Abstract
The detection of novel events and their identification is a basic prerequisite in a rapidly changing environment. Recently, the processing of novelty has been shown to rely on the hippocampus and to be associated with activity in reward‐related areas. The present study investigated the influence of spatial attention on neural processing of novel relative to frequently presented standard and target stimuli. Never‐before‐seen Mandelbrot‐fractals absent of semantic content were employed as stimulus material. Consistent with current theories, novelty activated a widespread network of brain areas including the hippocampus. No activity, however, could be observed in reward‐related areas with the novel stimuli absent of a semantic meaning employed here. In the perceptual part of the novelty‐processing network a region in the lingual gyrus was found to specifically process novel events when they occurred outside the focus of spatial attention. These findings indicate that the initial detection of unexpected novel events generally occurs in specialized perceptual areas within the ventral visual stream, whereas activation of reward‐related areas appears to be restricted to events that do possess a semantic content indicative of the biological relevance of the stimulus. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: novelty, substantia nigra, hippocampus, lingual gyrus, visual attention, fMRI
INTRODUCTION
The detection of novel events and their storage into memory are fundamental processes in daily life. Novel events capture attention and are more effectively encoded into memory than familiar events [Ranganath and Rainer,2003]. Previous studies [for review see Brown and Aggleton,2001; Grill‐Spector et al.,2006; Hasselmo and Stern,2006; Nyberg,2005; Ranganath and Rainer,2003] showed that novel stimuli activate a distributed network of areas including prefrontal and posterior association cortices, as well as the medial temporal lobe (MTL) [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Daselaar et al.,2006; Gur et al.,2007; Mahon et al.,2007; Menon et al.,2000; Schott et al.,2004; Strange and Dolan,2001; Strange et al.,1999, 2005; Yamaguchi et al.,2004]. According to a recent model, the hippocampus and the dopaminergic midbrain are at the heart of this distributed network, forming a functional loop designated to detect novel events and to ensure or control their entry into long‐term memory [Lisman and Grace,2005]. This theory is supported by recent work reporting concurrent activation of the hippocampus and the substantia nigra (SN) during novel stimulus processing [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Schott et al.,2006; Schott et al.,2004; Wittmann et al.,2007].
Importantly, most studies investigating novelty processing have typically employed never‐before‐seen pictures of real‐world objects [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Strange et al.,2005; Yamaguchi et al.,2004]. Despite their perceptual novelty, real‐world objects are not exemplarily novel, in that a semantic concept has been previously associated with the corresponding stimulus category. These concepts do affect visual object recognition even in cases when semantic retrieval is not explicitly required by the experimental task [Gauthier et al.,2003]. Furthermore event‐related brain potential (ERP) studies showed that the repetition of novel stimuli only affects a specific ERP component (novelty‐P3a) when the “novel” stimuli possess a semantic meaning [Cycowicz and Friedman,2007; Friedman and Cycowicz,2006; Kotz et al.,2007].
The detection of novelty especially when the spatial location in which novel events appear is unattended is mandatory for life. This implies the existence of a novelty detection instance at an early perceptual level that triggers subsequent processes like attentional orienting and/or capture [Yamaguchi et al.,2004]. The present study employed functional magnetic resonance imaging (fMRI) with a protocol previously shown to be especially sensitive to midbrain activity [Bunzeck and Duzel,2006; Bunzeck et al.,2007] to investigate the processing of exemplar novel stimuli presented at attended and unattended locations. The usage of Mandelbrot‐fractals as stimuli ensured that neither any semantic concepts, nor any biological meaning were associated with the novel events, permitting to investigate whether such stimuli also trigger a response in the reward system as meaningful stimuli do. Secondly, the study investigated the putative existence of a specific instance for the detection of novel events at unattended locations.
MATERIALS AND METHODS
Subjects
Eighteen healthy subjects (12 females), all with normal or corrected‐to‐normal vision, participated as paid volunteers in the study (mean age: 24.6 ± 3.2 (SD) years). All gave written informed consent before participation and the local ethics committee approved the study.
Stimuli and Experimental Design
We employed a visual‐selective attention task that allowed us to compare the neural responses upon novel, standard and target events (33% each) presented at attended or unattended locations. All stimuli were fractal pictures of 4.8° × 3°, randomly created by the open source program ChaosPro 2.1 (http://www.chaospro.de), which were presented in the upper left or right visual quadrant at 3.0° eccentricity (inner edge) and at 1.0° above a central fixation cross (see Fig. 1). The stimulus duration was 800 ms and the inter‐trial interval varied randomly between 0.7 and 5.2 s following a gamma function to allow trial separation in an event‐related analysis [Hinrichs et al.,2000]. The experiment consisted of 6 scanning sessions of 9 min, including 8 blocks of 24–26 trials each. At the beginning of each block, a white arrow (1.6 × 1°) pointing to the left or right (presented 1.9° above the fixation cross) indicated the location to be attended. The stimuli sequentially either occurred at the attended or the unattended location in a pseudo‐randomized sequence. The mean inter‐stimulus interval was 1.88 s and blocks lasted between 54 and 88 s (mean 67 s). Before fMRI data acquisition subjects were familiarized with the target and standard stimuli that consisted of one randomly picked fractal stimulus each in a practice session. Subjects with eye‐movements of more than 1° in this practice session were excluded from the experiment. The instruction was to sustain selective attention to the visual field indicated by the central arrow while maintaining central fixation. The subjects' task was to make a button‐press response as rapidly as possible upon the detection of that target stimulus in the attended visual field. Stimuli in the opposite hemifield were to be ignored.
Figure 1.
