Abstract
Recent evidence suggests that deficits of working memory may be a promising neurocognitive endophenotype of bipolar affective disorder. However, little is known about the neurobiological correlates of these deficits. The aim of this study was to determine possible pathophysiological trait markers of bipolar disorder in neural circuits involved in working memory. Functional magnetic resonance imaging was performed in 18 euthymic bipolar patients and 18 matched healthy volunteers using two circuit‐specific experimental tasks established by prior systematic neuroimaging studies of working memory. Both euthymic bipolar patients and healthy controls showed working memory‐related brain activations that were highly consistent with findings from previous comparable neuroimaging studies in healthy subjects. While these patterns of brain activation were completely preserved in the bipolar patients, only the patients exhibited activation of the right amygdala during the articulatory rehearsal task. In the same task, functional activation in right frontal and intraparietal cortex and in the right cerebellum was significantly enhanced in the patients. These findings indicate that the right amygdala is pathologically activated in euthymic bipolar patients during performance of a circuit‐specific working memory task (articulatory rehearsal). This pathophysiological abnormality appears to be a trait marker in bipolar disorders that can be observed even in the euthymic state and that seems to be largely independent of task performance and medication. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: brain imaging, limbic system, psychotic disorders, biological subtype, genetic effect
INTRODUCTION
Bipolar affective disorder is one of the most debilitating illnesses worldwide [Murray and Lopez, 1996]. It is characterized by recurrent episodes of mania and depression which gives rise to the assumption that mood instability and an impaired regulation of emotional states may be the core of the disorder [Phillips and Vieta, 2007]. Consequently, research into the pathophysiological basis of bipolar disorder has especially focused on possible abnormalities of brain areas involved in emotion processing by using paradigms known to activate those brain regions, e.g. facial expression identification tasks. The findings of these studies indicate quite consistently hyperactivation of brain regions subserving affective processing both in symptomatic (depressive or manic) and in asymptomatic (euthymic) bipolar patients [Blumberg et al., 2005; Lawrence et al., 2004; Malhi et al., 2004; Pavuluri et al., 2007; Rich et al., 2006; Yurgelun‐Todd et al., 2000].
Beside these findings from affective neuroscience, evidence from many neuropsychological studies converges to suggest that deficits in cognitive control processes, including attention and working memory, are also highly prevalent in bipolar patients [MacQueen et al., 2005]. As these deficits persist into the euthymic state they have been considered to be trait markers of bipolar disorder. Only few studies have investigated the neurobiological correlates of these deficits, and whether limbic brain areas also show abnormal hyperactivity during nonemotional, cognitive tasks. Some of these studies have used the Stroop task, which requires cognitive control processes to overcome prepotent response tendencies. Overall, findings from these studies are inconclusive by showing a mixed picture of hyper‐ and hypoactivations in several prefrontal subregions (in particular orbitofrontal cortex, medial prefrontal cortex including the anterior cingulate region, and dorsolateral prefrontal cortex) as well as in the basal ganglia [Blumberg et al., 2003a, b; Gruber et al., 2003, 2004; Kronhaus et al., 2006; Strakowski et al., 2005]. Few other neuroimaging studies addressed working memory dysfunctions which recently have been proposed to represent promising neurocognitive endophenotypes of bipolar affective disorder [Glahn et al., 2004], i.e. biomarkers that may help to identify genetic and other neurobiological factors in the pathogenesis of the disorder. These neuroimaging studies on working memory in bipolar disorder did not reveal a consistent picture either. In particular, brain regions that are known to be crucially involved in working memory, e.g. prefrontal, parietal, and frontomedian cortical areas, have been reported to be either hypoactive [Frangou, 2005; Lagopoulos et al., 2007] or hyperactive [Adler et al., 2004; Chang et al., 2004] in bipolar patients.
One possible reason for the discrepant results of previous studies may be the relatively low specificity of some of the experimental tests applied in these studies with respect to selective engagement of neural systems in the tasks. For instance, this is the case for the n‐back task which besides basic working memory functions requires more complex cognitive processes such as memory for serial order, sequencing and updating of the contents of working memory, leading to coactivation of several different neural networks [Gruber and von Cramon, 2003].
To achieve a more selective testing of the functional integrity of different brain systems involved in human working memory, for the present neuroimaging study we adapted more refined and specific established paradigms from experimental psychology. In a systematic reevaluation of the functional neuroanatomy of human working memory, a series of prior fMRI studies using these paradigms in healthy subjects had consistently demonstrated that two at least partially dissociable brain systems underlie verbal working memory in humans [Gruber, 2001; Gruber and von Cramon, 2001, 2003]. One of these systems is represented by a left‐lateralized network of brain regions, including Broca's area, left lateral and medial premotor cortex, intraparietal cortex, and the contralateral (right) cerebellum, which are involved in the articulatory rehearsal of phonological information. The other, more bilateral brain system comprises the anterior middle frontal gyrus, the inferior parietal lobule, deep frontal opercular cortex, medial frontal cortices and the cerebellum, and subserves the nonarticulatory maintenance of the same phonological information, e.g. if subjects are prevented from using the (more efficient) articulatory mechanism (see Methods). In the present fMRI investigation the same experimental tasks were applied to assess pathophysiological abnormalities in these two subsystems of verbal working memory in bipolar patients. Because only trait markers may qualify as endophenotypic markers for the disorder, we included only patients with a currently euthymic state into this study.
