Abstract
The neural simulation theory assumes that motor imagery and motor execution draw on a shared set of mechanisms underlying motor cognition. Evidence is accumulating that motor imagery and motor execution have many common features. The extent of the similarity and whether it spreads into the preparation phase is however unclear. This study used electroencephalographic recordings to compare the effects of providing advance information about upcoming movements on preparatory processing in a motor imagery and execution paradigm. Event‐related potential data were recorded in a priming task where participants were cued to perform simple or complex finger movements. We hypothesized that a high degree of functional similarity of motor imagery and motor execution should be reflected in similar alterations of lateralized preparatory activity. Lateralized preparatory activity was indeed very similar, showing both motor‐related (lateralized readiness potential, LRP) and cognitive components (anterior directing‐attention negativity or ADAN, late directing‐attention positivity or LDAP). Dipole analysis revealed that LRP, ADAN, and LDAP sources were very comparable for motor imagination and execution. Results generally support the idea of common underlying functional networks subserving both the preparation for execution and imagery of movements. They also provide a broader context for this notion by revealing similarities in cognitive components associated with the movement tasks. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: electroencephalography, fingers, movement preparation, motor imagery, lateralized readiness potential, ADAN, LDAP, LRP, dipole source localisation
INTRODUCTION
It has been repeatedly demonstrated that motor imagery and overt motor execution share many commonalities in terms of performance and underlying neural substrates [for a review see Jeannerod, 2001]. On the basis of these findings, Jeannerod [2001] proposed the neural simulation theory, which states that motor imagery is a covert action that differs from an overt action only in that the action is not executed. Moreover, he points out that irrespective of whether an action is overt or covert, it involves a covert stage during which the action is prepared, or simulated. In this study, we aimed to further investigate this hypothesis by focusing on the study of preparatory brain activity for overt and covert actions.
A tool ideally suited to investigate preparatory motor activity is the motor priming paradigm [Rosenbaum and Kornblum, 1982] in combination with event‐related potentials (ERP). In the motor priming paradigm a first stimulus (S1, prime) informs the participants about particular aspects of an upcoming movement to be carried out after a second stimulus (S2, response cue). In the S1‐S2 interval (the foreperiod), in the ERP a rising cortical negativity is observed contralateral to the effector, the readiness potential (RP). The lateralized aspect of the RP is reflected in the lateralized readiness potential (LRP), which is the mean of the differences between contra and ipsilateral electrode sites for left‐ and right‐hand responses [Coles, 1989]. The LRP is thought to be an index of motor preparation specific to the response hand [Leuthold et al., 1996; Masaki et al., 2004; Müller‐Gethmann et al., 2000]. The source of the LRP has been attributed to primary motor cortex [Böcker et al., 1994; Carrillo‐de‐la‐Pena et al., 2008; Praamstra et al., 1999] and lateral premotor areas (PMA) [Leuthold and Jentzsch, 2002; Mathews et al., 2006; Praeg et al., 2006]. The involvement of primary motor areas in motor preparation is still a matter of debate, however, as several fMRI studies have failed finding primary motor activation [Hanakawa et al., 2008; Mars et al., 2008].
Initial evidence derived from two experiments comparing imagination‐ and execution‐related LRPs suggests that lateralized activity is also generated during motor imagery conditions, but that the LRP amplitudes are smaller [Carrillo‐de‐la‐Pena et al., 2006; Galdo‐Alvarez and Carrillo‐ de‐la‐Pena, 2004]. Amplitude attenuation during motor imagery has also been reported for the Bereitschafts potential [Cunnington et al., 1996], a rising cortical negativity that precedes self‐paced voluntary movements [Deecke et al., 1969]. In general, the attenuation of preparatory motor potentials for imagined movements as described earlier is attributed to a reduced involvement of primary motor cortex [Caldara et al., 2004; Cunnington et al., 1996; Jankelowitz and Colebatch, 2002].
Preparatory motor potentials are also affected by various task characteristics. For instance, larger amplitudes have been found for complex as compared to simple tasks [Cui et al., 2000; Hackley and Miller, 1995], highly informative as compared to ambiguous S1 [Jentzsch et al., 2004; Ulrich et al., 1998; Wild‐Wall et al., 2003], and proximal as compared to distal movements [Jankelowitz and Colebatch, 2002]. To date, only the latter manipulation has been studied in motor imagery, and results indicate effects similar to those found in the execution condition. Taken together theses studies demonstrate that the amount of information provided but also the parameters of the task itself can affect late preparatory processing. In addition, the study by Jankelowitz and Colebatch [2002] provides first evidence that task characteristics affect the preparatory phase in both an execution and imagination context, supporting the idea of common functional networks underlying both modes of movement.
Lateralized ERP in motor priming tasks are not restricted to motor activity, however. We recently reported the presence of two early lateralized components in a motor priming paradigm [Mathews et al., 2006]. These two sets of lateralized activity are known as ADAN (anterior directing‐attention negativity) and LDAP (late directing‐attention positivity). ADAN and LDAP have been traditionally observed in studies where S1 covertly directs attention to the left or right hemifield [Eimer and Driver, 2001; Nobre et al., 2000; Praamstra and Seiss, 2005]. In our previous study [Mathews et al., 2006], and in earlier work [Eimer et al., 2005; Verleger et al., 2000], centrally presented S1 stimuli priming a unimanual response (and not explicitly shifting attention) also elicited these components. This suggests that a cue for the preparation/selection of a response triggers attention‐related activity. These findings are generally seen as supportive evidence for the premotor theory of attention, which suggests that shared sensorimotor mechanisms underly shifts of attention and selection/programming of a motor response [Rizzolatti et al., 1987]. ADAN and LDAP have been elicited in tasks where spatial attention shifts occur in various modalities, suggesting that they may demonstrate activity of a supramodal attentional network [Eimer et al., 2002]. However evidence for this is equivocal, as recently [Green and McDonald, 2006; Green et al., 2005] it was found that an intramodal auditory attention task did not elicit an early ADAN component [but see Seiss et al., 2007].
Sources of ADAN activity have been localized anterior to sources of motor‐related preparation in the premotor cortex [Mathews et al., 2006], leading to speculation that attentional and response preparation/selection mechanisms are closely linked both functionally and physiologically [Eimer et al., 2005; Praamstra et al., 2005]. ADAN has also been speculated to reflect processing in the frontal eye field area (FEF), drawing on evidence that links visuospatial attention with oculomotor processing [see Corbetta, 1998]. Indeed, studies have shown comparable early frontocentral ERLs in preparation of saccades and finger movements [van der Lubbe et al., 2000; Wauschkuhn et al., 1997]. Van der Lubbe et al. [2006] also implicated FEF as the generator of ADAN activity but, in a new interpretation, suggested that it represented the suppression of an unwanted saccade in a task requiring central fixation.
