Abstract
Coordinated hand use is an essential component of many activities of daily living. Although previous studies have demonstrated age‐related behavioral deficits in bimanual tasks, studies that assessed the neural basis underlying such declines in function do not exist. In this fMRI study, 16 old and 16 young healthy adults performed bimanual movements varying in coordination complexity (i.e., in‐phase, antiphase) and movement frequency (i.e., 45, 60, 75, 90% of critical antiphase speed) demands. Difficulty was normalized on an individual subject basis leading to group performances (measured by phase accuracy/stability) that were matched for young and old subjects. Despite lower overall movement frequency, the old group “overactivated” brain areas compared with the young adults. These regions included the supplementary motor area, higher order feedback processing areas, and regions typically ascribed to cognitive functions (e.g., inferior parietal cortex/dorsolateral prefrontal cortex). Further, age‐related increases in activity in the supplementary motor area and left secondary somatosensory cortex showed positive correlations with coordinative ability in the more complex antiphase task, suggesting a compensation mechanism. Lastly, for both old and young subjects, similar modulation of neural activity was seen with increased movement frequency. Overall, these findings demonstrate for the first time that bimanual movements require greater neural resources for old adults in order to match the level of performance seen in younger subjects. Nevertheless, this increase in neural activity does not preclude frequency‐induced neural modulations as a function of increased task demand in the elderly. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: aging, humans, bimanual coordination, hand, fMRI, motor neuroscience, sensorimotor function
INTRODUCTION
Many activities of daily living, such as eating with utensils and dressing, commonly require use of both hands in a coordinated fashion. Based on behavioral research, it is known that old adults have increased difficulty performing bimanual coordination tasks compared with young adults [Lee et al.,2002; Serrien et al.,1996;2000; Swinnen et al.,1998; Wishart et al.,2000]. These studies have relied upon a well‐established bimanual coordination paradigm comparing the performance of two intrinsic coordination tasks. Specifically, “in‐phase” coordination tasks are midline symmetric and involve simultaneous contractions of homologous muscle pairs. Such movements are performed quite easily across a wide range of speeds by subjects of all ages. In contrast, midline asymmetric “antiphase” coordination tasks, which require simultaneous contractions of nonhomologous muscle pairs, are performed less accurately than in‐phase movements by young, and even more so, old adults. Antiphase coordination tasks are also more prone to phase transitions (i.e., a spontaneous shift to the in‐phase coordination mode) when performed at high movement frequencies, with the “critical frequency” at which this transition occurs being lower for old compared with young individuals.
In addition to the bimanual deficits described above, recent data show that bimanual movements occur more than twice as often as those of either hand in isolation [Rinehart et al.,2009; Vega‐Gonzalez and Granat,2005], providing a strong impetus for exploring the neural basis of bimanual coordination in the elderly. Although, currently, studies that have attempted to make a substantive link between bimanual performance and neural activation in old adults do not exist, previous studies involving other forms of movement control in the elderly provide some basis for forming a prediction. First, studies involving unimanual finger responses for speeded tapping [Riecker et al.,2006] and motor sequence learning [Daselaar et al.,2003] have suggested that minimal or no differences exist in the neural control of elderly movement. In this case, known bimanual deficits in elderly behavior would appear to be the result of age‐related differences in nonneural factors such as muscular strength and/or biomechanics. Alternatively, there is mounting evidence that elderly subjects have greater neural activation compared with young individuals (i.e., “overactivation”) when performing motor‐related tasks [for review, see Ward,2006]. Indeed, such overactivation has been reported for a wide range of movement tasks including auditorily paced thumb to index finger tapping [Calautti et al.,2001], finger abduction/adduction [Hutchinson et al.,2002], wrist flexion/extension [Hutchinson et al.,2002], sequential finger presses [Mattay et al.,2002; Wu and Hallett,2005], hand force production [Ward and Frackowiak,2003; Ward et al.,2008], and hand/foot coordination [Heuninckx et al.,2005,2008; Van Impe et al.,2009].
Of the aforementioned studies, the work of Heuninckx et al. [2005,2008] is particularly relevant to the neural control of bimanual coordination in the elderly. In these studies, interlimb coordination was assessed during hand and foot movements that were either isodirectional or nonisodirectional. These movement patterns are analogous to the in‐phase and antiphase tasks that have already been shown to induce behavioral bimanual coordination deficits in the elderly. Heuninckx et al. showed that coordination of the hand and foot is characterized by greater activation for the elderly compared with young subjects. This increase in activation was evident even though the relative task difficulty was matched by allowing old subjects to move at a slower, more comfortable speed. Areas of greater activation for elderly subjects spanned brain areas typically associated with both motor and nonmotor functions (see Tables III and IV in the article by Heuninckx et al. [2005]). Further, using correlation approaches, it was shown that at least some of the age‐related increases in activation appeared to have a compensatory role as they correlated positively with task performance (see Table III in the article by Heuninckx et al. [2008]). Interestingly, these results are compatible with a number of studies in the cognitive aging domain [Cabeza et al.,2002; Grady,2000,2008; Reuter‐Lorenz and Lustig,2005].
Table III.
Areas showing significant modulation of activity with changes in movement frequency for both young and old individuals
| Activation peak location | Side | x | y | z | t‐value |
|---|---|---|---|---|---|
| CLUSTER#1 – 5,820 voxels | |||||
| Central sulcus (SM1, BA 3/4) | L | −24 | −26 | 58 | 8.77 |
| R | 26 | −26 | 56 | 6.66 | |
| Middle frontal gyrus (SMA proper, BA 6) | L | −2 | −8 | 64 | 6.13 |
| R | 2 | −14 | 56 | 5.12 | |
| Precentral gyrus (PMd, BA 6) | L | −42 | −12 | 60 | 4.6 |
| R | 46 | −10 | 48 | 3.41 | |
| Middle cingulate cortex (BA 23) | L | −8 | −24 | 50 | 3.85 |
| CLUSTER#2 – 655 voxels | |||||
| Cerebellar vermis (IV–V) | R | 2 | −46 | −4 | 4.4 |
| Cerebellar vermis (VI) | 0 | −66 | −12 | 4.36 | |
| Cerebellar hemisphere (IV–V) | R | 8 | −60 | −12 | 4.08 |
| Cerebellar hemisphere (VI) | R | 24 | −48 | −22 | 3.4 |
In this study, age‐related differences in brain activation during bimanual coordination tasks were determined for the first time. Based on previous studies of coordinative ability in the elderly [Heuninckx et al.,2005,2008] it was hypothesized that old individuals would demonstrate greater neural activity than young subjects in both motor and nonmotor regions. From a motor standpoint, age‐related increases were expected in areas known to be important for bimanual coordination, such as the supplementary motor area (SMA) and cingulate motor cortex [Carson,2005; Paus,2001; Swinnen and Wenderoth,2004]. With respect to nonmotor regions, it was thought that elderly individuals might recruit areas associated with higher‐order sensory feedback processing such as secondary somatosensory cortex (SII) and/or motor attention regions such as dorsolateral prefrontal cortex (DLPFC) and inferior frontal gyrus (IFG).
Given the influence of movement frequency on bimanual coordination, an additional aim of this study was to examine systematic responses to parametric changes in movement frequency during bimanual tasks for young and old adults. This analysis provided information regarding the extent to which brain areas could modulate neural activity in accordance with changes in movement rate. Previous work in young subjects has shown an extensive network of sensorimotor regions including primary somatosensory cortex and cerebellum that increase in activity with increased movement frequency [Blinkenberg et al.,1996; Deiber et al.,1999; Jancke et al.,1998a,b; Rao et al.,1996; Sadato et al.,1996; Schlaug et al.,1996; Turner et al.,2003; Van Meter et al.,1995]. Interestingly, in the only known study to examine this phenomenon in old adults, no differences were found between young individuals and a small group of very high‐functioning elderly [Riecker et al.,2006]. This study expands this initial finding by assessing such modulation in a larger, more typical, group of elderly subjects.