MATERIALS AND METHODS
Subjects
18 euthymic patients with bipolar affective disorder according to both ICD‐10 and DSM‐IV classification systems and 18 healthy comparison subjects participated in this study. All subjects were consistent right‐handers as assessed by the Edinburgh Inventory. After completing the description of the study to the subjects, written informed consent was obtained.
Patients were recruited at the outpatients departments of the Central Institute of Mental Health in Mannheim and the Saarland University Hospital in Homburg. The diagnosis of bipolar I disorder was confirmed by using the German language version of the Structural Clinical Interview for DSM‐IV. Exclusion criteria were substance abuse or dependence, other current comorbid psychiatric disorders, acute suicidal tendency, and a history of neurological illness or severe brain injury. Severity of psychopathology was evaluated with the Young Mania Rating Scale (YMRS), and the Hamilton Depression Scale (HAMD) or the Montgomery Asberg Depression Rating Scale (MADRS). “Euthymia” was defined as scores of less than 7 on these scales. All patients were in a remitted state for at least one month. Patients had to be either free from medication or on a stable dose of medication for at least 2 weeks prior to the experiment. Most of the investigated patients were taking medication at the time of the study: 12 were receiving mood stabilizers (5 lithium, 5 valproic acid, 2 carbamazepine, and 2 lamotrigine), 4 were taking neuroleptics (3 atypical, 1 typical), 6 were receiving antidepressants (3 SSRIs, 3 mirtazapine, 2 venlafaxine), and 4 were taking benzodiazepines. Three bipolar patients were currently free from any medication.
Healthy control subjects were recruited from hospital staff, medical students, and the community, and were balanced for age, gender, and level of education. All patients and control subjects were Caucasian. The demographic characteristics and working memory performance rates of both groups are displayed in Table I.
Table I.
Sample demographic variables and working memory performance
| Bipolar disorder | Control sample | |
|---|---|---|
| No. of subjects | 18 | 18 |
| Gender (% female) | 44.4 | 61.1 |
| Age at evaluation | 38.2 (9.9) | 33.9 (11.5) |
| Education (y) | 14.4 (3.1) | 15.8 (2.2) |
| YMRS | 2.3 (0.9) | — |
| HAMD (n = 13) | 0.3 (0.2) | — |
| MADRS (n = 5) | 5.2 (0.7) | — |
| Performance rates (% correct) | ||
| Articulatory rehearsal | 86.7 (11.0) | 91.9 (6.6) |
| Nonarticulatory maintenance | 83.4 (12.7) | 88.2 (7.0) |
| Response times (ms) | ||
| Articulatory rehearsal | 1,180 (151) | 1,114 (267) |
| Nonarticulatory maintenance | 1,178 (167) | 1,136 (282) |
Experimental Design
Subjects performed two established variants of a verbal delayed matching to sample task, which had been demonstrated by previous studies to reliably activate two different brain systems that together make up the dual architecture of verbal working memory in humans [Gruber, 2001; Gruber and von Cramon, 2001, 2003]. In both task variants, four different letters were visually presented for 2 s, followed by a delay of 4 s during which a fixation cross was displayed. Then a probe letter was presented for 1.5 s, followed by a 1.5‐s blank screen. Within this 3 s response window subjects had to indicate via button press whether the probe letter matched one of the target letters presented before or not. With regard to this visual presentation of stimulus material (see Fig. 1) all of the conditions (working memory and letter‐case judgment tasks) were completely matched. The two working memory task variants differed only with respect to the precise instructions that were given to the subjects prior to the respective session on how to perform the working memory task. In one variant they were instructed to intensely use (sub)articulatory rehearsal (sometimes referred to as “inner speech”) to remember the letters presented. Performance of this articulatory rehearsal task reliably activates a left‐lateralized network of brain regions including Broca's area, left lateral and medial premotor cortex, intraparietal cortex, and the contralateral (right) cerebellum [Gruber, 2001; Gruber and von Cramon, 2003]. By contrast, in the other task variant participants were instructed to use a nonarticulatory phonological memory strategy, i.e. to remember the phonological identity of the letters without using articulatory rehearsal (phonological maintenance task). This latter strategy was forced by the usage of the articulatory suppression technique, which has been applied in multiple studies to prevent human subjects from using articulatory rehearsal for working memory [Baddeley et al., 1984; Murray, 1968]. Like in prior functional neuroimaging studies, the subjects had to subvocalize “one, two, three, four, one, two …” in a repetitive and rapid manner, paced by tones that were presented throughout the delay interval (in each experimental condition). Using exactly the same technique, these preceding neuroimaging studies had repeatedly shown that, when articulatory mechanisms are not available for working memory purposes because they are needed for simple concurrent articulations, performance of the working memory task relies on a second, bilateral brain system that comprises the anterior middle frontal gyrus, the inferior parietal lobule, deep frontal opercular cortex, medial frontal cortices, and the cerebellum [for details see Gruber, 2001; Gruber and von Cramon, 2001, 2003; Henseler et al., 2008].
Figure 1.
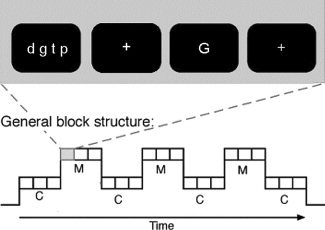
Example for the general design of single trials (of either the verbal delayed matching to sample task or the letter‐case judgment task) and the block structure of the experiment. M stands for blocks comprising working memory trials and C for blocks comprising control (letter‐case judgment) trials.
