Abstract
Trigeminal neuralgia (TN) is a pain state characterized by intermittent unilateral pain attacks in one or several facial areas innervated by the trigeminal nerve. The somatosensory cortex is heavily involved in the perception of sensory features of pain, but it is also the primary target for thalamic input of nonpainful somatosensory information. Thus, pain and somatosensory processing are accomplished in overlapping cortical structures raising the question whether pain states are associated with alteration of somatosensory function itself. To test this hypothesis, we used functional magnetic resonance imaging to assess activation of primary (SI) and secondary (SII) somatosensory cortices upon nonpainful tactile stimulation of lips and fingers in 18 patients with TN and 10 patients with TN relieved from pain after successful neurosurgical intervention in comparison with 13 healthy subjects. We found that SI and SII activations in patients did neither depend on the affected side of TN nor differ between operated and nonoperated patients. However, SI and SII activations, but not thalamic activations, were significantly reduced in patients as compared to controls. These differences were most prominent for finger stimulation, an area not associated with TN. For lip stimulation SI and SII activations were reduced in patients with TN on the contra‐ but not on the ipsilateral side to the stimulus. These findings suggest a general reduction of SI and SII processing in patients with TN, indicating a long‐term modulation of somatosensory function and pointing to an attempt of cortical adaptation to potentially painful stimuli. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: primary somatosensory cortex, secondary somatosensory cortex, nonpainful tactile stimulation, pain, trigeminal nerve, functional magnetic resonance imaging
INTRODUCTION
Trigeminal neuralgia (TN) is a pain state characterized by severe, mostly unilateral intermittent lancinating pain in one or several territories innervated by the trigeminal nerve. Pain attacks can be triggered by normally innocuous stimulation of the affected area, such as touch, shaving, or washing. TN occurs in 60% of the cases on the right side, affects mostly patients older than 50 years, females twice as often as males and is usually not associated with neurological deficits [Katusic et al.,1991; Zakrzewska and Lopez,2005]. In most cases, TN is caused by a vascular compression of the trigeminal nerve near its root‐entry‐zone at the brain stem [Kress et al.,2005; Lovely and Jannetta,1997; McLaughlin et al.,1999; Rasche et al.,2006; Tronnier et al.,2001; Zorman and Wilson,1984]. Although initial treatment is always based on anticonvulsants [Tenser,1998; Wiffen et al.,2000; Zakrzewska and Lopez,2005], neurosurgery can be an option in selected patients irresponsive to medical therapy. Microvascular decompression using Jannetta technique, interposing a prosthesis between the trigeminal nerve and the irritating vessel, has proved to be particularly effective in the treatment of TN with success rates of long‐term pain relief up to 80% [Barker et al.,1996; Jannetta,1985; Lovely and Jannetta,1997; Tenser,1998; Tronnier et al.,2001; Tyler‐Kabara et al.,2002; Zorman and Wilson,1984].
A number of functional imaging studies have dealt with the topic of cortical pain processing in human subjects and identified a variety of cortical regions encompassed in the so called “pain matrix” [Apkarian et al.,2005; Davis,2000; Derbyshire,2000; Ingvar,1999; Jones et al.,2002; Schnitzler and Ploner,2000; Tracey,2005; Treede et al.,1999]. While bilateral SII activation was consistently found during the application of painful stimuli [Brooks et al.,2002; Bushnell et al.,1999; Coghill et al.,1999; Ferretti et al.,2003; Talbot et al.,1991], the implication of SI is more controversial [Borsook et al.,2004; Bromm,2001; Bushnell et al.,1999; Peyron et al.,2000]. It is, however, currently believed that somatosensory cortices are responsible for the perception of sensory features of pain [Apkarian et al.,2005; Bushnell et al.,1999; Tracey,2005] and involved in emotional aspects of pain perception via a cortico‐limbic somatosensory pathway [Gracely et al.,2004; Porro et al.,2002; Price,2000; Sawamoto et al.,2000]. Although information from touch and pain receptors is relayed in the spinal cord and brain stem over distinct pathways, there is convergence of inputs in the thalamus and overlapping processing and integration of tactile and painful information at the level of somatosensory cortex [Basbaum and Jessell,2000]. This implies that pain perception might not only be associated with altered processing within the “pain matrix” [Derbyshire,2000], but also with modulation of somatosensory function itself.
In our previous study, we used functional magnetic resonance imaging (fMRI) to address functional connectivity of the somatosensory cortex in healthy subjects, providing a methodological and interpretational tool for the investigation of somatosensory BOLD‐signals under pathological conditions [Blatow et al.,2007]. Here, we examined tactile somatosensory function in patients with TN and patients relieved from pain by successful surgical treatment in comparison with healthy subjects. We used fMRI with unilateral tactile stimulation of lips and fingers, to probe one sensory area that is involved in trigeminal pain (lips) and another that is not (fingers) and asked the following questions: (i) Is fMRI with tactile stimulation feasible in patients with TN? (ii) Does unilateral tactile stimulation in patients with TN elicit different somatosensory activations depending on the affected body side? (iii) Are somatosensory activations in patients with TN different before and after successful pain relief? (iv) Are there fundamental changes in somatosensory processing in patients with TN compared to healthy subjects?
MATERIALS AND METHODS
Study Population
In total, 28 right‐handed patients and 13 volunteers (aged 25–70 years, 5 men, 8 women) participated in the study after written informed consent to the study protocol, which was approved by the Heidelberg Medical Faculty ethics committee and in line with the Declaration of Human Rights, Helsinki, 2002. Handedness was assessed using a modified version of the Annett questionnaire [Annett,1970]. Data from the control group was used in part for a previous publication [Blatow et al.,2007].
First patient group (before neurosurgery)
Eighteen patients (aged 48–73 years, 4 men, 14 women) were selected for the first group, of which 11 had TN on the right side and 7 had TN on the left side. TN was due to neurovascular compression as revealed on high‐resolution structural MRI. Pain location affected the maxillary (V2) and mandibular (V3) branch of the trigeminal nerve in 9 patients, only V2 in 8 and only V3 in 1 patient. Patients for the first group were only selected if they fulfilled the criteria for neurosurgical intervention, e.g., they had a pain history of several years and suffered from regular pain attacks refractory to their medication. None of the patients had a sensory deficit of the affected face area as measured by clinical neurological examination. Medication of patients is summarized in Table I.
Table I.
