Abstract
The “overlapping systems” theory of language function argues that linguistic meaning construction crucially relies on contextual information provided by “nonlinguistic” cognitive systems, such as perception and memory. This study examines whether linguistic processing of spatial relations established by reading sentences call on the same posterior parietal neural system involved in processing spatial relations set up through visual input. Subjects read simple sentences, which presented two agents in relation to each other, and were subsequently asked to evaluate spatial (e.g., “Was he turned towards her?”) and equally concrete nonspatial content (e.g., “Was he older than her?”). We found that recall of the spatial content relative to the nonspatial content resulted in higher BOLD response in a dorsoposterior network of brain regions, most significantly in precuneus, strikingly overlapping a network previously shown to be involved in recall of spatial aspects of images depicting similar scenarios. This supports a neurocognitive model of language function, where sentences establish meaning by interacting with the perceptual and working memory networks of the brain. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: space, working memory, language
INTRODUCTION
Language comprehension depends on the monitoring of context. This is clearly seen in the case of personal pronouns like “he/she” or “him/her.” Personal pronouns are among the most frequently used words in English [Leech et al., 2001], but they only become understandable in context, since on their own they can reference almost anything (e.g. “It is her. She is him. He is it.”). To understand who “he/she/it” refers to, we need additional information. This information can only come from online perception or from memory of what was said or perceived previously. One interpretation of this observation is an “overlapping systems” [Pulvermuller, 2005; Talmy, 2000] or “modality‐specific re‐enactment” [Barsalou et al., 2003] account of language processing. These theories are further analogous to feature‐based theories of categorization [Martin et al., 2000; Noppeney, 2004] suggesting that category‐specific deficits observed in patients [Warrington and McCarthy, 1987; Warrington and Shallice, 1984] reflect an underlying stratification of neural processes profiled by different categories due to their semantic link to different sensory‐motor systems.
What this implies is that when language “works,” that is, evokes in the reader or listener some understanding, it interacts with specific cognitive functions (e.g. memory and perception) also involved in nonlinguistic processing of similar experiences. This predicts that probing spatial memory should activate an overlapping network of spatially related brain regions, irrespective of whether the memory was caused by linguistic input or visual input.
Processing of spatial information is a highly suitable test case for examining an “overlapping systems” model in a neurocognitive framework. Spatial prepositions (e.g. “over,” “in,” “toward”) are the most important carriers of spatial information in language. But like the personal pronouns this small class of words only carries a very schematic [Talmy, 2000] notion of the spatial relations they depict. This allows for spatiodynamic “metaphors” [Lakoff and Johnson, 1980] to be widely used in language to express even abstract, nonspatial scenarios (e.g. “The result approaches significance”) as well as more concretely spatial scenarios. Cognitive linguists have, on the basis of these observations, named spatial cognition as one of the basic constituents of semantics [Fauconnier, 1997; Lakoff and Johnson, 1980; Langacker, 1991; Talmy, 2000]. From a neurocognitive point of view, much is already known about the neuronal underpinnings of spatial cognition, due to decades of study in both humans and in animal models [Baddeley and Hitch, 1974; Burgess et al., 2001; Hartley et al., 2003; O'Keefe and Nadel, 1978; Ungerleider and Mishkin, 1982; White and McDonald, 2002]. Particularly, posterior parietal cortex has been found to play an important role in spatial working memory [Burgess et al., 1999; Ungerleider et al., 1998; Wallentin et al., 2006] as part of the “dorsal stream” network for spatiodynamic processing [Goodale and Milner, 1992].
Evidence that the spatial working memory system is also selectively involved in the processing of spatial meaning in language [Mellet et al., 2002, 1996; Wallentin et al., 2005b] is supported by studies of patients suffering from Williams syndrome [Williams et al., 1961]. Williams syndrome patients exhibit impoverished spatial processing but relatively spared language skills, with the exception of language with spatial meaning, like prepositions [Bellugi et al., 1999; Phillips et al., 2004]. This impairment is consistent with dysfunctional dorsal stream processing [Atkinson et al., 1997; Paul et al., 2002], and may be related to abnormalities in parietal cortex [Meyer‐Lindenberg et al., 2004].
In a previous study [Wallentin et al., 2006], we demonstrated that linguistically cued recall of different aspects of a previously presented visual scene depicting two avatars, yielded a differentiated BOLD‐response according to which of the brain's complementary memory systems [White and McDonald, 2002] the question accessed. In concordance with the literature on spatial working memory, [Baddeley and Hitch, 1974; Smith and Jonides, 1998], we showed that asking questions about the spatial aspects of the scene (“Was X in front of Y?”), relative to nonspatial aspects (“Was X older than Y?) of the same concrete scene, caused an increased dorsoposterior parietal response, most predominantly in the precuneus. This clearly demonstrated that linguistic expressions may guide cognition in accessing different memory components, and this lends support to an “overlapping systems” model of language function.