Letter‐case judgment tasks performed on the single probe letters either with or without preceding articulatory suppression served as well‐matched comparison conditions and allowed to dissociate activations related to working memory from more general activations emanating from other (sensory, (sub)articulatory and motor) components of the tasks.
The experiment consisted of two runs, each being composed of 2 × 6 alternating 30‐s‐blocks of one variant of the verbal working memory task and its corresponding control condition (see Fig. 1). The order of the two sessions, i.e. of the two working memory task variants (articulatory rehearsal and phonological maintenance) was systematically varied across subjects and balanced across groups. Prior to each run, subjects were verbally instructed which task variant they had to perform during the following run (i.e. with or without articulatory suppression during the delay intervals). Blocks consisted of three trials of the same task type (3 × 9 s), and a 3 s‐cue at the beginning of each block indicated whether memory tasks or judgment tasks had to be performed in the upcoming block (see Fig. 1). All stimuli were generated using ERTS software (Experimental run time system, Version 3.11, BeriSoft cooperation, Frankfurt am Main, Germany).
FMRI Data Acquisition
All stimuli were visually presented on a screen as white stimuli on black background, except for the task cues, which were presented in yellow color. Subjects underwent fMRI at 1.5 T (Siemens Vision; voxel size 3.6 × 3.6 × 4 mm3, interscan interval 2,500 ms, TE 50 ms, distance factor 12%, flip angle 90°, field of view 230 mm, 64 × 64 matrix). There were two experimental runs during each of which a total of 271 functional image volumes were acquired, each consisting of 26 axial slices parallel to the AC‐PC plane (slice acquisition in ascending order). Functional imaging was synchronized with stimulus presentation by means of ERTS (Experimental run time system, Version 3.11, BeriSoft cooperation, Frankfurt am Main, Germany). Additionally, a high‐resolution, T1‐weighted 3D anatomical set (MPRAGE sequence, TE 4.42 ms, TR 11.9 ms, flip angle 15°, field of view 256 × 256 mm2, voxel size 1 × 1 × 1 mm3, 176 consecutive slices) was collected for each subject.
Data Preprocessing and Statistical Analysis
Demographic and behavioral data were analyzed using SPSS (version 15.0). The analysis of between‐group differences in these variables was conducted by means of one‐way ANOVA.
Functional imaging data were processed using the SPM2 software package (http://www.fil.ion.ucl.ac.uk/spm/spm2.html). The first five volumes of each run were discarded. Preprocessing comprised coregistration, corrections for motion artifacts, time differences in slice acquisition, global signal intensity variation, and low‐frequency fluctuations (high‐pass filter with 128‐s cutoff), normalization into standard stereotactic space, and spatial smoothing with a Gaussian kernel (FWHM = 12 mm). To examine working memory‐related brain activity in individual subjects, we computed voxel‐wise t‐statistics for each working memory task compared to its control condition using the general linear model with the four experimental conditions (working memory with/without articulatory suppression, letter‐case judgment with/without articulatory suppression) as regressors (regressors were convolved with the standard HRF). For group statistics, random effects analyses were performed on these single subject contrast images.
Significant brain activations in each group were searched for using a statistical threshold of P < 0.001, uncorrected. Corrections for multiple comparisons were performed at the voxel‐level using false discovery rate‐ (FDR‐) correction at P < 0.05 (activations that also exceeded the stricter criterion of family‐wise error‐correction are highlighted in the results section). Subsequently, we statistically determined significant group differences in task‐related brain activation between patients and control subjects with an ANOVA. These between‐group comparisons were restricted to those brain regions (i.e. voxels), which were also found to be significantly activated (P < 0.05, FDR‐corrected at the voxel‐level) by working memory performance in the patient or the control group, by using these within‐group effects as a mask for the between‐group statistics (i.e. control group within‐group effects as a mask for the contrast “controls>bipolars”, and patients' group within‐group effects as a mask for the contrast “bipolars>controls”). In this way, we were able to focus the statistical analysis of group differences on brain regions that were associated with performance of the working memory tasks in the respective group, and to exclude possible group differences related to activations in the control tasks from further analysis. For these between‐group effects, again a statistical threshold of P < 0.001, uncorrected, with a correction for false discovery rate at P < 0.05 (voxel‐level) was applied.
RESULTS
Demographic Measures and Behavioral Analysis
Demographic characteristics and working memory performance of the patient and control groups are shown in Table I. As a result of prior balancing of groups, the groups did not differ from each other with regard to age (F(1,34) = 1.46, P = 0.24), gender (F(1,34) = 0.98, P = 0.33), or years of education (F(1,34) = 2.45, P = 0.13).
Behavioral performance data were normally distributed according to Kolmogorov‐Smirnov test (articulatory rehearsal: Z = 0.74, P = 0.64; nonarticulatory maintenance of phonological information: Z = 0.78, P = 0.57). One‐way ANOVA revealed no significant between‐group effects in working memory task performance, but only a slight trend for minor performance rates of bipolar patients in the articulatory rehearsal task (articulatory rehearsal: F(1,34) = 2.84, P = 0.10; nonarticulatory maintenance of phonological information: F(1,34) = 2.02, P = 0.16). Furthermore, in both working memory tasks, response times were not significantly different between groups (articulatory rehearsal: F(1,34) = 0.82, P = 0.37; nonarticulatory maintenance of phonological information: F(1,34) = 0.30, P = 0.59).