Patient medication
| Medication | Dosage (mg/d) | |
|---|---|---|
| Patients with TN | ||
| 1 | Amitriptyline | 50 |
| 2 | Carbamazepine | 300 |
| 3 | Carbamazepine | 200 |
| 4 | Amitriptyline | 50 |
| 5 | Gabapentin | 1,000 |
| 6 | Carbamazepine | 800 |
| 7 | Gabapentin, Phenytoin | 1,500, 300 |
| 8 | Gabapentin, Phenytoin | 800, 200 |
| 9 | Gabapentin | 900 |
| 10 | Gabapentin | 1,800 |
| 11 | Amitriptyline | 50 |
| 12 | Carbamazepine, Valproic acid | 1,200, 2,000 |
| 13 | Carbamazepine, Amitriptyline | 300, 25 |
| 14 | Carbamazepine, Amitriptyline | 800, 50 |
| 15 | Carbamazepine | 800 |
| 16 | Carbamazepine | 1,200 |
| 17 | Carbamazepine | 600 |
| 18 | Gabapentin | 500 |
| Patients post‐OP | ||
| 1 | Carbamazepine | 100 |
| 2 | Carbamazepine | 100 |
| 3 | Carbamazepine | 200 |
| 4 | None | |
| 5 | None | |
| 6 | None | |
| 7 | None | |
| 8 | None | |
| 9 | None | |
| 10 | None | |
Second patient group (after neurosurgery)
Ten patients (aged 44–76 years, 4 men, 6 women) were selected for the second group, all with a history of TN on the right side, relieved from pain for at least 4 months (range between 4 and 12 months) by successful surgical treatment (microvascular decompression using Jannetta technique). None of the patients had a postoperatively acquired sensory deficit of the face. Seven patients received no medication and 3 patients received minimal medication (Table I). Of the 11 patients with right side TN from the first group only 6 were considered for the postoperative group because of either residual pain after surgery (n = 2), subthreshold activation in fMRI (n = 1) or nonattendance at the follow‐up fMRI for unknown reasons (n = 2).
Functional and Morphological MRI
To keep experimental conditions as similar as possible for patients and controls, all subjects were prepared for the experiments in the same way, provided with earplugs and instructed to gaze at a fixation point during the measurements, which were performed at approximately the same time of the day for all patients and controls. A visual analog scale was used before every fMRI measurement to make sure that patients were not having a pain attack in the moment of the experiment. Only patients with VAS = 0 were measured. Subjects were positioned in a clinical 1.5 Tesla MR‐imager (Siemens Magnetom Symphony) using a conventional birdcage head‐coil. Movement artifacts were reduced by relaxed positioning of the extremities and fixing the head with preformed foam cushions. Data sets contaminated with movement artifacts lager than 1 mm (translation) or 1 degree (rotation) were excluded from evaluation. After the measurement patients were specifically questioned about the occurrence of painful sensations during the stimulation, which, however, none of the patients reported. Standardized block‐designed BOLD‐fMRI was performed with fully automated nonpainful pneumatically‐driven unilateral tactile stimulation (duration = 50 ms, frequency = 4 Hz, air pressure = 3 bar, contact force = 100 mN/cm2) of fingers (digits 1 and 2 simultaneously) and lips (upper and lower) using high‐resistance pneumatic tubes (length 3.5 m, diameter 2 mm) and finger clips/face mask with a flexible membrane (BTI, San Diego, CA). Stimulations were applied for each body side and location separately (see Fig. 1). During somatotopic mapping, each volunteer had a total of four different standardized block‐designed whole brain BOLD‐fMRI measurements (somatosensory stimulations: fingers left, fingers right, lips left, lips right) using a single‐shot, blipped gradient echo Echo‐Planar‐Imaging sequence (GE‐EPI, TR = 3,000 ms, TE = 80 ms, FOV = 256 × 256 mm2, matrix = 128 × 128 voxels, flip angle = 90°, 22 contiguous axial images, slice thickness 5 mm, gap 1 mm). Each single measurement consisted of one offset, six baseline, and five stimulation intervals (duration 30 s each) resulting in a scanning time of 360 s. Individual T1‐weighted structural 3D‐MRI data sets (RF‐spoiled FLASH sequence, TR = 30 ms, TE = 4.4 ms, 144 sagittal slices, slice thickness 1 mm) were acquired to superimpose functional on structural images [Blatow et al.,2007; Stippich et al.,2004,2005].
Figure 1.
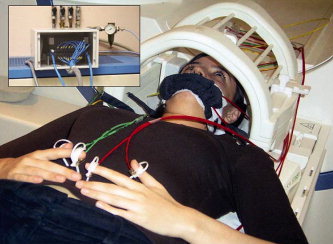
Pneumatic stimulation device for fMRI. Fully automated nonpainful pneumatically‐driven tactile stimulation using high resistance pneumatic tubes and finger clips/face mask with a flexible membrane was applied for each body side and location separately. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Standardized Analysis of fMRI Data
BrainVoyager® QX (Version 1.4; BrainInnovation, Maastricht, Netherlands) was used for standardized processing and analysis of structural as well as functional MRI‐data that included motion correction, spatial and temporal smoothing and a voxel‐wise calculation of BOLD‐activation using linear cross‐correlations. Data processing was fully standardized and automated except for the manual overlay of functional on structural MR‐images and for the individual definition of reference points required for spatial normalization. All structural and functional datasets were transformed to Talairach‐space [Talairach and Tournoux,1988] and evaluated on an individual basis. Six different regions of interest (ROIs) were defined for each experiment, namely the primary somatosensory areas of finger or lip representations, respectively, located in the postcentral gyrus of both hemispheres, the corresponding secondary somatosensory areas located caudally in the parietal operculum and the thalamus in both hemispheres. In each ROI, the exact anatomical correlates of functional activations were assessed on transverse, sagittal, and coronal sections. The Euclidean coordinates of the center of gravity (COG) of each BOLD‐cluster were determined along with the corresponding BOLD‐signal characteristics (correlation of the measured BOLD‐signal to the applied hemodynamic reference function (hrf) = r; relative BOLD‐signal change = ΔS (%)). To precisely analyze each individual functional dataset a standardized evaluation routine was used applying a dynamic statistical threshold [Blatow et al.,2007; Stippich et al.,2004,2005,2007]: a cluster size of ∼36 mm3 was used as the standard for data evaluation to achieve a precise determination of the anatomical correlates of the different functional activations by also eliminating very small clusters in the activation maps. At first, a very high statistical threshold value for the correlation (r) between the measured BOLD‐signals and the hrf was selected so that no functional activation was displayed (empty map). This threshold was then continually reduced. As a result, the activation with the highest correlation to the hrf that exceeded the cluster size of 36 mm3 was displayed first. By further reduction of the threshold, activations in other functional areas with lower correlations between the measured BOLD‐signals and the hrf appeared progressively. This procedure was continued until activations were identified in all ROIs. A threshold of r = 0.25 with P < 0.05 (Bonferoni corrected) was established as lower limit to ensure that BOLD‐signals were clearly distinguishable from background noise. If no BOLD‐signal was displayed in a ROI within the lower limit, this was evaluated as “no activation.” Likewise, BOLD‐signals with a relative change of ΔS > 4% were not included in the evaluation because such high‐level somatosensory activation is likely to originate from draining veins rather than from capillaries. All BOLD‐signals were evaluated and statistically compared on an individual basis; however, for Figures 3, 4, 5 group‐level fMRI maps were used for display to visualize group effects.
Figure 3.