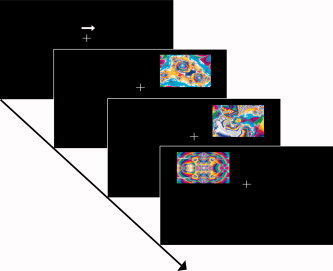
Schematic illustration of the experimental design. A central cue (white arrow pointing to the left or to the right) indicated the visual field to be attended. The stimuli consisted of fractal pictures presented to the upper left or right visual field in relation to a central fixation cross. Stimuli occurred at the attended as well as at the unattended location. One of the fractal pictures served as standard or target stimulus, respectively. Standards and targets were presented repeatedly across the experiment each accounting for 33.3% of the trials. Novel fractal pictures were presented only once during the entire experiment. Subjects were required to make a button press upon the occurrence of the target picture at the attended location.
fMRI Data Acquisition
During the scanning session the stimuli were presented via a projector‐mirror system. fMRI data acquisition was performed on a 3‐Tesla MR scanner (Siemens Magnetom Trio, Erlangen, Germany) using an 8‐channel head coil. Twenty four slices (thickness = 3 mm, in plane resolution 64 × 64 mm, FoV 192 × 192 mm, gap = 0.3 mm, resulting voxel size = 3 × 3 × 3 mm) parallel to the brainstem were acquired with a T2*‐weighted echo planar imaging (EPI) gradient echo sequence (TR = 1500 ms, TE = 30 ms, flip angle = 75°) in an odd‐even interleaved sequence. Each scanning session consisted of 279 partial volumes covering the hippocampus, amygdala, brainstem, cerebellum and surrounding cortical regions [Bunzeck and Duzel,2006]. In a structural session, sagittal whole‐head T1‐weighted images (60 slices, thickness = 3 mm, 64 × 64 matrix, FoV 192 × 192 mm, gap = 0.3 mm, spatial resolution = 3 × 3 × 3 mm) were collected using inversion recovery prepared EPI (IR‐EPI) sequences (TE = 33 ms; TI = 1450 ms; TR = 15000 ms).
fMRI Data Analysis
Data analysis was performed using SPM5 software (Wellcome Department of Cognitive Neurology, University College London, UK) and MATLAB 7.0 (The Mathwork, Inc.). Following correction for differences in timing of slice acquisition, EPI volumes were realigned to the first volume and resliced using SINC interpolation. Spatial normalization to a standard T1‐weighted SPM template [Ashburner and Friston,1999] was performed by warping the subjects anatomical IR‐EPI to the SPM template and application of the obtained parameters to the functional images. The normalized images were spatially smoothed with a 4‐mm isotropic Gaussian kernel. Statistical analysis of the data was performed, by applying the standard hemodynamic‐response function implemented in SPM5 in an event‐related design for each subject. The rigid‐body translations and the rotations from the realignment procedure were included in the models as covariates. Contrasts of parameter estimates comparing novel vs. standard stimuli, target vs. standard stimuli and novel vs. target stimuli were separately calculated for each attention condition. Group data were analyzed with random‐effects analysis by use of one‐sample t‐tests applied to the contrast images from the single‐subject analysis. The coordinates for voxels with maximal z values within activation clusters and locations are reported in the MNI standard space.
To compare the magnitude of hemodynamic modulations between the different contrasts a region of interest (ROI) analysis was performed using the MarsBar toolbox in SPM5 [Brett et al.,2002]. Four bilaterally represented ROIs (the fusiform gyrus (FG), the lingual gyrus (LG), the anterior/medial portion of the hippocampus (anterior hippocampus) and the posterior parahippocampal gyrus (posterior PHG)) were functionally defined using the main effect of a 2 × 3 factorial ANOVA (attention × stimulus categories). Because activations of the substantia nigra (SN) were neither observed in the 2 × 3 ANOVA nor in any of the one‐sample t‐test group‐analyses, the two SN‐ROIs (left and right SN) were defined based on anatomical constraints using a magnetization transfer template averaged over 33 subjects as described by Bunzeck and Duzel [2006]. In an additional analysis individual SN‐ROIs were defined on anatomical landmarks separately for each subject and the subsequent ROI‐analysis was performed on un‐normalized data modeled with the canonical HRF and its temporal derivative. For the identification of attention‐sensitive regions that process novel or target stimuli, an interaction analysis of attention x stimulus type combined with a subtraction analysis of stimulus type [Yamaguchi et al.,2004] was performed. In this analysis, regions determined in the stimulus‐type comparison, were masked by regions displaying a significant stimulus type x attention condition interaction (uncorrected, P < 0.05). Regions that were either activated by novel stimuli with inclusive interaction masking were considered as attention sensitive, whereas the regions activated by ignored novels or targets with exclusive interaction masking were defined as attention insensitive. This analysis revealed one bilaterally represented region located in the basal portion of the FG to be attention sensitive. Therefore the previously defined FG‐ROIs were further subdivided into an attention sensitive (FG+) and an attention insensitive portion (FG−).
For all ROIs (anterior hippocampus, FG+, FG−, LG, posterior PHG, SN), mean beta values were extracted from the individual subjects' data for all conditions. These values were subjected to a repeated measures analysis of variance (repeated measures ANOVA) with the factors region, hemisphere (left vs. right), attention condition (attended vs. unattended) and stimulus type (standards, targets and novels). The significance threshold was set to P < 0.05 following Greenhouse‐Geisser correction for non‐sphericity. Because no significant main effect was observed for the side of stimulus presentation, data were collapsed over both sides of stimulus presentation for separate analysis of each individual ROI. The data for each ROI were separately subjected to a repeated measures ANOVA with the factors attention condition and stimulus type. If statistical significance (P < 0.05) was obtained, a paired t test (Bonferroni corrected for multiple comparisons) was applied for post hoc comparisons between attention conditions and/or stimulus categories.
RESULTS
Behavioral Results
Mean reaction times (RTs; min/max: 307/1000 ms; mean ± standard error of the mean (SEM): 629 ± 12 ms) and the percentage of correct responses (min/max: 69.15/100%; mean ± SEM: 93.49 ± 1.18%) were separately submitted to a repeated measures ANOVA with the factor target location (left vs. right). These analyses revealed neither a significant main effect of the target location on RTs (F(1, 17) = 2.700, P = 0.119) nor on the hit rate (F(1, 17) = 0.617, P = 0.443).
fMRI Results
Brain activations elicited by stimulus novelty
In comparison to standards, attended novel stimuli elicited an increased hemodynamic activity in the anterior hippocampus, the bilateral fusiform gyrus (FG) and the posterior parahippocampal gyrus (posterior PHG) as well as in the left supramarginal gyrus (SMG). When presented to the unattended visual field novel stimuli induced activations in the FG, the posterior PHG and the lingual gyrus (LG) in both hemispheres (Fig. 2 and Table I). The analysis with inclusive interaction masking (see methods section) revealed reduced activations in distinct areas of the FG in both hemispheres, indicating that these regions are sensitive to voluntary deployment of attention (FG−; Fig. 2 and Table I). The direct comparison between activity elicited by novel events and target‐related activity revealed that the FG (T max = 9.85), the posterior PHG (T max = 6.83), the anterior hippocampus (T max = 6.91) and the medial temporal gyrus (T max = 7.05) were activated by attended novel stimuli.
