Abstract
The brain is a plastic entity that can undergo dynamic changes throughout the lifespan as a result of training. Attention‐deficit/hyperactivity disorder (ADHD) is commonly treated with psychostimulant medication, and the prevalence of ADHD medication prescription is a topic of heated scientific debate. In addition, cognitive training is frequently provided to patients with ADHD. Although psychostimulant effects have been thoroughly investigated, no previous studies have assessed the neural effects of cognitive training in ADHD. We applied fMRI‐paradigms of response inhibition and selective attention to chart the effects of a 10‐day cognitive training program in 19 unmedicated ADHD children receiving either cognitive or control training. The two resulting longitudinal datasets were analyzed using whole‐brain random‐effects general linear models. Although we observed no increases of activity in the control group, both fMRI‐datasets revealed enhanced activity after cognitive training in neural structures closely related to ADHD pathophysiology. On the inhibition paradigm, our results indicated increases in orbitofrontal, superior frontal, middle temporal, and inferior frontal cortex. The attentional task was characterized by increased activity in the cerebellum, which correlated with improvement on in‐scanner measures of attention. Our findings provide preliminary evidence that cognitive training enhances activity in neural structures typically affected by the disorder. Similar results have been obtained following methylphenidate administration, suggesting that training of cognitive functions may mimic the effects of psychostimulant medication on the brain. These findings postulate a neural account for the potency of cognitive training in ADHD, and hold clinical implications, supporting the inclusion of training programs in standard ADHD‐treatment. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: attention deficit hyperactivity disorder, training, cerebellum, frontal lobe, fMRI, attention, inhibition
INTRODUCTION
Attention‐deficit/hyperactivity disorder (ADHD), a neurodevelopmental disorder characterized by symptoms of inattention, impulsivity, and hyperactivity, bears a considerable impact on brain structure and function. Converging evidence has pinpointed a constellation of focal biochemical, morphological, and functional aberrations in the ADHD brain, potentially reflecting a primary disruption in catecholaminergic neurotransmission [for reviews, see Biederman et al., 2005; Giedd et al., 2001; Nigg et al., 2005]. These neural deficits have predominantly been localized to the frontal lobes, striatum, and cerebellum, implicating dysregulation of the frontostriatal and cerebellar circuits in ADHD pathophysiology [Biederman et al., 2005; Giedd et al., 2001; Nigg et al., 2005].
ADHD is commonly treated with psychostimulant medication, and prevalence rates of ADHD medication prescription are a topic of heated debate in the scientific community [e.g. Basu, 2006; Bonn, 1999; Breggin, 2001; Faraone et al., 2001; Lancet Editors, 2007; Waschbusch et al., 2004]. In addition to pharmacological remedies, psychiatric treatment frequently comprises training programs, and advances on training‐induced brain plasticity advocate their pertinence. Research findings indicate that the nervous system can undergo dynamic modifications in structure and function based on learning throughout the lifespan, which allow the brain to process, encode, and implement new knowledge and skills [Buonomano et al., 1998; Poldrack 2000]. Animal studies have shown that sensory experience can induce macro‐ and microscopic changes in the brain, including alterations in gene transcription, receptor expression, synaptic density, and cortical map organization [Buonomano et al., 1998]. In humans, neuroimaging studies indicate that training of cognitive functions and basic skills can render alterations in gray matter volumes and synaptic activity, which can be quantified using magnetic resonance imaging (MRI) [Draganski et al., 2004; Olesen et al., 2004; Pascual‐Leone et al., 2005].
In this study, we applied functional MRI paradigms to assess the effects of short‐term cognitive training on neural activity. Cognitive training is a type of training that directly targets cognitive skills, originally developed to enhance rehabilitation after brain damage [Cicerone et al., 2000]. It comprises one of the standard training methods provided to ADHD subjects, and behavioral studies have indicated improvement in ADHD symptoms and cognitive performance [for review, see Toplak et al., 2008]. This is the first study to assess the neural effects of cognitive training in ADHD subjects. We analyzed blood oxygenation‐dependent (BOLD) signals, as an indicator of neural activity, in a sample of 19 unmedicated ADHD children of the combined subtype, who were subjected to 10 daily 45‐min sessions of either control or cognitive training (see Methods). MRI sessions were performed before and after the training period. The MRI acquisitions incorporated an fMRI paradigm of response inhibition and an fMRI paradigm of selective attention, rendering two longitudinal fMRI datasets. We hypothesized that cognitive training would modulate activity in neural structures that are (1) implicated in ADHD pathophysiology; (2) involved in the cognitive task assessed by the respective fMRI‐paradigm.
