Abstract
The ability to detect and learn contingencies between fearful stimuli and their predictive cues is an important capacity to cope with the environment. Contingency awareness refers to the ability to verbalize the relationships between conditioned and unconditioned stimuli. Although there is a heated debate about the influence of contingency awareness on conditioned fear responses, neural correlates behind the formation process of contingency awareness have gained only little attention in human fear conditioning. Recent animal studies indicate that the ventral striatum (VS) could be involved in this process, but in human studies the VS is mostly associated with positive emotions. To examine this question, we reanalyzed four recently published classical fear conditioning studies (n = 117) with respect to the VS at three distinct levels of contingency awareness: subjects, who did not learn the contingencies (unaware), subjects, who learned the contingencies during the experiment (learned aware) and subjects, who were informed about the contingencies in advance (instructed aware). The results showed significantly increased activations in the left and right VS in learned aware compared to unaware subjects. Interestingly, this activation pattern was only found in learned but not in instructed aware subjects. We assume that the VS is not involved when contingency awareness does not develop during conditioning or when contingency awareness is unambiguously induced already prior to conditioning. VS involvement seems to be important for the transition from a contingency unaware to a contingency aware state. Implications for fear conditioning models as well as for the contingency awareness debate are discussed. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: fear conditioning, contingency awareness, differential classical conditioning, nucleus accumbens, striatum
INTRODUCTION
In a differential classical fear conditioning paradigm, a neutral stimulus (the conditioned stimulus; CS+) is repeatedly paired with an aversive stimulus (the unconditioned stimulus, UCS) such as an electric shock, while another stimulus (CS−) is never paired with the UCS. After only some trials the CS+ compared to the CS− elicits conditioned responses (CRs) such as increased skin conductance responses, increased heart rate, increased startle amplitudes, and also increased activity in the fear‐network including the amygdala [e.g. Büchel and Dolan, 2000; Knight et al., 2004; Olsson and Phelps, 2007].
There is an agreement that the amygdala plays a crucial role in fear conditioning [Büchel et al., 1998; LeDoux, 2000; Öhman, 2005; Olsson and Phelps, 2007]. However, the development of contingency awareness does most likely not depend on amygdala activity. For instance, patients with amygdala lesions were able to report CS‐UCS relationships (i.e., contingency aware) but failed to show conditioned fear responses [LaBar et al., 1995].
In the last decade, there has been a heated debate about the influence of contingency awareness on CRs. Contingency awareness can be defined as the explicit knowledge of the CS/UCS associations. In differential fear conditioning this means the knowledge that the CS+ is followed by an aversive event (UCS) whereas the CS− is not. To achieve this, subjects have to learn to differentiate between the CS+ and the CS−. Some authors report contingency awareness as essential for eliciting CRs [e.g. Dawson et al., 2007; Lovibond and Shanks, 2002; Pleyers et al., 2007], others found that CRs can also be measured in contingency unaware subjects [e.g. De Houwer et al., 2005; Öhman et al., 2007].
Regarding the neural substrates underlying contingency awareness, previous studies have identified several brain structures: for instance, Carter et al. [2006] reported a correlation of middle frontal gyrus (MFG) activation with contingency awareness on a trial‐by‐trial measurement. Another study by Carter et al. [2003] showed the middle prefrontal cortex (MPFC) to be involved in working memory processes, which are important for contingency awareness. In a current study with a sophisticated and new approach to identify brain structures important for contingency learning, Knight et al. [2009] reported increased hippocampal activity during aware trials. However, Knight et al. [2009] did not observe increased MFG activity in aware trials. The involvement of the hippocampus in contingency awareness was further supported by a lesion study [Bechara et al., 1995; for a review see Clark et al., 2002].
Some studies, however, point to a crucial role of the ventral striatum (VS) for the development of contingency awareness. For example, Jensen et al. [2003, 2007] and Menon et al. [2007] reported enhanced VS activity to a stimulus predicting an aversive event in contingency aware subjects. In their highly influential article, Phelps et al. [2004] also found enhanced striatal activity during fear conditioning, but did not explicitly relate it to contingency awareness. Further support for an involvement of the VS in the development of contingency awareness in humans comes from animal fear conditioning studies: Young et al. [1993] detected an increase of dopamine the Nucleus Accumbens (NAcc) during CS presentation, which could be linked to the CS‐UCS contingency. Fenu et al. [2001] observed a failure of fear acquisition, when D1 receptors in the NAcc were blocked. Further, Schwienbacher et al. [2004] reported a similar result during temporary NAcc inactivation. Remarkably, this result seems to be independent of CS and UCS input modalities (e.g. taste aversion learning or tone‐footshock learning). Summarizing these results, Pezze and Feldon [2004] suggest that “dopamine transmission in the NAcc, acting via D1 receptors, is essential for the association between a CS and a UCS” (p. 312). In sum, these studies strongly suggest that the VS is involved in contingency learning during fear conditioning. However, classical human fear conditioning studies have not yet investigated this issue in detail.