Figure 2.

Brain regions activated by novel and target stimuli. y‐ or z‐coordinates in MNI‐space are depicted above each slice series. The respective panels show brain activations upon attended novel (upper panel), attended target (second panel) and unattended novel stimuli (third panel).
Table I.
Regions exhibiting significant activity to novel or target stimuli
| Region | L/R | x (mm) | y (mm) | z (mm) | T max |
|---|---|---|---|---|---|
| Contrast: Attended novels > Attended standards | |||||
| FG | L | −30 | −50 | −10 | 7.94 |
| R | 30 | −40 | −16 | 6.70 | |
| Posterior PHG | L | −36 | −30 | −12 | 6.42 |
| R | 30 | −30 | −8 | 5.08 | |
| SMG | L | −64 | −32 | 40 | 5.53 |
| Anterior Hippocampus | L | −30 | −12 | −22 | 4.18 |
| R | 38 | −12 | −20 | 3.55 | |
| Contrast: Attended targets > Attended standards | |||||
| FG | L | −24 | −50 | −16 | 7.39 |
| Cerebellum, Region 8 | L | −32 | −52 | −42 | 5.53 |
| Cerebellum, Region 6 | R | 32 | −52 | −26 | 6.05 |
| Anterior Putamen | R | 24 | 8 | −6 | 7.66 |
| Posterior Putamen | L | −34 | −32 | 2 | 6.53 |
| R | 30 | −20 | 4 | 8.30 | |
| Insula | R | 42 | −4 | 16 | 6.22 |
| Inferior frontal operculum | L | −52 | 8 | 14 | 7.52 |
| SMG | R | 56 | −24 | 22 | 6.36 |
| Inferior parietal gyrus | R | 58 | −34 | 52 | 6.10 |
| Precentral Gyrus | L | −44 | 16 | 40 | 4.96 |
| Contrast: Unattended novels | |||||
| FG | L | −34 | −48 | −2 | 7.06 |
| R | 26 | −32 | −12 | 6.42 | |
| Posterior PHG | L | −30 | −34 | −20 | 5.80 |
| R | 36 | −32 | −12 | 6.09 | |
| LG | L | −22 | −50 | 0 | 5.19 |
| R | 28 | −46 | −2 | 3.88 | |
| Contrast: Unattended targets > Unattended standards | |||||
| Inferior frontal operculum | L | −32 | 14 | 32 | 4.25 |
Values represent coordinates in mm in MNI‐space and maximum T‐values.
Abbreviations: FG, fusiform gyrus; LG, lingual gyrus; PHG, parahippocampal gyrus; SMG, supramarginal gyrus.
Coordinates: x, left/right; y, posterior/anterior; z, inferior/superior in the reference frame of MNI space.
Brain activations elicited by target stimuli
In comparison to attended standards, attended target stimuli induced hemodynamic activity in several motor‐related brain regions, including the cerebellum, precentral gyrus, the inferior frontal operculum, the insula and distinct parts of the putamen. Moreover, activations were found in the right SMG, right inferior parietal gyrus and—as elicited by novel stimuli, in the left FG (Fig. 2 and Table I). A similar pattern was also found for the comparison between target‐ and novelty‐related activities within the attended visual field (data not shown). In contrast, presentation of the target stimulus at unattended locations only elicited hemodynamic activity in the inferior frontal operculum when compared to standard stimuli (T max = 4.25).
A region of interest (ROI) analysis was performed to compare the magnitude of neural modulations elicited by the different stimulus categories presented within/outside the focus of attention (Fig. 3 and Table II).
Figure 3.
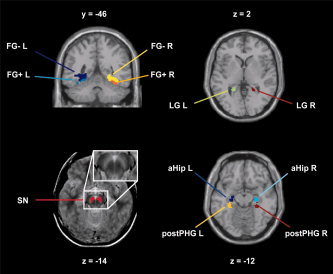
Location of the regions of interest. y‐ or z‐coordinates in MNI‐space are depicted above or below each slice. Abbreviations: FG+, attention sensitive portion of the fusiform gyrus; FG−, attention insensitive portion of the fusiform gyrus; LG, lingual gyrus; aHip, anterior/medial portion of the hippocampus; postPHG, posterior parahippocampal gyrus, SN, substantia nigra; L, left; R, right.
Table II.
MNI coordinates of the regions of interest
| Region | L/R | x | y | z | Number of voxels |
|---|---|---|---|---|---|
| FG− | L | −31 ± 13 | −42 ± 10 | −9 ± 11 | 284 |
| R | 32 ± 10 | −44 ± 8 | −9 ± 9 | 245 | |
| FG+ | L | −30 ± 12 | −46 ± 6 | −13 ± 9 | 120 |
| R | 33 ± 7 | −46 ± 6 | −16 ± 4 | 63 | |
| LG | L | −21 ± 9 | −42 ± 8 | 1 ± 11 | 143 |
| R | 20 ± 4 | −46 ± 4 | 2 ± 4 | 80 | |
| ant Hip | L | −26 ± 4 | −27 ± 5 | −14 ± 4 | 82 |
| R | 25 ± 5 | −27 ± 5 | −15 ± 5 | 82 | |
| post PHG | L | −30 ± 4 | −31 ± 5 | −15 ± 5 | 98 |
| R | 30 ± 4 | −31 ± 5 | −15 ± 5 | 76 | |
| SN | L | −5 ± 7 | −10 ± 16 | −15 ± 7 | 76 |
| R | 5 ± 7 | −10 ± 16 | −15 ± 7 | 83 |
The values represent coordinates in mm in MNI‐space.