The LDAP has been attributed to occipito‐temporal areas [Mathews et al., 2006; Praamstra et al., 2005], but tentatively also to the ventral intraparietal sulcus [van der Lubbe et al., 2006]. At present, there is no consensus about the functional significance of the LDAP. Accounts include that the LDAP represents activation of the extrastriate body area (EBA) [Praamstra et al., 2005], known to be activated by observation of body parts. Another suggestion is that it represents activity from an area anterior to the EBA, the action‐related region (ARR), that has been specifically related to the execution of motor actions [Peelen and Downing, 2005].
The important aspect of these findings is that an S1‐S2 paradigm requiring execution of a motor response elicits specific attention‐related lateralized components in the foreperiod when S1 provides information about response side. This raises the question of whether using the same paradigm with an equivalent motor imagery task would produce the same effects. If imagery and execution of movements are supported by similar underlying networks, it would be reasonable to assume that a prime for action in either an execution or imagery context would elicit comparable lateralized foreperiod activity. Conversely, it is possible that the differences observed in the late stages of imagery and execution preparation [Caldara et al., 2004; Cunnington et al., 1996; Jankelowitz and Colebatch, 2002] may also be reflected in attention‐related lateralized potentials.
In this study, we tested the assumption of similar underlying neural networks in overt and covert actions [Jeannerod, 2001] by studying the associated lateralized preparatory brain activity in a motor priming paradigm. In different sessions, participants prepared either imagined or executed sequential finger‐thumb oppositions. Finger‐thumb oppositions could either be simple or complex so as to manipulate the level of task complexity. S1 always informed about response hand and in addition would either inform about the complexity of the upcoming movement or not. Both task complexity and information content have been found to affect the preparation of overtly executed movements. If movement execution and imagination are indeed functionally similar these experimental manipulations should similarly affect imagined movements, which would support Jeannerod's view. In accordance with the neural simulation theory and previous research, we predicted lateralized potentials for both execution and imagination preparation. For LRP, ADAN, and LDAP, respectively, a high degree of functional similarity between imagination and execution would be reflected in highly similar component characteristics in terms of amplitude, neural generators, and sensitivity to task manipulations. On the other hand, functional differences would be reflected in dissimilar component characteristics.
MATERIALS AND METHODS
Subjects
Twelve right‐handed volunteers (four male, mean age 24.6, SD 5.6) participated in two 2‐h recording sessions conducted on consecutive days. Handedness was assessed using the Edinburgh Handedness Inventory [Oldfield, 1971], where a handedness score of 100 indicates complete right handedness and a score of −100 indicates complete left handedness. All participants were predominantly right handed with handedness scores of 100 in nine participants and scores of 25, 60, and 79 in respectively one participant. The mean handedness quotient of the group was 88.7; the median handedness quotient was 100. An hourly rate of £5 was paid for participation, plus a £5 bonus for good adherence to task instructions. The study was approved by the University of Surrey research ethics committee and complied with the Declaration of Helsinki. Written informed consent was taken prior to participation. All participants had normal or corrected‐to‐normal vision.
Procedure
The study consisted of two sessions on consecutive days. In one session participants executed sequential finger‐thumb oppositions with the left or right hand, in the other session participants were asked to imagine performing the same movements. The order of the sessions was counterbalanced across participants. In the imagination session participants were instructed to use kinaesthetic rather than visual imagery. This was done as kinaesthetic imagery appears to be more closely related to overt movement than to visual imagery [Lim et al., 2006; Neuper et al., 2005].
Participants sat in a dimly lit room at a viewing distance of 70 cm from a screen. They placed their hands in a relaxed, comfortable position on the desk in front of them with their palms faced upwards to avoid tactile stimulation of the finger tips by the desk. Trials began with a 1 s presentation of a central fixation cross after which S1 was presented to instruct participants whether to prepare for movement or, in the other session, imagination. The preparatory period was 1,300 ms, after which the imperative stimulus (S2) was presented to cue the movement (or imagination) onset. A varying time interval (2.5–4 s) elapsed before presentation of the next trial (see Fig. 1). Participants were instructed to keep their eyes on the fixation cross and to minimize blinks.
Figure 1.
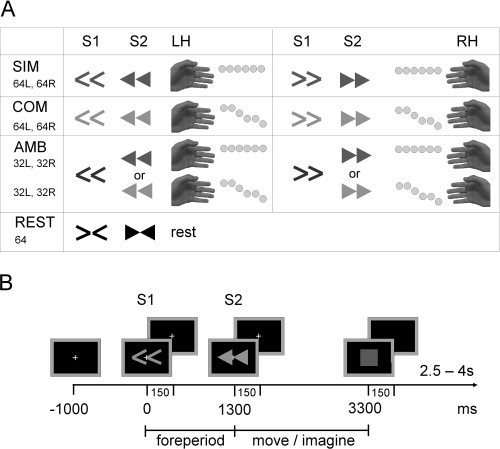
Experimental design. (A) Experimental conditions and associated stimuli. S1 and S2 arrow stimuli are shown followed by the required tapping sequence. Shades of gray represent different arrow colors (blue, pink, and green) per condition (counterbalanced across participants). Total trial counts are shown below the condition name for left (L) and right (R) hands. Gray dots next to the fingers indicate the thumb‐finger tap sequence in each condition. (B) An example trial sequence showing a complex left hand trial.
S1 were colored thin arrows. There were three preparation conditions: simple (SIM), complex (COM), and ambiguous (AMB). In the SIM and COM conditions, full information about the upcoming movement was provided with arrow direction and color 100% predictive. In the AMB condition, arrow direction was predictive but color was uninformative. A simple or complex movement followed with equal likelihood in the AMB condition. The assignment of color to preparation condition was counterbalanced across participants. An additional control condition (REST) was used where S1 and S2 were white arrows, which pointed inward toward each other. Participants were instructed simply to watch the screen and remain motionless during these trials. All stimuli were presented centrally for a duration of 150 ms, the fixation cross remained on‐screen throughout stimulus presentation.
S2 were colored block arrows. The direction of the arrows specified movement with the left or right hand and the color indicated either a simple or complex movement. Simple movements were six repetitions of an index finger to thumb opposition. Complex movements were a sequence of thumb‐finger oppositions: index finger twice, middle finger once, ring finger twice, little finger once. Movement duration was 2 s, after which a red square indicated that movement should be stopped. Participants were instructed to strictly adhere to the stop signal.
