Abstract
Convergent experimental evidence points to the cerebellum as a key neural structure mediating adaptation to visual and proprioceptive perturbations. In a previous study, we have shown that activity in the anterior cerebellum varies with the rate of learning, with fast learners exhibiting more activity in this region than slow learners. Here, we investigated whether this variability in behavior may partly reflect inter‐individual differences in the structural properties of cerebellar white‐matter output tracts. For this purpose, we used diffusion‐weighted magnetic resonance imaging to estimate fractional anisotropy (FA), and correlated the FA with the rate of adaptation to an optical rotation in 11 subjects. We found that FA in a region consistent with the superior cerebellar peduncle (SCP), containing fibers connecting the cerebellar cortex with motor and premotor cortex, was positively correlated with the rate of adaptation but not with the general level of performance or the initial deviation. The same pattern was observed in a region of the lateral posterior cerebellum. In contrast, FA in the angular gyrus of the posterior parietal cortex correlated positively both with the rate of adaptation and the overall level of performance. Our results show that the rate of learning a visuomotor task is associated with FA of cerebellar pathways. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: cerebellum, visuomotor adaptation, motor learning, motor control, diffusion weighted imaging, fractional anisotropy
INTRODUCTION
Our bodies change and so does our environment. The fact that we manage to perform accurate movements despite this variability is of major interest to the field of motor control. Convergent experimental evidence from human and nonhuman primates points to the cerebellum as a key structure in adapting to visual and proprioceptive perturbations of the environment. Extensive cerebellar damage compromises both the rate of adaptation and the magnitude of the after‐effect [Martin et al., 1996; Maschke et al., 2004; Smith and Shadmehr, 2005; Weiner et al., 1983; Werner et al., 2009]. Neurophysiological evidence suggests that during adaptation activity of climbing fibers, carrying information from the spinal cord, change the efficacy of parallel fibers, carrying information from the cerebellar cortex, at the Purkinje synapses [Gilbert and Thach, 1977]. These findings are consistent with the theory that the cerebellum is the site where motor memories are formed and maintained [Albus, 1971; Marr, 1969].
The rate of adaptation and the initial movement error vary across the population [e.g. Della‐Maggiore et al., 2008; Lackner, 1994]. Neuroimaging studies show that functional activity in the cerebellum covaries with the rate of learning and/or motor error. For example, total motor output correlates positively with activity in the anterior cerebellum during recall of an internal model 5.5 h after achieving adaptation to a force field [Shadmehr and Brashers‐Krug, 1997]. Motor error, measured as the maximal deviation of the arm when interacting with a force field, correlates positively with activity in the deep cerebellar nuclei during adaptation and negatively with activity in the anterior cerebellum 29 days later [Nezafat et al., 2001]. On the other hand, the rate of visuomotor adaptation when using a mouse with an optical rotation to track a visual target is associated with bilateral increments of activity around the posterior superior cerebellar fissure [Imamizu et al., 2000]. In summary, functional imaging studies have demonstrated that behavioral variation is associated with differences in activation patterns across the population.
The aim of the present study was to investigate whether inter‐individual variability in visuomotor adaptation may partly reflect differences in the structural microstructure of cerebellar white‐matter tracts. White‐matter structure have previously been shown to predict performance in different tasks such as learning of foreign speech sounds [Golestani et al., 2007] and visual memory [Begre et al., 2007]. Microstructural properties of white matter, such as axon caliber and myelin thickness, influence functionally relevant properties of axonal transduction, such as conduction velocity [Gillespie and Stein, 1983], and may thereby influence behavior. Here, we used diffusion‐weighted magnetic resonance imaging to characterize structural properties of white‐matter tracts through fractional anisotropy (FA), a voxel‐wise measure that is modulated by local factors such as myelin thickness, axon caliber, and packing density [Beaulieu, 2002]. Subjects performed a visuo‐motor adaptation task and we tested for correlation between behavioral and FA measures. We hypothesized that higher adaptation rates would be associated with greater FA values in white‐matter tracts within the cerebellum and/or those containing cerebellar projections to the motor regions of the cerebral cortex. Our previous work provides evidence that allows us to generate anatomically‐specific hypotheses for where such correlations may be found for this specific task. Previously, we have shown that activity of a motor network including the anterior cerebellum, the contralateral sensorimotor cortex and the cingulate motor area correlates with the rate of learning during the same adaptation task used here, with fast learners showing more activity in these areas than slow learners (Della‐Maggiore and McIntosh, 2005). The results of the current study show that the rate of learning a visuomotor task, but not the overall level of performance or the initial motor error, is associated with greater FA of cerebellar pathways.
