Abstract
Objective. So far, there have been no attempts to integrate the growing number of all brain volumetric magnetic resonance imaging studies in depression. In this comprehensive meta‐analysis the magnitude and extent of brain volume differences between 2,418 patients with major depressive disorder and 1,974 healthy individuals from 64 studies was determined. Methods. A systematic research was conducted for volumetric magnetic resonance imaging studies of patients with major depressive disorder in relation to healthy control subjects. Studies had to report sufficient data for computation of effect sizes. For each study, the Cohen's d was calculated. All analyses were performed using the random effects model. Additionally, meta‐regression analyses were done to explore the effects of potential sources of heterogeneity. Results. Patients showed large volume reductions in frontal regions, especially in the anterior cingulate and orbitofrontal cortex with smaller reductions in the prefrontal cortex. The hippocampus, the putamen and caudate nucleus showed moderate volume reductions. Conclusions. This is the first comprehensive meta‐analysis in major depressive disorder demonstrating structural brain abnormalities, particularly in those brain areas that are involved in emotion processing and stress‐regulation. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: major depressive disorder, meta‐analysis, magnetic resonance imaging, morphometry
INTRODUCTION
With an estimated life‐time risk of at least 10% [Kessler et al.,2003; Weissman et al.,1996] major depressive disorder (MDD) is one of the most common psychiatric illnesses. Moreover, depressive symptoms are highly prevalent among other psychiatric disorders such as schizophrenia [Häfner et al.,2005b], alcohol and drug abuse [Kessler et al.,1996; Regier et al.,1990], and post‐traumatic stress disorder [Kilpatrick et al.,2003]. Although brain abnormalities have been identified in MDD, the number of neuroimaging studies in patients with this illness pale in comparison to those performed in schizophrenia (for meta‐analyses see: [Boos et al.,2007; Honea et al.,2008; Steen et al.,2006; Wright et al.,2000]). Despite an incomplete understanding of the neural circuitry underlying MDD, there is growing consensus that several brain areas are involved in depression [Beyer and Krishnan,2002; Campbell and MacQueen,2006; Drevets,2000; Sheline,2003; Videbech,1997]. Although reviews have appeared summarizing the results of neuroimaging studies of the hippocampus [Campbell et al.,2004; Videbech and Ravnkilde,2004] and anterior cingulate cortex [Hajek et al.,2008], there have been no attempts to integrate all volumetric neuroimaging studies in depression to date. Since such an effort could clarify the role of specific brain areas in the pathogenesis of MDD, we conducted a meta‐analysis to determine the magnitude and extent of brain volume differences between patients with MDD and healthy individuals as measured with magnetic resonance imaging (MRI).
METHODS
Data Sources
Magnetic resonance imaging studies that examined differences in brain volumes between patients with MDD and healthy control subjects were obtained through computerized database searches, including PubMED, Embase, Medline, Psychinfo, and the Cochrane library. The keywords used in the computerized search were: (major) depression/‐ive, unipolar, mood (disorder), affective (disorder), MRI, imaging, (brain) volume(s), morphometry, limbic (system) and gray/white matter. Titles and abstracts of the articles were examined to see whether they fulfilled the inclusion criteria. Bibliographies of included articles were checked for primary studies that might be of relevance.
Study Selection
One hundred and four studies were identified as potential candidates for the meta‐analysis. Studies were included if they (1) were MRI studies of brain structures in MDD published before January 2008, (2) compared patients with MDD with a healthy control group, (3) were published in the English language, (4) reported sufficient data to obtain an effect size (means, standard deviations, exact P‐values, or exact F‐values for a 2‐group comparison), (5) reported brain volumes (from multiple slices) rather than an area (from a single slice) and (6) if the mean age in either the MDD or comparison group was above 18 years. Twenty‐one studies had to be excluded from the meta‐analysis because they did not present sufficient data to compute the Cohen's d values [Ballmaier et al.,2004a; Ballmaier et al.,2004b; Bilder et al.,1999; Buchsbaum,1986; Chen et al.,2007; Frodl et al.,2004b; Frodl et al.,2007; Greenwald et al.,1997; Inagaki et al.,2004; Janssen et al.,2007; Kumar et al.,2000; Kumar et al.,2000; Lacerda et al.,2005; Lampe et al.,2003; Lavretsky et al.,2007; Lewine et al.,1995; Munn et al.,2007; Rabins et al.,1991; Rabins et al.,2000; Salloway et al.,1996; Shah et al.,1998]. Five studies were excluded because no healthy control group was included or patients with MDD had a life threatening comorbid disease [Dahabra et al.,1998; Ebmeier et al.,1997; Kumar et al.,1999; Nakano et al.,2002; Simpson et al.,2001]. Finally, 10 studies were excluded because they examined brain volumes in children and/or adolescents [Caetano et al.,2007; Gabbay et al.,2007; MacMaster et al.,2006; MacMaster et al.,2007; MacMaster and Kusumakar,2004; MacMillan et al.,2003; Nolan et al.,2002; Rosso et al.,2005; Steingard et al.,1996; Steingard et al.,2002]. Brain structures were only evaluated when volumes were available in (three or more) independent studies, therefore four studies had to be excluded (overlapping samples: [Frodl et al.,2004a; Lai et al.,2000; Sheline et al.,1999]; less than three studies: [Rubin et al.,1996]), resulting in 64 suitable studies that reported volumes of 130 brain structures in a total of 2,418 patients and 1,974 control subjects. Table I lists the included articles and the brain structures that were analyzed.
Table I.
Summary of 64 studies included in the meta‐analysis
| Source | No. of patients | No. of controls | Included brain volumes |
|---|---|---|---|
| Almeida et al. [2003] | 51 | 37 | TB, PFC |
| Ashtari et al. [1999] | 40 | 46 | TB, Hip |
| Axelson et al. [1993] | 19 | 30 | TB, Hip |
| Ballmaier et al. [2004c] | 24 | 19 | IC, TB, OFC, ACC |
| Ballmaier et al. [2007] | 14 | 10 | Hip |
| Botteron et al. [2002] | 30 | 8 | TB, ACC |
| Brambilla et al. [2002] | 18 | 38 | ACC |
| Bremner et al. [2000] | 16 | 16 | IC, PFC, Hip, Amyg, Caud |
| Bremner et al. [2002] | 15 | 20 | TB, OFC, ACC |
| Caetano et al. [2001] | 17 | 39 | Thal |
| Caetano et al. [2004] | 31 | 31 | IC, Temp, Hip, Amyg |
| Caetano et al. [2006] | 31 | 31 | ACC |
| Colla et al. [2007] | 24 | 14 | IC, Hip |
| Coryell et al. [2005] | 10 | 10 | ACC |
| Drevets et al. [1997] | 17 | 21 | ACC |
| Dupont et al. [1995] | 30 | 26 | Caud |
| Frodl et al. [2002b] | 30 | 30 | IC, TB, Hip |
| Frodl et al. [2002a] | 30 | 30 | Amyg |
| Frodl et al. [2003]a | 27 | 27 | Amyg |
| Frodl et al. [2006] | 34 | 34 | IC, PFC, Hip |
| Hannestad et al. [2006] | 182 | 64 | Caud |
| Hastings et al. [2004] | 18 | 18 | ACC, Amyg |
| Hickie et al. [2005] | 66 | 20 | IC, Hip |
| Hickie et al. [2007] | 45 | 16 | Amyg, Caud |
| Husain et al. [1991] | 44 | 44 | TB, Puta |
| Janssen et al. [2004] | 28 | 41 | IC, TB, OFC, Hip |
| Krishnan et al. [1992] | 50 | 50 | TB, Caud |
| Krishnan [1993] | 25 | 20 | TB, Thal, Puta, Caud |
| Kumar et al. [1998] | 53 | 30 | IC, TB, PFC, Temp |
| Lacerda et al. [2003] | 25 | 48 | Puta, Caud |
| Lacerda et al. [2004] | 31 | 34 | OFC |
| Lange and Irle [2004] | 17 | 17 | TB, Hip, Amyg |
| Lavretsky et al. [2004] | 41 | 41 | IC, PFC, OFC |
| Lee et al. [2003]b | 41 | 41 | IC, OFC |
| Lenze and Sheline [1999] | 24 | 24 | Puta, Caud |
| Lloyd et al. [2004] | 51 | 39 | Hip |
| MacQueen et al. [2003] | 37 | 37 | Hip |
| Maller et al. [2007] | 45 | 30 | Hip |
| Mervaala et al. [2000] | 34 | 17 | Hip, Amyg |
| Monkul et al. [2007] | 17 | 17 | OFC, ACC, Hip, Amyg |
| Naismith et al. [2002] | 47 | 20 | Caud |
| Neumeister et al. [2005] | 31 | 57 | Hip |
| O'Brien et al. [2004] | 61 | 40 | TB, Hip |
| Pantel et al. [1997] | 19 | 13 | IC, TB, PFC, Temp |
| Parashos et al. [1998] | 32 | 32 | TB, PFC, OFC, Thal, Puta, Caud |
| Pillay et al. [1997] | 38 | 20 | TB |
| Pillay et al. [1998] | 38 | 20 | Caud |
| Posener et al. [2003] | 27 | 42 | TB, Hip |
| Rusch et al. [2001] | 25 | 15 | Hip |
| Salokangas et al. [2002] | 37 | 19 | PFC, Temp |
| Saylam et al. [2006] | 24 | 24 | IC, Hip |
| Sheline et al. [1996] | 10 | 10 | TB, Hip |
| Sheline et al. [1998] | 20 | 20 | TB, Amyg |
| Sheline et al. [2003] | 38 | 38 | Hip |
| Steffens et al. [2000] | 66 | 18 | Hip |
| Steffens et al. [2003]b | 30 | 40 | TB, OFC |
| Taylor et al. [2005] | 135 | 83 | Hip |
| Taylor et al. [2007] | 226 | 144 | OFC |
| Vakili et al. [2000] | 38 | 20 | Hip |
| Velakoulis et al. [2006] | 19 | 87 | Hip, Amyg |
| von Gunten et al. [2000] | 14 | 14 | Hip, Amyg |
| Vythilingam et al. [2002] | 32 | 14 | Hip |
| Vythilingam et al. [2004] | 38 | 33 | Hip |
| Weniger et al. [2006] | 21 | 23 | IC, TB, Hip, Amyg |
IC, intracranial; TB, total brain; PFC, prefrontal cortex; ACC, anterior cingulate; Temp, temporal cortex; ofc, orbitofrontal cortex; Hip, hippocampus; Amyg, amygdala; Puta, putamen; Caud, caudate nucleus; Thal, thalamus.