Lastly, this study sought to determine if coordinative ability was reflected by the level of activation in regions that were found to be more active in old compared with young subjects. Activation in these regions were correlated with performance to provide a direct link between brain and behavior, revealing the extent to which compensatory recruitment mechanisms might underlie the neural control of bimanual movements in the elderly. Overall, the results of this study not only afford a better understanding of the neural basis of bimanual movement in elderly individuals but also provide a starting point for future clinical interventions aimed at the enhancement of compensatory neural recruitment for increased coordinative performance.
MATERIALS AND METHODS
Participants
Sixteen old adults (mean age = 68.3 years; range, 61.1–78.7 years; eight females, eight males) and 16 young adults (mean age = 25.7 years; range, 21.0–30.9 years; eight females, eight males) were recruited from the local community. Participants were right‐handed as indicated by laterality quotients of greater than +90 on the Edinburgh Handedness Inventory [Oldfield,1971]. Subjects were free of neuromuscular impairment at the time of testing and were not under psychoactive or vasoactive medication. General assessment of cognitive function was conducted using the Mini‐Mental State Examination [Folstein et al.,1975], upon which all subjects scored within normal limits (i.e., score ≥27). Informed consent was obtained for all participants and procedures were conducted following guidelines established by the ethics committee of Biomedical Research at Katholieke Universiteit Leuven in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Experimental Design
Task procedures
Subjects performed alternating 21‐s blocks of three task conditions over four runs (i.e., time series) in a functional magnetic resonance imaging (fMRI) scanner. In the first task condition, involving in‐phase coordination (Fig. 1A), subjects made wrist flexion and extension movements in a mirror symmetric fashion with respect to body midline (i.e., with simultaneous activation of homologous muscles). In contrast, the second task condition involved antiphase coordination (Fig. 1B), with parallel motions of the hands via simultaneous flexion of one wrist and extension of the other, and then visa versa. In the third task, a rest condition was included where subjects abstained from moving either hand.
Figure 1.
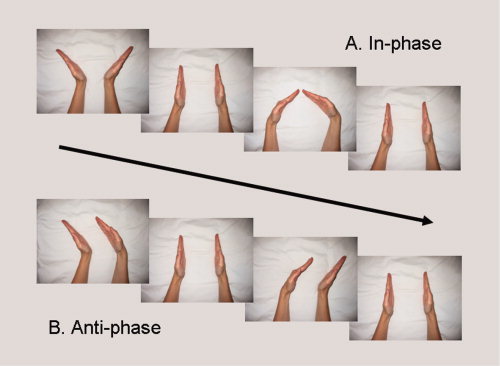
Wrist coordination tasks involving alternating flexion/extension of the wrists according to the midline symmetric, in‐phase pattern (A) and the midline asymmetric, antiphase pattern (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The three tasks were performed in the presence of clearly audible pacing tones, which were used to set movement frequency for the in‐phase and antiphase task conditions. Subjects were instructed to move smoothly and continuously, while timing each peak flexion/extension wrist movement with the occurrence of a tone. The frequency of tone presentation varied between blocks in a balanced fashion and corresponded to each subject's relative capability in the antiphase coordination task (i.e., speeds of 45, 60, 75, and 90% of critical frequency). Subcritical speeds were chosen to prevent phase transitions. Critical frequency was calculated as the maximum speed where subjects were able to maintain antiphase task performance within ±45° of relative phase for at least 3 s. This was determined from three frequency ramping trials performed prior to testing, which involved antiphase movements being performed at progressively faster speeds starting at 0.5 Hz and increasing by 0.125 Hz every 5 s.
The normalization procedure described above was primarily employed as a conservative approach to determine age‐related increases in brain activation during sensorimotor tasks. With this procedure it is less likely that potential overactivation in the elderly simply reflects the fact that old individuals perform the task closer to their maximum capacity than young subjects. It is anticipated that matching task difficulty will result in young subjects making, on average, more movements during a scan run than old individuals. Thus, brain activation primarily related to the movement frequency would actually be slightly underestimated in the old compared with the young individuals.
Scanning procedures
Within 2 days before all scanning sessions, 45 min of practice was given in a “dummy scanner” to familiarize subjects with task procedures and the scan environment. On the actual day of testing, subjects were placed head‐first and supine in the scanner with arms placed along the trunk and elbows flexed at ∼45°. This position was maintained throughout the scanning procedure with the aid of supportive cushioning. A bite‐bar was used to minimize movements of the head, and a mirror was utilized to allow vision of images from an LCD projection system displayed on a screen mounted above the shoulders. This setup was used to cue the different task conditions during each scan run, preventing subjects from seeing their hands during the movement task. Subjects wore headphones for communication with the experimenter and for hearing auditory pacing tones. Orthoses, which limited wrist movement to flexion and extension, were attached to the left and right arms along the forearm and hand segments. These devices were equipped with nonferromagnetic optical shaft encoders (sampled at 100 Hz) that measured joint displacement at a spatial resolution of 0.09°. The displacement signal was available in real‐time to the researchers during scanning, allowing them to ensure that subjects were complying with task instructions. This signal was also recorded for off‐line analysis following the experimental session.
Image acquisition was achieved using a Siemens 3‐T Magnetom Trio MRI scanner (Siemens, Erlangen, Germany) with standard head coil. Each scanning session included a high‐resolution T1‐weighted image (MPRAGE; TR = 2,300 ms, echo time [TE] = 2.98 ms, 1 × 1 × 1.1 mm voxels, field of view: 240 × 256, 160 sagittal slices) for anatomical detail. Functional (fMRI) data were acquired over four time series (i.e., runs) with an interleaved EPI pulse sequence for T2*‐weighted images (TR = 3,000 ms, TE = 30 ms, flip angle = 90°, 50 oblique slices each 2.8 mm thick, interslice gap 0.028 mm, in‐plane resolution 2.5 × 2.5 mm, 80 × 80 matrix). Three “dummy” scans at the beginning of each run were discarded from analysis to allow for scanner equilibration. Each run lasted 378 s (6.3 min) consisting of six blocks of the three task conditions with each condition lasting 21 s (i.e., seven whole brain images). The order of conditions within a block was randomized across time series and the auditorily paced movement frequency for each block was randomized according to a balanced presentation across all blocks. Rest periods of ∼3 min were inserted following each time series.
Kinematic Data Analyses
Coordinative ability was determined using the relative phase (φ) between left and right wrists, as calculated according to the following formula described by Scholz and Kelso [1989]:
whereby R and L refer to the right and left wrists, respectively; θ is the mean phase of the limb at each point in the movement cycle; X is the position of the limb (rescaled between −1 and 1); and dX/dt is the instantaneous velocity (rescaled between −1 and 1). From this measure, coordination accuracy (i.e., mean phase error) was determined as the average absolute deviation between the obtained relative phase and the target relative phase for in‐phase (i.e., 0°) or antiphase (i.e., 180°) movements. The standard deviation of mean phase error was then quantified to provide a measure of coordination stability (i.e., phase variability).
