Abstract
Movement execution strongly relies on precise sensorimotor synchronization. In a finger‐tapping task that requires subjects to synchronize their finger taps to regular pacing signal synchronization accuracy varies with respect to pacing signal's modality. This study aimed at elucidating functional brain dynamics associated with modality specific behavioral synchronization accuracy. To this end, 10 right‐handed subjects performed a finger‐tapping task with respect to regular auditory and visual pacing, respectively, whereas neuromagnetic activity was recorded using a 122‐channel whole‐head neuromagnetometer. Visual pacing was associated with significantly reduced tap‐to‐pacer asynchrony and increased intertap variability as compared to auditory pacing. The brain dynamics associated with task execution were analyzed using the frequency domain beamformer approach dynamic imaging of coherent sources (DICS). Both tasks were shown to be associated with comparable networks. However, during visual pacing involvement of the ventral premotor cortex (PMv) was shown, whereas during auditory pacing the dorsal premotor cortex (PMd) was concerned with task execution. Synchronization with respect to visual pacing was associated with significantly increased functional interaction between thalamus and PMv at beta frequency as compared to functional interplay between thalamus and PMd during auditory pacing. Auditory synchronization was associated with increased functional interaction between left superior temporal gyrus and PMd at alpha frequency. Furthermore, functional interaction between thalamus and premotor cortex at beta frequency was significantly correlated with synchronization accuracy. All in all the present data suggest that modality specific synchronization differences are associated with frequency and connectivity specific changes of functional interaction in distinct brain networks. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: oscillations, coherence, movement, motor control, network, brain, MEG
INTRODUCTION
Most of our everyday actions involve the effective planning and control of coordinated movements that strongly rely on the precise integration of sensory and motor information. The impact of sensory information on motor control has been particularly evidenced in tasks requiring exact motor timing like sensorimotor synchronization [reviewed in Aschersleben,2002; Repp,2005]. In such tasks subjects are instructed to synchronize their own finger taps with respect to a regular external pacing signal. It is well established that despite the impression of being exactly in time with the external cue, the finger tap usually precedes the pacing signal by some tens of milliseconds [for review see Aschersleben,2002; Repp,2005]. It has been shown that removal of sensory information yields impairment of movement timing accuracy as has been evidenced in patients with peripheral somatosensory deafferentation [Billon et al.,1996; Drewing et al.,2004; Stenneken et al.,2002] as well as in healthy volunteers following local anesthesia of the finger tip [Aschersleben et al.,2001]. Besides the significance of sensory re‐afferent information resulting from movement execution, previous data imply that accuracy of movement timing additionally depends on the pacing signal's modality [Jäncke et al.,2000; Kolers and Brewster,1985; Penhune et al.,1998]. Particularly, movement timing with respect to a regular visual pacing signal results in reduced tap‐to‐pacer asynchrony and increased intertap variability as compared to auditory cues. These data imply that different timing strategies might be employed depending on the pacing signal's modality. A previous functional magnetic resonance imaging (fMRI) study [Jäncke et al.,2000] revealed first evidence for the assumption that these modality dependent behavioral differences might be due to distinct brain networks subserving task execution. In particular, these data suggest that auditory paced movements might rely on a brain network associated with internal motor control, whereas visually cued movements might be controlled by a network associated with processing of the pacing stimuli. However, a direct investigation of functional network interaction is still missing.
Functional networks can be investigated by means of coherence and phase synchronization [for review see Fries,2005; Schnitzler and Gross,2005; Varela et al.,2001]. Previous studies have evidenced that functional interaction associated with motor control varies with learning [Andres and Gerloff,1999; Andres et al.,1999; Serrien and Brown,2003] and with specific task requirements like movement rate [Toma et al.,2002], task complexity [Manganotti et al.,1998], presence or absence of the pacing signal [Gerloff et al.,1998], regularity of the pacing signal [Pollok et al.,2008], and with hand speed in visuomotor tracking [Jerbi et al.,2007]. Thus, these data support the hypothesis that functional interaction in a given network is dynamic and varies with task requirements. Because the neural foundations of modality dependent synchronization differences are poorly understood, this study aims to establish a direct relation between functional brain networks and sensorimotor synchronization as a function of the pacing signal's modality. In particular, the study was designed to identify the neural signature of modality specific behavioral differences.