Only patients with recurrent MDD and their matched healthy controls were selected from this study, data regarding the first episode patients was not included.
OFC volumes were excluded in the analyses due to overlapping samples with Taylor et al [2007].
Data Extraction
The brain structures that were suitable for analysis included intracranium, total brain, prefrontal cortex, anterior cingulate, temporal cortex, orbitofrontal cortex, hippocampus, amygdala, caudate nucleus, putamen and thalamus. If sufficient data was present, analyses were extended to examine the effect in the two hemispheres separately. When brain volumes were only reported per hemisphere total volume was calculated by summarizing the left and right brain volumes. To obtain the total standard deviation (SDTo), the following formula was used:
| (1) |
with SDLe/Ri being the standard deviation of the volume of the particular left/right brain structure and CorLe,Ri being the correlation between the left and right volume of the brain structure. Although it is reasonable to assume that left and right brain volumes are (highly) correlated, it is unlikely that correlations are exactly “1” (e.g., due to the influence of handedness on brain volume [Geschwind et al.,2002]). However, to correct for rounding off errors and to be conservative in our estimation, we set the correlation at “1” and hence allowed larger estimated SD's for total volumes.
The key to a meta‐analysis is defining an effect size statistic capable of representing the quantitative findings of a set of research studies in a standardized form that permits meaningful comparison and analyses across the studies [Lipsey and Wilson,2001]. Therefore, for each study in this meta‐analysis, the effect size statistic Cohen's d was calculated. In this analysis, the mean volume of a specific brain structure for patients with MDD was subtracted from the mean volume for comparison subjects and divided by the pooled standard deviation of both. When means and standard deviations were not available, d‐values were calculated from exact P‐values, t‐values, or F‐values. Meta‐analytic methods were applied to obtain a combined effect size, which indicated the magnitude of the association across all studies [Hedges and Olkin,1985]. A t‐test was subsequently performed on the null hypothesis that the d value is 0.00, which we report together with the associated P‐value. According to Cohen [Cohen,1988], d values of 0.2 represent small effects, values between 0.4 and 0.6 moderate effects, and d values of 0.8 or higher large effects.
A second measure of effect size was used to calculate a percentage difference between both groups. We used the ratio of the mean volume in the depression group divided by the mean volume in the comparison group. Specifically, for each region in study i (i = 1,2,3,…k) we used the weighted ratio effect size (EffRW) defined as:
| (2) |
where pt refers to patients with MDD, nc to the control group, w to the weights per study and M to the group regional volume mean. To control for sample size differences, for each region the EffRW is multiplied with the specific weights per study derived from our meta‐analyses. By adding the separate weights, an average weighted percentage volume difference is obtained.
Next to the effect size the variance between and within studies has to be explored. All analyses were performed with a random‐effects model using the statistical package Comprehensive Meta‐analysis V2 [Borenstein and Rothstein,1999]. If there is significant heterogeneity among the results of the included studies, random effects models will give wider confidence intervals than fixed effect models [DerSimonian and Kacker,2007; DerSimonian and Laird,1986]. For each brain region, a test of homogeneity (Cochran's Q test) was performed to test whether the studies could be assumed to share a common population effect size. A significant Q statistic indicates heterogeneity of the individual study effect sizes, which poses a limitation to a reliable interpretation of the results. Additionally, we also calculated I 2 to provide a more interpretable measure of consistency between studies in this meta‐analysis [Higgins et al.,2003; Huedo‐Medina et al.,2006]. The I 2 index can be interpreted as the percentage of the total variability in a set of effect sizes due to true heterogeneity, that is, to between‐studies variability. The I 2 is placed between 0 and 100% where a value of 0% indicates no observed heterogeneity and larger values imply increasing heterogeneity. Since MDD is a heterogeneous psychiatric illness we expected a significant result in the homogeneity test. Therefore, a meta‐regression analysis was planned to explore the effects of potential sources of heterogeneity, by regressing effect sizes against mean age, gender, duration of illness, illness onset, number of depressive episodes, medication intake or symptom scores. The random effects regression analysis was performed using the unrestricted maximum likelihood model in Comprehensive Meta‐analysis [Borenstein and Rothstein,1999].
To examine the possibility of publication bias, we computed a fail‐safe number of studies [Orwin,1983; Rosenthal,1991]. Publication bias implies that studies with no effect may not be published, posing a threat to the stability of the obtained effect size. The fail‐safe number (NFS) of studies indicates the number of unpublished studies with null effects that must reside in file drawers to reduce the observed effect size to a negligible level. The statistic can be calculated with the use of the formula given by Orwin [1983] and Lipsey and Wilson [2001]:
| (3) |
with k being the number of studies, ESk, the mean weighted effect size; and ESc, the criterion effect size (which we set at a d value of 0.10).
RESULTS
Meta‐Analyses
As presented in Table II, the results of the meta‐analysis indicate brain volume decreases in patients with MDD as compared with healthy control subjects. The 95% confidence intervals were methodologically stringent for all significant findings. In addition, the fail‐safe number of studies for all analyses was large enough to lend credence to our findings.
Table II.
