Abstract
A quantitative, voxel‐wise meta‐analysis was performed to investigate the cortical control of water and saliva swallowing. Studies that were included in the meta‐analysis (1) examined water swallowing, saliva swallowing, or both, and (2) reported brain activation as coordinates in standard space. Using these criteria, a systematic literature search identified seven studies that examined water swallowing and five studies of saliva swallowing. An activation likelihood estimation (ALE) meta‐analysis of these studies was performed with GingerALE. For water swallowing, clusters with high activation likelihood were found in the bilateral sensorimotor cortex, right inferior parietal lobule, and right anterior insula. For saliva swallowing, clusters with high activation likelihood were found in the left sensorimotor cortex, right motor cortex, and bilateral cingulate gyrus. A between‐condition meta‐analysis revealed clusters with higher activation likelihood for water than for saliva swallowing in the right inferior parietal lobule, right postcentral gyrus, and right anterior insula. Clusters with higher activation likelihood for saliva than for water swallowing were found in the bilateral supplementary motor area, bilateral anterior cingulate gyrus, and bilateral precentral gyrus. This meta‐analysis emphasizes the distributed and partly overlapping cortical networks involved in the control of water and saliva swallowing. Water swallowing is associated with right inferior parietal activation, likely reflecting the sensory processing of intraoral water stimulation. Saliva swallowing more strongly involves premotor areas, which are crucial for the initiation and control of movements. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: meta‐analysis, functional magnetic resonance imaging, positron emission tomography, magnetoencephalography, swallowing, sensorimotor cortex, premotor cortex, insula, lateralization
INTRODUCTION
Swallowing is a complex sensorimotor function that is controlled by cortical, subcortical, and brainstem mechanisms [Miller, 1999] and recruits the coordinated activity of orofacial, pharyngeal, laryngeal, respiratory, and esophageal muscles [Doty and Bosma, 1956]. Swallowing comprises a voluntary oral preparatory phase during which ingested material is manipulated within the oral cavity and moved posteriorly over the tongue surface toward the pharynx. This is followed by a semi‐autonomic pharyngeal phase in which the airway is closed as the bolus is transported through the pharynx and into the esophagus [Dodds et al., 1990].
The importance of understanding swallowing neural control relates in part to the fact that the swallowing motor sequence is produced by paired muscles, which are organized about the midline. Little is known about the neural control of this class of movements because limb movements have formed the basis for most current concepts of motor control and motor neuroplasticity. However, brain‐mapping studies have suggested that oropharyngeal and swallowing neural control is distinct from limb motor control in terms of the extent to which muscles are represented in both hemispheres [Muellbacher et al., 1999], as well as the neuroplastic effects of central injury [Hamdy et al., 1998; Mistry et al., 2007]. Thus, an understanding of the neural basis of swallowing is important in terms of basic neuroscience. Swallowing neural control is also important from a clinical perspective because injury to the central nervous system frequently results in significant swallowing impairment [Logemann, 1996]. Indeed, brain injury can give rise to severe and protracted swallowing problems, which necessitate tube feeding. Current understanding of the neuropathophysiology of swallowing impairment, the neuroplastic mechanisms underlying swallowing recovery, as well as the principles of swallowing rehabilitation is limited [for review, see Martin, 2008].
Functional brain imaging has greatly advanced our understanding of the neural basis of swallowing. In humans, functional imaging substantiated the crucial role of the cerebral cortex for the control of voluntary and automatic swallowing [Hamdy et al., 1999a, b; Kern et al., 2001b; Martin et al., 2001, 2004, 2007; Mosier et al., 1999a, b; Zald and Pardo, 1999], corroborating earlier findings obtained by electrophysiologic techniques in awake primates [Martin and Sessle, 1993; Martin et al., 1997, 1999; Narita et al., 1999; Yao et al., 2002]. However, the exact neuroanatomy and functional significance of swallowing‐related networks in humans are not entirely clear. Although some cortical swallowing foci have consistently been identified, discrepancies have also emerged. For example, some studies have reported lateralization of sensorimotor cortical activation for swallowing [Dziewas et al., 2003; Martin et al., 2004; Teismann et al., 2008], whereas others have identified more bilateral activation [Hamdy et al., 1999b; Zald and Pardo, 1999]. Similarly, swallow‐related activation of the insula has varied with respect to the left [Dziewas et al., 2003] and right [Martin et al., 2001] hemisphere and anterior [Hamdy et al., 1999a] versus posterior [Suzuki et al., 2003] insular location.
These discrepancies may reflect methodological variation across studies. Functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and magnetoencephalography (MEG), the imaging techniques most frequently used to investigate the neural basis of swallowing, represent different aspects of brain activity. fMRI detects local task‐related changes in cerebral blood oxygenation, closely reflecting the underlying neural activity [Logothetis et al., 2001]. PET uses radioactive tracers to study local changes of neurophysiological parameters, such as brain perfusion (H2O PET) and glucose metabolism (18F‐fluorodeoxyglucose PET). MEG, finally, measures the small magnetic field changes corresponding to electrical brain activity. Each of these imaging modalities presents inherent challenges for swallowing research. Functional imaging of swallowing using fMRI is challenging because of the potential artifacts associated with swallowing‐related head movement and magnetic susceptibility phenomena due to movements outside the field of view (in particular movements of the jaw and the tongue) [Birn et al., 1998]. MEG of swallowing‐related brain activity may be affected by myo‐electric discharges, which are considerably larger than the magnetic brain activity under investigation [Loose et al., 2001; Sörös et al., 2003].
Functional imaging studies of swallowing are also heterogenous in terms of the swallowing tasks studied (voluntary or reflexive water swallowing; voluntary or naïve saliva swallowing), and the demographics of the participants. Even within tasks, experimental designs have differed between studies regarding the frequency and the total number of swallows, and, for water swallowing, bolus delivery methods and bolus volume, and block versus single event‐related imaging paradigm, all of which are potential confounding variables in individual studies. Given the potential bias introduced by these discrepant methodologies, it would be advantageous to identify swallowing‐related brain activity that is common across studies and thus relatively less affected by distinct experimental designs. Meta‐analysis is a statistical integration approach that examines the concordance of results across a corpus of studies and extracts the most significant finding [Egger and Smith, 1997]. Brain‐imaging studies are well suited to meta‐analysis because their findings are typically reported in standard stereotaxic coordinates.
This study employed a novel meta‐analysis technique based on activation likelihood estimation (ALE) [Chein et al., 2002; Turkeltaub et al., 2002]. ALE is a quantitative voxel‐wise meta‐analysis technique that pools the results of several studies, expressed by the standard space coordinates of activation maxima, estimates the activation likelihood in a given voxel, and rigorously tests the significance of the ALE statistic [Laird et al., 2005a]. ALE has been successfully used for meta‐analyses of imaging data from different neurofunctional systems and cognitive domains [e.g., Brown et al., 2005; Price et al., 2005].
The aim of this meta‐analysis of swallowing‐related brain activity was to identify and compare brain activation associated with two behaviorally distinct swallowing tasks, voluntary water swallowing and voluntary saliva swallowing.
MATERIALS AND METHODS
Search Strategies
To identify functional brain imaging studies on swallowing, literature searches were performed using four different approaches: (1) The databases PubMed and ISI Web of Science were searched. Key phrases and keywords included: “functional magnetic resonance imaging,” fMRI, “positron emission tomography,” PET, MEG, swallow, swallowing; (2) The references found in all identified articles were examined; (3) Using the ISI Web of Science database, articles citing the identified studies were examined and (4) Recent articles on the neurophysiology [Ertekin and Aydogdu, 2003] and functional neuroimaging of swallowing [Humbert and Robbins, 2007] were consulted.