MATERIALS AND METHODS
Subjects and Paradigm
Ten right‐handed subjects aged between 19 and 39 years (27.1 ± 1.7 years; mean ± s.e.m.; 3 male) participated in this study. Handedness was assessed using the Edinburgh Handedness Inventory [Oldfield,1971]. In two consecutive runs a visual and auditory pacing signal was presented with a regular interstimulus interval (ISI) of 800 ms and a length of 10 ms. Runs lasted for 4 min, respectively. The auditory signal was a sine wave tone with a frequency of 1,000 Hz. Loudness was adjusted individually. The visual signal was a red dot centred on a projection screen. The dot was 3 cm in diameter and the distance between subjects and projection screen was 70 cm. Volunteers were instructed to synchronize their finger taps with respect to the respective pacing signal. To this end, subjects performed brisk flexions and extensions of their right index finger. Session order was counterbalanced across subjects. The presentation of stimuli was performed using E‐prime (Psychology Software Tools). All subjects gave their written informed consent prior to the study that was approved by the local ethics committee and was in accordance with the declaration of Helsinki.
Data Collection
Subjects were comfortably seated in a magnetically shielded room while performing their tasks. Both arms rested on wooden panels fixed laterally to the chair. A short training period preceded the MEG measurement. The onset of finger‐taps was determined by a photoelectric barrier mounted on a pad. As behavioral measures the tap‐to‐pacer asynchrony and its standard deviation as a measure of intertap variability was determined individually.
Neuromagnetic activity was measured with a helmet‐shaped 122‐channel whole‐head neuromagnetometer (NeuromagTM). Simultaneously, we recorded muscle activity using surface EMGs placed on the right extensor digitorum communis muscle (EDC). MEG and EMG signals were recorded with a band‐pass filter of 0.03–330 Hz, digitized with 1,000 Hz, and stored digitally for off‐line analysis. Eye blinks were controlled by vertical electrooculogram (EOG).
High‐resolution T1‐weighted magnetic resonance images (MRI) were obtained from each subject. Coregistration between MRI and MEG data was achieved by localizing three anatomical landmarks (nasion, left and right preauricular points) in each individual and measuring the magnetic signals of four coils placed on the scalp. EMG signals were high‐pass filtered at 20 Hz to remove movement artifacts and rectified to enhance the firing rate information of muscle activity [Myers et al.,2003].
Data Analysis
For the detection of the oscillatory network associated with task execution, we used the analysis tool dynamic imaging of coherent sources [DICS; Gross et al.,2001]. Using a spatial filter algorithm and a realistic head model, DICS allows the detection of cerebromuscular and cerebrocerebral coherence within the entire brain. After applying a Hanning window, fast Fourier transform (FFT) was applied to all EMG and MEG signals using the matlab FFT function (http://www.mathworks.com). Values were calculated with a resolution of 1.3 Hz. Windows overlapped with half the FFT size (i.e., 125 samples). Cross‐spectral density was computed to all signal combinations and averaged across the whole measurement period. Finally, a spatial filter was applied to voxels of the entire brain to create tomographic maps of coherent activity. Voxel size was 6 × 6 × 6 mm. In a first step we identified the brain area showing strongest coherence to the EDC at movement frequency, corresponding to 1.3 Hz. In addition, coherence towards the respective pacing signal was calculated at 1.3 Hz. With respect to these sources brain areas showing significant cerebrocerebral coherence were identified. Coupling between brain areas was calculated at alpha (8–12 Hz) and beta (13–24 Hz) frequency, respectively. These frequency ranges were chosen because coupling as well as power at both frequencies have been shown to be closely related to motor control [for review see Fries,2005; Schnitzler and Gross,2005]. To determine differences between the two pacing conditions, we compared absolute power and coherence values associated with auditory and visual pacing.
For cerebromuscular as well as for cerebrocerebral coherence, the voxel showing strongest coherence towards the reference region was identified from local maxima of individual coherence maps and used for coherence analysis. To estimate a level of significance for cerebrocerebral coupling, confidence limits was computed from surrogate data by randomly shuffling the original time courses, destroying all actual coherence. Only sources exceeding a 95% confidence level were taken into account for further analysis. The exact DICS procedure has been described elsewhere [Gross et al.,2001]. The final source model was restricted to sources showing significant coherence towards at least one another brain area within the network. Sources have been identified separately for each pacing condition. We identified the position of each source in the individual brain. For visualization, mean localization maps of identified sources were calculated after normalization of individual anatomic and functional data using SPM99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm). For all statistics nonparametric test procedures were chosen. Paired comparisons were calculated using Wilcoxon test for dependent samples. For correlation analyses we used Spearman rank order correlation. All statistics were calculated two‐tailed. P‐values were not corrected for multiple testing.