FMRI Analysis
Within‐group analysis
Significant brain activations during performance of the two working memory tasks (in comparison to the corresponding control tasks) are shown for each group in Table II. Overall, activations were highly consistent with findings from prior studies using the same verbal delayed matching to sample tasks in healthy volunteers [Gruber, 2001; Gruber and von Cramon 2001, 2003]. Both in healthy subjects and in the euthymic bipolar patients, articulatory rehearsal of verbal information in working memory was associated with activation of a network of lateral and medial frontal, intraparietal and cerebellar cortical as well as subcortical (caudate nucleus) areas (Table IIA, Fig. 2). During nonarticulatory maintenance of phonological information in working memory, both groups showed activation in a bilateral network of (particularly anterior) lateral and medial prefrontal as well as parietal cortices, in the deep frontal opercular cortex, the cerebellum and the caudate nucleus (Table IIB, Fig. 3). While these overall patterns of brain activation associated with performance of the two verbal working memory tasks appeared to be completely preserved in the bipolar patients, patients exhibited additional activations in the right amygdala and the left anterior fusiform gyrus during articulatory rehearsal, which—even at a lowered statistical criterion of P < 0.05, uncorrected—were not present in the control subjects.
Table II.
Brain activations elicited by verbal working memory task performance in healthy controls and in euthymic bipolar patients
| Region | Statistical effects (T value) | |
|---|---|---|
| Control subjects | Bipolar patients | |
| (A) Activations during articulatory rehearsal | ||
| L inferior frontal gyrus (Broca) | −56 8 20 (6.37**) | −56 4 16 (8.15**) |
| (pre‐)SMA | −4 4 60 (5.72**) | 0 8 56 (7.41**) |
| L precentral gyrus | −48 −4 44 (7.00**) | −52 0 44 (9.30**) |
| R precentral gyrus | 56 0 44 (5.75**) | 56 −4 44 (7.87**) |
| L intraparietal cortex | −24 −64 40 (4.65**) | −24 −60 56 (6.30**) |
| R intraparietal cortex | 28 −48 36 (2.70*) | 28 −48 52 (4.70**) |
| L cerebellum | −20 −52 −32 (4.04) | −20 −60 −28 (5.44**) |
| R cerebellum | 24 −64 −28 (5.69**) | 24 −64 −28 (7.73**) |
| L caudate nucleus | −16 −12 24 (3.57) | −24 −16 24 (3.07) |
| R caudate nucleus | 20 4 20 (3.92) | 20 −12 20 (3.81) |
| L anterior fusiform gyrus | n.s. | −32 4 −40 (4.36) |
| L superior temporal gyrus | −52 −40 12 (4.86**) | 68 −36 8 (3.34) |
| L inferior temporal gyrus | −48 −48 −24 (3.54) | −48 −48 −28 (4.42) |
| R frontal eye field | 32 0 48 (1.73*) | 20 −8 56 (4.92**) |
| R middle frontal gyrus | 40 48 28 (1.86*) | 36 40 32 (4.02) |
| R amygdala | n.s. | 28 0 −24 (3.09) |
| (B) Activation during nonarticulatory phonological maintenance | ||
| L anterior middle frontal gyrus | −36 52 12 (4.34) | −32 52 12 (4.10) |
| R anterior middle frontal gyrus | 36 44 28 (6.00**) | 40 40 28 (5.34**) |
| L inferior parietal lobule | −44 −40 40 (4.68**) | −40 −40 44 (4.92**) |
| R inferior parietal lobule | 48 −40 40 (3.18) | 44 −40 44 (4.21) |
| (pre‐)SMA/ACC | 0 12 52 (7.74**) | 0 8 56 (5.30**) |
| L intraparietal cortex | −28 −60 40 (6.18**) | −28 −60 56 (4.64**) |
| R intraparietal cortex | 32 −56 44 (5.50**) | 44 −40 44 (4.21) |
| L deep frontal opercular cortex | −40 16 −4 (5.64**) | −36 20 12 (6.36**) |
| R deep frontal opercular cortex | 32 24 −4 (6.49**) | 32 24 −4 (5.95**) |
| L cerebellum | −36 −56 −36 (4.89**) | −32 −56 −36 (2.44*) |
| R cerebellum | 24 −64 −28 (5.16**) | 28 −60 −32 (3.55) |
| L caudate nucleus | −16 −4 16 (4.41) | −16 8 8 (4.85**) |
| R caudate nucleus | 20 4 16 (3.87) | 16 4 4 (5.15**) |
| L precentral/inferior frontal cortex | −44 8 24 (7.63**) | −40 4 28 (7.08**) |
| L inferior temporal gyrus | −48 −56 −16 (4.81**) | −48 −52 −24 (3.19) |
| L brainstem | −4 −24 −16 (5.95**) | −4 −24 −12 (2.55*) |
The values given are the stereotactic (MNI) coordinates and (in parentheses) the T values of the activation maxima within each anatomical region (as determined by the statistical contrasts between each working memory task and its corresponding control task).