In a 5‐min training period, participants familiarized themselves with the stimulus–response combinations and practiced the timing executing/imaging movements. Prior to the imagination sessions, participants were further trained to perform the imagination task without inducing muscle contractions.
Both sessions comprised eight blocks of trials with 16 trials of each preparation condition (split equally into left‐ and right‐hand movements) and eight rest trials presented in a random order. To control for attention an additional four catch trials were presented at random times within each block. Here, a question mark was presented instead of the red square following the imagination period. This instructed participants to press a key with the finger they last imagined to be in contact with the thumb. For consistency, catch trials were also included in the execution session.
Electrophysiological Recording and Processing
Electroencephalographic (EEG) signals were continuously recorded from Ag/AgCl electrodes using a 72‐channel QuickAmp amplifier (Brain Products; http://www.brainproducts.com). Electrodes were positioned according to the international 10‐10 system at midline sites Fpz, AFz, Fz, FCz, Cz, CPz, Pz, POz, Oz, and at lateral sites Fp1/2, AF3/4, AF7/AF8, F1/F2, F3/F4, F5/F6, F7/8, FC1/FC2, FC3/4, FC5/6, FT7/8, FT10, C1/2, C3/4, C5/6, T7/8, CP1/2, CP3/4, CP5/6, TP7/8, TP9/10, P1/2, P3/4, P5/6, P7/8, PO3/4, PO7/8, O1/2. Electrodes were recorded against an average reference calculated by the amplifier hardware. Vertical (VEOG) and horizontal (HEOG) electrooculographic signals were recorded bipolarly using electrodes above and below the left eye and from the left and right outer canthi, respectively. EMG was recorded bipolarly from electrodes positioned over the right and left forearm (flexor digitorum). Data were sampled at 500 Hz and recorded in DC mode. Electrode impedances were kept below 5 kΩ. Data were analyzed offline using BrainVision Analyzer (Brain Products) software. EMG was digitally filtered (high‐pass 30 Hz, low‐pass 50 Hz, 12 dB/oct). Eye‐related artifacts were removed from EEG signals using ICA analysis [Jung et al., 2000]. The data was segmented into condition‐specific 8 s epochs from 2,500 ms pre‐S2 to 5,500 ms post‐S2. Epochs were visually inspected and rejected if contaminated by artifacts. In addition, epochs were rejected if EMG activity was present during the foreperiod and, in the imagination session, if present in the imagination period. An automatic detection algorithm was used to determine the presence of EMG activity using a threshold method [Hodges and Bui, 1996]. Per epoch, the mean and standard deviation (SD) of EMG activity in the baseline period (1 s prior to S1) were calculated. A sliding 25 ms window was used in the test period to calculate mean EMG activity. If mean activity within the window lay outside a specified multiple of SDs from the baseline mean, this was considered significant EMG activity. The SD multiple in the calculation (range 2–3) was tailored for each participant by calibrating the algorithm using their overt execution period. EMG activity flagged by this algorithm was also manually checked for false positives. On completion of artifact rejection, a minimum of 77% of trials were retained for further analyses, yielding, on average, 98 epochs per preparation condition (split equally into left‐ and right‐hand trials) and 49 rest epochs per participant.
Event‐Related Lateralized Potentials and Source Analysis
Epochs were digitally filtered (high‐pass 0.01 Hz, low‐pass 25 Hz, 24 dB/oct) and baseline‐corrected using a 200 ms period pre‐S1. Event‐related lateralization (ERL) was calculated using a two‐step calculation [Coles, 1989]. First, activity at electrodes sites ipsilateral to the response hand was subtracted from that of homologous electrodes on the contralateral side. This difference waveform represents asymmetric activity for one hand. Second, the difference waveforms for each pair (e.g. C3/C4) are averaged across left and right tasks to remove asymmetric activity that is common to the two conditions. The resulting average waveform captures only activity that is specific to a particular response hand.
Source analysis was performed on the foreperiod ERL data using the antisymmetric method described by Praamstra et al. [1996]. This method allows the fitting of symmetrical dipoles for the averaged difference waveforms. To test whether data were indeed antisymmetric and thus to ensure that the antisymmetric method could by validly applied, the symmetry of the left‐ and right‐task difference waveforms was assessed. To this end, a single left minus right‐task subtraction was calculated at homologous electrode sites. Amplitude differences (e.g. at C3/C4) were statistically tested [paired t‐test, cf. Oostenveld et al., 2003] across hemispheres, and amplitudes at midline sites were tested for deviation from baseline. These tests showed that the assumption of antisymmetry, that is, equal magnitude on homologous electrodes but with opposite sign, was not violated [Oostenveld et al., 2003]. In accordance with the antisymmetric method, the averaged difference waveforms that had been derived as described earlier were then copied back to the original electrodes for each electrode pairing with polarity reversed for one hemisphere. For example, data from the C3/C4 pairing was assigned to C3 and the inverted data assigned to C4. This results in antisymmetrically distributed scalp data with an improved signal‐to‐noise ratio of √2 that were used for the subsequent source analysis.
In parallel to previous studies on source modelling of lateralized preparatory activity including one study by our own group [Leuthold and Jentzsch, 2002; Mathews et al., 2006; Praamstra et al., 1996], source models and waveforms were obtained using Brain Electromagnetic Source Analysis software (BESA, version 5.1; http://www.besa.de). A four‐shell spherical head model (brain, skull, cerebrospinal fluid, and scalp) was used as an approximation for dipole fitting. Digitized electrode positions were recorded from each participant using a Polhemus Fastrak Digitizer (http://www.polhemus.com). Three dimensional locations for each electrode site were averaged across participants and imported into BESA for dipole localization. Source locations are described in Talairach‐Tournoux coordinates. The detailed process of deriving the foreperiod source model is described in the results.
Data Analysis
ERL data were analyzed using mean amplitudes in selected time windows pooled from electrode clusters at three sites: anterior (F3/4, F5/6, FC3/4, FC5/6), frontocentral (FC1/2, FC3/4, C1/2, C3/4), and posterior (P3/4, P5/6, PO3/4, PO6/7). Clusters were based on grand average electrical foci of distribution of components (see Fig. 2). Time windows were selected as 100 ms intervals around peak component activity: ADAN (350–450 ms), LDAP (500–600 ms), and motor‐related (1,200–1,300 ms). A four‐way ANOVA (session by site by condition by window) assessed lateralized component differences. This was followed by single‐sample contrast t‐tests to test individual ERL amplitude deviations from baseline. ANOVA was Huynh‐Feldt adjusted where necessary (corrected df reported).
