Abstract
Does impairment of cholinergic systems represent an important factor in the development of amnesic mild cognitive impairment (aMCI), as a preclinical stage of Alzheimer's disease (AD)? Here we tested the hypothesis that electroencephalographic (EEG) rhythms, known to be modulated by the cholinergic system, may be particularly affected in aMCI patients with lesions along the cholinergic white‐matter tracts. Eyes‐closed resting EEG data were recorded in 28 healthy elderly (Nold) and 57 aMCI patients. Lesions along the cholinergic white‐matter tracts were detected with fluid‐attenuated inversion recovery sequences on magnetic resonance imaging. The estimation of the cholinergic lesion was performed with a validated semi‐automatic algorithm pipeline after registration to a stereotactic template, image integration with stereotactic masks of the cholinergic tracts, and normalization to intracranial volume. The aMCI patients were divided into two groups of high (MCI Ch+; N = 29; MMSE = 26.2) and low cholinergic damage (MCI Ch−; N = 28; MMSE = 26.6). EEG rhythms of interest were delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), and beta 2 (20–30 Hz). Cortical EEG generators were estimated by LORETA software. As main results, (i) power of occipital, parietal, temporal, and limbic alpha 1 sources was maximum in Nold, intermediate in MCI Ch−, and low in MCI Ch+ patients; (ii) the same trend was true in theta sources. These results are consistent with the hypothesis that damage to the cholinergic system is associated with alterations of EEG sources in aMCI subjects. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: amnesic mild cognitive impairment (aMCI), Alzheimer's disease (AD), electroencephalography (EEG), magnetic resonance imaging (MRI), white‐matter vascular lesion, cholinergic system
INTRODUCTION
It has been shown that modifications of resting electroencephalogram (EEG) across physiological aging in humans points to gradual changes in EEG spectral power as mainly represented by a pronounced amplitude decrease of dominant EEG oscillations, namely rhythms in the alpha range from 8 to 13 Hz [Hartikainen et al., 1992; Klass and Brenner, 1995; Klimesch, 1999; Markand, 1990; Pollock et al., 1990]. A recent study in a large sample of healthy subjects (N = 185; 18–85 years) has confirmed an age‐dependent power decrement of low‐frequency alpha rhythms (8–10.5 Hz) in parietal, occipital, and temporal regions [Babiloni et al., 2006a].
Modifications of resting EEG can be observed not only during physiological but also pathological aging. When compared to healthy elderly (Nold) subjects, Alzheimer's disease (AD) patients have been characterized by high power of delta (0–4 Hz) and theta (4–7 Hz) rhythms, and low power of posterior alpha (8–12 Hz) and/or beta (13–30 Hz) rhythms [Babiloni et al., 2004; Dierks et al., 2000; Jeong, 2004; Ponomareva et al., 2003; Koenig et al., 2005]. These EEG abnormalities were associated with altered regional cerebral blood flow/metabolism and with impaired global cognitive function as evaluated by mini mental state examination [MMSE Jeong, 2004; Rodriguez et al., 1998, 1999a, b; Sloan et al., 1995]. Furthermore, posterior alpha rhythms showed a power decrement even in subjects with amnesic mild cognitive impairment (MCI), a clinical state between elderly normal cognition and AD in which subjects present objective evidence of memory decline in some cases together with other cognitive impairment [Babiloni et al., 2006b; Jelic et al., 2000; Koenig et al., 2005; Zappoli et al., 1995].
With these data in mind, a logical question is “Which is the physiological mechanism at the basis of alpha rhythms decrement in amnesic MCI and AD subjects?” It has been reported that in AD patients, early pathological processes include loss of cholinergic basal forebrain neurons projecting to hippocampal and fronto‐parietal areas, and that alpha and slower EEG rhythms can be modulated by these neurons [Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004]. In contrast, brainstem cholinergic innervation of the thalamus would be relatively spared in AD [Geula and Mesulam, 1989, 1996, 1999; Mesulam et al., 2004]. Furthermore, it has been reported the effect of a cholinergic antagonist (i.e., scopolamine) on resting magnetoencephalograpic rhythms in normal subjects [Osipova et al., 2003]. The cholinergic antagonist modulated the power of alpha and theta rhythms and the coherence of theta rhythms, mimicking the typical effects of AD on brain rhythms of healthy subjects [Osipova et al., 2003].
In our study, we tested the hypothesis that cortical sources of resting EEG alpha rhythms, which are affected by AD processes [Babiloni et al., 2004, 2006b], are related to white‐matter lesions of cholinergic routs in amnesic MCI subjects.
METHODS
Subjects and Diagnostic Criteria
In this study, 57 amnesic MCI patients were enrolled. Furthermore, 28 cognitively normal elderly (Nold) subjects were recruited as a control group. Part of the individual data sets was used for previous EEG studies [Babiloni et al., 2004, 2006a, b, c, d, e, f] that did not deal with the evaluation of the relationships between cortical sources of EEG and white‐matter vascular load (i.e., the present study issue).
Local institutional ethics committees approved the study. All experiments were performed with the informed and overt consent of each participant or caregiver, in line with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Author's Institutional Review Board.
The present inclusion and exclusion criteria for amnesic MCI were based on previous seminal studies [Albert et al., 1991; Devanand et al., 1997; Flicker et al., 1991; Petersen et al., 1995, 1997, 2001; Portet et al., 2006; Rubin et al., 1989; Zaudig, 1992] defining elderly persons with objective cognitive deficits, especially in the memory domain, who did not meet criteria for a diagnosis of dementia. The inclusion criteria were as follows: (i) objective memory impairment on neuropsychological evaluation, as defined by performances ≥1.5 standard deviation below the mean value of age and education‐matched controls for a test battery including Busckhe–Fuld and Memory Rey tests; (ii) normal activities of daily living as documented by the history and evidence of independent living; and (iii) clinical dementia rating score of 0.5. The exclusion criteria included: (i) mild AD, as diagnosed by standard protocols including NINCDS‐ADRDA [McKhann et al., 1984]; (ii)evidence of concomitant dementia such as frontotemporal, vascular dementia, reversible dementias (including pseudo‐depressive dementia), fluctuations in cognitive performance, and/or features of mixed dementias; (iii) evidence of concomitant extra‐pyramidal symptoms; (iv) clinical and indirect evidence of depression as revealed by Geriatric Depression Scale scores higher than 13; (v) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence, and use of psychoactive drugs including acetylcholinesterase inhibitors or other drugs enhancing brain cognitive functions; and (vi) current or previous uncontrolled or complicated systemic diseases (including diabetes mellitus) or traumatic brain injuries. Of note, benzodiazepines, antidepressant and/or antihypertensive drugs when present, were withdrawn for about 24 h before the EEG recordings.
The Nold subjects were recruited mostly among nonconsanguineous patients' relatives. All Nold subjects underwent physical and neurological examinations as well as cognitive screening. Subjects affected by chronic systemic illnesses, those receiving psychoactive drugs, or with a history of neurological or psychiatric disease were excluded. All Nold subjects had a GDS score lower than 14 (no depression).
Magnetic Resonance Imaging
MRI scans were acquired with a 1.0 T Philips Gyroscan at the Neuroradioloy Unit of the Città di Brescia hospital, Brescia. The following sequences were used to measure white matter hyperintensity (WMH) volumes within the cholinergic pathways: a high‐resolution gradient echo T1‐weighted sagittal 3D sequence (TR = 20 ms, TE = 5 ms, flip angle = 30°, field of view = 220 mm, acquisition matrix = 256 × 256, slice thickness = 1.3 mm), and a fluid‐attenuated inversion recovery (FLAIR) sequence (TR = 5,000 ms, TE = 100 ms, flip angle = 90°, field of view = 230 mm, acquisition matrix = 256 × 256, slice thickness = 5 mm). The FLAIR images were used to segment WMHs and the high‐resolution sequences to register the segmented WMHs to a stereotactic template.