RESULTS
Behavioral Data
The handedness test revealed a mean laterality quotient of 97.0 ± 0.9 (range 95.0–100.0) indicating that all subjects were strictly right‐handed. During both pacing conditions subjects demonstrated the well‐known tap‐to‐pacer asynchrony with finger‐taps preceding the pacing signal. Mean values were −63.8 ± 16.9 ms (auditory pacing) and −27.4 ± 10.1 ms (visual pacing). Values differed significantly between conditions (Z = −2.5, P = 0.01). The intertap variability was 47.0 ± 4.4 ms during auditory pacing and 69.8 ± 8.9 ms during visual pacing (Z = −2.5, P = 0.01). Again, values differed significantly. Behavioral data are summarized in Figure 1.
Figure 1.
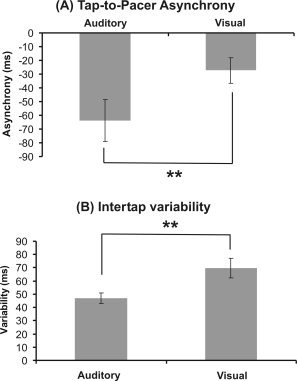
Behavioural data. (A) Tap‐to‐pacer asynchrony during auditory and visual synchronization. (B) Intertap variability depending on the modality of the pacing signal. Error bars indicate standard error of mean (s.e.m.). Asterisks indicate significant differences (**P = 0.01).
The Oscillatory Network
The analysis of brain dynamics revealed a brain network comprising contralateral sensorimotor cortex (S1/M1), lateral premotor cortex (PMC), supplementary motor area (SMA), posterior parietal cortex (PPC), thalamus, and ipsilateral cerebellum associated with both pacing conditions. Sources have been identified in all subjects. In line with the previous data [Butz et al.,2006; Gross et al.,2005], cerebrocerebral coherence analysis revealed discernible peaks at alpha and at beta frequency.
S1/M1 was localized with respect to EDC at 1.3 Hz corresponding to movement frequency. No further brain areas being consistently coherent to EDC have been found. During auditory pacing, sources within bilateral superior temporal gyrus (STG) were detected coupling at 1.3 Hz with the pacing signal. Along the same line, during visual pacing a source within the occipital cortex (OC) at 1.3 Hz was localized. Sources within PMC, SMA, PPC, thalamus, and cerebellum were identified with S1/M1 as reference region at alpha as well as at beta frequencies. Defining sources within STG and OC as reference region did not yield detection of additional brain sources.
Because localization accuracy in brain areas remote from the MEG sensors is reduced, we investigated variation of individual thalamus coordinates as determined by the spatial distance between the individual source and its mean localization. In the auditory condition this distance varied to 0.6–23.2 mm for x‐axis, 1.1–16.8 mm for y‐axis, and 0.7–8.9 mm for z‐axis. In the visual condition coordinates varied 1.4–10.2 mm for x‐axis, 0.3–17.7 mm for y‐axis, and 0.7–12.3 mm for z‐axis.
Coordinates of the lateral PMC differed with respect to the pacing signal's modality. Although auditory pacing was associated with oscillatory activity of the dorsal PMC (PMd), visually paced movements yielded involvement of its ventral part (PMv; [Picard and Strick,2001]. Statistical analysis of individual sources revealed that during visual pacing PMC was localized significantly more inferior as compared to auditory pacing (Wilcoxon test: Z = −2.09, P = 0.03). Other source localizations did not differ significantly (P > 0.5). Mean source localizations are illustrated in Figure 2. Table I summarizes the appendant coordinates according to Talairach and Tournoux [1988] and the respective Brodmann Areas.
Figure 2.