Figure 2.
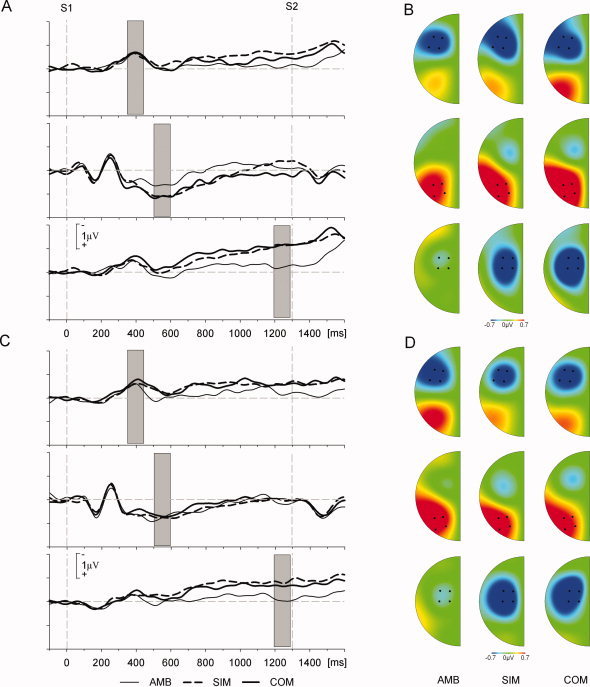
Waveforms and topographies of foreperiod lateralized components. (A,C) ERL waveforms in the execution (A) and imagination sessions (C) respectively pooled over four electrodes at anterior (top), posterior (middle), and frontocentral (bottom) sites. Gray bars indicate the100 ms windows around peak component activity that were used for statistical analysis of ADAN, LDAP, and LRP. (B,D) Scalp distributions of the lateralized ERPs averaged across the 100 ms windows used for statistical analysis in the execution (B) and imagination sessions (D), respectively (cf. Fig. 2A,C). Top maps in (B) and (D) show the topographical distribution of the ADAN, middle maps of the LDAP, and bottom maps of the LRP. Plotted electrodes are those used for statistical analysis of the lateralized ERPs and on which the waveforms shown in (A) and (C) are based. Maps were produced using Brain Vision Analyzer by spherical spline interpolation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
Event‐Related Lateralized Potentials
The imagination and execution sessions showed a similar pattern of lateralized activity in each condition (see Fig. 2). Three lateralized components were identified with distinct latencies and topographies during the 1,300 ms foreperiod. At anterior electrodes a contralateral negativity was present with onset ∼300 ms and peak ∼400 ms (ADAN). At posterior electrodes a contralateral positivity occurred with onset ∼400 ms and peak ∼550 ms (LDAP). Finally, in the SIM and COM conditions only, at frontocentral electrodes a rising contralateral negativity with onset ∼650 ms peaked after S2 presentation (motor‐related).
Differences in these lateralized components were formally tested using a four‐way repeated‐measures ANOVA with factors session (execution, imagination), site (anterior, frontocentral, posterior), condition (AMB, SIM, COM), and window (three levels). As the three components occur at different latencies and electrode sites, the important results from this analysis are any interactions involving factors site and window. There was a significant window by site interaction [F(2.7, 29.3) = 4.2, P < 0.01] confirming that, across sessions and conditions, the distribution of activity differed in the three time windows. There was no session by window by site interaction (P = 0.63) or a session by condition by window by site interaction (P = 0.92). Finally, there was a significant condition by window by site interaction [F(6.3, 69.3) = 2.2, P < 0.05] showing that, across sessions, the pattern of foreperiod activity was different per condition. Post‐hoc tests for each of the three time windows showed that this condition effect was restricted to the latest, i.e., the LRP, time, window, however. Only for this window the two‐way ANOVA with factors site and condition indicated a significant site by condition interaction [F(1.6, 17.3) = 16.8, P < 0.001]. Paired t‐tests (two‐tailed) for the three conditions conducted at all three sites confirmed that at anterior and frontocentral sites the LRP was significantly attenuated in the AMB condition as compared to the SI and COM conditions [all t(11) > 3.0, all P < 0.05]. For the first (ADAN) and second (LDAP) time windows main effects of the factor site in the post‐hoc two‐way ANOVAS confirmed the components' frontal and posterior distributions, respectively [F(1.9, 21.1) = 30.5, P < 0.001 and F(1.2, 13.6) = 36.4, P < 0.001].
To further investigate the differences within the time windows, one‐sample contrast t‐tests were used to test ERL amplitude deviations from baseline. Mean ERL amplitudes per window and condition are shown for each session in Figure 3. Significant deviations from baseline are indicated at each of the three electrode clusters. This shows the expected pattern whereby, in both sessions, absolute component amplitudes are significantly above baseline at their respective sites and windows in all conditions. The exception to this is the AMB condition where there is no significant activity in the latest time window at any site. This accounts for the three‐way interaction effect in the ANOVA described previously. Figure 3 also highlights some overlapping of components. In the latest time window anterior electrodes showed attenuated but significant activity in addition to the expected frontocental activity. This is most likely due to the spatial overlap between anterior and frontocentral electrode locations––an explanation that is supported by the corresponding lack of anterior activity in the AMB condition in this window. Finally, there was posterior activity in the earliest time window reflecting the temporal overlap of ADAN and LDAP components (cf. Figs. 2 and 3). This posterior activity was significant for all but the AMB condition in the execution session. As this session effect was not sufficiently reliable to be reflected in the ANOVA results as well it was not further focused on.
Figure 3.
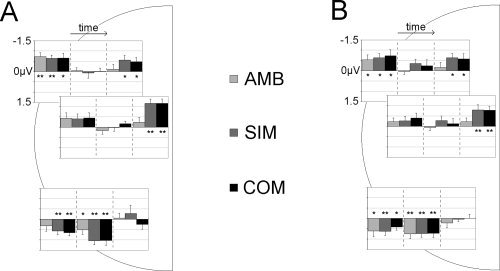
Mean ERL amplitudes per condition. Results are shown for (A) execution and (B) imagination sessions. Mean ERL amplitudes are depicted for the three electrode clusters used for statistical analysis, i.e., anterior (top inset), frontocentral (middle inset), and posterior (bottom inset) electrode sites. Insets read left to right from earliest to latest time window: 350–450 ms (ADAN, left), 500–600 ms (LDAP, middle), and 1200–1300 ms (LRP, right). Significant deviations from baseline amplitude are marked at the 0.05 (*) and 0.01 (**) level.