Therefore, the main focus of this study was to elucidate the role of the VS in the development of contingency awareness. To investigate this, we reanalyzed recent classical fear conditioning studies conducted in our laboratory.
This reanalysis includes subjects, who learned the contingencies during conditioning referred to as “learned aware subjects,” subjects, who did not learn the contingencies at all referred to as “unaware subjects” [Klucken et al., 2009; Stark et al., 2006; Tabbert et al., 2005, 2006], and subjects, who were instructed about the contingencies prior to conditioning [Tabbert et al., 2006] referred to as “instructed aware subjects.”
Derived from the literature cited earlier, we assume that the VS is involved in the development of contingency awareness. However, it remains unclear whether the differential VS activity still occurs once contingency awareness has been established. Thus, we expected differential VS activity to CS+ and CS− in learned aware subjects, with higher responses to the CS+. In unaware subjects, we did not expect significant differences in VS activity. Analysis of conditioning related VS activity in instructed aware subjects was explorative.
METHODS AND OVERVIEW
The present reanalysis includes data from four fear conditioning experiments in our laboratory [Klucken et al., 2009; Stark et al., 2006; Tabbert et al., 2005, 2006, see Table I]. This section gives a short overview of the studies used in the current reanalysis to point out similarities and differences between the experiments. Detailed methodological parameters can be found in the original articles.
Table I.
Overview of the four included fear conditioning studies forming the basis of this reanalysis
| Study | Subjects | CS duration | UCS | Trials per CS | |
|---|---|---|---|---|---|
| Classical fear conditioning | Tabbert et al. [2005] | 6 unaware; 12 learned aware | 8 s | Electric stimulation | 30 |
| Stark et al. [2006] | 14 unaware; 20 learned aware | 8 s | Electric stimulation | 30 | |
| Klucken et al. [2009] | 18 unaware; 14 learned aware | 8 s | Aversive pictures | 20 | |
| Instructed fear conditioning | Tabbert et al. [2006] | 17 unaware; 16 instructed aware | 8 s | Electric stimulation | 30 |
Sample Characteristics
One hundred seventeen (63 female) subjects were included in the present reanalysis. The mean age of all subjects in the different studies was comparable: 25.33 years [Tabbert et al., 2005], 24.2 years [Stark et al., 2006], 23.36 [Tabbert et al., 2006], and 23.26 years [Klucken et al., 2009]. All subjects had normal or corrected‐to‐normal vision. Most of the participants were university students, who had been recruited via announcements at bulletin boards at the campus. None of them was taking regular medication and nobody had a history of psychiatric or neurological treatment. They signed an informed consent that they could terminate the experiment at any time. Every subject participated only in one of the studies. All studies were approved by the ethics committee of the German Psychological Society.
Conditioned Stimuli
All studies used two simple geometric figures (a rhomb and a square) serving as CS+ and CS−. Both visual stimuli were grey in color and had identical luminance. For visual stimulation inside the scanner, an LCD‐projector (Model EPSON EMP‐7250) was used, which projected pictures onto a screen at the end of the scanner (visual field = 18°). Pictures were viewed by means of a mirror mounted to the head coil.
Each of the studies used a differential fear conditioning paradigm, in which the UCS always appeared with the CS+ (100% reinforcement) and the CS− was never paired with the UCS. In every study, the CS was presented for 8 s. For each participant, a pseudo randomized stimulus order was used with the restrictions that (1) no more than two successive presentations of the same CS occurred and that (2) the CS+ and CS− were equally distributed within each half of the acquisition period. The rhomb and the square were counterbalanced as CS+ between participants.
