Abstract
The origin of within‐subject variability in perceptual experiments is poorly understood. We here review evidence that baseline brain activity in the areas involved in sensory perception predict subsequent variations in sensory awareness. We place these findings in light of recent findings on the architecture of spontaneous BOLD fluctuations in the awake human brain, and discuss the possible origins of the observed baseline brain activity fluctuations. Hum Brain Mapp 2008. © 2008 Wiley‐Liss, Inc.
Keywords: functional MRI, consciousness, pain, somatosensory
INTRODUCTION
In perceptual experiments, within‐subject variability in perception is commonly observed across multiple presentations of the same stimuli [Sergent et al., 2005]. In parallel, trial‐to‐trial variability in event‐related BOLD responses magnitude has been shown to be relevant to human perception and behavior [Fox et al., 2006]. In many cases, this inter‐trial inconsistency cannot be attributed to the variability in stimuli [Pessoa et al., 2002; Pessoa and Padmala, 2005; Ress and Heeger, 2003]. Despite its demonstrated relevance for human behavior, the sources of these event‐related BOLD responses (and related perception) variability are only partially understood [Dehaene and Changeux, 2005; Fox et al., 2006].
We recently made the hypothesis that variability in perception of identical stimuli could be due to differences in prestimulus baseline brain activity, in areas related to stimulus perception [Boly et al., 2007]. We will here review this work, beginning with summarizing the existing knowledge about neural correlates of self and external awareness. After reviewing data suggesting the presence of spontaneous neural activity fluctuations in the areas involved in the genesis of awareness, we will discuss our results suggesting that spatially‐specific differences in baseline brain activity influence subsequent external stimulus perception. We will finally mention possible sources of these baseline brain activity fluctuations.
NEURAL CORRELATES OF CONSCIOUS PERCEPTION
Consciousness is a multifaceted concept, which can be divided in two main components—arousal, or the level of consciousness, and awareness—or the contents of consciousness. Usually, arousal and awareness are positively correlated: when your arousal decreases, so does your awareness [Laureys, 2005]. A recent effort has been made in identifying the neural correlates of awareness. In particular, a contrastive approach has been extensively used in neuroimaging studies, comparing states of physiological or pathological unawareness to normal awake state in healthy volunteers. The common finding of these studies is that a widespread frontoparietal associative network, both on lateral and midline sides show a consistent functional impairment in various altered states of consciousness like sleep, coma, general anaesthesia, generalized seizures [Baars et al., 2003]. These areas also show a consistent impairment in dissociated unconsciousness states, where awareness is absent while arousal is preserved, encompassing the vegetative state, absence seizures, complex partial seizures, and somnambulism [Laureys, 2005]. These results emphasize the role of frontoparietal network in the genesis of awareness, and are in line with the global workspace theory as proposed by Baars et al. [ 2003] (see Fig. 1).
Figure 1.
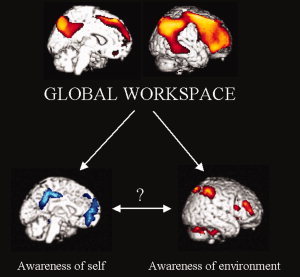
Two components of awareness. In healthy awake volunteers as in disorders of consciousness studies, awareness has been related to the activity of a widespread frontoparietal associative network, both on the convexity and the midline. These findings are in line with the “global workspace” theory of consciousness. Awareness can in turn be divided in two main components, external and self awareness. Similarly, the awareness related frontoparietal network can be partitioned in two nonoverlapping subsystems, involved in processing the self and external‐related components of awareness. At present, the relationships between self and external awareness remain poorly understood. Upper image adapted from [Laureys, 2005], represents statistically significant hypometabolic areas as measured by FDG‐PET in the vegetative state as compared with conscious waking. Lower images are adapted from [Boly et al., 2007], showing the default network (in blue) and the lateral frontoparietal network (in red) as identified in this study by fMRI. The color code refers to voxel‐wise T values, ranging respectively from red to yellow or from blue to white.
