Abstract
Sleep propensity increases sharply at night. Some evidence implicates the pineal hormone melatonin in this process. Using functional magnetic resonance imaging, brain activation during a visual search task was examined at 22:00 h (when endogenous melatonin levels normally increase). The relationships between brain activation, endogenous melatonin (measured in saliva), and self‐reported fatigue were assessed. Finally, the effects of exogenous melatonin administered at 22:00 h were studied in a double blind, placebo‐controlled crossover manner. We show that brain activation patterns as well as the response to exogenous melatonin significantly differ at night from those seen in afternoon hours. Thus, activation in the rostro‐medial and lateral aspects of the occipital cortex and the thalamus diminished at 22:00 h. Activation in the right parietal cortex increased at night and correlated with individual fatigue levels, whereas exogenous melatonin given at 22:00 h reduced activation in this area. The right dorsolateral prefrontal cortex, an area considered to reflect homeostatic sleep debt, demonstrated increased activation at 22:00 h. Surprisingly, this increase correlated with endogenous melatonin. These results demonstrate and partially differentiate circadian effects (whether mediated by melatonin or not) and homeostatic sleep debt modulation of human brain activity associated with everyday fatigue at night. Hum Brain Mapp, 2009. © 2007 Wiley‐Liss, Inc.
Keywords: melatonin, circadian, homeostat, fMRI, fatigue, night
INTRODUCTION
Consolidated sleep at night and daytime wakefulness are remarkably standardized in humans. These bouts reflect an interaction between the homeostatic sleep debt, that is manifested by increase in sleep propensity after sleep deprivation and decrease during sleep, and the circadian pacemaker [Borbely, 1982], located in the hypothalamic suprachiasmatic nuclei (SCN) [reviewed in Zisapel, 2007]. It has been proposed that a circadian wakefulness‐promoting signal maintains wakefulness in the face of increasing homeostatic sleep debt, thereby consolidating wakefulness during the circadian day [Borbely, 1982, 1998]. A major increase in sleep propensity at night, known as the “opening of the sleep gate”, precedes the onset of the nocturnal sleep period [Lavie, 1986] and most probably reflects the decline in the circadian wakefulness‐promoting signal [Dijk and Lockley, 2002].
Several lines of evidence implicate the pineal hormone melatonin (N‐acetyl‐5‐methoxytryptamine) in the “opening of the sleep gate” at night. Melatonin serves as a time cue (signal of darkness) to the organism [Reiter, 1991]. It is produced during the “nocturnal” phase of the SCN activity and has an inhibitory effect on the SCN [Liu et al., 1997]. The dim light melatonin onset (DLMO; defined as the time of initial surge in melatonin at night under low‐light conditions) is considered a reliable and consistent marker of the internal circadian phase [Lewy, 1999]. The DLMO typically occurs 14 h after spontaneous wakeup which would correspond to 21:00 h in a person who wakes up at 07:00 h [Lewy, 1999] and 2 h before the nocturnal sleep gate [Lewy, 1999; Shochat et al., 1997; Tzischinsky et al., 1993; Wirz‐Justice et al., 2004]. Thus, the circadian rhythm in synthesis and secretion of melatonin is closely associated with the sleep rhythm in both sighted and blind subjects [Lockley et al., 1997; Nakagawa et al., 1992]. Daytime administration of exogenous melatonin (when it is not present endogenously) promotes sleep in humans [Cajochen et al., 2002; Wyatt et al., 2006], presumably by inhibiting circadian wakefulness mechanisms [Liu et al., 1997; Shochat et al., 1997].
In a previous study we demonstrated, using functional magnetic imaging (fMRI), that melatonin administration during afternoon hours (i.e., when endogenous melatonin levels are minimal) resulted in modified brain activity compatible with sleep anticipation [Gorfine et al., 2006]. Particularly, during a visual search task, melatonin caused robust reductions in task‐related activation in the rosto‐medial part of the occipital cortex without modulating activity in the more caudal and lateral parts of the occipital cortex as well as the parietal cortex and the thalamus. Thus, exogenously administered melatonin modulates brain activity while subjects are awake in a manner resembling actual sleep. Notably, reduced activation correlated with increased fatigue (feeling of weariness, tiredness, or lack of energy) and sleepiness (feeling drowsy with a tendency to actually fall asleep) caused by the exogenous melatonin. In contrast, sleep deprivation experiments (employing a variety of visual tasks) have consistently demonstrated changes in activation of the parietal and prefrontal regions but not of the rostro‐medial occipital cortex [Chee and Choo, 2004; Chee et al., 2006; Drummond et al., 1999, 2005]. In other words, experiments performed during actual sleep as well as following melatonin administration, using a variety of tasks including both visual and auditory stimuli, identify a robust change in the rostro‐medial occipital cortex whereas sleep deprivation experiments do not report such an effect. Thus, even considering the differences in tasks used, it is reasonable to assume that there are profound differences in the effects of melatonin and actual sleep and those of sleep deprivation (enforced sleep debt) on brain activation patterns.
This study is designed to explore brain activation at 22:00 h, around the time of the “opening of the sleep gate” at night in reference to brain activation patterns in the afternoon (16:00 h). Furthermore, we attempted to differentiate the role of the circadian system, melatonin (as a downstream mediator of this system) and the normal increase in sleep debt at night in these changes.
We hypothesized that the increase in endogenous melatonin at night will result in reduced activation in the rostro‐medial part of the occipital cortex compared with the afternoon (similar to that demonstrated with the exogenous melatonin in the afternoon). In addition, we hypothesized that the normal increase in homeostatic sleep debt in the evening would cause changes in parietal and prefrontal activations (as demonstrated following sleep deprivation).
Furthermore, it is well established that exogenous melatonin is most effective when endogenous levels are low [e.g., during daytime Anton‐Tay et al., 1971; Lieberman et al., 1984] and in elderly insomnia patients [Leger et al., 2004; Zisapel, 2001a] presumably because the presence of the endogenous hormone obviates its effects. Likewise, night‐time melatonin administration has been shown to have no effect on self‐rated fatigue, sleep latency, sleep consolidation, sleep efficiency, and the spectral composition of sleep EEG [Cajochen et al., 2003; Nickelsen et al., 1989; Pires et al., 2001; Stone et al., 2000; Wyatt et al., 2006]. We thus hypothesized that while exogenous melatonin effectively affects behavior and brain activation when given during the day, it will not do so at night in excess of the effects of the endogenous hormone.
To test these hypotheses we conducted an fMRI study assessing subjective fatigue and sleepiness and brain activation at night as compared with those found in the afternoon. Endogenous melatonin levels, measured in saliva, were used to evaluate the contribution of the endogenous hormone to the changes in brain activation and in addition, as a surrogate marker of internal biological night.