Brain structures included in Meta‐analysis and Results
| Brain structure | No. of studies | No. of patients | No. of controls | Mean weighted effect size Cohen's d (95% CI) | P‐value for d | Average weighted percentage difference in % | Within‐ category homogeneity statistic Q | P‐value for Q | I 2 in % | NFS |
|---|---|---|---|---|---|---|---|---|---|---|
| Intracranium | 14 | 452 | 364 | 0.03 (−0.13 to 0.20) | 0.37 | NA | 17.69 | 0.17 | 26.53 | 10 |
| Total brain | 22 | 703 | 622 | −0.06 (−0.16 to 0.05) | 0.29 | NA | 15.89 | 0.78 | 0.00 | 35 |
| Amygdala | ||||||||||
| Left | 13 | 321 | 361 | 0.07 (−0.28 to 0.43) | 0.68 | NA | 49.53 | <0.001 | 75.72 | 4 |
| Right | 13 | 321 | 361 | 0.14 (−0.11 to 0.40) | 0.27 | NA | 26.00 | 0.011 | 53.85 | 6 |
| Total | 14 | 366 | 377 | 0.05 (−0.25 to 0.35) | 0.76 | NA | 42.58 | <0.001 | 69.47 | 7 |
| Anterior cingulate cortex | ||||||||||
| Left | 8 | 183 | 171 | −1.11 (−1.88 to −0.34) | 0.005 | −12.19 | 74.83 | 0.005 | 90.65 | 97 |
| Right | 7 | 166 | 150 | −0.62 (−0.88 to −0.37) | <.001 | −10.71 | 7.29 | 0.30 | 17.65 | 52 |
| Total | 8 | 181 | 170 | −0.769 (−1.32 to −0.22) | 0.006 | −11.91 | 44.58 | <0.001 | 84.30 | 71 |
| Caudate nucleus | ||||||||||
| Left | 5 | 285 | 172 | −0.04 (−0.24 to 0.16) | 0.67 | NA | 0.33 | 0.52 | 0.00 | 8 |
| Right | 5 | 285 | 172 | 0.00 (−0.20 to 0.20) | 0.99 | NA | 1.27 | 0.87 | 0.00 | 5 |
| Total | 10 | 467 | 316 | −0.31 (−0.58 to −0.04) | 0.024 | −6.74 | 26.89 | 0.001 | 70.19 | 41 |
| Hippocampusa | ||||||||||
| Left | 30 | 1083 | 914 | −0.37 (−0.52 to −0.23) | <0.001 | −4.71 | 65.43 | <0.001 | 55.68 | 142 |
| Right | 30 | 1083 | 914 | −0.41 (−0.54 to −0.28) | <0.001 | −5.12 | 58.14 | 0.001 | 50.12 | 153 |
| Total | 31 | 1114 | 991 | −0.41 (−0.54 to −0.28) | <0.001 | −5.07 | 60.67 | <0.001 | 50.55 | 158 |
| Orbitofrontal cortex | ||||||||||
| Left | 5 | 326 | 255 | −0.52 (−0.85 to −0.18) | 0.002 | −9.48 | 10.76 | 0.029 | 62.82 | 31 |
| Right | 5 | 326 | 255 | −0.47 (−0.79 to −0.15) | 0.004 | −8.71 | 9.87 | 0.043 | 59.48 | 29 |
| Total | 7 | 373 | 204 | −0.43 (−0.78 to −0.09) | 0.014 | −9.18 | 21.95 | 0.001 | 72.67 | 38 |
| Prefrontal cortex | ||||||||||
| Left | 5 | 157 | 119 | −0.22 (−0.44 to −0.01) | 0.045 | −2.1 | 1.55 | 0.82 | 0.00 | 17 |
| Right | 5 | 157 | 119 | −0.22 (−0.44 to 0.00) | 0.053 | −1.21 | 1.94 | 0.75 | 0.00 | 16 |
| Total | 7 | 242 | 181 | −0.34 (−0.52 to −0.16) | <0.001 | −3.35 | 4.82 | 0.57 | 0.00 | 31 |
| Putamen | ||||||||||
| Left | 3 | 90 | 116 | −.32 (−0.88 to 0.23) | 0.26 | NA | 7.53 | 0.023 | 73.42 | 13 |
| Right | 3 | 90 | 116 | −.34 (−0.95 to 0.27) | 0.27 | NA | 9.05 | 0.011 | 77.90 | 14 |
| Total | 6 | 192 | 184 | −0.48 (−0.80 to −0.16) | 0.003 | −11.28 | 11.77 | 0.038 | 57.52 | 35 |
| Temporal cortex | ||||||||||
| Left | 3 | 87 | 63 | 0.07 (−0.24 to 0.37) | 0.67 | NA | 0.44 | 0.80 | 0.00 | 2 |
| Right | 3 | 87 | 63 | 0.28 (−0.03 to 0.58) | 0.076 | NA | 0.81 | 0.67 | 0.00 | 6 |
| Total | 4 | 140 | 93 | −0.03 (−0.40 to 0.34) | 0.88 | NA | 6.85 | 0.077 | 56.20 | 6 |
| Thalamus | 3 | 74 | 91 | −0.17 (−0.55 to 0.20) | 0.37 | NA | 3.08 | 0.21 | 35.10 | 9 |
CI, confidence intervals; NFS, fail‐safe number: number of unpublished studies with null effects that must reside in file drawers to reduce the observed effect size to a negligible level; I 2: percentage of the total variability due to true heterogeneity; NA, not applicable.
Since exclusion of studies examining medication naive and medication free (at least six weeks of medication) patients did not change the results, the full sample results are displayed.
Areas in the Frontal Lobe
The largest effect was found for the anterior cingulate cortex, with smaller volumes in patients with MDD compared with healthy control subjects (Fig. 1). Eight studies were included with a total group size of 181 patients with MDD and 170 healthy controls, resulting in a combined‐effect Cohen's d of −0.769 (P = 0.006). Excluding the studies that used specific subregions of the anterior cingulate cortex [Botteron et al.,2002; Brambilla et al.,2002; Bremner et al.,2002; Drevets et al.,1997; Hastings et al.,2004] did not change the results. The effect was most pronounced in the left anterior cingulate cortex volume (k = 8; d = −1.11; P = 0.005; right anterior cingulate cortex volume: k = 7; d = −0.624; P < 0.001).
Figure 1.
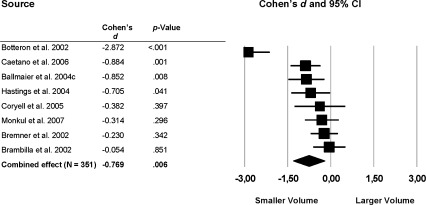
Mean total anterior cingulate cortex volume. Error bars indicate 95% confidence interval.
Other areas in the frontal lobe that showed reduced volumes in patients with MDD were the orbitofrontal cortex (total: k = 7; d = −0.433; P = 0.014; left: k = 5; d = −0.516; P = 0.002; right: k = 5; d = −0.469; P = 0.004; Fig. 2) and the left (k = 5) and total (k = 7) prefrontal cortex (left: d = −0.223; P < 0.005), total: d = −0.342; P < 0.0001). The right prefrontal cortex volume difference between patients with MDD and healthy controls was significant at trend level (d = −0.216; P = 0.053; Fig. 3).
Figure 2.
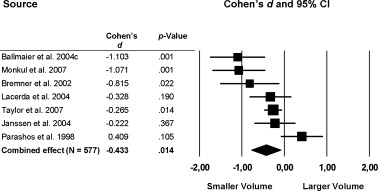
Mean total orbitofrontal cortex volume. Error bars indicate 95% confidence interval.
Figure 3.
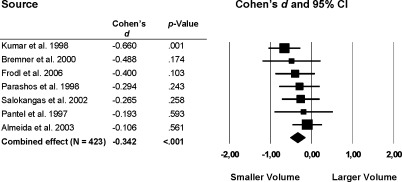
Mean total prefrontal cortex volume. Error bars indicate 95% confidence interval.
Hippocampus
The hippocampus was the brain structure studied most often (k > 30), with a total group size of 1,114 (left/right: 1,083) patients with MDD and 991 (left/right: 914) control subjects. Reduced hippocampal volumes were found in patients with combined‐effect sizes of −0.373 (P < 0.0001), −0.408 (P < 0.0001) and −0.408 (P < 0.0001) for the left, right and total hippocampus volume, respectively (Fig. 4). Excluding the studies that examined medication naive or medication free (at least 6 weeks free of medication) patients [MacQueen et al.,2003; Neumeister et al.,2005; Saylam et al.,2006; Vythilingam et al.,2004] did not change the results (total: k = 27; d = −0.409; P < 0.001). We also examined hippocampal volume of these studies with untreated patients to elucidate differences as compared with the whole sample with treated patients, but findings in this group are comparable (k = 4; d = −0.362; P = 0.008).
Figure 4.
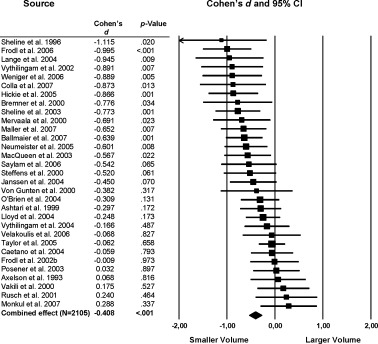
Mean total hippocampus cortex volume. Error bars indicate 95% confidence interval.
Striatum
Significant volume reductions of the striatum were found in the patient group compared with healthy individuals (Fig. 5, 6). Specifically, a moderate effect was found for the total putamen volume (k = 6; d = −0.48; P = 0.003) and a small effect was found in the total caudate nucleus volume, (d = −0.31, P = 0.024).
Figure 5.
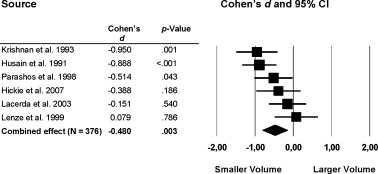
Mean total putamen volume. Error bars indicate 95% confidence interval.
Figure 6.
