window viewed in the sagittal, coronal, and transverse sections (x = 25.7, y = 24.4, z = 35.9). The bottom left panel is the fitted dipole within the 240–300 ms window viewed in the sagittal, coronal, and transverse sections (x = 0.5, y = 8, z = 32.3). The bottom right panel is the fitted dipole within the 340–400 ms window viewed in the sagittal, coronal, and transverse section s (x = 25.7, y = 25, z = 24.9).
The same source analysis procedure was used to localize sources for the c3−c1 difference wave within 240–300 and 340–400 ms windows. The first PCA component for the 240–300 ms window explained 96.3% of variance and that for the 340–400 ms window explained 96.94% of the variance; thus one dipole was used for source localization for these two windows. The dipole for the 240–300 ms window was located in the ACC and accounted for the variance with a residual of 11.31%. The dipole for the 340–400 ms window was located in the right inferior PFC and there was a residual of 13.485%. The locations of these two dipoles are shown in Figure 7.
DISCUSSION AND CONCLUSION
During the PIC task, participants form a categorization concept before the probe is presented, which determines which feature they will attend to in the probe. Consequently, the relevant feature in the probe is attended to and irrelevant features are omitted from attentional processing. After the probe is presented, participants only pay attention to the feature in accordance with their anticipation. One, two, and three features were attended to in c1, c2, and c3, respectively.
Early Processing
In general, attention is reflected by N1 amplitude [e.g., Hillyard and Anllo‐Vento,1998; Mangun,1995; Sabri et al.,2006], which was modulated in the present study by the number of features attended. N1 amplitude in c2 and c3 were lager than c1, but did not differ between c2 and c3. These results suggest that N1 amplitude is related to whether attention is distributed rather than the scope of its distribution. While N1 amplitude differed between the conditions requiring distributed attention (c2 and c3) and the condition that did not (c1), the difference between the extent of the distribution of attention did not result in significant differences in N1 amplitude between c2 and c3. The P2 is believed to relate to perceptual processing [Bigman and Pratt,2004; Hillyard and Anllo‐Vento,1998] and we found a main effect of condition on P2 amplitude, but not latency. The results of Pairwise Comparisons of Means showed that the P2 reflected the magnitude of the difference in feature perception. The perceptual processing reflected by the P2 provides a basis for subsequent cognitive processing.
Conflict Detection and Control
Unlike the fixed dimension in which the conflict feature occurs [e.g., Carter et al.,2000], in the present study, the conflict information was randomly assigned to a dimension. For example, in the c2 condition, if the concept is “red” – “triangle”, the conflict feature could appear in either the color or shape dimension. In this case, even if the participant knows that the conflict information will occur in the forthcoming probe, he or she can not prepare in advance for which dimension the conflict will be seen in because there are two possibilities. Instead, only after perceiving the probe and comparing the features presented with the concept can conflict be judged and further control take place. Here the judgment of whether a feature is conflicting or not represents conflict detection; therefore, conflict detection can happen only after the perception of the probe.
Following the N1 and P2 early processing components, the coming N2 was elicited in all three conditions, and previous research suggests that the N2 indicates conscious processing of information [e.g., Lumer et al.,1998; Marois,2005; Sergent et al.,2005]. It is likely that the participants consciously analyzed the features of the probe to compare them to the anticipated features in accordance with the categorization concept. Categorization takes place and conflict is elicited if the features do not match. The N2 may also reflect the appearance of conflict [e.g., Lange et al.,1998; Liotti et al.,2000; Nieuwenhuis et al.,2003; van Veen and Carter,2002; Yeung and Cohen,2006; Yeung et al.,2004], which is manifested in the N2 amplitudes of the three conditions: the c2 and c3 conditions, which have conflicting information, had larger amplitudes than the c1 condition, which did not. These results show that participants detect conflict about 280 ms after probe onset.