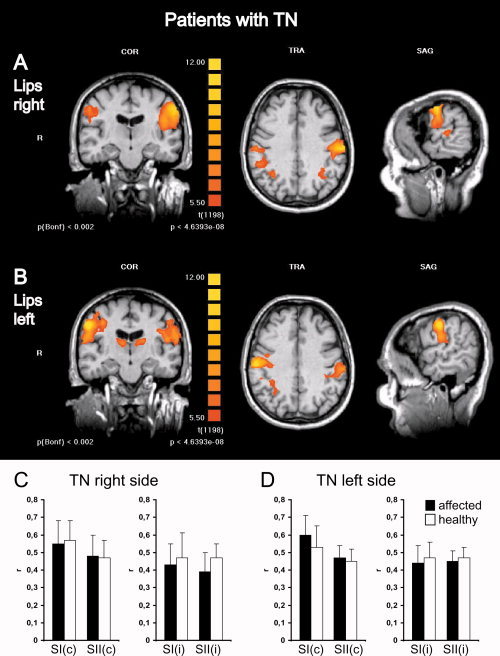
Somatosensory activations in patients with TN show no differences depending on stimulation of the affected or unaffected body side. Group level fMRI of bilateral somatosensory activations in the postcentral gyrus and parietal operculum in patients with TN on the right side are shown for stimulation of the right (A) and left (B) lips. To conserve anatomical details, group level BOLD‐activation maps (n = 11) were overlaid onto anatomical images of one individual subject. Left panels show a coronal view (COR) through the plane of the postcentral gyrus, middle panels a transversal view (TRA) through the plane of SI activations and left panels a sagittal view (SAG) through the plane of contralateral activations. R, right, L, left. For display of group data (different from Fig. 2) colors depicting the t‐statistic with uncorrected and Bonferoni corrected P‐values were chosen to facilitate comparison with other studies where mostly t‐values are presented. Each activation map had a false discovery rate (FDR) < 0.001. Statistical thresholds were deliberately kept equal to allow comparison of activation maps. No major differences in activation levels can be observed depending on the stimulation side. Note bilateral thalamic activations in (B) (left panel) and coactivations in the superior parietal lobules in (A) and (B) (middle panel). Histograms depict quantitative analyses of r‐values (mean ± SD) comparing SI and SII activations elicited by lip stimulation on the affected (black bars) or the healthy side (white bars) for patients with TN on the right side (C) and left side (D) separately. c, contralateral; i, Ipsilateral; r, correlation of the measured BOLD‐signal to the applied hemodynamic reference function.
Figure 4.
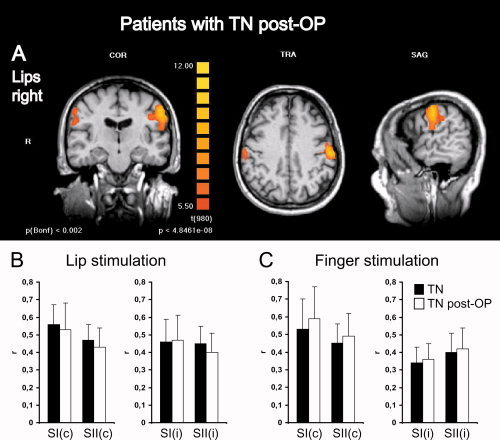
Somatosensory activations in patients with TN show no differences before and after neurosurgical treatment and prolonged pain relief. (A) Group level fMRI of bilateral somatosensory activations in the postcentral gyrus and parietal operculum in patients with TN on the right side after successful microvascular decompression by Jannetta technique are shown for stimulation of the right lips. To conserve anatomical details, group level BOLD‐activation maps (n = 10) were overlaid onto anatomical images of one individual subject. Left panels show a coronal view (COR) through the plane of the postcentral gyrus, middle panels a transversal view (TRA) through the plane of SI activations and left panels a sagittal view (SAG) through the plane of contralateral activations. R, right; L, left. Colors depict the t‐statistic with uncorrected and Bonferoni corrected P‐values. Statistical thresholds were chosen as in Figure 3 to allow comparison of activation maps. Histograms depict quantitative analyses of r‐values (mean ± SD) comparing SI and SII activations in patients with TN before (black bars) and after pain relief (white bars) for lip (B) and finger (C) stimulations. c, contralateral; i, ipsilateral; r, correlation of the measured BOLD‐signal to the applied hemodynamic reference function.
Figure 5.
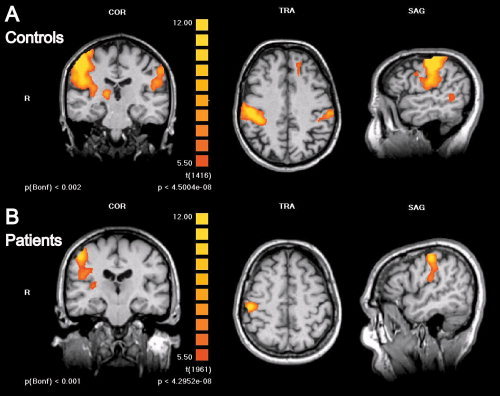
Somatosensory activations are significantly reduced in patients with TN as compared to healthy subjects. Group level fMRI of bilateral somatosensory activations in the postcentral gyrus and parietal operculum in control subjects (n = 13) (A) and patients with TN (n = 18) (B) are shown for stimulation of the right fingers. Activation maps are presented as in Figures 3 and 4 and statistical thresholds for image display were kept constant for easier comparison. BOLD‐activation is visibly reduced in patients versus controls; at the chosen threshold no ipsilateral SI/SII activation is displayed in the patients' fMRI. Note contralateral thalamic activation in (A) (left panel).
Statistical Analysis
Descriptive analysis (calculation of the mean and the standard deviation) was made for correlations of BOLD‐signals to the applied hemodynamic reference function (r), relative BOLD‐signal changes (ΔS), and Euclidean coordinates of COGs (x, y, z). All calculations were made for each region of interest in individual subjects. Statistical analysis was performed on r‐values. ΔS‐values usually showed the same trend as r‐values, but displayed a stronger variability. In our experience correlations of BOLD‐signals to the applied hemodynamic reference function (r‐values) are a more suitable parameter for the quality of fMRI activations than relative BOLD‐signal changes (ΔS‐values) in particular for quantitative comparison, because r‐values are less variable between subjects and more reliable in consecutive measurements in the same subject. In addition using a dynamic statistical threshold for data analysis, r‐values allow a more precise determination of COGs of BOLD‐activations [Blatow et al.,2007; Stippich et al.,2004,2005,2007]. For the assessment of the statistical significance of differences, data were first analyzed for normal distribution using the Lilliefors Test. In 14% of data sets normal distribution could not be confirmed, presumably due to the small sample size. To account for this and for variable sample size, nonparametrical Wilcoxon Rank‐Sum Test was used for all comparisons. To test for correlation of r‐values with age regression analysis was performed using the Pearson's correlation coefficient R plotting z(r) against age with z(r) = (r − r mean)/SD. The coefficient of determination R 2 = 0.01 indicated no correlation of r‐values with age. Statistical calculations were done in MATLAB 6.5 (MathWorks, Natick, MA).