In turn, awareness can be divided in two components—self awareness and external awareness. External awareness has to date been extensively studied in the human brain, especially in the visual domain. Recent reviews suggest that necessary and sufficient conditions to elicit conscious sensory perception would be the combination of a distributed representation of the perceptual scene in specialized sensory areas, plus lateral parietal, and frontal associative cortices activity [Rees, 2007]. In this view, the presence of external awareness would be linked to the activation of lateral fronto‐parietal areas, while neural activity in early sensory areas would determine the contents of external awareness per se.
Neural correlates of self awareness are to date much less understood. Several authors have linked self‐referential processes with the absence of task or so‐called “resting‐state” brain activity [Baars et al., 2003; Mazoyer et al., 2001]. This view came from the curious finding of consistent activity decreases in a unique set of areas during goal‐directed tasks compared to a passive resting baseline [Shulman et al., 1997]. This “default network” [Gusnard and Raichle, 2001] encompasses bilateral posterior cingulate/precuneus, mesiofrontal cortices, and temporo‐parietal junctions (and for some authors inferior temporal cortices and parahippocampal gyri). These regions were further implicated in task‐independent thoughts [McKiernan et al., 2006] and various aspects of self‐referential processing [Fox et al., 2005]. Although it is currently regarded that self and environmental consciousness share different brain processes, the relationships between self and environment awareness and their related neural mechanisms are yet poorly characterized.
BASELINE ACTIVITY FLUCTUATIONS IN SELF AND EXTERNAL AWARENESS NETWORKS
Even in the absence of sensory inputs, structured patterns of ongoing spontaneous activity can be observed in cortical and thalamic neurons [Dehaene and Changeux, 2005]. Spontaneous brain activity has been shown to be characterized by the coalescence of different brain rhythms—ranging from very slow to very fast frequencies. In parallel, recent functional magnetic resonance imaging (fMRI) studies have identified spontaneous fluctuations in neural activity in the resting human brain. Slow blood oxygen level dependent (BOLD) fluctuations (in the range of 0.01–0.1 Hz) are not random but coherent within specific neuroanatomical systems [Fox and Raichle, 2007], including sensory systems, and self and external awareness networks. Spontaneous BOLD signal fluctuations may also be linked to infra‐slow electrophysiological oscillations as observed by direct current electro‐encephalography, which were suggested to possibly modulate ongoing cortical excitability [Vanhatalo et al., 2004].
In parallel, other resting‐state fMRI studies have shown anticorrelated patterns of spontaneous fluctuations, in regions with apparent opposing functionality. In particular, two independent groups [Fox et al., 2005; Fransson, 2005] recently showed that even in the absence of any task or behavior, in the so called “conscious resting state” of the human brain, two sets of areas very similar to self and external awareness networks show a pattern of anticorrelated activity. Another recent fMRI study showed that auditory, visual and somatosensory systems exhibited significant negative correlation with self awareness or “default mode” network during the awake resting state [Tian et al., 2007]. The functional significance of these slow neural activity fluctuations remain only partly understood.
These studies show that even in the absence of any task or sensory stimulation, the activity in the areas involved in self and external awareness is not constant, but continuously fluctuates. The frequential characteristics of spontaneous BOLD oscillations were furthermore reported to be the same as those observed in human behavioral variability [Fox and Raichle, 2007]. In the work reviewed below, we hypothesized that such slow baseline brain activity fluctuations in areas involved in perception would be likely to influence subsequent sensory stimulus perception.