Beyond these phase measures, mean cycling frequency and movement amplitude were calculated. Cycle frequency was defined as the number of total movement cycles per second (Hz) made by a particular hand. Movement amplitude corresponded to the peak‐to‐peak displacement of the wrist joint during each half cycle of movement. Statistical analysis of all kinematic measures (i.e., φ accuracy, φ stability, frequency, amplitude) involved multivariate analysis of variance (MANOVA) with the following factors: age group (i.e., young, old), coordination task (i.e., in‐phase, antiphase), and movement frequency (i.e., 45, 60, 75, 90% critical). Post‐hoc analyses were performed using Tukey's honestly significant difference test. Statistical significance was considered at P < 0.05.
fMRI Data Analyses
Imaging data were processed using Statistical Parametric Mapping (SPM) 5 software (Wellcome Department of Imaging Neuroscience, London, UK) implemented in MatLab 7.4 (MathWorks, Natick, MA). For each subject, EPI volumes were realigned to the first image in the first time series and a mean image was created from the realigned volumes. No subject in either group moved more than 2 mm in any direction over the course of a time series; however, some task‐related movement was observed. To account for this, realigned images underwent an “unwarp” procedure to remove some of the unwanted movement‐related variance independent of variance related to the task conditions [Andersson et al.,2001]. Normalization of the resulting images was performed using a standardized EPI template based on the Montreal Neurological Institute reference brain in Talairach space [Talairach and Tournaux,1998]. Voxels were subsampled at 2 × 2 × 2 mm and smoothed with a Gaussian kernel (10 mm FWHM).
All statistical analyses were performed in accordance with the general linear model [Friston et al.,1995]. For each subject, a first level model was specified with four regressors in total. Regressors for in‐phase and antiphase movement were modeled with boxcar functions representing periods of movement and rest with onset and duration calculations based on the kinematics recorded during scanning. Thus, activation during movement conditions was compared with an implicit (rest) baseline. Additionally, parametric modulation of relative movement frequency in the in‐phase and antiphase conditions was added to estimate linear change about the mean response. Positive estimates indicated brain areas that increased activation linearly with increasing frequency and vice versa. Regressors were convolved with the canonical hemodynamic response function provided in SPM and subjected to high‐pass filtering (1/128 Hz) to remove low‐frequency drifts.
First‐level contrasts for in‐phase and antiphase were entered into a second‐level, random effects analysis of variance (ANOVA) with the following factors: group (young, old) and task (in‐phase, antiphase). Separate ANOVAs were run for the mean and parametric (i.e., linear relationship with frequency) responses. A masking procedure was utilized [Heuninckx et al.,2005], which restricted analyses to voxels with significant activation during either the in‐phase or antiphase task in either group with family wise error <0.05. This procedure reduces the search volume on the basis of an independent contrast which has been shown to be both valid and advantageous compared with functional localizer approaches [Friston et al.,2006].
Common activations between young and old subjects
For both the mean and parametric response ANOVAs, a conjunction analysis previously described by Heuninckx et al. [2005,2008] was utilized to determine areas that were active, or that modulated activation with frequency, in a similar fashion between both young and old individuals during coordination tasks. Conjunctions were determined according to the method of Nichols et al. [2005] whereby significant activation reflects that both groups exceeded the threshold level. For all analyses, a false discovery rate (FDR) was applied to ensure P < 0.01 across groups. Only cluster sizes with an extent of at least 20 voxels were considered.
Age‐related differences in activation for young and old subjects
Differential activations between young and old individuals in terms of mean and parametric responses were identified using post‐hoc t comparisons within the framework of the ANOVA model. FDR correction and cluster extents were as described earlier.
Correlation of areas with age‐related activation differences and performance
When areas were found to have greater activation for one group, multiple linear regression analyses were conducted to determine significant correlations between increased activity and task performance. To accomplish this, a constrained search [Friston et al.,2006] was again performed based on the results of the independent contrasts conducted in the previous methods step (i.e., the contrast images for each of the main effects of bimanual movement), using areas that were significantly more active in one group versus the other. Next, a second level model was defined, with a performance covariate of interest for each subject as well as age and critical movement frequency covariates of no interest. For the performance covariate, the inverse of coordination accuracy was chosen, simplifying interpretation of the results such that positive effects indicated increased performance with increased activation [for a similar procedure, see Heuninckx et al.,2008]. To increase sensitivity, this analysis was first conducted at an uncorrected threshold of P < 0.05 within SPM 5 [cf. Colcombe et al.,2005]. Then, subsequent correlation tests between cluster‐level percent signal change (PSC) (marsbar toolbox) [Brett et al.,2002] and performance were conducted to obtain measures of relationship strength (r‐values), and significance (P < 0.01, Bonferroni corrected), respectively.
RESULTS
Kinematic Data
As expected, the mean critical frequency of young subjects (1.8 Hz) was significantly greater (F (1,15) = 135.6, P < 0.001) than that of elderly individuals (1.5 Hz). Importantly, neither bimanual coordination accuracy (Fig. 2A) nor stability (Fig. 2B) differed between groups at any of the relative movement frequencies. For both groups, coordinative ability was significantly decreased during antiphase versus in‐phase tasks (accuracy: F (1,15) = 66.8, P < 0.001; stability: F (1,15) = 97.6, P < 0.001). Lower accuracy and stability was also observed for both groups at the 45% compared with 75% and 90% critical frequencies (Tukey's HSD). Small, but significant (F (1,15) = 5.3, P < 0.01), decreases in movement amplitude were seen with increased movement frequency. However, movement amplitude was not different between left and right hands and did not significantly differ between age groups.
Figure 2.
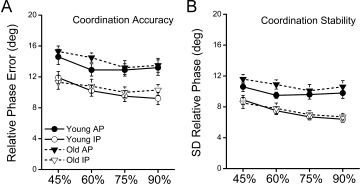
Coordination accuracy (A) and stability (B) for young and old individuals in each task (IP, in‐phase; AP, antiphase) and frequency (45, 60, 75, 90% critical) condition. Task performance did not significantly differ between groups. However, in‐phase accuracy (P > 0.001) and stability (P > 0.001) was greater in the in‐phase compared with antiphase condition across young and old subjects.
Imaging Data
Activations during bimanual coordination tasks
Brain areas significantly activated during bimanual coordination tasks compared with rest are provided in Figure 3. The ANOVA model did not reveal a main effect of task between the in‐phase and antiphase conditions, and thus, these results reflect both in‐phase and antiphase conditions. Activations common to young and old subjects (represented in yellow in Fig. 3; peak coordinates in Table I) included areas typically observed during motor coordination tasks. Specifically, significant activations were seen bilaterally in the sensorimotor cortices (SMI), SMA, cerebellum, and dorsal premotor cortices (PMd), as well as in the right ventral premotor cortex (PMv). Additionally, there was activation along the left and right lateral fissures involving regions such as secondary somatosensory area (SII), primary auditory cortex (AI), and the IFG (pars opercularis).
Figure 3.
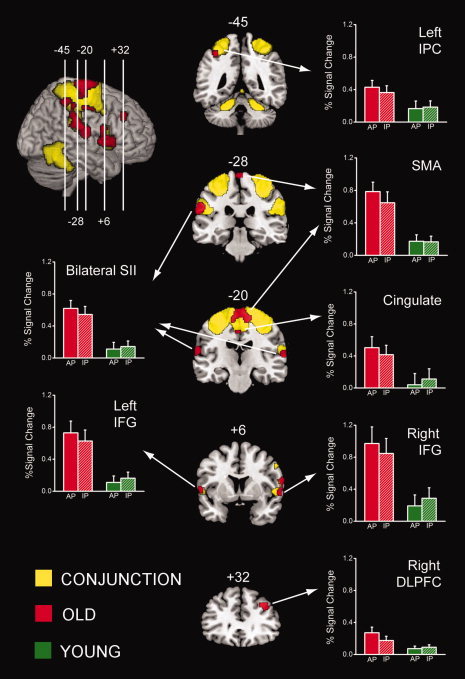
Mean activations during bimanual coordination tasks versus rest, overlaid on a standard MNI template brain. Areas activated by both young and old individuals (common) are shown in yellow, whereas greater activations by old subjects are depicted in red. Bar plots represent BOLD responses measured in percent signal change for the overactivated areas during in‐phase (IP) and antiphase (AP) tasks, in old (red) and young (green) subjects.