All activations were significant at P < 0.05, FDR‐corrected for multiple comparisons at the voxel‐level, if not indicated otherwise (*P < 0.05, uncorrected; **P < 0.05, FWE‐corrected).
Figure 2.
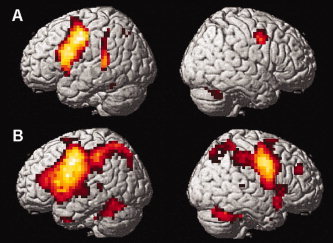
Brain activations associated with articulatory rehearsal in verbal working memory in (A) healthy controls and (B) euthymic bipolar patients. For illustration purposes, activation maps were statistically thresholded at P < 0.001, uncorrected, and displayed on the rendered surface of the standard MNI‐template. Local activation maxima in all depicted brain regions reached a threshold of P < 0.05, FDR‐corrected; see Table IIA for coordinates of activation foci and statistical significances. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
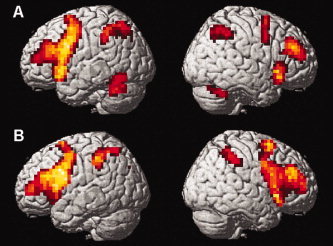
Brain activations associated with nonarticulatory phonological maintenance in verbal working memory in (A) healthy controls and (B) euthymic bipolar patients. For illustration purposes, activation maps were statistically thresholded at P < 0.001, uncorrected, and displayed on the rendered surface of the standard MNI‐template. Local activation maxima in all depicted brain regions reached a threshold of P < 0.05, FDR‐corrected; see Table IIB for coordinates of activation foci and statistical significances. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Between‐group analysis
Between‐group analysis was performed to statistically confirm the descriptive group differences of the within‐group analyses, and to detect possible further, merely quantitative differences in the brain activations elicited by the two verbal working memory tasks. This analysis confirmed the significance of the described group differences with regard to the abnormal amygdala activation in bipolar patients (see Table III, Fig. 4), but not with regard to the additional activation of left anterior fusiform gyrus observed in the patient group. Furthermore, significant quantitative differences in the extent of activation of those brain areas, which were actually found to be activated in both groups during articulatory rehearsal, showed up right‐lateralized in the precentral gyrus, the intraparietal cortex, the cerebellum, and the frontal eye field (Table III).
Table III.
Significant group differences in brain activation during verbal working memory task performance
| Region | MNI coordinates | T value |
|---|---|---|
| Articulatory rehearsal: Bipolar patients > healthy controls | ||
| R amygdala | 24 0 −24 | 3.52 |
| R precentral gyrus | 56 −12 32 | 3.70 |
| R intraparietal cortex | 12 −52 64 | 3.45 |
| R cerebellum | 24 −40 −20 | 3.36 |
| R frontal eye field | 24 −12 60 | 3.28 |
| Articulatory rehearsal: Healthy controls > bipolar patients | ||
| No significant hypoactivations in bipolar patients | ||
| Non‐articulatory phonological maintenance | ||
| No significant group differences in brain activation | ||
Results are based on statistical contrasts between each working memory task and its corresponding control task.
All activations were significant at P < 0.05, FDR‐corrected for multiple comparisons.
Figure 4.
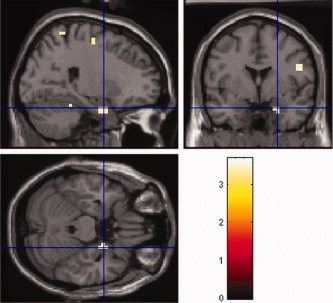
Pathological activation of the right amygdala as found in euthymic patients with bipolar affective disorder during articulatory rehearsal in verbal working memory. Depicted is the direct comparison between bipolar patients and controls at a statistical threshold of P < 0.001, uncorrected, masked with the within‐group effect in bipolar patients at P < 0.05, FDR‐corrected at the voxel‐level (see Table III for coordinates of activation foci and statistical significances). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Correlation analyses
To test whether the observed hyperactivations were correlated with individual differences in working memory performance, additional correlation analyses were performed between the strength of individual activation in the hyperactivated brain regions as indexed by the beta values (i.e. effect sizes derived from the contrast articulatory rehearsal minus control task) within the respective peak voxel of activation, on the one side, and the individual performance rates (accuracy), on the other. Pearson's correlation coefficients between performance rate and activation in the amygdala, right premotor, and right intraparietal cortex were 0.098 (P = 0.58), −0.161 (P = 0.36), and −0.118 (P = 0.51), respectively.
Moreover, correlation analyses were also performed between the strength of individual activation in the amygdala, on the one side, and in the right premotor and intraparietal cortex, on the other. Activity in the amygdala was significantly correlated both with activity in the right premotor cortex (ρ = 0.434, P = 0.009) and with activity in the right intraparietal cortex (ρ = 0.375, P = 0.026), although the strongest correlation was found between right premotor and right intraparietal cortex (ρ = 0.605, P < 0.001).