It is important to note that a certain variability in the internal (free‐running) period of the circadian clock exists even among healthy individuals [Czeisler et al., 1999]. This will translate into intersubject variability in the internal biological time when subjects are entrained to the 24‐h light–dark cycle and consequently in the timing of melatonin onset and sleep [Wright et al., 2005]. We therefore aimed to use the intersubject variability in internal biological time to distinguish the effects of melatonin per se (which would be expected to correlate with the endogenous melatonin levels and/or be demonstrated upon administration of the exogenous hormone) and the circadian system (which would be expected to differ between subjects who have crossed and not crossed the DLMO at the time of observation, but not correlate with endogenous melatonin levels).
DLMO has been defined as the first interpolated value to exceed a fixed threshold of 10 pg/ml in plasma [Lewy, 1999]. Recent work has shown that saliva melatonin levels reliably reflect the plasma profile of the hormone and correspond to 30% of serum melatonin levels, and the salivary DLMO was thus defined by a 3‐pg/ml threshold crossing [Wirz‐Justice et al., 2004] While subjects who are low‐melatonin secretors might attain any predefined threshold melatonin levels later than the actual melatonin onset, the 3‐pg/ml salivary melatonin threshold crossing is useful to identify those who already make significant amounts of melatonin at the time of observation. Thus, we compared the change in nocturnal brain activation between subjects who crossed and did not cross the predefined 3‐pg/ml salivary melatonin threshold at 22:00 h. Correlation between individual endogenous melatonin levels and brain activation was also examined. Finally, the effects of melatonin administration at night on fatigue and brain activity were assessed in a double blind, placebo controlled manner.
METHODS
Subjects
Fourteen subjects (9 men, 5 women, body mass 23.2 ± 2 kg/m2; age 26 ± 2 year) were enrolled. The selection was based on an interview with the investigator in which specific questions addressed sleep habits and travel history of the subjects as well as history of sleep and mood disorders. All subjects who were entered into the study reported on habitual night sleep from 22:00 to 24:00 until 06:00–08:00 h. None had any sleep disorders or recent disturbances in the sleep–wake cycle. Other exclusion criteria were reports of psychiatric disorders, medication or drug consumption, and alcohol abuse.
The study protocol was approved by the Tel Aviv Medical Center Human Sciences Ethics Committee. All subjects received a detailed explanation of the study and gave written informed consents.
As a reference group, we used the baseline data obtained in the course of the study on the effects of melatonin versus placebo given at 16:00 h (2 men, 10 women, body mass 21.3 ± 2.3 kg/m2; age 26 ± 5 year) [Gorfine et al., 2006].
Experimental Design
Subjects were instructed to abstain from alcohol for 24 h, maintain a regular sleep–wake schedule (sleep 23:00–07:00 h) on the night prior to the trial, and abstain from caffeine during the trial. The study had a double‐blind, crossover design, balanced according to the order of treatment. Each subject attended two trials (melatonin and placebo), separated by at least 10 days.
Baseline measurements, which were intended for the evaluation of the effect of time of day on brain activation, were done for the first time the subject performed the task in the magnet to avoid practice related changes. Each trial lasted 2–3 h and comprised two fMRI sessions, one before (baseline) and the second 1 h after (treatment) drug administration to allow the ingested melatonin to reach maximal levels in the blood. Melatonin (2 mg in 100 ml of 1% ethanol in water) or placebo (100 ml of 1% ethanol in water) was given orally by drinking. After given melatonin or placebo, subjects were instructed to stay awake in the ambient dim light room (<300 lux).
Psycho‐Behavioral Assessment
Fatigue and sleepiness were assessed using the Bond‐Lader visual analogue questionnaire previously used to demonstrate fatigue‐inducing effects of melatonin in humans [Gorfine et al., 2006; Terlo et al., 1997]. This is a self‐report form that includes 16, 10‐cm visual analogue scales and provides the subjective ratings of mood fatigue and sleepiness. Questionnaires were filled twice in each trial, once before the baseline fMRI session and once after the treatment (melatonin or placebo) session.
Saliva Melatonin Levels
Saliva specimens were collected at 22:00 h using the Sali‐Saver (American Laboratory Products Company, Windham, NH). Saliva melatonin was determined by a Direct Saliva Melatonin Enzyme Linked Immunoabsorbent Assay kit from Buhlman Laboratories (Allschwil, Switzerland). Aliquots of at least 200 μl of centrifuged saliva from each collection time were used for the analysis. Assay functional sensitivity was 1.3 pg/ml, and the maximum intra‐ and interassay coefficients of variability were 6.5% (n = 12) and 11.3% (n = 12), respectively (in the range of concentrations of melatonin between 1 and 81 pg/ml).
Experimental Task
A visual search task (“pop out,” target detection) was used (see Fig. 1). Subjects were presented with a matrix of 20 half‐circular forms with only one form pointing in the opposite direction (the outlier). Task block included five sets of such matrices. Each matrix appeared for 1 s followed by 0.8‐s fixation. Subjects were asked to attempt to identify the outlier in each matrix. The protocol was block designed with six task blocks of 9 s interleaved with fixation baseline blocks of either 6 or 9 s.
Figure 1.
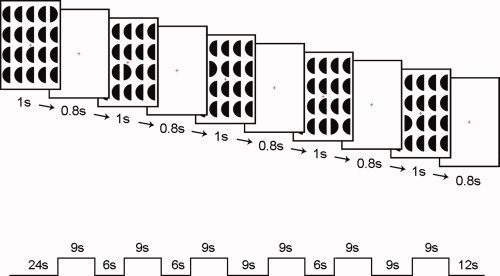
Visual search task. Schematic illustration showing task matrices used and presentation timing. In the first matrix, in this example, the outlier to be identified is located at the right upper corner. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Image Acquisition
Imaging was performed on a 1.5T GE MRI system. Functional imaging employs a gradient‐echo, echo planar imaging (EPI) sequence. Parameters were TR = 3,000 ms, TE = 50 ms, 27 4‐mm thick axial slices with no gaps, acquisition matrix dimensions 80 × 80 (reconstructed to 128 × 128), field of view 240 × 240 mm2 (acquired resolution of 3 × 3 × 4 mm3). For each subject a 3D T1 SPGR sequence was performed for anatomical overlay. Acquisition parameters for the SPGR were as follows: TR/TE = 30/9 ms, flip angle of 15° with resolution of 0.93 × 0.93 × 2 mm3.
Data Analysis
Behavioral Variables
Subjective parameters of fatigue and sleepiness were measured by the Bond‐Lader visual analogue scales. At 22:00 h, fatigue and sleepiness (previously shown to be increased following melatonin intake) were compared between subjects with endogenous melatonin levels above and below the 3‐pg/ml salivary melatonin threshold level and correlated with individual levels of melatonin. Statistical analysis used a two‐tailed, unpaired, Student's t‐test. Significance was set at P < 0.05.
For the analysis of effect of exogenous melatonin administration at night, the mean differences (delta) between baseline and treatment values in the melatonin and placebo trials were compared using a two‐tailed, paired, Student's t‐test.