Inclusion Criteria for Articles
Of the 30 studies identified, 10 were included in this meta‐analysis (summarized in Table I). Inclusion criteria were: (1) Studies included the contrasts voluntary water swallowing1 (wet swallowing) versus rest, saliva swallowing (dry swallowing) versus rest, or both. (2) Studies reported their results in stereotaxic coordinates (either based on the brain template by Talairach and Tournoux [1988] or the Montreal Neurological Institute (MNI). The included studies analyzed data from the entire cerebrum and, in part, from the cerebellum. None of those studies was based on previously defined regions of interest.
Table I.
Studies included in the meta‐analysis
| Article | Imaging modality | n | Age (yrs) mean (range) | Cue | Number of swallows | Foci |
|---|---|---|---|---|---|---|
| Water swallow | ||||||
| Fraser et al., 2002 | fMRI (1.5 T) | 8 | 26 (23–34) | Visual | 96 | 8 |
| Hamdy et al., 1999a | fMRI (1.5 T) | 10 | 32 (22–61) | Self‐paced | 20 | 24 |
| Martin et al., 2001 | fMRI (4 T) | 14 | 28 | Self‐paced | 5–17 | 9 |
| Martin et al., 2007 | fMRI (4 T) | 9 | 74 | Visual | 10 | 32 |
| Furlong et al., 2004 | MEG | 8 | NA (26–45) | Visual | 20 | 5 |
| Hamdy et al., 1999b | H2O PET | 8 | 48 (35–65) | Visual | 180 | 9 |
| Harris et al., 2005 | FDG PET | 8 | NA (29–37) | Visual | 90 | 17 |
| Saliva swallow | ||||||
| Martin et al., 2001 | fMRI (4 T) | 14 | 28 | Self‐paced | 4–12 | 6 |
| Martin et al., 2004 | fMRI (4 T) | 14 | 28 | Visual | 18 | 16 |
| Martin et al., 2007 | fMRI (4 T) | 9 | 74 | Visual | 10 | 24 |
| Suzuki et al., 2003 | fMRI (1.5 T) | 11 | NA (24–42) | Auditory | 15 | 5 |
| Zald and Pardo, 1999 | H2O PET | 8 | 30 (20–51) | Self‐paced | 22 | 21 |
fMRI, functional magnetic resonance imaging; MEG, magnetoencephalography; PET, positron emission tomography; NA, not available.
In Furlong et al. [2004], the included foci represent MEG activity in the 5–15 Hz frequency band during the swallowing phase. In Hamdy et al. [1999], the included foci represent areas of increased regional cerebral blood flow. In Harris et al. [2005], the median age is 32 years (mean age not available). In this study, swallowing was performed during the uptake period of F‐fluorodeoxyglucose (FDG) before the PET scan.
The 20 articles not fulfilling both criteria are listed in the Appendix. Most of these studies had to be excluded because activated brain regions or Brodmann areas were reported rather than stereotaxic coordinates.
Conditions
In seven studies, water swallowing served as an experimental condition. Voluntary saliva swallowing was used in five studies (two studies used both water and saliva swallowing [Martin et al., 2001, 2007]. For water swallowing, 3–5 ml of water was given via a syringe or a continuous infusion. For water delivery, four studies [Hamdy et al., 1999a, b; Martin et al., 2001, 2007] used a length of tubing that was held between the subject's lips at midline (in the three remaining studies, no information on tubing placement was given). In two studies [Martin et al., 2001, 2007], the tube extended only a few millimeters beyond the lips, which allowed the participant to gather the liquid on or below the tongue surface, similar to the bolus containment reported in healthy controls [Dodds et al., 1990]. Intervals between water boli were between 30 s [Hamdy et al., 1999a] and 60 s [Martin et al., 2001]. The infusion speed varied between 2.5 ml/s [Furlong et al., 2004] and 0.25 ml/s [Harris et al., 2005].
For saliva swallowing, participants were asked to swallow their accumulated saliva. Intervals between single swallows varied between 30 s [Suzuki et al., 2003] and ≥120 s [Martin et al., 2004]. To increase the homogeneity of groups, reflexive water swallowing, triggered by a pharyngeal water injection [Dziewas et al., 2003], and naïve saliva swallowing (the subject is unaware of functional data acquisition [Martin et al., 2001]) were excluded.
Within‐Condition Meta‐Analysis
Quantitative voxel‐wise ALE meta‐analyses were performed for each contrast separately as described previously [Laird et al., 2005a; Turkeltaub et al., 2002]. Each included focus (i.e., an activation maximum in standard space) is modeled by a 3‐D Gaussian distribution, defined by a user‐specified full‐width half‐maximum (FWHM). All data processing was done in a fully automated multi‐step procedure with GingerALE version 1.1 (http://brainmap.org/ale/index.html).
In brief, the following steps were performed: (1) The ALE value of each voxel of the brain in Talairach space was calculated, using a matrix of 2 mm × 2 mm × 2 mm and a FWHM of 10 mm. (2) A permutation test with 5,000 permutations was performed to determine the significance of the ALE statistic at each voxel, returning an ALE map with a P value for each voxel. (3) This ALE map was thresholded using the false discovery rate algorithm [Genovese et al., 2002] with a false discovery rate of 0.05. (4) A cluster analysis of the thresholded maps was performed with a minimum cluster size of 100 mm. Anatomical labels for these clusters were provided by the Talairach Daemon (http://www.talairach.org/) [Lancaster et al., 2000]. In one study, foci were reported in MNI space [Hamdy et al., 1999b]. Before inclusion into the meta‐analysis, these coordinates were transformed to Talairach space [Talairach and Tournoux, 1988] using the icbm2tal conversion algorithm [Lancaster et al., 2007]. The coordinates of all other studies were reported in Talairach space. ALE clusters were overlaid onto an optimized version of the International Consortium for Brain Mapping single subject MRI anatomical template (colin1.1, available at http://brainmap.org/ale/colin1.1.nii) [Kochunov et al., 2002] using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/).
Between‐Condition Meta‐Analysis
To determine the differences between the ALE maps for saliva and water swallowing, the ALE values for the saliva swallowing versus rest contrast were subtracted from the ALE values for the water swallowing versus rest contrast in each voxel [Laird et al., 2005a]. A permutation test (5,000 permutations) was done to determine the statistical significance of the observed differences. The subsequent analysis steps, in particular thresholding with a false discovery rate of 0.05 and cluster analysis with a minimum cluster size of 100 mm, were identical to those used in the within‐condition meta‐analyses. For the visualization of the results of the between‐condition meta‐analysis, a three‐dimensional surface reconstruction of the colin1.1 brain template was performed using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/) [Dale et al., 1999].
RESULTS
Voluntary Water Swallowing
The meta‐analysis of brain activation associated with water swallowing resulted in 12 clusters of significant activation likelihood. Table II identifies the coordinates of the voxel with the highest local activation likelihood in each cluster and the corresponding brain region. Figure 1 illustrates clusters of significant activation likelihood, in particular in the left (cluster no. 1) and right sensorimotor cortex (2), right inferior parietal lobule (3), and right anterior insula (6).
Table II.
Location of significant ALE maxima: water swallow
| Cluster | Brain region | BA | x | y | z | Volume (mm) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| 1 | L Precentral gyrus | 4 | −54 | −8 | 24 | 1,912 | 17.76 |
| 2a | R Postcentral gyrus | 43 | 58 | −8 | 20 | 1,576 | 14.85 |
| 2b | R Inferior frontal gyrus | 44 | 54 | 6 | 16 | 1,576 | 12.39 |
| 3 | R Inferior parietal lobule | 40 | 38 | −56 | 36 | 1,056 | 19.57 |
| 4 | L Cingulate gyrus | 24 | −10 | 0 | 46 | 392 | 11.42 |
| 5 | L Paracentral lobule | 4 | −6 | −34 | 66 | 392 | 12.40 |
| 6 | R Insula | 13 | 40 | 4 | 8 | 272 | 11.06 |
| 7 | L Medial frontal gyrus | 10 | 0 | 52 | 6 | 224 | 10.45 |
| 8 | L Cingulate gyrus | 31 | −8 | −44 | 28 | 208 | 9.62 |
| 9 | R Middle frontal gyrus | 9 | 52 | 18 | 26 | 160 | 8.99 |
| 10 | L Putamen | – | −24 | 4 | 2 | 144 | 9.23 |
| 11 | L Cuneus | 18 | −20 | −74 | 18 | 144 | 8.42 |
| 12 | L Culmen | – | −12 | −60 | −4 | 112 | 8.30 |
BA, Brodmann area.