Table I.
Areas with significant activation in old and young individuals during bimanual coordination tasks compared with rest
| Activation peak location | Side | x | y | z | t‐value |
|---|---|---|---|---|---|
| CLUSTER#1 – 11,237 voxels | |||||
| Precentral gyrus (MI, BA 4) | L | −30 | −30 | 58 | 14.24 |
| R | 34 | −24 | 52 | 11.54 | |
| Postcentral gyrus (SI, BA 1/3) | L | −36 | −40 | 70 | 8.76 |
| R | 38 | −30 | 66 | 11.03 | |
| Middle frontal gyrus (SMA, BA 6) | L | −4 | −10 | 60 | 13.09 |
| R | 6 | −12 | 58 | 12.22 | |
| Precentral gyrus (PMd, BA 6) | L | −36 | −12 | 66 | 9.98 |
| R | 28 | −16 | 64 | 11.3 | |
| CLUSTER#2 – 2,242 voxels | |||||
| Cerebellar hemisphere (IV–V) | L | −20 | −46 | −24 | 8.05 |
| R | 24 | −44 | −26 | 8.56 | |
| Cerebellar vermis (IV–V) | L | −2 | −60 | −16 | 8.4 |
| R | 2 | −48 | −4 | 6.51 | |
| CLUSTER#3 – 475 voxels | |||||
| Parietal operculum (SII, BA 43) | L | −46 | −28 | 22 | 6.88 |
| Superior temporal gyrus (AI, BA 41) | L | −46 | −36 | 24 | 6.8 |
| Supramarginal gyrus (SI, BA 2) | L | −56 | −26 | 36 | 3.92 |
| CLUSTER#4 – 259 voxels | |||||
| IFG (pars opercularis, BA 44) | R | 60 | 10 | 12 | 4.11 |
| CLUSTER#5 – 748 voxels | |||||
| Parietal operculum (SII, BA 43) | R | 46 | −26 | 20 | 6.08 |
| Superior temporal gyrus (AI, BA 41) | R | 58 | −30 | 20 | 5.73 |
| Supramarginal gyrus (SI, BA 2) | R | 54 | −32 | 32 | 5.64 |
| CLUSTER#6 – 21 voxels | |||||
| Precentral gyrus (PMv, BA 6) | R | 58 | 8 | 40 | 5.89 |
| CLUSTER#7 – 31 voxels | |||||
| IFG (pars opercularis, BA 44) | L | −54 | 4 | 0 | 2.75 |
BA, Brodmann area.
With respect to group differences, a main effect of age was found revealing several areas that were significantly more active with age (represented in red on Fig. 3; peak coordinates in Table II). These areas included more extensive activation of the SMA and areas along the left and right lateral fissures (SII, IFG pars opercularis), as well as unique activations in bilateral middle cingulate cortex, secondary auditory area (AII), left inferior parietal cortex (IPC), and right DLPFC. No areas that showed significantly more activity in young compared with old subjects were found.
Table II.
Areas with significant overactivation in old versus young individuals during bimanual coordination tasks compared with rest
| Activation peak location | Side | x | y | z | t‐value |
|---|---|---|---|---|---|
| CLUSTER#1 – 517 voxels | |||||
| Superior frontal gyrus (SMA, BA 6) | L | −10 | −18 | 64 | 3.18 |
| R | 12 | −20 | 66 | 3.87 | |
| CLUSTER#2 – 313 voxels | |||||
| Parietal operculum (SII, BA 43) | L | −60 | −28 | 28 | 4 |
| Superior temporal gyrus (AII, BA 22) | L | −66 | −16 | 12 | 4.7 |
| CLUSTER#3 – 120 voxels | |||||
| Parietal operculum (SII, BA 43) | R | 68 | −12 | 14 | 4.25 |
| Superior temporal gyrus (AII, BA 22) | R | 66 | −14 | 10 | 4.42 |
| CLUSTER#4 – 86 voxels | |||||
| IFG (pars opercularis, BA 44) | L | −58 | 12 | 2 | 4.22 |
| CLUSTER#5 – 196 voxels | |||||
| IFG (pars opercularis, BA 44) | R | 62 | 20 | 4 | 3.96 |
| CLUSTER#6 – 78 voxels | |||||
| Inferior parietal cortex (BA 40) | L | −40 | −44 | 52 | 3.28 |
| CLUSTER#7 – 69 voxels | |||||
| Middle frontal gyrus (DLPFC, BA 46) | R | 34 | 34 | 38 | 3.57 |
| CLUSTER#8 – 48 voxels | |||||
| Middle cingulate cortex (BA 23) | L | −2 | −16 | 44 | 3.44 |
| R | 4 | −8 | 42 | 3.19 |
Modulated neural activity in accordance with movement frequency
Parametric analyses revealed several areas that linearly modulated activity with changes in movement frequency (see Fig. 4). A main effect of task was again not evident and so the results pertain to both the in‐phase and antiphase tasks. Compared with rest, parametrically modulated activations common to both young and old individuals (represented in yellow in Fig. 4; peak coordinates in Table III) involved a variety of motor execution areas. These included left and right SMI, SMA, PMd, cerebellum, and left middle cingulate cortex. Only one group difference was found between young and old subjects whereby slightly more extensive modulation of SMA (represented in red in Fig. 4; peak coordinates: x = −4, y = −20, z = 64; t = 3.19) was seen in old compared with young individuals.
Figure 4.
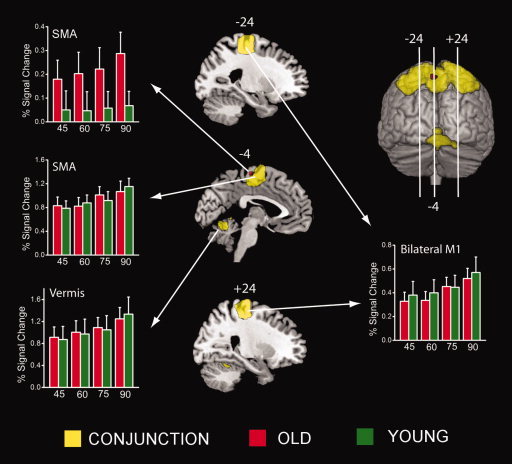
Areas showing parametric modulation of brain activity in accordance with changes in movement frequency. Similar responses for young and old individuals (common) are shown in yellow, whereas modulation by elderly, but not young, is depicted in red. Bar plots represent BOLD responses measured in percent signal change across tasks for old (red) and young (green) subjects in each frequency (45, 60, 75, 90% critical) condition. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Correlations between areas more active in the elderly and coordination performance
Based on the regression analysis, two clusters were identified in old subjects that demonstrated a positive relationship between coordinative ability and degree of activation. In this case, these areas were specific to the more complex antiphase versus in‐phase task condition. As shown in Figure 5, the clusters were located in SMA (peak coordinates: x = −4, y = −22, z = 72; t = 2.41) and SII (peak coordinates: x = −60, y = −26, z = 30; t = 1.98), respectively. Post‐hoc correlations involving PSC and task performance demonstrated that the strength of this relationship was r = 0.57 (P < 0.01) for SMA and r = 0.69 (P < 0.01) for SII. No significant brain–behavior correlations were observed for young individuals.
Figure 5.