METHODS
Experimental Procedures
Twelve right‐handed healthy participants (range 21–45 year, mean ± SD = 30 ± 8) were recruited for the experiment. Participants were screened to ensure none suffered from medical, neurological, or psychiatric disorders. During the experiment, participants used their right hand to track a target moving randomly on a screen with a joystick; a cursor represented the movement of the hand. After performing this task for 5 min, an optical perturbation evidenced by a rotation in the cursor direction relative to the hand was applied [for details see Della‐Maggiore and McIntosh, 2005]. Subjects performed six 5‐min blocks of the task with the perturbation and a final 5‐min block without the perturbation to measure after‐effects. The distance between target and cursor was sampled at 200 Hz and averaged for each block; it was used as measure of visuomotor error.
MRI Acquisition
Magnetic resonance brain images were acquired on a 1.5 Tesla Sonata scanner (Siemens, Erlangen, Germany) with a one‐channel head coil; this dataset has also been used in a different study [Johansen‐Berg et al., 2007]. Three sets of whole‐brain diffusion‐weighted images were acquired (60 directions, b‐value 1,000 s mm−2, 2.2 × 2.2 × 2.2 mm3, 60 slices). In addition, six images without diffusion weighting were acquired (Bo). Images were eddy‐current corrected and aligned to the first nondiffusion weighted image using the FSL toolbox (http://www.fmrib.ox.ac.uk/fsl).
Statistical Analysis
The FSL toolbox was used for statistical analysis. Tract‐based spatial statistics was used to compute correlations between behavioral measures and FA [Smith et al., 2006]. Although our working hypothesis focused on the cerebellum, we decided to compute the correlations across the whole brain so that we could also identify other brain tracts that may contribute to inter‐individual variability in adaptation. Briefly, FA maps were calculated for each individual using FDT and then aligned nonlinearly to FSL's FA template (generated form 58 FA maps) using FNIRT. The aligned images were averaged to generate a mean FA image. This mean FA image was “thinned” and thresholded at an FA value of 0.2 to generate a tract skeleton that included only the major white‐matter pathways. Finally, for each subject the FA values of local tract centers were projected onto this invariant tract representation.
Three behavioral measures were used to compute correlations with FA: the initial visuomotor error (IVE), the rate of visuomotor adaptation, and the level of performance. The rate of adaptation was quantified by fitting the time course of the visuomotor error (six data points in total) with a power function (y = a * × ∩b), of which b was the learning rate. The initial visuomotor error was the average of the error obtained during the first adaptation block. The level of performance during adaptation was inferred from the standard error (SE) of the regression, assuming that higher standard errors would reflect lower levels of performance than lower standard errors [Keating and Thach, 1990]. We tested for correlation between IVE, the rate of visuomotor adaptation “b,” the level of performance (SE) and FA in the whole brain after covarying for age, which has significant effects on FA [Pfefferbaum et al., 2000] and on the number of Purkinje cells after 40 years of age [Andersen et al., 2003]. We performed an exploratory, whole‐brain analysis, for which we used a t threshold of 3.169 (10° of freedom; P < 0.005 uncorrected one‐tail) and a cluster extent threshold greater than 15 voxels [Boorman et al., 2007] to define clusters of positive correlation.