RESULTS
Detection of SI, SII, and Thalamic Activations in Patients With TN and Healthy Control Subjects
Unilateral tactile stimulation of lips and fingers elicited BOLD‐activations of contra‐ and ipsilateral SI and SII cortex in the somatotopically corresponding representations of the postcentral gyrus in patients with TN and healthy control subjects. In each subject significant BOLD‐activations were clearly distinguishable between SI and SII regions (see Fig. 2). In the case of lip stimulation SII activations were located 10–15 mm caudally of SI activations; for finger stimulation SII activations were located 20–25 mm caudally of SI activations. During lip stimulation occurrence probability of contralateral somatosensory activation was 100% for SI and 88% for SII in controls, 97% for SI and 92% for SII in patients with TN, and 100% for SI and 85% for SII in TN post‐OP patients. In addition, we observed ipsilateral somatosensory activation in 100% for SI and 81% for SII in controls, 78% for SI and 94% for SII in patients with TN, and 50% for SI and 100% for SII in TN post‐OP patients (Table II). Thalamic activity was recorded to monitor input function to the somatosensory cortex in all three groups (Fig. 2; Table II). During finger stimulation occurrence probability of contralateral SI and SII activation was 96% for SI and 100% for SII in controls, 89% for SI and 92% for SII in patients with TN, and 100% for SI and 85% for SII in TN post‐OP patients. Ipsilateral SI and SII activation could be measured in 69% for SI and 96% for SII in controls, 42% for SI and 83% for SII in patients with TN, and 30% for SI and 90% for SII in TN post‐OP patients (Table II). Correlations of the measured BOLD‐signals to the applied hemodynamic reference function (r), relative BOLD‐signal changes (ΔS), and Euclidean coordinates of COGs were measured for individual significant activations and are summarized in Table III. These results suggest that nonpainful sensory paradigms can be successfully and reliably applied in patients with TN yielding comparable qualitative somatosensory BOLD‐activation patterns and success rates as in healthy control subjects.
Figure 2.
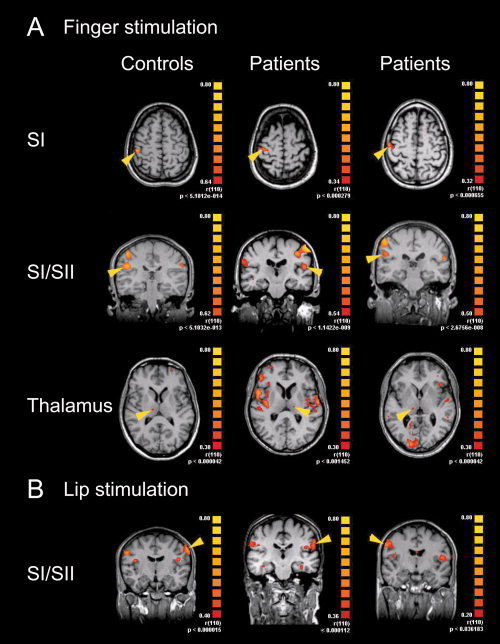
Representative fMRI of SI, SII, and thalamic activations during finger and lip stimulations in individual subjects. Unilateral stimulation of left or right fingers (A) or lips (B) elicited BOLD‐activations in SI and SII areas and thalamus in controls (left panel), patients with TN (middle panel) and patients with TN after neurosurgical intervention (right panel). Representative examples are chosen for left or right stimulations in transversal or coronal views, yellow arrowheads point to activations contralateral to the stimulus. For display of individual data colors depicting the r‐statistic with uncorrected P‐values for the entire activation map were chosen to facilitate comparison with r‐values presented in this study. Each cluster fulfilled criteria of significance with a lower threshold of r > 0.25 and P < 0.05 (Bonferoni corrected; see methods section).
Table II.
Occurrence probabilities of SI, SII, and thalamic activations
| Subjects | Stimulation | Representation | |||||
|---|---|---|---|---|---|---|---|
| SI (c) | SII (c) | Thalamus (c) | SI (i) | SII (i) | Thalamus (i) | ||
| Controls (n = 13) | Lips | 100 | 88 | 81 | 100 | 81 | 65 |
| Fingers | 96 | 100 | 92 | 69 | 96 | 65 | |
| Patients with TN (n = 18) | Lips | 97 | 92 | 72 | 78 | 94 | 64 |
| Fingers | 89 | 92 | 61 | 42 | 83 | 47 | |
| Patients post‐OP (n = 10) | Lips | 100 | 85 | 70 | 50 | 100 | 50 |
| Fingers | 100 | 85 | 85 | 30 | 90 | 60 | |
Occurrence probabilities (in %) of contra‐ (c) and ipsilateral (i) SI and SII and thalamic activations elicited by each different sensory paradigm in control subjects, patients with TN (right and left side) and patients with TN after neurosurgical intervention (post‐OP).
Table III.
BOLD‐signal characteristics and Euclidean coordinates of SI, SII, and thalamic activations
| Subjects | Stimulation | Activation | n | r | ΔS | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Controls (n = 13) | Lips | SI (c) | 26 | 0.63 ± 0.10 | 1.05 ± 0.46 | 54 ± 3 | −15 ± 7 | 37 ± 5 |
| SII (c) | 23 | 0.50 ± 0.09 | 0.73 ± 0.51 | 52 ± 7 | −21 ± 9 | 21 ± 6 | ||
| Thalamus (c) | 21 | 0.40 ± 0.09 | 0.52 ± 0.30 | 15 ± 6 | −19 ± 7 | 7 ± 5 | ||
| SI (i) | 26 | 0.50 ± 0.09 | 0.83 ± 0.45 | 55 ± 4 | −14 ± 10 | 35 ± 6 | ||
| SII (i) | 21 | 0.44 ± 0.09 | 0.62 ± 0.23 | 53 ± 7 | −23 ± 10 | 21 ± 5 | ||
| Thalamus (i) | 17 | 0.38 ± 0.08 | 0.51 ± 0.41 | 13 ± 2 | −20 ± 7 | 7 ± 5 | ||
| Fingers | SI (c) | 25 | 0.72 ± 0.11 | 1.34 ± 0.69 | 49 ± 4 | −18 ± 10 | 46 ± 4 | |
| SII (c) | 26 | 0.58 ± 0.13 | 0.87 ± 0.34 | 48 ± 6 | −19 ± 6 | 18 ± 6 | ||
| Thalamus (c) | 24 | 0.40 ± 0.09 | 0.40 ± 0.20 | 14 ± 5 | −18 ± 6 | 7 ± 4 | ||
| SI (i) | 18 | 0.49 ± 0.17 | 0.73 ± 0.47 | 47 ± 7 | −19 ± 7 | 48 ± 6 | ||
| SII (i) | 25 | 0.47 ± 0.12 | 0.59 ± 0.28 | 52 ± 6 | −20 ± 7 | 20 ± 6 | ||
| Thalamus (i) | 17 | 0.33 ± 0.09 | 0.32 ± 0.12 | 11 ± 4 | −18 ± 6 | 8 ± 4 | ||
| Patients with TN (n = 18) | Lips | SI (c) | 35 | 0.56 ± 0.12 | 0.66 ± 0.33 | 52 ± 5 | −16 ± 5 | 38 ± 8 |
| SII (c) | 33 | 0.47 ± 0.09 | 0.46 ± 0.24 | 50 ± 8 | −21 ± 9 | 22 ± 8 | ||
| Thalamus (c) | 26 | 0.37 ± 0.09 | 0.32 ± 0.12 | 13 ± 4 | −15 ± 8 | 6 ± 6 | ||
| SI (i) | 28 | 0.46 ± 0.13 | 0.47 ± 0.21 | 52 ± 6 | −17 ± 4 | 38 ± 8 | ||
| SII (i) | 34 | 0.45 ± 0.10 | 0.55 ± 0.39 | 52 ± 9 | −22 ± 9 | 24 ± 8 | ||
| Thalamus (i) | 23 | 0.39 ± 0.09 | 0.38 ± 0.18 | 13± 5 | −15 ± 6 | 8 ± 6 | ||
| Fingers | SI (c) | 32 | 0.53 ± 0.17 | 0.72 ± 0.37 | 48 ± 8 | −19 ± 5 | 47 ± 5 | |