The weaker N2 in c1 may reflect feature matching, but stronger N2s in c2 and c3 may also reflect feature conflict. The difference waves c2–c1 and c3–c1 theoretically reflect the processing of feature conflict. The dipole source analysis conducted on the two difference waves in the 240–300 ms time window showed that two dipoles were localized in the ACC, which is consistent with previous results that the encephalic source of the N2 is the ACC [Lange et al.,1998; Liotti et al.,2000; van Veen and Carter,2002]. Because of the close relationship between the ACC and conflict detection [Botvinick et al.,2004; Fincham and Anderson,2006; Kerns et al.,2004; Swick and Turken,2002], it may be concluded that the stronger N2 in the c2 and c3 conditions reflects the detection of conflict.
Detected conflicting information must be inhibited or it would disturb task‐relevant processing, i.e., categorization judgment. Thus, the P3 after the N2 may reflect inhibition processing. Some previous research suggests that P3 amplitude elicited by a condition involving inhibition is less than that elicited by a condition without inhibition [Markela‐Lerenc et al.,2004; Qiu et al.,2006; Ramautar et al.,2006]. The pattern of P3 amplitude reported here is consistent with these findings: P3 amplitude in both c2 and c3 were larger than that in c1, but did not differ between c2 and c3, suggesting that the P3 in c2 and c3 reflects inhibition.
Many studies suggest that the P3 reflects categorization processing [Batty and Taylor,2002; Donchin,1981; Mecklinger and Ullsperger,1993]. In this case, however, there was only one matching feature in all three conditions, making categorization in all three conditions equivalent. According to the task design, only categorization took place in c1, while inhibition control was also elicited in c2 and c3. Accordingly, the difference waves in the time windows of P3 would reflect inhibition control. The dipole source analysis conducted on the two difference waves in the 340–400 ms time window showed that two dipoles were localized in the right PFC, which is consistent with the result that P3 amplitude in the right hemisphere was larger than that in the left. Research has suggested that the PFC is involved in inhibition in cognitive control [e.g., Botvinick,2004; Botvinick et al.,1999; Kerns et al.,2004; MacDonald et al.,2000], thus the dipole results support that the P3 in c2 and c3 reflect inhibition.
It is worth noting that the waves elicited by c2 and c3 did not show significant differences in amplitude and latency of the main ERP components, and while the reaction times did not differ between the two conditions. Because these two conditions differed significantly from the c1 condition with regard to the main ERP components, reaction time, and accuracy, it could be suggested that the brain is sensitive to whether conflict information appears, but not to the magnitude of the conflict, which is consistent with some previous findings [e.g., Egner and Hirsch,2005; Nieuwenhuis and Yeung,2005]. It may be interpreted that whatever the magnitude of the conflict, the brain simply inhibits or rejects it. However, this problem requires further research.
In the present study, the N2 and ACC activity reflecting conflict detection happened at about 280 ms after the onset of the probe, and the P3 and PFC activity reflecting inhibition control happened at about 370 ms. Importantly, the successive activities happened within one trial. In contrast to the ACC and PFC activation occurring in different trials according to previous research [e.g., Kerns et al.,2004], the results of the present study provide direct support for the monitoring hypothesis of cognitive control [Botvinick et al.,2001].
Conflict Detection vs. Working Memory Load
In this PIC study, conflict detection could be confounded with task load or difficulty. In fact, there is a one unit WM load difference and a crucial difference in conflict (with/without) between c1 and c2. There is also a one unit WM load difference and a weak difference in conflict (level difference) between c2 and c3. If we assume that the WM load difference acts as the main factor, we should find significant N2/P3 differences for both situations (c1 vs. c2 and c2 vs. c3). However, the differences between c2 and c3 were not significant. In contrast, if we assume that the crucial difference in conflict (with/without) was the main factor, we may find a significant difference between c1 and c2, and a non‐significant difference between c2 and c3. Our results were consistent with the latter assumption.