DISCUSSION
Two neural networks that together underlie verbal working memory in humans were assessed in 18 euthymic bipolar patients and 18 well‐matched healthy controls, which so far is one of the largest samples of bipolar patients studied with fMRI. Because verbal working memory disturbances have been proposed to represent promising endophenotypic markers of bipolar disorder [Glahn et al., 2004], the detection of neuropathophysiological changes associated with these disturbances may provide further insight into this important disorder. Both the bipolar patients and the healthy control subjects reliably activated the brain systems, which had been demonstrated by several previous studies to subserve the articulatory and the nonarticulatory mechanism of verbal working memory [Gruber, 2001; Gruber and von Cramon, 2001, 2003]. Despite a slight trend towards minor performance in the articulatory rehearsal task, the bipolar patients did not show any significant hypoactivation during task performance. Instead, activation was significantly enhanced in right‐hemispherical cerebral and cerebellar cortex, in particular in the precentral gyrus, the intraparietal cortex, and the frontal eye field, which may be interpreted in terms of a reduced cortical efficiency resulting in stronger, possibly compensatory activation [for critical discussions see Callicott et al. 2000; Henseler et al., 2008; Hillary, 2008; Tan et al. 2006]. Most interestingly, however, only the patients exhibited a (pathological) activation of the right amygdala during working memory task performance (Fig. 4, Table II), which so far had never been observed neither in healthy controls nor in patients suffering from other psychiatric disorders, e.g. obsessive‐compulsive disorder or schizophrenia [Henseler et al., 2008, 2009]. At a statistical threshold of P < 0.05, this pathological amygdala activation was present in 13 out of 18 bipolar patients suggesting that it may be specific to the group (or at least to a subgroup) of patients with bipolar disorder, although this remains to be directly tested in future work.
These results extend previous findings by showing that in patients with bipolar affective disorder the amygdala is not only hyperreactive in response to affective stimulation, but shows abnormal hyperactivity also during cognitive processing in working memory tasks. Because the patients included in the present investigation were studied in the remitted state, our findings further suggest that this amygdala hyperactivity may represent a trait rather than a state marker of bipolar disorder.
The Role of Task Performance and Medication
Beside the pathophysiological changes in bipolar disorder themselves, other factors that may have influenced the pattern and the extent of brain activation in the current study include differences in task performance and medication effects. Overall, task performance of the euthymic bipolar patients examined in this study did not differ significantly from the performance of the healthy volunteers. The slight trend for minor performance in the articulatory rehearsal task cannot account for the significant hyperactivations observed in the patients. Additional correlation analyses between the strength of individual activation (in the amygdala, the right premotor, and the right intraparietal cortex) and the individual performance rates provided further evidence that activity in these brain regions was not correlated with individual differences in working memory performance. Furthermore, as indicated in the sample description, medication of the patients in this study was quite variable, which makes it unlikely that the group differences in brain activation may have resulted from a systematic effect of one specific drug. In particular, only a minority of patients received antidepressant (SSRIs) or antipsychotic medication. Both SSRI antidepressant and antipsychotic medication has been found to be associated with decreased amygdala activation [Del‐Ben et al., 2005; Harmer et al., 2006; Takahashi et al., 2005], i.e. it cannot explain the amygdala hyperactivation found in our patients. The same is true for mood‐stabilizing medications and benzodiazepines, which have also been shown to rather decrease the (pathologically elevated) amygdala activity than to lead to increased activation [Blumberg et al., 2005; Drevets et al., 2002; Paulus et al., 2005]. Furthermore, single subject analyses revealed abnormal amygdala activation even in bipolar patients without current medication as well as in those who had never received treatment with lithium or lamotrigin [which has been reported to possibly influence neuroimaging results; see Foland et al., 2008; Haldane et al., 2008]. Taken together, these findings strongly suggest that the abnormal amygdala activation observed in the bipolar patients did not result from differences in task performance or medication effects. Nevertheless, further studies are needed to directly address the potential effects of different types of psychotropic medications on this abnormal pattern of neural activity.
Brain Activation and Brain Volume
Our finding of amygdala activation in bipolar patients during working memory performance must also be discussed in the light of possible morphological differences as it is conceivable that hyperactivations may sometimes simply result from increased brain volumes. First, it is important to note that the amygdala finding in the present investigation is not simply a quantitative difference in the sense of an exaggerated brain activation that, in principle, is also elicited by the working memory task in normal healthy volunteers. Rather, this activation represents a qualitative abnormality since amygdala activation has never been observed during performance of these working memory tasks neither in healthy controls nor in patients suffering from other psychiatric disorders, e.g. schizophrenia or obsessive‐compulsive disorder [Henseler et al., 2008, 2009]. While volumetric differences may in fact account for relative differences in activation that is normally elicited by a given (e.g. emotional) task, it is very unlikely that they might also be able to produce a completely abnormal activation of the respective brain area in an experimental condition that normally does not activate this brain region at all, i.e. during working memory performance. Second, although some studies have reported increased amygdala volumes in bipolar patients, findings are inconsistent with other investigations describing decreased or unchanged amygdala volumes in bipolar affective disorder. In a meta‐analysis of 26 carefully selected MRI studies larger right lateral ventricle was the only significant volumetric difference in bipolar patients, whereas no significant difference was obtained in total brain volumes or in regions of particular interest like limbic structures among them the amygdala [McDonald et al., 2004]. Similarly, our own data from a larger sample of 35 bipolar patients and 35 matched controls including some, but not all of the patients and controls from the present sample investigated by fMRI, did not provide evidence for volumetric changes of the amygdala in bipolar disorder [Scherk et al., 2008]. Together, these findings support the assumption that the abnormal hyperactivation of the amygdala observed in the present study represents a neuropathophysiological characteristic of bipolar patients rather than being the consequence of volumetric amygdala changes.