RESULTS
Behavioral Results
One of the 12 participants was excluded from the analysis because the adaptation rate deviated more than three standard deviations from the sample mean.
Adaptation rate computed as the regression coefficient (b) and the IVE (first symbol) are shown for all subjects in Figure 1 (n = 11; df = 5; R 2 ranged from 0. 78 to 0.95). Statistical significance for each regression coefficient was assessed for each subject using t tests (P values ranged between 0.02 and 0.001). All subjects adapted to the perturbation. After‐effects, assessed by averaging the visuomotor error during the first minute of the last 5‐min block, were also present in all subjects (df = 10; t = 6.502, P < 0.0001), a finding consistent with the modification or generation of an internal model for the perturbation [Shadmehr and Mussa‐Ivaldi, 1994].
Figure 1.
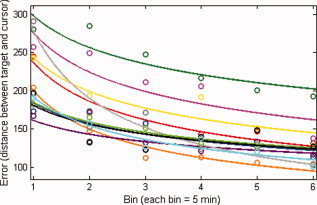
Behavioral results. Shown is the visuomotor error, computed as the distance between target and cursor, corresponding to the average of 5‐min blocks for all 11 subjects. Individual data was fit using a power function (y = a * × ∩b). Each color represents a different subject.
DWI Results
The TBSS analysis yielded four significant clusters in which FA correlated positively with the rate of adaptation (Fig. 2a). Two of these clusters were located in the ipsilateral (i.e. right) cerebellum; the first one in a region of the anterior cerebellar lobe consistent with the superior cerebellar peduncle (r = 0.75; 34 voxels; max t = 6.81; x = 6, y = −48, z = −26, t > 3.169, P < 0.005), and a second cluster, in the posterior cerebellar lobe, near the horizontal fissure (Crus I and Crus II; r = 0.82; 18 voxels; max t = 3.85; x = 32, y = −71, z = −30, t > 3.169, P < 0.005). The third cluster showing a positive correlation between FA and the rate of adaptation was located in the left inferior parietal lobule, in a region consistent with the angular gyrus (r = 0.92; 20 voxels, max t = 6.38, x = −21, y = −56, z = 28, t > 3.169, P < 0.005). Finally, a fourth cluster was identified in the left external capsule (r = 0.9; 28 voxels, max t = 4.94, x = −31, y = −20, z = 1, t > 3.169, P < 0.005).
Figure 2.
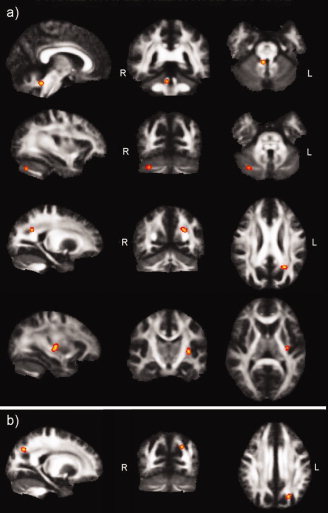
Tract‐based spatial statistics. (a) Clusters exhibiting a positive correlation between FA and the rate of visuomotor adaptation. Top panel shows the superior cerebellar peduncle cluster; second panel shows the cluster identified on the lateral cerebellum; third panel shows the cluster identified in the angular gyrus of the posterior parietal cortex and forth panel shows the cluster identified in the external capsule. (b) Clusters exhibiting a positive correlation between the overall level of performance and FA. Shown is the cluster identified in the angular gyurs, in the vicinity of that identified in (a).
As for the correlation between FA and the level of performance, only a region in the vicinity of the left parietal cluster (26 voxels, max t = 5.46, x = −18, y = −60, z = 37, t > 3.169, P < 0.005) exhibited a positive correlation between FA and the level of performance measured as the SE of the regression (Fig. 2b), suggesting that FA values in this area of the angular gyrus are not specific to the rate of learning but also contribute to the ability to perform the task. Fractional anisotropy in the cerebellum did not correlate with the SE of the regression or the IVE at the defined statistical threshold.