Differences Between the Studies
Instructed contingency awareness versus learned contingency awareness
Three of the four studies [Klucken et al., 2009; Stark et al., 2006; Tabbert et al., 2005] used a classical fear conditioning paradigm, in which subjects were not informed about the conditioning paradigm (and of course not about the contingencies). After each of the experiments subjects filled out a short forced‐choice paper‐pencil recognition test for contingency awareness. Dawson and Reardon [1973] showed that this kind of recognition test is one of the most valid and sensitive instruments to verify contingency awareness [see also: Dawson et al., 2007; Lovibond and Shanks, 2002]. Subjects had to choose one of the following statements: “Before the aversive pictures there was”: (a) “…always a picture of a rhomb”; (b) “…sometimes a picture of a rhomb”; (c) “…never a picture of a rhomb”; (d) “I do not know.” The same statement was used for the second stimulus (only the word “rhomb” was replaced by the word “square”). For classification as aware, subjects had to state the correct answer (answer “c”) for the CS‐ and answers “a” or “b” for the CS+. If subjects stated answer “d” for both CS‐types, they were classified as unaware.
In the remaining forth study by Tabbert et al. [2006] contingency awareness was manipulated by a written and oral instruction, which explained the CS‐UCS contingencies to half of the subjects before the acquisition phase. The other group was not informed about the contingencies. To avoid deviations from this manipulation all subjects had to engage in a distractor task (2‐back‐task) during the conditioning procedure. The manipulation of contingency awareness was controlled by the recognition questionnaire described above. Hence, the aware group in Tabbert et al. [2006] did not have to learn contingencies, which constitutes a major difference to the aware participants in the other three studies. The contingency aware group in Tabbert et al. [2006] will be referred to as the instructed aware group. Aware subjects from the other three studies had to learn the contingencies between CS and UCS during the experiment and will be addressed as the learned aware group. The remaining subjects, who did not learn the contingencies, will be labeled as unaware subjects (see Table I).
Tabbert et al. [2006] used a recognition questionnaire similar to the ones in the other studies. The participants had to choose one of the following completions for the statement: “The electrical stimulation was presented after”: (a) “all presentations,” (b) “some presentations,” (c) “no presentation,” (d) “I can't remember.” Next to each answer, the respective CS was pictured. Manipulation of awareness of stimulus contingencies was regarded as being successful if participants in the aware group correctly identified the contingencies for CS+ and CS− and if participants in the unaware group did not recognize the correct relationship for CS+ and CS−. Two unaware participants stated other combinations (e.g. one of them stated answer “b” for the CS+ and the non‐C). Nonetheless, they remained in the original analyses and in the current analyses, as their awareness was at the most partial [cf. Tabbert et al., 2006]. In the study by Klucken et al. [2009], none of the subjects recognized the CS+/UCS contingency but not the CS‐/non‐UCS contingency (i.e. “CS+ aware” but not “CS‐unaware”) or vice versa.
Influence of hormones
The main purpose of the study by Stark et al. (2006) was the investigation of the influence of the stress hormone cortisol on fear conditioning. Thirty milligram of hydrocortisone was administered to half of the participants, while the other half received visually identical placebos. Because this manipulation did not have an effect on VS activity, both groups were converged into one.
Unconditioned Stimuli
Three of the four studies used a mild electrical shock as UCS. Stimulus intensity was set individually for each participant to an “unpleasant but not painful” level using a gradually increasing rating procedure. A custom‐made impulse‐generator (833 Hz) provided transcutaneous electrical stimulation to the left shin through two silver/silverchlorid electrodes. Each UCS was applied for 100 ms during the conditioning procedures.
The remaining study [Klucken et al., 2009] used highly aversive pictures as UCS (e.g. mutilations). In this experiment, the UCS presentation time was 4 s.
Statistical Analyses
For all statistical analyses the following parameters were used: Blood Oxygen Level Dependent signal change (BOLD‐response) was analyzed using Statistical Parametric Mapping (SPM2, Wellcome Departement of Cognitive Neurology, London, UK) implemented in MatLab 6.5 (Mathworks, Sherbourn, MA). All preprocessing (realignment, slice time correction, normalization, and smoothing) analyses were conducted in a comparable manner as specified in the original articles. All experimental conditionings were modeled by a stick function convolved with the canonical hemodynamic response function (hrf) in the general linear model, as implemented in SPM2. In the first level analysis, the following contrasts were computed for the entire acquisition phase: CS+ > CS− and CS− > CS+. As described in the original articles, the data reported in Tabbert et al. [2006] were based on the first block of the acquisition phase and in Tabbert et al. [2005] on the second block. The current reanalyses were conducted with the full acquisition phase for all studies to ensure comparability. Region of interest (ROI) were performed using the small volume correction in SPM2 (α < 0.05, FWE‐corrected). A minimum cluster size of five voxel was required.