However, the study did not examine whether this effect of language extends from the probing of recent visual experiences to include recent linguistic experiences as well. Previous language studies have focused on imagery components, contrasting sentences with highly imageable content with sentences with abstract content (e.g. Just et al., 2004; Wallentin et al., 2005b]. These studies have not, however, contrasted spatial content with equally imageable nonspatial content. This was the aim of the present study, which was carefully designed as a “language only” version of our previous experiment. We first exposed subjects to simple written sentences that depicted two agents who were both spatially and nonspatially related to each other. We then used personal pronouns in simple verbal questions to “script” [Jack and Roepstorff, 2002] the subjects to access either spatial or nonspatial aspects of the sentence. In accordance with an “overlapping systems” account of language, we hypothesized that recall of spatial relations (e.g. “Was he turned towards her?”) relative to recall of nonspatial relations (e.g. “Was he older than her?”) would involve the same dorsoposterior parietal network, irrespective of whether the content was established through images or through words.
MATERIALS AND METHODS
Stimuli
Stimuli consisted of 30 event sequences. In each sequence, subjects were asked to read a sentence in Danish, which was projected onto a screen for 4,500 ms. Each sentence contained information about a man and a woman and their relative relationship along one spatial and two nonspatial axes (e.g. “Ved siden af hinanden står en rødmende studine og en olding med fuldskæg.” [Next to each other stand a blushing female student and an oldster with a beard.]). After a short delay, subjects were presented with three questions (delivered through headphones) related to the content of the sentence: a spatial question (“Was he/she turned towards him/her?”), and two nonspatial questions, one relational (NonSpace1), related to age differences (“Was he/she older than him/her?”) and one nonrelational (NonSpace2), related to some feature of one of the characters (e.g. “Was she blushing?”). The spatial questions and the age‐questions (NonSpace1) all consisted of five words, whereas the feature‐question (NonSpace2) all were made of three words. The two possible space questions had five and six syllables, whereas NonSpace1 questions were made of six and seven syllables and NonSpace2 questions had on average 4.9 syllables (see appendix for a full list of stimuli). Contrasting our spatial questions to both the NonSpace1 and the NonSpace2 conditions enabled us to rule out any possible effects of these differences. If our findings were significant relative to both control tasks then neither number of words nor number of syllables would be able to explain away such a difference. All questions were recorded to last exactly 2,000 ms. Subjects were asked to respond “yes” or “no” as quickly and as accurately as possible by button‐press, using the right index or middle finger. Question order and delay period between reading and hearing questions (SOA range 4,000–8,000 ms) was randomized across sentences and subjects. Fifty percent of each question type required a “yes” response.
Stimuli were presented using Cogent 2000 software, a Matlab toolbox developed at the Functional Imaging Laboratory and Institute of Cognitive Neuroscience, UCL, London. Sentences were projected onto a screen using a video projector placed in the control room, shooting through the window onto a screen placed at the end of the scanner bed, seen by the subject through the scanner mirror. Auditory stimuli were delivered through an AVOTEC sound system.
Subjects and Acquisition Parameters
Twenty three volunteers (13 women, 10 men; mean age 25 ± 3 years (std) were scanned using a General Electric 3T MR system. One subject was subsequently excluded from the study due to the discovery of a cerebral low pressure cavernous angioma. Three hundred and thirty volumes with 38 axial slices (4 mm) and an in‐plane resolution of 3 mm × 3 mm were acquired in each subject with TR: 2,600 ms, TE: 30 ms, flipangle: 90°.
Data Analysis
Scanning data were spatially realigned [Friston et al., 1995a], unwarped [Andersson et al., 2001], slice time‐corrected, normalized [Ashburner and Friston, 1999], and smoothed (10 mm FWHM) using SPM5. Task‐related BOLD‐responses for each subject were estimated using a general linear model [Friston et al., 1995b] with a 128‐s high‐pass filter, global scaling and AR(1) modeling of serial correlation in SPM5 with four regressors modeling onsets for: (1) Reading/Encoding; (2) Space Recall; (3) NonSpace Recall 1 (Age); NonSpace Recall 2 (feature), convolved with the canonical heamodynamic response function implemented in SPM5. six regressors, including parameters from the motion correction procedure, were added to regress out motion artefacts in the 1st level analysis.
Contrast‐estimates of Space vs NonSpace1 and NonSpace2 were submitted to a 2nd level RFX group analysis [Friston et al., 1999] using a one‐sample T‐test. For comparison, individual T‐tests were also conducted on the Space‐NonSpace1 contrast and the Space‐NonSpace2 contrast (Fig. 1, bottom). Significance threshold was set to P < 0.05, FDR‐corrected for multiple comparisons.