| SII (c) | 33 | 0.44 ± 0.11 | 0.47 ± 0.19 | 48 ± 7 | −23 ± 10 | 21 ± 6 | ||
| Thalamus (c) | 22 | 0.39 ± 0.10 | 0.38 ± 0.18 | 12 ± 4 | −17 ± 5 | 5 ± 4 | ||
| SI (i) | 15 | 0.34 ± 0.09 | 0.35 ± 0.16 | 48 ± 7 | −20 ± 5 | 45 ± 6 | ||
| SII (i) | 30 | 0.40 ± 0.11 | 0.43 ± 0.27 | 50 ± 8 | −22 ± 12 | 22 ± 7 | ||
| Thalamus (i) | 17 | 0.36 ± 0.10 | 0.37 ± 0.25 | 12 ± 6 | −16 ± 6 | 7 ± 5 | ||
| Patients post‐OP (n = 10) | Lips | SI (c) | 20 | 0.53 ± 0.16 | 0.77 ± 0.56 | 54 ± 6 | −15 ± 6 | 40 ± 8 |
| SII (c) | 17 | 0.43 ± 0.11 | 0.41 ± 0.13 | 51 ± 8 | −19 ± 7 | 26 ± 7 | ||
| Thalamus (c) | 14 | 0.37 ± 0.10 | 0.52 ± 0.43 | 12 ± 5 | −16 ± 6 | 7 ± 6 | ||
| SI (i) | 10 | 0.47 ± 0.14 | 0.64 ± 0.23 | 56 ± 3 | −17 ± 7 | 38 ± 4 | ||
| SII (i) | 20 | 0.40 ± 0.11 | 0.38 ± 0.14 | 54 ± 5 | −21 ± 12 | 27 ± 7 | ||
| Thalamus (i) | 10 | 0.37 ± 0.10 | 0.38 ± 0.35 | 12 ± 5 | −19 ± 6 | 7 ± 6 | ||
| Fingers | SI (c) | 20 | 0.59 ± 0.18 | 0.96 ± 0.56 | 47 ± 7 | −20 ± 5 | 49 ± 6 | |
| SII (c) | 17 | 0.49 ± 0.13 | 0.58 ± 0.26 | 48 ± 8 | −22 ± 7 | 22 ± 6 | ||
| Thalamus (c) | 17 | 0.36 ± 0.06 | 0.35 ± 0.15 | 14 ± 5 | −15 ± 3 | 7 ± 4 | ||
| SI (i) | 6 | 0.36 ± 0.09 | 0.45 ± 0.22 | 49 ± 11 | −23 ± 6 | 47 ± 4 | ||
| SII (i) | 18 | 0.42 ± 0.12 | 0.43 ± 0.20 | 53 ± 9 | −23 ± 12 | 27 ± 7 | ||
| Thalamus (i) | 12 | 0.36 ± 0.06 | 0.34 ± 0.06 | 12 ± 4 | −15 ± 5 | 7 ± 4 |
Correlations of the measured BOLD‐signals to the applied hemodynamic reference function (r). Relative BOLD‐signal changes (ΔS%) and Euclidean coordinates of COGs (x, y, z) are depicted for contra‐ (c) and ipsilateral (i) SI, SII, and thalamic activations for each sensory paradigm in control subjects, patients with TN (right and left side) and patients with TN after neurosurgical intervention (post‐OP). Data from right and left stimulation sides were pooled and X‐coordinates all transferred into positive values. Data are given as mean ± SD.
Somatosensory Activations in Patients With TN Show No Differences Depending on Stimulation of the Affected or Unaffected Body Side
Because TN is in most cases a strictly unilateral phenomenon, our first hypothesis was that unilateral tactile stimulation may have lateralized effects on somatosensory activation depending on whether the stimulus was applied to the affected or unaffected body side. If there was a lateralized effect, it might be more pronounced for stimulation of the lips, being an area involved in trigeminal pain. To test this hypothesis we compared correlations of the measured BOLD‐signals to the applied hemodynamic reference function (r‐values) in patients with TN between right and left stimulation sides. As an internal control patients with TN on the right side and on the left side were treated independently. Wilcoxon Rank‐Sum test was used for all comparisons, the resulting P‐values were evaluated with a level of significance at α = 5% (i.e. 5% of all P‐values had to be smaller than 0.05 to assume a difference). The analysis revealed no significant P‐values in both TN right and left patient groups for both lip and finger stimulation (see Fig. 3). Thus, fMRI could not detect a lateralized effect on activation in SI and SII depending on the affected body side, neither for lip nor for finger stimulation. Therefore, for further analysis patients with TN on the right and left side were evaluated together.
Somatosensory Activations in Patients With TN Show No Differences Before and After Neurosurgical Treatment and Prolonged Pain Relief
For the second patient group of this study, patients who had undergone microvascular decompression using Jannetta technique and were free of pain and medication for at least 4 months were selected. This allowed us to ask the question whether successful treatment of pain had an influence on somatosensory activation in patients with TN. To address this issue, we compared r‐values of BOLD‐activations between patients with TN from the first group (with ongoing pain) with those from the second group (free of pain). No significant differences were found comparing the two groups (see Fig. 4), indicating that there was no measurable effect of medication or neurosurgical treatment on somatosensory activation in these patients.
Somatosensory Activations Are Significantly Reduced in Patients With TN as Compared to Healthy Subjects
The next question was whether somatosensory processing was generally different in patients with TN as compared to healthy control subjects. Because no significant differences of activations were found within and between the patient groups, we chose to examine all patients together versus the control group. However, we also analyzed the groups individually and obtained similar results. Comparing r‐values of BOLD‐activations between patients with TN and controls revealed a significant reduction of both SI and SII activations in patients with TN (see Fig. 5). These differences were most obvious for finger stimulation: SI activations contralateral to the stimulus had significantly lower r‐values in patients with TN (r = 0.55 ± 0.17) as compared to controls (r = 0.72 ± 0.11; P < 0.00001); likewise contralateral SII activations had significantly lower r‐values in patients with TN (r = 0.46 ± 0.12) as compared to controls (r = 0.58 ± 0.13; P = 0.00002). Also ipsilateral activations had significantly lower r‐values in patients with TN, although the differences were less marked: for ipsilateral SI (patient, r = 0.34 ± 0.08; control, r = 0.49 ± 0.17; P = 0.003) and for ipsilateral SII (patient, r = 0.41 ± 0.11; control, r = 0.47 ± 0.12; P = 0.03). Thalamic activation, however, was comparable between patients and controls (Fig. 6A).
Figure 6.