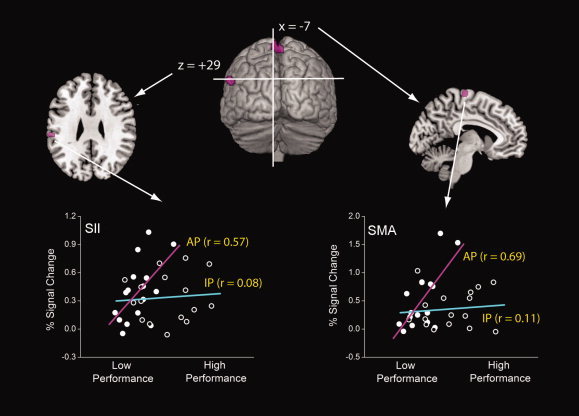
Areas demonstrating a positive relationship between activation and performance in elderly subjects during the antiphase task. Cross‐plots show strength of relationship within the in‐phase (cyan) and antiphase (violet) conditions. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This study represents the first definitive analysis of age‐related differences in the neural activation patterns underlying bimanual movements. By normalizing movement frequencies across age groups, it was shown that, even when coordination performance was similar for young and old individuals, elderly brain function was characterized by greater age‐related activation in multiple motor, sensory, and cognitive regions. These areas included, importantly, activity in SMA and left SII that correlated positively with performance in the more complex antiphase task condition and suggest a compensatory recruitment strategy. Additionally, parametric brain responses associated with increased movement frequency showed that old and young subjects have similar capacities for modulating brain activity with altered movement frequency demands. This unexpected and encouraging result indicates that greater age‐related brain activity does not preclude frequency‐induced neural modulation as a function of increased task demand in the elderly.
Areas of Increased Activation for Elderly Bimanual Movements
The in‐phase and antiphase tasks utilized in this study represent base patterns of coordination for many activities of daily living such as opening a box, swimming, dancing, or swinging one's arms during gait. As such, our results provide important information regarding age‐related differences in the neural control of bimanual movement and the general “resiliency features” that support it. More specifically, it appears that bimanual coordination in the elderly is accomplished through age‐related activation in regions that span the motor, sensory, and cognitive domains.
From a motor standpoint, brain areas along the medial wall (SMA and cingulate motor areas) seem particularly relevant because these regions appear to play a vital role in the temporal organization and execution of bimanual tasks [Carson,2005; Paus,2001; Swinnen,2002; Swinnen and Wenderoth,2004]. Indeed, early studies involving nonhuman primates have shown that SMA lesions lead to enduring deficits in the ability to use the arms in a cooperative fashion [Brinkman,1981,1984]. More recently, medical imaging studies contrasting unimanual versus bimanual tasks have shown that SMA [Debaere et al.,2004; Goerres et al.,1998; Immisch et al.,2001; Sadato et al.,1997; Stephan et al.,1999; Toyokura et al.,1999,2002; Ullen et al.,2003; Wenderoth et al.,2004] and cingulate motor areas [Debaere et al.,2004; Immisch et al.,2001; Stephan et al.,1999; Ullen et al.,2003; Wenderoth et al.,2005] are more extensively involved during bimanual movements. These activations are especially striking during bimanual tasks requiring increased levels of coordination complexity.
In addition to the medial motor areas, old adults showed greater activation in various sensory feedback processing regions along lateral sulci of the left and right hemispheres. These activations included the regions of SII, AII, and the frontal opercula. Activation in SII, as defined by Eickhoff et al. [2006], is known to have multiple somatosensory feedback functions including the perception of movement‐related proprioceptive feedback [Naito et al.,2005,2007]. Given that movements in this study were made without visual guidance, SII activation, therefore, likely reflected greater proprioceptive monitoring of the hands by elderly subjects to maintain the desired coordination patterns. Similarly, increased activation in AII may also reflect increased monitoring of sensory information but, in this case, related to the auditory modality (i.e., metronome pacing). Although AII has traditionally been associated with verbal information processing, recent studies have clearly demonstrated a role for this area in other aspects of audition including, for example, memory for pitch [Gaab et al.,2003,2006]. In accord with SII and AII activations, more extensive activation during coordinated movement was seen for older individuals in the frontal opercula. This region is thought to play an important role in the higher‐order processing of auditory information [Bamiou et al.,2003; Platel et al.,1997; Thaut,2003], particularly as it relates to the synchronization of movement to an auditory tone [Lewis et al.,2004; Thaut,2003]. Taken together, the overactivated areas described above suggest a greater reliance on sensory information processing mechanisms for elderly subjects to perform rhythmic bimanual actions.
Old individuals in this study also showed overactivations in brain areas typically ascribed to more cognitive aspects of performance. Greater activation for old adults in DLPFC and IPC, for example, have previously been implicated as part of a compensatory frontoparietal network in tasks requiring visual attention [Cabeza et al.,2004, Grady et al.,1994; Madden et al.,1997,2007]. Further, right DLPFC has received increasing attention in the aging literature due to its likely involvement in memory retrieval [D'Esposito et al.,1999; Rypma and D'Esposito,2000] and the formation of action‐oriented representations derived from sensory information [Jueptner et al.,1997; Toni and Passingham,1999; Toni et al.,2001]. In the latter case, DLPFC activation is thought to reflect “attention to action,” as activity is decreased in this area when task performance becomes automated. With respect to left IPC, greater activation for old adults in this area was in a similar location as that described by Rushworth et al. [1997,2001a,b] for motor attentional processes. Such studies have provided convincing evidence that activity in more anterior regions of left parietal cortex relate to ongoing monitoring of actions to be performed, in the absence of any (visual) orienting cues. To this extent, movements made by elderly individuals in this study appear to have necessitated greater mental effort than those of young subjects.
The greater bilateral activations found in inferior frontal gyri (p. opercularis) for elderly subjects during bimanual coordination might also reflect a cognition‐based difference between young and old subjects. Specifically, it has been shown that a similar pattern of activation is characteristic of response inhibition tasks such as “go/no go” [Langenecker and Nielson,2003; Nielson et al.,2002] and “Stroop” [Langenecker et al.,2004]. In this case, young and elderly individuals activate comparable regions across the brain when presented with a response conflict. However, old individuals have greater, and sometimes more extensive, activations in frontal regions including, especially, the left and right inferior frontal gyri.
Age‐Resistant Parametric Responses to Altered Movement Frequency
Contrary to our original hypothesis, young and old subjects showed similar responses to changes in movement frequency with only slightly more extensive SMA activation for elderly individuals. Although the relationship between neural activation and frequency‐related movement parameters has been explored in depth for young subjects [Blinkenberg et al.,1996; Deiber et al.,1999; Jancke et al.,1998a,b; Rao et al.,1996; Sadato et al.,1996; Schlaug et al.,1996; Turner et al.,2003; Van Meter et al.,1995], little is known regarding older individuals. In the only other aging study assessing changes in brain activation with movements of increasing speed [Riecker et al.,2006] it was also shown that old and young subjects did not differ in brain areas demonstrating frequency‐related neural modulation. The study by Riecker et al. [2006] was restricted, however, to young and elderly subjects who could perform finger tapping movements at an extremely high rate (6 Hz, one tap every 167 ms). In contrast, the frequency normalization procedure utilized in this study likely allowed for a more representative sample of the elderly population to be tested. In this case, it appears that this aspect of elderly neural function is a relatively robust phenomenon and future research is no doubt necessary to determine the extent to which this relatively age‐resistant aspect of neural function extends beyond the sensorimotor domain.
Age‐Related Increases in Activation as a Compensatory Mechanism for Bimanual Movements
Although overactivation itself may reflect a general form of neural compensation in the elderly, positive correlations between task performance and areas of increased activation for old adults in SMA and left SII provide the clearest evidence of a compensatory strategy employed by elderly individuals. As previously stated, SMA is known to be a critical area for bimanual coordination (especially during tasks requiring increased coordination demands), and therefore, it is perhaps not surprising that greater recruitment of this area resulted in improved antiphase coordination. With respect to SII, its role in higher‐order processing of somatosensory function also makes it a feasible substrate for enhancing bimanual movement performance, given that the tasks performed were proprioceptively guided.