Figure 1.
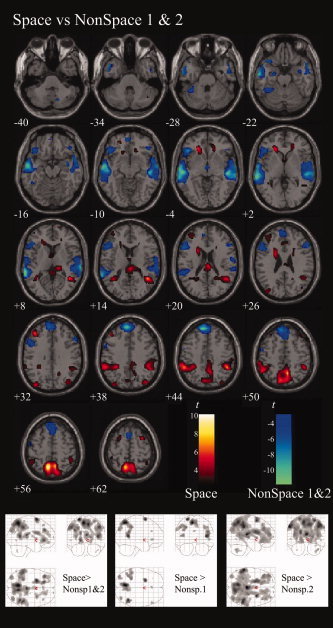
Recall of space relative to recall of two nonspatial tasks, thresholded at P < 0.05, FDR‐corrected. Main contrast of space is seen against the collapsed effect of the two nonspatial contrasts. Effects, however, are similar when contrasted only with one or the other. Glass brains show: LEFT: Space‐NonSpace1 & 2; MIDDLE: Space‐NonSpace 1; RIGHT: Space‐NonSpace2, all at P < 0.05, FDR‐corrected.
Putative anatomical regions were located using WFU (Wake Forest University School of Medicine) Pickatlas [Maldjian et al., 2003, 2004] referencing the aal atlas [Tzourio‐Mazoyer et al., 2002].
RESULTS
Behavioral Results
Average response time across subjects (mean of medians) was: Space: 2,586 ± 302 ms (std); NonSpace1: 2,487 ± 331 ms (std); NonSpace2: 2,649 ± 336 ms (std). There was no significant response time difference between conditions when tested using a one‐way ANOVA (n = 22, P = 0.25). Median percentage correct responses across subjects were: Space: 92%; NonSpace1: 93%; NonSpace2: 93%. There was no significant difference between percentage correct responses when compared using a nonparametric ANOVA (Kruskal–Wallis) test of equal medians (n = 22, P = 0.77).
Scanning Results
Space>NonSpace1 & 2
Recall of spatial linguistic information relative to the two nonspatial recall conditions revealed a distinct dorso‐parietal pattern of brain responses, predominantly in precuneus and superior parietal lobule (Fig. 1), strikingly similar (Fig. 2) to that found with verbally cued recall of nonlinguistic spatial information [Wallentin et al., 2006]. Peak regions include, as in the previous study, precuneus bilaterally (MNI [−8 −60 56] and [8 −56 52]), superior frontal gyrus/frontal eye fields (MNI [−24 6 70] and [30 6 62]), middle temporal gyrus (MNI 42 −52 12]) and temporo‐occipito‐parietal (TOP) junction, also bilaterally (MNI: [−38 −84 30] and [38 −78 36]) (Fig. 1 and Table I).
Figure 2.
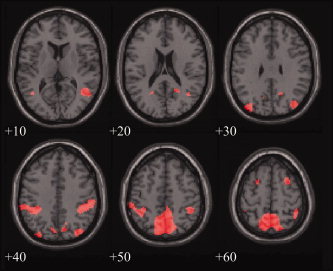
Recall of spatial content from language relies on the same network of brain regions found to be involved in recall of spatial aspects of an image [Wallentin et al., 2006]. The figure shows overlapping voxels from the [Space>NonSpace1 & NonSpace2] contrast from this study and the [Space>NonSpace] contrast from Wallentin et al. [ 2006] study, both thresholded at P < 0.05, FDR‐corrected. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
P < 0.05, FDR‐corrected for multiple comparisons
| Contrast | Putative anatomical region | Peak MNI | Z‐score |
|---|---|---|---|
| Space > NonSpace 1 & 2 | L Precuneus–BA 7 | [−8 −60 56] | 5.69 |
| R Precuneus–BA 7 | [8 −56 52] | 5.31 | |
| R Mid. Temp. Gyrus–BA 21 | [42 −52 12] | 4.9 | |
| R Supramarginal Gyrus–BA 40 | [46 −42 42] | 4.86 | |
| R Precuneus–BA 7 | [12 −72 56] | 4.31 | |
| L Sup. Front. Gyrus/Front. Eye Fields–BA 6 | [−24 6 70] | 5.39 | |
| R Sup. Front. Gyrus/Front. Eye Fields–BA 6 | [30 6 62] | 4.14 | |
| R Post. Cingulate/Corpus Callosum | [2 −30 16] | 5.03 | |
| L Caudate/ White matter | [−20 32 −4] | 4.78 | |
| L Caudate/ White matter | [−20 2 24] | 4.29 | |
| L Inf. Parietal Lobule–BA 40 | [−52 −44 42] | 4.75 | |
| L Mid. Front. Gyrus–BA 9 | [−40 34 34] | 4.65 | |
| L TOP Junction–BA 19/39 | [−38 −84 30] | 4.11 | |
| R TOP Junction–BA 19/39 | [38 −78 36] | 3.94 | |
| NonSpace 1 & 2 > Space | R Mid. Temp. Gyrus–BA 21/22 | [−62 −44 10] | 5.96 |
| R Mid. Temp. Gyrus–BA 21 | [−56 −22 −49] | 5.76 | |
| R Mid. Temp. Gyrus–BA 21 | [−54 −8 −14] | 5.56 | |
| L Inf. Front. Triangularis–BA 45 | [−42 28 −2] | 5.19 | |
| L Temp. Pole–BA 38 | [−46 12 −34] | 4.94 | |
| L Inf. Front. Gyrus–BA 47 | [−36 24 −22] | 4.4 | |
| L Sup. Med. Front. Gyrus–BA 8/9 | [−10 46 44] | 5.86 | |
| R Mid. Temp. Gyrus–BA 21 | [68 −28 8] | 5.52 | |