DISCUSSION
In this study, we explored the possibility that inter‐individual differences in cerebellar structure may in part explain inter‐individual variability in visuomotor adaptation. We found evidence in favor of this hypothesis. Fast learners showed higher values of fractional anisotropy than slow learners in the posterior cerebellum and in the superior cerebellar peduncle. By contrast, correlation between FA and the level of performance yielded no significant clusters in the cerebellum, indicating that anatomical variations in these areas relate to the rate of learning rather than an overall ability to perform the task. Factors that influence FA include myelination of axons, axonal diameter, or packing density of axons [Beaulieu, 2002] and any of these structural features could influence speed or efficiency of neural signals propagation, thereby influencing behavior. Our results show that the speed of visuomotor adaptation may be determined partly by cerebellar anatomy.
Neurophysiological studies point to the cortex as the site of motor‐memory storage in the cerebellum [Gilbert and Thach, 1977; Keating and Thach, 1990]. Our results identified a cluster in the posterior cerebellum, near the horizontal fissure (between Crus I and Crus II: HVIIA). Two different hand‐representations have been described in the cerebellum using fMRI [Grodd et al., 2001]. The larger is located in the anterior cerebellum (Lobules IV, V). The second smaller representation is located in the posterior cerebellum (HVIII), and is nearby the posterior cluster identified with TBSS. Given its anatomical location in the lateral cerebellum, this cluster may be part of a pathway connecting the cerebellar cortex with the dentate nucleus. The second cluster is located in the superior cerebellar peduncle. The SCP contains the main efferent connections to the thalamus. Most fibers passing through the SCP originate in the dentate nucleus, continue rostrally to the brain stem and decussate at the level of the inferior colliculus [Dum and Strick, 2003]. In addition, some of the fibers that pass through the SCP originate in the interposed nucleus. The majority of SCP fibers connect the cerebellum with motor and premotor areas of the cerebral cortex via the thalamus [Rouiller et al., 1994].
Although abundant experimental evidence points to a critical role of the cerebellum in adaptation, the site of adaptation in the cerebellar cortex is controversial. Neuroimaging studies looking for functional correlates of the rate of adaptation to proprioceptive perturbations have shown increased activity in regions corresponding to the anterior cerebellum and/or the dentate nucleus [Nezafat et al., 2001; Shadmehr and Holcomb, 1997]. On the other hand, adaptation to visual perturbations has been associated with activation of the anterior cerebellum [Della‐Maggiore and McIntosh, 2005], bilateral activation of the area around the posterior cerebellar fissure [Imamizu et al., 2000], and the posterior cerebellum [Krakauer et al., 2004]. Given that only the most anterior part of the cerebellum was imaged in our previous functional study [Della‐Maggiore and McIntosh, 2005], we cannot rule out the possibility that more posterior cerebellar areas may also take part in adaptation. The location of the posterior cluster identified here is more consistent with the results from the visuomotor functional studies by Imamizu et al. and Krakauer et al. although ipsilateral to the active hand.