Activated brain regions, stereotaxic coordinates according to Talairach and Tournoux [1988] of the voxel with highest local activation likelihood (ALE) in a cluster, cluster volumes and activation likelihood of the local maximum.
Figure 1.
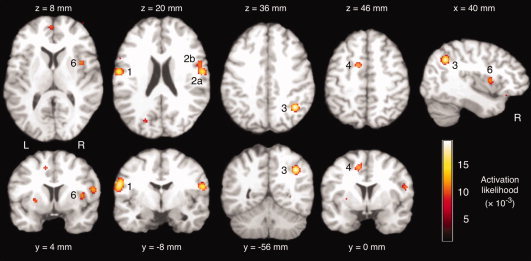
Activation likelihood estimation (ALE) meta‐analysis of brain activity associated with water swallowing versus rest. Significant activation clusters included the left precentral gyrus (1), right postcentral (2a) and inferior frontal gyrus (2b), right inferior parietal lobule (3), left cingulate gyrus (4), and right insula (6). Axial and coronal images are oriented in neurological convention (the left hemisphere is shown on the left side of the figure). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Saliva Swallowing
For saliva swallowing, the meta‐analysis identified seven activation clusters of significant activation likelihood (Table III). Figure 2 depicts significant activation clusters. Highest ALE values were found in the bilateral cingulate gyrus (1a, 1b), the right supplementary motor area (1a), left pre‐ and postcentral gyrus (2a, 2b), and right posterior insula (3).
Table III.
Location of significant ALE maxima: saliva swallow
| Cluster | Brain region | BA | x | y | z | Volume (mm) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| 1a | R Medial frontal gyrus | 6 | 2 | −2 | 48 | 3,464 | 17.28 |
| 1b | R Cingulate gyrus | 32 | 6 | 8 | 40 | 3,464 | 13.44 |
| 1c | L Cingulate gyrus | 32 | −8 | 6 | 40 | 3,464 | 12.93 |
| 2a | L Postcentral gyrus | 43 | −56 | −6 | 14 | 3,184 | 19.86 |
| 2b | L Precentral gyrus | 4 | −52 | −10 | 28 | 3,184 | 10.82 |
| 3 | R Insula | 13 | 36 | −8 | 16 | 632 | 11.65 |
| 4 | L Putamen | – | −20 | 8 | 4 | 616 | 11.67 |
| 5 | R Precentral gyrus | 6 | 46 | −10 | 36 | 616 | 13.47 |
| 6 | R Inferior frontal gyrus | 44 | 56 | 4 | 16 | 440 | 9.42 |
| 7a | L Thalamus | – | −10 | −16 | 4 | 160 | 8.13 |
| 7b | L Thalamus | – | −8 | −12 | 2 | 160 | 8.00 |
BA, Brodmann area.
Activated brain regions, stereotaxic coordinates according to Talairach and Tournoux [1988] of the voxel with highest local activation likelihood (ALE) in a cluster, cluster volumes and activation likelihood of the local maximum.
Figure 2.
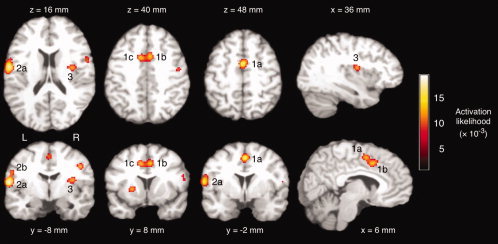
ALE meta‐analysis of brain activity associated with saliva swallowing versus rest. Significant activation clusters included the right medial frontal gyrus (1a), right (1b) and left cingulate gyrus, left postcentral (2a) and precentral gyrus (2b), and right insula (3). Axial and coronal images are oriented in neurological convention (the left hemisphere is shown on the left side of the figure). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Water Versus Saliva Swallowing
The comparison between brain activity associated with water and saliva swallowing revealed five clusters with significantly higher ALE values for water than saliva swallowing (Table IV). In Figure 3, clusters with significantly higher ALE values for water swallowing are shown in blue and include the right inferior parietal lobule (1), right postcentral gyrus (2), and right anterior insula (3). Clusters with significantly higher ALE values for saliva swallowing (Fig. 3, red) include the right supplementary motor area (6c) bilateral anterior cingulate gyrus (6a, 6b), and bilateral precentral gyrus (7, 8). Figure 4 illustrates the locations of these clusters on the 3D‐reconstructed template brain.
Table IV.
Water versus saliva swallowing: location of significant ALE maxima
| Cluster | Brain region | BA | x | y | z | Volume (mm) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| Water swallowing > saliva swallowing | |||||||
| 1 | R Inferior parietal lobule | 40 | 38 | −56 | 36 | 864 | 19.56 |
| 2 | R Postcentral gyrus | 43 | 58 | −8 | 20 | 560 | 14.18 |
| 3 | R Insula | 13 | 40 | 4 | 8 | 224 | 10.86 |
| 4 | L Paracentral lobule | 4 | −6 | −36 | 64 | 200 | 11.11 |
| 5 | L Medial frontal gyrus | 10 | 0 | 52 | 6 | 136 | 10.45 |
| Saliva swallowing > water swallowing | |||||||
| 6a | R Cingulate gyrus | 32 | 4 | 8 | 40 | 1,144 | −10.12 |
| 6b | L Cingulate gyrus | 32 | −8 | 8 | 40 | 1,144 | −11.68 |
| 6c | R Medial frontal gyrus | 6 | 2 | −2 | 48 | 1,144 | −14.00 |
| 7 | L Precentral gyrus | 43 | −56 | −6 | 12 | 568 | −14.17 |
| 8 | R Precentral gyrus | 6 | 46 | −10 | 36 | 328 | −13.42 |
BA, Brodmann area.
Activated brain regions, stereotaxic coordinates according to Talairach and Tournoux [1988] of the voxel with highest local activation likelihood (ALE) in a cluster, cluster volumes and activation likelihood of the local maximum.
Figure 3.
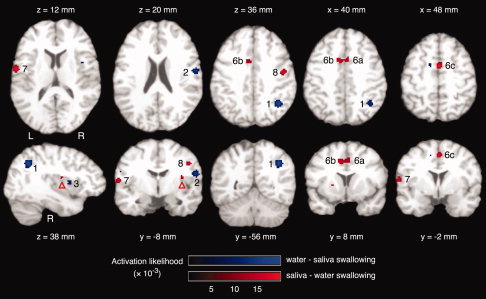
Comparison between the ALE maps for water swallowing versus rest (see Fig. 1) and saliva swallowing versus rest (see Fig. 2). Clusters with significantly higher activation likelihood for water swallowing versus rest are shown in blue, including the right inferior parietal lobule (1), right postcentral gyrus (2), and right insula (3). Clusters with significantly higher activation likelihood for saliva swallowing versus rest are shown in red, including the right (6a) cingulate gyrus, left cingulate gyrus (6b), right medial frontal gyrus (6c), left precentral gyrus (7), and right precentral gyrus (8). In the right insula, a small cluster is visible (red arrow head) that did not fulfill the minimum volume criterion. Axial and coronal images are oriented in neurological convention (the left hemisphere is shown on the left side of the figure). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.

Overlay of the between‐condition ALE map (see Fig. 3) on a 3D‐reconstruction of the colin 1.1 brain template. Clusters with significantly higher activation likelihood for water swallowing versus rest are shown in blue, including the right inferior parietal lobule (1), right postcentral gyrus (2), and right insula (3). Clusters with significantly higher activation likelihood for saliva swallowing versus rest are shown in red, including the left (7) and right precentral gyrus (8). Insular clusters are located below the frontal and parietal opercula. The cluster representing higher activation likelihood for water swallowing versus rest is marked by the blue arrow head (3). The cluster representing higher activation likelihood for saliva swallowing versus rest is marked by the red arrow head. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
A conjunction analysis was performed to determine voxels that showed a significant activation likelihood in both contrasts (see Fig. 5). An overlap of significant voxels was found in the bilateral pericentral cortex, left more than right.