| R Inf. Front. Triangularis–BA 45 | [56 24 28] | 4.25 | |
| L Mid. Orb. Front. Gyrus–BA 11 | [−4 52 −10] | 4.06 | |
| L Cerebellum | [−38 −44 −28] | 4.05 | |
| L Inf. Front. Operculum–BA 44 | [−52 14 0] | 3.46 |
In this contrast responses from both nonspatial tasks are collapsed. Results, however, were comparable when the contrast was limited to either of the two nonspatial responses (Fig. 1, bottom).
DISCUSSION
The study confirmed our hypothesis that probing linguistically generated spatial memories activates a distinct posterior parietal network most significantly in precuneus (Fig. 1). This is an extension of our previous findings, [Wallentin et al., 2006], which showed that verbally cued recall of visually established spatial relations, relative to recall of nonspatial information, calls on the same network (Fig. 2). Nonspatial information activated more “ventral” regions, primarily in the temporal lobe, well‐known to be involved in semantic processes [e.g. see Price, 2000]. Since our main hypothesis involved the spatial “dorsal” system, and precuneus in particular, we will focus our discussion on this.
Precuneus
Studies have suggested that posterior parietial regions are part of a secondary perceptual system for spatiodynamic processing [Burgess et al., 1999; Ungerleider and Mishkin, 1982], involving short‐term memory processes related to spatial relations [Baddeley and Hitch, 1974; Casey et al., 1998; Ungerleider et al., 1998; Wallentin et al., 2006], virtual reality navigation [Burgess et al., 2001; Hartley et al., 2003; Iaria et al., 2003], and imagery [Fletcher et al., 1995; Kosslyn et al., 1997; Mellet et al., 1998, 2002], extending to processing of words [Jessen et al., 2000] and sentences [Just et al., 2004; Wallentin et al., 2005b] with a concrete meaning relative to sentences with abstract meaning [but see Kemmerer, 2006 for a slightly different view.]
Damage to parietal cortex has been found to lead to deficits such as neglect [Mesulam, 1981], including representational space [Bisiach and Luzzatti, 1978], simultagnosia [Coslett and Saffran, 1991] or optical apraxia [Perenin and Vighetto, 1988], all deficits tied to visuospatial processing.
We extend these findings by showing that posterior parietal regions are also specifically involved when spatial relations, created solely through language, are contrasted with equally concrete nonspatial information. Unlike other studies that looked at differences between concrete and abstract content [Just et al., 2004; Wallentin et al., 2005b], the BOLD response difference observed in this study therefore cannot be interpreted as reflecting a unitary imagery system [Pavio, 1995], as all questions refer to the same concrete linguistic context. Whether or not subjects used a task‐solving strategy involving imagery, our results suggest that their strategy distinguished between spatial and nonspatial content, and if imagery played a role as a strategy then imagery must be understood as a multicomponential phenomenon, in which case the only difference between an imagery strategy and a “re‐enactment” strategy [Barsalou et al., 2003] may relate to whether the experience is necessarily conscious or not [Barsalou, 1999].
The present study was conducted in Danish, whereas our previous image recall study [Wallentin et al., 2006] was conducted in English. It therefore also seems unlikely that low‐level phonological similarities between the two studies may have caused the observed overlap in neural activity. The overlap, it seems, can therefore only be attributed to differences in working memory processes between the spatial and nonspatial questions in both studies. This distinction may explain why some studies of imagery have failed to find posterior parietal activation [Mellet et al., 1998; Noppeney and Price, 2004; Tyler et al., 2001].