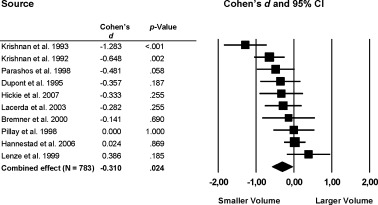
Mean total caudate nucleus volume. Error bars indicate 95% confidence interval.
Other Brain Areas
Analyses of volumes of the intracranial space, total brain, temporal cortex, (bilateral) amygdala, and thalamus did not show significant differences between the groups.
Meta‐Regression
Significant heterogeneity among studies was detected for several structures that showed significant differences between the groups (Table II lists the Cochran's Q‐coefficients and I 2 for the homogeneity tests). A priori it was assumed that inter‐study differences in age, gender, age of onset, duration of illness or symptom scores could explain some of the surplus in variance. Unfortunately, most studies provided insufficient data regarding medication intake or number of episodes; therefore we were unable to perform the meta‐regression with these variables. A significant effect of age (z = −2.38; P = 0.017) was found on total putamen volume (Q model = 5.33; P = 0.02 vs. Q residual = 6.73; P = 0.15). None of the other moderator effects reached significance.
DISCUSSION
This meta‐analysis integrated the results of 64 magnetic resonance imaging studies that compared the volumes of various brain structures in patients with MDD (N = 2,418) with those of healthy control subjects (N = 1,974). The results indicate pronounced brain volume reductions in specific brain areas in patients with MDD.
Frontal Lobe
Several lines of evidence suggest the presence of specific neural circuits within the limbic‐cortical system that mediate stress‐responsiveness, mood and emotional regulation [Brody et al.,2001; Seminowicz et al.,2004; Tekin and Cummings,2002]. Interestingly, the results of this meta‐analysis revealed the presence of structural brain abnormalities in patients with MDD in many of the regions involved in emotional processing and stress‐responsiveness. Of particular interest are the prominent volume reductions in frontal regions (anterior cingulate, orbitofrontal and prefrontal cortex) which are known to control emotion regulation by inhibiting the activity of limbic regions such as the hippocampus and the amygdala [Beauregard et al.,2001; Hariri et al.,2000]. Evidence from postmortem studies suggests cell loss and cell atrophy in subgenual prefrontal and orbitofrontal cortex in patients with MDD [Rajkowska,2000]. The results of this meta‐analysis also showed a pronounced volume reduction of the left anterior cingulate cortex relative to the right side. This finding is consistent with previous studies reporting larger left than right subgenual anterior cingulate cortex volumes reductions [Chen et al.,2007; Drevets et al.,1997].
Limbic System and Frontal Lobe
In addition to the decreased volumes of prefrontal regions, hippocampal volumes were also reduced in patients with MDD. Given the putative relationship between increased sensitivity to stress and affective disorders [Swaab et al.,2005], it is important to note that the hippocampus, amygdala and prefrontal cortex are also involved in Hypothalamus‐Pituitary‐Adrenal (HPA)‐axis regulation. This is relevant, since MDD has been linked to disrupted HPA‐axis activity and increased levels of cortisol [Bao et al.,2008; Swaab et al.,2005] which in turn has been postulated to effect hippocampal volume through the inhibition of neurogenesis in this brain structure [Czeh and Lucassen,2007; Henn and Vollmayr,2004; Sapolsky,2000]. Several mechanisms have been proposed to explain how prolonged stress can result in limbic and prefrontal abnormalities, such as decreased dendritic branching [Radley and Morrison,2005], decreased neurogenesis [Duman,2004], loss of neurons, or decreased expression of brain derived neurotrophic factor [Duman et al.,1997; Radley and Morrison,2005]. Evidence for stress‐induced brain abnormalities in MDD is also provided by studies examining genetic variations in the glucocorticoid receptor gene. Especially functional polymorphisms of the NR3C1 gene (Nuclear Receptor Subfamily 3, Group C, Member 1) are associated with increased susceptibility to MDD [van Rossum et al.,2006; van West et al.,2006]. Of particular interest are the findings of a recent study reporting an association of four illness‐related polymorphisms of the NR3C1 gene with overall smaller hippocampal volumes in patients with MDD [Zobel et al.,2008]. This suggests that “at‐risk”‐alleles of the NR3C1 gene influence hippocampal volume.
Amygdala
The meta‐analysis revealed no significant volumetric abnormalities in the amygdala. However, the amygdala findings are highly inconsistent with a broad range of effect sizes. Although the anatomical boundaries of the amygdala are difficult to outline on MRI images, all studies reported high intrarater correlation coefficients suggesting reliably measured volumes. Also, no association was found between choice of segmentation protocol and amygdala volume increases or decreases. An explanation for the discrepancy between studies may relate to genetic differences between subjects and patients samples. For instance, reduced gray matter volume in the amygdala is more pronounced in those subjects carrying the s‐allele of the serotonin transporter promoter polymorphism [Heinz et al.,2005; Pezawas et al.,2005]. Indeed, individuals carrying the s‐allele tend to have increased anxiety‐related temperamental traits, which in turn are related to increased risk for developing depression [Lotrich and Pollock,2004]. Only a few studies examined the association between the serotonin transporter polymorphism and brain structures in patients with MDD, showing mixed results with respect to amygdala, hippocampus and caudate nucleus volumes [Frodl et al.,2004b; Hickie et al.,2007; O'hara et al.,2007; Taylor et al.,2005]. Finally, it is unknown if other factors, such as whether a patient is in a current episode or in remission as well as duration of illness contributes to differences in amygdala volume. In addition, it is unclear to what extent the amygdala is affected by antidepressant medication.
Striatum
The findings from this meta‐analysis support the presence of volume reductions in the striatum, primarily in total putamen volume. The striatum has been associated with mood, cognitive processes, motivation and regulation of movement and is also part of several neuroanatomic circuits that are involved in mood regulation [Alexander et al.,1986; Drevets,2001; Rogers et al.,1998; Tekin and Cummings,2002]. Further evidence of striatal involvement in MDD is provided by a postmortem study showing reduced putamen and pallidum volumes in patients with MDD compared with nonpsychiatric subjects [Baumann et al.,1999]. Interestingly, lesions of the putamen and of the caudate nucleus have been associated with a higher prevalence of MDD and/or depressive symptoms in post‐stroke depression [Starkstein and Robinson,1989; Vataja et al.,2004], Huntington disease [Slaughter et al.,2001a] and Parkinson disease [Slaughter et al.,2001b].
Antidepressant Medication
Most studies included in this meta‐analysis examined brain volumes in patients on antidepressant treatment; this hampered our ability to analyze effects of antidepressant medication on brain volumes in depression. Moreover, definition of medication free status varied greatly among studies. Although the effect of antidepressants on the brain is obviously important, so far only a few studies evaluated this in patients with MDD. In geriatric patients with depression, antidepressant exposure was associated with larger orbitofrontal gray matter volume compared with medication naïve patients [Lavretsky et al.,2005]. In contrast, other studies have reported improved memory and decreased symptom severity, but failed to find an association with hippocampal or orbitofrontal cortex volume [Janssen et al.,2007; MacQueen et al.,2003; Vythilingam et al.,2004]. However, one must bear in mind that with the exception of one study [Vythilingam et al.,2004], all studies were cross‐sectional, and none of these studies corrected for cumulative or life‐time medication intake.
Overlap and Differences With Schizophrenia and Bipolar Disorder
Major depressive disorder, schizophrenia and bipolar disorder share important clinical features, i.e. depressive symptoms, anhedonia, memory deficits, and lack of motivation [Häfner et al.,2005b; Häfner et al.,2005a; Lake,2008]. Moreover, during the course of schizophrenia the prevalence of depression ranges widely, from 6 to 75% [Siris and Bench,2003]. While there is considerable overlap in risk factors and precursors in these disorders, the overlap in brain volume abnormalities is less clear. Recent meta‐analyses indicate mild ventricular enlargement in bipolar disorder [Kempton et al.,2008; McDonald et al.,2004] and reduced cerebral, temporal lobe and amygdala volumes, and enlarged lateral and third ventricles, and basal ganglia volumes in schizophrenia [Boos et al.,2007; Wright et al.,2000] compared with healthy controls. Interestingly, based on our results and the previous meta‐analyses in schizophrenia, patients with MDD and schizophrenia both show reduced hippocampal [Nelson et al.,1998], prefrontal and anterior cingulate cortex volumes [Baiano et al.,2007; Wright et al.,2000], suggesting that brain regions regulating stress response are affected in both disorders. Indeed, psychosocial stress is a well‐established precipitant of depressive episodes as well as psychotic relapses [Walker, 2008]. Moreover, stressors such as life events and high expressed emotion have been found to precede the onset and recurrence of depression [Kessler,1997] and psychotic disorder [Bebbington et al.,1996]. Therefore, it may well be that the phenotypic overlap of reduced brain volumes (on the basis of the previous mentioned meta‐analyses) in MDD and schizophrenia could be explained by a common genetic vulnerability to stress. Indeed, this vulnerability may be phenotypically expressed as a decrease in hippocampal volume. In fact, evidence that schizophrenia and MDD share common genetic background is found in the original DISC1‐gene study (DISC1 = Disrupted in Schizophrenia‐1 family)[Millar et al.,2000]. Usually, DISC1 is considered to be a major risk gene for schizophrenia but affected individuals in this family displayed a range of diagnoses, with the majority being diagnosed with either schizophrenia or MDD [Blackwood et al.,2001; Hashimoto et al.,2006]. In addition, the DISC1‐gene is involved in structural and functional alterations in hippocampal formation and probably adult neurogenesis in the dentate gyrus [Callicott et al.,2005].