Figure 5.
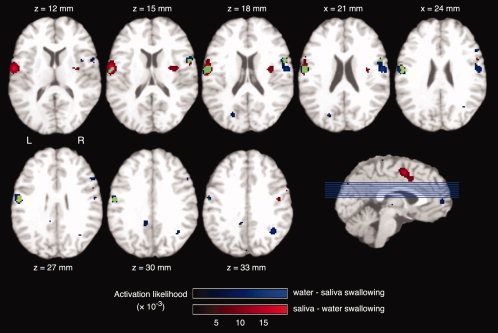
Conjunction analysis of the ALE meta‐analyses of water swallowing versus rest and saliva swallowing versus rest. Voxels with significant activation likelihood for water swallowing versus rest are displayed in blue, voxels with significant activation likelihood for saliva swallowing versus rest in red, and voxels with significant activation likelihood in both contrasts in green. The axial images are oriented in neurological convention (the left hemisphere is shown on the left side of the figure). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We present the first quantitative voxel‐wise meta‐analysis of swallowing‐related brain activity. This meta‐analysis emphasizes that distributed, partly overlapping, but clearly distinct neural networks are involved in the central nervous control of voluntary water and saliva swallowing. The principal strength of our quantitative meta‐analysis is that it is based on 10 published studies with a total of 98 participants [Fraser et al., 2002; Furlong et al., 2004; Hamdy et al., 1999a, b; Harris et al., 2005; Martin et al., 2001, 2004, 2007; Suzuki et al., 2003; Zald and Pardo, 1999]. Each of these studies is unique, characterized by a particular imaging technique (fMRI, H2O PET, 18F‐fluorodeoxyglucose PET, and MEG), experimental settings (e.g., number and frequency of swallows), and demographics of the participants (age and gender). Thus, our group average swallowing‐related brain activation maps are expected to be more robust than those of any one individual imaging study.
Activation Associated With Water and Saliva Swallowing: Sensorimotor Cortex and Adjacent Parietal Operculum
In this meta‐analysis, water and saliva swallowing were associated with clusters of high activation likelihood in the lateral precentral, lateral postcentral, and premotor cortices, as well as in the adjacent parietal opercular region. Moreover, the conjunction analysis showed that activation of a region of the lateral pericentral cortex, particularly within the left hemisphere, was common to both water and saliva swallowing. These findings underscore the fundamental role of the lateral pericentral and perisylvian cortex in swallowing. They corroborate the findings from electrophysiological studies in awake primates showing that swallowing can be evoked by intracortical microstimulation (ICMS) applied to the lateral aspects of the tongue primary motor cortex (MI), face primary somatosensory cortex (SI), as well as the lateral premotor cortex (BA 6) and the frontal operculum of the primate [Martin et al., 1999]. It is also consistent with studies showing that single neurons in the primate tongue MI fire in relation to swallowing [Martin et al., 1997]. Reversible inactivation by cooling of the ICMS‐defined face MI impairs preswallow phase activity during chewing [Yamamura et al., 2002], whereas swallowing execution is impaired by inactivation of the ICMS‐defined swallow MI [Narita et al., 1999].
Swallowing involves not only the preparation and execution of a motor sequence, but also the sensory stimulation of intraoral and oropharyngeal structures, and the esophageus. Pure sensory stimulation of the tongue using water [Zald and Pardo, 2000], the posterior oral cavity using short air pulses [Sörös et al., 2008], and the esophageus [Dziewas et al., 2005], activated fronto‐parietal areas, including the primary motor and somatosensory cortex in humans. Similarly, a recent MEG study showed that oropharyngeal anesthesia resulted in reduced activity of not only SI but also MI [Teismann et al., 2007]. These findings in humans are supported by primate studies indicating that neurons in both the lateral precentral and lateral postcentral gyri have orofacial mechanoreceptive fields [Lin et al., 1994; Martin et al., 1997; Murray and Sessle, 1992], and emphasize the importance of somatosensory afference from oropharyngeal structures in sensorimotor functions such as swallowing.
In this study, significant activation likelihood was found in the parietal operculum (BA 40, 43), the putative anatomical correlate of the secondary somatosensory cortex (SII) [Eickhoff et al., 2006]. SII is activated, together with SI, by a variety of somatosensory stimuli, such as light touch of the lips, face, hand, trunk, and foot as shown by MEG [Disbrow et al., 2000] and fMRI [Hodge et al., 1998; Malinen et al., 2006]. For visceral stimulation, however, SII is regarded as the primary cortical projection area [Aziz et al., 2000]. This area has also been implicated in taste sensation in monkeys and humans [Cerf et al., 1998; Faurion et al., 1998].
Swallowing and overt speech production involve similar oro‐pharyngeal structures and are also supposed to rely on similar neural resources. Quantitative meta‐analyses of overt speech production [Brown et al., 2005] and overt single‐word reading [Turkeltaub et al., 2002] found significant activation likelihood in the bilateral sensorimotor cortex and perisylvian areas, very similar to the results of this meta‐analysis of swallowing‐related brain activity. Similarly, the regions of the SMA and cingulate motor areas with significant activation likelihood for saliva swallowing in this study correspond with regions identified in previous quantitative meta‐analyses of overt speech production [Brown et al., 2005] and overt single‐word reading [Turkeltaub et al., 2002]. These findings suggest that the neural control of swallowing and speaking relies on partly overlapping neural circuits.
Although the involvement of the pericentral and premotor cortex is highly consistent among studies on swallowing‐related brain activity, the question whether the cortical control of swallowing is lateralized to one hemisphere remains unsettled. Some studies have indicated that lateralization of swallowing‐related sensorimotor cortical activation varies across individuals [Martin et al., 2004; Mosier et al., 1999a]. Although dysphagia can occur after strokes of either hemisphere [Barros et al., 2006], left‐ and right‐hemispheric cortical lesions may affect distinct components of swallowing [Daniels et al., 2006]. Lesion studies [Daniels et al., 1996; Robbins et al., 1993] and a study on task interference [Daniels et al., 2006] suggest that the oral phase of swallowing is preferentially mediated by left and the pharyngeal phase by the right hemisphere. A recent MEG study by Teismann et al. [2007] also showed a time‐varying shift of cortical processing from left to right hemisphere in association with the oral and pharyngeal phases of swallowing.
In our meta‐analysis, significant activation likelihood was seen in the bilateral sensorimotor cortex in the saliva swallowing versus rest and the water swallowing versus rest contrasts (Figs. 1 and 2). Sensorimotor processing in both tasks, however, relied on partially distinct cortical areas (see Fig. 5). The results of the between‐condition meta‐analysis revealed significantly higher activation likelihood in the left parietal operculum (i.e., BA 43) for saliva swallowing, and in the right parietal operculum (i.e., BA 40, 43) for water swallowing (see Fig. 3). Most water swallowing studies included in this meta‐analysis [Hamdy et al., 1999a, b; Martin et al., 2001, 2007] used a tubing that was held between the subject's lips at midline for water infusion. Thus, we are confident that the lateralization of water swallow activation in the pericentral and opercular cortex does not simply reflect the infusion methods but, rather, reflects an inherent hemispheric dominance for water swallowing.
The differences in sensorimotor processing between tasks might be explained, at least in part, by the different properties of saliva and water used to facilitate water swallowing, and/or by the properties of the motor responses required to swallow these distinct fluids. Water, given as a bolus or an infusion, was of greater volume and colder than saliva. In most studies, moreover, undistilled water was used, which may have evoked gustatory sensations. In any case, the present finding of different lateralization of sensorimotor activation for water and saliva swallowing suggests that lateralization of swallow‐related brain activity depends on the specific behavioral context of the swallowing act.