WMHs segmentation was performed using previously described algorithms [De Carli et al., 2005]. Briefly, the procedure includes (i) filtering of FLAIR images to exclude radiofrequency inhomogeneities, (ii) segmentation of brain tissue from cerebrospinal fluid, (iii) modelling of brain intensity histogram as a Gaussian distribution, and (iv) classification of the voxels whose intensities were >3.5 standard deviations (SDs) above the mean as WMHs [DeCarli et al., 1995]. To place each subject's WMHs map onto a common template, a set of linear and nonlinear transformations were applied with the protocol described in the following. The FLAIR images were coregistered to the high‐resolution T1 image using a nine‐parameters affine transformation [i.e., including rotation, translation, and scaling; Maes et al., 1997], and the same alignment parameters were applied to the WMHs mask image created at the previous step. T1 images were intensity corrected in order to reduce adverse impact of the WMH voxel values on the accuracy of the nonlinear warping algorithm. This step involved estimating the normal white matter mean intensities surrounding the voxels classified as WMHs and replacing voxels in the T1 image corresponding to WMHs by the estimated values. T1 intensity‐corrected images were normalized onto a standard template by mean of high‐dimensional cubic B‐spline warp [Otte, 2001]. The template image used in this study was created from the MRI scans of a population of 88 elderly subjects with a mix of diagnoses distributed homogeneously (26 AD, 29 MCI, and 33 normal), and was therefore the one minimizing the amount of distortion necessary to nonlinearly align individual MRIs [Yoshita et al., 2005]. The parameters computed from nonlinear warping were then used to warp each subject's FLAIR image and WMHs mask onto the template [DeCarli et al., 2005]. At the end of the process, anatomical regions are accurately matched between subjects, and WMH voxels are in locations analogous to their original locations in the subjects' image.
Regions of interest (ROIs) located along the corticopetal cholinergic tracts were manually traced on the template based on published immunohistochemical tracings of the medial and lateral cholinergic pathways in human brains [Selden et al., 1998]. The medial pathway originates from the nucleus basalis of Meynert, supplies the orbitofrontal cortex, courses along the cingulate gyri, and enters the retrosplenial cortices. The lateral pathway branches were subdivided into a perisylvian component, traveling within the claustrum to supply the opercular and insular cortices, and a capsular component traveling adjacent to the putamen and within the external capsule to supply the remaining cortical areas [Selden et al., 1998]. Figure 1 illustrates these pathways.
Figure 1.
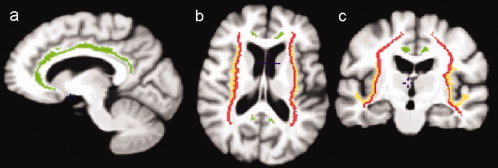
The trajectories of the cholinergic pathways superimposed onto the MRI scan of the template. (a) Midsagittal, (b) axial and (c) coronal views of the tracing of the pathways. The medial pathway, shown in green, originates from the nucleus basalis of Meynert and travels within the cingulum to supply the orbitofrontal, subcallosal, cingulate and retrosplenial cortices (a). The perisylvian lateral pathway courses within the claustrum to supply the opercular and insular cortices (b,c) and is represented in yellow. The capsular lateral pathway, shown in red, travels into the externale capsule and uncinate fasciculus (b,c) to supply the frontoparietal cortex, the middle and inferior temporal gyri, the inferotemporal cortex and the parahippocampal gyrus [Selden et al., 1998]. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The volume of WMHs within the cholinergic pathways was computed by counting the number of voxels segmented as WMHs in the defined ROIs, and multiplying that number by voxel size (1.44 mm3). Group differences within the cholinergic pathways were compared as a percentage of the size of each ROI. Voxels segmented as WMHs and outside the ROIs were classified as noncholinergic.
Composition of MCI Patient Groups
The amnesic MCI patients were divided into two sub‐groups of high and low cholinergic lesion load, based on the 50th percentile of the distribution of WMH volume within the cholinergic tracts: namely, 29 subjects with low and 28 subjects with high degree of cholinergic lesion load (MCI Ch− and MCI Ch+); the lesion threshold separating the two groups was set to 6.5 mm3. Noteworthy, the two sub‐groups of amnesic MCI patients were comparable for demographic and clinical features. Table I summarizes the relevant demographic and clinical data of Nold, MCI Ch− and MCI Ch+ participants. The subjects' age, education, and gender were used as covariates in the statistical evaluation of the cortical sources of EEG rhythms, to remove possible confounding effects.
Table I.
Demographic and neuropsychological data of healthy elderly (Nold) and amnesic mild cognitive impairment (MCI) subjects
| MCI C+ | MCI C− | Nold | |
|---|---|---|---|
| N | 28 | 29 | 28 |
| Age (years) | 77 (± 1.0 SE) | 73 (± 1.5 SE) | 73.2 (±1.2 SE) |
| Education (years) | 8 (± 0.6 SE) | 8 (± 0.8 SE) | 8.2 (± 0.8 SE) |
| Gender (M/F) | 12/16 | 14/15 | 12/16 |
| MMSE | 26.6 (± 0.3 SE) | 26.2 (± 0.3 SE) | 28.4 (± 0.2 SE) |
| ApoE4 (%) | 28 | 38 | — |
| Lesion of cholinergic white matter (cm3) | 0.933 (± 0.180 SE) | 0.027 (± 0.005 SE) | — |
Of note, the MCI group was divided in two sub‐groups: the MCI subjects with low degree of cholinergic lesion (MCI Ch−, cholinergic lesional load <0.0065) and MCI subjects with high degree of cholinergic lesion (MCI Ch+, cholinergic lesional load >0.0065).
EEG Recordings
The EEG data were recorded by specialized clinical units in the Nold, MCI Ch− and MCI Ch+ subjects at resting state (eyes‐closed). The EEG recordings were performed (0.3–70 Hz bandpass; cephalic reference) from 19 electrodes positioned according to the International 10–20 System (i.e. Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2). To monitor eye movements, the horizontal and vertical electrooculogram (0.3–70 Hz bandpass) was also collected. All data were digitized in continuous recording mode (5 min of EEG; 128–256 Hz sampling rate). The recordings were performed in the late morning. To keep constant the level of vigilance, an experimenter controlled on‐line the subject and the EEG traces. He verbally alerted the subject any time there were signs of behavioral (i.e., neck muscle relaxation) and/or EEG (i.e., slowing) drowsiness and/or sleeping.
Of note, the duration of the EEG recording (5 min) allowed the comparison of the present results with several previous AD studies using either EEG recording periods shorter than 5 min [Babiloni et al. 2004, 2006a, b, c, d; Pucci et al., 1999; Rodriguez et al., 2002; Szelies et al., 1999] or about 1 min [Dierks et al., 2000]; longer epochs would have reduced data variability but increased risks for dropping vigilance and arousal.
The recorded EEG data were analyzed and fragmented off‐line in consecutive epochs of 2 s. The EEG epochs with ocular, muscular, and other types of artifact were preliminarily identified by a computerized automatic procedure. The EEG epochs with sporadic blinking artifacts (less than 10% of the total) were corrected by an autoregressive method [Moretti et al., 2003]. Two independent experimenters blind to the diagnosis manually confirmed the EEG segments accepted for further analysis.
Spectral Analysis of the EEG Data
The digital FFT‐based power spectrum analysis (Welch technique, Hanning windowing function, no phase shift) was evaluated in order to calculate the individual alpha frequency (IAF) peak, defined as the frequency associated with the strongest EEG power at the extended alpha range [Klimesch, 1999]. Mean IAF peak was 9.3 Hz (±0.2 standard error, SE) in the Nold subjects, 9.1 Hz (±0.2 SE) in the MCI Ch− subjects, and 9.0 Hz (±0.2 SE) in the MCI Ch+ subjects. To control for the effect of IAF on the EEG comparisons between these three groups, the IAF peak was used as a covariate (together with age, education and gender) for further statistics.
The standard frequency bands of interest were delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), beta 2 (20–30 Hz), and gamma (30–40 Hz). Choice of the fixed EEG bands did not account for IAF peak. However, this should not affect the results, since more than 90% of the subjects had IAF peaks within the alpha 1 band (8–10.5 Hz), and IAF peak was used as a covariate in the statistical analysis.