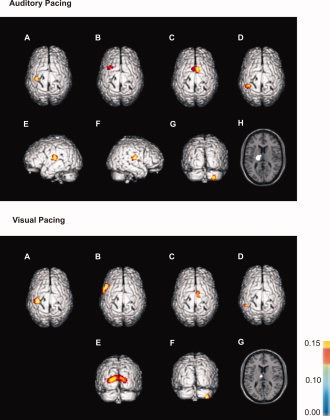
Mean source localizations identified during auditory and visual synchronization as determined by SPM99. Upper panel: (A) primary sensorimotor cortex (S1/M1), (B) dorsal premotor cortex (PMd), (C) supplementary motor area (SMA), (D) posterior parietal cortex (PPC), (E/F) superior temporal gyrus, (G) ipsilateral cerebellum, (H) thalamus. Lower panel: (A) primary sensorimotor cortex (S1/M1), (B) ventral premotor cortex (PMv), (C) supplementary motor area (SMA), (D) posterior parietal cortex (PPC), (E) occipital cortex (OC), (F) ipsilateral cerebellum, (G) thalamus. Please note that the source within S1/M1 was localized with respect to EDC. Sources within superior temporal sulcus and occipital cortex were localized with respect to the respective pacing signal. All other sources were identified with S1/M1 as reference region. Coherence strength is color coded: yellow indicates stronger coherence, whereas blue indicates weaker coherence. Please note that SPM99 has been used for visualization of mean source localizations, only. Maps do not represent any statistical comparison between the two pacing conditions.
Table I.
Talairach coordinates
| X | Y | Z | BA | |||||
|---|---|---|---|---|---|---|---|---|
| Auditory | Visual | Auditory | Visual | Auditory | Visual | Auditory | Visual | |
| S1/M1 | −36 | −38 | −22 | −22 | 60 | 62 | 4 | 4 |
| PMC | −32 | −56 | 14 | 8 | 56 | 38 | 6 | 6 |
| SMA | 6 | 10 | 6 | 10 | 68 | 72 | 6 | 6 |
| PPC | −44 | −44 | −42 | −44 | 62 | 62 | 5 | 5 |
| Thalamus | −16 | −6 | −18 | −12 | 12 | 12 | — | — |
| Cerebellum | 32 | 38 | −82 | −80 | −46 | −50 | — | — |
| STG left | −52 | — | −14 | — | 18 | — | 42 | — |
| STG right | 60 | — | −16 | — | 16 | — | 42 | — |
| OC | — | −18 | — | −96 | — | 10 | — | 18 |
Talairach coordinates of identified brain sources subserving synchronization with respect to auditory and visual pacing and the respective Brodmann area.
At pacing frequency power of bilateral STG was decreased during the visual condition as compared to auditory pacing. Vice versa, power of the OC source was decreased during auditory pacing in comparison to visual synchronization. No further significant power differences as a measure of local activity were evident at pacing frequency or at the alpha or beta range.
The analysis of the functional network interplay at alpha frequency suggested significantly stronger coherence between left STG and PMd during auditory pacing (Z = −2.1; P = 0.04) as compared to coherence between OC and PMv during visual pacing. In addition, coherence between left STG and PMd during auditory pacing was significantly increased as compared to visual synchronization (Z = −2.1; P = 0.03). At beta frequency, coherence between thalamus and PMv was significantly increased during visual pacing as compared to coherence between thalamus and PMd during auditory synchronization (Z = −2.2, P = 0.03). Accordingly, thalamus‐PMv coherence during visual pacing was significantly increased in comparison to auditory pacing (Z = −2.4; P = 0.01). Absolute values of coherence strength are summarized in Figure 3. No further significant differences of brain dynamics were evident.
Figure 3.
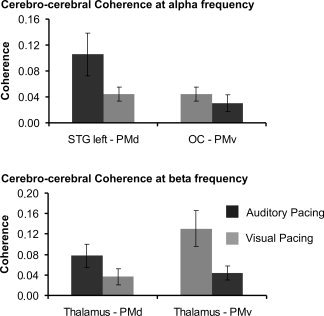
Mean cerebrocerebral coherence associated with auditory and visual synchronization as a measure of functional interaction. Error bars indicate s.e.m. The figure indicates absolute coherence values for those connections showing significant differences between visual and auditory synchronization.
To investigate the relation between coherence and power on the one hand and behavioral synchronization accuracy on the other hand correlation analyses were calculated across data from both synchronization conditions. The analysis reveals a linear relationship between coherence strength between thalamus and PMC at beta frequency and tap‐to‐pacer asynchrony (Rho = 0.51, P = 0.03; Fig. 4). No further significant correlation was evident.
Figure 4.
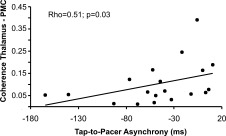
Correlation between thalamus–PMC coherence at beta frequency and tap‐to‐pacer asynchrony. Please note that for the analysis data from auditorily and visually paced movements have been pooled.