MATERIALS AND METHODS
Subjects and Treatment Protocol
Twenty‐seven children diagnosed with ADHD combined subtype, referred from outpatient clinics at Vall d'Hebron hospital, were recruited for this study. All subjects met DSM‐IV diagnostic criteria for ADHD combined subtype, as assessed by semistructured diagnostic interviews conducted by a team of psychologists and psychiatrists. In addition, Conner's scales were administered to both parents and teachers (mean ± SD: father, 92.36 ± 5.46; mother, 88.06 ± 11.75; teacher, 90.20 ± 11.63) [Conners 1990; Farré‐Riba et al., 1997]. Exclusion criteria comprised comorbidity with neurological disorders, other psychiatric disorders, cerebral damage, extreme prematurity and low IQ's (<80, WISC‐R). The subjects had never been exposed to cognitive training, and they were either medication‐naive or medication‐free for at least 15 days prior to their participation. Five subjects had to be excluded from the sample because of inability to attend all training and MRI sessions. In addition, upon visual inspection of the MRI data by a radiologist, three subjects were removed from the data and two subjects could only be included in one of the functional tasks because of movement‐related artifacts (ghosting) occurring in either one of the sessions.
This reduced our final subject group to 19 children for each functional task. The sample consisted of two ADHD groups, with matching demographic characteristics for age, IQ, gender, Conner's scores and prior exposure to medication (Mann–Whitney mean ± SD: Contr, 11.22 ± 3.11; 110.67 ± 16.4; 8M/1F; 87.46 ± 12.13; three medication‐naive patients. Exp. selective‐attention: 11.10 ± 2.56; 108.50 ± 18.67; 8M/2F; 91.14 ± 4.89; three medication‐naive patients. Exp. inhibition: 11.10 ± 2.56; 108.50 ± 18.67; 8M/2F; 90.00 ± 4.89; three medication‐naive patients; all P‐values >0.4).
Both ADHD groups were subjected to 10 daily 45‐min training sessions, performed in an ambulatory setting. One group was provided with cognitive training, a type of training that directly targets cognitive skills, which was originally developed to enhance cognitive rehabilitation after cerebral damage [Cicerone et al., 2000]. This method comprises one of the standard training programs provided to patients with ADHD, and behavioral studies have observed improvement in symptoms and cognitive performance in ADHD patients after cognitive training, which were maintained at a 3‐month follow‐up [Klingberg et al., 2005; Toplak et al., 2008].
During the training sessions, the children performed paper and pencil exercises, under guidance of a trained cognitive training therapist. The cognitive training program implemented exercises that stimulate working memory, cognitive flexibility, attention, planning and problem solving, offered in order of increasing difficulty [García‐Sánchez, 2001; García‐Sánchez et al., 2002]. The tasks were not arranged according to the targeted cognitive process but were presented intermixedly. Some examples of tasks employed during these sessions are labyrinths (planning), word list recall (memory), detecting the missing numbers from numerical lists (attention), creating lists of objects sharing certain characteristics (cognitive flexibility), and code deciphering (problem solving). The training sessions did not encompass exercises of the kind applied during the in‐scanner fMRI paradigms.
The other subject group participated in a series of social training sessions, described in detail by Inglés [Inglés Saura 2003], which focused on the comprehension and application of social norms and standards. The main goals of this program are to explain the meaning and value of interpersonal skills, to aid in the differentiation between aggressive, passive, and assertive behavior, and to improve the capacity to say no and express negative emotions such as aggravation or uneasiness. The sessions included the presentation of information on social rules and standards (e.g. watching informative videos), and performing exercises to implement these rules (e.g. role‐playing exercises). This type of training, employed as control training in our study, is sometimes applied to improve interpersonal abilities in ADHD children. Treatment was conducted by one of two trained therapists [V. Tremols; J. Kyra], according to the treatment manuals [García‐Sánchez, 2001; García‐Sánchez et al., 2002; Inglés Saura, 2003]. The study was approved by the Hospital Universitari Vall d'Hebron Ethics Committee, and informed consent was obtained from the children and the parents or legal guardians.