BASELINE BRAIN ACTIVITY FLUCTUATIONS PREDICT CONSCIOUS PERCEPTION
As previously noted, the origin of intra‐subject variability in perceptual experiments remains poorly understood. The aim of our study [Boly et al., 2007] was to test whether differences in prestimulus baseline brain activity could predict subsequent differences in subjective perception of external stimuli. We tested this hypothesis using an event‐related thulium‐YAG laser fMRI paradigm, assessing somatosensory perception (contrasting perceived versus unperceived identical stimuli) and pain‐intensity perception (contrasting identical stimuli perceived as more or less painful). Therefore, subjects received laser stimuli on the dorsum of their left hand. After each stimulus, subjects rated their sensory perception on a five point scale: P0, no stimulus perceived; P1, warm but not painful sensation; P2, P3, and P4, increasingly painful sensations. P2 was defined as mild pain, P4 as very painful. P3 was defined as an intermediate pain between P2 and P3. Stimuli were afterwards matched for their intensity in unperceived (P0 score) versus perceived (P1 score) conditions, and comparing two pain levels (P2 and P3 scores). Our main analysis looked for areas in which differences in brain activity shortly before the presentation of the stimuli (i.e., 3 s) could predict changes in subsequent stimulus perception, i.e., in stimulus awareness for the sensory range, or in pain intensity level for the painful range of intensities. We empirically chose to investigate baseline brain activity at a delay of 3 s before the onset of the stimulus, for reasons of haemodynamic physiology [Henson, 2003] and according to the time scale of spontaneous slow brain fluctuations (below 0.1 Hz) observed in previous resting‐state fMRI studies [Cordes et al., 2001; Fox et al., 2005].
Our first important finding was that conscious perception of near‐threshold sensory stimuli was predicted by increased baseline neural activity in lateral fronto–parietal network, 3 s before stimulus occurrence. This finding emphasizes the importance of fronto–parietal cortices activity in sensory awareness. Our results can also be related to the preferential metabolic impairment of higher order fronto–parietal regions, compared to the relative preservation of primary sensory cortices, in various states of altered consciousness [Laureys et al., 2004]. On the other hand, we also showed a facilitatory effect of high baseline activity in medial thalamus on external stimulus perception. This area has repeatedly been reported to be the interface between alertness and cognition in humans [Foucher et al., 2004; Kinomura et al., 1996]. These results are in line with a recently proposed model of external awareness [Dehaene and Changeux, 2005], predicting a facilitatory effect of an arousal‐related increase in regional spontaneous activity on external stimuli processing.
Conversely, we showed that sensory stimuli were predicted to be unperceived when preceding baseline activity is increased in self awareness network. These results might reflect a competition between conscious access to external stimuli and self‐referential processes. In other words, if the activity 3 s prior to stimulation in the lateral fronto–parietal network is high, the subject is in a state of high receptivity to the external world leading to higher chances of perception of the applied low intensity laser stimuli. Inversely, if the activity is high 3 s prior to stimulation in self awareness network, the subject is putatively more likely to be in a state of higher introspection or self‐oriented thoughts, and will have fewer chances to subsequently report the stimuli as consciously perceived. Our findings go against a widespread idea in theories of subjective awareness positing a self‐related “observer” function as an essential component without which awareness can not emerge [Baars et al., 2003]. In line with a recent study on sensorimotor processing [Goldberg et al., 2006], these results suggest that self‐related processes are not necessarily engaged in sensory awareness. In contrast to this view, they would rather be in competition with external stimulus perception.
Finally, with regard to pain, we showed that enhanced activity in bilateral insula and the posterior part of anterior cingulate cortex (part of the so‐called “pain matrix”) prior to high intensity laser stimuli predicts these stimuli to be scored as more painful. In other words, if the baseline cerebral activity in the pain‐related anterior cingulate cortex and insula is high prior to noxious stimulation, the subject is in a state of high receptivity to pain and is more likely to experience intensity‐matched laser stimuli as highly painful. On the contrary, low baseline activity in pain matrix decreases the likelihood to rate stimuli as painful. Our results pertain to findings in chronic pain patients [Hsieh et al., 1995], as well as previous findings by our and other laboratories on nonpharmacological cognitive modulation of pain during the hypnotic state [Kupers et al., 2005].