DISCUSSION
Timing accuracy in sensorimotor synchronization relies on the modality of the pacing signal. In particular, auditorily cued movements are related to larger tap‐to‐pacer asynchrony and smaller intertap variability as compared to visually paced movements. The present data suggest a functional dissociation of the premotor cortex by showing involvement of the PMv during visually paced synchronization and PMd during auditory paced movements. This hypothesis is further supported by the observation that brain dynamics vary with respect to the pacing signal's modality. In particular, visually cued movements were shown to be associated with stronger thalamus‐PMv coherence at beta frequency, whereas auditory pacing was associated with stronger left STG‐PMd interaction at alpha frequency. Thalamus‐PMC coherence at beta frequency was correlated with tap‐to‐pacer asynchrony indicating that thalamo‐premotor interplay subserves synchronization accuracy. These results suggest that modality dependent differences of sensorimotor synchronization are related to functional interaction in distinct brain networks.
Behavioral Data
During both synchronization conditions the well‐known tap‐to‐pacer asynchrony was evident. Although several studies tried to shed light on the foundations of this phenomenon, the underlying neurophysiological mechanisms are still poorly understood [for review see Aschersleben,2002; Repp,2005]. The present data reveal increased tap‐to‐pacer asynchrony during auditory pacing and increased intertap variability during visual pacing replicating previous findings [Jäncke et al.,2000; Kolers and Brewster,1985; Penhune et al.,1998]. Interestingly, during visually cued synchronization subjects usually report stronger effort to correct for errors than during sensorimotor synchronization with respect to auditory stimuli [Kolers and Brewster,1985]. Thus, it has been argued that during auditory pacing task execution might rely on the prediction of the pacing signal without explicit attention to single stimuli [Jäncke et al.,2000]. Conversely, during visual pacing subjects might particularly pay attention to the exact occurrence of the pacing signal suggesting that motor control in visually paced movements might be based on sensory processing. Accordingly, the significance of auditory cues for timed movements has been demonstrated during rehabilitation of patients with certain movement disorders like Parkinson's disease [McIntosh et al., 1997] suggesting that auditory cues may facilitate rhythmic movement execution. Interestingly enough, visual cues have been shown to be less effective in such facilitation [Patel et al.,2005; Repp and Penel,2004].
Although the significance of the modality of external cues on motor behavior has been well established, the neural foundations of behavioral differences depending on the pacing signal's modality are less well understood.
The Functional Brain Network
Synchronization of one's own movements with respect to a regular auditory pacing signal is associated with a cerebello‐thalamo‐cortical network as evidenced by fMRI [Chen et al.,2006; Jäncke et al.,2000; Lutz et al.,2000; Rao et al.,1997; Sadato et al.,1996] as well as by MEG studies [Pollok et al.,2005a,b). In this study, task execution yields a functional network comprising bilateral STG, contralateral S1/M1, PMC, SMA, PPC, and thalamus as well as the ipsilateral cerebellum replicating these previous findings. During visual pacing a source within the occipital cortex was detected instead of bilateral STG indicating that the pacing signal yields involvement of brain areas subserving its sensory processing. Although involvement of the cerebellum and thalamus in motor control has been evidenced in this study, we would like to stress that localization accuracy in brain areas remote from the MEG sensors is reduced as compared to the cortex. Thus, the exact localization within these structures should be interpreted with caution. However, previous studies have evidenced the feasibility of detecting coherent sources even in deep brain areas like thalamus and the cerebellum [e.g. Butz et al.,2006; Dalal et al.,2008].
Despite the similarities of brain networks underlying task execution, the present data suggest that depending on the pacing signal's modality different parts of PMC are involved. Although auditory synchronization involved PMd, during visually paced movements a PMC source located inferior to the PMC source during auditory pacing was detected, suggesting functional dissociation of the premotor cortex.