Dipole Source Analysis
Source localization is best suited to data with a good signal‐to‐noise ratio and due to the similarity of components in the SIM and COM conditions (see Fig. 2) the two conditions were averaged to provide data for the source modeling. The components and time windows for dipole source analysis were chosen based on visual analysis of the lateralized ERPs. This approach was justified as the components of interest in this study, i.e. ADAN, LDAP, and LRP are well‐established components and were clearly identifiable in the ERP. In addition, these lateralized ERPs were very similar to our previous dipole source analysis study in which we used a similar foreperiod and setup [Mathews et al., 2006]. To model the early overlapping components ADAN and LDAP, two symmetrical dipole pairs were fitted simultaneously over the interval 300–550 ms covering the onset and peak of both components. Initial component source locations prior to fitting were seeded from the source model previously reported by our group for an identical foreperiod length, S1 stimuli, and similar experimental task [Mathews et al., 2006]. These dipole pairs localized initially to frontal and occipitotemporal areas. Sources were subsequently fine tuned by fitting each pair individually (with the other held constant) over intervals from component onset to peak (300–400 ms and 400–550 ms, respectively). Another symmetric dipole pair was fitted over a late foreperiod time window (1,050–1,250 ms). Because of the spatial overlap of the ADAN and motor‐related distributions this last pair was fitted with the early ADAN source pair disabled. This approach is justified by the clear temporal separation of the components (Figs. 2 and 3).
The resulting source models for the execution and imagination sessions are shown in Figure 4A,B. The similarity between sessions in source locations and waveforms for each component is evident, accounting for foreperiod data with a residual variance of 6.2% and 7.4% in the execution and imagination sessions, respectively. The ADAN source was located in lateral premotor areas [EX: x = ±30, y = 1, z = 51; IM: x = ±27, y = 2, z = 53]. The LDAP source was located in an occipitotemporal region [EX: x = ±33, y = −58, z = 4; IM: x = ±37, y = −54, z = −2]. The motor‐related source was located in lateral sensorimotor areas anterior to the central sulcus [EX: x = ±39, y = −15, z = 53; IM: x = ±37, y = −15, z = 61]. The motor‐related source pair is the most dissimilar between the execution and imagination session with the imagination source residing in more dorsal areas. This difference along the Z axis was statistically tested using a jackknife subsampling method [Leuthold and Jentzsch, 2002; Miller et al., 1998]. Twelve subsamples of lateralized ERP average waveforms were calculated, each omitting one participant. The motor‐related source‐pair was refitted holding the LDAP source constant and disabling the ADAN source (see earlier). The jacknife‐based standard error was calculated for the difference between execution and imagination Z coordinates of the motor‐related sources. This jacknife‐based standard error and the location difference of the grand mean average Z coordinates were submitted to a t‐test. Despite the tight clustering of jacknife‐based sources around the original sources (cf. Fig. 4C), the one‐tailed t‐test did not indicate a significant difference between the motor‐related sources of the imagination and the execution sessions (P = 0.24).
Figure 4.
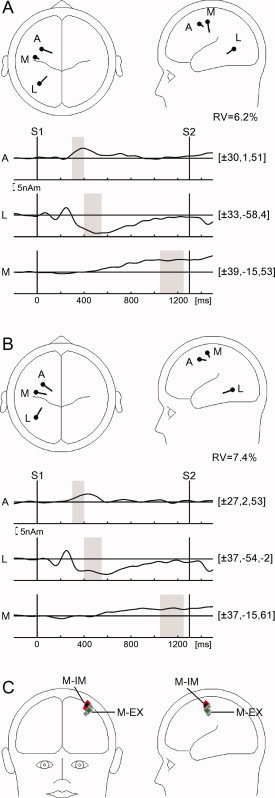
Results of dipole source analysis of foreperiod lateralized activity for (A) execution and (B) imagination sessions. Top insets in (A) and (B) show source locations in an average BESA head model for the ADAN (A), LDAP (L), and motor‐related (M) components. Sources in one hemisphere only are shown for ERL data. Bottom insets depict source waveforms for the ADAN (A), LDAP (L), and motor‐related (M) components. Source locations are shown as [x, y, z] in Talairach‐Tournoux coordinates. Source waveforms in the figure are low‐pass filtered at 6 Hz. Shaded bars indicate the time windows for which dipoles were fitted (for details see text). (C) Jacknife‐based motor‐related dipole sources for the imagination and execution sessions and grand‐mean based motor‐related dipole sources. Red diamonds indicate sources derived from jackknife subsamples (see text) for the imagination session, the black diamond represents the location of the grand mean motor‐related source for this session [(±37, −15, 61); cf. Fig. 4A]. Gray diamonds indicate sources derived from jackknife subsamples for the execution session, the green diamond represents the location of the grand‐mean motor‐related source for this session [(±39, −15, 53); cf. Fig. 4B]. Despite the tight clustering of jackknife sources around the respective grand‐mean source were the location difference between execution and imagination sources not statistically significant (see text). For the purpose of clarity of the figure all sources are shown without orientation information. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
EMG
EMG recording was used to test that participants did not demonstrate anticipatory movements in the foreperiod of the execution session or make inadvertent movements any time during the imagination session. Figure 5 shows the grand average EMG traces separately for the left and right hand, pooled over all left and right hand trials, respectively. This figure shows the presence of EMG activity in the movement period of the execution session only as desired.
Figure 5.
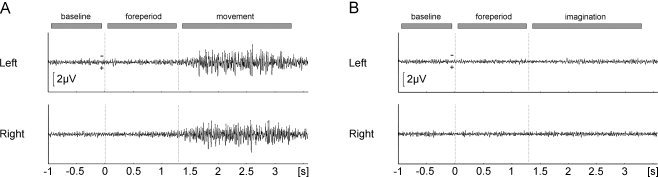
Grand average EMG traces. Results are shown for (A) execution and (B) imagination sessions. Traces correspond to left and right hand EMG data, pooled over all left and right hand trials, respectively.