Cortical Source Analysis of the EEG Rhythms by LORETA
Low resolution electromagnetic source tomography (LORETA) was used for the estimation of cortical sources of resting EEG power [version available at http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm; Pascual‐Marqui and Michel, 1994, Pascual‐Marqui et al., 1999, 2002]. LORETA is a functional imaging technique belonging to a family of linear inverse solution procedures [Valdes et al., 1998] modeling 3D distributions of EEG cortical sources [Pascual‐Marqui et al., 2002]. With respect to the moving equivalent current dipole modeling of EEG cortical sources, no a priori decision on dipole starting position and number is required by the investigators using LORETA. In a previous review paper, it has been shown that LORETA is quite efficient when compared to other linear inverse algorithms like minimum norm solution, weighted minimum norm solution or weighted resolution optimization [Pascual‐Marqui et al., 1999; Phillips et al., 2002; Yao and He, 2001]. Finally, LORETA has been successfully used in recent EEG studies on pathological brain aging [Babiloni et al., 2004, 2006a, b, c, d, e; Dierks et al., 2000].
LORETA computes 3D linear solutions (LORETA solutions) for the EEG inverse problem within a three‐shell spherical head model including scalp, skull, and brain compartments. The brain compartment is restricted to the cortical gray matter/hippocampus of a head model coregistered to the Talairach probability brain atlas and digitized at the Brain Imaging Center of the Montreal Neurological Institute [Talairach and Tournoux, 1988]. This compartment includes 2,394 voxels (7 mm resolution), each voxel containing an equivalent current dipole.
LORETA can be used from EEG data collected by low spatial sampling of 10–20 system (19 electrodes) when cortical sources are estimated from resting EEG rhythms, since these rhythms are generated by largely distributed cortical sources that can be accurately investigated by this way [Anderer et al., 2000, 2003, 2004; Babiloni et al., 2004, 2006a, b, c, d, e; Laufer and Pratt, 2003a, b; Mulert et al., 2001; Veiga et al., 2003; Winterer et al., 2001]. LORETA solutions consisted of voxel current density values able to predict EEG spectral power density at scalp electrodes; noteworthy, these solutions are reference‐free, in that one obtains the same LORETA source distribution for EEG data referenced to any reference electrode including common average. A normalization of the data was obtained by normalizing the LORETA current density at each voxel with the power density averaged across all frequencies (0.5–45 Hz) and across all 2,394 voxels of the brain volume. After the normalization, the solutions lost the original physical dimension and were represented by an arbitrary unit scale. This has been successfully used in previous EEG studies. The general procedure fitted the LORETA solutions in a Gaussian distribution and reduced inter‐subject variability [Babiloni et al., 2004, 2006a, b, c, d, e; Leuchter et al., 1993; Nuwer, 1988]. Other methods of normalization using the principal component analysis are effective for estimating the subjective global factor scale of the EEG data [Hernández et al., 1994]. These methods are not available in the LORETA package, so they were not used in our study.
Solutions of the EEG inverse problem are under‐determined and ill conditioned when the number of spatial samples (electrodes) is lower than that of the unknown samples (current density at each voxel). To properly address this problem, the cortical LORETA solutions predicting scalp EEG spectral power density were regularized to estimate distributed rather than punctual EEG source patterns [Pascual‐Marqui and Michel, 1994, Pascual‐Marqui et al., 1999, 2002]. In line with the low spatial resolution of the adopted technique, we used customized MATLAB software to collapse the voxels of LORETA solutions at frontal, central, parietal, occipital, temporal, and limbic regions of the brain model coded into Talairach space. The Brodmann areas listed in Table II formed each of these ROIs.
Table II.
Brodmann areas included in the cortical regions of interest (ROIs) of the present study
| Frontal | 8, 9, 10, 11, 44, 45, 46, 47 |
| Central | 1, 2, 3, 4, 6 |
| Parietal | 5, 7, 30, 39, 40, 43 |
| Temporal | 20, 21, 22, 37, 38, 41, 42 |
| Occipital | 17, 18, 19 |
| Limbic | 31, 32, 33, 34, 35, 36 |
LORETA solutions were collapsed in frontal, central, parietal, occipital, temporal, and limbic ROIs.
Finally, the main advantage of the regional analysis of LORETA solutions‐using an explicit source model coregistered into Talairach space‐was that our modeling could disentangle rhythms of contiguous cortical areas (namely EEG rhythms from the occipital source were disentangled with respect to those of the contiguous parietal and temporal sources, etc).
Statistical Analysis of the LORETA Solutions
The main statistical analysis aimed at evaluating the working hypothesis that cortical sources of alpha rhythms show difference in amplitude among Nold, MCI Ch−, and MCI Ch+ subjects, because of their dependence by the functional integrity of cholinergic tracts. To this aim, the regional normalized LORETA solutions from Nold, MCI Ch−, and MCI Ch+ subjects were used as an input for ANOVA analysis. Subjects' age, education, gender and IAF peak served as covariates. Mauchly's test evaluated the sphericity assumption. Correction of the degrees of freedom was made with the Greenhouse‐Geisser procedure. The ANOVA analysis used the factors Group (Nold, MCI Ch−, MCI Ch+; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (central, frontal, parietal, occipital, temporal, limbic). The hypothesis would be confirmed by the following two statistical results: (i) a statistical ANOVA effect including the factor Group (P < 0.05) and (ii) a post hoc test indicating statistically significant differences of the (LORETA) alpha sources with the pattern Nold ≠ MCI Ch− ≠ MCI Ch+ (Duncan test, P < 0.05).
To avoid that the statistical results were emphasize by the presence of the Nold group, we also performed a statistical control analysis restricted to the two MCI sub‐groups.
RESULTS
Topography of the EEG Cortical Sources as Estimated by LORETA
For illustrative purposes, Figure 2 maps the grand average of the LORETA solutions (i.e., relative current density at cortical voxels) modeling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in the Nold, MCI Ch− and MCI Ch+ groups. The Nold group presented alpha 1 source with the maximal values of amplitude distributed in parietal, occipital, and temporal regions. Delta, theta, and alpha 2 sources had moderate amplitude values when compared to alpha 1 sources. Finally, beta 1, beta 2, and gamma sources were characterized by lowest amplitude values. Compared to the Nold group, both MCI Ch− and MCI Ch+ groups showed a decrease in amplitude of the parietal, occipital, limbic, and temporal alpha 1 sources. This decrement was stronger in the MCI Ch+ than MCI Ch− group.
Figure 2.
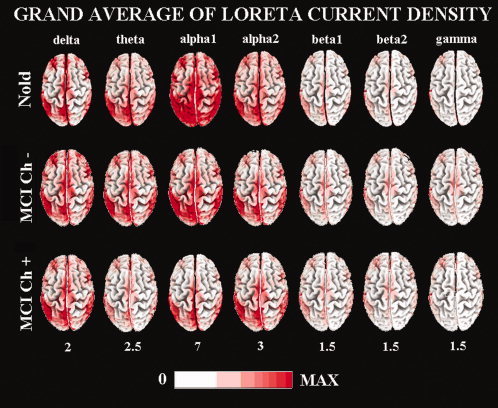
Grand average of LORETA solutions (i.e. normalized relative current density at the cortical voxels) modeling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in Nold, MCI C− (cholinergic lesion load <0.0065) and MCI Ch+ (cholinergic lesion load >0.0065). The left side of the maps (top view) corresponds to the left hemisphere. Legend: LORETA, low resolution brain electromagnetic tomography. Color scale: all power density estimates were scaled based on the averaged maximum value (i.e. alpha 1 power value of occipital region in Nold). The maximal value of power density is reported under each column. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Statistical Comparisons of LORETA EEG Sources
Figure 3 shows mean regional normalized LORETA solutions (distributed EEG sources) relative to a statistical ANOVA interaction (F(60,2460) = 5.44; MSe = 0.4; P < 0.00001) among the factors Group (Nold, MCI Ch−, MCI Ch+), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (central, frontal, parietal, occipital, temporal, limbic). In the figure, the LORETA solutions had the shape of EEG relative power spectra. Notably, profile and magnitude of these spectra in the Nold, MCI Ch− and MCI Ch+ groups differed across diverse cortical macro‐regions, thus supporting the idea that scalp EEG rhythms are generated by a distributed pattern of EEG cortical sources. The planned post hoc testing showed that a source pattern Nold > MCI Ch− > MCI Ch+ was fitted by the following four normalized regional LORETA solutions: parietal, occipital, temporal, and limbic alpha 1 sources (P < 0.00001). Furthermore, parietal theta sources were greater in amplitude in the MCI Ch− than MCI Ch+ group.