Functional Dissociation Between PMv and PMd
In general, the lateral PMC––in contrast to mesial parts––seems to be concerned with movements executed with respect to external stimuli [Gerloff et al.,1998; Halsband et al.,1994]. Anatomical [Jackson and Husain,1996; Picard and Strick,2001] as well as functional dissociation of PMd and PMv has been evidenced for visuomotor tasks using fMRI [Debaere et al.,2003; Hoshi and Tanji,2006] and transcranial magnetic stimulation [TMS; Davare et al.,2006]. Although it should be stressed that the precise boundaries of PMv in humans are less well‐defined than those of PMd [Picard and Strick,2001], anatomical connections led to the hypothesis that PMv and PMd might be part of distinct networks underlying different aspects of motor control [Jackson and Husain,1996]. Along this line it has been argued that PMd might play a role in movement preparation, whereas PMv might be particularly involved in the execution of visually guided movements [Jackson and Husain,1996]. However, this explanation is at odds with the study of Jäncke et al. [2000] indicating increased PMv activation during auditory synchronization. Thus, these data do not support the hypothesis that PMv is exclusively related to visuomotor control. Alternatively, PMd has been related to movement planning and PMv to the online control of movement execution [for review see Jackson and Husain,1996]. Along this line, a previous study investigating the neural substrates of visuomotor learning [Grafton et al.,2008] suggests that the PMd is part of a network associated with predictive motor control, whereas PMv is stronger activated when movement execution relies on feedback. The present data are in line with the hypothesis of a functional dissociation of the premotor cortex. Moreover, this study reveals further support for this assumption by showing distinct interaction patterns of PMd and PMv, respectively. In particular, during auditory pacing auditory–PMd interaction at alpha frequency was stronger as compared to visual pacing, whereas during visual pacing stronger thalamus–PMv coherence was evident in comparison to auditory pacing. Auditory‐premotor interaction during synchronization with respect to musical rhythms has been demonstrated using fMRI [Chen et al.,2006,2008). These data indicate that with increasing metric salience of the auditory cue local activity of the PMd and the STG as well as the functional connectivity between both areas increases [Chen et al.,2006] leading to the hypothesis that PMd plays a crucial role for accurate timing of movements with respect to auditory cues. The present data corroborate the specific significance of auditory‐premotor interaction for auditorily paced movements. As a second result, visual synchronization was associated with stronger coherence between thalamus and PMv at beta frequency as compared to auditory pacing. It has been suggested that thalamo‐cortical loops are crucial for decoding temporal information provided by sensory information [Klimesch et al.,2007]. Accordingly, visual synchronization might be related to sensory information processing by thalamo‐premotor functional interaction.
All in all, the data imply that during auditory pacing subjects might rely on the prediction of the pacing signal, whereas during visual pacing subjects may pay stronger attention to the actual occurrence of the pacing signal instead of its prediction.
Functional Significance of Coherence at Alpha and Beta Frequency
Interestingly, differences of functional interaction between left STG and PMd occurred at alpha frequency, whereas those between thalamus and PMv were evident at beta frequency. The exact functional significance of different frequency ranges is still a matter of debate. Traditionally, oscillations of the sensorimotor and occipital cortex at alpha frequency have been related to an idling state [for review see Miller,2007]. But, growing evidence gave rise to the hypothesis that oscillatory coupling at this frequency might be key for coding of relevant information processing in the brain as well [reviewed in Miller,2007]. The precise functional significance of oscillations at the alpha range for motor control has yet to be solved. Although previous data do not support a specific significance for movement execution [Klostermann et al.,2007], another study gave rise to the hypothesis that coherence at frequencies between 6 and 9 Hz––a frequency range that is quite close to that measured in the present data––might indicate a mechanism for intermittent motor control [Gross et al.,2002]. Interestingly, it was shown that during early stages of motor learning coherence at beta frequency prevails and decreases during the course of learning [Andres et al.,1999]. Thus, one might argue that functional interaction at beta frequency is related to control of complex movements, whereas coupling at alpha frequency might represent a marker of motor control associated with the execution of simple motor tasks––possibly based on predictive motor control. This interpretation is in line with the observation that visual synchronization is reported to be more demanding than auditory synchronization. Furthermore, it fits the hypothesis that auditory synchronization relies on the generation of an internal movement rhythm [Jäncke et al.,2000]. Further evidence for this assumption comes from the present data showing a significant correlation between thalamus–PMv interaction and tap‐to‐pacer asynchrony at 20 Hz. Thus, stronger thalamus–PMv interaction at the beta range seems to be associated with more precise synchronization supporting the hypothesis that 20 Hz coherence might reflect feedback related motor control.
CONCLUSION
The present data support the hypothesis that modality dependent differences of synchronization accuracy are associated with functional interaction in distinct brain networks. Although auditory synchronization involves dorsal parts of the premotor cortex, visually paced movements are associated with ventral premotor cortex involvement. Thus, the present data suggest that involvement of PMd might reflect predictive motor control, although PMv may subserve feedback related motor control.
Acknowledgements
We would like to thank Erika Rädisch for her technical support during MRI scans.