DISCUSSION
The neural simulation theory [Jeannerod, 2001] incorporates the assumption that both overt and covert actions are preceded by a covert stage, that “includes the goal of the action, the means to reach it, and its consequences on the organism and the external world” [Jeannerod, 2001, p. S103). In this study, we tested whether the covert stage is indeed comparable for overt and covert actions by comparing lateralized foreperiod activity in an S1‐S2 paradigm. Task complexity and information content of S1 were manipulated. We followed the rationale that a high degree of functional similarity of motor imagination and motor execution as proposed in the neural simulation theory [Jeannerod, 2001] would be reflected in highly similar lateralized foreperiod activity. In particular, we were interested in the ADAN and LDAP as components reflecting cognitive processes, i.e., attentional shifts, during unimanual response preparation, and in the LRP as an indicator of motor preparation as such. Highly similar ADAN, LDAP, and LRP ERPs were observed for both execution and imagination in the two conditions where S1 provided all the information of the upcoming task. Dipole analysis confirmed the similarity between imagination and execution in that it revealed very comparable sources for LRP, ADAN, and LDAP: ADAN activity was localized in lateral premotor areas, and LDAP activity in the occipitotemporal region. The motor‐related activity was localized in lateral sensorimotor areas just anterior to the central sulcus. Thus, in sum, the findings of this study provide direct evidence that motor imagination and motor execution do not just share similar mechanisms directly linked to the preparation of a motor action, but also more cognitive processes as reflected in the ADAN and LDAP.
Cognitive Components––ADAN and LDAP
ADAN and LDAP components have been consistently found in studies of spatial attention shifts [Eimer and Driver, 2001; Nobre et al., 2000; Praamstra et al., 2005] and have been interpreted as reflecting the directing of attention to one side of perceptual space. The presence of these components in a motor paradigm requiring central fixation has led to speculation that spatial attention processing may be strongly linked to preparation/selection of response hand [Eimer et al., 2005; Mathews et al., 2006], a finding generally seen as supporting the premotor theory of attention [Rizzolatti et al., 1987].
The distribution of the ADAN shows a somewhat similar pattern to that of later motor‐related activity (see Fig. 2). Eimer [1995] speculated that this early component may in fact reflect an early automatic evocation of the later LRP with this activity being inhibited during the middle part of the foreperiod. This interpretation has been challenged by the finding that the topographical distributions of the two components are significantly different [Mathews et al., 2006; Praamstra et al., 2005; Verleger et al., 2000] and, as we could show here and in earlier work [Mathews et al., 2006], by that dipole localization shows sources to have separate locations along the anterior–posterior axis. Another alternative account of the ADAN is that it reflects saccade preparation/inhibition [van der Lubbe et al., 2006] or aspects of attentional orienting common to the preparation of saccades and the planning of lateral movement [van der Lubbe et al., 2000; Verleger et al., 2000].
The LDAP component partially overlaps in time with the ADAN and is characterized by an occipitotemporal positivity contralateral to the primed direction. Dipole analysis of LDAP activity in the present as well as a previous study [Mathews et al., 2006] localized this activity in visual area MT+. This is consistent with fMRI studies of spatial attention, which show activation in this region [Gitelman et al., 1999; Rosen et al., 1999]. These findings suggest that activity in visual areas may be modulated by attentional processing, an interpretation that is supported by similar results in fMRI work using concurrent tactile [Macaluso et al., 2000] and auditory [Berman and Colby, 2002] stimuli. The attention‐related interpretation of the LDAP is also accepted by authors who interpret the ADAN as a reflection of saccade preparation/inhibition rather than attentional orienting [van der Lubbe et al., 2006]. Praamstra et al. [2005], on the other hand, challenge the attention‐related account of the LDAP in favor of a more general function. They speculate that LDAP activity within the MT+ region may represent activation of the extrastriate body area (EBA), an area hypothesized to integrate visual, spatial attention, and motor signals [Astafiev et al., 2004].
This study was not aimed to solve the ongoing debate about the functional significance of ADAN and LDAP, but rather used ADAN and LDAP as tools to study the functional equivalence of motor imagery and motor execution. However, our results nevertheless contribute to this discussion. First, they show that attentional orienting processes as reflected in LDAP and ADAN are elicited, despite the explicit knowledge that the prepared movement will not be overtly executed. Second, results demonstrate that in a motor priming paradigm attentional shifts can be triggered in the absence of lateralized preparatory motor activity, and hence, presumably, M1 involvement. This finding indicates that in motor priming paradigms, lateralized motor preparation and attentional orienting do not necessarily go hand in hand. Thereby, the current data provide further evidence for the ADAN and LRP representing separate phenomena as discussed earlier. They also support the notion of ADAN and LDAP reflecting some rather general attentional orienting mechanism [Eimer et al., 2002; Seiss et al., 2007].
Motor‐Related Component––LRP
LRPs were observed in the SIM and COM conditions irrespective of whether the movement was imagined or executed. This indicates that if hand‐specific preparation is possible it is comparable for overt and covert movements, which supports Jeannerod's [2001] view. Neither in the execution nor in the imagination session were the lateralized ERPs affected by task complexity. At least for the LRP this was not in line with our expectations, as previous studies on motor preparation have reported increased preparatory activity for more complex tasks [Cui et al., 2000; Hackley and Miller, 1995]. On the other hand, we did find a very clear effect of the information given by the cue, with ambiguous information regarding task complexity but not response hand leading to an absent LRP. In our opinion this pattern of results strongly suggests that the ERLs are relevant for movement preparation, but depend stronger on whether task specific preparation is possible than on the complexity of the movement prepared for. Why others [Cui et al., 2000; Hackley and Miller, 1995] did observe an effect of task complexity on the LRP during motor preparation remains an open question. Reasons may include the absence of an ambiguous condition in these studies or differences in the studied tasks. Another issue might be that effects of task complexity in the ERP are not or only weakly lateralized in nature and therefore disappear when calculating ERLs. This is supported by previous research [Cui et al., 2000] and the analysis of nonlateralized electrical activity of the present data set which shows an effect of task complexity (Kranczioch et al., submitted for publication). Critical is the fact that task complexity did neither affect ERLs in the imagination nor in the execution session. This is further evidence for the high degree of similarity in the neural mechanisms underlying imagination and execution.
The absence of the LRP in the AMB condition, despite response hand being fully specified in this condition was unexpected. This result is inconsistent with previous findings using primes that specify hand only, where foreperiod ERL activity was still present albeit attenuated compared with a prime specifying full movement information [Leuthold and Jentzsch, 2001; Ulrich et al., 1998]. The reason for the lack of lateralized motor‐related activity in the AMB condition is not clear. Results of an analysis of the same data focusing on nonlateralized preparatory activity suggest that, despite not knowing the upcoming movement complexity, participants still show late preparatory activity in the AMB condition. One explanation is that our task used relatively intricate sequential movements, whereas previous studies employed simple transient movements. When information about response hand but not specific movement is known effector‐specific preparation is limited by the lack of knowledge about what movement to actually make. However, if movements are very similar (for example a simple flexion or extension of the index finger) presumably there are common effector‐specific preparatory mechanisms that can be invoked in an ambiguous condition. Perhaps, the two movement tasks used here are sufficiently disparate that effector‐specific preparation is not possible without full knowledge of the upcoming task. In other words, our results suggest that constellations exist in which motor preparation is only possible if the required movement is fully specified despite the effector being known. What is again most striking about the comparison of the execution and imagery data is that this unexpected result in the AMB condition is present in both sessions. This is good evidence that the underlying mechanisms preventing hand‐specific preparation in the AMB condition for overt movements also place a similar restriction on preparation for imagined movements.