MRI Sessions
MRI acquisitions were performed on a Philips head‐only 1.5T scanner, equipped with a standard quadrature radiofrequency coil. For anatomical reference, a high‐resolution T1‐weighted image was acquired using a fast spoiled gradient recalled pulse sequence (TR = 20 ms, TE = 4.6 ms, FA = 30°, matrix size: 256 × 256, 100 slices, voxel size: 0.86 mm × 0.86 mm × 1.4 mm, acquisition time 3′30″). An echo‐planar sequence was employed to create T2*‐weighted functional volumes, each comprising 30 four‐mm‐thick slices acquired approximately parallel to the bicommissural plane (TR = 3,000 ms, TE = 50 ms, FA = 90°, in‐plane resolution 3.59 × 3.59, gap = 0.5, matrix size: 64 × 64, FOV = 230 mm, acquisition time 8′08″).
During the fMRI acquisitions, we employed fMRI‐suitable functional paradigms of response inhibition and selective attention, which have previously been applied to children of the same age [Booth et al., 2003]. Presentation software (version 9.10; http://www.neurobs.com) was used to present figures on the screen, depicting red triangles (targets), blue triangles, or red trapezoids (distracters).
To measure attentional processes, we employed a discrimination task of visuospatial selective attention during the functional acquisition, involving the visual search for a conjunction target in a field of distracters [Booth et al., 2003]. Subjects were instructed to press either the left or the right button, depending on whether the red triangle was present in the screen (50% probability). The task involved blocks of selective attention, comprising trials with multiple figures (a 3 × 3 matrix of nine figures), and blocks of nonselective attention, during which only trials with one figure were presented. Each condition was preceded by a 3‐s instruction screen. A total of 108 stimuli was provided during this functional paradigm. The stimuli were displayed for 1,400 ms, followed by a variable interstimulus interval between 450 and 750 ms, resulting in a mean trial duration of 2,000 ms.
Response inhibition was assessed with a stimulus‐controlled Go‐NoGo paradigm [Booth et al., 2003]. The task comprised Go‐ and NoGo‐trials, and involved one response‐button and picture stimuli representing a 3 × 3 matrix of nine figures. During the Go‐condition, which was applied to create a pre‐potent response tendency, the participants pressed the button every time a stimulus appeared on the screen. For the NoGo‐condition, the participants were instructed to press the button for all stimuli, but withhold their response if the red triangle was presented on the screen (50% probability). The Go‐NoGo paradigm comprised 108 stimuli, and the pictures (1,400 ms duration) were alternated with the presentation of a blank screen, which lasted between 450 and 750 ms. Preceding each condition, a 3‐s instruction screen was presented.
Participants were encouraged to respond as quickly as possible. Before entering the scanner, all subjects received instructions and performed a practice session outside the scanner.
Data Analyses
MRI image processing and statistical analyses were conducted with Statistical Parametric Mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab (version 6.5; http://www.mathworks.com). The first three volumes of each session were discarded to remove nonsteady‐state effects. Subsequently, we performed a series of temporal interpolation over each voxel's time course using sinc functions. Spatial interpolation was applied to correct for head motion, employing parameters derived from a six‐parameter rigid body transformation with a least squares algorithm. Analyses of variance (ANOVA) indicated no significant difference between the two subject groups on any of the translation or rotation movement parameters in either session (all means between −1 and 1 mm, P values >0.2). For each subject, the T1‐weighted image was registered to the mean functional EPI, and subsequently segmented, rendering gray matter, white matter and cerebrospinal fluid partitions. During the segmentation step, sample‐specific tissue prior probability maps were applied to replace the default adult‐based tissue priors, which are less suitable for pediatric samples. A 12‐parameter affine transformation algorithm followed by nonlinear warping was then employed to register the gray matter segment to a gray matter ADHD children template created for this study, to optimize the transformation for the tissue of interest and ensure high‐resolution transformation. The resulting transformation matrix was applied to the EPI timeseries, interpolating the images to 2 × 2 × 2 cubic voxels. Finally, EPI images were smoothed by imposing a 10‐mm FWHM isotropic kernel on the space domain.
At the first level of analysis, voxel‐wise changes in BOLD response across conditions were assessed for each subject and session separately, according to the general linear model. Time courses for selective attention/non‐selective attention and Go/NoGo conditions were introduced into separate models, comprising two regressors of interest for each of the functional paradigms, and additional regressors were included to account for residual effects of movement and temporal inhomogeneities. To maximize sensitivity, we employed distributed sampling during the acquisition phase and applied regressors based on temporal basis functions, as recommended by Price et al. [Henson 2004; Price et al., 1999]. The regressors of interest were convolved with the canonical hemodynamic response function implemented in SPM, and optimal parameter estimates were computed using a least squares function. The linear contrasts “selective attention > non‐selective attention” and “NoGo > Go” were applied to estimate effect sizes for each participant and generate statistical parametric maps.