Abbreviations: FG+, attention sensitive portion of the fusiform gyrus; FG−, attention insensitive portion of the fusiform gyrus; LG, lingual gyrus; aHip, anterior/medial portion of the hippocampus; postPHG, posterior parahippocampal gyrus, SN, substantia nigra; L, left; R, right.
Coordinates: x, left/right; y, posterior/anterior; z, inferior/superior in the reference frame of MNI space.
Region of Interest Analyses
For quantification and comparison of the neural activity during the different experimental conditions beta weights were extracted for each subject and each ROI. These modulations are shown in Figure 4 (FG+, FG−), Figure 5 (anterior hippocampus and posterior PHG) and Figure 6 (SN and LG). For the SN the betas were extracted from the group analysis (group‐SN‐ROI) and in addition also from individually determined ROIs for each subject (individual‐SN‐ROI).
Figure 4.

Activity in the fusiform gyrus ROIs elicited by the different stimulus categories in the two attention conditions. The bar color indicates the particular stimulus category (black: targets; grey: standards; white: novels). The particular attention conditions are depicted below each bar plot. Abbreviations: FG+, attention sensitive portion of the fusiform gyrus; FG−, attention insensitive portion of the fusiform gyrus; R, right. Coordinates: y, posterior/anterior in the reference frame of MNI space.
Figure 5.
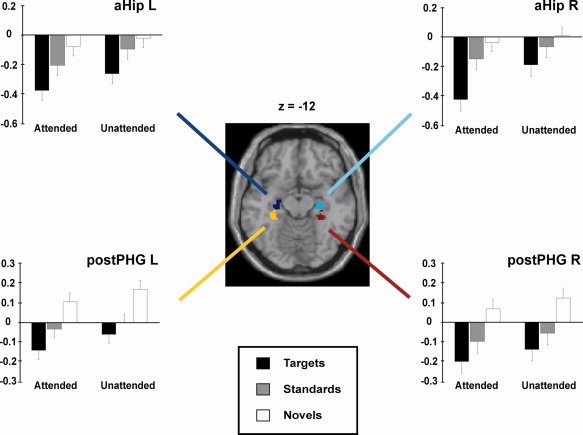
Activity in the anterior/middle hippocampus (aHip) and in the posterior parahippocampal gyrus (postPHG) elicited by the different stimulus categories in the two attention conditions. The bar color indicates the particular stimulus category (black: targets; grey: standards; white: novels). The particular attention conditions are shown below each bar plot. Abbreviations: aHip, anterior/middle hippocampus; postPHG, posterior parahippocampal gyrus; L, left; R, right. Coordinates: z, inferior/superior in the reference frame of MNI space.
Figure 6.

Activity in the lingual gyrus (LG) and in the substantia nigra (SN) elicited by the different stimulus categories in the two attention conditions. The bar color indicates the particular stimulus category (black: targets; grey: standards; white: novels). The particular attention conditions are depicted below each bar plot. Abbreviations: LG, lingual gyrus; SN, substantia nigra; L, left; R, right. Coordinates: z, inferior/superior in the reference frame of MNI space.
Main effects and interactions
For statistical analysis the extracted β‐values were submitted to a repeated measures ANOVA with the factors region (FG+, FG−, anterior hippocampus, posterior PHG, SN and LG), attention condition (attended vs. unattended), stimulus category (targets, standards, and novels) and hemisphere (left vs. right). Significant main effects were observed for the factors region (F(5, 85) = 50.043, P < 0.0001) and stimulus category (F(2, 34) = 25.866, P < 0.0001), as well as a four‐way interaction between region, attention condition, stimulus category and hemisphere (F(10, 170) = 7.289, P < 0.0001).
For direct comparison of attention/stimulus specific effects the data for each ROI were separately subjected to a repeated measures ANOVA with the factors attention condition (attended vs. unattended) and stimulus category (targets, standards and novels). When statistical significance (P < 0.05) was observed, a paired t test (Bonferroni corrected) was applied for post hoc comparisons. Table III shows a detailed list of the significance values for the comparison of the individual attention/stimulus category conditions within each ROI.
Table III.
Significance values for the post hoc comparisons (Bonferroni corrected) between categories for each ROI
| FG+R | Attended targets vs. | Unattended standards | 0.05 |
| Unattended targets | 0.05 | ||
| Attended novels vs. | Unattended standards | 0.001 | |
| unattended targets | 0.005 | ||
| Unattended novels vs. | Unattended standards | 0.01 | |
| Unattended targets | 0.01 | ||
| FG+L | Attended targets vs. | Attended standards | 0.001 |
| Unattended standards | 0.001 | ||
| Unattended targets | 0.001 | ||
| Attended novels vs. | Unattended standards | 0.01 | |
| Unattended targets | 0.01 | ||
| Unattended novels vs. | Unattended standards | 0.05 | |
| Unattended targets | 0.05 | ||
| FG−R | Attended novels vs. | Attended standards | 0.05 |
| Unattended standards | 0.05 | ||
| Unattended targets | 0.01 | ||
| Unattended novels vs. | Attended standards | 0.005 | |
| Unattended standards | 0.05 | ||
| Unattended targets | 0.01 | ||
| FG−L | Attended novels vs. | Attended standards | 0.05 |
| Unattended standards | 0.005 | ||
| Unattended targets | 0.05 | ||
| Unattended novels vs. | Attended standards | 0.001 | |
| Attended targets | 0.001 | ||
| Unattended standards | 0.001 | ||
| Unattended targets | 0.001 | ||
| aHip R | attended targets vs. | Attended novels | 0.001 |
| Attended standards | 0.05 | ||
| Unattended novels | 0.001 | ||
| Unattended standards | 0.001 | ||
| aHip L | Attended targets vs. | Attended novels | 0.005 |
| Unattended novels | 0.001 | ||
| Unattended standards | 0.01 | ||
| Unattended targets vs. | Unattended novels | 0.05 | |
| PostPHG R | Attended novels vs. | Attended targets | 0.005 |
| Unattended targets | 0.05 | ||
| Unattended novels vs. | Attended standards | 0.05 | |
| Attended targets | 0.001 | ||
| Unattended targets | 0.005 | ||
| PostPHG L | Attended novels vs. | Attended targets | 0.005 |
| Unattended novels vs. | Attended standards | 0.05 | |
| Attended targets | 0.001 | ||
| Unattended targets | 0.005 | ||
| LG R | Unattended novels vs. | Attended standards | 0.05 |
| Attended targets | 0.001 | ||
| attended targets vs. | Unattended standards | 0.05 | |
| LG L | Unattended novels vs. | Attended novels | 0.005 |
| Attended standards | 0.005 | ||
| Attended targets | 0.001 | ||
| Unattended standards | 0.005 | ||
| Unattended targets | 0.001 |
Abbreviations: FG+, attention sensitive portion of the fusiform gyrus; FG−, attention insensitive portion of the fusiform gyrus; LG, lingual gyrus; aHip, anterior/medial portion of the hippocampus; postPHG, posterior parahippocampal gyrus, SN, substantia nigra; L, left; R, right.