REFERENCES
- Andres FG,Gerloff C ( 1999): Coherence of sequential movements and motor learning. J Clin Neurophysiol 16: 520–527. [DOI] [PubMed] [Google Scholar]
- Andres FG,Mima T,Schulman AE,Dichgans J,Hallett M,Gerloff C ( 1999): Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855–870. [DOI] [PubMed] [Google Scholar]
- Aschersleben G ( 2002): Temporal control of movements in sensorimotor synchronization. Brain Cognit 48: 66–79. [DOI] [PubMed] [Google Scholar]
- Aschersleben G,Gehrke J,Prinz W ( 2001): Tapping with peripheral nerve block. Exp Brain Res 136: 331–339. [DOI] [PubMed] [Google Scholar]
- Billon M,Semjen A,Cole J,Gauthier G ( 1996): The role of sensory information in the production of periodic finger‐tapping sequences. Exp Brain Res 110: 117–130. [DOI] [PubMed] [Google Scholar]
- Butz M,Timmermann L,Gross J,Pollok B,Dirks M,Hefter HAS ( 2006): Oscillatory coupling in writing and writer's cramp. J Physiol (Paris) 99: 14–20. [DOI] [PubMed] [Google Scholar]
- Chen JL,Zatorre RJ,Penhune VB ( 2006): Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage 32: 1771–1781. [DOI] [PubMed] [Google Scholar]
- Chen JL,Penhune VB,Zatorre RJ ( 2008): Moving on time: brain network for auditory‐motor synchronization is modulated by rhythm complexity and musical training. J Cognit Neurosci 20: 226–239. [DOI] [PubMed] [Google Scholar]
- Dalal SS,Guggisberg AG,Edwards E,Sekihara K,Findlay AM,Canolty RT,Berger MS,Knight RT,Barbaro NM,Kirsch HE, Nagarajan SS ( 2008): Five‐dimensional neuroimaging: Localization of the time‐frequency dynamics of cortical activity. Neuroimage 40: 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M,Andres M,Cosnard G,Thonnard JL,Olivier E ( 2006): Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci 26: 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F,Wenderoth N,Sunaert S,Van Hecke P,Swinnen SP ( 2003): Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19: 764–776. [DOI] [PubMed] [Google Scholar]
- Drewing K,Stenneken P,Cole J,Prinz W,Aschersleben G ( 2004): Timing of bimanual movements and deafferentation: Implications for the role of sensory movement effects. Exp Brain Res 158: 50–57. [DOI] [PubMed] [Google Scholar]
- Fries P ( 2005): A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cognit Sci 9: 474–480. [DOI] [PubMed] [Google Scholar]
- Gerloff C,Richard J,Hadley J,Schulman AE,Honda M,Hallett M. ( 1998): Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531. [DOI] [PubMed] [Google Scholar]
- Grafton ST,Schmitt P,Van Horn J,Diedrichsen J ( 2008): Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage 39: 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J,Kujala J,Hamalainen M,Timmermann L,Schnitzler A,Salmelin R ( 2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J,Timmermann L,Kujala J,Dirks M,Schmitz F,Salmelin R,Schnitzler A ( 2002): The neural basis of intermittent motor control in humans. Proc Natl Acad Sci USA 99: 2299–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J,Pollok B,Dirks M,Timmermann L,Butz M,Schnitzler A ( 2005): Task‐dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage 26: 91–98. [DOI] [PubMed] [Google Scholar]
- Halsband U,Matsuzaka Y,Tanji J ( 1994): Neuronal activity in the primate supplementary, pre‐supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res 20: 149–155. [DOI] [PubMed] [Google Scholar]
- Hoshi E,Tanji J ( 2006): Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616. [DOI] [PubMed] [Google Scholar]
- Jackson SR,Husain M ( 1996): Visuomotor functions of the lateral pre‐motor cortex. Curr Opin Neurobiol 6: 788–795. [DOI] [PubMed] [Google Scholar]
- Jäncke L,Loose R,Lutz K,Specht K,Shah NJ ( 2000): Cortical activations during paced finger‐tapping applying visual and auditory pacing stimuli. Cogn Brain Res 10: 51–66. [DOI] [PubMed] [Google Scholar]
- Jerbi K,Lachaux JP,N′Diaye K,Pantazis D,Leahy RM,Garnero L,Baillet S ( 2007): Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc Natl Acad Sci USA 104: 7676–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W,Sauseng P,Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Klostermann F,Nikulin VV,Kuhn AA,Marzinzik F,Wahl M,Pogosyan A,Kupsch A,Schneider GH,Brown P,Curio G ( 2007): Task‐related differential dynamics of EEG alpha‐ and beta‐band synchronization in cortico‐basal motor structures. Eur J Neurosci 25: 1604–1615. [DOI] [PubMed] [Google Scholar]