Subsequently, the first‐level parameter estimates for the selective attention > non‐selective attention and NoGo > Go contrasts were entered into a second‐level repeated measures ANOVA, comprising a random‐effects analysis. We applied a whole‐brain approach to extract the changes in neural activity after the training period (group × session interaction effects). The whole‐brain functional activation maps were constructed by selecting clusters with per‐voxel probability value below 0.001 and a minimum spatial extent of 80 mm3 (corresponding to 10 contiguous 2 × 2 × 2 cubic voxels), a frequently employed threshold adopted from the source article of our in‐scanner tasks [Booth et al., 2003; Forman et al., 1995].
To assess if the changes in neural activity observed after cognitive training were associated with cognitive improvement, we performed regression analyses on the post‐predifference variables of the experimental group and applied regions of interest of the functional clusters significantly enhanced by cognitive training in the second‐level ANOVAs. These regression analyses incorporated the individual post‐pre difference variable of the behavioral measures that were improved after the training period in the group receiving cognitive training. We analyzed the behavioral variables in SPSS (version 12; http://www.spss.com) by means of nonparametric tests (normality assumptions assessed with Shapiro–Wilk), and the error rates for target and nontarget trials for each of the paradigms were extracted with a Presentation script. In addition to the behavioral measures, the individual post‐pre contrast images (computed from the pre‐ and post‐NoGo > Go and selective attention > non‐selective attention images) were entered into the regression models. To correct for multiple comparisons, we applied FWE‐correction to the region of interest analyses (P < 0.05).
RESULTS
To map the effects of cognitive training on brain activity, we applied whole‐brain random‐effects repeated‐measures analyses to the EPI timeseries. These analyses incorporated the NoGo > Go and selective attention > non‐selective attention contrast images computed from the pre‐ and posttraining MRI data.
For the task of response inhibition, we observed exclusive changes of neural activity in the right inferior frontal cortex, left medial orbitofrontal cortex, left superior frontal cortex, and right middle temporal cortex (Group × session interaction effects: inferior frontal: 56x14y18z, F1,36 = 26.09, P < 0.001, 360 mm3; orbitofrontal: −2x50y–12z, F 1,36 = 28.20, P < 0.001, 136 mm3; superior frontal: −18x54y4z, F 1,36 = 25.83, 216 mm3; middle temporal: 48x–38y–10z, F 1,36 = 27.61, P < 0.001, 336 mm3), and main‐effects contrasts indicate that these changes in neural activity correspond to the results for the main‐effects contrast “post‐cognitive training > pre‐cognitive training” (Table I and Fig. 1A,C).
Table I.
Full Results of Whole‐Brain Random‐Effects Analyses
| Training | Brain region | Talairach | Cluster size (mm3) | t | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Response inhibition | |||||||
| Cognitive Training | |||||||
| Decrease | — | ||||||
| Increase | R frontal inferior cortex* | 58 | 14 | 20 | 912 | 5.53 | <0.001 |
| L frontal superior cortex* | −18 | 56 | 8 | 456 | 4.58 | <0.001 | |
| L frontal med. orbital cortex* | −4 | 50 | −14 | 160 | 4.76 | <0.001 | |
| R temporal mid. cortex* | 50 | −38 | −12 | 544 | 4.98 | <0.001 | |
| Control training | |||||||
| Decrease | — | ||||||
| Increase | — | ||||||
| Selective Attention | |||||||
| Cognitive Training | |||||||
| Decrease | L precuneus | −10 | −72 | 52 | 184 | 4.01 | <0.001 |
| R precuneus | 18 | −60 | 48 | 928 | 4.34 | <0.001 | |
| R superior parietal cortex | 12 | −68 | 54 | 928 | 4.41 | <0.001 | |
| Increase | R superior posterior cerebellum* | 30 | −46 | −22 | 200 | 4.09 | <0.001 |
| Midbrain | −6 | −10 | −22 | 496 | 5.24 | <0.001 | |
| 2 | −14 | −22 | 4.64 | <0.001 | |||
| Control Training | |||||||
| Decrease | R superior posterior cerebellum* | 8 | −78 | −20 | 216 | 4.12 | <0.001 |
| Increase | — | ||||||
The table describes the complete results of the whole‐brain random‐effects analyses of the selective attention and response inhibition fMRI‐paradigms, as well as the direction of the changes in neural activity after training (df 1,36). Main‐effects changes in BOLD signal corresponding to group x session interaction effects are indicated with an asterisk, and results in italic reflect the main‐effects changes that did not surface in the group x session interaction contrast.