Limitations
Some limitations of this meta‐analysis should be noted. First, structures other than those that have been evaluated in this meta‐analysis may also be affected in patients with MDD. For instance, separated gray and white matter volumes of the cerebrum are scarcely reported. Moreover, only two studies [Parashos et al.,1998; Salokangas et al.,2002] examined ventricular volumes, while increased ventricular volume is the most replicated finding in schizophrenia (interestingly, both studies reported volume increases in MDD patients)[Wright et al.,2000]. Second, although we found significant decreases in brain volumes, almost all analyses resulted in significant heterogeneity which hampers a reliable interpretation and may have influenced the results [Hedges and Vevea,1998]. However, all significant findings indicate brain volume reductions with reliable confidence intervals in patients with MDD relative to healthy control subjects. In addition, the meta‐regression analyses did not show differences between studies, except for a small effect of age on the putamen. Unfortunately, many studies included in this meta‐analysis did not provide sufficient data to examine the effects of antidepressant medication. Moreover, most studies lack information on number of depressive episodes and scores on symptom scales. Thus, the possibility that some of the significant effects are confounded by these factors cannot be ruled out.
Future Directions
So far, most studies used a region of interest approach, however, the use of voxel‐based morphometry allows comprehensive and global assessment of brain structures without a priori identification of regions of interest [Ashburner and Friston,2000]. Although, voxel‐based morphometry studies are sparse in MDD [Bell‐McGinty et al.,2002; Chen et al.,2007; Shah et al.,1998; Tang et al.,2007; Vasic et al.,2008], findings are in line with those found in our meta‐analysis. Of interest is also the measurement of cortical thickness, i.e. a measure to determine the thickness of the gray matter of the human cerebral cortex [Davatzikos and Bryan,1996; Fischl and Dale,2000; Kabani et al.,2001; Thompson and Toga,1996]. Cortical thinning is frequently regionally specific and can therefore provide important additional information for characterizing disease‐specific neuroanatomical changes. Thus far, there have been no published studies that measured cortical thickness in MDD.
Relatively new MRI techniques such as diffusion tensor imaging and magnetization transfer imaging are used to study the orientation of white matter tracts in vivo and yield an index of microstructural integrity [Basser et al.,1994; Wolff and Balaban,1989]. Interestingly, findings from these studies in MDD indicate microstructural white matter abnormalities in widespread prefrontal and limbic regions [Alexopoulos et al.,2008; Bae et al.,2006; Gunning‐Dixon et al.,2008; Li et al.,2007; Murphy et al.,2007; Nobuhara et al.,2004; Nobuhara et al.,2006; Taylor et al.,2004; Yang et al.,2007]. Integration of white matter volume measurements and these MRI techniques may improve our understanding of the neural circuitry involved in MDD.
In addition, longitudinal imaging studies can clarify whether the volume reductions are static or progressive over time and to what extent the volume (change) is affected by the effects of antidepressant medication, illness severity or age. Future studies should also include relatives of patients with MDD and (discordant) monozygotic and dizygotic twin‐pairs to examine the relationship between genetic vulnerability to develop the illness and brain morphology. Such studies have proven to be valuable in schizophrenia and bipolar disorder in clarifying some of the underlying mechanisms of the brain volume abnormalities in probands [Baaré et al.,2001; Boos et al.,2007; Brans et al.,2008; Lawrie et al.,2008; van der Schot et al., 2008; Whalley et al.,2007]. Finally, future studies ought to focus on the search for susceptibility genes in relation to brain abnormalities by using linkage and association methods.
CONCLUSION
In summary, our results show that structural brain abnormalities are present in patients with MDD. Since MDD is characterized by abnormalities in emotion regulation and stress‐responsiveness, the majority of studies in our meta‐analysis focused on investigating those areas that are involved in these processes. Our findings indeed provide evidence that many, but not all, of those areas, show volume reductions in patients with MDD. Some of the brain abnormalities in depression are similar to those reported in patients with schizophrenia, such as the declines in hippocampal and frontal volumes with comparable effect sizes. Finally, this meta‐analysis confirms the preservation of global cerebral and temporal cortex volume in MDD patients, which is in line with findings in patients with bipolar disorder, but in contrast to the slight but significant reduction found in schizophrenia patients. Our results strongly suggest that studying brain structure in MDD will contribute to understanding the pathogenesis of this disease.
Acknowledgements
The authors would like to thank Heleen B.M. Boos for her valuable comments and discussions in the preparation of the manuscript.
REFERENCES
- Alexander GE, DeLong MR, Strick PL ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357– 381. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning‐Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ ( 2008): Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 165: 238– 244. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Burton EJ, Ferrier N, McKeith IG, O'Brien JT ( 2003): Depression with late onset is associated with right frontal lobe atrophy. Psychol Med 33: 675– 681. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805– 821. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer‐Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S ( 1999): Hippocampal/amygdala volumes in geriatric depression. Psychol Med 29: 629– 638. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH Jr, Krishnan KR ( 1993): Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 47: 163– 173. [DOI] [PubMed] [Google Scholar]
- Baaré WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS ( 2001): Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry 58: 33– 40. [DOI] [PubMed] [Google Scholar]
- Bae JN, Macfall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD ( 2006): Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late‐life depression. Biol Psychiatry 60: 1356– 1363. [DOI] [PubMed] [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P ( 2007): Anterior cingulate volumes in schizophrenia: A systematic review and a meta‐analysis of MRI studies. Schizophr Res 93: 1– 12. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Kumar A, Thompson PM, Narr KL, Lavretsky H, Estanol L, Deluca H, Toga AW ( 2004a): Localizing gray matter deficits in late‐onset depression using computational cortical pattern matching methods. Am J Psychiatry 161: 2091– 2099. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, Welcome SE, Deluca H, Toga AW ( 2004b): Mapping brain size and cortical gray matter changes in elderly depression. Biol Psychiatry 55: 382– 389. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A ( 2004c): Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI‐based parcellation of the prefrontal cortex. Am J Psychiatry 161: 99– 108. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin‐Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A ( 2007): Hippocampal morphology and distinguishing late‐onset from early‐onset elderly depression. Am J Psychiatry 165: 229– 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF ( 2008): The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res Rev 57: 531– 553. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D ( 1994): MR diffusion tenser spectroscopy and imaging. Biophys J 66: 259– 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B ( 1999): Reduced volume of limbic system‐affiliated basal ganglia in mood disorders: Preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci 11: 71– 78. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebbington P, Wilkins S, Sham P, Jones P, van OJ, Murray R, Toone B, Lewis S ( 1996): Life events before psychotic episodes: Do clinical and social variables affect the relationship? Soc Psychiatry Psychiatr Epidemiol 31: 122– 128. [DOI] [PubMed] [Google Scholar]
- Bell‐McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF III, Becker JT ( 2002): Brain morphometric abnormalities in geriatric depression: Long‐term neurobiological effects of illness duration. Am J Psychiatry 159: 1424– 1427. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR ( 2002): Volumetric brain imaging findings in mood disorders. Bipolar Disord 4: 89– 104. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Ashtari M, Robinson D, Woerner M, Lieberman JA, Degreef G ( 1999): Cerebral volume asymmetries in schizophrenia and mood disorders: A quantitative magnetic resonance imaging study. Int J Psychophysiol 34: 197– 205. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St CD, Porteous DJ, Muir WJ ( 2001): Schizophrenia and affective disorders—Cosegregation with a translocation at chromosome 1q42 that directly disrupts brain‐expressed genes: Clinical and P300 findings in a family. Am J Hum Genet 69: 428– 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol HE, Kahn RS ( 2007): Brain volumes in relatives of patients with schizophrenia: A meta‐analysis. Arch Gen Psychiatry 64: 297– 304. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Rothstein H ( 1999): A computer program for research synthesis. Englewood: BioStat, Inc. [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD ( 2002): Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 51: 342– 344. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2002): Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology 27: 792– 799. [DOI] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Pol HE ( 2008): Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry 65: 1259– 1268. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS ( 2000): Hippocampal volume reduction in major depression. Am J Psychiatry 157: 115– 118. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS ( 2002): Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 51: 273– 279. [DOI] [PubMed] [Google Scholar]
- Brody AL, Barsom MW, Bota RG, Saxena S ( 2001): Prefrontal‐subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry 6: 102– 112. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS ( 1986): Brain imaging in the search for biological markers in affective disorder. J Clin Psychiatry 47 Suppl: 7– 12. [PubMed] [Google Scholar]