Image Processing
Analysis was performed using BrainVoyager 4.9 and QX software (Brain Innovation, Masstricht, The Netherlands).
Preprocessing included 3D motion correction, linear trend removal, and high‐pass filtering (filtering out up to three cycles in time course). The first six functional volumes, before signal stabilization, were excluded from analysis. Functional 2D data was manually aligned and coregistered with 3D anatomical data and transformed into Talairach space. The Talairach coordinates were in the LPI convention (right hemisphere has positive X values and the anterior part has positive Y values). Spatial smoothing (Gaussian 4‐mm kernel) was applied.
Task‐related activation was defined in correlation to predictors of the used protocol. To account for a hemodynamic response, predictors were shifted one or two TRs. The shift was selected to result in maximized correlation between individual blood oxygen level dependant (BOLD) signal intensity time course and predictors. A separate predictor for the mean of each study (confound predictor) was added to account for differences in the mean level of signal time course at a voxel between studies. Z‐normalization of the time course of each study was performed to address differences in the variance of voxel time courses between studies.
Time of Day Effects on Brain Activity
Two separate multisubject general linear models (GLM) were designed. One included the 14 subjects from the night trial and the other included the 12 subjects of the reference group (afternoon trial). Group activation maps were generated using random effect and false discovery rate (FDR) correction for multiple comparisons, P‐corrected < 0.01. Activation maps were directly compared and any region demonstrating a difference (e.g., task‐related activation at 16:00 but not 22:00 h) was quantitatively analyzed using individual parameter estimated (regression coefficients) from the clusters identified. This analysis compared individual parameter estimates (average regression coefficients) from the identified cluster as well as a similar cluster in the complementary map.
Brain regions that demonstrated task‐related activity at 22:00 but not 16:00 h, were further analyzed. This analysis aimed to investigate whether the demonstrated effect could be related to circadian phase or melatonin levels. Thus, we compared parameter estimates between the night subgroups (subjects with above and below melatonin threshold levels) and performed a correlation analysis between individual subjects' melatonin levels and corresponding brain activation parameters obtained at 22:00 h. In addition, a correlation analysis was performed between parameter estimates derived from these brain regions and individual self‐reported fatigue.
All statistical analysis used a two‐tailed, unpaired, Student's t‐test. Significance was set at P < 0.05.
Effect of Exogenous Melatonin Administered at Night
The GLM model included all trials: before melatonin intake; after melatonin intake; before placebo intake; after placebo intake (14 subjects in a crossover design, altogether 56 sessions). Drug effect was defined by a conjunction analysis of two contrasts: activation after melatonin intake > activation before melatonin intake and activation after melatonin intake > activation after placebo intake. This analysis identifies regions that are affected by melatonin, while excluding changes resulting from placebo or second examination effects. In addition, this analysis identifies regions that show greater activation after melatonin but not placebo intake and regions that show lesser activation after melatonin but not placebo intake. A hypothesis‐driven region of interest (ROI) analysis was performed using a lenient statistical threshold set at P < 0.05. A cluster size of 50 continuous voxels was included. No other correction for multiple comparisons was used. The use of a lenient P‐value maximizes statistical power so as to minimize the risk for a negative finding (as is our hypothesis regarding the lack of drug effect at night). It also allows an accurate evaluation of decreased as opposed to abolished task‐related activation. In agreement, with our hypothesis and the designed task our ROI included the visual and parietal cortices as well as the thalamus. Brain regions within ROI demonstrating melatonin but not placebo effect were further analyzed using individual parameter estimates. Drug effect, placebo effect, and the differences (delta) between baseline and treatment values in melatonin compared with placebo trials were examined using a two‐tailed, paired, Student's t‐test. Significance was set at P < 0.05. In addition, an effect of melatonin (when demonstrated) was further verified using two separate GLM for pre‐ and postmelatonin sessions using random effect and FDR correction.
RESULTS
Melatonin Levels at 22:00 h
Eight of the 14 subjects in the night group had salivary melatonin above melatonin threshold levels at 22:00 h (Mean + SD 6.4 ± 2.3 pg/ml; range 3.3–13.8 pg/ml). The other six subjects had lower than melatonin threshold levels at this time (1 ± 0.6 pg/ml; range < 0.5–1.5 pg/ml).
Psycho‐Behavioral Variables
The comparison of psycho‐behavioral variables between subjects who crossed and did not cross the melatonin threshold level at 22:00 h are depicted in Table I. At 22:00 h subjects who have crossed the melatonin threshold level reported greater fatigue when compared with those who have not crossed the threshold (Mean + SD 6 + 1.8 vs. 2.7 + 1.7 cm; P = 0.005). Sleepiness (as well as all other psycho‐behavioral variables, except for a slight increase in feeling of calmness), was not significantly different between these groups (Table I). To further examine the above result, fatigue level at 22:00 h in the total group and in the subgroup of subjects with higher than melatonin threshold levels was compared with the mean level measured in the reference group (16:00 h). Mean subjective sleepiness, fatigue, and mood parameters at 22:00 h were not statistically different from those reported at 16:00 h. However, those subjects that have crossed the melatonin threshold at 22:00 h do report significantly greater fatigue relative to subjects studied at 16:00 h (Mean + SD 6 + 1.8 vs. 3.4 + 2.2 cm; P = 0.01). It should be noted that for subjects with below melatonin threshold levels at 22:00 h, self‐reported fatigue levels subsequently increased (as assessed at 24:00 h both during melatonin and placebo trials), thus ruling out that these subjects had problems in perceiving fatigue.
Table I.
Comparison of Psycho‐Behavioral Variables Between Subjects who Crossed and Did Not Cross the Melatonin Threshold at 22:00 h
| Bond‐Lader parameter | Mean + SD cm at 22:00 h | P (t‐test) | |
|---|---|---|---|
| Below threshold melatonin levels N = 6 | Above threshold melatonin levels N = 8 | ||
| Sleepy–alert | 6.2 + 2.1 | 5.8 + 2 | 0.7 |
| Excited–calm | 8 + 1.3 | 7.5 + 1.3 | 0.04 |
| Weak–vigor | 7.7 + 1.3 | 6.5 + 1.5 | 0.2 |
| Lucid–confused | 0.9 + 1.2 | 2.5 + 3 | 0.2 |
| Clumsy–well coordinated | 6.2 + 2.2 | 7.9 + 2.5 | 0.2 |
| Energetic–fatigue | 2.7 + 1.7 | 6 + 1.8 | 0.005 |
| Satisfied–dissatisfied | 8.2 + 1.8 | 6.8 + 2.5 | 0.3 |
| Calm–worried | 1.5 + 1.6 | 1.6 + 1.4 | 0.9 |
| Quick thinking–slow thinking | 2.1 + 1.9 | 2 + 1.7 | 0.9 |
| Relaxed–tense | 3.2 + 2 | 2.2 + 2.2 | 0.4 |
| Dreamy–concentrated | 7.9 + 0.9 | 7.5 + 0.5 | 0.4 |
| Very efficient–inefficient | 2.7 + 1.4 | 2 + 1.2 | 0.3 |
| Sad–happy | 7.2 + 2 | 7.2 + 2 | 1 |
| Friendly–hostile | 1 + 1.3 | 0.7 + 0.8 | 0.7 |
| Bored–interested | 8 + 1 | 7.9 + 1.7 | 0.9 |
| Sociable–reclusive | 1.7 + 1.6 | 2.4 + 3 | 0.6 |
Regarding the effect of exogenous melatonin administered at night, fatigue and sleepiness increased during both placebo and melatonin trials, and melatonin administration had no effect over that of placebo on these or any of the other behavioral parameters assessed.