Figure 1.
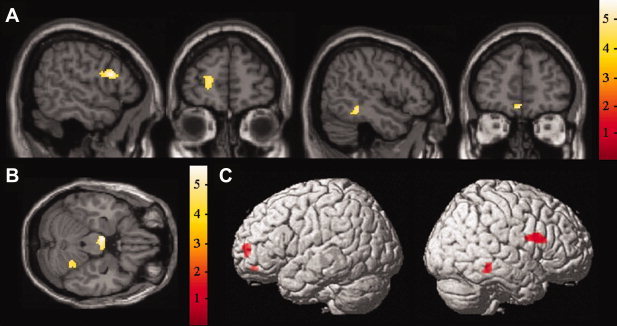
Statistical parametric maps depicting increases in BOLD response after cognitive training. Whole‐brain random effects analyses. (A) Regions of increased activation on the task of response inhibition after cognitive training; (B) regions of increased activation on the task of selective attention after cognitive training; (C) regions of increased activation on the task of response inhibition displayed on surface maps.
The task of selective attention was characterized by a change in BOLD signal in the right superior posterior lobule of the cerebellum (group × session interaction effect: 10x‐78y‐18z, F 1,36 = 18.66, P < 0.001, 112 mm3), a region demonstrating effects of opposite direction in the training groups, with a decrease in the control group, but an increase after cognitive training (Fig. 1B,C and Table I). The ADHD group receiving control training did not display increases of activity in any region on either of the two functional paradigms. Mean and individual parameter estimates and contrast values before and after cognitive training, as well as individual percent signal changes, are provided in Supporting Information Tables I–III and Supporting Information Figures 1 and 2.
To assess if within‐group changes in neural activity co‐varied with the degree of cognitive improvement, we performed regression analyses on variables quantifying the change after the cognitive training period. These variables constituted subtraction images of the post and pre contrast images (NoGo > Go and selective attention > non‐selective attention) and the post‐pre difference variable of the in‐scanner measures of cognitive performance that were improved after the period of cognitive training.
Behavioral analyses on the measures of in‐scanner task performance indicated reductions in error rates for the conditions of interest of both in‐scanner fMRI paradigms after cognitive training, albeit not bonferroni‐corrected, but no changes in the control group (Wilcoxon signed ranks, mean ± SD, inhibition paradigm: omission: pre: 8.10 ± 10.74, post: 1.90 ± 2.03; Z 1,18 = −2.02, P < 0.05; selective attention paradigm: target incorrect: pre: 5.90 ± 4.28, post: 4.00 ± 3.56; Z 1,18 = −1.97, P < 0.05; target omission: pre: 1.30 ± 1.42, post: 0.80 ± 1.23; Z 1,18 = −2.24, P < 0.05. Analyses of the other measures did not render significant results (Inhibition paradigm: commission errors. Selective attention paradigm: non‐target errors (omission and incorrect responses), P > 0.05). The post‐pre difference variables of the behavioral measures were entered into the regression models.
The results of the regression analyses revealed a negative correlation between the increase of activity after cognitive training in the right superior posterior cerebellum and the decreased measure of incorrect responses on the task of selective attention in the experimental group (Fig. 2, 24x‐52y‐20z, R 1,8 = −0.84, 168 mm3, P < 0.05), suggesting that enhancement of the activity in this region is associated with behavioral improvement after cognitive training. No significant correlation was found for the improved measure of inhibition performance.
Figure 2.