Patients suffering from Williams syndrome [Williams et al., 1961] have been used as primary evidence for a separate language module [Pinker, 1994]. Among other deficits, Williams syndrome patients exhibit impoverished spatial processing but have relatively spared language skills. Both structural and functional parietal abnormalities have been found in Williams syndrome patients [Meyer‐Lindenberg et al., 2004]. Our results, however, are consistent with more detailed language studies showing that the spatial deficit in Williams syndrome patients extends to processing of language with spatial meaning [Bellugi et al., 1999; Phillips et al., 2004].
The spatial questions in this study probed the relationship between two objects, i.e. incorporating an allocentric viewpoint (i.e. the grammatical object of the question), whereas our feature‐related questions (NonSpace2) did not necessarily involve a viewpoint shift because these questions did not incorporate a second object against which the first were to be judged (i.e. these questions contained no grammatical object). In NonSpace1 the question did contain a grammatical object, which could be argued to lead to allocentric perspective construction, but this sentence was nonspatial. Our finding that the contrast between Space and NonSpace2 tends to yield a more significant result than the Space‐NonSpace1 contrast (Fig. 1, bottom) is therefore consistent with studies showing a higher precuneus activation during construction of a spatial third person perspective relative to a first person perspective [David et al., 2006; Vogeley et al., 2004]. Further studies are needed to investigate whether these differences reflect independent spatial and perspectival processes working in the precuneus. In a reanalysis of the [Wallentin et al., 2006] study [Wallentin et al., Frontal eye fields involved in construction of new spatial viewpoint in imagery, submitted], looking at the allocentric and egocentric spatial dichotomy, we did not see an effect of perspective in precuneus, but this may be due to the very conservative analytic approach taken to avoid a task difficulty difference confound.
CONCLUSIONS
Our results demonstrate that probing spatial relations in linguistically established memories activates a posterior network of brain regions similar to that activated when probing visually established memories. This extends previous work on spatial working memory [Burgess et al., 1999; Ungerleider et al., 1998; Wallentin et al., 2006] and linguistic concreteness effects [Jessen et al., 2000; Just et al., 2004; Wallentin et al., 2005b]. Further, it is in concordance with an “overlapping systems” model of language, where the meaning embedded in actual sentences is evoked through an interaction with relevant cognitive systems that are not in themselves strictly linguistic [Barsalou et al., 2003; Pulvermuller, 2005; Talmy, 2000]. The attempt to ground the human language faculty in a separate, context‐free cognitive module [Fodor, 1983], although interesting in itself, has implied a shift in focus away from the functional aspects of language, i.e. the role it plays within a concrete communicative situation. Taken together with our previous experiments [Wallentin et al., 2005a,b, 2006; Wallentin et al., Frontal eye fields involved in construction of new spatial viewpoint in imagery, submitted], this study suggests that when linguistic expressions “work,” i.e. convey meaning in a communicative situation, they do so through fine‐grained interaction with other relevant cognitive systems. This may pave the way for understanding language not only as an abstract system, but also as a pragmatic communicative tool [e.g. see Tomasello 2003]. Further, it may point toward the development of linguistic means for investigating different short term memory processes and deficits in a seamless and naturalistic way as part of an understanding of how interacting minds relate to resonating brains [Roepstorff and Frith, 2004].
Sentences for Encoding
1. Med ryggen til hinanden står en gammel kutteklædt mand og en ung kvinde.
1. [Back to back stand an old cloak‐dressed man and a young woman.]
2. Med front mod hinanden står en tyk ældre mand og en rødhåret pige.
2. [Facing each other stand a fat elderly man and a red‐haired girl]
3. Ved siden af hinanden står en gråhåret herre og en skolepige.
3. [Next to each other stand a grey‐haired gentleman and a schoolgirl.]
4. En krumbøjet mand står med front mod sin sortklædte lillesøsters ryg.
4. [A bent man stands with his front towards his black‐dressed little sister's back.]
5. En gammel støder i brun jakke står vendt mod en fotomodels nakke.
5. [An old fart in a brown jacket stands turned towards the back of the head of a model.]
6. En fyr, godt oppe i årene står ryg mod ryg med en ung dame med røde sko på.
6. [A fellow, well into his golden years, stands back to back with a young lady in red shoes.]
7. En tynd mand står vendt mod sin datter, som grædende har vendt sig bort.
7. [A thin man stands turned toward his daughter, who, crying, has turned away.]
8. Med ryggen til hinanden står en bleg fyr med kasket og hans smilende mor.
8. [Back to back stand a pale young man with a cap and his smiling mother.]