Possible Genetic Effects on Amygdala Activation
A relatively common variant (5‐HTTLPR) of the human serotonin transporter gene (SLC6A4) that influences transporter availability has been found in several functional neuroimaging studies to modulate amygdala reactivity to environmental stressors [Bertolino et al., 2005; Brown and Hariri, 2006; Canli et al. 2005, 2006; Dannlowski et al., 2007, 2008; Domschke et al., 2006; Furmark et al., 2004; Hariri et al., 2002, 2005; Heinz et al., 2005, 2007; Munafo et al., 2008; Rao et al., 2007; Smolka et al., 2007]. In line with these findings, this genetic polymorphism has been connected with increased vulnerability for affective disorders [Caspi et al., 2003; Hariri et al., 2002, 2005; Lesch and Mossner, 1998; Pezawas et al., 2005]. Recently, another functional polymorphism in the COMT gene (val158met), which has been associated with higher risk for anxiety‐related behaviors, has also been reported to have an impact on limbic system and particularly amygdala activation [Smolka et al., 2005]. As we did not obtain genetic data from all participants included into our study, we could not exactly quantify the influence that these two functional polymorphisms might have taken on amygdala activation in the present study. However, single subject analyses of our fMRI data revealed that even those bipolar patients who carried the genetic variants that have been reported to be associated with lower amygdala reactivity to affective stimulation [5‐HTTLPR: L/L; COMT val158met: val/val; see Munafo et al., 2008; Smolka et al., 2005] did show significant (abnormal) amygdala activation during working memory task performance, whereas none of the healthy control subjects exhibited such activation. While these data do not necessarily contradict prior findings of significant genetic effects on amygdala reactivity, which were obtained using explicit affective stimulation [Hariri et al., 2005; Smolka et al., 2005], they suggest that the abnormal amygdala activation as found in the present sample of bipolar patients during performance working memory tasks may be a pathophysiological marker that is largely independent of these genetic effects.
CONCLUSION
The present findings suggest a more general role of pathological amygdala activation during articulatory rehearsal in working memory as a potential trait characteristic that can be observed at least in a subgroup of patients with bipolar disorder, even in the euthymic state and largely independent of task performance, medication, and some genetic factors like the 5‐HTTLPR and COMT (val158met) polymorphisms. To unequivocally determine the cause for this (relative) hyperactivation of the amygdala in bipolar patients, future studies should provide an absolute quantification of blood flow in the amygdala in both bipolar patients and healthy controls in corresponding experimental conditions and, in addition, in the resting state. Further studies in healthy relatives of patients with bipolar disorder are necessary to clarify whether this pathophysiological abnormality may also qualify as an endophenotypic marker that may be related to (other) genetic factors involved in the etiology of the disorder. In any case, the identification of such potential pathophysiological trait markers represents an important step towards the distinction of different biological subtypes of the probably heterogeneous group of bipolar affective disorders. Focusing on more homogeneous patient groups may result in more consistent clinical research findings and may pave the way for a future classification of affective and psychotic disorders based on pathophysiological and pathogenetic processes.
Acknowledgements
This study was conducted in cooperation with the Department of Neuroradiology at Saarland University Hospital, Homburg. The authors thank Prof. Dr. Wolfgang Reith and Dr. Christoph Krick for their support in data acquisition, and Bastian Krauss for support in data analysis.
REFERENCES
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM ( 2004): Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 6: 540–549. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Lewis V, Vallar G ( 1984): Exploring the articulatory loop. Quart J Exp Psychol Sect A Hum Exp Psychol 36: 233–252. [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T ( 2005): Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry 57: 1517–1525. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS ( 2003a): A functional magnetic resonance imaging study of bipolar disorder—State‐ and trait‐related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60: 601–609. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS ( 2003b): Frontostriatal abnormalities in adolescents with bipolar disorder: Preliminary observations from functional MRI. Am J Psychiatry 160: 1345–1347. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH ( 2005): Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord 7: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Hariri AR ( 2006): Neuroimaging studies of serotonin gene polymorphisms: Exploring the interplay of genes, brain, and behavior. Cogn Affective Behav Neurosci 6: 44–52. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR ( 2000): Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10: 1078–1092. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP ( 2005): Beyond affect: A role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA 102: 12224–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP ( 2006): Neural correlates of epigenesis. Proc Natl Acad Sci USA 103: 16033–16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R ( 2003): Influence of life stress on depression: Moderation by a polymorphism in the 5‐HTT gene. Science 301: 386–389. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A ( 2004): Anomalous prefrontal‐subcortical activation in familial pediatric bipolar disorder—A functional magnetic resonance imaging investigation. Arch Gen Psychiatry 61: 781–792. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T ( 2007): Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav 6: 672–676. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T ( 2008): 5‐HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology 33: 418–424. [DOI] [PubMed] [Google Scholar]
- Del‐Ben CM, Deakin JFW, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM ( 2005): The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: An fMRI study. Neuropsychopharmacology 30: 1724–1734. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, Hohoff C, Kersting A, Engelien A, Arolt V, Heindel W, Deckert J ( 2006): Association of the functional‐1019C/G 5‐HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol 9: 349–355. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME ( 2002): Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav 71: 431–447. [DOI] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM ( 2008): Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 19: 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S ( 2005): The maudsley bipolar disorder project. Epilepsia 46: 19–25. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L, Fredrikson M ( 2004): Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett 362: 189–192. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA ( 2004): The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord 6: 171–182. [DOI] [PubMed] [Google Scholar]