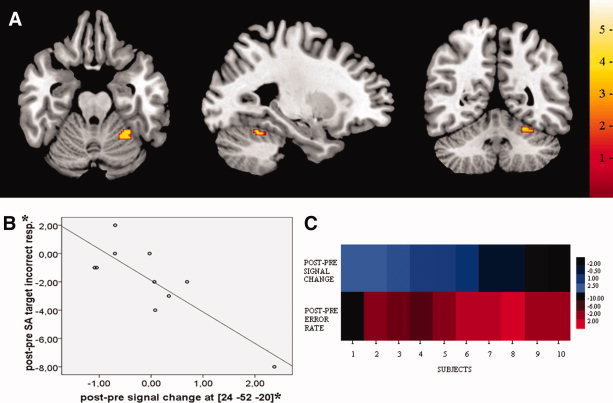
Correlations between cognitive performance and brain activity. Statistical parametric map (A) and scatterplot (B) depicting a negative correlation between the post‐pre signal change in the right superior posterior cerebellum after cognitive training (post‐pre contrast image) and decreased error rate (post‐pre difference) on the task of selective attention after cognitive training. For illustrative purposes, the functional activation map was thresholded at P < 0.05 and masked with ROI of posttraining change in cerebellar activity. (C) An intensity color plot illustrating the distribution of the change in neural activity and degree of behavioral improvement in voxel [24, −52, −20] across subjects (Cluster 3.0, Java Treeview). Blue and red colors reflect individual post‐pre error rate and post‐pre MRI signal change after cognitive training respectively, with the intensity indicating the relative strength of the change. The left to right intensity range, from light blue to dark blue on one hand and from dark red to light red on the other hand, illustrates the opposite distribution of the two variables across subjects. *Individual parameter estimates and behavioral values before and after cognitive training are provided in Supporting Information Table I.
DISCUSSION
On the paradigm of response inhibition, our results indicate increases of activity after cognitive training in the left orbitofrontal, right middle temporal, and bilateral dorsolateral prefrontal cortex, more specifically the left superior frontal and right inferior frontal cortex. The frontal lobe plays a key role in ADHD, and converging research findings demonstrate aberrations in frontal grey matter volume, metabolism and hemodynamics, predominantly pinpointed to orbitofrontal and inferior frontal regions [Aron et al., 2005; Biederman et al., 2005; Giedd et al., 2001; Nigg et al., 2005; Van't Ent et al., 2007].
Response inhibition relies on frontal lobe functioning, and various frontal regions have been implicated in inhibitory control [Dillon et al., 2007]. However, the right inferior frontal cortex is generally regarded as the core structure of the neural system regulating response inhibition, and right inferior frontal hypoactivation is considered the neural correlate of a less efficient inhibitory system [for review, see Aron et al., 2004]. In ADHD subjects, this structure exhibits especially severe structural and functional abnormalities, and right inferior hypoactivity is associated with the inhibitory deficits characteristic for ADHD patients [for review, see Aron et al., 2005].
On the task of selective attention, we found increases of activity after cognitive training in the right superior posterior cerebellum, which were associated with improvement on in‐scanner measures of attention, and a decrease of activity in this cerebellar subdivision in the control group. The cerebellum is a structure thought to be directly related to AHDH pathophysiology, and ADHD patients are characterized by stable and progressive gray matter volume loss in this structure and considerable metabolic and hemodynamic abnormalities [Giedd et al., 2001; Nigg et al., 2005].
Although the cerebellum has traditionally been regarded as a neural device dedicated to motor control, research findings indicate a role for the posterior cerebellum in the acquisition and modulation of cognitive processes by adjusting the responsiveness in other brain systems [Akshoomoff et al., 1997; Steinlin, 2007]. Attention is one of the cognitive processes thought to operate under cerebellar control, and neuroimaging studies have pointed to cerebellar involvement in the coordination and direction of selective attention [Akshoomoff et al., 1997; Le et al., 1998]. The finding of decreased activity in the cerebellum in the control group was unexpected, as we hypothesized the control children would not display any changes of activity on the fMRI tasks. The source of this change can only be speculated upon, but considering the role of the posterior cerebellum in cognitive learning and modulation, we postulate this reflects a reduction in the required modulation and acquisition of attentional functions when performing the same task a second time without training of these functions in between.
Albeit not evident in the interaction results, additional parietal and mesencephalic changes of activity suggest alterations in the posterior attention system, which modulates processes of selective attention by regulating coordinated interactions between frontal, parietal and midbrain systems [Posner et al., 1990]. The cerebellum is tightly linked to this network and forms part of a complete loop connecting the cerebellum and parietal cortex via cerebello‐thalamico and cortico‐pontine‐cerebellar circuits.
Taken together, both our fMRI‐datasets indicate increases of activity after training of cognitive functions. Although we cannot completely exclude the contribution of other factors besides the training type to these results, we have undertaken steps to minimize the bias introduced by potential confounding factors: (A) to account for the effect of training per se, a control training program of identical duration and frequency was implemented; (B) to minimize the habituation effect, the training programs did not encompass exercises of the kind applied during the fMRI paradigms; (C) to address the impact of task repetition, a well‐matched control group undergoing identical fMRI paradigms was included. Therefore, we attribute the observed changes in activity to the training type factor.