9. Med front mod hinanden står en dreng og en voksen kvinde og spiser is.
9. [Facing each other stand a boy and an adult woman and eat ice‐cream.]
10. Ved siden af hinanden står en yngre tyk mand og en ældre dame med stok.
10. [Next to each other stand a young, fat man and an elderly woman with a cane.]
11. En knægt med regnfrakke står med front mod en kvindelig pensionists ryg.
11. [A boy with a rain‐coat stands facing a female retiree's back.]
12. En ung mand med store øjne står vendt mod en gammel dames bagdel.
12. [A young man with large eyes stands turned toward an old lady's behind.]
13. I køen står en yngre herre i jeans foran en kvinde af ældre model.
13. [In the checkout line stands a young man in front of a woman who is getting up in years.]
14. En skoledreng står vendt mod sin kvindelige lærer, som har vendt sig bort.
14. [A schoolboy stands turned toward his female teacher, who has turned away from him.]
15. En beskidt fyr står ansigt til ansigt med sin renvaskede storesøster.
15. [A grubby youth stands face to face with his newly bathed older sister.]
16. En ældre kvinde og en mandlig studerende står med ryggen til hinanden.
16. [An elderly woman and a male student stand back to back.]
17. En bedstemor og en skoledreng med shorts står vendt mod hinanden.
17. [A grandmother and a schoolboy in shorts stand turned toward each other.]
18. En bleg pige og hendes lillebror står ved siden af hinanden.
18. [A pale girl and her little brother stand next to each other.]
19. En yngre dame står med front mod sin sorthårede lillebrors ryg.
19. [A young lady stands facing her dark‐haired little brother's back]
20. En fornøjet gammel tante står vendt mod en yngre fyrs nakke.
20. [A contented old aunt stands turned toward a young fellow's back.]
21. En solbrændt kvinde står ansigt til ansigt med sin behårede lillebror.
21. [A suntanned woman stands face to face with her hairy younger brother.]
22. En kvinde står vendt mod sit barnebarns solbrændte ryg.
22. [A woman stands turned toward her grandchild's suntanned back.]
23. Med ryggen til hinanden står en rasende pige og hendes forkølede far.
23. [With their backs to each other stand a furious girl and her flu‐stricken father.]
24. Med front mod hinanden står en purung dame og en nedslidt stodder.
24. [Facing each other stand a very young lady and used‐up old fogey.]
25. Ved siden af hinanden står en rødmende studine og en olding med fuldskæg.
25. [Next to each other stand a blushing female student and an oldster with a beard.]
26. En langhåret pige står med front mod en distingveret herres ryg.
26. [A long‐haired girl stands facing a distinguished gentleman's back.]
27. En svedende ballerina står vendt mod en gammel knarks bagdel.
27. [A sweating ballerina stands turned toward an old geezer's rump.]
28. En spinkel pige står vendt mod sin lillebror, som distræt har vendt sig bort.
28. [A slender girl stands turned toward her little brother, who has absent‐mindedly turned away.]
29. I køen står en buttet skolepige bag sin urolige mandlige lærer.
29. [In the checkout line stands a plump schoolgirl behind her restless male teacher.]
30. En kvinde i 20erne står vendt mod sin farfars skaldede baghoved.
30. [A woman in her 20's stands turned toward the back of her grandfather's bald head.]
Questions for Recall
SPACE
Var han vendt mod hende? [Was he turned towards her?]
Var hun vendt mod ham? [Was she turned towards him?]
Average number of words: 5
Average number of syllables: 5.5
NONSPACE1 (AGE)
Var han ældre end hende? [Was he older than her?]
Var hun ældre end ham? [Was she older than him?]
Average number of words: 5
Average number of syllables: 6.5
NONSPACE2 (FEATURE)