Activation Associated Preferentially With Water Swallowing: Parietal and Insular Cortex
The regions of cortex activated preferentially by water swallowing, the right inferior parietal lobule, right postcentral gyrus, and right anterior insula (see Fig. 3) have been implicated in various aspects of sensory processing.
The posterior parietal cortex is a sensory integration area that receives multimodal sensory information [Buneo and Andersen, 2006] and which is reciprocally connected with motor areas in the frontal lobe [Rizzolatti et al., 1998]. Activation of the posterior parietal cortex may represent the integration of thermal, gustatory, and somatosensory information during water swallowing.
The insula is a cytoarchitectonically and functionally diverse area, integrating information from several distinct regions of the brain. Converging evidence shows that the insula is functionally separated by the central sulcus of the insula, which delimits the anterior and posterior lobules. The anterior insula is involved in the processing of vibrotactile stimulation [Sörös et al., 2007], gustation [Smits et al., 2007], olfaction [Poellinger et al., 2001], emotions such as anxiety [Etkin and Wager, 2007], and disgust [Stark et al., 2007]. The anterior insula also mediates visceral sensations of the pharynx, esophagus [Binkofski et al., 1998], and gastrointestinal tract [Vandenbergh et al., 2005] through an ascending pathway involving the parabrachial nucleus and the VPL/VPM thalamus. The activation of the anterior insula through water swallowing, as suggested by this meta‐analysis, is supported by the results of lesion studies [Daniels and Foundas, 1997; Riecker et al., in press].
The posterior insula, in contrast, is believed to be involved in cardiovascular regulation, including heart rate [Kimmerly et al., 2005; Oppenheimer et al., 1992] and respiration [Corfield et al., 1995], and the processing of painful and nonpainful somesthetic afferents [Cereda et al., 2002; Ostrowsky et al., 2002].
The rat insula has multiple reciprocal anatomic connections with brain structures implicate in swallowing. The rat insula receives primary vagal afferents [Ito, 1994], and the mouse insula projects to the nucleus tractus solitarius and brain stem vasomotor regions [Shipley, 1982], suggesting a mechanism through which higher cortical activity may influence autonomic function.
The distinct activation of the anterior (water swallowing) and posterior insula (saliva swallowing) presumably reflects behavioral differences between tasks. To facilitate water swallowing, a small water bolus or a continuous water infusion were given. Oral stimulation with pure water, although tasteless, is associated with bilateral insular activation [Zald and Pardo, 2000]. Activation of these brain areas would be consistent with the mechanical and possibly thermal and gustatory stimulation of the oral cavity and pharynx in the water swallow, compared with the saliva swallow. The water bolus used in most studies was of greater volume and, at room temperature, would be perceived as colder than saliva. Furthermore, the use of undistilled water may have evoked gustatory sensations.
A possible explanation for the activation of the posterior insula during saliva swallowing compared with water swallowing may be that the two tasks have differential cardiovascular effects. Differences in cardiovascular regulation may be a consequence of swallow‐related respiratory alterations [Martin et al., 1994], which might be distinct for the different swallowing tasks.
Activation Associated Preferentially With Saliva Swallowing: Premotor Areas
The regions preferentially activated by voluntary saliva swallowing, including the supplementary motor area (BA 6) and anterior cingulate gyrus (BA 32, Fig. 2), have been implicated in the programming, execution and control of fine, sequential movements [Dum and Strick, 2002; Roland et al., 1980].
Brodmann area 6, on the mesial wall of the hemisphere, contains two functionally distinct areas, the SMA (proper) in the caudal portion of BA 6 and the pre‐SMA in the rostral portion, separated by the anterior commissure line [Geyer et al., 2000]. The SMA, but not the pre‐SMA, is directly connected to MI and the spinal cord [Dum and Strick, 1991], and is regarded as a premotor area based on anatomical and functional characteristics [Picard and Strick, 2001]. In this meta‐analysis, voxels with significant activation likelihood were found in the caudal portion of the bilateral BA 6, corresponding to the SMA proper (see Fig. 2).
Activation of the bilateral SMA is a consistent finding in imaging studies on voluntary orofacial movements, such as whistling [Dresel et al., 2005], chewing [Onozuka et al., 2002], tongue elevation [Martin et al., 2004], and speaking [Sörös et al., 2006]. Tasks that activate the cingulate motor areas include finger movements [Paus et al., 1993] and overt speech production [Paus et al., 1993; Sörös et al., 2006]. With its involvement in cognitive function and attentional control, the anterior cingulate cortex is regarded as an interface between intention and motor execution [Paus, 2001]. The involvement of the SMA and cingulate motor areas in saliva swallowing as shown by this meta‐analysis is very similar to the results of quantitative meta‐analyses of overt speech production [Brown et al., 2005] and overt single‐word reading [Turkeltaub et al., 2002]. This finding, together with the similarities in sensorimotor activation, emphasizes that the neural control of swallowing and speaking relies on partly overlapping neural circuits.
The role of the SMA in swallowing is further supported by neuroimaging studies that were not included in this meta‐analysis using fMRI [Mosier and Bereznaya, 2001; Mosier et al., 1999b; Toogood et al., 2005] and EEG [Satow et al., 2004]. Similarly, studies not included here corroborate the role of the anterior cingulate cortex for the control of swallowing [Kern et al., 2001a, b; Mosier and Bereznaya, 2001].
Saliva swallowing is characterized by increased muscle activity during the volitional oral phase of swallowing compared with water swallowing and may require additional effort for the initiation and planning of movements, probably reflected by increased activity of premotor and cingulate motor areas. Collecting saliva before swallowing is associated with activity in the tongue, and the masseter and submental muscles, not present in water swallowing [Vaiman et al., 2004]. Water swallow, in contrast, appears to involve stronger contractions of pharyngeal and laryngeal muscles [Gupta et al., 1996; Kleinjan and Logemann, 2002]. Both, water and saliva swallowing, require complex tongue movements to press the liquid against the hard palate [Ono et al., 2004] and propel it through the pharynx into the esophagus [McConnell et al., 1988]. Furthermore, although both the saliva and water swallowing tasks used a visual cue in most of the studies included in our meta‐analysis, it is likely that subjects initiated the two types of swallows qualitatively differently, given the oral stimulation by water in the water swallow. That is, initiation of the voluntary saliva swallow may have been more directly contingent on the visual cue, with related requirements for initiation and planning in relation to the visual cue. Initiation of the water swallow, in contrast, may have occurred more in relation to the water stimulation, even though a visual cue was presented simultaneous with the water bolus.
It is also noteworthy that a high activation likelihood was found within the thalamus for the saliva swallow, but not for the water swallow. The thalamus (especially nucleus ventralis lateralis, pars oralis, VLo) is believed to represent a relay for basal ganglia outflow to the SMA. MI and SI, each send topographic projections to putamen, which projects to VLo, which in turn, projects to SMA. The present finding of high activation likelihood within the putamen, thalamus, and SMA during saliva swallow is consistent with saliva swallowing being mediated by this basal ganglia‐thalamocortical motor circuit [Schell and Strick, 1984; Wiesendanger and Wiesendanger, 1985].
Cerebellum
In this meta‐analysis, no activation of the cerebellar hemispheres were found most likely because of a restricted field of view, which did not cover the entire cerebellum in most of the included studies (for a study on cerebellar activation in saliva swallowing, see [Suzuki et al., 2003]. In several studies of water and saliva swallowing that did not report stereotaxic coordinates, and thus had to be excluded from this meta‐analysis, activation of the cerebellar hemispheres was seen [Mosier and Bereznaya, 2001; Zald and Pardo, 1999]. Corroborating these findings, functional neuroimaging of chewing [Onozuka et al., 2002], orofacial movements [Sörös et al., 2006], lip and tongue movements [Grodd et al., 2001], and whistling [Dresel et al., 2005] also demonstrated activation of the cerebellar hemispheres.