Figure 3.
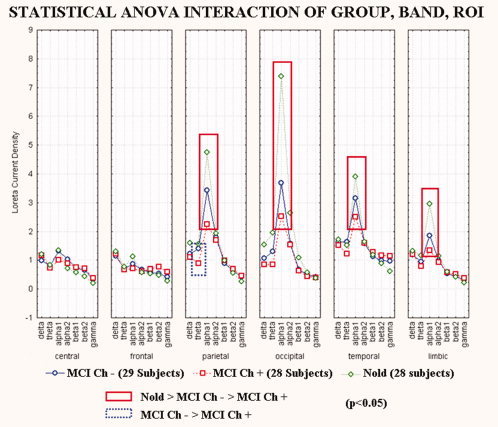
Regional normalized LORETA solutions (mean across subjects) relative to a statistical ANOVA interaction (F(60,2460) = 5.44; MSe = 0.4; P < 0.00001) among the factors Group (Nold, MCI Ch−, MCI Ch+), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (central, frontal, parietal, occipital, temporal, limbic). This ANOVA design used the regional normalized LORETA solutions as a dependent variable. Subjects' age, education, gender and individual alpha frequency peak (IAF) were used as covariates. Regional normalized LORETA solutions modeled the EEG relative power spectra as revealed by a sort of “virtual” intracranial macro‐electrodes located on the macrocortical regions of interest. Legend: the rectangles indicate the cortical regions and frequency bands in which LORETA solutions presented statistically significant LORETA patterns Nold > MCI Ch− > MCI Ch+ (P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To avoid that the statistical results were affected by the total amount of WM lesion, we performed a statistical control analysis including just the two MCI sub‐groups (i.e. MCI Ch−, MCI Ch+), with age, gender, IAF, and total amount of WM lesion as covariates. Figure 4 shows the results of this analysis. In particular, there was a statistical ANOVA interaction (F(30,1650) = 1.60; MSe = 0.2; P < 0.0216) among the factors Group (MCI Ch−, MCI Ch+; independent variables), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (central, frontal, parietal, occipital, temporal, limbic). The planned post hoc testing showed that the source pattern MCI Ch− > MCI Ch+ was fitted by the five normalized regional LORETA solutions: central, parietal, occipital, temporal, and limbic alpha 1 sources (P < 0.0216) as well as by the three normalized regional LORETA solutions: parietal, occipital and temporal theta sources (P < 0.0216). Therefore, the modulation of the theta and alpha 1 sources in amnesic MCI subjects depends on the peculiar amount of cholinergic lesion in the white matter as revealed by WMH.
Figure 4.
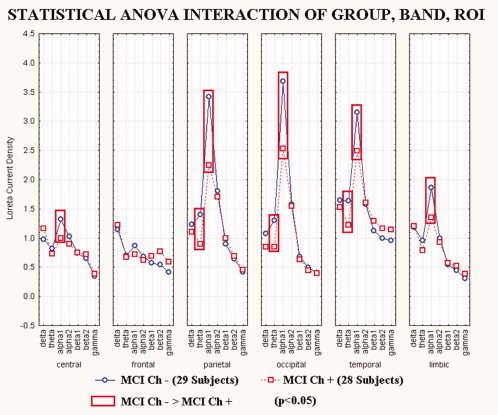
Regional normalized LORETA solutions (mean across subjects) relative to a statistical ANOVA interaction (F(30,1650) = 1.60; MSe = 0.2; P < 0.0216) among the factors Group (MCI Ch, MCI Ch+), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (central, frontal, parietal, occipital, temporal, limbic). This ANOVA design used the regional normalized LORETA solutions as a dependent variable. Subjects' age, education, gender and individual alpha frequency peak (IAF) were used as covariates. Regional normalized LORETA solutions modeled the EEG relative power spectra as revealed by a sort of “virtual” intracranial macro‐electrodes located on the macrocortical regions of interest. Legend: the rectangles indicate the cortical regions and frequency bands in which LORETA solutions presented statistically significant LORETA patterns MCI Ch− > MCI Ch+ (P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Control Analysis
In the earlier analysis, we used a broad‐band whole‐head normalization procedure for the evaluation of EEG source power. Consequently, the alpha source power values had only relative meaning. To complement such an analysis, we evaluated the LORETA solutions relative to the absolute values of alpha 1 source power. These regional LORETA solutions were used as an input for an ANOVA analysis using the factors Group (Nold, MCI) and ROI (central, frontal, parietal, occipital, temporal, limbic). We found a statistical interaction (F(5,400) = 2.23, P < 0.05) between the two factors. Furthermore, the post hoc testing indicated that the absolute LORETA power values of occipital alpha 1 sources were higher in the Nold than in the MCI subjects (P < 0.01), in line with the main results.
DISCUSSION
This EEG study was performed in amnesic MCI patients to evaluate the relationships between cortical sources of resting EEG power and functional integrity of cholinergic tracts to cortex, as revealed by white‐matter hyperintensities. The topographic location of the hyperintensities was defined based on the seminal study by Selden et al. [1998], where they located the projections of cholinergic basal forebrain to cingulate and neighbors (medial cholinergic pathway) as well as to frontal, temporal, parietal, and occipital cortices (lateral capsular and lateral perisylvian). Specifically, we considered the total amount of cholinergic lesion in these medial and lateral pathways, because of the intrinsic limitation of spatial resolution of the present EEG techniques. Based on the measurement of this lesion, the recruited amnesic MCI patients were classified as having low or high white‐matter cholinergic vascular lesion (MCI Ch− or MCI Ch+, respectively). Independently of the total amount of white‐matter vascular lesion (i.e., summing up white‐matter lesion in the cholinergic and noncholinergic compartments), the power of parietal, temporal, occipital, and limbic low‐frequency alpha sources (8–12.5 Hz) was higher in the MCI Ch− than MCI Ch+ group; the same trend was true for parietal, temporal, and occipital theta sources. In the evaluation of the results, at least three methodological remarks should be taken into account. Firstly, the present “cholinergic” bundles [Selden et al., 1998] may include a minority of dopaminergic and noradrenergic projections as previously suggested [Gritti et al., 1993]. Secondly, the spatial resolution of the method was limited by the voxel matrix and slice thickness (i.e., around 1 mm in the coronal plane and 1.5 mm along the rostrocaudal axis), and by the deformations of the digitalized brain images; to minimize these deformations, the present warping technique stretched the brain images using both outer boundary and ventricular wall of the high‐resolution T1 MRIs as reference points [Yoshita et al., 2005]. Thirdly, the EEG electrode positions were not coregistered to individual brain source models; unlikely, the official LORETA package (see Methods section) did not include software to do so and we could not obtain the digitalization of the electrode position from our clinical units.