To investigate the differences between learned aware and unaware subjects, the contrast CS+ > CS− was analyzed across the relevant three studies [Klucken et al., 2009; Stark et al., 2006; Tabbert et al., 2005] via analysis of variance with six groups (three learned aware vs. three unaware groups) implemented in SPM2. To further disentangle potential effects, one sample t‐tests for the contrast CS+ > CS− were computed for each of the six groups separately. To investigate differential responses to CS+ and CS− in instructed aware subjects, one sample t‐tests were performed. Finally, to investigate potential differences between learned aware and instructed aware subjects as well as between instructed aware and unaware subjects, two‐sample t‐tests between the respective groups were conducted. For all analyses, the opposite contrast CS− > CS+ was also investigated.
ROI analyses were performed for the following structures: medial frontal gyrus (MFG), medial prefrontal cortex (MPFC), hippocampus, and ventral striatum. Masks for the MFG, the hippocampus were taken from the current “Harvard‐Oxford cortical and subcortical structural atlases” provided by the Harvard Center for Morphometric Analysis (http://www.cma.mgh.harvard.edu/) with the probability threshold at 0.5. VS and MPFC masks were taken from the Human Brain Project Repository database (THOR Center for Neuroinformatics; http://hendrix.ei.dtu.dk/; labeled as ventral striatum region and prefrontal medial and superior region) with the threshold at 0.5. The original data for the masks described above was based on the BrainMap database [Fox and Lancaster, 1994; Nielsen and Hansen, 2002].
RESULTS
We found increased activity in the MFG, the hippocampus, and the MPFC for the contrast CS+ > CS− in the learned aware group. In the instructed aware group we only found increased activity in the MPFC (see Table II). No significant activations were found in the unaware group in any of the ROIs. In addition, no significant enhanced activation could be observed in the opposite contrast CS− > CS+ in any group.
Table II.
Significant activations to the contrast CS+ > CS−
| Group | Brain structures | Side | x | y | z | T max | P |
|---|---|---|---|---|---|---|---|
| Learned aware | MFG | Right | 45 | 3 | 57 | 4.73 | 0.002 |
| Left | −42 | 6 | 48 | 5.10 | 0.001 | ||
| Hippocampus | Right | 24 | −36 | 3 | 3.86 | 0.013 | |
| MPFC | Right | 9 | 18 | 36 | 6.59 | 0.000 | |
| Instructed aware | MPFC | 0 | 42 | 15 | 4.78 | 0.049 | |
| Unaware | No significant activations | n.s. | n.s | ||||
| Instructed aware > learned aware | MPFC | Right | 3 | 48 | 39 | 4.13 | .019 |
All coordinates are given in MNI space. n.s.: no significant activations.
Significantly increased MFG and MPFC activity was found in learned aware as compared to unaware subjects. Further, enhanced MPFC activity was found in instructed aware as compared to learned aware (see Table II) as well as compared to unaware subjects. However, no significant differences could be obtained in learned aware compared to instructed aware subjects.
Effects of Contingency Awareness in the Ventral Striatum
Analysis of variance revealed significantly higher responses in the contrast CS+ > CS− in the left (t = 3.07; P < 0.05) and the right (t = 3.80; P < 0.001) VS in learned aware subjects compared to unaware subjects. Additionally, significantly enhanced differentiation of CS+ and CS− in the same direction was observed in learned aware versus instructed aware subjects (see Table III and Figs. 1 and 2).
Table III.
Main effect of awareness with increased VS activation for the learned aware compared to the unaware group and the instructed aware group in the contrast CS+ > CS−
| Main effect | Post‐hoc test | Side | x | y | z | T max | P |
|---|---|---|---|---|---|---|---|
| Awareness | Learned aware > unaware | Right | 18 | 3 | −3 | 3.80 | 0.006 |
| Left | −21 | 9 | −3 | 3.07 | 0.038 | ||
| Learned aware > instructed aware | Right | 15 | 12 | −9 | 3.10 | 0.022 | |
| Left | −12 | 18 | −3 | 2.74 | 0.072 | ||
| Unaware > learned aware | Right | n.s. | |||||
| Left | n.s. |
Figure 1.
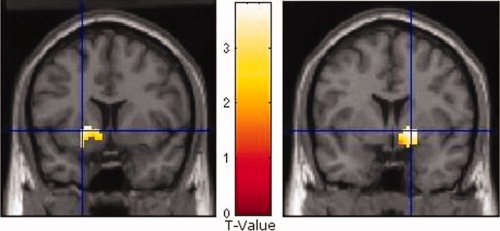
Neural activation (in T‐values) of the learned aware > unaware group for the left and the right VS in the contrast CS+ > CS−. Statistical parametric maps are overlaid on a T1 template (depicted from the SPM2 package). Because of illustration reasons the blue crosses were set at the peak voxel of the left VS (coordinates: −21, 9, −3) and of the right VS (coordinates: 18, 3, −3). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 2.