Methodological Considerations
ALE meta‐analyses allow the quantitative, voxel‐wise, and statistically sound integration of functional neuroimaging data found in original research. The ALE approach as implemented in GingerALE, however, has methodological limitations (for a comprehensive discussion see [Laird et al., 2005a]). In particular, this approach does not take into account the number of subjects and the number of reported activation foci in single studies. Another limitation of the approach is that it excludes studies reporting their results other than in stereotaxic coordinates. It would be advantageous to have these studies included as well. Algorithms that address these limitations, though, are not publicly available.
In this meta‐analysis, studies included between 8 and 14 individuals. The number of reported foci per study ranged from 5 to 32 for water swallowing and from five to 24 for saliva swallowing (Table I). We cannot rule out the possibility that the studies with fewer participants and with higher numbers of foci (possibly reflecting lower statistical thresholds) are overrepresented in the final results of the meta‐analysis.
This meta‐analysis is based on a relatively small number of studies compared to other ALE meta‐analyses of different neurofunctional systems [e.g., Farrell et al., 2005; Laird et al., 2005b]. There are, however, meta‐analyses based on a similar number of original studies that successfully employed the ALE approach [e.g., Brown et al., 2005]. We were able to cross‐validate the results of the quantitative meta‐analysis with the original results of those studies that did not fulfill the inclusion criteria (summarized in the Appendix). Although three of the five studies included in the saliva swallow analysis came from a single laboratory, these three studies examined different subject samples, including young and older adults, and employed different experimental paradigms and imaging protocols. Taken together, we are confident that this meta‐analysis provides a close representation of the neural control of swallowing as assessed by functional neuroimaging.
CONCLUSIONS
This meta‐analysis indicates that voluntary swallowing of water and voluntary swallowing of saliva are represented within distributed neural networks that are distinct yet overlapping. The distinction of these two networks suggests fundamental differences in the neural control of voluntary water, and voluntary saliva, swallowing. The significance of this meta‐analysis is that, by pooling data from a number of swallowing brain‐imaging studies, it provides a more robust representation of swallowing‐related brain activation than can be expected from any single study.
ADDENDUM
After the completion of this meta‐analysis, Lowell et al. [2008] published an fMRI study on intraoral somatosensory stimulation and (overt and covert) saliva swallowing. Activated areas during overt saliva swallowing included the bilateral sensorimotor cortex, the SMA, the cingulate gyrus, and insula.
Table .
Functional brain imaging studies not included in the meta‐analysis
| Article | Imaging modality | Task |
|---|---|---|
| Abe et al., 2003 | MEG | Water swallowing |
| Birn et al., 1998 | fMRI (3 T) | Saliva swallowing |
| Birn et al., 1999 | fMRI (3 T) | Saliva swallowing |
| Dziewas et al., 2003 | MEG | Voluntary and reflexive water swallowing |
| Hartnick et al., 2001 | fMRI (3 T) | Saliva swallowing |
| Kern et al., 2001a | fMRI (1.5/3 T) | Saliva swallowing |
| Kern et al., 2001b | fMRI (1.5/3 T) | Saliva swallowing, reflexive water swallowing |
| Mosier et al., 1999b | fMRI (1.5 T) | Saliva and water swallowing |
| Mosier et al., 1999a | fMRI (1.5 T) | Saliva and water swallowing |
| Mosier and Bereznaya, 2001 | fMRI (1.5 T) | Saliva and water swallowing |
| Satow et al., 2004 | MRCP | Water swallowing |
| Teismann et al., 2007 | MEG | Water swallowing |
| Teismann et al., 2008 | MEG | Water swallowing |
| Toogood et al., 2005 | fMRI (4 T) | Saliva swallowing |
| Watanabe et al., 2004 | MEG | Water swallowing |
MEG, magnetoencephalography; fMRI, functional magnetic resonance imaging; MRCP, move‐ment‐related cortical potentials.
Footnotes
Voluntary water swallowing (as opposed to reflexive water swallowing) will be termed “water swallowing” in this article.
REFERENCES
- Abe S,Wantanabe Y,Shintani M,Tazaki M,Takahashi M,Yamane G,Ide Y,Yamada Y,Shimono M,Ishikawa T ( 2003): Magnetoencephalo‐graphic study of the starting point of voluntary swallowing. Cranio 21: 46–49. [DOI] [PubMed] [Google Scholar]
- Aziz Q,Schnitzler A,Enck P ( 2000): Functional neuroimaging of visceral sensation. J Clin Neurophysiol 17: 604–612. [DOI] [PubMed] [Google Scholar]
- Barros A,Fábio S,Furkim A ( 2006): Relation between clinical evaluation of deglutition and the computed tomography in acute ischemic stroke patients. Arq Neuropsiquiatr 64: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Schnitzler A,Enck P,Frieling T,Posse S,Seitz R,Freund H ( 1998): Somatic and limbic cortex activation in esophageal distention: A functional magnetic resonance imaging study. Ann Neurol 44: 811–815. [DOI] [PubMed] [Google Scholar]
- Birn R,Bandettini P,Cox R,Jesmanowicz A,Shaker R ( 1998): Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med 40: 55–60. [DOI] [PubMed] [Google Scholar]
- Birn R,Bandettini P,Cox R,Shaker R ( 1999): Event‐related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S,Ingham R,Ingham J,Laird A,Fox P ( 2005): Stuttered and fluent speech production: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo C,Andersen R ( 2006): The posterior parietal cortex: Sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606. [DOI] [PubMed] [Google Scholar]
- Cereda C,Ghika J,Maeder P,Bogousslavsky J ( 2002): Strokes restricted to the insular cortex. Neurology 59: 1950–1955. [DOI] [PubMed] [Google Scholar]
- Cerf B,Lebihan D,Van de Moortele PF,Mac Leod P,Faurion A ( 1998): Functional lateralization of human gustatory cortex related to handedness disclosed by fMRI study. Ann NY Acad Sci 855: 575–578. [DOI] [PubMed] [Google Scholar]
- Chein J,Fissell K,Jacobs S,Fiez J ( 2002): Functional heterogeneity within Broca's area during verbal working memory. Physiol Behav 77: 635–639. [DOI] [PubMed] [Google Scholar]
- Corfield DR,Fink GR,Ramsay SC,Murphy K,Harty HR,Watson JD,Adams L,Frackowiak RS,Guz A ( 1995): Evidence for limbic system activation during CO2‐stimulated breathing in man. J Physiol 488: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM,Fischl B,Sereno MI ( 1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Daniels S,Foundas A,Iglesia G,Sullivan M ( 1996): Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis 6: 30–34. [DOI] [PubMed] [Google Scholar]
- Daniels SK,Foundas AL ( 1997): The role of the insular cortex in dysphagia. Dysphagia 12: 146–156. [DOI] [PubMed] [Google Scholar]
- Daniels S,Corey D,Fraychinaud A,DePolo A,Foundas A ( 2006): Swallowing lateralization: The effects of modified dual‐task interference. Dysphagia 21: 21–27. [DOI] [PubMed] [Google Scholar]
- Disbrow E,Roberts T,Krubitzer L ( 2000): Somatotopic organization of cortical fields in the lateral sulcus of homo sapiens: Evidence for SII and PV. J Comp Neurol 418: 1–21. [DOI] [PubMed] [Google Scholar]
- Dodds WJ,Stewart ET,Logemann JA ( 1990): Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol 154: 953–963. [DOI] [PubMed] [Google Scholar]
- Doty RM,Bosma JF ( 1956): An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60. [DOI] [PubMed] [Google Scholar]
- Dresel C,Castrop F,Haslinger B,Wohlschlaeger A,Hennenlotter A,Ceballos‐Baumann A ( 2005): The functional neuroanatomy of coordinated orofacial movements: Sparse sampling fMRI of whistling. Neuroimage 28: 588–597. [DOI] [PubMed] [Google Scholar]