Figure 2 shows mean group peristimulus time histograms of baseline activity in regions detected as presenting predictive brain signals to stimulus perception, illustrating that in these areas, BOLD signal behave differently before the occurrence of the stimulus. In fact, a complementary analysis (Table I) showed us that the part of the baseline haemodynamic response function that is orthogonal to the BOLD response to the following stimulus was sufficient to explain our main results, both for stimulus awareness and for pain intensity perception. These data reinforce the evidence that our results originate from modifications in baseline brain activity before the presentation of stimuli, independently of stimuli presentation per se. According to Sapir et al. [ 2005] and Raichle and Gusnard [ 2005], the presence of endogenous predictive signals is the strongest evidence that can be obtained using correlational methods, for a causal link between neural activity and perception or behavior.
Figure 2.
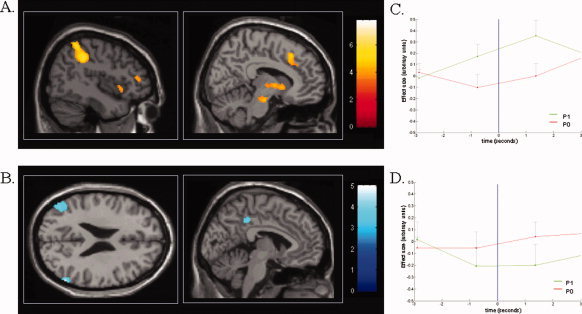
Baseline brain activity predicting conscious perception of subsequent somatosensory stimuli. (A) Increased baseline brain activity in medial thalamus (Th), dorsolateral prefrontal cortex (DLPF), intraparietal sulcus/posterior parietal cortex (IPS), and anterior cingulate cortex (ACC) 3 s before stimulus presentation predicts perception of low intensity sensory stimuli. (B) Decreased baseline activity in the default brain network encompassing posterior cingulate/precuneus (Pr) and bilateral temporo–parietal junctions (TP) exert a facilitatory effect on perception of subsequent somatosensory stimuli. Color scales refer to T‐values of individual voxels. Reproduced with permission from [Boly et al., 2007). (C and D) Graphical illustration of mean group peristimulus time histograms extracted from peak areas of significance for stimulus awareness contrasts, using a finite impulse response model approach as implemented in SPM2. Results are mean effect sizes (arbitrary units) with 95% confidence intervals. In intraparietal sulcus, baseline BOLD signal before the occurrence of the stimulus presentation (blue line) is higher for events that will be perceived (P1 scores, in green) as compared to events that will be unperceived (P0 scores, in red). An inverse response shape is observed in lateral temporoparietal junction.
Table I.
Complementary analysis confirming the predictor effect of baseline brain activity on subsequent stimulus perception
| Brain regions | Side | x | y | z | Z‐values | Uncorrected p | SVC corrected p |
|---|---|---|---|---|---|---|---|
| Facilitatory baseline activity effect on low intensity stimuli perception | |||||||
| Middle frontal gyrus | L | −58 | 6 | 32 | 3.77 | <0.001 | 0.008 |
| Intraparietal sulcus/PPC | R | 28 | −52 | 38 | 3.51 | <0.001 | 0.017 |
| L | −48 | −34 | 52 | 3.26 | 0.001 | 0.034 | |
| Anterior ACC | 4 | 22 | 40 | 3.77 | <0.001 | 0.008 | |
| Preventive baseline activity effect on low intensity stimuli perception | |||||||
| Temporo‐parietal junctions | R | 50 | −74 | 34 | 2.84 | 0.002 | 0.090 |
| L | −40 | −70 | 34 | 2.53 | 0.006 | 0.166 | |
| Parahippocampal gyrus | L | −30 | −28 | −20 | 2.43 | 0.008 | 0.198 |
| Inferior temporal cortex | L | −60 | −42 | −18 | 2.67 | 0.004 | 0.128 |
| Facilitatory baseline activity effect on high pain intensity perception | |||||||
| Posterior ACC | 8 | 8 | 42 | 3.14 | 0.001 | 0.046 | |
ACC, anterior cingulate cortex; PPC, posterior parietal cortex; SVC, small‐volume corrected.