In previous studies, the effect of WM load on ERPs was mainly indexed by positive slow waves (PSW; e.g., García‐Larrea and Cézanne‐Bert,1998], not the N2 and P3 which showed significant differences among conditions in the present study. The dipole localization results revealed that the neural structure that generated the N2 activation was the ACC, whereas typical WM results have not found a role for the ACC [Ungerleider et al.,1998]. Therefore, the differences in N2 and P3 in the present study are not likely to reflect differences in working memory load.
Monitoring Hypothesis vs. Regulative Hypothesis
Besides the monitoring hypothesis, the regulative hypothesis has also received substantial support. The study by Markela‐Lerenc et al. [2004] had a similar recording and analysis approach as the present study. However, they adopted a classic Stroop task while the present study adopted a novel PIC task. We note that the conflict in PIC is perceptual whereas the conflict in the Stroop is between semantic automatic processing and color naming. In PIC, participants have clear expectations about the forthcoming features so that incongruence between the expected feature and the probe feature on a specific attribute elicits conflict immediately after the early perceptual processing stage. In contrast, participants lack clear expectations in the Stroop task and the processing of word meaning takes place after the perceptual processing of word shape; hence, conflict would not emerge as early as in our PIC task. In contrast to our findings, Markela‐Lerenc et al. [2004] observed no difference in N2 amplitude between congruent and incongruent conditions. Instead, the waveform difference appeared in the P3 component, which has also been observed in other ERP studies focusing on cognitive control using the Stroop paradigm [e.g., Qiu et al.,2006]. As the P3 appears only after the completion of evaluation of a stimulus [Kok,2001; Polich,2004], the difference in the P3 should reflect information processing at a higher level, such as the comparison between word meaning and color naming. Because the PFC is related to working memory processing [Braver et al.,1997; Miller and Cohen,2001], the semantic conflict may be recognized in PFC and recurrently the ACC was activated, and such a process may result in a PFC activation preceding the ACC activation.
Using an arrow‐word Stroop task, Roelofs et al. [2006] found that ACC activity on incongruent trials was greater than congruent trials when responding to words, but no difference existed between the two conditions when responding to arrows. Although the authors did not discuss this result, it could be interpreted that conflict in the Stroop task takes place after understanding the meaning of the words, but participants had enough time (about 400 ms) before understanding the words to perceptually detect the arrows. In responding to arrows, the response could take place just after their detection, though it is impossible to understand words in such a short time (about 200 ms). Therefore, no conflict may have taken place even in incongruent trials.
Recently, a study using single‐unit activity (SUA) recording in monkeys found that ACC activity is related to top‐down control [Johnston et al.,2007], which is consistent with the regulative hypothesis. In fact, there is evidence that the ACC may be involved in both control [Badgaiyan,2000; Johnston et al.,2007; Markela‐Lerenc et al.,2004; Roelofs et al.,2006; Swick and Turken,2002] and conflict detection [Botvinick et al.,1999; Carter et al.,2000; Kerns et al.,2004; MacDonald et al.,2000; Matsumoto and Tanaka,2004]. According to Rainer [2007], in conflict detection, the detectable neurophysiological change in the ACC is found in the local field potential (LFP), while in control, the change is mainly reflected by SUA. Meanwhile, according to neurophysiological studies, scalp EEG/ERP recordings are closely related to the LFP [Kühn et al.,2004; Speckmann and Elger,2004]. These facts suggest a reason why the work with SUA shows a role in control [Johnston et al.,2007] and the current work with ERPs shows a role in conflict detection for the ACC.
Conclusion
In the present study, an inverse pattern of N2 and P3 was identified within each trial: the N2 amplitude in the conflict conditions were greater than that in the nonconflict condition, and the P3 amplitude in the conflict conditions were less than that in the nonconflict condition. The dipole analysis of the difference waves related to the conflict detection and control processing showed that the dipoles in the N2 and P3 time windows were localized to the ACC and PFC, respectively. Moreover, the behavioral performance results showed that accuracy in the conflict conditions was less than the nonconflict condition and the reaction time in the former was longer than in the latter. These results were consistent with the monitoring hypothesis. In our experimental paradigm, successive conflict detection and conflict control was elicited by the PIC task, which provides a new approach for studying the neural processing of cognitive control.