fMRI
Time of Day Effects on Brain Activity
A number of brain regions that demonstrated significant task‐related activation at 16:00 did not demonstrate such activations at 22:00 h (Fig. 2a). These regions comprised an extensive cluster located at the rostro‐medial occipital cortex including mainly the cuneus but extending into the lingual gyrus (2,133 voxels, center talarich coordinates: 3, −71, 15), bilateral superior and middle occipital gyrus (right: 2,350 voxels, center talarich coordinates: 24, −86, 25; left: 1,379 voxels, center talarich coordinates: −23, −85, 20) and the right pulvinar of the thalamus (122 voxels, center talarich coordinates: 7, −22, 3). Parameter estimates from these regions verify a decrease in activation at 22:00 h compared with 16:00 h (Fig. 2b).
Figure 2.
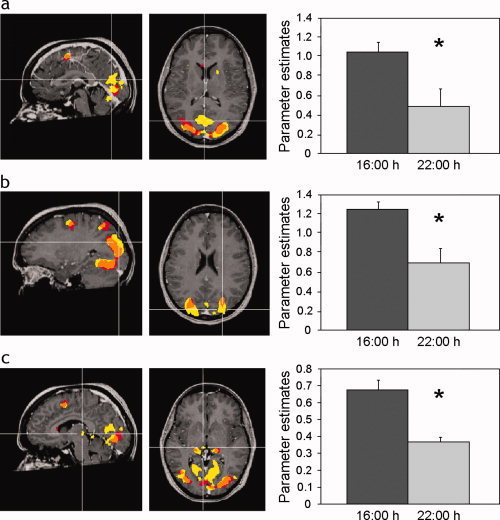
Brain areas demonstrating significant task related activity at 16:00 h but not 22:00 h. Statistical parametric maps (P < 0.01 corrected) demonstrating task related activation at 16:00 h (yellow) and 22:00 h (red). Orange denotes overlapped activation. Graphs show mean (+SEM) activation parameter estimates derived from the respective regions marked by intersecting lines: (a) rostro‐medial aspect of the occipital cortex (center talarich coordinates: 3, −71, 15); (b) left lateral aspect of the occipital cortex (center talarich coordinates: −23, −85, 20); (c) pulvinar of the thalamus (center talarich coordinates: 7, −22, 3).
Because these areas do not demonstrate task‐related activation at 22:00 h no further analysis was performed.
A number of brain regions that demonstrated significant task‐related activation at 22:00 did not demonstrate activation at 16:00 h. These regions comprised an area at the right inferior parietal gyrus (Fig. 3a) (1,332 voxels, center talarich coordinates: 37, −40, 50) and the right dorsolateral prefrontal (DLPF) cortex (428 voxels, center talarich coordinates: 47, 6, 36) (Fig. 4a).
Figure 3.
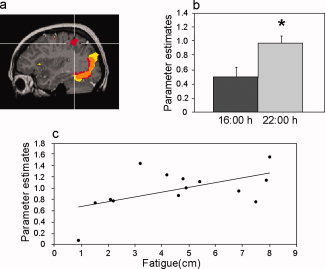
Task related activation and correlation to fatigue in the right parietal cortex. (a) Statistical parametric map (P < 0.01 corrected) demonstrating task related activation at 16:00 h (yellow) and 22:00 h (red). Orange denotes overlapped activation. Intersecting lines denote activation in the right parietal cortex (center talarich coordinates: 37, −40, 50) demonstrated at 22:00 but not 16:00 h. (b) Mean (+SEM) parameter estimates. (c) Correlation between fatigue levels (higher numbers denote greater fatigue) reported at 22:00 h and activation parameters from the parietal cluster.
Figure 4.
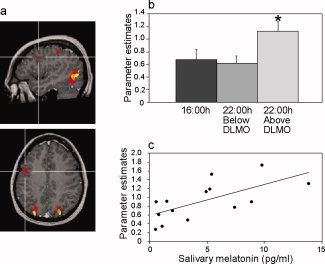
Task related activation and correlation to endogenous melatonin in the right DLPF cortex. (a) Statistical parametric maps (P < 0.01 corrected) demonstrating task related activation at 16:00 h (yellow) and 22:00 h (red). Orange denotes overlapped activation. Intersecting lines denote activation in the right DLPF cortex (center talarich coordinates: 47, 6, 36) demonstrated at 22:00 but not 16:00 h. (b) Mean (+SEM) parameter estimates. (c) Correlation between endogenous melatonin levels at 22:00 h and activation parameters from the DLPF.
Parameter estimates from the right parietal cortex verified increased activation at 22:00 h in this region (P = 0.008) (Fig. 3b). Further analysis found no significant difference in activation in this region between subjects with higher and lower than melatonin threshold levels at 22:00 h and no correlation between brain activation and individual endogenous melatonin levels. A significant correlation was found between subjective fatigue and parietal activation at 22:00 h (r = 0.55, P = 0.04) (Fig. 3c). Namely, greater fatigue reported at 22:00 h, was associated with higher activation within the right parietal cortex.
Significant task‐related activation was demonstrated in the right DLPF cortex at 22:00 but not 16:00 h (Fig. 4a). However, the mean parameter estimates in this region for the entire group at 22:00 h did not differ significantly from those at 16:00 h (P = 0.2). On the other hand, a significant difference was found in parameter estimates from the right DLPF cortex between subjects with higher and lower than melatonin threshold levels at 22:00 h (Fig. 4b). Namely, activation at 22:00 h was higher in subjects with higher than melatonin threshold levels relatively to subjects with below these levels (P = 0.02). Furthermore, a highly significant correlation is demonstrated between individual endogenous melatonin levels at 22:00 h and brain activation in this region (r = 0.66, P = 0.009) (Fig. 4c). In addition, higher activation in this region tended to correlate with higher subjective fatigue at 22:00 h (r = 0.52, P = 0.058).