The lesion literature is also controversial; small cerebellar lesions tend to yield no deficit whereas lesions or brain damage over large cortical areas tend to impair adaptation dramatically [Thach et al., 1992]. A few studies carried out in nonhuman primates suggest that the lateral cerebellar cortex, which projects to the dentate nucleus, is critical for adaptation. Inactivation of the somatosensory representation of this area (Lobules III, IV, and V and lateral within Crus I) slows the rate of visuomotor adaptation but does not affect the level of performance [Keating and Thach, 1990, 1995]. Reduced learning rate has also been observed in a patient with stroke of the ipsilateral superior cerebellar artery when performing the same task [Keating and Thach, 1990]. Yet, a different study from the same laboratory has shown that the rate of adaptation to reversed prisms is impaired in patients with stroke of the posterior cerebellar artery, which supplies the posterior cerebellar cortex and the inferior cerebellar peduncle, but not in patients with stroke of the superior cerebellar artery, which supplies the SCP, the dentate nucleus, and the anterior cerebellar cortex. Recent findings by Rabe et al. [ 2009] indicate that the topography of the lesion may affect differently force‐field and visuomotor adaptation. Cerebellar atrophy affecting more significantly the anterior cerebellum interferes with adaptation to a proprioceptive perturbation but not as much with adaptation to a visual perturbation. The opposite pattern is observed for lesions affecting more significantly the posterior cerebellum. The posterior cerebellar cluster identified in our study is consistent with the results from the last two patient studies [Martin et al., 1996; Rabe et al., 2009] whereas the SCP cluster is inconsistent with the findings from Martin et al. but is in agreement with those from Keating and Thach [ 1990]. Differences in the motor demands associated with each task (optical rotation when moving a cursor likely requires less coordinated control than adapting while throwing a ball with the arm) may account for the differences in functional topography.
What aspects of cerebellar processing could relate to the structural properties of these cerebellar clusters? The neural mechanism through which the cerebellum mediates adaptation remains unknown. It has been postulated that information about the motor plan conveyed by mossy fibers is contrasted with sensory feedback information conveyed by climbing fibers [Gilbert and Thach, 1977; Watanabe, 1984]. This error could be used to update the forward model so that predictions of the sensory consequences of movement based on an efferent copy of the motor command are accurate (forward learning, [Martin et al., 1996; Mazzoni and Krakauer, 2006; Miall et al., 2007; Tseng et al., 2007], or could be transformed into motor corrections that act as teaching signals for the brain (feedback learning [Kawato, 1996; Thoroughman and Shadmehr, 1999]). As mentioned previously, most efferent fibers passing through the SCP originate in the dentate nucleus. On the other hand, the posterior cerebellar cluster identified in this study is located in the lateral cerebellum, which in turn projects to the dentate nucleus. Higher FA may reflect, among other things, higher thickness of the myelin sheath [Beaulieu, 2002], which can influence conduction velocity [Gillespie and Stein, 1983]. Given that the dentate nucleus appears to be involved early in movement production, we can speculate that faster transduction of nervous signals might be beneficial to forward learning by increasing the likelihood of synaptic plasticity. For example faster conduction of cerebral signals to lateral cerebellar cortex could contribute to more effective synaptic plasticity at the parallel fiber‐Purkinje synapse. This would lead to a progressive reduction in error and may explain the contribution of the posterior cluster. Likewise for the SCP, faster conduction speed of output signals from the cerebellum may be essential for synaptic plasticity at the level of the motor cortex, which may also contribute to faster learning. Further examination using a longitudinal design may help elucidate the role of these cerebellar regions in adaptation.
Acknowledgements
The authors thank Kate Watkins and Steve Smith for aiding in the initial processing of the data.