Contributor Information
Dezhong Yao, Email: dyao@uestc.edu.cn.
Hong Li, Email: lihong@swu.edu.cn.
REFERENCES
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD ( 2000): Executive control, willed actions, and nonconscious processing. Hum Brain Mapp 9: 38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo F, Escera C, Corral MJ, Perianez JA ( 2006): Task switching and novelty processing activate a common neural network for cognitive control. J Cogn Neurosci 18: 1734–1748. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor MJ ( 2002): Visual categorization during childhood: An ERP study. Psychophysiology 39: 482–490. [DOI] [PubMed] [Google Scholar]
- Bigman Z, Pratt H ( 2004): Time course and nature of stimulus evaluation in category induction as revealed by visual event‐related potentials. Biol Psychol 66: 99–127. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD ( 1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick MM, Ross LL, Stenger VA, Noll D, Cohen JD ( 2000): Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS ( 2000): Anterior cingulate and prefrontal cortex: Who's in control? Nat Neurosci 3: 421–423. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ, Clarke AR ( 2003): Inhibitory motor control in children with attention‐deficit/hyperactivity disorder: Event‐related potentials in the stop‐signal paradigm. Biol Psychiatry 54: 1345–1354. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ ( 2006): The auditory‐evoked N2 and P3 components in the stop‐signal task: Indices of inhibition, response‐conflict or error‐detection? Brain Cogn 62: 98–112. [DOI] [PubMed] [Google Scholar]
- Donchin E ( 1981): Surprise…Surprise? Psychophysiology 18: 493–513. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): Cognitive control mechanisms resolve conflict through cortical amplification of task‐relevant information. Nat Neurosci 8: 1784–1790. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Anderson JR ( 2006): Distinct roles of the anterior cingulate and prefrontal cortex in the acquisition and performance of a cognitive skill. Proc Natl Acad Sci USA 103: 12941–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK ( 2001): Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291: 312–316. [DOI] [PubMed] [Google Scholar]
- García‐Larrea L, Cézanne‐Bert G ( 1998): P3, positive slow wave and working memory load: A study on the functional correlates of slow wave activity. Electroencephalogr Clin Neurophysiol 108: 260–273. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT ( 2000): Prefrontal–cingulate interactions in action monitoring. Nat Neurosci 3: 516–520. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo‐Vento L ( 1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH ( 2002): The neural basis of human error processing: Reinforcement learning, dopamine, and the error‐related negativity. Psychol Rev 109: 679–709. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S ( 2007): Top‐down control‐signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron 53: 453–462. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS ( 2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kok A ( 2001): On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 38: 557–577. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar J, de Ruiter M, Band GPH, Ridderinkhof KR ( 2004): ERP components associated with successful and unsuccessful inhibition in a stop‐signal task. Psychophysiology 41: 9–20. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider G, Yarrow K, Brown P ( 2004): Event‐related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 127: 735–746. [DOI] [PubMed] [Google Scholar]
- Lange JJ, Wijers AA, Mulder LJM, Mulder G ( 1998): Color selection and location selection in ERPs: Differences, similarities and “neural specificity”. Biol Psychol 48: 153–182. [DOI] [PubMed] [Google Scholar]
- Linden DJ ( 2005): The P300: Where in the brain is it produced and what does it tell us? Neuroscientist 11: 563–576. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS ( 2000): An ERP study of the temporal course of the Stroop color–word interference effect. Neuropsychologia 38: 701–711. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ ( 2006): Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task‐switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Luck SJ ( 2005): An introduction to event‐related potentials and their neural origins In: Luch SJ, editor. An Introduction to the Event‐Related Potential Technique. Cambridge, MA: MIT; P 107. [Google Scholar]