The encountered increases of activity after cognitive training concern brain structures typically affected by the disorder. Although results on ADHD have not always been consistent, converging research findings appoint the frontal lobes, striatum, and cerebellum as the primary sites of impairment in the ADHD brain, harboring a predominant share of the established syndrome‐associated neural deficits [Biederman et al., 2005; Carmona et al., 2009; Giedd et al., 2001; Nigg et al., 2005]. Our findings of fronto‐temporo‐cerebellar increase provide preliminary evidence that cognitive training targets critical syndrome‐associated neural structures, and suggest it alleviates cognitive deficits in ADHD children by enhancing the activity in dysregulated brain circuits. These results hold clinical implications, advocating the inclusion of training programs targeting cognitive functions in standard ADHD‐treatment, and stress the need for large‐scale clinical studies to further define the behavioral impact of cognitive training methods.
The results obtained in our study are in line with previous neuroimaging research on learning‐induced plasticity [Olesen et al., 2004], demonstrating increases in neural activity after training of higher‐order cognitive functions. The signal changes were observed after a 2‐week training period, indicating that alterations in synaptic activity can already be detected after a relatively short exposure to cognitive training. These results illustrate the capacity of the nervous system for rapid adjustments in response to changes in cognitive demands.
Interestingly, the structures of enhanced activity in our study comprise targets of psychostimulant medication, which is thought to exert its clinical effects by enhancing cerebello‐thalamo‐frontal circuits [Volkow et al., 2002], and our results pose the possibility that cognitive training and psychostimulant medication act on similar neural circuitry. Frontostriatal structures constitute major binding sites of methylphenidate, which exerts its primary impact via d‐threo‐methylphenidate binding to dopamine transporters [Volkow et al., 2002]. In addition, molecular imaging studies have shown consistent increases in cerebellar blood flow and metabolism after methylphenidate administration, potentially reflecting methylphenidate affinity for noradrenergic and dopaminergic transporters[Volkow et al., 2002]. Neuroimaging studies assessing psychostimulant impact in ADHD children have also demonstrated increases of neural activity in frontal and cerebellar structures after methylphenidate administration, which correlate with the degree of symptom improvement, suggesting that these methylphenidate‐induced increases underlie the therapeutic efficacy of psychostimulant medication [e.g. Anderson et al., 2002; Epstein et al., 2007; Loo et al., 2004; Vaidya et al., 1998]. Of special interest is a study by Vaidya et al., [1998], who assessed methylphenidate effects in very similar experimental conditions, employing a stimulus‐controlled Go‐NoGo fMRI paradigm and ADHD children of the same age. Similar to our results, their findings included BOLD signal increases in superior frontal, inferior frontal and orbitofrontal cortex, regions also affected by cognitive training in our study. Hence, our findings provide preliminary indications that training of cognitive functions in ADHD patients may mimic the neural effects of psychostimulant medication. However, future research should establish methylphenidate and cognitive training response within the same experimental setup to accurately assess the similarity and relative extent of these effects as well as the interaction between the treatment methods.
To resume our findings, both datasets revealed enhanced activity after cognitive training in neural structures closely related to ADHD pathophysiology. On the task of response inhibition, we found exclusive increases in middle temporal, orbitofrontal, superior frontal and inferior frontal regions. The right inferior frontal cortex, which exhibits especially severe deficits in ADHD subjects, is considered the core structure of the neural inhibition device. The task of selective attention was characterized by increased activity in the cerebellum, a structure implicated in the coordination and direction of attentional focus, and this cerebellar increase was associated with improvement on in‐scanner measures of attention.
This is the first study to establish neural effects of a cognitive training program in ADHD. Our findings provide preliminary evidence that training of cognitive functions targets critical syndrome‐associated structures, and indicate it may improve cognitive performance by enhancing dysregulated fronto‐cerebellar circuits. Interestingly, similar results have been demonstrated following methylphenidate administration, suggesting that cognitive training may mimic the effects of psychostimulant medication on the brain. On the whole, our results postulate a neural account for the potency of cognitive training in ADHD patients, and hold clinical implications, supporting the pertinence of training programs as part of standard ADHD‐treatment. Large‐scale studies are needed to confirm our findings and further elucidate the effects of cognitive training on brain activity.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Materials.
Acknowledgements
We thank Joanna Kyra for performing training sessions with the subjects. The funding organizations did not participate in any component of the study.