The earlier results confirmed the hypothesis that cortical sources of posterior alpha rhythms are more preserved in amnesic MCI patients in whom the global cognitive status is challenged by, at least in part, processes outside the cholinergic basal forebrain pathways conveying arousing signals to cerebral cortex. In this sense, these rhythms appeared to be considered as a marker sensitive to efficiency of basal cholinergic systems in amnesic MCI subjects, in agreement with previous evidence showing that posterior alpha rhythms are more affected in AD patients than in subjects with sub‐cortical vascular dementia [Babiloni et al., 2004], and in AD than in amnesic MCI subjects [Babiloni et al., 2006c]. Keeping in mind these data and considerations, it can be speculated that posterior alpha rhythms are generally more abnormal in AD subjects than in sub‐cortical vascular dementia subjects, since these EEG rhythms are sensitive to cholinergic systems, AD neurodegeneration is deeply developed into cholinergic systems, and cerebrovascular lesions are generally not focused on cholinergic systems. Furthermore, posterior alpha rhythms are generally more abnormal in MCI subjects having marked vascular lesions in the cholinergic white matter tracts than in MCI subjects having slight vascular lesions in the cholinergic white matter tracts.
Sensitivity of brain rhythms to cholinergic systems is emphasized by the fact that our MCI groups differed in terms of cholinergic white matter lesions but not in terms of global cognitive functioning. This suggests a non‐linear relationship between the functional impairment of cholinergic systems and global cognitive status along pathological aging, as a possible effect of concomitant factors including non‐cholinergic systems, cognitive reserve, and neuroprotective agents [Mortimer et al., 2005]. In this framework, EEG rhythms and their magnetoencephalographic counterpart might probe efficiency of cholinergic systems in pathological aging better than standard clinical and neuropsycholological assessment. Indeed, previous magnetoencephalographic evidence has shown that cholinergic antagonist modulated the power of alpha and theta rhythms and the coherence of theta rhythms in healthy subjects, mimicking the typical effects of AD on brain rhythms [Osipova et al., 2003].
A crucial question is then: “What are the functional relationships among basal cholinergic systems, low‐band alpha rhythms, and amnesic MCI subjects?” At this early stage of research, the present results allow just an initial view on these relationships. In the condition of wakening rest, low‐band (8–10.5 Hz) alpha rhythms might mainly reflect time‐varying inputs of forebrain cholinergic pathways to cortex [Ricceri et al., 2004]. Therefore, it can be speculated that resting low‐band alpha rhythms in amnesic MCI subjects might reflect the efficiency by which cholinergic basal forebrain neurons convey signals trough white‐matter to cerebral cortex, in order to modulate the transmission and processing of cortical information [Manshanden et al., 2002; Nunez et al., 2001]. Several lines of evidence have shown that experimental lesions of cholinergic basal forebrain affected the amplitude of EEG rhythms including alpha frequencies [Buzsaki et al., 1988; Ray and Jackson, 1991]. The same was true in AD patients supposed to have an impairment of cholinergic basal forebrain [Babiloni et al., 2004; Dierks et al., 2000; Mesulam et al., 2004; Moretti et al., 2004; Rodriguez et al., 1999a, b], but a relatively spared thalamic cholinergic innervation from the brainstem [Geula and Mesulam 1989, 1996, 1999; Mesulam et al., 2004]. Furthermore, it has been reported that cholinergic basal forebrain was more structurally impaired in AD [Teipel et al., 2005], especially in non responders to cholinergic therapy [Tanaka et al., 2003]. Finally, posterior alpha rhythms other than delta rhythms were found to be modulated by long‐term cholinergic therapy in AD subjects [Babiloni et al., 2006f]. However, it should be remarked that the above data do not mean that clinical and cognitive deficits in amnesic MCI and AD subjects just depend on an impairment of cholinergic systems. Previous findings have shown higher values of choline acetyl transferase as a marker of enhanced cholinergic tone in the hippocampus of MCI subjects DeKosky et al., 2002]. In the same vein, it has been demonstrated that neocortical cholinergic deficits characterize severe but not mild AD patients [Davis et al., 1999], and that monoaminergic [Dringenberg, 2000] and non‐NMDA vs. NMDA glutamatergic unbalance [Di Lazzaro et al., 2004] might affect cortical excitability and EEG rhythms in amnesic MCI and AD subjects. To reconcile the above findings, a reasonable explanation is that clinical and cognitive deficits in amnesic MCI and AD subjects may be explained by complex non‐linear relationships among different neuromodulatory systems rather than a major impairment of cholinergic systems. In this theoretical framework, posterior cortical EEG rhythms may reflect the functional integrity of cholinergic pathways.
Another important question is “Why is white‐matter cholinergic vascular lesion related to posterior theta other than alpha rhythms in amnesic MCI subjects?” A tentative explanation is that posterior theta rhythms are affected by some interaction involving the cholinergic basal forebrain pathways projecting to hippocampus/amygdala (mainly modulating theta rhythms), the cholinergic basal forebrain pathways directly projecting to cerebral cortex (mainly modulating alpha rhythms), and modulatory GABAergic systems. Such an explanation is grounded on the following well‐known statements on theta rhythms: (i) theta rhythms functionally connect the activity of hippocampal/amygdala systems and cortical mantle [Buzsaki and Draguhn, 2004]; (ii) they are supposed to sub‐serve focused attention, working memory, encoding processes of episodic memory, and the control of action [Gevins and Smith, 2000, Klimesch et al., 2001, 2006; Klimesch, 1999]; (iii) hippocampal theta rhythms depend on cholinergic and GABAergic interactions [Andersen et al., 2007; Kimura, 2000; Vertes, 2005]; (iv) pathological brain oscillations including theta rhythms are related to the atrophy of hippocampus, rhinal paleocortex, and temporo‐parietal neocortex in MCI and AD subjects [Fernandez et al., 2003; Killiany et al., 1993].
A final question is “What is the relationship between the integrity of cholinergic basal forebrain projections and cerebrovascular function in amnesic MCI subjects? A recent view called ”cholinergic‐vascular hypothesis“ posits that human cholinergic systems not only arouse cerebral cortex but also contribute to event‐related enhancement of cerebral blood flow at the basis of cognitive functions [Claassen and Jansen, 2006]. Such a relationship may be non‐linear, depending on vasomotor reactivity of cerebral circulation to cognitive demands [Claassen and Jansen, 2006; Silvestrini et al., 2006], severity of AD [Davis et al., 1999], and early compensatory responses within cholinergic system [DeKosky et al., 2002]. An alternative view proposes that cerebrovascular and AD lesions represent additive or synergistic factors in the development of cognitive impairment across AD [Nagy et al., 1997; Snowdon et al., 1997; van Oijen M et al., 2007; Zekry et al., 2002]. Unlikely, the results of the present study cannot unveil the relationship between the integrity of cholinergic basal forebrain projections and cerebrovascular function in amnesic MCI subjects. The contribution of the present study is to support the view that the vascular lesions of cholinergic white matter tracts especially affect the neural synchronization mechanisms at the basis of the generation of cortical alpha rhythms in MCI subjects.
CONCLUSIONS
The involvement of cholinergic systems in the development of amnesic MCI, as a preclinical stage of AD at group level, is currently debated. In our study, we tested the hypothesis that cortical sources of EEG rhythms, which are affected by AD processes, are especially affected in amnesic MCI subjects in whom the cognitive decline is mainly explained by cholinergic lesions in white‐matter due to vascular factors. As main results, power of occipital, parietal, temporal, and limbic parietal alpha 1 sources was maximum in Nold, intermediate in MCI Ch− (low cholinergic lesion in white matter), and low in MCI Ch+ group (high cholinergic lesion in white matter). Furthermore, the same trend was true in theta sources. These results are in line with the hypothesis that an impairment (white matter) of cholinergic systems is associated with marked alterations of cortical sources of EEG rhythms in the amnesic MCI status as a pre‐clinical stage of AD. Future studies should correlate posterior theta and alpha rhythms with fine structural features of basal forebrain, cholinergic pathways, and mesial‐temporal cortex in amnesic MCI subjects.
Acknowledgements
The authors thank Drs. Michele Bonetti, Orazio Zanetti, Carlo Miniussi, Mario Ortega, Stefania Tilgher, and Giuliano Binetti for their precious help in the development of the present study.