- Dum RP,Strick PL ( 1991): The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum R,Strick P ( 2002): Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682. [DOI] [PubMed] [Google Scholar]
- Dziewas R,Sörös P,Ishii R,Chau W,Henningsen H,Ringelstein E,Knecht S,Pantev C ( 2003): Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144. [DOI] [PubMed] [Google Scholar]
- Dziewas R,Sörös P,Ishii R,Chau W,Henningsen H,Ringelstein EB,Knecht S,Pantev C ( 2005): Cortical processing of esophageal sensation is related to the representation of swallowing. Neuroreport 16: 439–443. [DOI] [PubMed] [Google Scholar]
- Egger M,Smith GD ( 1997): Meta‐analysis. Potentials and promise. BMJ 315: 1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S,Amunts K,Mohlberg H,Zilles K ( 2006): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279. [DOI] [PubMed] [Google Scholar]
- Ertekin C,Aydogdu I ( 2003): Neurophysiology of swallowing. Clin Neurophysiol 114: 2226–2244. [DOI] [PubMed] [Google Scholar]
- Etkin A,Wager T ( 2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M,Laird A,Egan G ( 2005): Brain activity associated with painfully hot stimuli applied to the upper limb: A meta‐analysis. Hum Brain Mapp 25: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurion A,Cerf B,Le Bihan D,Pillias AM ( 1998): fMRI study of taste cortical areas in humans. Ann N Y Acad Sci 855: 535–545. [DOI] [PubMed] [Google Scholar]
- Fraser C,Power M,Hamdy S,Rothwell J,Hobday D,Hollander I,Tyrell P,Hobson A,Williams S,Thompson D ( 2002): Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 34: 831–840. [DOI] [PubMed] [Google Scholar]
- Furlong P,Hobson A,Aziz Q,Barnes G,Singh K,Hillebrand A,Thompson D,Hamdy S ( 2004): Dissociating the spatio‐temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage 22: 1447–1455. [DOI] [PubMed] [Google Scholar]
- Genovese C,Lazar N,Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Geyer S,Matelli M,Luppino G,Zilles K ( 2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol 202: 443–474. [DOI] [PubMed] [Google Scholar]
- Grodd W,Hülsmann E,Lotze M,Wildgruber D,Erb M ( 2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V,Reddy NP,Canilang EP ( 1996): Surface EMG measurements at the throat during dry and wet swallowing. Dysphagia 11: 173–179. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Aziz Q,Rothwell JC,Power M,Singh KD,Nicholson DA,Tallis PC,Thompson DG ( 1998): Recovery of swallowing after dysphagia stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115: 1104–1112. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Mikulis D,Crawley A,Xue S,Lau H,Henry S,Diamant N ( 1999a): Cortical activation during human volitional swallowing: An event‐related fMRI study. Am J Physiol 277: G219–G225. [DOI] [PubMed] [Google Scholar]
- Hamdy S,Rothwell J,Brooks D,Bailey D,Aziz Q,Thompson D ( 1999b): Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol 81: 1917–1926. [DOI] [PubMed] [Google Scholar]
- Harris M,Julyan P,Kulkarni B,Gow D,Hobson A,Hastings D,Zweit J,Hamdy S ( 2005): Mapping metabolic brain activation during human volitional swallowing: A positron emission tomography study using 18Fluorodeoxyglucose. J Cereb Blood Flow Metab 25: 520–526. [DOI] [PubMed] [Google Scholar]
- Hartnick C,Rudolph C,Willging J,Holland S ( 2001): Functional magnetic resonance imaging of the pediatric swallow: Imaging the cortex and the brainstem. Laryngoscope 111: 118–1191. [DOI] [PubMed] [Google Scholar]
- Hodge CJJ,Huckins SC,Szeverenyi NM,Fonte MM,Dubroff JG,Davuluri K ( 1998): Patterns of lateral sensory cortical activation determined using functional magnetic resonance imaging. J Neurosurg 89: 769–779. [DOI] [PubMed] [Google Scholar]
- Humbert I,Robbins J ( 2007): Normal swallowing and functional magnetic resonance imaging: A systematic review. Dysphagia 22: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S ( 1994): Electrophysiological evidence for projections of myelinated and non‐myelinated primary vagal afferents to the rat insular cortex. Neurosci Lett 179: 29–32. [DOI] [PubMed] [Google Scholar]
- Kern M,Birn R,Jaradeh S,Jesmanowicz A,Cox R,Hyde J,Shaker R ( 2001a): Swallow‐related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280: G531–G538. [DOI] [PubMed] [Google Scholar]
- Kern M,Jaradeh S,Arndorfer R,Shaker R ( 2001b): Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360. [DOI] [PubMed] [Google Scholar]
- Kimmerly D,O'Leary D,Menon R,Gati J,Shoemaker J ( 2005): Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan K,Logemann J ( 2002): Effects of repeated wet and dry swallows in healthy adult females. Dysphagia 17: 50–56. [DOI] [PubMed] [Google Scholar]
- Kochunov P,Lancaster J,Thompson P,Toga A,Brewer P,Hardies J,Fox P ( 2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Laird A,Fox P,Price C,Glahn D,Uecker A,Lancaster J,Turkeltaub P,Kochunov P,Fox P ( 2005a): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A,McMillan K,Lancaster J,Kochunov P,Turkeltaub P,Pardo J,Fox P ( 2005b): A comparison of label‐based review and ALE meta‐analysis in the Stroop task. Hum Brain Mapp 25: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J,Tordesillas‐Gutierrez D,Martinez M,Salinas F,Evans A,Zilles K,Mazziotta J,Fox P ( 2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J,Woldorff M,Parsons L,Liotti M,Freitas C,Rainey L,Kochunov P,Nickerson D,Mikiten S,Fox P ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LD,Murray GM,Sessle BJ ( 1994): Functional properties of single neurons in the primate face primary somatosensory cortex. I. Relations with trained orofacial motor behaviors. J Neurophysiol 71: 2377–2390. [DOI] [PubMed] [Google Scholar]
- Logemann JA ( 1996): Screening, diagnosis, and management of neurogenic dysphagia. Semin Neurol 16: 319–327. [DOI] [PubMed] [Google Scholar]
- Logothetis NK,Pauls J,Augath M,Trinath T,Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Loose R,Hamdy S,Enck P ( 2001): Magnetoencephalographic response characteristics associated with tongue movement. Dysphagia 16: 183–185. [DOI] [PubMed] [Google Scholar]
- Lowell SY,Poletto CJ,Knorr‐Chung BR,Reynolds RC,Simonyan K,Ludlow CL ( 2008): Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 42: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen S,Schürmann M,Hlushchuk Y,Forss N,Hari R ( 2006): Improved differentiation of tactile activations in human secondary somatosensory cortex and thalamus using cardiac‐triggered fMRI. Exp Brain Res 174: 297–303. [DOI] [PubMed] [Google Scholar]
- Martin BJ,Logemann JA,Shaker R,Dodds WJ ( 1994): Coordination between respiration and swallowing: Respiratory phase relationships and temporal integration. J Appl Physiol 76: 714–723. [DOI] [PubMed] [Google Scholar]
- Martin R,Barr A,MacIntosh B,Smith R,Stevens T,Taves D,Gati J,Menon R,Hachinski V ( 2007): Cerebral cortical processing of swallowing in older adults. Exp Brain Res 176: 12–22. [DOI] [PubMed] [Google Scholar]
- Martin R,Goodyear B,Gati J,Menon R ( 2001): Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950. [DOI] [PubMed] [Google Scholar]
- Martin R,Kemppainen P,Masuda Y,Yao D,Murray G,Sessle B ( 1999): Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol 82: 1529–1541. [DOI] [PubMed] [Google Scholar]
- Martin R,MacIntosh B,Smith R,Barr A,Stevens T,Gati J,Menon R ( 2004): Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443. [DOI] [PubMed] [Google Scholar]
- Martin RE,Murray GM,Kemppainen P,Masuda Y,Sessle BJ ( 1997): Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol 78: 1516–1530. [DOI] [PubMed] [Google Scholar]
- Martin R ( 2008): Neuroplasticity and swallowing. Dysphagia, in press. [DOI] [PubMed] [Google Scholar]