- Kolers PA,Brewster JM ( 1985): Rhythms and responses. J Exp Psychol Hum Percept Perform 11: 150–167. [DOI] [PubMed] [Google Scholar]
- Lutz K,Specht K,Shah NJ,Jäncke L ( 2000): Tapping movements according to regular and irregular visual timing signals investigated with fMRI. Neuroreport 11: 1301–1306. [DOI] [PubMed] [Google Scholar]
- Manganotti P,Gerloff C,Toro C,Katsuta H,Sadato N,Zhuang P,Leocani L,Hallett M ( 1998): Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62. [DOI] [PubMed] [Google Scholar]
- McIntosh GC,Brown SH,Rice RR,Thaut MH ( 1997): Rhythmic auditory‐motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 62: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R ( 2007): Theory of the normal waking EEG: From single neurones to waveforms in the alpha, beta and gamma frequency ranges. Int J Psychophysiol 64: 18–23. [DOI] [PubMed] [Google Scholar]
- Myers LJ,Lowery M,O'Malley M,Vaughan CL,Heneghan C,St Clair Gibson A,Harley YX,Sreenivasan R ( 2003): Rectification and non‐linear pre‐processing of EMG signals for cortico‐muscular analysis. J Neurosci Methods 124: 157–165. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Patel AD,Iversen JR,Chen Y,Repp BH ( 2005): The influence of metricality and modality on synchronization with a beat. Exp Brain Res 163: 226–238. [DOI] [PubMed] [Google Scholar]
- Penhune VB,Zatorre RJ,Evans AC ( 1998): Cerebellar contributions to motor timing: A PET study of auditory and visual rhythm reproduction. J Cognit Neurosci 10: 753–765. [DOI] [PubMed] [Google Scholar]
- Picard N,Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Pollok B,Gross J,Müller K,Aschersleben G,Schnitzler A ( 2005a): The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage 24: 646–655. [DOI] [PubMed] [Google Scholar]
- Pollok B,Sudmeyer M,Gross J,Schnitzler A ( 2005b): The oscillatory network of simple repetitive bimanual movements. Brain Res Cogn Brain Res 25: 300–311. [DOI] [PubMed] [Google Scholar]
- Pollok B,Gross J,Kamp D,Schnitzler A ( 2008): Evidence for anticipatory motor control within a cerebello‐diencephalic‐parietal network. J Cognit Neurosci 20: 828–840. [DOI] [PubMed] [Google Scholar]
- Rao S,Harrington DL,Haaland KY,Bobholz JA,Cox RW,Binder JR ( 1997): Distributed neural systems underlying the timing of movements. J Neurosci 17: 5528–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp BH ( 2005): Sensorimotor synchronization: A review of the tapping literature. Psychon Bull Rev 12: 969–992. [DOI] [PubMed] [Google Scholar]
- Repp BH,Penel A ( 2004): Rhythmic movement is attracted more strongly to auditory than to visual rhythms. Psychol Res 68: 252–270. [DOI] [PubMed] [Google Scholar]
- Sadato N,Campbell G,Ibanez V,Deiber M,Hallett M ( 1996): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A,Gross J ( 2005): Normal and pathological oscillatory communication in the brain. Nat Neurosci Rev 6: 285–296. [DOI] [PubMed] [Google Scholar]
- Serrien DJ,Brown P ( 2003): The integration of cortical and behavioural dynamics during initial learning of a motor task. Eur J Neurosci 17: 1098–1104. [DOI] [PubMed] [Google Scholar]
- Stenneken P,Aschersleben G,Cole J,Prinz W ( 2002): Self‐induced versus reactive triggering of synchronous movements in a deafferented patient and control subjects. Psychol Res 66: 40–49. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P. 1988. Co‐planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System––An Approach to Cerebral Imaging. New York: Thieme Medical Publishers. [Google Scholar]
- Toma K,Mima T,Matsuoka T,Gerloff C,Ohnishi T,Koshy B,Andres F,Hallett M ( 2002): Movement rate effect on activation and functional coupling of motor cortical areas. J Neurophysiol 88: 3377–3385. [DOI] [PubMed] [Google Scholar]
- Varela F,Lachaux JP,Rodriguez E,Martinerie J ( 2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2: 229–239. [DOI] [PubMed] [Google Scholar]