It is worthy of note that evidence now exists in young subjects that proprioceptive processing in SII is relatively lateralized to the right hemisphere—a phenomenon which may underlie contralateral left arm advantages for proprioceptive arm position monitoring [Goble and Brown,2007,2008a,b,c,2009; Goble et al.,2005,2006,2009a]. A similar asymmetry was also seen for old adults in this study (right S2 PSC = 0.80; left S2 PSC = 0.39), which might allow for a parallel to be drawn between our findings and the hemispheric asymmetry reduction in old adults (i.e., HARold) model of aging [Cabeza,2002; Cabeza et al.,2002; Dolcos et al.,2002]. In this case, known deficits in the proprioceptive sensibility of elderly individuals [Goble et al.,2009b] may have been compensated for in successfully performing elderly by a reduction in the degree of right lateralized S2 activity. However, given that our experiment was not designed to address such questions of laterality, some caution should be exercised with respect to the interpretation of this result.
Additional Considerations and Future Work
Although a tendency was noted for greater neural activation in antiphase versus in‐phase movement conditions, statistical significance was not achieved at the rather stringent threshold selected. The lack of differences seen may simply be related to the imposed movement frequency requirements in this study, which dictated that movements be performed at stable, subcritical speeds. This design was necessary to equate subject performance and to ensure the coordination tasks of interest were performed correctly, while maintaining the desired target frequencies. In future work, it may be interesting to look at critical and/or supracritical speeds to elucidate activations related to a transition from the antiphase to in‐phase movement. Despite this caveat, however, task‐related differences were evident in kinematic measures of movement performance (i.e., phase accuracy/stability) and in the analysis of compensatory overactivation. Interestingly, if absolute frequencies were to be maintained across all subjects, it is most likely that the age‐related increase in activation reported here would actually have been even greater and more extensive in nature. Similarly, an underestimation of overactivation may also have occurred based on age‐related differences in neurovascular coupling. Indeed, although old subjects are known to have a similar peak BOLD response as young subjects, it has been shown to be more variable and of longer duration [D'Esposito et al.,1999].
Study Relevance
It is important to investigate bimanual coordination from both fundamental and clinical perspectives. On the one hand, the principles governing interlimb coordination are not simple extrapolations of those obtained in single‐limb movements. This has been shown for several pathological populations who can easily perform tasks involving a single arm, but have great difficulty when performing activities with both arms [Swinnen,2002]. On the other hand, there have been increasing attempts to use bimanual synergies as a tool for the training and treatment of individuals with unilateral sensorimotor dysfunction including, especially, individuals with hemiparetic stroke. For example, using active–passive bilateral therapy, Stinear et al. [2008] were recently able to demonstrate that a relative “rebalancing” of the affected and less affected hemispheres is possible using 10–15 min of passive bilateral movement prior to active movement training. In addition, it has been shown on a number of occasions that it is possible to exploit the inherent neural coupling underlying bimanual movement in such a way that simultaneous movement of the unaffected arm with the affected arm can improve the level of affected arm performance [Cauraugh et al.,2009; Goble,2006; Stewart et al.,2006; Summers et al.,2007]. Given the promise of this work, studies such as the present one will assist in further informing researchers of the neural status underlying bimanual coordination ability under normal aging conditions.
CONCLUSIONS
Bimanual coordination is a frequent and necessary component of many activities of daily living [Swinnen,2002; Swinnen and Wenderoth,2004], and declines in bimanual performance are characteristic of old versus younger individuals [Lee et al.,2002; Serrien et al.,1996,2000; Swinnen et al.,1998; Wishart et al.,2000]. This study provided the first known assessment of the neural correlates associated with bimanual coordination in the elderly. Age‐related increases in activation, observed in old compared with young subjects, extend recent work showing that complex movements require greater neural resources with aging, including those related to sensorimotor and cognitive functions. Positive correlations between the SMA and left SII with movement performance demonstrate that age‐associated increases in neural activity are, at least in part, compensatory for bimanual tasks. A surprising, yet encouraging, finding was the ability of old adults to modulate neural activity with changes in movement frequency to a similar degree as their young counterparts. Overall, it appears from these results that aging is associated with a shift from more automatic to feedback‐dependent control of bimanual movements. This may come at the cost of performing other, nonmotor activities, as has been evident in various dual‐tasking paradigms (e.g., Doumas et al.,2008,2009, Heuninckx et al.,2004]. In this way, our results have both theoretical as well as clinical relevance to the field of gerontology.
REFERENCES
- Andersson JL,Hutton C,Ashburner J,Turner R,Friston K ( 2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–919. [DOI] [PubMed] [Google Scholar]
- Bamiou DE,Musiek FE,Luxon LM ( 2003): The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev 42: 143–154. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M,Bonde C,Holm S,Svarer C,Andersen J,Paulson OB,Law I ( 1996): Rate dependence of regional cerebral activation during performance of a repetitive motor task: A PET study. J Cereb Blood Flow Metab 16: 794–803. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton JL,Valabregue R,Poline JB ( 2002): Region of interest analysis using an SPM toolbox. Presented at the Eighth International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June 2–6.
- Brinkman C ( 1981): Lesions in supplementary motor area interfere with a monkey's performance of a bimanual coordination task. Neurosci Lett 27: 267–270. [DOI] [PubMed] [Google Scholar]
- Brinkman C ( 1984): Supplementary motor area of the monkey's cerebral cortex: Short‐ and long‐term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R,Anderson ND,Locantore JK,McIntosh AR ( 2002): Aging gracefully: Compensatory brain activity in high‐performing older adults. Neuroimage 17: 1394–1402. [DOI] [PubMed] [Google Scholar]
- Cabeza R,Daselaar SM,Dolcos F,Prince SE,Budde M,Nyberg L ( 2004): Task‐independent and task‐specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375. [DOI] [PubMed] [Google Scholar]
- Calautti C,Serrati C,Baron JC ( 2001): Effects of age on brain activation during auditory‐cued thumb‐to‐index opposition: A positron emission tomography study. Stroke 32: 139–146. [DOI] [PubMed] [Google Scholar]
- Carson RG ( 2005): Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev 49: 641–662. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH,Coombes SA,Lodha N,Naik SK,Summers JJ ( 2009): Upper extremity improvements in chronic stroke: Coupled bilateral load training. Restor Neurol Neurosci 27: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ,Kramer AF,Erickson KI,Scalf P ( 2005): The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging 20: 363–375. [DOI] [PubMed] [Google Scholar]