- Lumer ED, Friston KJ, Rees G ( 1998): Neural correlations of perceptual rivalry in the human brain. Science 280: 1930–1934. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mangun GR ( 1995): Neural mechanisms of visual selective attention. Psychophysiology 32: 4–18. [DOI] [PubMed] [Google Scholar]
- Markela‐Lerenc J, Ille N, Kaiser S, Fiedler P, Mundt C, Weisbrod M ( 2004): Prefrontal‐cingulate activation during executive control: Which comes first? Cogn Brain Res 18: 278–287. [DOI] [PubMed] [Google Scholar]
- Marois R ( 2005): Two‐timing attention. Nat Neurosci 8: 1285–1286. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K ( 2004): Conflict and cognitive control. Science 303: 969–970. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman‐Rakic P ( 1997): Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 77: 1630–1644. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Ullsperger P ( 1993): P3 varies with stimulus categorization rather than probability. Electroencephalogr Clin Neurophysiol 86: 395–407. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N ( 2005): Neural mechanisms of attention and control: Losing our inhibitions? Nat Neurosci 8: 1631–1633. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR ( 2003): Electrophysiological correlates of anterior cingulate function in a go/no‐go task: Effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci 3: 17–26. [DOI] [PubMed] [Google Scholar]
- Polich J ( 2004): Neuropsychology of P3a and P3b: A theoretical overview In: Moore NC, Arikan K, editors. Brainwaves and Mind: Recent Developments. Wheaton, IL: Kjellberg; pp 15–29. [Google Scholar]
- Posner MI, DiGirolamo GJ ( 1998): Executive attention: Conflict, target detection and cognitive control In: Parasuraman R, editor. The Attentive Brain. Cambridge, MA: MIT; pp 401–423. [Google Scholar]
- Qiu J, Luo YJ, Wang QH, Zhang FH, Zhang QL ( 2006): Brain mechanism of Stroop interference effect in Chinese characters. Brain Res 1072: 186–193. [DOI] [PubMed] [Google Scholar]
- Rainer G ( 2007): Behavioral flexibility and the frontal lobe. Neuron 53: 321–323. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR ( 2006): Effects of stop‐signal modality on the N2/P3 complex elicited in the stop‐signal paradigm. Biol Psychol 72: 96–109. [DOI] [PubMed] [Google Scholar]
- Roelofs A ( 2003): Goal‐referenced selection of verbal action: Modeling attentional control in the Stroop task. Psychol Rev 110: 88–125. [DOI] [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MG ( 2006): Anterior cingulate cortex activity can be independent of response conflict in Stroop‐like tasks. Proc Natl Acad Sci USA 103: 13884–13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Liebenthal E, Waldron EJ, Medler DA, Binder JR ( 2006): Attentional modulation in the detection of irrelevant deviance: A simultaneous ERP/fMRI study. J Cogn Neurosci 18: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S ( 2005): Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 8: 1391–1400. [DOI] [PubMed] [Google Scholar]
- Speckmann E, Elger CE ( 2004): Introduction to the neurophysiologicl basis of the EEG and DC potentials Ernst Niedermeyer. Fernando Lopes da Silva. (Eds). Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins, Baltimore, USA, 2004, P 17–29. [Google Scholar]
- Swick D, Turken AU ( 2002): Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA 99: 1635–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C ( 1996): Speed of processing in the human visual system. Nature 381: 520–522. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D ( 1999): Response selection in the human anterior cingulate cortex. Nat Neurosci 2: 920–924. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV ( 1998): A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS ( 2002): The timing of action‐monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14: 593–602. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD ( 2006): The impact of cognitive deficits on conflict monitoring. Predictable dissociations between the error‐related negativity and N2. Psychol Sci 17: 164–171. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM ( 2004): The neural basis of error detection: Conflict monitoring and the error‐related negativity. Psychol Rev 111: 931–959. [DOI] [PubMed] [Google Scholar]