Brain areas where the part of the baseline haemodynamic response function orthogonal to the BOLD response related to stimulus presentation show a significant difference depending on subsequent stimulus perception. Results are reported at uncorrected P‐value, and at P‐values corrected for multiple comparisons in a 10 mm‐radius small volumes of interest centered on a priori coordinates from (Boly, et al. 2007). This analysis confirms a significant (corrected P < 0.05) predictor effect of baseline brain activity of fronto–parieto–cingulate cortices on conscious stimuli perception. Though a similar trend was observed for default network areas, results did not reach statistical significance. Our results also confirm a significant predictor effect of pain‐related anterior cingulate cortex baseline activity on pain intensity perception. Coordinates are defined in Montreal National Institute space.
As a whole, our results thus show that stimulus‐unrelated, spontaneous baseline brain activity fluctuations in self and external awareness networks are likely to profoundly modify our conscious perception of the external world [Boly et al., 2007]. The source of spontaneous baseline brain activity fluctuations, observed in our study as in resting state fMRI experiments, remains to be assessed. We will next discuss possible sources of such spontaneous brain activity fluctuations.
SOURCES OF BRAIN ACTIVITY BASELINE FLUCTUATIONS
Because of the intrinsic temporal smoothing inherent to BOLD haemodynamic response function, our fMRI analysis necessarily focused on relatively slow baseline brain activity fluctuations. MEG studies also showed prestimulus baseline changes related to perception [Linkenkaer‐Hansen et al., 2004; Palva et al., 2005] in a higher frequency range. Our study was not designed to identify the source of baseline brain activity fluctuations observed, which remains to be assessed in future experiments. We here discuss possible origins of these fluctuations.
A first possible source of the baseline brain activity fluctuations we observed in our study could be fluctuations of the arousal level of our subjects throughout the experiment. As reported above, high medial thalamus activity predicts intensity‐matched stimuli to be perceived. Medial thalamus is known to be involved in vigilance and alertness [Kinomura et al., 1996; Portas et al., 1998]. This area has also been involved in attentional effects varying with the level of arousal [Coull et al., 2004]. In parallel, a sleep deprivation study in healthy volunteers showed that increases in alertness is also associated with increased regional metabolism in bilateral fronto‐parieto‐cingulate cortices [Thomas et al., 2000], both on the convexity and the midline. This vigilance‐related effect could thus potentially explain the facilitatory effect of thalamus and lateral frontoparietal network activity on perception. However, the facilitatory effect of decreased baseline default network activity on stimulus perception cannot be explained by vigilance modifications, since as for the lateral frontoparietal areas, activity in the default network is likely to increase in parallel with the subjects' arousal level [Thomas et al., 2000].
A second possible source of slow neural activity fluctuations would be spontaneous changes in selective attention. Our results demonstrate that high lateral fronto–parietal activity predicts increases in chances of perception of somatosensory stimuli. These findings are in line with a number of studies showing enhanced lateral fronto–parietal activity during increased visual attention (e.g., [Buschman and Miller, 2007; Weissman et al., 2006]). Our findings could thus possibly be interpreted to reflect attentional fluctuations in a stimulus‐awareness related network preceding stimulus presentation. On the other hand, several studies have shown that as attentional demands in cognitive tasks are increased, activity in the default system is decreased [McKiernan et al., 2003; Weissman et al., 2006], suggesting that spontaneous modifications in attention could explain our current findings. Finally, attention to pain has also been shown to increase BOLD signal in the pain matrix encompassing the anterior cingulate cortex and insula [Koyama et al., 2005].