REFERENCES
- Albus J ( 1971): A theory of cerebellar function. Math Biosci 10: 25–61. [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B ( 2003): Aging of the human cerebellum: A stereological study. J Comp Neurol 466: 356–365. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15: 435–455. [DOI] [PubMed] [Google Scholar]
- Begré S, Frommer A, von Känel R, Kiefer C, Federspiel A ( 2007): Relation of white matter anisotropy to visual memory in 17 healthy subjects. Brain Res 1168: 60–66. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen‐Berg H ( 2007): Individual differences in white‐matter microstructure reflect variation in functional connectivity during choice. Curr Biol 17: 1426–1431. [DOI] [PubMed] [Google Scholar]
- Della‐Maggiore V, McIntosh AR ( 2005): Time course of changes in brain activity and functional connectivity associated with long‐term adaptation to a rotational transformation. J Neurophysiol 93: 2254–2262. [DOI] [PubMed] [Google Scholar]
- Della‐Maggiore V, Johansen‐Berg H, Paus T ( 2008): The Rate of Visuomotor Adaptation is Associated With White Matter Integrity of Cerebellar Projections to Motor Cortices, Washington DC. Abstr Soc Neurosci 166: 10. [Google Scholar]
- Dum RP, Strick PL ( 2003): An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89: 634–639. [DOI] [PubMed] [Google Scholar]
- Gilbert PF, Thach WT ( 1977): Purkinje cell activity during motor learning. Brain Res 128: 309–328. [DOI] [PubMed] [Google Scholar]
- Gillespie MJ, Stein RB ( 1983): The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res 259: 41–56. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C ( 2007): Brain structure predicts the learning of foreign speech sounds. Cereb Cortex 17: 575–582. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M ( 2001): Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M ( 2000): Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T ( 2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. Neuroimage 36 ( Suppl 2): T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M ( 1996): Learning internal models of the motor apparatus In: Bloedel JRET, Wise SP, editors. The Acquisition of Motor Behaviors in Vertebrates. Cambridge, MA: MIT Press; pp 409–430. [Google Scholar]
- Keating JG, Thach WT ( 1990): Cerebellar motor learning: Quantitation of movement adaptation and performance in rhesus monkeys and humans implicates cortex as the site of adaptation. Abstr Soc Neurosci 16: 762. [Google Scholar]
- Keating JG, Thach WT ( 1995): Nonclock behavior of inferior olive neurons: Interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol 73: 1329–1340. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C ( 2004): Differential cortical and subcortical activations in learning rotations and gains for reaching: A PET study. J Neurophysiol 91: 924–933. [DOI] [PubMed] [Google Scholar]
- Lackner JRDP ( 1994): Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72: 299–313. [DOI] [PubMed] [Google Scholar]
- Marr D ( 1969): A theory of cerebellar cortex. J Physiol 202: 437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT ( 1996): Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119 ( Part 4): 1183–1198. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J ( 2004): Hereditary cerebellar ataxia progressively impairs force adaptation during goal‐directed arm movements. J Neurophysiol 91: 230–238. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW ( 2006): An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Christensen LO, Cain O, Stanley J ( 2007): Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5: e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH ( 2001): Long‐term adaptation to dynamics of reaching movements: A PET study. Exp Brain Res 140: 66–76. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M ( 2000): Age‐related decline in brain white matter anisotropy measured with spatially corrected echo‐planar diffusion tensor imaging. Magn Reson Med 44: 259–268. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O ( 2009): Adaptation to visuomotor rotation and force field perturbations is correlated to different brain areas in patients with cerebellar degeneration. J Neurophys 101: 1061–1971. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang F, Babalian A, Moret V, Wiesendanger M ( 1994): Cerebellothalamocortical and pallidothalamocortical projections to the primary and supplementary motor cortical areas: A multiple tracing study in macaque monkeys. J Comp Neurol 345: 185–213. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa‐Ivaldi FA ( 1994): Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb ( 1997): Neural correlates of motor memory consolidation. Science 277: 821–825. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R ( 2005): Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG ( 1992): The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15: 403–442. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R ( 1999): Electromyographic correlates of learning an internal model of reaching movements. J Neurosci 19: 8573–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ ( 2007): Sensory prediction errors drive cerebellum‐dependent adaptation of reaching. J Neurophysiol 98: 54–62. [DOI] [PubMed] [Google Scholar]
- Watanabe E ( 1984): Neuronal events correlated with long‐term adaptation of the horizontal vestibulo‐ocular reflex in the primate flocculus. Brain Res 297: 169–174. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH ( 1983): Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33: 766–772. [DOI] [PubMed] [Google Scholar]
- Werner S, Bock O, Timmann D ( 2009): The effect of cerebellar cortical degeneration on adaptive plasticity and movement control. Exp Brain Res 193: 189–196. [DOI] [PubMed] [Google Scholar]