Effect of Exogenous Melatonin Administered at Night
Results of the effect of exogenous melatonin administered during afternoon hours were described elsewhere [Gorfine et al., 2006]. Unlike melatonin intake during afternoon hours, which demonstrated a robust decrease in activation in a large cluster within the rostro‐medial part of the occipital cortex, melatonin intake at night demonstrates a diffuse, nonspecific pattern. Thus, an effect of exogenous melatonin compared with placebo was demonstrated in a few relatively small clusters within the occipital cortex. Two clusters in the rostro‐medial part of the occipital cortex (320 voxels, center talarich coordinates: 15, −63, 4, and 220 voxels, center talarich coordinates: 3, −64, −1) demonstrate reduced task‐related activation after melatonin intake compared with placebo, but, the effect is much smaller, in size and significance, than that witnessed during afternoon trials. Other clusters showing different activations after melatonin compared with placebo were found bilateral in the fussiform cortex (380 voxels, center talarich coordinates: 20, −68, −19, and 343 voxels, center talarich coordinates: −14, −71, −18). No effect of exogenous melatonin was witnessed in the thalamus.
While exogenous melatonin does not affect parietal cortex activation when given in the afternoon [Gorfine et al., 2006], exogenous melatonin administration at night (22:00) resulted in decreased activation within the right parietal cortex (183 voxels, center talarich coordinates: 33, −48, 48) from baseline at this hour and compared with the parallel placebo treatment (see Fig. 5).
Figure 5.
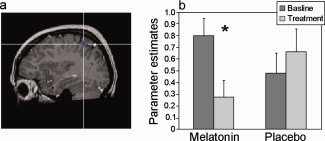
The effect of exogenous melatonin administration at 22:00 h on activation in the right parietal cortex. (a) Statistical parametric map of drug versus placebo effect (conjunction analysis, P < 0.05 uncorrected). Reduced activation in the right parietal cortex following melatonin but not placebo intake is denoted by the intersecting lines. (b) Mean (+SEM) parameter estimates of activation.
DISCUSSION
This study confirms the role of melatonin and the internal clock in the rise in fatigue at night. Thus, subjects who had higher than threshold melatonin levels reported higher levels of fatigue than those who had not attained melatonin threshold at 22:00. The latter reported on similar fatigue levels to levels reported by subjects studied at 16:00 h. As each of the subjects who had lower than threshold melatonin levels at 22:00 h reported on increased fatigue levels at 24:00 h, it is unlikely that these individual had difficulties in perceiving fatigue. On the other hand, fatigue levels did not correlate with individual salivary melatonin levels. This lack of correlation may have several explanations. First, it might potentially be attributed to interindividual variability in endogenous melatonin production capacities leading to differences in the proportion of circulating melatonin found in saliva [Laakso et al., 1990]. Secondly, there might be differences in levels of melatonin needed to elicit a recognizable level of fatigue among individuals. Alternatively, the increase in fatigue might be associated with the endogenous biological time, not necessarily melatonin levels. In such case, higher fatigue level might potentially be due to increase in sensitivity of the brain to melatonin or to humoral sleep‐inducing factors that accumulate in the brain during prolonged wakefulness [e.g., prostaglandin D2 and adenosine Huang et al., 2007].
It is interesting that despite the expected “opening of the sleep gate” as well as accumulation of sleep debt during 14 h of wakefulness, and increased fatigue, there was no rise in sleepiness at night as compared with afternoon hours, and more so, between subjects who have crossed the threshold melatonin levels at 22:00 and those who have not. This observation is in line with previous reports [Cajochen et al., 2002; Dijk and Lockley, 2002; Wyatt et al., 2006] showing that sleepiness levels are quite stable during the first 16 h of wakefulness. An increase in sleepiness was reported at 24:00 h both during melatonin and placebo trials, most probably due to a further rise in endogenous melatonin levels as well as accumulating sleep dept. Apparently, under everyday conditions the perceived fatigue is more sensitive to sleep debt, biological timing, and melatonin than sleepiness and therefore occurs earlier in the evening.
In parallel, clear and significant changes in brain activation patterns occur at night. Thus, extensive areas in rostro‐medail and lateral aspects of the visual cortex as well as a cluster located at the pulvinar of the thalamus exhibit lower task‐related activation at night compared with the afternoon hours. In contrast, the right parietal and right DLPF cortices demonstrate higher task‐related activation at night compared with afternoon hours. It should be pointed out that differences in activation between hours may result from either a change in task or baseline activity (e.g., decreased activation could result from decreased activity during task or from increased activity during baseline).
Reduced task‐related activity in the rostro‐medial part of the occipital cortex has been demonstrated after melatonin administration during afternoon hours [Gorfine et al., 2006]. Decreased activity in this specific area has also been consistently demonstrated during sleep upon visual and auditory stimuli [Altman and Bernal, 2001; Born et al., 2002; Czisch et al., 2002]. Contrary, sleep deprivation, using a variety of visual tasks have consistently demonstrated a different pattern [Bell‐McGinty et al., 2004; Choo et al., 2005; Drummond et al., 1999, 2000, 2001]. Thus, in line with our hypothesis, a connection to circadian rather than homeostatic sleep debt effect is favored with respect to the changes in activation of the rostro‐medial part of the occipital cortex. A limitation to this claim is that activation in this region was reduced at 22:00 h for the entire night group, whereas not all subjects attained melatonin threshold levels at this hour. Possibly, reduced activity in this area precedes both the conventional definition of melatonin threshold levels and subjective perception of fatigue. Compatible with our hypothesis, the robust, highly significant, decrease in activity in the rostro‐medial aspect of the occipital cortex seen after melatonin intake in the afternoon did not occur after melatonin administration at night. This lack of effect is also compatible with reduced activation already demonstrated in this area at 22:00 h. Interestingly, diffuse small clusters in the visual cortex showed decreased activity after melatonin but not placebo intake at 22:00 h. These changes should however be regarded with caution because these areas are relatively small and scattered within extensive task‐related activity in the visual cortex. Notably, there is an intriguing lack of referral to this specific region in wakefulness experiments. Further studies are warranted to elucidate its function and importance for fatigue induction and sleep/wake regulation.
A different picture is presented in the lateral parts of the occipital cortex. We found decreases in task‐related activation bilateral in the superior and middle occipital gyri at 22:00 h. Such changes have been described in several studies after sleep deprivation [Bell‐McGinty et al., 2004; Choo et al., 2005; Habeck et al., 2004] but did not occur after melatonin administration during afternoon hours [Gorfine et al., 2006]. Thus decreased activation in the lateral parts of the occipital cortex appears to be related to the homeostatic sleep debt rather than a circadian effect. A limitation to our study is that in the evaluation of the influence of the homeostatic sleep debt we could only make reference to sleep deprivation experiments, in which the accumulated sleep debt may be supra‐physiological.
Relative glucose metabolism has been previously reported to be lower in the evening than in the morning in several clusters in the occipital lobe including both medial and lateral parts [Buysse et al., 2004]. These reductions were hypothesized to reflect increased homeostatic sleep debt. Our study points toward differential influences of the circadian versus homeostatic sleep debt in the occipital cortex, where the medial parts are more probably connected to circadian regulation and the more lateral parts are connected to the homeostatic sleep debt.