CONCLUSION
The results of this study support the theory that motor imagery and execution share common features and demonstrate unequivocally that these similarities extend into the preparation phase. Commonalities concern both motor‐related preparatory processing and processing associated with preparatory shifts of spatial attention. In a wider context, our data support the theoretical notion that motor control is not primarily a reflection of cortical motor neuron functionality but that motor control processes are heavily interlinked with cognitive processes. The latter is particularly important in a clinical context where motor deficits are by and large conceptualized as a disturbance of motor execution.
Acknowledgements
SM was supported by a PhD studentship from the ESRC. We are grateful for Stefan Debener's contributions to the dipole source analyses and for the helpful comments of two anonymous reviewers.
REFERENCES
- Astafiev SV,Stanley CM,Shulman GL,Corbetta M ( 2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 542–548. [DOI] [PubMed] [Google Scholar]
- Berman RA,Colby CL ( 2002): Auditory and visual attention modulate motion processing in area MT+. Cognit Brain Res 14: 64–74. [DOI] [PubMed] [Google Scholar]
- Böcker KB,Brunia CH,Cluitmans PJ ( 1994): A spatio‐temporal dipole model of the readiness potential in humans. I. Finger movement. Electroencephalogr Clin Neurophysiol 91: 275–285. [DOI] [PubMed] [Google Scholar]
- Caldara R,Deiber MP,Andrey C,Michel CM,Thut G,Hauert C ( 2004): Actual and mental motor preparation and execution: A spatiotemporal ERP study. Exp Brain Res 159: 389–399. [DOI] [PubMed] [Google Scholar]
- Carrillo‐de‐la‐Pena MT,Lastra‐Barreira C,Galdo‐Alvarez S ( 2006): Limb (hand vs foot) and response conflict have similar effects on event‐related potentials (ERPs) recorded during motor imagery and overt execution. Eur J Neurosci 24: 635–643. [DOI] [PubMed] [Google Scholar]
- Carrillo‐de‐la‐Pena MT,Galdo‐Alvarez S,Lastra‐Barreira C ( 2008): Equivalent is not equal: Primary motor cortex (MI) activation during motor imagery and execution of sequential movements. Brain Res 1226: 134–143. [DOI] [PubMed] [Google Scholar]
- Coles MG ( 1989): Modern mind‐brain reading: psychophysiology, physiology, and cognition. Psychophysiology 26: 251–269. [DOI] [PubMed] [Google Scholar]
- Corbetta M ( 1998): Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems. Proc Natl Acad Sci USA 95: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui RQ,Egkher A,Huter D,Lang W,Lindinger G,Deecke L ( 2000): High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non‐motor task. Clin Neurophysiol 111: 1847–1859. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Iansek R,Bradshaw JL,Phillips JG ( 1996): Movement‐related potentials associated with movement preparation and motor imagery. Exp Brain Res 111: 429–436. [DOI] [PubMed] [Google Scholar]
- Deecke L,Scheid P,Kornhuber HH ( 1969): Distribution of readiness potential, pre‐motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res 7: 158–168. [DOI] [PubMed] [Google Scholar]
- Eimer M ( 1995): Stimulus‐response compatibility and automatic response activation: Evidence from psychophysiological studies. J Exp Psychol Hum Perc Perf 21: 837–854. [DOI] [PubMed] [Google Scholar]
- Eimer M,Driver J ( 2001): Crossmodal links in endogenous and exogenous spatial attention: Evidence from event‐related brain potential studies. Neurosci Biobehav Rev 25: 497–511. [DOI] [PubMed] [Google Scholar]
- Eimer M,van Velzen J,Driver J ( 2002): Cross‐modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cognit Neurosci 14: 254–271. [DOI] [PubMed] [Google Scholar]
- Eimer M,Forster B,van Velzen J,Prabhu G ( 2005): Covert manual response preparation triggers attentional shifts: ERP evidence for the premotor theory of attention. Neuropsychologia 43: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdo‐Alvarez S,Carrillo‐de‐la‐Pena MT ( 2004): ERP evidence of MI activation without motor response execution. NeuroReport 15: 2067–2070. [DOI] [PubMed] [Google Scholar]
- Gitelman DR,Nobre AC,Parrish TB,LaBar KS,Kim Y,Meyer JR,Mesulam MM ( 1999): A large‐scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122: 1093–1106. [DOI] [PubMed] [Google Scholar]
- Green JJ,McDonald JJ ( 2006): An event‐related potential study of supramodal attentional control and crossmodal attention effects. Psychophysiology 43: 161–171. [DOI] [PubMed] [Google Scholar]
- Green JJ,Teder‐Salejarvi WA,McDonald JJ ( 2005): Control mechanisms mediating shifts of attention in auditory and visual space: A spatio‐temporal ERP analysis. Exp Brain Res 166: 358–369. [DOI] [PubMed] [Google Scholar]
- Hackley SA,Miller J ( 1995): Response complexity and precue interval effects on the lateralized readiness potential. Psychophysiology 32: 230–241. [DOI] [PubMed] [Google Scholar]
- Hanakawa T,Dimyan MA,Hallett M ( 2008): Motor planning, imagery, and execution in the distributed motor network: A time‐course study with functional MRI. Cereb Cortex 18: 2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW,Bui BH ( 1996): A comparison of computer‐based methods for the determination of onset of muscle contraction using electromyography. Electroenceph Clin Neurophysiol 101: 511–519. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK,Colebatch JG ( 2002): Movement‐related potentials associated with self‐paced, cued and imagined arm movements. Exp Brain Res 147: 98–107. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 2001): Neural simulation of action: A unifying mechanism for motor cognition. NeuroImage 14: S103–S109. [DOI] [PubMed] [Google Scholar]
- Jentzsch I,Leuthold H,Ridderinkhof KR ( 2004): Beneficial effects of ambiguous precues: Parallel motor preparation or reduced premotoric processing time? Psychophysiology 41: 231–244. [DOI] [PubMed] [Google Scholar]
- Jung T,Makeig S,Humphries C,Lee T,McKeown MJ,Iragui V,Sejnowski TJ ( 2000): Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37: 163–178. [PubMed] [Google Scholar]