1. Var han kutteklædt? [Was he wearing a cloak?]
2. Var hun lyshåret? [Was she blonde?]
3. Var hun skolepige? [Was she a schoolgirl?]
4. Var hun krumbøjet? [Was she bent?]
5. Var han brunjakket? [Was he wearing a brown jacket?]
6. Var han rødskoet? [Was he wearing red shoes?]
7. Var hun grædende? [Was she crying?]
8. Var han solbrændt? [Was he tanned?]
9. Var hun isspisende? [Was she eating an ice‐cream?]
10. Var han med stok? [Was he using a cane?]
11. Var hun pensionist? [Was she retired?]
12. Var hun storøjet? [Did she have big eyes?]
13. Var han jeansklædt? [Was he wearing jeans?]
14. Var han advokat? [Was he a lawyer?]
15. Var hun renvasket? [Was she washed clean?]
16. Var han pensionist? [Was he retired?]
17. Var han shortsklædt? [Was he wearing shorts?]
18. Var hun mørklødet? [Was she dark‐skinned?]
19. Var han sorthåret? [Was he black‐haired?]
20. Var hun rasende? [Was she furious?]
21. Var han behåret? [Was he hairy?]
22. Var han bleghudet? [Was he pale?]
23. Var hun rasende? [Was she furious?]
24. Var han purung? [Was he very young?]
25. Var hun rødmende? [Was she blushing?]
26. Var han langhåret? [Was he long‐haired?]
27. Var hun svedende? [Was she sweating?]
28. Var hun kraftig? [Was she plump?]
29. Var han urolig? [Was he restless?]
30. Var han hårfager? [Was he covered with hair?]
Average number of words: 3
Average number of syllables: 4.9
REFERENCES
- Andersson JLR, Hutton C, Ashburner J, Turner R, Friston K ( 2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–919. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Map 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, King JA, Braddick O, Nokes L, Anker S, Braddick F ( 1997): A specific deficit of dorsal stream function in Williams' syndrome. Neuroreport 8: 1919–1922. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ ( 1974): Working memory In: Bower GA, editor. Recent Advances in Learning and Motivation. New York: Academic Press; pp 47–90. [Google Scholar]
- Barsalou LW ( 1999): Perceptual symbol systems. Behav Brain Sci 22: 577–609; discussion 610–660. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD ( 2003): Grounding conceptual knowledge in modality‐specific systems. Trends Cogn Sci 7: 84–91. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR ( 1999): Bridging cognition, the brain and molecular genetics: Evidence from Williams syndrome. Trends Neurosci 22: 197–207. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C ( 1978): Unilateral neglect of representational space. Cortex 14: 129–133. [DOI] [PubMed] [Google Scholar]
- Burgess N, Jeffery KJ, O'Keefe J ( 1999): Integrating hippocampal and parietal functions: A spatial point of view In: Burgess N, Jeffery KJ, O'Keefe J, editors. The Hippocampal and Parietal Foundations of Spatial Cognition. Oxford: Oxford University Press; pp 3–29. [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J ( 2001): A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14: 439–453. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O'Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR ( 1998): Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage 8: 249–261. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran E ( 1991): Simultanagnosia. To see but not two see. Brain 114(Part 4): 1523–1545. [DOI] [PubMed] [Google Scholar]
- David N, Bewernick BH, Cohen MX, Newen A, Lux S, Fink GR, Shah NJ, Vogeley K ( 2006): Neural representations of self versus other: Visual‐spatial perspective taking and agency in a virtual ball‐tossing game. J Cogn Neurosci 18: 898–910. [DOI] [PubMed] [Google Scholar]
- Fauconnier G ( 1997): Mappings in Thought and Language. Cambridge: Cambridge University Press. [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ ( 1995): The mind's eye–precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Fodor J ( 1983): The Modularity of Mind. Bradford Books; Cambridge: MIT Press. [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RS ( 1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS ( 1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD ( 1992): Separate visual pathways for perception and action. TINS 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N ( 2003): The well‐worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 37: 877–888. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD ( 2003): Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci 23: 5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AI, Roepstorff A ( 2002): Introspection and cognitive brain mapping: From stimulus‐response to script‐report. TICS 6: 333–339. [DOI] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath D‐O, Klose U, Papassotiropoulos A, Grodd W ( 2000): The concreteness effect: Evidence for dual coding and context availability. Brain Lang 74: 103–112. [DOI] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA ( 2004): Imagery in sentence comprehension: An fMRI study. Neuroimage 21: 112–124. [DOI] [PubMed] [Google Scholar]
- Kemmerer D ( 2006): The semantics of space: Integrating linguistic typology and cognitive neuroscience. Neuropsychologia 44: 1607–1621. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Alpert NM ( 1997): Neural systems shared by visual imagery and visual perception: A positron emission tomography study. Neuroimage 6: 320–334. [DOI] [PubMed] [Google Scholar]
- Lakoff J, Johnson M ( 1980): Metaphors We Live By. Chicago: University of Chicago Press. [Google Scholar]
- Langacker RW ( 1991): Concept, Image, and Symbol. The Cognitive Basis of Grammar. Berlin: Mouton de Gruyter. [Google Scholar]