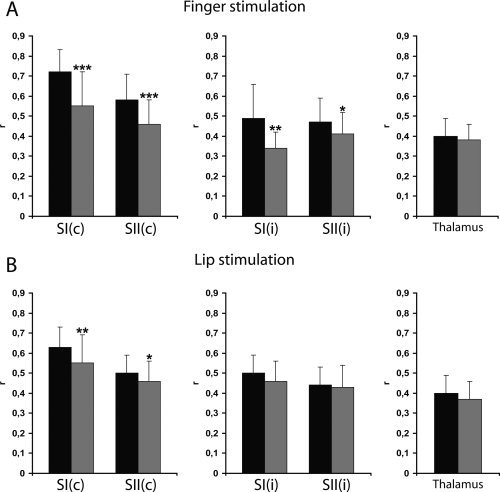
Quantitative assessment of somatosensory activations in patients with TN and healthy subjects. Correlations of BOLD‐signals to the applied hemodynamic reference function (r; mean ± SD) are plotted for contra‐ and ipsilateral SI and SII and contralateral thalamic activations for finger (A) and lip (B) stimulations in healthy controls (black bars) and patients with TN (gray bars). c, contralateral; i, ipsilateral. Levels of statistical significance: ***P < 0.0001, **P < 0.01, *P < 0.05.
For lip stimulation, patients with TN had lower r‐values in contralateral SI (r = 0.55 ± 0.14) and SII (r = 0.46 ± 0.10) activations than healthy subjects (SI: r = 0.63 ± 0.10; P = 0.007; SII: r = 0.50 ± 0.09; P < 0.05), but no significant differences were found between ipsilateral SI and SII or thalamic activations in patients with TN versus controls (Fig. 6B). These results show a general reduction of SI and SII activation in patients with TN upon unilateral tactile stimulation. This reduction does not seem to be present at the level of the thalamus.
DISCUSSION
The main findings of this study can be summarized as follows: (i) nonpainful tactile stimulation of lips and fingers in patients with TN yielded qualitatively comparable somatosensory activation patterns as in healthy subjects. (ii) SI and SII activations in patients with TN showed no differences depending on whether the stimulation concerned the affected or the unaffected body side. (iii) SI and SII activations in patients with TN did not change after pain relief by neurosurgical intervention. (iv) However, SI and SII activations in patients with TN were significantly reduced as compared to healthy subjects.
This is the first study that uses fMRI to investigate tactile somatosensory function in patients with TN. TN is distinctive in that pain can be triggered by normally innocuous stimuli, pointing to a pathological interference between sensory and pain information. In terms of its pathophysiology, TN results most likely from demyelinization of the trigeminal nerve at its root‐entry‐zone leading to nerve atrophy [Herweh et al.,2007; Kress et al.,2005] and cross‐excitation between axons mediating touch and pain sensation [Love et al.,1998]. This is in contrast to the pathophysiology of neuropathic pain which is a consequence of deafferentiation after traumatic or other nerve injury [Jones,2000]. Accordingly, clinical appearances of neuralgic and neuropathic pain are markedly different. While patients with TN typically experience a sharp pain that lasts for seconds only and may recur many times a day, patients with neuropathic pain are in constant pain [Tronnier et al.,2001]. In addition, TN is typically not associated with sensory deficits or other neurological symptoms, whereas neuropathic pain goes along with hypesthesia, hyperalgesia, or allodynia of the painful territory. Although lateralized alteration of somatosensory processing was observed in previous studies in patients with various neuropathic pain disorders associated with unilateral peripheral sensory deficits [Apkarian et al.,2005; Becerra et al.,2006; Juottonen et al.,2002; Maihofner et al.,2005; Peyron et al.,2004; Pleger et al.,2006], one would not necessarily expect lateralized effects in this study, because patients with TN have no neurological deficits and were all examined outside pain attacks. Furthermore, none of the patients reported the occurrence of painful sensations during the experiments.
Effects of cortical reorganization comprising the somatosensory cortex can be seen soon after nerve injury [Jones,2000]. In TN, most likely demyelinization leads to pathological peripheral input [Love et al.,1998]. Considering the long pain history of most patients with TN, changes at the cortical level have many years to evolve. Interestingly, pain relief after neurosurgery is usually immediate [Tronnier et al.,2001], which cannot be attributed to a remyelinization of axons. In a later study at 3 Tesla, we found a case of possible remyelinization of the trigeminal nerve as demonstrated by diffusion tensor imaging in a patient with TN 5 months after pain relief [Herweh et al.,2007]. In this study, the pain‐free time interval in the postoperative patient group between surgery and fMRI was between 4 and 12 months. This time may be sufficient for axonal remyelinization, but possibly not for the reversal of cortical changes, and studies at even later time points would be required to address this issue.
BOLD signals can be altered due to psychoactive drugs, as in the case of anticonvulsive medication in TN. However, it is difficult to predict the influence of medication in this study. Jokeit et al. [2001] demonstrated a dose‐dependent reduction of BOLD signals after application of Carbamazepine in the mesial temporal lobes in a memory retrieval task. In a novel study, Governo et al. [2008] showed a differential influence (positive and negative effects) of various doses of Gabapentin on BOLD signals in nociceptive brain regions in rats. One may therefore speculate that Carbamazepine could be responsible for a reduction, whereas Gabapentin could produce an increase of BOLD signals in somatosensory cortex. Because our patients from the first group received both these drugs alone or in combination with other substances at various doses, the summative effect on the BOLD signals cannot be assessed. Therefore, for the second group we selected only patients with no or minimal medication. Our results showed no significant differences between somatosensory activations in patients from the first and the second group. Hence, it seems justified to conclude that the observed reduction of activation as compared to healthy subjects was most likely not a result of medication.
The observed reduction of somatosensory activation in patients with TN as compared to healthy subjects was not only bilateral but also extended to the finger representation, a representation not involved in trigeminal pain. This is a strong indicator for a general alteration of somatosensory processing in TN. A possible pathophysiological process could look as follows: the nerve‐vessel conflict progressively leads to demyelinization and cross‐excitation of axons mediating tactile and painful information, thereby resulting in a unilateral pathological peripheral input [Herweh et al.,2007; Kress et al.,2005; Love et al.,1998]. It follows that a tactile input may be relayed to thalamic neurons responsive to painful stimulation. It is known that receptive fields of these neurons in rats are often bilateral, larger and more variable than those of neurons responsive to tactile stimulation [Lamour et al.,1983; Monconduit et al.,2006]. Further, it was demonstrated that thalamocortical inputs following noxious stimulation are not clearly segregated into anatomically distinct regions in rats [Monconduit et al.,2006], and accordingly a less confined somatotopy for noxious compared to innocuous inputs has been observed in human somatosensory cortex [Apkarian et al.,2005]. In addition, there is now growing evidence for bilateral representations of tactile information not only in SII but also in SI in humans [Blatow et al.,2007]. Thus, information derived from sensory inputs is relayed to bilateral somatosensory cortices and may in the case of TN expand to representations other than the stimulated one. In line with this assumption are studies in a rat model for neuropathic pain showing bilateral changes in somatosensory cortex not restricted to the affected somatotopic area [Mao et al.,1993; Paulson et al.,2000]. Benoist et al. proposed a rat model for TN using loose chronic constriction injury of the infraorbital nerve and found a profound disturbance in the somatosensory cortical somatotopy contra‐ and ipsilateral to the affected side and an attenuation of cortical responsiveness [Benoist et al.,1999], which might parallel the findings in this study.