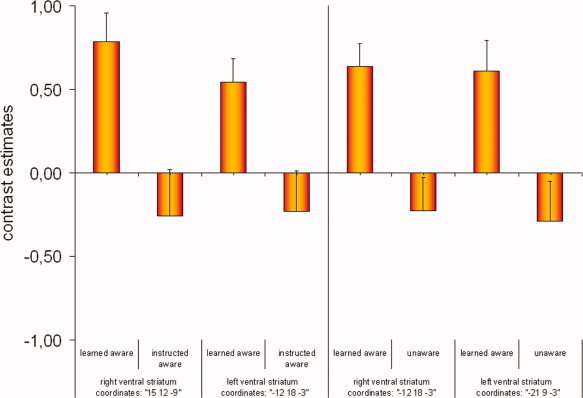
Mean (SE) of the contrast estimates in the ventral striatum (CS+ > CS−) for the peak voxels from the group comparisons (see Table III) of learned aware versus instructed aware and learned aware versus unaware. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In addition, one sample t‐tests showed significant VS activation in all learned aware groups for the contrast CS+ > CS− [except for the right VS in Tabbert et al., 2005], indicating that the observed main effect was not caused by a single learned aware group (see Table IV).
Table IV.
Ventral striatal activations in the learned und instructed aware groups for the contrast CS+ > CS−, separately for each study
| Study | Side | x | y | z | T max | P | |
|---|---|---|---|---|---|---|---|
| Classical fear conditioning | Tabbert et al. [2005] | Right | n.s. | ||||
| Left | −9 | 18 | −9 | 4.92 | 0.019 | ||
| Stark et al. [2006] | Right | 12 | 6 | −15 | 5.76 | 0.001 | |
| Left | −21 | 3 | −3 | 4.69 | 0.005 | ||
| Klucken et al. [2009] | Right | 18 | 6 | −15 | 6.53 | 0.001 | |
| Left | −18 | 6 | −12 | 5.90 | 0.004 | ||
| Instructed fear conditioning | Tabbert et al. [2006] | Right | n.s. | ||||
| Left | n.s. |
No increased VS activation could be obtained for the contrast CS+ > CS− in the instructed aware group as well as in the unaware groups (all P > 0.1). In addition, no significant VS activation could be observed for the opposite contrast CS− > CS+ in any of the groups.
DISCUSSION
In the current reanalysis, data of four classical fear conditioning experiments were analyzed with an emphasis on the role of VS activity for the development of contingency awareness. Subjects were classified into three distinct categories: participants who did not learn the CS‐UCS contingencies (unaware), participants who learned the contingencies during the experiment (learned aware), and participants who were informed about the contingencies prior to the experiment (instructed aware).
Despite our focus on the VS, several other ROIs were also included in our analyses. These analyses revealed higher activity towards the CS+ in comparison to the CS− in the MPFC in the instructed as well as the learned aware, but not in the unaware groups. Further, group comparisons yielded significant differences for the contrast CS+ > CS− with higher MPFC contrast values in the instructed aware compared to the learned aware group. Considering these results and the findings of other studies, we assume that the MPFC is involved in the maintenance or recall of CS‐UCS contingencies [e.g. Quirk and Mueller, 2008; Sotres‐Bayon et al., 2006] and/or more generally in the coding of emotional salience [e.g. Phan et al., 2003]. The hippocampus and the MFG showed higher responses to the CS+ compared to the CS− in the learned aware groups but not in the instructed aware and the unaware groups. However, group comparisons did not reveal significant differences. These results are in line with previous studies [Bechara et al., 1995; Carter et al., 2006; Clark et al., 2002; Knight et al., 2009]. Yet, the present findings cannot unequivocally clarify the exact role of these structures in contingency awareness because group comparisons between learned aware and instructed aware subjects were not significant.