FG+
For the FG+ data the repeated measures ANOVA revealed a significant main effect for the attention condition (F(1, 17) = 45.001, P < 0.0001 for FG+ L and F(1, 17) = 13.751, P < 0.005 for FG+ R) and stimulus category (F(2, 34) = 25.551, P < 0.0001 for FG+ L and F(2, 34) = 18.181, P < 0.0001 for FG+ R) as well as a significant interaction between both factors (F(2, 34) = 17.222, P < 0.0001 for FG+ L and F(2, 34) = 4.157, P < 0.05 for FG+ R). Post hoc analyses revealed significantly higher modulations for attended and unattended novel as well as attended target stimuli in comparison to unattended standards and targets. Moreover, in the left hemisphere attended targets also elicited a significantly higher neural modulation in comparison to attended standards.
FG−
Analysis of the FG− data by repeated measures ANOVA only revealed a significant main effect for stimulus category (F(2, 34) = 40.297, P < 0.0001 for FG− L and F(2, 34) = 40.250, P < 0.0001 for FG− R) and a significant interaction between attention condition and stimulus category for FG− L (F(2, 34) = 4.367, P < 0.05) but not for FG− R (F(2, 34) = 0.487, P = 0.618). In contrast to the FG+ data, no main effect could be observed for the factor attention condition for both hemispheres (F(1,17) = 0.391, P = 0.540 for FG− L and F(1,17) = 0.548, P = 0.469 for FG− R). Pairwise comparison showed that the highest modulations in both hemispheres were elicited by novel stimuli irrespective of the attentional state.
Anterior Hippocampus
The repeated measures ANOVA applied to the anterior hippocampus ROIs showed significant main effects for attention condition (F(1, 17) = 7.018, P < 0.05 for the left anterior hippocampus and F(1,17) = 14.907, P < 0.001 for the right anterior hippocampus) and stimulus category (F(2, 34) = 15.499, P < 0.0001 for the left anterior hippocampus and F(2, 34) = 17.343, P < 0.0001 for the right anterior hippocampus) and an interaction between both factors for the right anterior hippocampus (F(2, 34) = 3.505, P < 0.05) but not for the left anterior hippocampus (F(2, 34) = 0.261, P = 0.771). For the right‐hemispheric ROI the detailed analysis revealed that the beta values elicited by attended targets were significantly decreased in comparison to all other conditions. In the left hemisphere the hemodynamic modulations elicited by attended targets were significantly decreased compared to those elicited by attended and unattended novel stimuli as well as by unattended standards. In addition, unattended targets showed a decreased modulation in comparison to unattended novel stimuli. Given that targets and standard stimuli both belong to the stimulus category “old” the main effect of stimulus category can be regarded as an effect due to stimulus novelty.
Posterior PHG
The repeated measures ANOVA on the data from the posterior PHG ROIs revealed significant main effects for attention condition (F(1, 17) = 5.523, P < 0.05 for the posterior PHG L and F(1, 17) = 6.836, P < 0.05 for the posterior PHG R) and stimulus category (F(2, 34) = 23.375, P < 0.0001 for posterior the PHG L and F(2, 34) = 22.290, P < 0.0001) but no attention condition x stimulus category interaction (F(2, 34) = 0.304, P = 0.740 for the posterior PHG L and F(2, 34) = 0.065, P = 0.937 for the posterior PHG R). Pairwise comparison showed these effects to depend on an increased modulation to novel stimuli regardless of the attention condition (For detailed description of the statistical values see Table III).
SN
Group SN‐ROI
The repeated measures ANOVA on the SN data showed no significant main effects or interactions between factors for the SN L (attention condition: F(1, 17) = 0.698, P = 0.415; stimulus category: F(2, 34) = 0.648, P = 0.529 and attention condition × stimulus category interaction: F(2, 34) = 1.003, P = 0.377). For the SN R the analysis also showed no main effects for attention condition (F(1, 17) = 0.076, P = 0.786) or stimulus category (F(2, 34) = 0.034, P = 0.967), but a marginal significant interaction between both factors (F(2, 34) = 4.728, P < 0.05). No significant differences emerged between any of the conditions during post hoc comparison of the SN R data, even when the analysis was performed without correction for multiple comparisons (Fisher's least significant difference test).
Individual SN‐ROIs
The repeated measures ANOVA applied to the data from the individually defined SN‐ROIs showed a significant main effect for the factor stimulus category (F(1, 17) = 5.088, P < 0.05 for SN L and F(1, 17) = 5.179, P < 0.05 for SN R) and an interaction between attention condition and stimulus category (F(2, 34) = 15.573, P < 0.0001 for SN L and F(2, 34) = 16.566, P < 0.0001 for SN R). In contrast, no main effects were observed for the factor attention condition (F(1, 17) = 1.393, P = 0.254 for SN L and F(1, 17) = 1.488, P < 0.239 for SN R). The Post hoc analysis showed an increased modulation to attended targets in comparison to all other stimulus categories except unattended novels for both hemispheres that was responsible for the interaction (see Supporting Information Fig. S1 and Table S1).