If a unilateral pathological input leads to bilateral cortical changes they must originate either from the thalamus or from the cortex itself. One argument for the cortical origin in this study is the absence of significant differences in thalamic activations between patients and controls although admittedly thalamic activations in fMRI are small und accordingly difficult to quantify. Another argument is the fact that there are nearly ten times as many fibers projecting back from SI to the thalamus as there are in the forward direction from thalamus to cortex implying a powerful endogenous control of the processing of sensory information originating from SI [Briggs and Usrey,2008; Monconduit et al.,2006]. This cortico‐thalamic feedback network comprises both glutamatergic excitatory and GABAergic inhibitory components and is thought to gate sensory stimuli. It can furthermore enable the cortex to dynamically modulate thalamic activity and determine the nature of its own input [Briggs and Usrey,2008]. A GABAergic hypofunction of this network followed by pain hypersensitivity and delayed loss of sensory neurons was proposed as an explanation for neuropathic pain [Canavero and Bonicalzi,1998; DaSilva et al.,2008; Woolf and Salter,2000]. Recent studies have shown under experimental conditions in rats that a decrease of glutamatergic excitatory or alternatively an increase of GABAergic inhibitory drive from SI would reduce thalamic responsiveness to painful stimulation [Monconduit et al.,2006; Wang et al.,2007]. Thus, the observed cortical signal reduction in the present study may reflect a top‐down mechanism to reduce pain related relay. The fact that the changes were less prominent for stimulation of the affected than the nonaffected area might reflect a reduced efficiency of the mechanism in the affected system, in particular in ipsilateral cortices involved in the integration of attentional or emotional components of the stimulus. In line with this idea are results from fMRI studies using painful stimulation showing increased ipsilateral SI and SII responses for pain catastrophizing [Gracely et al.,2004].
Finally, the comparison of BOLD signals has methodological limitations because fMRI is not suitable to provide absolute quantifications of neuronal activity. We tried to improve the validity of our data by first conducting a detailed analysis of somatosensory activation patterns in healthy subjects, providing a methodological and interpretational tool for the investigation of somatosensory BOLD‐signals in TN [Blatow et al.,2007].
CONCLUSION
This study shows a general reduction of somatosensory cortical fMRI activations following nonpainful stimulation in TN. We propose a decreased activity of the cortico‐thalamic feedback network as a putative mechanism for long‐term adaptation to painful stimulation.
Acknowledgements
M. B. was supported by the Olympia‐Morata‐Program of the University of Heidelberg Medical Faculty. E. N. was supported by the Dietmar‐Hopp‐Foundation (Neurocognition and Behavioral Neurology Group, University of Heidelberg). The authors wish to thank Drs. T. Hahn and S. Garbade for help with statistical analysis.
REFERENCES
- Annett M ( 1970): A classification of hand preference by association analysis. Br J Psychol 61: 303–321. [DOI] [PubMed] [Google Scholar]
- Apkarian AV,Bushnell MC,Treede RD,Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Barker FG II,Jannetta PJ,Bissonette DJ,Larkins MV,Jho HD ( 1996): The long‐term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 334: 1077–1083. [DOI] [PubMed] [Google Scholar]
- Basbaum AI,Jessell TM ( 2000): The perception of pain In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw‐Hill; pp 472–491. [Google Scholar]
- Becerra L,Morris S,Bazes S,Gostic R,Sherman S,Gostic J,Pendse G,Moulton E,Scrivani S,Keith D,Chizh B,Borsook D ( 2006): Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 26: 10646–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist JM,Gautron M,Guilbaud G ( 1999): Experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve: Changes in neuronal activities in the somatosensory cortices corresponding to the infraorbital nerve. Exp Brain Res 126: 383–398. [DOI] [PubMed] [Google Scholar]
- Blatow M,Nennig E,Durst A,Sartor K,Stippich C ( 2007): fMRI reflects functional connectivity of human somatosensory cortex. Neuroimage 37: 927–936. [DOI] [PubMed] [Google Scholar]
- Borsook D,Burstein R,Becerra L ( 2004): Functional imaging of the human trigeminal system: Opportunities for new insights into pain processing in health and disease. J Neurobiol 61: 107–125. [DOI] [PubMed] [Google Scholar]
- Briggs F,Usrey WM ( 2008): Emerging views of corticothalamic function. Curr Opin Neurobiol 18: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B ( 2001): Brain images of pain. News Physiol Sci 16: 244–249. [DOI] [PubMed] [Google Scholar]
- Brooks JC,Nurmikko TJ,Bimson WE,Singh KD,Roberts N ( 2002): fMRI of thermal pain: Effects of stimulus laterality and attention. Neuroimage 15: 293–301. [DOI] [PubMed] [Google Scholar]
- Bushnell MC,Duncan GH,Hofbauer RK,Ha B,Chen JI,Carrier B ( 1999): Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96: 7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S,Bonicalzi V ( 1998): The neurochemistry of central pain: evidence from clinical studies, hypothesis and therapeutic implications. Pain 74: 109–114. [DOI] [PubMed] [Google Scholar]
- Coghill RC,Sang CN,Maisog JM,Iadarola MJ ( 1999): Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol 82: 1934–1943. [DOI] [PubMed] [Google Scholar]
- DaSilva AF,Becerra L,Pendse G,Chizh B,Tully S,Borsook D ( 2008): Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS ONE 3: e3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD ( 2000): The neural circuitry of pain as explored with functional MRI. Neurol Res 22: 313–317. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW ( 2000): Exploring the pain “neuromatrix”. Curr Rev Pain 4: 467–477. [DOI] [PubMed] [Google Scholar]
- Ferretti A,Babiloni C,Gratta CD,Caulo M,Tartaro A,Bonomo L,Rossini PM,Romani GL ( 2003): Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: An fMRI study. Neuroimage 20: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Governo RJ,Morris PG,Marsden CA,Chapman V ( 2008): Gabapentin evoked changes in functional activity in nociceptive regions in the brain of the anaesthetized rat: an fMRI study. Br J Pharmacol 153: 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH,Geisser ME,Giesecke T,Grant MA,Petzke F,Williams DA,Clauw DJ ( 2004): Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127 (Part 4): 835–843. [DOI] [PubMed] [Google Scholar]
- Herweh C,Kress B,Rasche D,Tronnier V,Troger J,Sartor K,Stippich C ( 2007): Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology 68: 776–778. [DOI] [PubMed] [Google Scholar]
- Ingvar M ( 1999): Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci 354: 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannetta PJ ( 1985): Microsurgical management of trigeminal neuralgia. Arch Neurol 42: 800. [DOI] [PubMed] [Google Scholar]