- Gruber O ( 2001): Effects of domain‐specific interference on brain activation associated with verbal working memory task performance. Cerebral Cortex 11: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Gruber O, von Cramon DY ( 2001): Domain‐specific distribution of working memory processes along human prefrontal and parietal cortices: A functional magnetic resonance imaging study. Neurosci Lett 297: 29–32. [DOI] [PubMed] [Google Scholar]
- Gruber O, von Cramon DY ( 2003): The functional neuroanatomy of human working memory revisited—Evidence from 3‐T fMRl studies using classical domain‐specific interference tasks. Neuroimage 19: 797–809. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun‐Todd DA ( 2003): Differential activation of the anterior cingulate and prefrontal cortex in schizophrenic and bipolar patients: An fMRI study. Schizophrenia Res 60: 219–219. [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun‐Todd DA ( 2004): Decreased activation of the anterior cingulate in bipolar patients: An fMRI study. J Affective Disord 82: 191–201. [DOI] [PubMed] [Google Scholar]
- Haldane M, Jogia J, Cobb A, Kozuch E, Kumari V, Frangou S ( 2008): Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur Neuropsychopharmacol 18: 48–54. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR ( 2002): Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400–403. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana LS, Mattay VS, Egan MF, Weinberger DR ( 2005): A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 62: 146–152. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM ( 2006): Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C ( 2005): Amygdala‐prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8: 20–21. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, Buchel C, Hariri AR, Weinberger DR ( 2007): Serotonin transporter genotype (5‐HTTLPR): Effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry 61: 1011–1014. [DOI] [PubMed] [Google Scholar]
- Henseler I, Gruber O, Kraft S, Krick C, Reith W, Falkai P ( 2008): Compensatory hyperactivations as markers of latent working memory dysfunctions in patients with obsessive‐compulsive disorder: An fMRI study. J Psychiatry Neurosci 33: 209–215. [PMC free article] [PubMed] [Google Scholar]
- Henseler I, Falkai P, Gruber O ( 2009): A systematic fMRI investigation of the brain systems subserving different working memory components in schizophrenia. Eur J Neurosci, in press. [DOI] [PubMed] [Google Scholar]
- Hillary FG ( 2008): Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. J Int Neuropsy Soc 14: 526–534. [DOI] [PubMed] [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SCR, Andrew CM, Phillips ML ( 2006): Stroop performance in bipolar disorder: Further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord 8: 28–39. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Ivanovski B, Malhi GS ( 2007): An event‐related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci 32: 174–184. [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML ( 2004): Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55: 578–587. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mossner R ( 1998): Genetically driven variation in serotonin uptake: Is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry 44: 179–192. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Hajek T, Alda M ( 2005): The phenotypes of bipolar disorder: Relevance for genetic investigations. Mol Psychiatry 10: 811–826. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Ward PB, Kumari V, Mitchell PB, Parker GB, Ivanovski B, Sachdev P ( 2004): Cognitive generation of affect in bipolar depression: An fMRI study. Eur J Neurosci 19: 741–754. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe‐Hesketh S, Ellison‐Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N ( 2004): Meta‐analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56: 411–417. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR ( 2008): Serotonin transporter (5‐HTTLPR) genotype and amygdala activation: A meta‐analysis. Biol Psychiatry 63: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD ( 1996): Evidence‐based health policy—Lessons from the global burden of disease study. Science 274: 740–743. [DOI] [PubMed] [Google Scholar]
- Murray DJ ( 1968): Articulation and acoustic confusability in short‐term memory. J Exp Psychol 78: 679–684. [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB ( 2005): Dose‐dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 62: 282–288. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA ( 2007): Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 62: 158–167. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR ( 2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Vieta E ( 2007): Identifying functional neuroimaging biomarkers of bipolar disorder: Toward DSM‐V. Schizophrenia Bull 33: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HY, Gillihan SJ, Wang JJ, Korczykowski M, Sankoorikal GMV, Kaercher KA, Brodkin ES, Detre JA, Farah MJ ( 2007): Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry 62: 600–606. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson‐Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E ( 2006): Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 103: 8900–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherk H, Kemmer C, Usher J, Reith W, Falkai P, Gruber O ( 2008): No change to grey and white matter volumes in bipolar I disorder patients. Eur Arch Psychiatry Clin Neurosci 258: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A ( 2005): Catechol‐O‐methyltransferase val(158)met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci 25: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, Zimmer A, Wrase J, Flor H, Mann K, Braus DF, Goldman D, Heinz A ( 2007): Gene‐gene effects on central processing of aversive stimuli. Mol Psychiatry 12: 307–317. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC ( 2005): Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting stroop interference task. Am J Psychiatry 162: 1697–1705. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, Okubo Y ( 2005): Effects of dopaminergic and serotonergic manipulation on emotional processing: A pharmacological fMRI study. Neuroimage 27: 991–1001. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer‐Lindenberg A, Egan MF, Weinberger DR, Callicott JH ( 2006): Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry 163: 1969–1977. [DOI] [PubMed] [Google Scholar]
- Yurgelun‐Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD ( 2000): fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2: 237–248. [DOI] [PubMed] [Google Scholar]