- Daselaar SM,Rombouts ARB,Veltman DJ,Raaijmakers JGW,Jonker C ( 2003): Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Age 24: 1013–1019. [DOI] [PubMed] [Google Scholar]
- Debaere F,Wenderoth N,Sunaert S,Van Hecke P,Swinnen SP ( 2004): Cerebellar and premotor function in bimanual coordination: Parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21: 1416–1427. [DOI] [PubMed] [Google Scholar]
- Deiber MP,Honda M,Ibanez V,Sadato N,Hallett M ( 1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J Neurophysiol 81: 3065–3077. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Zarahn E,Aguirre GK,Rypma B ( 1999): The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10: 6–14. [DOI] [PubMed] [Google Scholar]
- Dolcos F,Rice HJ,Cabeza R ( 2002): Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26: 819–825. [DOI] [PubMed] [Google Scholar]
- Doumas M,Smolders C,Krampe RT ( 2008): Task prioritization in aging: Effects of sensory information on concurrent posture and memory performance. Exp Brain Res 187: 275–281. [DOI] [PubMed] [Google Scholar]
- Doumas M,Rapp MA,Krampe RT ( 2009): Working memory and postural control: Adult age differences in potential for improvement, task priority and dual tasking. J Gerontol B Psychol Sci Soc Sci 64: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB,Amunts K,Mohlberg H,Zilles K ( 2006): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279. [DOI] [PubMed] [Google Scholar]
- Folstein MF,Folstein SE,McHugh PR ( 1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Poline JB,Grasby PJ,Williams SC,Frackowiak RS,Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Rotshtein P,Geng JJ,Sterzer P,Henson RN ( 2006): A critique of functional localisers. Neuroimage 30: 1077–1087. [DOI] [PubMed] [Google Scholar]
- Gaab N,Gaser C,Zaehle T,Jancke L,Schlaug G ( 2003): Functional anatomy of pitch memory—An fMRI study with sparse temporal sampling. Neuroimage 19: 1417–1426. [DOI] [PubMed] [Google Scholar]
- Gaab N,Gaser C,Schlaug G ( 2006): Improvement‐related functional plasticity following pitch memory training. Neuroimage 31: 255–263. [DOI] [PubMed] [Google Scholar]
- Goble DJ ( 2006): The potential for utilizing inter‐limb coupling in the rehabilitation of upper limb motor disability due to unilateral brain injury. Disabil Rehabil 28: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Brown SH ( 2007): Task‐dependent asymmetries in the utilization of proprioceptive feedback for goal‐directed movement. Exp Brain Res 180: 693–704. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Brown SH ( 2008a): Reply to Dr. Derakhshan. J Neurophysiol 100: 3459. [Google Scholar]
- Goble DJ,Brown SH ( 2008b): The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neurosci Biobehav Rev 32: 598–610. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Brown SH ( 2008c): Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol 99: 3063–3074. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Brown SH ( 2009): Dynamic proprioceptive target matching behavior in the upper limb: Effects of speed, task difficulty and arm/hemisphere asymmetries. Behav Brain Res 200: 7–14. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Lewis CA,Hurvitz EA,Brown SH ( 2005): Development of upper limb proprioceptive accuracy in children and adolescents. Hum Mov Sci 24: 155–170. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Lewis CA,Brown SH ( 2006): Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res 168: 307–311. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Hurvitz EA,Brown SH ( 2009a): Deficits in the ability to use proprioceptive feedback in children with hemiplegic cerebral palsy. Int J Rehabil Res 32: 276–279. [DOI] [PubMed] [Google Scholar]
- Goble DJ,Coxon JP,Wenderoth N,Van Impe A,Swinnen SP ( 2009b): Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic‐adaptive processes. Neurosci Biobehav Rev 33: 271–278. [DOI] [PubMed] [Google Scholar]
- Goerres GW,Samuel M,Jenkins IH,Brooks DJ ( 1998): Cerebral control of unimanual and bimanual movements: An H2(15)O PET study. Neuroreport 9: 3631–3638. [DOI] [PubMed] [Google Scholar]
- Grady CL ( 2000): Functional brain imaging and age‐related changes in cognition. Biol Psychol 54: 259–281. [DOI] [PubMed] [Google Scholar]
- Grady CL ( 2008): Cognitive neuroscience of aging. Ann NY Acad Sci 1124: 127–144. [DOI] [PubMed] [Google Scholar]
- Grady CL,Maisog JM,Horwitz B ( 1994): Age‐related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14: 1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S,Debaere F,Wenderoth N,Verschueren S,Swinnen SP ( 2004): Ipsilateral coordination deficits and central processing requirements associated with coordination as a function of aging. J Gerontol B Psychol Sci Soc Sci 59: 225–232. [DOI] [PubMed] [Google Scholar]
- Heuninckx S,Wenderoth N,Debaere F,Peeters R,Swinnen SP ( 2005): Neural basis of aging: The penetration of cognition into action control. J Neurosci 25: 6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S,Wenderoth N,Swinnen SP ( 2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S,Kobayashi M,Horkan CM,Pascual‐Leone A,Alexander MP,Schlaug G ( 2002): Age‐related differences in movement representation. Neuroimage 17: 1720–1728. [DOI] [PubMed] [Google Scholar]
- Immisch I,Waldvogel D,van GP,Hallett M ( 2001): The role of the medial wall and its anatomical variations for bimanual antiphase and in‐phase movements. Neuroimage 14: 674–684. [DOI] [PubMed] [Google Scholar]
- Jancke L,Peters M,Schlaug G,Posse S,Steinmetz H,Muller‐Gartner H ( 1998a): Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res 6: 279–284. [DOI] [PubMed] [Google Scholar]
- Jancke L,Specht K,Mirzazade S,Loose R,Himmelbach M,Lutz K,Shah NJ ( 1998b): A parametric analysis of the ‘rate effect’ in the sensorimotor cortex: A functional magnetic resonance imaging analysis in human subjects. Neurosci Lett 252: 37–40. [DOI] [PubMed] [Google Scholar]
- Jueptner M,Stephan KM,Frith CD,Brooks DJ,Frackowiak RS,Passingham RE ( 1997): Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Langenecker SA,Nielson KA ( 2003): Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage 20: 1384–1392. [DOI] [PubMed] [Google Scholar]
- Langenecker SA,Nielson KA,Rao SM ( 2004): fMRI of healthy older adults during Stroop interference. Neuroimage 21: 192–200. [DOI] [PubMed] [Google Scholar]
- Lee TD,Wishart LR,Murdoch JE ( 2002): Aging, attention, and bimanual coordination. Can J Aging 21: 549–557. [Google Scholar]
- Lewis PA,Wing AM,Pope PA,Praamstra P,Miall RC ( 2004): Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia 42: 1301–1312. [DOI] [PubMed] [Google Scholar]
- Madden DJ,Turkington TG,Trovenzale JM,Hawk TC,Hoffman JM,Coleman RE ( 1997): Selective and divided visual attention: Age‐related changes in regional cerebral blood flow measured by H2O15 PET. Hum Brain Mapp 5: 389–409. [DOI] [PubMed] [Google Scholar]
- Madden DJ,Spaniol J,Whiting WL,Bucur B,Provenzale JM,Cabeza R,White LE,Huettel SA ( 2007): Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol Aging 28: 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS,Fera F,Tessitore A,Hariri AR,Das S,Callicott JH,Weinberger DR ( 2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58: 630–635. [DOI] [PubMed] [Google Scholar]
- Naito E,Roland PE,Grefkes C,Choi HJ,Eickhoff S,Geyer S,Zilles K,Ehrsson HH ( 2005): Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol 93: 1020–1034. [DOI] [PubMed] [Google Scholar]
- Naito E,Nakashima T,Kito T,Aramaki Y,Okada T,Sadato N ( 2007): Human limb‐specific and non‐limb‐specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci 25: 3476–3487. [DOI] [PubMed] [Google Scholar]