LG
Significant main effects for attention condition (F(1, 17) = 16.214, P < 0.001 for LG L and F(1, 17) = 22.331, P < 0.0001 for LG R) and stimulus category (F(2, 34) = 12.658, P < 0.0001 for LG L and F(2, 34) = 11.043, P < 0.0001 for LG R) for both hemispheres were observed in a repeated measures ANOVA applied to the LG data. Moreover the analysis showed a significant interaction between these factors for the left‐hemispheric ROI (F(2, 34) = 5.912, P < 0.01) but not for the LG R data (F(2, 34) = 0.400, P = 0.674). Post hoc analysis showed an increased neural activity upon novel stimuli that were presented to the unattended visual hemifield. In the left hemisphere unattended novels differed significantly from all other conditions, whereas in the LG R they elicited significantly higher neural modulations only in comparison to all attended stimuli irrespective of the stimulus category.
DISCUSSION
The present study investigated the influence of spatial attention on neural processing of novel relative to frequently presented standard and target stimuli. To ensure perceptual and exemplar novelty, Mandelbrot‐fractals were employed as stimulus material. The spatial attention of the subjects was directed to one visual hemifield while stimuli could occur at the attended or the unattended location. This design permitted to specifically assess the activity elicited by novel, standard and target stimuli that were presented within and outside the focus of attention. Consistent with current theories novelty activated a widespread network of brain areas including regions located in the ventral visual stream, the parahippocampal gyrus and the hippocampus. Importantly, no activity was observed in reward‐related areas with novel fractal‐stimuli, which are absent of a semantic meaning. An important functional segregation was observed in visual ventral stream. The FG was found to have an attention sensitive and insensitive part. The LG was specifically activated by stimulus novelty occurring in the spatially unattended visual field, thereby acting as a novelty detector at early perceptual level.
The results are consistent with findings from previous studies showing that novel visual stimuli are processed in a distributed cortical network of ventral visual stream, medial temporal lobe (MTL) and frontal regions [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Daselaar et al.,2006; Gur et al.,2007; Menon et al.,2000; Schott et al.,2004; Strange and Dolan,2001; Strange et al.,1999, 2005; Yamaguchi et al.,2004]. In the perceptual part of the network a functional segregation was observed in the ventral visual stream, in which a region located in the anterior portion of the LG exclusively responded to novel stimuli presented at unattended locations (Fig. 6 and Table I). In contrast, novelty‐related activations within the FG and posterior PHG (see Fig. 2 and Table I) occurred irrespective of attention while activity in the left SMG was exclusively induced by novel stimuli occurring at attended locations. The FG−activations by novel stimuli irrespective of the attention condition (see Figs. 2 and 4 and Table I) extend previous findings [Yamaguchi et al.,2004], showing that the novelty responsive region within the FG in fact consists of an attention sensitive (FG+) and an attention insensitive (FG−) portion. The present ROI analysis indicates that the attention effect within the FG+ was mainly due to an increased response to attended targets and not to an attentional modulation of the novelty‐response per se (see Fig. 4).
Numerous studies have shown that attention increases the neuronal modulations within those brain regions that process the perceptual attributes of the attended stimuli [e.g. Chawla et al.,1999; Schoenfeld et al.,2003, 2007]. Typically, the highest modulations occur if the stimuli are presented at attended locations irrespective of their constituent features [Stoppel et al.,2007]. In contrast, in situations that require spatial attention with a high attentional load, events that occur at an unattended part of space are, if at all, only subject to minimal perceptual analysis [Schoenfeld et al.,2003]. The detection of novel events is of fundamental importance in daily life, especially in those cases when the locations where the events occur are unattended. It has been argued that foraging species must have a drive to explore novelty [Panksepp,1998], which therefore acts as an ‘exploration bonus’ to motivate an organism to explore novel environmental conditions [Kakade and Dayan,2002]. Indeed, novel events typically trigger an automatic orienting response leading to an involuntary capture of attention followed by enhanced processing at that location [Escera et al.,1998; Sokolov,1963]. The current results point out to the major role of the lingual gyrus, being the starting point of the novelty‐processing network in charge of the detection of novel perceptual events especially when they occur outside the focus of attention. The size of the receptive fields of the neurons in this region is ideally suited to process novel stimuli at unattended locations preattentively [Duncan et al.,2009], especially when the majority of processing resources is devoted to stimulus processing at the attended part of the space. The receptive fields in hierarchically earlier visual areas are much smaller, implying the risk to miss novel events that occur at unattended locations.
With regard to the attentional modulations of activity in the visual cortex, like in the present study, it has to be kept in mind that eye‐movements could be a confounding factor, especially because eye‐movements were not directly monitored during the fMRI experiment. Nevertheless, prior to the scanning subjects were tested in a practice session outside the scanner to become familiar with the task. In this session we did monitor eye‐movements and only those subjects who were able to properly maintain fixation took part in the fMRI experiment. This approach does not exclude the eye‐movement confound completely, but minimizes the probability that the observed attention effects are caused by eye‐movements.
Recent studies described concurrent activations of the SN and MTL structures in course of the presentation of novel stimuli [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Schott et al.,2004, 2006; Wittmann et al.,2007], supporting a model proposed by Lisman and Grace [2005] in which novelty promotes memory formation by means of a functional loop between the hippocampus and the SN. In this model, the hippocampus is thought to generate a novelty signal by comparison of the incoming information with stored memories [Kumaran and Maguire,2007; Vinogradova,2001]. After being relayed to the midbrain via subcortical connections, this signal activates the SN. Finally, closing the hippocampal‐SN loop, the novelty signal is routed back in terms of hippocampal dopamine release, thereby enhancing the memorization of the novel stimulus [Lisman and Grace,2005]. One goal of the present study was to enquire whether and to what extent stimuli absent of semantic content like Mandelbrot‐fractals are also processed in the hippocampus‐SN loop. We especially employed an fMRI protocol previously shown to be sensitive to hippocampal and especially to midbrain activity [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Wittmann et al.,2007].
In line with previous investigations, within the MTL enhanced neural activity to novel stimuli was observed in the anterior hippocampus and in the posterior PHG (see Fig. 2) [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Daselaar et al.,2006; Dolan and Fletcher,1997; Duzel et al.,2003; Escera et al.,1998; Fenker et al.,2008; Gur et al.,2007; Hasselmo and Stern,2006; Kohler et al.,2002, 2005; Lepage et al.,1998; Menon et al.,2000; Saykin et al.,1999; Schacter and Wagner,1999; Schott et al.,2004, 2006; Strange et al.,1999; Wittmann et al.,2007]. Both, the posterior PHG and the anterior hippocampus displayed broad novelty‐related activations in comparison to standard as well as to target stimuli, stimulus categories that were repeatedly presented in the present study and therefore belonging to the stimulus category “old.” Importantly, despite the evident hippocampal activations no concomitant novelty‐related activity could be observed in the SN (see Fig. 6).