- McConnel FMS,Cerenko D,Mendelsohn MS ( 1988): Manofluorographic analysis of swallowing. Otolaryngol Clin North Am 21: 625–636. [PubMed] [Google Scholar]
- Miller A ( 1999): The Neuroscientific Principles of Swallowing and Dysphagia. San Diego: Singular Publishing Group Inc. [Google Scholar]
- Mistry S,Verin E,Singh S,Jefferson S,Rothwell JC,Thompson DG,Hamdy S ( 2007): Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals funcional asymmetry in the hemispheric projections to human swallowing. J Physiol 585: 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K,Bereznaya I ( 2001): Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140: 280–289. [DOI] [PubMed] [Google Scholar]
- Mosier K,Liu W,Maldjian J,Shah R,Modi B ( 1999a): Lateralization of cortical function in swallowing: A functional MR imaging study. AJNR Am J Neuroradiol 20: 1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Mosier K,Patel R,Liu W,Kalnin A,Maldjian J,Baredes S ( 1999b): Cortical representation of swallowing in normal adults: Functional implications. Laryngoscope 109: 1417–1423. [DOI] [PubMed] [Google Scholar]
- Muellbacher W,Artner C,Mamoli B ( 1999): The role of the intact hemisphere in recovery of midline muscles after recent momohemispheric stroke. J Neurol 246: 250–256. [DOI] [PubMed] [Google Scholar]
- Murray GM,Sessle BJ ( 1992): Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. J Neurophysiol 67: 747–758. [DOI] [PubMed] [Google Scholar]
- Narita N,Yamamura K,Yao D,Martin RE,Sessle BJ ( 1999): Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res 824: 140–145. [DOI] [PubMed] [Google Scholar]
- Ono T,Hori K,Nokubi T ( 2004): Pattern of tongue pressure on hard palate during swallowing. Dysphagia 19: 259–264. [DOI] [PubMed] [Google Scholar]
- Onozuka M,Fujita M,Watanabe K,Hirano Y,Niwa M,Nishiyama K,Saito S ( 2002): Mapping brain region activity during chewing: A functional magnetic resonance imaging study. J Dent Res 81: 743–746. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S,Gelb A,Girvin J,Hachinski V ( 1992): Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K,Magnin M,Ryvlin P,Isnard J,Guenot M,Mauguiere F ( 2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12: 376–385. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Paus T,Petrides M,Evans A,Meyer E ( 1993): Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Picard N,Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Poellinger A,Thomas R,Lio P,Lee A,Makris N,Rosen B,Kwong K ( 2001): Activation and habituation in olfaction‐an fMRI study. Neuroimage 13: 547–560. [DOI] [PubMed] [Google Scholar]
- Price C,Devlin J,Moore C,Morton C,Laird A ( 2005) Meta‐analyses of object naming: Effect of baseline. Hum Brain Mapp 25: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A,Gastl R,Kühnlein P,Kassubek J,Prosiegel M ( 2008): Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. Jul 10. DOI 10.1007/s00455‐008‐9164‐1. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Luppino G,Matelli M ( 1998): The organization of the cortical motor system: New concepts. Electroencephalogr Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Robbins J,Levine R,Maser A,Rosenbek J,Kempster G ( 1993): Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 74: 1295–1300. [DOI] [PubMed] [Google Scholar]
- Roland P,Larsen B,Lassen N,Skinhoj E ( 1980): Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 43: 118–136. [DOI] [PubMed] [Google Scholar]
- Satow T,Ikeda A,Yamamoto J,Begum T,Thuy D,Matsuhashi M,Mima T,Nagamine T,Baba K,Mihara T,Inoue Y,Miyamoto S,Hashimoto N,Shibasaki H ( 2004): Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: A movement‐related cortical potential study. Am J Physiol Gastrointest Liver Physiol 287: G459–G470. [DOI] [PubMed] [Google Scholar]
- Schell GR,Strick PL ( 1984): The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 4: 539–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT ( 1982): Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull 8: 139–148. [DOI] [PubMed] [Google Scholar]
- Smits M,Peeters R,van Hecke P,Sunaert S ( 2007): A 3 T event‐related functional magnetic resonance imaging (fMRI) study of primary and secondary gustatory cortex localization using natural tastants. Neuroradiology 49: 61–71. [DOI] [PubMed] [Google Scholar]
- Sörös P,Cornelissen K,Laine M,Salmelin R ( 2003): Naming actions and objects: Cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. Neuroimage 19: 1787–1801. [DOI] [PubMed] [Google Scholar]
- Sörös P,Sokoloff L,Bose A,McIntosh A,Graham S,Stuss D ( 2006): Clustered functional MRI of overt speech production. Neuroimage 32: 376–387. [DOI] [PubMed] [Google Scholar]
- Sörös P,Marmurek J,Tam F,Baker N,Staines W,Graham S ( 2007): Functional MRI of working memory and selective attention in vibrotactile frequency discrimination. BMC Neurosci 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörös P,Lalone E,Smith R,Stevens T,Theurer J,Menon R,Martin R ( 2008): Functional MRI of oropharyngeal air‐pulse stimulation. Neuroscience 153: 1300–1308. [DOI] [PubMed] [Google Scholar]
- Stark R,Zimmermann M,Kagerer S,Schienle A,Walter B,Weygandt M,Vaitl D ( 2007): Hemodynamic brain correlates of disgust and fear ratings. Neuroimage 37: 663–673. [DOI] [PubMed] [Google Scholar]
- Suzuki M,Asada Y,Ito J,Hayashi K,Inoue H,Kitano H ( 2003): Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 18: 71–77. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Georg Thieme. [Google Scholar]
- Teismann I,Dziewas R,Steinstraeter O,Pantev C ( 2008): Time‐dependent hemispheric shift of the cortical control of volitional swallowing. Hum Brain Mapp. Nov 2. DOI 10.1002/hbm.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann I,Steinstraeter O,Stoeckigt K,Suntrup S,Wollbrink A,Pantev C,Dziewas R ( 2007): Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood J,Barr A,Stevens T,Gati J,Menon R,Martin R ( 2005): Discrete functional contributions of cerebral cortical foci in voluntary swallowing: A functional magnetic resonance imaging (fMRI) “go, no‐go” study. Exp Brain Res 161: 81–90. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P,Eden G,Jones K,Zeffiro T ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Vaiman M,Eviatar E,Segal S ( 2004): Evaluation of normal deglutition with the help of rectified surface electromyography records. Dysphagia 19: 125–132. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J,Dupont P,Fischler B,Bormans G,Persoons P,Janssens J,Tack J ( 2005): Regional brain activation during proximal stomach distention in humans: A positron emission tomography study. Gastroenterology 128: 564–573. [DOI] [PubMed] [Google Scholar]
- Watanabe Y,Abe S,Ishikawa T,Yamada Y,Yamane G ( 2004): Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia 19: 100–108. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R,Wiesendanger M ( 1985): The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (Macaca fascicularis). Exp Brain Res 59: 91–104. [DOI] [PubMed] [Google Scholar]
- Yamamura K,Narita N,Yao D,Martin RE,Masuda Y,Sessle BJ ( 2002): Effects of reversible bilateral inactivation of face primary motor cortex on mastication and swallowing. Brain Res 944: 40–55. [DOI] [PubMed] [Google Scholar]
- Yao D,Yamamura K,Narita N,Martin RE,Murray GM,Sessle BJ ( 2002): Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol 87: 2531–2541. [DOI] [PubMed] [Google Scholar]
- Zald D,Pardo J ( 1999): The functional neuroanatomy of voluntary swallowing. Ann Neurol 46: 281–286. [PubMed] [Google Scholar]
- Zald D,Pardo J ( 2000): Cortical activation induced by intraoral stimulation with water in humans. Chem Senses 25: 267–275. [DOI] [PubMed] [Google Scholar]