Apart from the visual cortex, decreased activity at 22:00 h compared with 16:00 h was demonstrated in the pulvinar of the right thalamus. Several explanations may equally explain this finding. First, the thalamus and specifically the pulvinar participate in a network subserving alertness and attention [Coull et al., 2004; Kinomura et al., 1996; Petersen et al., 1987; Steriade, 2003; Sturm and Willmes, 2001]. Therefore, modulation in thalamic activation may be related to changes in attention secondary to reduced arousal at night. Second, increased sleep debt may affect thalamic activation. Imaging studies following sleep deprivation are equivocal regarding thalamic activity as some show increased [Chee and Choo, 2004; Habeck et al., 2004; Portas et al., 1998] and the other decreased [Chee et al., 2006; Thomas et al., 2000; Wu et al., 1991] activation. In any case, sleep deprivation may induce greater arousal levels and affect activity in the thalamus differently than the homeostatic sleep debt per se. In our study the amount of accumulated sleep debt was within the daily norm, thus, it cannot be ruled out that reduced thalamic activation is connected to the homeostatic sleep regulation.
A more plausible explanation connects reduced activity in the thalamus to the circadian system. Contemporary sleep/wake regulation assigns a key role for activation of thalamic nuclei by the arousal system in the brainstem (reticular formation) in wakefulness maintenance [Merica and Fortune, 2004; Pace‐Schott and Hobson, 2002]. Thalamic deactivation is the most reproducible pattern observed in human sleep stage 2 and slow wave sleep [Kaufmann et al., 2006; Maquet, 2000; Nofzinger et al., 2002]. Nevertheless, in this study subjects remained wakeful throughout each trial (2–3 h past 22:00 h). Therefore, a state transition to sleep is obviously not responsible for reduced thalamic activation at 22:00 compared with 16:00 h. On the other hand, the critical difference between the two time points may be related to differences in activity of the SCN. The SCN promotes wakefulness during the day but not in night hours. Consequently, the transition from day to night in SCN activity may be responsible for the demonstrated changes in thalamic activation.
The right parietal cortex demonstrates increased activity at 22:00 h regardless of the melatonin threshold crossing and without correlation to melatonin levels. In addition, the parietal cortex was not affected by melatonin administration in the afternoon [Gorfine et al., 2006]. Therefore it is reasonable to conclude that baseline changes in the parietal cortex at this hour are not related to circadian regulation. More probably, it may be related to increased homeostatic sleep debt. Indeed, while some studies show decreased activation in the parietal cortex after sleep deprivation [Chee et al., 2006; Drummond et al., 1999; Habeck et al., 2004] other clearly show increased parietal activation [Drummond et al., 2000, 2001, 2005]. Most interestingly, these studies demonstrate better performance in sleep deprived subjects associated with greater parietal activation, which led the authors to suggest that this region may represent the neurophysiologic substrate of initial compensatory changes. Notably, our results showed that at 22:00 h parietal cortex activation correlates with fatigue levels. Namely, the greater the fatigue reported at 22:00 h the greater the activation. This is also compatible with a compensatory reaction. It should be noted that for the entire group no change in mean fatigue level was found at night compared with afternoon hours. This may imply that parietal activation is sensitive to small individual changes in fatigue.
Beside the fatigue‐related increase in parietal cortex activation, at a similar location, exogenous melatonin administered at night resulted in decreased activation relative to placebo. As no such effect of melatonin was demonstrated following administration of melatonin in the afternoon, these findings suggest that the effect of melatonin in this area depended on the time of administration. Thus, increased parietal activation at 22:00 h may already be a manifestation of compensatory mechanisms related to increased homeostatic sleep debt; melatonin administration may result in an inability to sustain these compensatory mechanisms.
Significant activation in the right DLPF cortex is not demonstrated at 16:00 h. It is however demonstrated at 22:00 h but only in subjects that crossed melatonin threshold levels. What's more, brain activation in this region and individual melatonin levels are highly correlated. This result was not predicted by our previous study in which melatonin given in the afternoon did not provoke activation in the DLPF [Gorfine et al., 2006]. Furthermore, the prefrontal cortex has previously been closely linked to the homeostatic sleep debt [Dijk and Lockley, 2002].
A plausible explanation is that the DLPF is coupled to activity in another brain area which responds to melatonin in a circadian manner, e.g., SCN, hippocampus [Anis et al., 1989; Chaudhury et al., 2005; Musshoff et al., 2002; Poirel et al., 2002; Zisapel et al., 1988]. The hypothalamic SCN has a major role in the circadian regulation of sleep [Fuller et al., 2006]. The SCN has direct connections to the dorsomedial hypothalamic nucleus (DMH) that innervates the ventrolateral preoptic nucleus (VLPO). The VLPO of the hypothalamus contains a group of sleep‐active, galanin‐producing neurons that appears to be a critical component of sleep circuitry across multiple species. The VLPO presumably inhibits the major ascending monoaminergic arousal systems during sleep; lesions of the VLPO cause insomnia. The possibility that the SCN mediates all sleep anticipating effects of melatonin remains to be elucidated.
It should be noted that MT1 receptors have been localized in the SCN and hippocampus as well as other areas of the brain including the prefrontal cortex [Uz et al., 2005]. MT1 receptors exhibit diurnal rhythm with high levels occurring at night and low levels during the day. Therefore direct as well as indirect effects of melatonin on the DLPF are possible. Interestingly, in rodents as also in humans most areas that expressed MT1 receptors or responded to melatonin were linked to central dopaminergic pathways [Uz et al., 2005; Zisapel, 2001b].
To summarize, this study confirms the role of melatonin and the internal clock in the rise in fatigue at night, and demonstrates that in everyday life conditions brain activity at night compared with afternoon hours reflects both a change in the circadian phase (related and unrelated to melatonin) and increase in homeostatic sleep debt. The rostro‐medial part of the occipital cortex is a melatonin‐ responsive area that is apparently related to circadian sleep/wake regulation and may precede the sense of fatigue. The thalamus demonstrates reduced activity at night compared with afternoon hours which is possibly related to the internal circadian clock phase. Contrarily, the lateral visual and parietal cortices exhibit changes in activation at night which are compatible with the increase in homeostatic sleep debt. As increased activation in the parietal cortex correlates with fatigue level, a compensatory mechanism is suggested. Exogenous melatonin administered at night caused decreased activity in the same region suggesting that it overrides compensation. Surprisingly, increased activity was demonstrated in the right DLPF at 22:00 h which correlated with endogenous melatonin. As no such activity is seen with melatonin administered in the afternoon, we suspect that this change may be linked to the effect of melatonin on the DLPF or another area (e.g., SCN, hippocampus) that exhibits a circadian rhythm in response to melatonin. Whatever the case may be, this to our knowledge is the first report of melatonin modulation in the prefrontal cortex.