- Caetano SC, Sassi R, Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2001): MRI study of thalamic volumes in bipolar and unipolar patients and healthy individuals. Psychiatry Res 108: 161– 168. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2004): Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res 132: 141– 147. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, Mallinger AG, Keshavan MS, Kupfer DJ, Frank E, Soares JC ( 2006): Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry 59: 702– 706. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Fonseca M, Hatch JP, Olvera RL, Nicoletti M, Hunter K, Lafer B, Pliszka SR, Soares JC ( 2007): Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett 427: 142– 147. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer‐Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR ( 2005): Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA 102: 8627– 8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, MacQueen G ( 2006): An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry 19: 25– 33. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM ( 2004): Lower hippocampal volume in patients suffering from depression: A meta‐analysis. Am J Psychiatry 161: 598– 607. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo‐Pich E, Bullmore E ( 2007): Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 62: 407– 414. [DOI] [PubMed] [Google Scholar]
- Cohen J ( 1988): Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates. [Google Scholar]
- Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, Heuser I ( 2007): Hippocampal volume reduction and HPA‐system activity in major depression. J Psychiatr Res 41: 553– 560. [DOI] [PubMed] [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC ( 2005): Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: Diagnostic specificity and prognostic implications. Am J Psychiatry 162: 1706– 1712. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ ( 2007): What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 257: 250– 260. [DOI] [PubMed] [Google Scholar]
- Dahabra S, Ashton CH, Bahrainian M, Britton PG, Ferrier IN, McAllister VA, Marsh VR, Moore PB ( 1998): Structural and functional abnormalities in elderly patients clinically recovered from early‐ and late‐onset depression. Biol Psychiatry 44: 34– 46. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Bryan N ( 1996): Using a deformable surface model to obtain a shape representation of the cortex. IEEE Trans Med Imaging 15: 785– 795. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Kacker R ( 2007): Random‐effects model for meta‐analysis of clinical trials: An update. Contemp Clin Trials 28: 105– 114. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N ( 1986): Meta‐analysis in clinical trials. Control Clin Trials 7: 177– 188. [DOI] [PubMed] [Google Scholar]
- Drevets WC ( 2000): Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813– 829. [DOI] [PubMed] [Google Scholar]
- Drevets WC ( 2001): Neuroimaging and neuropathological studies of depression: Implications for the cognitive‐emotional features of mood disorders. Curr Opin Neurobiol 11: 240– 249. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME ( 1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824– 827. [DOI] [PubMed] [Google Scholar]
- Duman RS ( 2004): Depression: A case of neuronal life and death? Biol Psychiatry 56: 140– 145. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ ( 1997): A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597– 606. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC ( 1995): Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry 52: 747– 755. [DOI] [PubMed] [Google Scholar]
- Ebmeier KP, Prentice N, Ryman A, Halloran E, Rimmington JE, Best JK, Goodwin GM ( 1997): Temporal lobe abnormalities in dementia and depression: A study using high resolution single photon emission tomography and magnetic resonance imaging. J Neurol Neurosurg Psychiatry 63: 597– 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM ( 2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97: 11050– 11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ ( 2002a): Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 51: 708– 714. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ ( 2002b): Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159: 1112– 1118. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ ( 2003): Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry 53: 338– 344. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottlender R, Reiser M, Moller HJ ( 2004a): Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1‐year follow‐up. J Clin Psychiatry 65: 492– 499. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, Rupprecht R, Bondy B, Reiser M, Moller HJ ( 2004b): Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch Gen Psychiatry 61: 177– 183. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schaub A, Banac S, Charypar M, Jager M, Kummler P, Bottlender R, Zetzsche T, Born C, Leinsinger G, Reiser M, Moller HJ, Meisenzahl EM ( 2006): Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 31: 316– 323. [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Moller HJ, Meisenzahl EM ( 2007): Association of the brain‐derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 64: 410– 416. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Hess DA, Liu S, Babb JS, Klein RG, Gonen O ( 2007): Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: A proton MR spectroscopy study. Am J Psychiatry 164: 1881– 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D ( 2002): Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA 99: 3176– 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald BS, Kramer‐Ginsberg E, Bogerts B, Ashtari M, Aupperle P, Wu H, Allen L, Zeman D, Patel M ( 1997): Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later‐onset depression and Alzheimer's disease? Psychol Med 27: 421– 431. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher‐Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS ( 2008): Macromolecular white matter abnormalities in geriatric depression: A magnetization transfer imaging study. Am J Geriatr Psychiatry 16: 255– 262. [DOI] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Trendler G, an der HW, Schmidt M ( 2005a): The early course of schizophrenia and depression*. Eur Arch Psychiatry Clin Neurosci 255: 167– 173. [DOI] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Trendler G, an der HW, Schmidt M, Konnecke R ( 2005b): Schizophrenia and depression: Challenging the paradigm of two separate diseases—A controlled study of schizophrenia, depression and healthy controls. Schizophr Res 77: 11– 24. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kozeny J, Kopecek M, Alda M, Höschl C ( 2008): Reduced subgenual cingulate volumes in mood disorders: A meta‐analysis. J Psychiatry Neurosci 33: 91– 99. [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Taylor WD, McQuoid DR, Payne ME, Krishnan KR, Steffens DC, Macfall JR ( 2006): White matter lesion volumes and caudate volumes in late‐life depression. Int J Geriatr Psychiatry 21: 1193– 1198. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC ( 2000): Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 11: 43– 48. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, Chiba S, Noguchi H, Suzuki T, Iwata N, Ozaki N, Taguchi T, Kamiya A, Kosuga A, Tatsumi M, Kamijima K, Weinberger DR, Sawa A, Kunugi H ( 2006): Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet 15: 3024– 3033. [DOI] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ ( 2004): Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 29: 952– 959. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I ( 1985): Statistical Methods for Meta‐analysis. New York: Academic Press; [Google Scholar]
- Hedges LV, Vevea JL ( 1998): Fixed‐ and random‐effects models in meta‐analysis. Psychol Methods 3: 486– 504. [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C ( 2005): Amygdala‐prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8: 20– 21. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B ( 2004): Neurogenesis and depression: Etiology or epiphenomenon? Biol Psychiatry 56: 146– 150. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G ( 2005): Reduced hippocampal volumes and memory loss in patients with early‐ and late‐onset depression. Br J Psychiatry 186: 197– 202. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Naismith SL, Ward PB, Scott EM, Mitchell PB, Schofield PR, Scimone A, Wilhelm K, Parker G ( 2007): Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. J Affect Disord 98: 137– 142. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG ( 2003): Measuring inconsistency in meta‐analyses. BMJ 327: 557– 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Meyer‐Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH ( 2008): Is gray matter volume an intermediate phenotype for schizophrenia? A voxel‐based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 63: 465– 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo‐Medina TB, Sanchez‐Meca J, Marin‐Martinez F, Botella J ( 2006): Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychol Methods 11: 193– 206. [DOI] [PubMed] [Google Scholar]
- Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, Boyko OB, Nemeroff CB, Krishnan KR ( 1991): A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res 40: 95– 99. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Matsuoka Y, Sugahara Y, Nakano T, Akechi T, Fujimori M, Imoto S, Murakami K, Uchitomi Y ( 2004): Hippocampal volume and first major depressive episode after cancer diagnosis in breast cancer survivors. Am J Psychiatry 161: 2263– 2270. [DOI] [PubMed] [Google Scholar]
- Janssen J, Hulshoff Pol HE, Lampe IK, Schnack HG, de Leeuw FE, Kahn RS, Heeren TJ ( 2004): Hippocampal changes and white matter lesions in early‐onset depression. Biol Psychiatry 56: 825– 831. [DOI] [PubMed] [Google Scholar]
- Janssen J, Pol HE, Schnack HG, Kok RM, Lampe IK, de Leeuw FE, Kahn RS, Heeren TJ ( 2007): Cerebral volume measurements and subcortical white matter lesions and short‐term treatment response in late life depression. Int J Geriatr Psychiatry 22: 468– 474. [DOI] [PubMed] [Google Scholar]
- Kabani N, Le GG, MacDonald D, Evans AC ( 2001): Measurement of cortical thickness using an automated 3‐D algorithm: A validation study. Neuroimage 13: 375– 380. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM ( 2008): Meta‐analysis, database, and meta‐regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65: 1017– 1032. [DOI] [PubMed] [Google Scholar]