Regarding VS activity, we found strong bilateral activation in the contrast CS+ > CS− exclusively in the learned aware groups but not in the unaware groups and the instructed aware group. The specific role of the VS for the learned aware groups is further underlined by the fact that the contrast values of the contrast CS+ > CS− were significantly higher in the learned aware groups in comparison to the unaware groups as well as to the instructed aware group. Considering the present results, we speculate that the differential VS activity between CS+ and CS− is not involved when contingency awareness does not develop during conditioning or when contingency awareness is unambiguously present already prior to conditioning (e.g. in case of instructed awareness). In fact, VS involvement seems to be crucial for the transition from a contingency unaware to a contingency aware state. Consistent with this assumption, Jensen et al. [2007] also reported enhanced VS activation to a stimulus predicting an aversive event. Importantly, this activity was only found in participants, who learned the contingencies during the experiment. However, they did not include unaware participants in their data analyses, because the main purpose of their study was to investigate neural correlates of the prediction error rather than the mechanisms underlying the development of contingency awareness. Cooper and Knutson [2007] showed that the VS (or more specifically the NAcc) is not significantly activated in situations with predetermined aversive outcomes, but only in situations where participants did not have full certainty. In a very recent study, Schiller et al. [2008] found increased striatal activity when contingency awareness developed. These findings support our assumption that the VS is involved in the transition from being contingency unaware to contingency aware. In addition, they also found increased activity in the striatum when contingencies were suddenly changed and the new contingencies had to be learned.
Contingency awareness implies that participants respond differently towards the CS+ than the CS−. This indicates that the VS is involved in the differentiation process between fear‐cues (CS+) and non‐fear‐cues (CS−). It is crucial to differ between fear and non‐fear cues in order to react effectively towards aversive events. The difference between the activation towards the CS+ and the CS− is therefore due to the fact that an aversive event which follows the CS+ is more salient. Current fear conditioning theories emphasize this “functional role” of the CS+ (and in line with this, the awareness of the CS+/UCS contingency) to interact fast, effectively and adequately with the UCS [Domjan, 2005]. Cooper and Knutson [2007] showed that the NAcc is more activated to salient cues compared to less salient cues. Animal studies also provide evidence for this striatal role [for a review see: Pezze and Feldon, 2004]. Iordanova et al. [2006] also found that the ability to distinguish between reliable and unreliable fear cues (conditioned stimuli) was impaired, when D1 and D2 receptors in the NAcc were blocked. Although fMRI data cannot distinguish activity based on specific neurotransmitter release, dopamine transmission in the NAcc could be a mechanism involved in the development of contingency awareness in human classical aversive conditioning.
The current findings could be implemented in current two‐level account conditioning models [e.g. Hamm and Weike, 2005; Lovibond and Shanks, 2002]. According to these models, the pairing of CS and UCS ultimately leads to certain (automatic and unconscious) CRs (first process) and contingency awareness (second process), which are generated by widely independent processes. Our data suggest that the VS is involved in the second learning process.
Some limitations and open questions should be mentioned. First, due to the limited resolution of fMRI, our data do not unequivocally answer the question if the VS or adjacent structures contribute to contingency learning in fear conditioning. For instance, based on their animal studies Martinez et al. [2008] proposed that the NAcc should be subdivided in two different regions, each involved in fear conditioning but with distinct roles. Second, current studies showed a differential influence of contingency awareness on trace compared to delay conditioning [Knight et al., 2006; Weike et al., 2007]. This study however examined delay conditioning only. In addition, Knight et al. [2009] used a sophisticated subliminal procedure to avoid contingency learning. This procedure is very different to our conscious presentation of the CS. Finally, our data appear somewhat in contrast to Jensen et al. [2003] and Menon et al. [2007], who partly informed their participants about the contingencies and found increased VS activity during anticipation of aversive stimuli. This seems at first glance contrary to the result of our instructed aware group (no differential VS activity towards the CS+ compared to the CS−). Jensen et al. and Menon et al. only informed the participants about the presence of CS‐UCS contingencies (subjects were told that one CS would sometimes be followed by an aversive shock), but they were neither informed about which CS was going to be the danger signal nor about the exact reinforcement schedule (33% reinforcement). Thus, it might be conceivable that the experimental protocol of Jensen et al. led to contingency learning similar to our learned aware groups. In contrast, the instructed aware group in Tabbert et al. (2006) did not have to learn anything about the contingencies. In sum, our findings call for additional studies with trace conditioning and an investigation of the role of the VS in subliminal conditioning. Also, the activation time course of the VS activity could be analyzed in more detail. To investigate this issue, it might be useful to correlate VS activity with trial‐by‐trial measurements of UCS expectancy.
The ventral striatum has been ascribed various different functions such as reward prediction [e.g. Knutson et al., 2001], and more generally the processing of positive emotions [e.g. reviews: Burgdorf and Panksepp, 2006; Whittle et al., 2006]. Research in the field of addiction suggests that the nucleus accumbens might increase incentive salience attribution to conditioned cues that predict reward [Pecina, 2008]. Yet, there are several studies confirming the involvement of the VS in prediction errors for aversive outcomes also [McNally and Westbrook, 2006; Niv and Schoenbaum, 2008; Seymour et al., 2004]. The current findings add a further facet to the function of this very interesting structure by showing that the VS evidently plays a major role in the development of contingency awareness in classical fear conditioning experiments.