Alternatively, several fMRI studies recently identified structured patterns of spontaneous neural activity fluctuations despite the absence of awareness in the primate brain. In particular, a recent study demonstrated the preservation of coherent BOLD fluctuations in deeply anesthetized macaque monkeys [Vincent et al., 2007]. Correlations among brain regions involved with the default‐mode network were also shown to persist during light sleep [Horovitz et al., in press]. These results suggest that spontaneous resting‐state BOLD fluctuations are an aspect of brain functional organization that may transcend levels of consciousness. As the brain is a complex system of interacting units, it is bound to oscillate and thus to have self‐organizing connectivity patterns emerging spontaneously which temporally link neurons into assemblies. Even if at least part of these oscillations do not correspond to the presence of a conscious content, they are thought to be functionally relevant and have been potentially associated with input selection, synaptic plasticity, temporal representation, andlong‐term consolidation of information [Buzsaki and Draguhn, 2004].
CONCLUSION
Though the neural correlates of external awareness begin to be better understood, much less is known about self awareness and its relationships with environmental perception. On the other hand, even in the absence of cognitive tasks or sensory stimulation, baseline brain activity in the areas involved in the genesis of conscious perception continuously fluctuates in the human brain. We showed that these baseline brain activity fluctuations are likely to profoundly modify our perception of the external world. Moreover, our data suggest that self and external awareness are not concurrent; they would rather be in competition during the process of sensory perception. Taken as a whole, these data suggest that baseline brain activity fluctuations are likely to shape the contents of our ongoing “stream of consciousness” in a decisive manner. However, the origin and physiology of these fluctuations remains a mystery, and will be the subject of future studies.
Acknowledgements
MB, EB, CP, SL and PM are respectively Research Fellow, Post‐Doctoral Research Fellow, Research Associate, Senior Research Associate and Research Director at FNRS.
REFERENCES
- Baars BJ,Ramsoy TZ,Laureys S ( 2003): Brain, conscious experience and the observing self. Trends Neurosci 26: 671–675. [DOI] [PubMed] [Google Scholar]
- Boly M,Balteau E,Schnakers C,Degueldre C,Moonen G,Luxen A,Phillips C,Peigneux P,Maquet P,Laureys S ( 2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104: 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ,Miller EK ( 2007): Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862. [DOI] [PubMed] [Google Scholar]
- Buzsaki G,Draguhn A ( 2004): Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- Cordes D,Haughton VM,Arfanakis K,Carew JD,Turski PA,Moritz CH,Quigley MA,Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Coull JT,Jones ME,Egan TD,Frith CD,Maze M ( 2004): Attentional effects of noradrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. Neuroimage 22: 315–322. [DOI] [PubMed] [Google Scholar]
- Dehaene S,Changeux JP ( 2005): Ongoing spontaneous activity controls access to consciousness: A neuronal model for inattentional blindness. PLoS Biol 3: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher JR,Otzenberger H,Gounot D ( 2004): Where arousal meets attention: A simultaneous fMRI and EEG recording study. Neuroimage 22: 688–697. [DOI] [PubMed] [Google Scholar]
- Fox MD,Raichle ME ( 2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Corbetta M,Van Essen DC,Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Zacks JM,Raichle ME ( 2006): Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg II,Harel M,Malach R ( 2006): When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 50: 329–339. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Raichle ME ( 2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Henson RN ( 2003): Analysis of fMRI time series In: Frackowiak R,Friston K, Frith C, Dolan R, Price C, Zeki S, Ashburner J, Penny W, editors. Human Brain Function, 2nd ed. London: Academic Press. [Google Scholar]