The most important difference between the current study and previous investigations [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Schott et al.,2004, 2006; Wittmann et al.,2007] consists in the employed stimulus material. Most previous studies used never‐before‐seen pictures of real‐world objects or visual scenes [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Strange et al.,2005; Yamaguchi et al.,2004]. Given the large amount of visual information in consideration for subsequent memory storage the necessity to evaluate the importance of that information is mandatory. Current theories on object processing posit that real‐world objects are processed in a series of interactive stages, including the processing of their physical attributes and more importantly the semantic knowledge associated with them during previous experience [Bright et al.,2005; Humphreys and Forde,2001]. This semantic knowledge influences visual object recognition even when the task does not explicitly require semantic retrieval [Gauthier et al.,2003]. Furthermore, it even has been shown that novel stimuli only elicit the novelty‐P3a and ‐N400 ERP components in the case of a preexisting semantic memory representation [Cycowicz and Friedman,2007; Friedman and Cycowicz,2006; Kotz et al.,2007; Mecklinger et al.,1997]. The present results suggest that the semantic content of visual stimuli might be even more important for the storage of information into memory than for the retrieval. Real‐world objects do possess semantic information that the hippocampus can compare to information present in memory [Kumaran and Maguire,2007; Vinogradova,2001]. Subsequently a novelty signal triggering the SN can be sent out, that in turn enhances the memory formation of that object [Lisman and Grace,2005]. In contrast to this, the never‐before‐seen‐fractal pictures employed in the current study are absent of semantic content. The hippocampal comparison to stored information as reflected in the observed activation of the hippocampus for novel compared to old stimuli (targets and standards) fails to generate a novelty signal that triggers the SN. Therefore a consecutive enhancement of hippocampal activity promoting enhanced memory formation is missing with the further consequence that novel fractal stimuli would not be well encoded into memory. This has been found to be exactly the case, recognition memory performance was described to be clearly reduced for fractal stimuli compared to natural scenes or magazine covers [Cerf et al.,2007].
Nevertheless, the lack of activation in the SN in response to novel fractal stimuli absent of semantic content has to be interpreted with care, given that no semantic meaningful stimuli were employed as within‐subject manipulation in the present experiment. This is certainly a weakness of the experiment. However, previous studies from our laboratory that were performed in exactly the same MR‐scanner, and employed the identical fMRI‐acquisition protocol have repeatedly demonstrated activation of the SN in response to semantically meaningful novel stimuli [Bunzeck and Duzel,2006; Bunzeck et al.,2007; Fenker et al.,2008; Wittmann et al.,2007]. It should also be mentioned that we did not find a completely unresponsive SN. The analysis based on the individual ROIs respecting each subject's anatomy revealed responses in the SN to target stimuli after which a motor action (button press) was subsequently executed (see Supporting Information Fig. S1 and Table S1). This most likely reflects the participation of the SN in the generation of the motor response. Alternatively, targets might have acquired some kind of contextual/semantic representation due to their relevance for the task and could therefore elicit SN‐activations. This explanation is however rather unlikely, because if an acquired contextual/semantic representation produced a SN‐activation, this same activation would appear in response to both, attended and unattended target stimuli, which was not the case. The fact that we observed a response to targets in the SN increases the confidence that with the current approach SN activity to novel fractal stimuli would have been detected if it was present.
The current study investigated the processing of exemplar novelty and the influence of spatial attention on that processing. In the perceptual part of the novelty‐processing network a region in the lingual gyrus was found to specifically process novel events when they occur outside the focus of spatial attention. This region located in the ventral visual pathway can therefore be regarded as the perceptual novelty detection instance, that doesn't take care of the semantic content associated with the stimuli. Furthermore, novel events absent of semantic meaning and behavioral relevance were found to activate the hippocampus but not the SN which—according to the hippocampal‐SN loop model—promotes effective memory encoding. Hence, the semantic content appears to be a key feature for successful activation of the reward system biasing successful encoding into memory.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure S1 Activity in the SN elicited by the different stimulus categories in the two attention conditions. The figure shows the Betas from the analysis performed on un‐normalized data with individually defined anatomical SN‐ROIs for each subject. The bar color indicates the particular stimulus category (black: targets; grey: standards; white: novels). The particular attention conditions are depicted below each bar plot. Abbreviations: SN, substantia nigra; L, left; R, right.
Supplementary Figure for Reviewer 1
Supplementary Figure for Reviewer 2
Table S1 Significance values for the post hoc comparisons between categories for the individual SN‐ROI data. Abbreviations: SN, substantia nigra; L, left; R, right.
Acknowledgements
The authors thank Dr. Michael Scholz for technical advice. This work was supported by the following grants: Scho 1217/1‐1 and SFB 779/TP‐A1 from the Deutsche Forschungsgemeinschaft (DFG) awarded to M.A.S.
REFERENCES
- Ashburner J, Friston KJ ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254– 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J‐L, Valabregue R, Poline J‐B ( 2006): Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, Vol. 16. Sendai, Japan, 2–6 June 2002.