- Leech G, Rayson P, Wilson A ( 2001): Word Frequencies in Written and Spoken English: Based on the British National Corpus. London: Longman. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH ( 2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21: 450–455. [DOI] [PubMed] [Google Scholar]
- Martin A, Underleider LG, Haxby JV ( 2000): Category specificity and the brain: The sensory/motor model of semantic representations of objects In: Gazzaniga MS, editor. The New Cognitive Neurosciences, 2nd ed. Cambridge: MIT Press; pp 1023–1037. [Google Scholar]
- Mellet E, Tzourio N, Crivello F, Joliot M, Denis M, Mazoyer B ( 1996): Functional anatomy of spatial mental imagery generated from verbal instructions. J Neurosci 16: 6504–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellet E, Tzourio N, Denis M, Mazoyer B ( 1998): Cortical anatomy of mental imagery of concrete nouns based on their dictionary definition. Neuroreport 9: 803–808. [DOI] [PubMed] [Google Scholar]
- Mellet E, Bricogne S, Crivello F, Mazoyer B, Denis M, Tzourio‐Mazoyer N ( 2002): Neural basis of mental scanning of a topographic representation built from a text. Cereb Cortex 12: 1322–1330. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10: 309–325. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF ( 2004): Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron 43: 623–631. [DOI] [PubMed] [Google Scholar]
- Noppeney U ( 2004): The feature‐based model of semantic memory In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W, editors. Human Brain Function, 2nd ed. Elsevier; San Diego: Academic Press; pp 533–545. [Google Scholar]
- Noppeney U, Price CJ ( 2004): Retrieval of abstract semantics. Neuroimage 22: 164–170. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L ( 1978): The Hippocampus as a Cognitive Map. Oxford: Oxford University Press. [Google Scholar]
- Paivio A ( 1995): Imagery and Memory In: Gazzaniga MS, Bizzi E, Black IB, Blakemore C, Cosmides L, Kosslyn SM, LeDoux JE, Movshon JA, Pinker S, Posner MI. and others, (eds.). The Cognitive Neurosciences: MIT Press; pp. 977–986. [Google Scholar]
- Paul BM, Stiles J, Passarotti A, Bavar N, Bellugi U ( 2002): Face and place processing in Williams syndrome: Evidence for a dorsal‐ventral dissociation. Neuroreport 13: 1115–1119. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A ( 1988): Optic ataxia: A specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain 111(Part 3): 643–674. [DOI] [PubMed] [Google Scholar]
- Phillips CE, Jarrold C, Baddeley AD, Grant J, Karmiloff‐Smith A ( 2004): Comprehension of spatial language terms in Williams syndrome: Evidence for an interaction between domains of strength and weakness. Cortex 40: 85–101. [DOI] [PubMed] [Google Scholar]
- Pinker S ( 1994): The Language Instinct. Harmondsworth: Allen Lane. [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197(Part 3): 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F ( 2005): Brain mechanisms linking language and action. Nat Rev Neurosci 6: 576–582. [DOI] [PubMed] [Google Scholar]
- Roepstorff A, Frith C ( 2004): What's at the top in the top‐down control of action? Script‐sharing and ‘top‐top’ control of action in cognitive experiments. Psychol Res 68: 189–198. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1998): Neuroimaging analyses of human working memory. PNAS 95: 12061–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmy L ( 2000): Toward a Cognitive Semantics. Cambridge: MIT Press. [Google Scholar]
- Tomasello M ( 2003): Constructing a Language: A Usage‐based Theory of Language Acquisition. Harvard: Harvard University Press. [Google Scholar]
- Tyler LK, Russell R, Fadili J, Moss HE ( 2001): The neural representation of nouns and verbs: PET studies. Brain 124(Part 8): 1619–1634. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M ( 1982): Two cortical visual systems In: Ingle D, Goodale M, Mansfield R, editors. Analysis of Visual Behavior. Cambridge, MA: MIT‐Press. [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV ( 1998): A neural system for human visual working memory. PNAS 95: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR ( 2004): Neural correlates of first‐person perspective as one constituent of human self‐consciousness. J Cogn Neurosci 16: 817–827. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Lund TE, Ostergaard S, Ostergaard L, Roepstorff A ( 2005a): Motion verb sentences activate left posterior middle temporal cortex despite static context. Neuroreport 16: 649–652. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Østergaard S, Lund TE, Østergaard L, Roepstorff A ( 2005b): Concrete spatial language: See what I mean? Brain Lang 92: 221–233. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Roepstorff A, Glover R, Burgess N ( 2006): Parallel memory systems for talking about location and age in precuneus, caudate and Broca's region. Neuroimage 32: 1850–1864. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA ( 1987): Categories of knowledge. Further fractionations and an attempted integration. Brain 110(Part 5): 1273–1296. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T ( 1984): Category specific semantic impairments. Brain 107(Part 3): 829–854. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ ( 2002): Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 77: 125–184. [DOI] [PubMed] [Google Scholar]
- Williams JCP, Barratt‐Boyes BG, Lowe JB ( 1961): Supravalvular aortic stenosis. Circulation 24: 311–1318. [DOI] [PubMed] [Google Scholar]