Acknowledgements
We wish to thank the anonymous reviewers for their helpful comments.
REFERENCES
- Bechara A,Tranel D,Damasio H,Adolphs R,Rockland C,Damasio AR ( 1995): Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Büchel C,Dolan RJ ( 2000): Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol 10: 219–223. [DOI] [PubMed] [Google Scholar]
- Büchel C,Morris J,Dolan RJ,Friston KJ ( 1998): Brain systems mediating aversive conditioning: An event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Burgdorf J,Panksepp J ( 2006): The neurobiology of positive emotions. Neurosci Biobehav Rev 30: 173–187. [DOI] [PubMed] [Google Scholar]
- Carter RM,Hofstotter C,Tsuchiya N,Koch C ( 2003): Working memory and fear conditioning. Proc Natl Acad Sci USA 100: 399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM,O'Doherty JP,Seymour B,Koch C,Dolan RJ ( 2006): Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage 29: 1007–1012. [DOI] [PubMed] [Google Scholar]
- Clark RE,Manns JR,Squire LR ( 2002): Classical conditioning, awareness, and brain systems. Trends Cognit Sci 6: 524–531. [DOI] [PubMed] [Google Scholar]
- Cooper JC,Knutson B ( 2007): Valence and salience contribute to nucleus accumbens activation. NeuroImage 39: 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME,Reardon P ( 1973): Construct validity of recall and recognition postconditioning measures of awareness. J Exp Psychol 98: 308–315. [DOI] [PubMed] [Google Scholar]
- Dawson ME,Rissling AJ,Schell AM,Wilcox R ( 2007): Under what conditions can human affective conditioning occur without contingency awareness? Test of the evaluative conditioning paradigm. Emotion 7: 755–766. [DOI] [PubMed] [Google Scholar]
- De Houwer J,Baeyens F,Field AP ( 2005): Associative learning of likes and dislikes: some current controversies and possible ways forward. Cognit Emot 19: 161–174. [DOI] [PubMed] [Google Scholar]
- Domjan M ( 2005): Pavlovian conditioning: A functional perspective. Annu Rev Psychol 56: 179–206. [DOI] [PubMed] [Google Scholar]
- Fenu S,Bassareo V,Di Chiara G ( 2001): A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci 21: 6897–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT,Lancaster JL ( 1994): Neuroscience on the net. Science 266: 994–996. [DOI] [PubMed] [Google Scholar]
- Hamm AO,Weike AI ( 2005): The neuropsychology of fear learning and fear regulation: Neurobiology of fear and disgust. Int J Psychphysiol 57: 5–14. [DOI] [PubMed] [Google Scholar]
- Iordanova MD,Westbrook RF,Killcross AS ( 2006): Dopamine activity in the nucleus accumbens modulates blocking in fear conditioning. Eur J Neurosci 24: 3265–3270. [DOI] [PubMed] [Google Scholar]
- Jensen J,McIntosh AR,Crawley AP,Mikulis DJ,Remington G,Kapur S ( 2003): Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40: 1251–1257. [DOI] [PubMed] [Google Scholar]
- Jensen J,Smith AJ,Willeit M,Crawley AP,Mikulis DJ,Vitcu I,Kapur S ( 2007): Separate brain regions code for salience vs. valence during reward prediction in humans. Hum Brain Mapp 28: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T,Kagerer S,Schweckendiek J,Tabbert K,Vaitl D,Stark R ( 2009): Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture‐picture conditioning paradigm. Neuroscience 158: 721–731. [DOI] [PubMed] [Google Scholar]
- Knight DC,Cheng DT,Smith CN,Stein EA,Helmstetter FJ ( 2004): Neural substrates mediating human delay and trace fear conditioning. J Neurosci 24: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC,Nguyen HT,Bandettini PA ( 2006): The role of awareness in delay and trace fear conditioning in humans. Cogn Affect Behav Neurosci 6: 157–162. [DOI] [PubMed] [Google Scholar]
- Knight DC,Waters NS,Bandettini PA ( 2009): Neural substrates of explicit and implicit fear memory. NeuroImage 45: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B,Adams CM,Fong GW,Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS,LeDoux JE,Spencer DD,Phelps EA ( 1995): Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci 15: 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE ( 2000): Emotion circuits in the brain. Ann Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Lovibond PF,Shanks DR ( 2002): The role of awareness in pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol Anim Behav Processes 28: 3–26. [PubMed] [Google Scholar]
- Martinez RCR,Oliveira AR,Macedo CE,Molina VA,Brandão ML ( 2008): Involvement of dopaminergic mechanisms in the nucleus accumbens core, shell subregions in the expression of fear conditioning. Neurosci Lett 446: 112–116. [DOI] [PubMed] [Google Scholar]
- McNally GP,Westbrook RF ( 2006): Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem 13: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M,Jensen J,Vitcu I,Graff‐Guerrero A,Crawley A,Smith MA,Kapur S ( 2007): Temporal difference modeling of the blood‐oxygen level dependent response during aversive conditioning in humans: Effects of dopaminergic modulation. Biol Psychiat 62: 765–772. [DOI] [PubMed] [Google Scholar]
- Nielsen F,Hansen LK ( 2002): Automatic anatomical labeling of Talairach coordinates and generation of volumes of interest via the BrainMap database. NeuroImage, Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan. Available on CD‐Rom.