- Bright P, Moss HE, Stamatakis EA, Tyler LK ( 2005): The anatomy of object processing: The role of anteromedial temporal cortex. Q J Exp Psychol B 58: 361– 377. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP ( 2001): Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51– 61. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E ( 2006): Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron 51: 369– 379. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schutze H, Stallforth S, Kaufmann J, Duzel S, Heinze HJ, Duzel E ( 2007): Mesolimbic novelty processing in older adults. Cereb Cortex 17: 2940– 2948. [DOI] [PubMed] [Google Scholar]
- Cerf M, Cleary DR, Peters RJ, Einhauser W, Koch C ( 2007): Observers are consistent when rating image conspicuity. Vision Res 47: 3052– 3060. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ ( 1999): The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671– 676. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D ( 2007): Visual novel stimuli in an ERP novelty oddball paradigm: Effects of familiarity on repetition and recognition memory. Psychophysiology 44: 11– 29. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R ( 2006): Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. J Neurophysiol 96: 1902– 1911. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC ( 1997): Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388: 582– 585. [DOI] [PubMed] [Google Scholar]
- Duncan K, Curtis C, Davachi L ( 2009): Distinct memory signatures in the hippocampus: Intentional States distinguish match and mismatch enhancement signals. J Neurosci 29: 131– 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ ( 2003): Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci 23: 9439– 9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R ( 1998): Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci 10: 590– 604. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Frey JU, Schuetze H, Heipertz D, Heinze HJ, Duzel E. ( 2008): Novel scenes improve recollection and recall of words. J Cogn Neurosci 20: 1250– 1265. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM ( 2006): Repetition priming of possible and impossible objects from ERP and behavioral perspectives. Psychophysiology 43: 569– 578. [DOI] [PubMed] [Google Scholar]
- Gauthier I, James TW, Curby KM, Tarr MJ ( 2003): The influence of conceptual knowledge in visual discrimination. Cogn Neuropsychol 20: 507– 523. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A ( 2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10: 14– 23. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Elliott MA, Bilker WB, Arnold SE, Gur RE ( 2007): Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event‐related fMRI study. Hum Brain Mapp 28: 263– 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE ( 2006): Mechanisms underlying working memory for novel information. Trends Cogn Sci 10: 487– 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs H, Scholz M, Tempelmann C, Woldorff MG, Dale AM, Heinze HJ ( 2000): Deconvolution of event‐related fMRI responses in fast‐rate experimental designs: Tracking amplitude variations. J Cogn Neurosci 12( Suppl 2): 76– 89. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM ( 2001): Hierarchies, similarity, and interactivity in object recognition: “category‐specific” neuropsychological deficits. Behav Brain Sci 24: 453– 476; discussion 476–509. [PubMed] [Google Scholar]
- Kakade S, Dayan P ( 2002): Dopamine: Generalization and bonuses. Neural Netw 15: 549– 559. [DOI] [PubMed] [Google Scholar]
- Kohler S, Crane J, Milner B ( 2002): Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus 12: 718– 723. [DOI] [PubMed] [Google Scholar]
- Kohler S, Danckert S, Gati JS, Menon RS ( 2005): Novelty responses to relational and non‐relational information in the hippocampus and the parahippocampal region: A comparison based on event‐related fMRI. Hippocampus 15: 763– 774. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Opitz B, Friederici AD ( 2007): ERP effects of meaningful and non‐meaningful sound processing in anterior temporal patients. Restor Neurol Neurosci 25: 273– 284. [PubMed] [Google Scholar]
- Kumaran D, Maguire EA ( 2007): Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus 17: 735– 748. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E ( 1998): Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus 8: 313– 322. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA ( 2005): The hippocampal‐VTA loop: Controlling the entry of information into long‐term memory. Neuron 46: 703– 713. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A ( 2007): Action‐related properties shape object representations in the ventral stream. Neuron 55: 507– 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A, Opitz B, Friederici AD ( 1997): Semantic aspects of novelty detection in humans. Neurosci Lett 235: 65– 68. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL ( 2000): Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp 11: 117– 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L ( 2005): Any novelty in hippocampal formation and memory? Curr Opin Neurol 18: 424– 428. [DOI] [PubMed] [Google Scholar]
- Panksepp J. 1998. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press. [Google Scholar]
- Ranganath C, Rainer G ( 2003): Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci 4: 193– 202. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Johnson SC, Flashman LA, McAllister TW, Sparling M, Darcey TM, Moritz CH, Guerin SJ, Weaver J, Mamourian A ( 1999): Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: An fMRI study. Brain 122( Part 10): 1963– 1971. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD ( 1999): Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7– 24. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Woldorff M, Duzel E, Scheich H, Heinze HJ, Mangun GR ( 2003): Form‐from‐motion: MEG evidence for time course and processing sequence. J Cogn Neurosci 15: 157– 172. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, Heinze HJ, Hillyard SA ( 2007): Spatio‐temporal analysis of feature‐based attention. Cereb Cortex 17: 2468– 2477. [DOI] [PubMed] [Google Scholar]
- Schott BH, Sellner DB, Lauer CJ, Habib R, Frey JU, Guderian S, Heinze HJ, Duzel E ( 2004): Activation of midbrain structures by associative novelty and the formation of explicit memory in humans. Learn Mem 11: 383– 387. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Duzel E ( 2006): The dopaminergic midbrain participates in human episodic memory formation: Evidence from genetic imaging. J Neurosci 26: 1407– 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN ( 1963): Higher nervous functions; the orienting reflex. Annu Rev Physiol 25: 545– 580. [DOI] [PubMed] [Google Scholar]
- Stoppel CM, Boehler CN, Sabelhaus C, Heinze HJ, Hopf JM, Schoenfeld MA ( 2007): Neural mechanisms of spatial‐ and feature‐based attention: A quantitative analysis. Brain Res 1181: 51– 60. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ ( 2001): Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus 11: 690– 698. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ ( 1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034– 4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Duggins A, Heinze HJ, Dolan RJ ( 2005): Dissociating intentional learning from relative novelty responses in the medial temporal lobe. Neuroimage 25: 51– 62. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS ( 2001): Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11: 578– 598. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Duzel E ( 2007): Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage 38: 194– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT ( 2004): Rapid prefrontal‐hippocampal habituation to novel events. J Neurosci 24: 5356– 5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure S1 Activity in the SN elicited by the different stimulus categories in the two attention conditions. The figure shows the Betas from the analysis performed on un‐normalized data with individually defined anatomical SN‐ROIs for each subject. The bar color indicates the particular stimulus category (black: targets; grey: standards; white: novels). The particular attention conditions are depicted below each bar plot. Abbreviations: SN, substantia nigra; L, left; R, right.
Supplementary Figure for Reviewer 1
Supplementary Figure for Reviewer 2
Table S1 Significance values for the post hoc comparisons between categories for the individual SN‐ROI data. Abbreviations: SN, substantia nigra; L, left; R, right.