- Kranczioch C,Mathews S,Dean PJA,Sterr A: Task complexity differentially affects executed and imagined movement preparation. Evidence from movement‐related potentials. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold H,Jentzsch I ( 2001): Neural correlates of advance movement preparation: A dipole source analysis approach. Cognit Brain Res 12: 207–224. [DOI] [PubMed] [Google Scholar]
- Leuthold H,Jentzsch I ( 2002): Distinguishing neural sources of movement preparation and execution: An electrophysiological analysis. Biol Psychol 60: 173–198. [DOI] [PubMed] [Google Scholar]
- Leuthold H,Sommer W,Ulrich R ( 1996): Partial advance information and response preparation: Inferences from the lateralized readiness potential. J Exp Psychol Gen 125: 307–323. [DOI] [PubMed] [Google Scholar]
- Lim VK,Polych MA,Hollander A,Byblow WD,Kirk IJ,Hamm JP ( 2006): Kinesthetic but not visual imagery assists in normalizing the CNV in Parkinson's disease. Clin Neurophysiol 117: 2308–2314. [DOI] [PubMed] [Google Scholar]
- Macaluso E,Frith CD,Driver J ( 2000): Modulation of human visual cortex by crossmodal spatial attention. Science 289: 1206–1208. [DOI] [PubMed] [Google Scholar]
- Mars RB,Coles MG,Hulstijn W,Toni I ( 2008): Delay‐related cerebral activity and motor preparation. Cortex 44: 507–520. [DOI] [PubMed] [Google Scholar]
- Masaki H,Wild‐Wall N,Sangals J,Sommer W ( 2004): The functional locus of the lateralized readiness potential. Psychophysiology 41: 220–230. [DOI] [PubMed] [Google Scholar]
- Mathews S,Dean PJA,Sterr A ( 2006): EEG dipole analysis of motor‐priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol 117: 2675–2683. [DOI] [PubMed] [Google Scholar]
- Miller J,Patterson T,Ulrich R ( 1998): Jackknife‐based method for measuring LRP onset latency differences. Psychophysiology 35: 99–115. [PubMed] [Google Scholar]
- Müller‐Gethmann H,Rinkenauer G,Stahl J,Ulrich R ( 2000): Preparation of response force and movement direction: Onset effects on the lateralized readiness potential. Psychophysiology 37: 507–514. [PubMed] [Google Scholar]
- Neuper C,Scherer R,Reiner M,Pfurtscheller G ( 2005): Imagery of motor actions: Differential effects of kinesthetic and visual‐motor mode of imagery in single‐trial EEG. Cognit Brain Res 25: 668–677. [DOI] [PubMed] [Google Scholar]
- Nobre AC,Sebestyen GN,Miniussi C ( 2000): The dynamics of shifting visuospatial attention revealed by event‐related potentials. Neuropsychologia 38: 964–974. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oostenveld R,Stegeman DF,Praamstra P,van Oosterom A ( 2003): Brain symmetry and topographic analysis of lateralized event‐related potentials. Clin Neurophysiol 114: 1194–1202. [DOI] [PubMed] [Google Scholar]
- Peelen MV,Downing PE ( 2005): Is the extrastriate body area involved in motor actions? Nat Neurosci 8: 125–126. [DOI] [PubMed] [Google Scholar]
- Praamstra P,Seiss E ( 2005): The neurophysiology of response competition: Motor cortex activation and inhibition following subliminal response priming. J Cognit Neurosci 17: 483–493. [DOI] [PubMed] [Google Scholar]
- Praamstra P,Stegeman DF,Horstink MW,Cools AR ( 1996): Dipole source analysis suggests selective modulation of the supplementary motor area contribution to the readiness potential. Electroenceph Clin Neurophysiol 98: 468–477. [DOI] [PubMed] [Google Scholar]
- Praamstra P,Schmitz F,Freund HJ,Schnitzler A ( 1999): Magneto‐encephalographic correlates of the lateralized readiness potential. Brain Res Cognit Brain Res 8: 77–85. [DOI] [PubMed] [Google Scholar]
- Praamstra P,Boutsen L,Humphreys GW ( 2005): Frontoparietal control of spatial attention and motor intention in human EEG. J Neurophysiol 94: 764–774. [DOI] [PubMed] [Google Scholar]
- Praeg E,Esslen M,Lutz K,Jancke L ( 2006): Neuronal modifications during visuomotor association learning assessed by electric brain tomography. Brain Topogr 19: 61–75. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Riggio L,Dascola I,Umilta C ( 1987): Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25: 31–40. [DOI] [PubMed] [Google Scholar]
- Rosen AC,Rao SM,Caffarra P,Scaglioni A,Bobholz JA,Woodley SJ,Hammeke TA,Cunningham JM,Prieto TE,Binder JR ( 1999): Neural basis of endogenous and exogenous spatial orienting: A functional MRI study. J Cognit Neurosci 11: 135–152. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA,Kornblum S ( 1982): A priming method for investigating the selection of motor response. Acta Psychol (Amst) 51: 223–243. [Google Scholar]
- Seiss E,Gherri E,Eardley AF,Eimer M ( 2007): Do ERP components triggered during attentional orienting represent supramodal attentional control? Psychophysiology 44: 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R,Leuthold H,Sommer W ( 1998): Motor programming of response force and movement direction. Psychophysiology 35: 721–728. [PubMed] [Google Scholar]
- van der Lubbe RHJ,Wauschkuhn B,Wascher E,Niehoff T,Kompf D,Verleger R ( 2000): Lateralized EEG components with direction information for the preparation of saccades versus finger movements. Exp Brain Res 132: 163–178. [DOI] [PubMed] [Google Scholar]
- van der Lubbe RHJ,Neggers SFW,Verleger R,Kenemans JL ( 2006): Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res 1072: 133–152. [DOI] [PubMed] [Google Scholar]
- Verleger R,Vollmer C,Wauschkuhn B,van der Lubbe RH,Wascher E ( 2000): Dimensional overlap between arrows as cueing stimuli and responses? Evidence from contra‐ipsilateral differences in EEG potentials. Cogn Brain Res 10: 99–109. [DOI] [PubMed] [Google Scholar]
- Wauschkuhn B,Wascher E,Verleger R ( 1997): Lateralized cortical activity due to preparation of saccades and finger movements: A comparative study. Electroenceph Clin Neurophysiol 102: 114–124. [DOI] [PubMed] [Google Scholar]
- Wild‐Wall N,Sangals J,Sommer W,Leuthold H ( 2003): Are fingers special? Evidence about movement preparation from event‐related brain potentials. Psychophysiology 40: 7–16. [DOI] [PubMed] [Google Scholar]