- Kessler RC ( 1997): The effects of stressful life events on depression. Annu Rev Psychol 48: 191– 214. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ ( 1996): The epidemiology of co‐occurring addictive and mental disorders: Implications for prevention and service utilization. Am J Orthopsychiatry 66: 17– 31. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS ( 2003): The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS‐R). JAMA 289: 3095– 3105. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL ( 2003): Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psychol 71: 692– 700. [DOI] [PubMed] [Google Scholar]
- Krishnan KR ( 1993): Neuroanatomic substrates of depression in the elderly. J Geriatr Psychiatry Neurol 6: 39– 58. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB ( 1992): Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry 49: 553– 557. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G ( 1998): Late‐onset minor and major depression: Early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci USA 95: 7654– 7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Jin Z, Udupa J, Gottlieb G ( 1999): Age of onset of depression and quantitative neuroanatomic measures: Absence of specific correlates. Psychiatry Res 91: 101– 110. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Lavretsky H, Gottlieb G ( 2000): Volumetric asymmetries in late‐onset mood disorders: An attenuation of frontal asymmetry with depression severity. Psychiatry Res 100: 41– 47. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2003): Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res 124: 129– 140. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC ( 2004): Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry 55: 353– 358. [DOI] [PubMed] [Google Scholar]
- Lacerda AL, Brambilla P, Sassi RB, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2005): Anatomical MRI study of corpus callosum in unipolar depression. J Psychiatr Res 39: 347– 354. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR ( 2000): Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry 48: 971– 975. [DOI] [PubMed] [Google Scholar]
- Lake CR ( 2008): Disorders of thought are severe mood disorders: The selective attention defect in mania challenges the Kraepelinian dichotomy a review. Schizophr Bull 34: 109– 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe IK, Hulshoff Pol HE, Janssen J, Schnack HG, Kahn RS, Heeren TJ ( 2003): Association of depression duration with reduction of global cerebral gray matter volume in female patients with recurrent major depressive disorder. Am J Psychiatry 160: 2052– 2054. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E ( 2004): Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med 34: 1059– 1064. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kurbanyan K, Ballmaier M, Mintz J, Toga A, Kumar A ( 2004): Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry 12: 653– 657. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Roybal DJ, Ballmaier M, Toga AW, Kumar A ( 2005): Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry 66: 964– 967. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A ( 2007): Neuroanatomical characteristics of geriatric apathy and depression: A magnetic resonance imaging study. Am J Geriatr Psychiatry 15: 386– 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC ( 2008): Brain structure and function changes during the development of schizophrenia: The evidence from studies of subjects at increased genetic risk. Schizophr Bull 34: 330– 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, Krishnan KR ( 2003): Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry 54: 529– 533. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Sheline YI ( 1999): Absence of striatal volume differences between depressed subjects with no comorbid medical illness and matched comparison subjects. Am J Psychiatry 156: 1989– 1991. [DOI] [PubMed] [Google Scholar]
- Lewine RRJ, Hudgins P, Brown F, Caudle J, Risch SC ( 1995): Differences in qualitative brain morphology findings in schizophrenia, major depression, bipolar disorder and normal volunteers. Schizophr Res 15: 253– 259. [DOI] [PubMed] [Google Scholar]
- Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L ( 2007): Prefrontal white matter abnormalities in young adult with major depressive disorder: A diffusion tensor imaging study. Brain Res 1168: 124– 128. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB ( 2001): The way in which intervention studies have “personality” and why it is important to meta‐analysis. Eval Health Prof 24: 236– 254. [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O'Brien JT ( 2004): Hippocampal volume change in depression: Late‐ and early‐onset illness compared. Br J Psychiatry 184: 488– 495. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG ( 2004): Meta‐analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet 14: 121– 129. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V ( 2004): Hippocampal volume in early onset depression. BMC Med 2: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Taormina SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR ( 2006): Pituitary volume in treatment‐naive pediatric major depressive disorder. Biol Psychiatry 60: 862– 866. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, Lynch M, Rose M, Moore GJ, Rosenberg DR ( 2007): Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry 63: 385– 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, Banerjee SP, Rosenberg DR ( 2003): Increased amygdala: Hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol 13: 65– 73. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, Mcewen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT ( 2003): Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 100: 1387– 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB ( 2007): Hippocampal volumetrics in depression: The importance of the posterior tail. Hippocampus 17: 1023– 1027. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe‐Hesketh S, Ellison‐Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N ( 2004): Meta‐analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56: 411– 417. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen‐Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J ( 2000): Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 30: 117– 125. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson‐Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, Porteous DJ ( 2000): Disruption of two novel genes by a translocation co‐segregating with schizophrenia. Hum Mol Genet 9: 1415– 1423. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC ( 2007): Fronto‐limbic brain structures in suicidal and non‐suicidal female patients with major depressive disorder. Mol Psychiatry 12: 360– 366. [DOI] [PubMed] [Google Scholar]
- Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, Ratnanather JT, Huang H, Todd RD, Miller MI, Botteron KN ( 2007): Amygdala volume analysis in female twins with major depression. Biol Psychiatry 62: 415– 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CF, Gunning‐Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS ( 2007): White‐matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry 61: 1007– 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith S, Hickie I, Ward PB, Turner K, Scott E, Little C, Mitchell P, Wilhelm K, Parker G ( 2002): Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry 159: 2096– 2098. [DOI] [PubMed] [Google Scholar]
- Nakano T, Wenner M, Inagaki M, Kugaya A, Akechi T, Matsuoka Y, Sugahara Y, Imoto S, Murakami K, Uchitomi Y ( 2002): Relationship between distressing cancer‐related recollections and hippocampal volume in cancer survivors. Am J Psychiatry 159: 2087– 2093. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ ( 1998): Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta‐analytic study. Arch Gen Psychiatry 55: 433– 440. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC ( 2005): Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry 57: 935– 937. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T, Tajika A, Sugimoto T, Tamagaki C, Ikeda K, Sawada S, Kinoshita T ( 2004): Effects of electroconvulsive therapy on frontal white matter in late‐life depression: A diffusion tensor imaging study. Neuropsychobiology 50: 48– 53. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T ( 2006): Frontal white matter anisotropy and symptom severity of late‐life depression: A magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 77: 120– 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CL, Moore GJ, Madden R, Farchione T, Bartoi M, Lorch E, Stewart CM, Rosenberg DR ( 2002): Prefrontal cortical volume in childhood‐onset major depression: Preliminary findings. Arch Gen Psychiatry 59: 173– 179. [DOI] [PubMed] [Google Scholar]
- O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N ( 2004): A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 161: 2081– 2090. [DOI] [PubMed] [Google Scholar]
- O'hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, Weiner M, Kraemer HC, Noda A, Lin X, Gray HL, Hallmayer JF ( 2007): Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: Association and interaction with cortisol. Mol Psychiatry 12: 544– 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin RG ( 1983): A fail‐safe N for effect size in meta‐analysis. J Educ Stat 8: 157– 159. [Google Scholar]
- Pantel J, Schroder J, Essig M, Popp D, Dech H, Knopp MV, Schad LR, Eysenbach K, Backenstrass M, Friedlinger M ( 1997): Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 42: 69– 83. [DOI] [PubMed] [Google Scholar]
- Parashos IA, Tupler LA, Blitchington T, Krishnan KR ( 1998): Magnetic‐resonance morphometry in patients with major depression. Psychiatry Res 84: 7– 15. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR ( 2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8: 828– 834. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Yurgelun‐Todd DA, Bonello CM, Lafer B, Fava M, Renshaw PF ( 1997): A quantitative magnetic resonance imaging study of cerebral and cerebellar gray matter volume in primary unipolar major depression: Relationship to treatment response and clinical severity. Biol Psychiatry 42: 79– 84. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Renshaw PF, Bonello CM, Lafer BC, Fava M, Yurgelun‐Todd D ( 1998): A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: Relationship to treatment response and clinical severity. Psychiatry Res 84: 61– 74. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG ( 2003): High‐dimensional mapping of the hippocampus in depression. Am J Psychiatry 160: 83– 89. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K ( 1991): Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry 148: 617– 620. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Alyward E, Holroyd S, Pearlson G ( 2000): MRI findings differentiate between late‐onset schizophrenia and late‐life mood disorder. Int J Geriatr Psychiatry 15: 954– 960. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH ( 2005): Repeated stress and structural plasticity in the brain. Ageing Res Rev 4: 271– 287. [DOI] [PubMed] [Google Scholar]