- Nielson KA,Langenecker SA,Garavan H ( 2002): Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging 17: 56–71. [DOI] [PubMed] [Google Scholar]
- Nichols T,Brett M,Andersson J,Wager T,Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–423. [DOI] [PubMed] [Google Scholar]
- Platel H,Price C,Baron JC,Wise R,Lambert J,Frackowiak RS,Lechevalier B,Eustache F ( 1997): The structural components of music perception. A functional anatomical study. Brain 120( Part 2): 229–243. [DOI] [PubMed] [Google Scholar]
- Rao SM,Bandettini PA,Binder JR,Bobholz JA,Hammeke TA,Stein EA,Hyde JS ( 1996): Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 16: 1250–1254. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA,Lustig C ( 2005): Brain aging: Reorganizing discoveries about the aging mind. Curr Opin Neurobiol 15: 245–251. [DOI] [PubMed] [Google Scholar]
- Riecker A,Groschel K,Ackermann H,Steinbrink C,Witte O,Kastrup A ( 2006): Functional significance of age‐related differences in motor activation patterns. Neuroimage 32: 1345–1354. [DOI] [PubMed] [Google Scholar]
- Rinehart JK,Singleton RD,Adair JC,Sadek JR,Haaland KY ( 2009): Arm use after left or right Hemiparesis is influenced by hand preference. Stroke 40: 545–550. [DOI] [PubMed] [Google Scholar]
- Rushworth MF,Nixon PD,Renowden S,Wade DT,Passingham RE ( 1997): The left parietal cortex and motor attention. Neuropsychologia 35: 1261–1273. [DOI] [PubMed] [Google Scholar]
- Rushworth MF,Ellison A,Walsh V ( 2001a): Complementary localization and lateralization of orienting and motor attention. Nat Neurosci 4: 656–661. [DOI] [PubMed] [Google Scholar]
- Rushworth MF,Krams M,Passingham RE ( 2001b): The attentional role of the left parietal cortex: The distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci 13: 698–710. [DOI] [PubMed] [Google Scholar]
- Rypma B,D'Esposito M ( 2000): Isolating the neural mechanisms of age‐related changes in human working memory. Nat Neurosci 3: 509–515. [DOI] [PubMed] [Google Scholar]
- Sadato N,Ibanez V,Deiber MP,Campbell G,Leonardo M,Hallett M ( 1996): Frequency‐dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab 16: 23–33. [DOI] [PubMed] [Google Scholar]
- Sadato N,Yonekura Y,Waki A,Yamada H,Ishii Y ( 1997): Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17: 9667–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G,Sanes JN,Thangaraj V,Darby DG,Jancke L,Edelman RR,Warach S ( 1996): Cerebral activation covaries with movement rate. Neuroreport 7: 879–883. [DOI] [PubMed] [Google Scholar]
- Scholz JP,Kelso JA ( 1989): A quantitative approach to understanding the formation and change of coordinated movement patterns. J Mot Behav 21: 122–144. [DOI] [PubMed] [Google Scholar]
- Serrien DJ,Teasdale N,Bard C,Fleury M ( 1996): Age‐related differences in the integration of sensory information during the execution of a bimanual coordination task. J Mot Behav 28: 337–347. [DOI] [PubMed] [Google Scholar]
- Serrien DJ,Swinnen SP,Stelmach GE ( 2000): Age‐related deterioration of coordinated interlimb behavior. J Gerontol B Psychol Sci Soc Sci 55: 295–303. [DOI] [PubMed] [Google Scholar]
- Stephan KM,Binkofski F,Posse S,Seitz RJ,Freund HJ ( 1999): Cerebral midline structures in bimanual coordination. Exp Brain Res 128: 243–249. [DOI] [PubMed] [Google Scholar]
- Stewart KC,Cauraugh JH,Summers JJ ( 2006): Bilateral movement training and stroke rehabilitation: A systematic review and meta‐analysis. J Neurol Sci 244: 89–95. [DOI] [PubMed] [Google Scholar]
- Stinear CM,Barber PA,Coxon JP,Fleming MK,Byblow WD ( 2008): Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain 131: 1381–1390. [DOI] [PubMed] [Google Scholar]
- Summers JJ,Kagerer FA,Garry MI,Hiraga CY,Loftus A,Cauraugh JH ( 2007): Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. J Neurol Sci 252: 76–82. [DOI] [PubMed] [Google Scholar]
- Swinnen SP ( 2002): Intermanual coordination: From behavioural principles to neural‐network interactions. Nat Rev Neurosci 3: 348–359. [DOI] [PubMed] [Google Scholar]
- Swinnen SP,Wenderoth N ( 2004): Two hands, one brain: Cognitive neuroscience of bimanual skill. Trends Cogn Sci 8: 18–25. [DOI] [PubMed] [Google Scholar]
- Swinnen SP,Verschueren S,Bogaerts H,Dounskaia N,Lee TD,Stelmach GE,Serrien DJ ( 1998): Age‐related deficits in motor learning and differences in feedback processing during the production of a bimanual coordination pattern. Cogn Neuropsychol 15: 439–466. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournaux P ( 1998): Co‐planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Thaut MH ( 2003): Neural basis of rhythmic timing networks in the human brain. Ann NY Acad Sci 999: 364–373. [DOI] [PubMed] [Google Scholar]
- Toni I,Passingham RE ( 1999): Prefrontal‐basal ganglia pathways are involved in the learning of arbitrary visuomotor associations: A PET study. Exp Brain Res 127: 19–32. [DOI] [PubMed] [Google Scholar]
- Toni I,Ramnani N,Josephs O,Ashburner J,Passingham RE ( 2001): Learning arbitrary visuomotor associations: Temporal dynamic of brain activity. Neuroimage 14: 1048–1057. [DOI] [PubMed] [Google Scholar]
- Toyokura M,Muro I,Komiya T,Obara M ( 1999): Relation of bimanual coordination to activation in the sensorimotor cortex and supplementary motor area: Analysis using functional magnetic resonance imaging. Brain Res Bull 48: 211–217. [DOI] [PubMed] [Google Scholar]
- Toyokura M,Muro I,Komiya T,Obara M ( 2002): Activation of pre‐supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: An analysis using functional magnetic resonance imaging. J Neuroimaging 12: 172–178. [DOI] [PubMed] [Google Scholar]
- Turner RS,Desmurget M,Grethe J,Crutcher MD,Grafton ST ( 2003): Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol 90: 3958–3966. [DOI] [PubMed] [Google Scholar]
- Ullen F,Forssberg H,Ehrsson HH ( 2003): Neural networks for the coordination of the hands in time. J Neurophysiol 89: 1126–1135. [DOI] [PubMed] [Google Scholar]
- Van Impe A,Coxon JP,Goble DJ,Wenderoth N,Swinnen SP ( 2009): Ipsilateral coordination at preferred rate: Effects of age, body side and task complexity. Neuroimage: 47: 1854–1862. [DOI] [PubMed] [Google Scholar]
- Van Meter JW,Maisog JM,Zeffiro TA,Hallett M,Herscovitch P,Rapoport SI ( 1995): Parametric analysis of functional neuroimages: Application to a variable‐rate motor task. Neuroimage 2: 273–283. [DOI] [PubMed] [Google Scholar]
- Vega‐Gonzalez A,Granat MH ( 2005): Continuous monitoring of upper‐limb activity in a free‐living environment. Arch Phys Med Rehabil 86: 541–548. [DOI] [PubMed] [Google Scholar]
- Ward NS ( 2006): Compensatory mechanisms in the aging motor system. Ageing Res Rev 5: 239–254. [DOI] [PubMed] [Google Scholar]
- Ward NS,Frackowiak RS ( 2003): Age‐related changes in the neural correlates of motor performance. Brain 126: 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS,Swayne OBC,Newton JM ( 2008): Age‐dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiol Aging 29: 1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N,Debaere F,Sunaert S,Van Hecke P,Swinnen SP ( 2004): Parieto‐premotor areas mediate directional interference during bimanual movements. Cereb Cortex 14: 1153–1163. [DOI] [PubMed] [Google Scholar]
- Wenderoth N,Debaere F,Sunaert S,Swinnen SP ( 2005): The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci 22: 235–246. [DOI] [PubMed] [Google Scholar]
- Wishart LR,Lee TD,Murdoch JE,Hodges NJ ( 2000): Effects of aging on automatic and effortful processes in bimanual coordination. J Gerontol B Psychol Sci Soc Sci 55: 85–94. [DOI] [PubMed] [Google Scholar]
- Wu T,Hallett M ( 2005): The influence of normal human ageing on automatic movements. J Physiol 562: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]