REFERENCES
- Albert M,Smith LA,Scherr PA,Taylor JO,Evans DA,Funkenstein HH ( 1991): Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci 57: 167–178. [DOI] [PubMed] [Google Scholar]
- Anderer P,Saletu B,Pascual‐Marqui RD ( 2000): Effect of the 5‐HT(1A) partial agonist buspirone on regional brain electrical activity in man: a functional neuroimaging study using low‐resolution electromagnetic tomography (LORETA). Psychiatry Res 100: 81–96. [DOI] [PubMed] [Google Scholar]
- Anderer P,Saletu B,Semlitsch HV,Pascual‐Marqui RD ( 2003): Non‐invasive localization of P300 sources in normal aging and age‐associated memory impairment. Neurobiol Aging 24: 463–479. [DOI] [PubMed] [Google Scholar]
- Anderer P,Saletu B,Saletu‐Zyhlarz G,Gruber D,Metka M,Huber J,Pascual‐Marqui RD ( 2004): Brain regions activated during an auditory discrimination task in insomniac postmenopausal patients before and after hormone replacement therapy: Low‐resolution brain electromagnetic tomography applied to event‐related potentials. Neuropsychobiology 49: 134–153. [DOI] [PubMed] [Google Scholar]
- Andersen P,Morris R,Amaral D,Bliss T,O'Keefe J ( 2007): The Hippocampus Book. New York: Oxford University Press. [Google Scholar]
- Babiloni C,Binetti G,Cassetta E,Cerboneschi D,Dal Forno G,Del Percio C,Ferreri F,Ferri R,Lanuzza B,Miniussi C,Moretti DV,Nobili F,Pascual‐Marqui RD,Rodriguez G,Romani GL,Salinari S,Tecchio F,Vitali P,Zanetti O,Zappasodi F,Rossini PM ( 2004): Mapping distributed sources of cortical rhythms in mild Alzheimers disease. A multi‐centric EEG study. Neuroimage 22: 57–67. [DOI] [PubMed] [Google Scholar]
- Babiloni C,Binetti G,Cassarino A,Dal Forno G,Del Percio C,Ferreri F,Ferri R,Frisoni G,Galderisi S,Hirata K,Lanuzza B,Miniussi C,Mucci A,Nobili F,Rodriguez G,Romani GL,Rossini PM ( 2006a): Sources of cortical rhythms in adults during physiological aging: A multi‐centric EEG study. Hum Brain Mapp 27: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C,Binetti G,Cassetta E,Dal Forno G,Del Percio C,Ferreri F,Ferri R,Frisoni G,Hirata K,Lanuzza B,Miniussi C,Moretti DV,Nobili F,Rodriguez G,Romani GL,Salinari S,Rossini PM ( 2006b): Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: A multi‐centric study. Clin Neurophysiol 117: 252–268. [DOI] [PubMed] [Google Scholar]
- Babiloni C,Benussi L,Binetti G,Bosco P,Busonero G,Cesaretti S,Dal Forno G,Del Percio C,Ferri R,Frisoni G,Ghidoni R,Rodriguez G,Squitti R,Rossini PM ( 2006c): Genotype (cystatin C) and EEG phenotype in Alzheimer disease and mild cognitive impairment: A multicentric study. Neuroimage 29: 948–964. [DOI] [PubMed] [Google Scholar]
- Babiloni C,Benussi L,Binetti G,Cassetta E,Dal Forno G,Del Percio C,Ferreri F,Ferri R,Frisoni G,Ghidoni R,Miniussi C,Rodriguez G,Romani GL,Squitti R,Ventriglia MC,Rossini PM ( 2006d): Apolipoprotein E and α brain rhythms in mild cognitive impairment: A multicentric EEG study. Ann Neurol 59: 323–334. [DOI] [PubMed] [Google Scholar]
- Babiloni C,Frisoni G,Steriade M,Bresciani L,Binetti G,Del Percio C,Geroldi C,Miniussi C,Nobili F,Rodriguez G,Zappasodi F,Carfagna T,Rossini PM ( 2006e): Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer's disease. Clin Neurophysiol 117: 1113–1129. [DOI] [PubMed] [Google Scholar]
- Babiloni C,Cassetta E,Dal Forno G,Del Percio C,Ferreri F,Ferri R,Lanuzza B,Miniussi C,Moretti DV,Flavio Nobili F,Pascual‐Marqui RD,Rodriguez G,Romani GL,Salinari S,Zanetti O,Rossini PM ( 2006f): Donepezil effects on sources of cortical rhythms in mild Alzheimer's disease: Responders vs. non‐responders. Neuroimage 31: 1650–1665. [DOI] [PubMed] [Google Scholar]
- Buzsaki G,Draguhn A ( 2004): Neuronal oscillations in cortical networks. Science 304: 1926–1929. (Review). [DOI] [PubMed] [Google Scholar]
- Buzsaki G,Bickford RG,Ponomareff G,Thal LJ,Mandel R,Gage FH ( 1988): Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8: 4007–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA,Jansen RW ( 2006): Cholinergically mediated augmentation of cerebral perfusion in Alzheimer's disease and related cognitive disorders: The cholinergic‐vascular hypothesis. J Gerontol A Biol Sci Med Sci 61: 267–271. [DOI] [PubMed] [Google Scholar]
- Davis Davis KL,Mohs RC,Marin D,Purohit DP,Perl DP,Lantz M,Austin G,Haroutunian V ( 1999): Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 281: 1401–1406. [DOI] [PubMed] [Google Scholar]
- DeCarli C,Murphy DG,Tranh M,Grady CL,Haxby JV,Gillette JA,Salerno JA,Gonzales‐Aviles A,Horwitz B,Rapoport SI, et al. ( 1995): The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 45: 2077–2084. [DOI] [PubMed] [Google Scholar]
- DeCarli C,Fletcher E,Ramey V,Harvey D,Jagust WJ ( 2005): Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 36: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST,Ikonomovic MD,Styren SD,Beckett L,Wisniewski S,Bennett DA,Cochran EJ,Kordower JH,Mufson EJ ( 2002): Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 51: 145–155. [DOI] [PubMed] [Google Scholar]
- Devanand DP,Folz M,Gorlyn M,Moeller JR,Stem J ( 1997): Questionable dementia: Clinical course and predictors of outcome. J Am Geriatr Soc 45: 321–328. [DOI] [PubMed] [Google Scholar]
- Dierks T,Jelic V,Pascual‐Marqui RD,Wahlund LO,Julin P,Linden DEJ,Maurer K,Winblad B,Nordberg A ( 2000): Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG‐generators in Alzheimer's disease. Clin Neurophysiol 111: 1817–1824. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V,Oliviero A,Pilato F,Saturno E,Dileone M,Marra C,Daniele A,Ghirlanda S,Gainotti G,Tonali PA ( 2004): Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry 75: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC ( 2000): Alzheimer's disease: More than a ‘cholinergic disorder’—Evidence that cholinergic‐monoaminergic interactions contribute to EEG slowing and dementia. Behav Brain Res 115: 235–249. [DOI] [PubMed] [Google Scholar]
- Fernandez A,Arrazola J,Maestu F,Amo C,Gil‐Gregorio P,Wienbruch C,Ortiz T ( 2003): Correlations of hippocampal atrophy and focal low‐frequency magnetic activity in Alzheimer disease: Volumetric MR imaging‐magnetoencephalographic study. AJNR Am J Neuroradiol 24: 481–487. [PMC free article] [PubMed] [Google Scholar]
- Flicker CS,Ferris H,Reisberg B ( 1991): Mild cognitive impairment in the elderly: Predictors of dementia. Neurology 41: 1006–1009. [DOI] [PubMed] [Google Scholar]
- Geula C,Mesulam MM ( 1989): Cortical cholinerigc fibers in aging and Alzheimer's disease: A morphometirc study. Neuroscience 33: 469–481. [DOI] [PubMed] [Google Scholar]
- Geula C,Mesulam MM ( 1996): Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer's disease. Cereb Cortex 6: 165–177. [DOI] [PubMed] [Google Scholar]
- Geula C,Mesulam MM ( 1999): Cholinergic system in Alzheimer's disease In Terry RD,Katzman R, Bick KL, editors. Alzheimer Disease, 2nd ed. Philadelphia, PA: Lippincott, Williams and Wilkins; pp 69–292. [Google Scholar]