- Rajkowska G ( 2000): Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 48: 766– 777. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK ( 1990): Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA 264: 2511– 2518. [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Pantelis C, Phillips JG ( 1998): Frontostriatal deficits in unipolar major depression. Brain Res Bull 47: 297– 310. [DOI] [PubMed] [Google Scholar]
- Rosenthal R ( 1991): Meta‐analytic Procedures for Social Research. London: Sage Publications; [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun‐Todd DA ( 2005): Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 57: 21– 26. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Phillips JJ, McCracken JT, Sadow TF ( 1996): Adrenal gland volume in major depression: Relationship to basal and stimulated pituitary‐adrenal cortical axis function. Biol Psychiatry 40: 89– 97. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ ( 2001): Hippocampal morphometry in depressed patients and control subjects: Relations to anxiety symptoms. Biol Psychiatry 50: 960– 964. [DOI] [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Tung G, Richardson E, Thomas C, Westlake R ( 1996): MRI and neuropsychological differences in early‐ and late‐life‐onset geriatric depression. Neurology 46: 1567– 1574. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Cannon T, Van Erp T, Ilonen T, Taiminen T, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Kaljonen A, Syvalahti E, Vilkman H, Alanen A, Hietala J ( 2002): Structural magnetic resonance imaging in patients with first‐episode schizophrenia, psychotic and severe non‐psychotic depression and healthy controls. Results of the schizophrenia and affective psychoses (SAP) project. Br J Psychiatry Suppl 43: s58– s65. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM ( 2000): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925– 935. [DOI] [PubMed] [Google Scholar]
- Saylam C, Ucerler H, Kitis O, Ozand E, Gonul AS ( 2006): Reduced hippocampal volume in drug‐free depressed patients. Surg Radiol Anat 28: 82– 87. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi‐Tari S ( 2004): Limbic‐frontal circuitry in major depression: A path modeling metanalysis. Neuroimage 22: 409– 418. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM ( 1998): Cortical grey matter reductions associated with treatment‐resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry 172: 527– 532. [DOI] [PubMed] [Google Scholar]
- Sheline YI ( 2003): Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54: 338– 352. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW ( 1996): Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93: 3908– 3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL ( 1998): Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 9: 2023– 2028. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH ( 1999): Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19: 5034– 5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC ( 2003): Untreated depression and hippocampal volume loss. Am J Psychiatry 160: 1516– 1518. [DOI] [PubMed] [Google Scholar]
- Simpson SW, Baldwin RC, Burns A, Jackson A ( 2001): Regional cerebral volume measurements in late‐life depression: Relationship to clinical correlates, neuropsychological impairment and response to treatment. Int J Geriatr Psychiatry 16: 469– 476. [DOI] [PubMed] [Google Scholar]
- Siris SG, Bench C ( 2003) Depression and Schizophrenia In: Hirsch SR, Weinberger DR, editors. Schizophrenia. Oxford: Blackwell Publishing; pp 142– 167. [Google Scholar]
- Slaughter JR, Martens MP, Slaughter KA ( 2001a): Depression and Huntington's disease: Prevalence, clinical manifestations, etiology, and treatment. CNS Spectr 6: 306– 326. [DOI] [PubMed] [Google Scholar]
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP ( 2001b): Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. J Neuropsychiatry Clin Neurosci 13: 187– 196. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG ( 1989): Affective disorders and cerebral vascular disease. Br J Psychiatry 154: 170– 182. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA ( 2006): Brain volume in first‐episode schizophrenia: Systematic review and meta‐analysis of magnetic resonance imaging studies. Br J Psychiatry 188: 510– 518. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, Macfall JR, Krishnan KR ( 2000): Hippocampal volume in geriatric depression. Biol Psychiatry 48: 301– 309. [DOI] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Welsh‐Bohmer KA, Krishnan KR ( 2003): Left orbital frontal cortex volume and performance on the benton visual retention test in older depressives and controls. Neuropsychopharmacology 28: 2179– 2183. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Renshaw PF, Yurgelun‐Todd D, Appelmans KE, Lyoo IK, Shorrock KL, Bucci JP, Cesena M, Abebe D, Zurakowski D, Poussaint TY, Barnes P ( 1996): Structural abnormalities in brain magnetic resonance images of depressed children. J Am Acad Child Adolesc Psychiatry 35: 307– 311. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, Connor DF, Au TH, Yurgelun‐Todd DA ( 2002): Smaller frontal lobe white matter volumes in depressed adolescents. Biol Psychiatry 52: 413– 417. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ ( 2005): The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4: 141– 194. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP ( 2007): Reduced ventral anterior cingulate and amygdala volumes in medication‐naive females with major depressive disorder: A voxel‐based morphometric magnetic resonance imaging study. Psychiatry Res 156: 83– 86. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR ( 2004): Late‐life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry 161: 1293– 1296. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, Payne ME, Macfall JR, Marchuk DA, Svenson IK, Krishnan KR ( 2005): Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late‐life depression. Arch Gen Psychiatry 62: 537– 544. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR ( 2007): Orbitofrontal cortex volume in late life depression: Influence of hyperintense lesions and genetic polymorphisms. Psychol Med 37: 1763– 1773. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL ( 2002): Frontal‐subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res 53: 647– 654. [DOI] [PubMed] [Google Scholar]
- Thompson P, Toga AW ( 1996): A surface‐based technique for warping three‐dimensional images of the brain. IEEE Trans Med Imaging 15: 402– 417. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello‐Cintron CM, Yurgelun‐Todd DA ( 2000): Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol Psychiatry 47: 1087– 1090. [DOI] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brans R, van Haren NE, Koolschijn PC, Nuboer V, Schnack HG, van Baal GC, Boomsma DI, Nolen WA, Hulshoff Pol H.E., Kahn RS ( 2009): Influence of genes and environment on brain volumes in twin‐pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry 66: 142– 151. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F ( 2006): Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry 59: 681– 688. [DOI] [PubMed] [Google Scholar]
- van West D, Van Den EF, Del‐Favero J, Souery D, Norrback KF, Van DC, Sluijs S, Adolfsson R, Mendlewicz J, Deboutte D, Van BC, Claes S ( 2006): Glucocorticoid receptor gene‐based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology 31: 620– 627. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Hose A, Wolf RC ( 2008): Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: A voxel‐based morphometry study. J Affect Disord 109( 1–2): 107– 116. [DOI] [PubMed] [Google Scholar]
- Vataja R, Leppavuori A, Pohjasvaara T, Mantyla R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T ( 2004): Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci 16: 156– 162. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C ( 2006): Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first‐episode psychosis, and ultra‐high‐risk individuals. Arch Gen Psychiatry 63: 139– 149. [DOI] [PubMed] [Google Scholar]
- Videbech P ( 1997): MRI findings in patients with affective disorder: A meta‐analysis. Acta Psychiatr Scand 96: 157– 168. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B ( 2004): Hippocampal volume and depression: A meta‐analysis of MRI studies. Am J Psychiatry 161: 1957– 1966. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA ( 2000): A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci 12: 493– 498. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD ( 2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159: 2072– 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD ( 2004): Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry 56: 101– 112. [DOI] [PubMed] [Google Scholar]
- Walker E: Stress and the HPA axis activity in the developmental course of schizophrenia. Annu Rev Clin Psychol (in press) [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio‐Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK ( 1996): Cross‐national epidemiology of major depression and bipolar disorder. JAMA 276: 293– 299. [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E ( 2006): Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord 94: 219– 229. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Harris JC, Lawrie SM ( 2007): The neurobiological underpinnings of risk and conversion in relatives of patients with schizophrenia. Int Rev Psychiatry 19: 383– 397. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS ( 1989): Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 10: 135– 144. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe‐Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET ( 2000): Meta‐analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16– 25. [DOI] [PubMed] [Google Scholar]
- Yang Q, Huang X, Hong N, Yu X ( 2007): White matter microstructural abnormalities in late‐life depression. Int Psychogeriatr 19: 757– 766. [DOI] [PubMed] [Google Scholar]
- Zobel A, Jessen F, von WO, Schuhmacher A, Hofels S, Metten M, Rietschel M, Scheef L, Block W, Becker T, Schild HH, Maier W, Schwab SG ( 2008): Unipolar depression and hippocampal volume: Impact of DNA sequence variants of the glucocorticoid receptor gene. Am J Med Genet B Neuropsychiatr Genet 147B: 836– 843. [DOI] [PubMed] [Google Scholar]