- Jokeit H,Okujava M,Woermann FG ( 2001): Carbamazepine reduces memory induced activation of mesial temporal lobe structures: A pharmacological fMRI‐study. BMC Neurol 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG ( 2000): Cortical and subcortical contributions to activity‐dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci 23: 1–37. [DOI] [PubMed] [Google Scholar]
- Jones AK,Kulkarni B,Derbyshire SW ( 2002): Functional imaging of pain perception. Curr Rheumatol Rep 4: 329–333. [DOI] [PubMed] [Google Scholar]
- Juottonen K,Gockel M,Silen T,Hurri H,Hari R,Forss N ( 2002): Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 98: 315–323. [DOI] [PubMed] [Google Scholar]
- Katusic S,Williams DB,Beard CM,Bergstralh EJ,Kurland LT ( 1991): Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945–1984. Neuroepidemiology 10: 276–281. [DOI] [PubMed] [Google Scholar]
- Kress B,Schindler M,Rasche D,Hahnel S,Tronnier V,Sartor K,Stippich C ( 2005): MRI volumetry for the preoperative diagnosis of trigeminal neuralgia. Eur Radiol 15: 1344–1348. [DOI] [PubMed] [Google Scholar]
- Lamour Y,Willer JC,Guilbaud G ( 1983): Rat somatosensory (SmI) cortex. I. Characteristics of neuronal responses to noxious stimulation and comparison with responses to non‐noxious stimulation. Exp Brain Res 49: 35–45. [DOI] [PubMed] [Google Scholar]
- Love S,Hilton DA,Coakham HB ( 1998): Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol 8: 1–11; discussion 11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely TJ,Jannetta PJ ( 1997): Microvascular decompression for trigeminal neuralgia. Surgical technique and long‐term results. Neurosurg Clin N Am 8: 11–29. [PubMed] [Google Scholar]
- Maihofner C,Forster C,Birklein F,Neundorfer B,Handwerker HO ( 2005): Brain processing during mechanical hyperalgesia in complex regional pain syndrome: A functional MRI study. Pain 114: 93–103. [DOI] [PubMed] [Google Scholar]
- Mao J,Mayer DJ,Price DD ( 1993): Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci 13: 2689–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin MR,Jannetta PJ,Clyde BL,Subach BR,Comey CH,Resnick DK ( 1999): Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg 90: 1–8. [DOI] [PubMed] [Google Scholar]
- Monconduit L,Lopez‐Avila A,Molat JL,Chalus M,Villanueva L ( 2006): Corticofugal output from the primary somatosensory cortex selectively modulates innocuous and noxious inputs in the rat spinothalamic system. J Neurosci 26: 8441–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE,Morrow TJ,Casey KL ( 2000): Bilateral behavioral and regional cerebral blood flow changes during painful peripheral mononeuropathy in the rat. Pain 84: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R,Laurent B,Garcia‐Larrea L ( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Peyron R,Schneider F,Faillenot I,Convers P,Barral FG,Garcia‐Larrea L,Laurent B ( 2004): An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 63: 1838–1846. [DOI] [PubMed] [Google Scholar]
- Pleger B,Ragert P,Schwenkreis P,Forster AF,Wilimzig C,Dinse H,Nicolas V,Maier C,Tegenthoff M ( 2006): Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 32: 503–510. [DOI] [PubMed] [Google Scholar]
- Porro CA,Baraldi P,Pagnoni G,Serafini M,Facchin P,Maieron M,Nichelli P ( 2002): Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22: 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD ( 2000): Psychological and neural mechanisms of the affective dimension of pain. Science 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- Rasche D,Kress B,Stippich C,Nennig E,Sartor K,Tronnier VM ( 2006): Volumetric measurement of the pontomesencephalic cistern in patients with trigeminal neuralgia and healthy controls. Neurosurgery 59: 614–620. [DOI] [PubMed] [Google Scholar]
- Sawamoto N,Honda M,Okada T,Hanakawa T,Kanda M,Fukuyama H,Konishi J,Shibasaki H ( 2000): Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event‐related functional magnetic resonance imaging study. J Neurosci 20: 7438–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A,Ploner M ( 2000): Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 17: 592–603. [DOI] [PubMed] [Google Scholar]
- Stippich C,Romanowski A,Nennig E,Kress B,Hahnel S,Sartor K ( 2004): Fully automated localization of the human primary somatosensory cortex in one minute by functional magnetic resonance imaging. Neurosci Lett 364: 90–93. [DOI] [PubMed] [Google Scholar]
- Stippich C,Romanowski A,Nennig E,Kress B,Sartor K ( 2005): Time‐efficient localization of the human secondary somatosensory cortex by functional magnetic resonance imaging. Neurosci Lett 381: 264–268. [DOI] [PubMed] [Google Scholar]
- Stippich C,Blatow M,Durst A,Dreyhaupt J,Sartor K ( 2007): Global activation of primary motor cortex during voluntary movements in man. Neuroimage 34: 1227–1237. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotactic Atlas of the Human Brain: 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme. [Google Scholar]
- Talbot JD,Marrett S,Evans AC,Meyer E,Bushnell MC,Duncan GH ( 1991): Multiple representations of pain in human cerebral cortex. Science 251: 1355–1358. [DOI] [PubMed] [Google Scholar]
- Tenser RB ( 1998): Trigeminal neuralgia: Mechanisms of treatment. Neurology 51: 17–19. [DOI] [PubMed] [Google Scholar]
- Tracey I ( 2005): Nociceptive processing in the human brain. Curr Opin Neurobiol 15: 478–487. [DOI] [PubMed] [Google Scholar]
- Treede RD,Kenshalo DR,Gracely RH,Jones AK ( 1999): The cortical representation of pain. Pain 79: 105–111. [DOI] [PubMed] [Google Scholar]
- Tronnier VM,Rasche D,Hamer J,Kienle AL,Kunze S ( 2001): Treatment of idiopathic trigeminal neuralgia: Comparison of long‐term outcome after radiofrequency rhizotomy and microvascular decompression. Neurosurgery 48: 1261–1267; discussion 1267–1268. [PubMed] [Google Scholar]
- Tyler‐Kabara EC,Kassam AB,Horowitz MH,Urgo L,Hadjipanayis C,Levy EI,Chang YF ( 2002): Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: Comparison of results following microvascular decompression. J Neurosurg 96: 527–531. [DOI] [PubMed] [Google Scholar]
- Wang JY,Chang JY,Woodward DJ,Baccala LA,Han JS,Luo F ( 2007): Corticofugal influences on thalamic neurons during nociceptive transmission in awake rats. Synapse 61: 335–342. [DOI] [PubMed] [Google Scholar]
- Wiffen P,Collins S,McQuay H,Carroll D,Jadad A,Moore A ( 2000): Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev 3: CD001133. [DOI] [PubMed] [Google Scholar]
- Woolf CJ,Salter MW ( 2000): Neuronal plasticity: Increasing the gain in pain. Science 288: 1765–1769. [DOI] [PubMed] [Google Scholar]
- Zakrzewska JM,Lopez BC ( 2005): Trigeminal neuralgia. Clin Evid 14: 1669–1677. [PubMed] [Google Scholar]
- Zorman G,Wilson CB ( 1984): Outcome following microsurgical vascular decompression or partial sensory rhizotomy in 125 cases of trigeminal neuralgia. Neurology 34: 1362–1365. [DOI] [PubMed] [Google Scholar]