Acknowledgements
We are thankful to Mr. Ofer Pasternak who was responsible for the blinding and preparation of the blinded melatonin/placebo solutions, to Ms. Zila Shen‐Orr who skillfully assessed salivary melatonin, and to Dr. Yaniv Asaf and Dr. Talma Hendler for fruitful discussions. Dr. Tali Gorfine is a recipient of a Levie‐Edersheim‐Gitter Institute for Human Brain Mapping and Fred Schaoul Scholarships, and a laureate of the Dan David prize for PhD students. Dr. Nava Zisapel is an incumbent of the Michael GlucK Chair in Neuropharmacology and ALS Research.
REFERENCES
- Altman NR,Bernal B ( 2001): Brain activation in sedated children: Auditory and visual functional MR imaging. Radiology 221: 56–63. [DOI] [PubMed] [Google Scholar]
- Anis Y,Nir I,Zisapel N ( 1989): Diurnal variations in melatonin binding sites in the hamster brain: Impact of melatonin. Mol Cell Endocrinol 67: 121–129. [DOI] [PubMed] [Google Scholar]
- Anton‐Tay F,Diaz JL,Fernandez‐Guardiola A ( 1971): On the effect of melatonin upon human brain. Its possible therapeutic implications. Life Sci I 10: 841–850. [DOI] [PubMed] [Google Scholar]
- Bell‐McGinty S,Habeck C,Hilton HJ,Rakitin B,Scarmeas N,Zarahn E,Flynn J,DeLaPaz R,Basner R,Stern Y ( 2004): Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex 14: 496–502. [DOI] [PubMed] [Google Scholar]
- Borbely AA ( 1982): A two process model of sleep regulation. Hum Neurobiol 1: 195–204. [PubMed] [Google Scholar]
- Borbely AA ( 1998): Processes underlying sleep regulation. Horm Res 49: 114–117. [DOI] [PubMed] [Google Scholar]
- Born AP,Law I,Lund TE,Rostrup E,Hanson LG,Wildschiodtz G,Lou HC,Paulson OB ( 2002): Cortical deactivation induced by visual stimulation in human slow‐wave sleep. Neuroimage 17: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Buysse DJ,Nofzinger EA,Germain A,Meltzer CC,Wood A,Ombao H,Kupfer DJ,Moore RY ( 2004): Regional brain glucose metabolism during morning and evening wakefulness in humans: Preliminary findings. Sleep 27: 1245–1254. [DOI] [PubMed] [Google Scholar]
- Cajochen C,Wyatt JK,Czeisler CA,Dijk DJ ( 2002): Separation of circadian and wake duration‐dependent modulation of EEG activation during wakefulness. Neuroscience 114: 1047–1060. [DOI] [PubMed] [Google Scholar]
- Cajochen C,Krauchi K,Wirz‐Justice A ( 2003): Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol 15: 432–437. [DOI] [PubMed] [Google Scholar]
- Chaudhury D,Wang LM,Colwell CS ( 2005): Circadian regulation of hippocampal long‐term potentiation. J Biol Rhythms 20: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW,Choo WC ( 2004): Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci 24: 4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW,Chuah LY,Venkatraman V,Chan WY,Philip P,Dinges DF ( 2006): Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto‐parietal activation with performance. Neuroimage 31: 419–428. [DOI] [PubMed] [Google Scholar]
- Choo WC,Lee WW,Venkatraman V,Sheu FS,Chee MW ( 2005): Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage 25: 579–587. [DOI] [PubMed] [Google Scholar]
- Coull JT,Jones ME,Egan TD,Frith CD,Maze M ( 2004): Attentional effects of noradrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. Neuroimage 22: 315–322. [DOI] [PubMed] [Google Scholar]
- Czeisler CA,Duffy JF,Shanahan TL,Brown EN,Mitchell JF,Rimmer DW,Ronda JM,Silva EJ,Allan JS,Emens JS,Dijk DJ,Kronauer RE ( 1999): Stability, precision, and near‐24‐hour period of the human circadian pacemaker. Science 284: 2177–2181. [DOI] [PubMed] [Google Scholar]
- Czisch M,Wetter TC,Kaufmann C,Pollmacher T,Holsboer F,Auer DP ( 2002): Altered processing of acoustic stimuli during sleep: Reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage 16: 251–258. [DOI] [PubMed] [Google Scholar]
- Dijk DJ,Lockley SW ( 2002): Integration of human sleep‐wake regulation and circadian rhythmicity. J Appl Physiol 92: 852–862. [DOI] [PubMed] [Google Scholar]
- Drummond SP,Brown GG,Stricker JL,Buxton RB,Wong EC,Gillin JC ( 1999): Sleep deprivation‐induced reduction in cortical functional response to serial subtraction. Neuroreport 10: 3745–3748. [DOI] [PubMed] [Google Scholar]
- Drummond SP,Brown GG,Gillin JC,Stricker JL,Wong EC,Buxton RB ( 2000): Altered brain response to verbal learning following sleep deprivation. Nature 403: 655–657. [DOI] [PubMed] [Google Scholar]
- Drummond SP,Gillin JC,Brown GG ( 2001): Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res 10: 85–92. [DOI] [PubMed] [Google Scholar]
- Drummond SP,Meloy MJ,Yanagi MA,Orff HJ,Brown GG ( 2005): Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res 140: 211–223. [DOI] [PubMed] [Google Scholar]
- Fuller PM,Gooley JJ,Saper CB ( 2006): Neurobiology of the sleep‐wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms 21: 482–493. [DOI] [PubMed] [Google Scholar]
- Gorfine T,Assaf Y,Goshen‐Gottstein Y,Yeshurun Y,Zisapel N ( 2006): Sleep‐anticipating effects of melatonin in the human brain. Neuroimage 31: 410–418. [DOI] [PubMed] [Google Scholar]
- Habeck C,Rakitin BC,Moeller J,Scarmeas N,Zarahn E,Brown T,Stern Y ( 2004): An event‐related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed‐match‐to‐sample task. Brain Res Cogn Brain Res 18: 306–321. [DOI] [PubMed] [Google Scholar]
- Huang ZL,Urade Y,Hayaishi O ( 2007): Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol 7: 33–38. [DOI] [PubMed] [Google Scholar]
- Kaufmann C,Wehrle R,Wetter TC,Holsboer F,Auer DP,Pollmacher T,Czisch M ( 2006): Brain activation and hypothalamic functional connectivity during human non‐rapid eye movement sleep: An EEG/fMRI study. Brain 129: 655–667. [DOI] [PubMed] [Google Scholar]
- Kinomura S,Larsson J,Gulyas B,Roland PE ( 1996): Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271: 512–515. [DOI] [PubMed] [Google Scholar]
- Laakso ML,Porkka‐Heiskanen T,Alila A,Stenberg D,Johansson G ( 1990): Correlation between salivary and serum melatonin: Dependence on serum melatonin levels. J Pineal Res 9: 39–50. [DOI] [PubMed] [Google Scholar]