- Horovitz SG,Fukunaga M,de Zwart JA,van Gelderen P,Fulton SC,Balkin TJ,Duyn JH: Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG‐fMRI study. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC,Belfrage M,Stone‐Elander S,Hansson P,Ingvar M ( 1995): Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain 63: 225–236. [DOI] [PubMed] [Google Scholar]
- Kinomura S,Larsson J,Gulyas B,Roland PE ( 1996): Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271: 512–515. [DOI] [PubMed] [Google Scholar]
- Koyama T,McHaffie JG,Laurienti PJ,Coghill RC ( 2005): The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci USA 102: 12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R,Faymonville ME,Laureys S ( 2005): The cognitive modulation of pain: Hypnosis‐ and placebo‐induced analgesia. Prog Brain Res 150: 251–269. [DOI] [PubMed] [Google Scholar]
- Laureys S ( 2005): The neural correlate of (un)awareness: Lessons from the vegetative state. Trends Cogn Sci 9: 556–559. [DOI] [PubMed] [Google Scholar]
- Laureys S,Owen AM,Schiff ND ( 2004): Brain function in coma, vegetative state, and related disorders. Lancet Neurol 3: 537–546. [DOI] [PubMed] [Google Scholar]
- Linkenkaer‐Hansen K,Nikulin VV,Palva S,Ilmoniemi RJ,Palva JM ( 2004): Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci 24: 10186–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B,Zago L,Mellet E,Bricogne S,Etard O,Houde O,Crivello F,Joliot M,Petit L,Tzourio‐Mazoyer N ( 2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- McKiernan KA,Kaufman JN,Kucera‐Thompson J,Binder JR ( 2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- McKiernan KA,D'Angelo BR,Kaufman JN,Binder JR ( 2006): Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage 29: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S,Linkenkaer‐Hansen K,Naatanen R,Palva JM ( 2005): Early neural correlates of conscious somatosensory perception. J Neurosci 25: 5248–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L,Padmala S ( 2005): Quantitative prediction of perceptual decisions during near‐threshold fear detection. Proc Natl Acad Sci USA 102: 5612–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L,Gutierrez E,Bandettini P,Ungerleider L ( 2002): Neural correlates of visual working memory: Fmri amplitude predicts task performance. Neuron 35: 975–987. [DOI] [PubMed] [Google Scholar]
- Portas CM,Rees G,Howseman AM,Josephs O,Turner R,Frith CD ( 1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME,Gusnard DA ( 2005): Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol 493: 167–176. [DOI] [PubMed] [Google Scholar]
- Rees G ( 2007): Neural correlates of the contents of visual awareness in humans. Philos Trans R Soc Lond B Biol Sci 362: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D,Heeger DJ ( 2003): Neuronal correlates of perception in early visual cortex. Nat Neurosci 6: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A,d'Avossa G,McAvoy M,Shulman GL,Corbetta M ( 2005): Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA 102: 17810–17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C,Baillet S,Dehaene S ( 2005): Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 8: 1391–400. [DOI] [PubMed] [Google Scholar]
- Shulman GL,Fiez JA,Corbetta M,Buckner RL,Miezin FM,Raichle ME,Petersen SE ( 1997): Common blood flow changes across visual tasks. II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Thomas M,Sing H,Belenky G,Holcomb H,Mayberg H,Dannals R,Wagner H,Thorne D,Popp K,Rowland L,Welsh A,Balwinski S,Redmond D ( 2000): Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9: 335–352. [DOI] [PubMed] [Google Scholar]
- Tian L,Jiang T,Liu Y,Yu C,Wang K,Zhou Y,Song M,Li K ( 2007): The relationship within and between the extrinsic andintrinsic systems indicated by resting state correlational patterns of sensory cortices. Neuroimage 36: 684–690. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S,Palva JM,Holmes MD,Miller JW,Voipio J,Kaila K ( 2004): Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci USA 101: 5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL,Patel GH,Fox MD,Snyder AZ,Baker JT,Van Essen DC,Zempel JM,Snyder LH,Corbetta M,Raichle ME ( 2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86. [DOI] [PubMed] [Google Scholar]
- Weissman DH,Roberts KC,Visscher KM,Woldorff MG ( 2006): The neural bases of momentary lapses in attention. Nat Neurosci 9: 971–978. [DOI] [PubMed] [Google Scholar]