- Gevins A,Smith ME ( 2000): Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex 10: 829–839. [DOI] [PubMed] [Google Scholar]
- Gritti I,Mainville L,Jones BE ( 1993): Codistribution of GABA‐ with acetylcholine‐synthesizing neurons in the basal forebrain of the rat. J Comp Neurol 329: 438–457. [DOI] [PubMed] [Google Scholar]
- Hartikainen P,Soininen H,Partanen J,Helkala EL,Riekkinen P ( 1992): Aging and spectral analysis of EEG in normal subjects: a link to memory and CSF AChE. Acta Neurol Scand 86: 148–150. [DOI] [PubMed] [Google Scholar]
- Helkala EL,Hanninen T,Hallikainen M,Kononen M,Laakso MP,Hartikainen P,Soininen H,Partanen J,Partanen K,Vainio P,Riekkinen P Sr ( 1996): Slow‐wave activity in the spectral analysis of the electroencephalogram and volumes of hippocampus in subgroups of Alzheimer's disease patients. Behav Neurosci 110: 1235–1243. [DOI] [PubMed] [Google Scholar]
- Hernández JL,Valdés P,Biscay R,Virués T,Szava S,Bosch J,Riquenes A,Clark I ( 1994): A global scale factor in brain topography. Int J Neurosci 76: 267–278. [DOI] [PubMed] [Google Scholar]
- Holschneider DP,Waite JJ,Leuchter AF,Walton NY,Scremin OU ( 1999): Changes in electrocortical power and coherence in response to the selective cholinergic immunotoxin 192 IgG‐saporin. Exp Brain Res 126: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V,Johansson SE,Almkvist O,Shigeta M,Julin P,Nordberg A,Winblad B,Wahlund LO ( 2000): Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer's disease. Neurobiol Aging 21: 533–540. [DOI] [PubMed] [Google Scholar]
- Jeong J ( 2004): EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol 115: 1490–1505. [DOI] [PubMed] [Google Scholar]
- Killiany RJ,Moss MB,Albert MS,Sandor T,Tieman J,Jolesz F ( 1993): Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Arch Neurol 50: 949–954. [DOI] [PubMed] [Google Scholar]
- Kimura F ( 2000): Cholinergic modulation of cortical function: A hypothetical role in shifting the dynamics in cortical network. Neurosci Res 38: 19–26. [DOI] [PubMed] [Google Scholar]
- Klass DW,Brenner RP ( 1995): Electroencephalography of the elderly. J Clin Neurophysiol 12: 116–131. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1999): EEG α and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev 29: 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Stadler W,Pollhuber D,Sauseng P,Rohm D ( 2001): Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett 302: 49–52. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Hanslmayr S ( 2006): Upper α ERD and absolute power: Their meaning for memory performance. Prog Brain Res 159: 151–165. (Review). [DOI] [PubMed] [Google Scholar]
- Koenig T,Prichep L,Dierks T,Hubl D,Wahlund LO,John ER,Jelic V ( 2005): Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 26: 165–171. [DOI] [PubMed] [Google Scholar]
- Laufer I,Pratt H ( 2003a): Evoked potentials to auditory movement sensation in duplex perception. Clin Neurophysiol 114: 1316–1331. [DOI] [PubMed] [Google Scholar]
- Laufer I,Pratt H ( 2003b): The electrophysiological net response (‘F‐complex’) to spatial fusion of speech elements forming an auditory object. Clin Neurophysiol 114: 818–834. [DOI] [PubMed] [Google Scholar]
- Leuchter AF,Cook IA,Newton TF,Dunkin J,Walter DO,Rosenberg‐Tompson S,Lachenbruch PA,Weiner H ( 1993): Regional differences in brain electrical activity in dementia: use of spectral power and spectral ratio measures. Electroenceph clin Neurophysiol 87: 385–393. [DOI] [PubMed] [Google Scholar]
- Maes F,Collignon A,Vandermeulen D,Marchal G,Suetens P ( 1997): Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 16: 187–198. [DOI] [PubMed] [Google Scholar]
- Manshanden I,De Munck JC,Simon NR,Lopes da Silva FH ( 2002): Source localization of MEG sleep spindles and the relation to sources of α band rhythms. Clin Neurophysiol 113: 1937–1947. [DOI] [PubMed] [Google Scholar]
- Markand ON ( 1990): Alpha rhythms. J Clin Neurophysiol 7: 163–189. (Review). [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Mesulam M,Shaw P,Mash D,Weintraub S ( 2004): Cholinergic nucleus basalis tauopathy emerges early in the aging‐MCI‐AD continuum. Ann Neurol 55: 815–828. [DOI] [PubMed] [Google Scholar]
- Moretti DV,Babiloni F,Carducci F,Cincotti F,Remondini E,Rossini PM,Salinari S,Babiloni C ( 2003): Computerized processing of EEG‐EOG‐EMG artifacts for multicentirc studies in EEG oscillations and event‐related potentials. Int J Pshycophysiol 47: 199–216. [DOI] [PubMed] [Google Scholar]
- Moretti DV,Babiloni C,Binetti G,Cassetta E,Dal Forno G,Ferreri F,Ferri R,Lanuzza Bartolo,Miniussi C,Nobili F,Rodriguez G,Salinari S,Rossini PM ( 2004): Individual analysis of EEG frequency and band power in mild Alzheimer's Disease. Clin Neurophysiol 115: 299–308. [DOI] [PubMed] [Google Scholar]
- Mortimer JA,Borenstein AR,Gosche KM,Snowdon DA ( 2005): Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol 18: 218–223. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C,Gallinat J,Pascual‐Marqui R,Dorn H,Frick K,Schlattmann P,Mientus S,Herrmann WM,Winterer G ( 2001): Reduced event‐related current density in the anterior cingulate cortex in schizophrenia. Neuroimage 13: 589–600. [DOI] [PubMed] [Google Scholar]
- Nagy Z,Esiri MM,Jobst KA,Morris JH,King EM‐F,McDonald B,Joachim C,Litchfield S,Barnetson L,Smith AD ( 1997): The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56: 165–170. [DOI] [PubMed] [Google Scholar]
- Nunez PL,Wingeier BM,Silberstein RB ( 2001): Spatial‐temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 13: 125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR ( 1988): Quantitative EEG. I. Tecniques and problems of frequency analysis and topographic mapping. J Clin Neurophysiol 5: 1–43. [PubMed] [Google Scholar]
- Osipova D,Ahveninen J,Kaakkola S,Jaaskelainen IP,Huttunen J,Pekkonen E ( 2003): Effects of scopolamine on MEG spectral power and coherence in elderly subjects. Clin Neurophysiol 114: 1902–1907. [DOI] [PubMed] [Google Scholar]
- Otte M ( 2001): Elastic registration of fMRI data using Bezier‐spline transformations. IEEE Trans Med Imaging 20: 193–206. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Michel CM ( 1994): LORETA (low resolution brain electromagnetic tomography): New authentic 3D functional images of the brain. ISBET Newslett ISSN 5: 4–8. [Google Scholar]
- Pascual‐Marqui RD,Lehmann D,Koenig T,Kochi K,Merlo MC,Hell D,Koukkou M ( 1999): Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic‐naive, first‐episode, productive schizophrenia. Psychiatry Res 90: 169–179. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD,Esslen M,Kochi K,Lehmann D ( 2002): Functional imaging with low resolution brain electromagnetic tomography (LORETA): A review. Methods Find Exp Clin Pharmacol 24: 91–95. [PubMed] [Google Scholar]