- Lavie P ( 1986): Ultrashort sleep‐waking schedule. III. 'Gates' and 'forbidden zones' for sleep. Electroencephalogr Clin Neurophysiol 63: 414–425. [DOI] [PubMed] [Google Scholar]
- Leger D,Laudon M,Zisapel N ( 2004): Nocturnal 6‐sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am J Med 116: 91–95. [DOI] [PubMed] [Google Scholar]
- Lewy AJ ( 1999): The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Biol Signals Recept 8: 79–83. [DOI] [PubMed] [Google Scholar]
- Lieberman HR,Waldhauser F,Garfield G,Lynch HJ,Wurtman RJ ( 1984): Effects of melatonin on human mood and performance. Brain Res 323: 201–207. [DOI] [PubMed] [Google Scholar]
- Liu C,Weaver DR,Jin X,Shearman LP,Pieschl RL,Gribkoff VK,Reppert SM ( 1997): Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102. [DOI] [PubMed] [Google Scholar]
- Lockley SW,Skene DJ,Tabandeh H,Bird AC,Defrance R,Arendt J ( 1997): Relationship between napping and melatonin in the blind. J Biol Rhythms 12: 16–25. [DOI] [PubMed] [Google Scholar]
- Maquet P ( 2000): Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 9: 207–231. [DOI] [PubMed] [Google Scholar]
- Merica H,Fortune RD ( 2004): State transitions between wake and sleep, and within the ultradian cycle, with focus on the link to neuronal activity. Sleep Med Rev 8: 473–485. [DOI] [PubMed] [Google Scholar]
- Musshoff U,Riewenherm D,Berger E,Fauteck JD,Speckmann EJ ( 2002): Melatonin receptors in rat hippocampus: Molecular and functional investigations. Hippocampus 12: 165–173. [DOI] [PubMed] [Google Scholar]
- Nakagawa H,Sack RL,Lewy AJ ( 1992): Sleep propensity free‐runs with the temperature, melatonin and cortisol rhythms in a totally blind person. Sleep 15: 330–336. [DOI] [PubMed] [Google Scholar]
- Nickelsen T,Demisch L,Demisch K,Radermacher B,Schoffling K ( 1989): Influence of subchronic intake of melatonin at various times of the day on fatigue and hormonal levels: A placebo‐controlled, double‐blind trial. J Pineal Res 6: 325–334. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA,Buysse DJ,Miewald JM,Meltzer CC,Price JC,Sembrat RC,Ombao H,Reynolds CF,Monk TH,Hall M,Kupfer DJ,Moore RY ( 2002): Human regional cerebral glucose metabolism during non‐rapid eye movement sleep in relation to waking. Brain 125: 1105–1115. [DOI] [PubMed] [Google Scholar]
- Pace‐Schott EF,Hobson JA ( 2002): The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3: 591–605. [DOI] [PubMed] [Google Scholar]
- Petersen SE,Robinson DL,Morris JD ( 1987): Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25: 97–105. [DOI] [PubMed] [Google Scholar]
- Pires ML,Benedito‐Silva AA,Pinto L,Souza L,Vismari L,Calil HM ( 2001): Acute effects of low doses of melatonin on the sleep of young healthy subjects. J Pineal Res 31: 326–332. [DOI] [PubMed] [Google Scholar]
- Poirel VJ,Masson‐Pevet M,Pevet P,Gauer F ( 2002): MT1 melatonin receptor mRNA expression exhibits a circadian variation in the rat suprachiasmatic nuclei. Brain Res 946: 64–71. [DOI] [PubMed] [Google Scholar]
- Portas CM,Rees G,Howseman AM,Josephs O,Turner R,Frith CD ( 1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ ( 1991): Melatonin: The chemical expression of darkness. Mol Cell Endocrinol 79: C153–C158. [DOI] [PubMed] [Google Scholar]
- Shochat T,Luboshitzky R,Lavie P ( 1997): Nocturnal melatonin onset is phase locked to the primary sleep gate. Am J Physiol 273: R364–R370. [DOI] [PubMed] [Google Scholar]
- Steriade M ( 2003): The corticothalamic system in sleep. Front Biosci 8: d878–d899. [DOI] [PubMed] [Google Scholar]
- Stone BM,Turner C,Mills SL,Nicholson AN ( 2000): Hypnotic activity of melatonin. Sleep 23: 663–669. [PubMed] [Google Scholar]
- Sturm W,Willmes K ( 2001): On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14: S76–S84. [DOI] [PubMed] [Google Scholar]
- Terlo L,Laudon M,Tarasch R,Schatz T,Caine YG,Zisapel N ( 1997): Effects of low doses of melatonin on late afternoon napping and mood. Biol Rhythm Res 28: 2–16. [Google Scholar]
- Thomas M,Sing H,Belenky G,Holcomb H,Mayberg H,Dannals R,Wagner H,Thorne D,Popp K,Rowland L,Welsh A,Balwinski S,Redmond D ( 2000): Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9: 335–352. [DOI] [PubMed] [Google Scholar]
- Tzischinsky O,Shlitner A,Lavie P ( 1993): The association between the nocturnal sleep gate and nocturnal onset of urinary 6‐sulfatoxymelatonin. J Biol Rhythms 8: 199–209. [DOI] [PubMed] [Google Scholar]
- Uz T,Arslan AD,Kurtuncu M,Imbesi M,Akhisaroglu M,Dwivedi Y,Pandey GN,Manev H ( 2005): The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res 136: 45–53. [DOI] [PubMed] [Google Scholar]
- Wirz‐Justice A,Krauchi K,Cajochen C,Danilenko KV,Renz C,Weber JM ( 2004): Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J Pineal Res 36: 192–194. [DOI] [PubMed] [Google Scholar]
- Wright KP Jr,Gronfier C,Duffy JF,Czeisler CA ( 2005): Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC,Gillin JC,Buchsbaum MS,Hershey T,Hazlett E,Sicotte N,Bunney WE Jr ( 1991): The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep 14: 155–162. [PubMed] [Google Scholar]
- Wyatt JK,Dijk DJ,Ritz‐de Cecco A,Ronda JM,Czeisler CA ( 2006): Sleep‐facilitating effect of exogenous melatonin in healthy young men and women is circadian‐phase dependent. Sleep 29: 609–618. [DOI] [PubMed] [Google Scholar]
- Zisapel N ( 2001a): Circadian rhythm sleep disorders: Pathophysiology and potential approaches to management. CNS Drugs 15: 311–328. [DOI] [PubMed] [Google Scholar]
- Zisapel N ( 2001b): Melatonin‐dopamine interactions: From basic neurochemistry to a clinical setting. Cell Mol Neurobiol 21: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N ( 2007): Sleep and sleep disturbances: Biological basis and clinical implications. Cell Mol Life Sci 64: 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N,Nir I,Laudon M ( 1988): Circadian variations in melatonin‐binding sites in discrete areas of the male rat brain. FEBS Lett 232: 172–176. [DOI] [PubMed] [Google Scholar]