- Niv Y,Schoenbaum G ( 2008): Dialogues on prediction errors. Trends Cogn Sci 12: 265–272. [DOI] [PubMed] [Google Scholar]
- Öhman A ( 2005): The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinoly 30: 953–958. [DOI] [PubMed] [Google Scholar]
- Öhman A,Carlsson K,Lundqvist D,Ingvar M ( 2007): On the unconscious subcortical origin of human fear. Physiol Behav 92: 180–185. [DOI] [PubMed] [Google Scholar]
- Olsson A,Phelps EA ( 2007): Social learning of fear. Nat Neurosci 10: 1095–1102. [DOI] [PubMed] [Google Scholar]
- Pecina S ( 2008): Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol Behav 94: 675–680. [DOI] [PubMed] [Google Scholar]
- Pezze MA,Feldon J ( 2004): Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 74: 301–320. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Decker LR,Noll DC,Nichols TE,Britton JC,Liberzon I ( 2003): Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: A fMRI study. Biol Psychiat 53: 211–215. [DOI] [PubMed] [Google Scholar]
- Phelps EA,Delgado MR,Nearing KI,LeDoux JE ( 2004): Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- Pleyers G,Corneille O,Luminet O,Yzerbyt V ( 2007): Aware and (dis)liking: Item‐based analyses reveal that valence acquisition via evaluative conditioning emerges only when there is contingency awareness. J Exp Psych 33: 130–144. [DOI] [PubMed] [Google Scholar]
- Quirk GJ,Mueller D ( 2008): Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienbacher I,Fendt M,Richardson R,Schnitzler H ( 2004): Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear‐potentiated startle in rats. Brain Res 1027: 87–93. [DOI] [PubMed] [Google Scholar]
- Schiller D,Levy I,Niv Y,LeDoux JE,Phelps EA ( 2008): From fear to safety and back: reversal of fear in the human. J Neurosci 28: 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B,O'Doherty JP,Dayan P,Koltzenburg M,Jones AK,Dolan RJ,Friston KJ,Frackowiak RS ( 2004): Temporal difference models describe higher‐order learning in humans. Nature 429: 664–667. [DOI] [PubMed] [Google Scholar]
- Stark R,Wolf OT,Tabbert K,Kagerer S,Zimmermann M,Kirsch P,Schienle A,Vaitl D ( 2006): Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. NeuroImage 32: 1290–1298. [DOI] [PubMed] [Google Scholar]
- Sotres‐Bayon F,Cain CK,LeDoux JE ( 2006): Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol Psychiat 60: 329–336. [DOI] [PubMed] [Google Scholar]
- Tabbert K,Stark R,Kirsch P,Vaitl D ( 2005): Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int J Psychophysiol 57: 15–23. [DOI] [PubMed] [Google Scholar]
- Tabbert K,Stark R,Kirsch P,Vaitl D ( 2006): Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage 32: 761–770. [DOI] [PubMed] [Google Scholar]
- Weike AI,Schupp HT,Hamm AO ( 2007): Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology 44: 170–180. [DOI] [PubMed] [Google Scholar]
- Whittle S,Allen NB,Lubman DI,Yücel M ( 2006): The neurobiological basis of temperament: Towards a better understanding of psyhopthology. Neurosci Biobeh Rev 30: 511–525. [DOI] [PubMed] [Google Scholar]
- Young AM,Joseph MH,Gray JA ( 1993): Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience 54: 5–9. [DOI] [PubMed] [Google Scholar]