- Petersen RC,Smith GE,Ivnik RJ,Tangalos EG,Schaid SN,Thibodeau SN,Kokmen E,Waring SC,Kurland LT ( 1995): Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory‐impaired individuals. JAMA 273: 1274–1278. [PubMed] [Google Scholar]
- Petersen RC,Smith GE,Waring SC,Ivnik RJ,Kokmen E,Tangelos EG ( 1997): Aging, memory, and mild cognitive impairment. Int Psychogeriatr 9 ( Suppl 1): 65–69. [DOI] [PubMed] [Google Scholar]
- Petersen RC,Doody R,Kurz A,Mohs RC,Morris JC,Rabins PV,Ritchie K,Rossor M,Thal L,Winblad B ( 2001): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985–1992. [DOI] [PubMed] [Google Scholar]
- Phillips C,Rugg MD,Friston KJ ( 2002): Systemic regularization of linear inverse solutions of the EEG source localization problem. Neuroimage 17: 287–301. [DOI] [PubMed] [Google Scholar]
- Pollock VE,Schneider LS,Lyness SA ( 1990): EEG amplitudes in healthy, late‐middle‐aged and elderly adults: Normality of the distributions and correlations with age. Electroencephalogr Clin Neurophysiol 75: 276–288. [DOI] [PubMed] [Google Scholar]
- Ponomareva NV,Selesneva ND,Jarikov GA ( 2003): EEG alterations in subjects at high familial risk for Alzheimer's disease. Neuropsychobiology 48: 152–159. [DOI] [PubMed] [Google Scholar]
- Portet F,Ousset PJ,Visser PJ,Frisoni GB,Nobili F,Scheltens P,Vellas B,Touchon J; MCI Working Group of the European Consortium on Alzheimer's Disease ( 2006): Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry 77: 714–718. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci E,Belardinelli N,Cacchio G,Signorino M,Angeleri F ( 1999): EEG power spectrum differences in early and late onset forms of Alzheimer's disease. Clin Neurophysiol 110: 621–631. [DOI] [PubMed] [Google Scholar]
- Ray PG,Jackson WJ ( 1991): Lesions of nucleus basalis alter ChAT activity and EEG in rat frontal neocortex. Electroencephalogr Clin Neurophysiol 79: 62–68. [DOI] [PubMed] [Google Scholar]
- Ricceri L,Minghetti L,Moles A,Popoli P,Confaloni A,De Simone R,Piscopo P,Scattoni ML,di Luca M,Calamandrei G ( 2004): Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol 189: 162–172. [DOI] [PubMed] [Google Scholar]
- Rodriguez G,Nobili F,Rocca G,DeCarli F,Gianelli MV,Rosadini G ( 1998): Quantitative electroencephalography and regional cerebral blood flow: Discriminant analysis between Alzheimer's patients and healthy controls. Dement Geriatr Cogn Disord 9: 238–274. [DOI] [PubMed] [Google Scholar]
- Rodriguez G,Copello F,Nobili F,Vitali P,Perego G,Nobili F ( 1999a): EEG spectral profile to stage Alzheimer's disease. Clin Neurophysiol 110: 1831–1837. [DOI] [PubMed] [Google Scholar]
- Rodriguez G,Nobili F,Copello F,Vitali P,Gianelli MV,Taddei G,Catsafados E,Mariani G ( 1999b): 99mTc‐HMPAO regional cerebral blood flow and quantitative electroencephalography in Alzheimer's disease: A correlative study. J Nucl Med 40: 522–529. [PubMed] [Google Scholar]
- Rodriguez G,Vitali P,De Leo C,De Carli F,Girtler N,Nobili F ( 2002): Quantitative EEG changes in Alzheimer patients during long‐term donepezil therapy. Neuropsychobiology 46: 49–56. [DOI] [PubMed] [Google Scholar]
- Rubin EH,Morris JC,Grant EA,Vendegna T ( 1989): Very mild senile dementia of the Alzheimer type. I. Clinical assessment. Arch Neurol 46: 379–382. [DOI] [PubMed] [Google Scholar]
- Selden NR,Gitelman DR,Salamon‐Murayama N,Parrish TB,Mesulam MM ( 1998): Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 121 (Part 12): 2249–2257. [DOI] [PubMed] [Google Scholar]
- Silvestrini M,Pasqualetti P,Baruffaldi R,Bartolini M,Handouk Y,Matteis M,Moffa F,Provinciali L,Vernieri F ( 2006): Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 37: 1010–1015. [DOI] [PubMed] [Google Scholar]
- Sloan EP,Fenton GW,Kennedy NSJ,MacLennan JM ( 1995): Electroencephalography and single photon emission computed tomography in dementia: A comparative study. Psychol Med 25, 631–638. [DOI] [PubMed] [Google Scholar]
- Snowdon DA,Greiner LH,Markesbery WR ( 2000): Linguistic ability in early life and the neuropathology of Alzheimer's disease and cerebrovascular disease. Findings from the Nun Study. Ann N Y Acad Sci 903: 34–38. [DOI] [PubMed] [Google Scholar]
- Szelies B,Mielke R,Kessler J,Heiss WD ( 1999): EEG power changes are related to regional cerebral glucose metabolism in vascular dementia. Clin Neurophysiol 110: 615–620. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Tanaka Y,Hanyu H,Sakurai H,Takasaki M,Abe K ( 2003): Atrophy of the substantia innominata on magnetic resonance imaging predicts response to donepezil treatment in Alzheimer's disease patients. Dement Geriatr Cogn Disord 16: 119–125. [DOI] [PubMed] [Google Scholar]
- Teipel SJ,Flatz WH,Heinsen H,Bokde AL,Schoenberg SO,Stockel S,Dietrich O,Reiser MF,Moller HJ,Hampel H ( 2005): Measurement of basal forebrain atrophy in Alzheimer's disease using MRI. Brain 128 (Part 11): 2626–2644. [DOI] [PubMed] [Google Scholar]
- Valdès P,Picton TW,Trujillo N,Bosch J,Aubert E,Riera J ( 1998): Constraining EEG‐MEG source imaging with statistical neuroanatomy. Neuroimage 4: 635. [Google Scholar]
- van Oijen M,de Jong FJ,Witteman JC,Hofman A,Koudstaal PJ,Breteler MM ( 2007): Atherosclerosis and risk for dementia. Ann Neurol 61: 403–410. [DOI] [PubMed] [Google Scholar]
- Veiga H,Deslandes A,Cagy M,Fiszman A,Piedade RA,Ribeiro P ( 2003): Neurocortical electrical activity tomography in chronic schizophrenics. Arq Neuropsiquiatr 61: 712–717. [DOI] [PubMed] [Google Scholar]
- Vertes RP ( 2005): Hippocampal theta rhythm: A tag for short‐term memory. Hippocampus 15: 923–935. [DOI] [PubMed] [Google Scholar]
- Winterer G,Mulert C,Mientus S,Gallinat J,Schlattmann P,Dorn H,Herrmann WM ( 2001): P300 and LORETA: Comparison of normal subjects and schizophrenic patients. Brain Topogr 13: 299–313. [DOI] [PubMed] [Google Scholar]
- Yao D,He B ( 2001): A self‐coherence enhancement algorithm and its application to enhancing three‐dimensional source estimation from EEGs. Ann Biomed Eng 29: 1019–1027. [DOI] [PubMed] [Google Scholar]
- Yoshita M,Fletcher E,DeCarli C ( 2005): Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging. Top Magn Reson Imaging 16: 399–407 (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappoli R,Versari A,Paganini M,Arnetoli G,Muscas GC,Gangemi PF,Arneodo MG,Poggiolini D,Zappoli F,Battaglia A ( 1995): Brain electrical activity (quantitative EEG and bit‐mapping neurocognitive CNV components), psychometrics and clinical findings in presenile subjects with initial mild cognitive decline or probable Alzheimer‐type dementia. Ital J Neurol Sci 16: 341–376. (Review). [DOI] [PubMed] [Google Scholar]
- Zaudig M ( 1992): A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD‐10 and DSM‐III‐R criteria. Int Psychogeriatr 4 ( Suppl 2): 203–219. [DOI] [PubMed] [Google Scholar]
- Zekry D,Duyckaerts C,Moulias R,Belmin J,Geoffre C,Herrmann F,Hauw JJ ( 2002): Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 103: 481–487. [DOI] [PubMed] [Google Scholar]