REFERENCES
- Akshoomoff NA, Courchesne E, Townsend J ( 1997): Attention coordination and anticipatory control. Int Rev Neurobiol 41: 575–598. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH ( 2002): Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. Am J Psychiatry 159: 1322–1328. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA ( 2005): The cognitive neuroscience of response inhibition: Relevance for genetic research in attention‐deficit/hyperactivity disorder. Biol Psychiatry 57: 1285–1292. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Basu P ( 2006): Addictive drugs still best option for attention deficit disorder. Nat Med 12: 374. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV ( 2005): Attention‐deficit hyperactivity disorder. Lancet 366: 237–248. [DOI] [PubMed] [Google Scholar]
- Bonn D ( 1999): Debate on ADHD prevalence and treatment continues. Lancet 354: 2139. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM ( 2003): Neural development of selective attention and response inhibition. Neuroimage 20: 737–751. [DOI] [PubMed] [Google Scholar]
- Breggin PR ( 2001): Questioning the treatment for ADHD. Science 291: 595. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM ( 1998): Cortical plasticity: From synapses to maps. Annu Rev Neurosci 21: 149–186. [DOI] [PubMed] [Google Scholar]
- Carmona S, Proal E, Hoekzema EA, Gispert JD, Picado M, Moreno I, Soliva JC, Bielsa A, Rovira M, Hilferty J, Bulbena A, Casas M, Tobeña M, Vilarroya O: Ventro‐striatal reductions underpin symptoms of hyperactivity and impulsivity in attention‐deficit/hyperactivity disorder. Biol Psychiatry 66: 972–977. 0000. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, Felicetti T, Giacino JT, Harley JP, Harrington DE, Herzog J, Kneipp S, Laatsch L, Morse PA ( 2000): Evidence‐based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil 81: 1596–1615. [DOI] [PubMed] [Google Scholar]
- Conners CK. 1990. Conners' Rating Scales Manual. Toronto: Multi‐Health Systems. [Google Scholar]
- Dillon DG, Pizzagalli DA ( 2007): Inhibition of action, thought, and emotion: A selective neurobiological review. Appl Prev Psychol 12: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A ( 2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J ( 2007): ADHD‐ and medication‐related brain activation effects in concordantly affected parent‐child dyads with ADHD. J Child Psychol Psychiatry 48: 899–913. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J ( 2001): ADHD: Disorder or discipline problem? Science 291: 1488–1489. [DOI] [PubMed] [Google Scholar]
- Farré‐Riba A, Narbona J ( 1997): Escalas de Conners en la evaluación del trastorno por déficit de atención con hiperactividad: nuevo estudio factorial en niños españoles. Revista de Neurología 25: 200–204. [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- García‐Sánchez C. 2001. El juego de la atención. Barcelona: Lebon. [Google Scholar]
- García‐Sánchez C, Estévez‐González A. 2002. Estimulación Cognitiva‐II. Barcelona: Lebon. [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX ( 2001): Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci 931: 33–49. [DOI] [PubMed] [Google Scholar]
- Henson RN ( 2004): Analysis of fMRI time series: Linear time‐invariant models, event‐related fMRI, and optimal experimental design In: Frackowiak RS, Friston KJ, Frith C, Dolan R, Mazziotta JC, editors. Human Brain Function. San Diego: Elsevier; pp 793–823. [Google Scholar]
- Inglés Saura CJ. 2003. Enseñanza de habilidades interpersonales para adolescentes. Madrid: Piramide. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H ( 2005): Computerized training of working memory in children with ADHD‐‐a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44: 177–186. [DOI] [PubMed] [Google Scholar]
- Lancet Editors ( 2007): Promoting optimum management for ADHD. Lancet 369: 880. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X ( 1998): 4 T‐fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Loo SK, Hopfer C, Teale PD, Reite ML ( 2004): EEG correlates of methylphenidate response in ADHD: Association with cognitive and behavioral measures. J Clin Neurophysiol 21: 457–464. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ ( 2005): An integrative theory of attention‐deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 17: 785–806. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T ( 2004): Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7: 75–79. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Amedi A, Fregni F, Merabet LB ( 2005): The plastic human brain cortex. Annu Rev Neurosci 28: 377–401. [DOI] [PubMed] [Google Scholar]
- Poldrack RA ( 2000): Imaging brain plasticity: Conceptual and methodological issues—A theoretical review. Neuroimage 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE ( 1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Price CJ, Veltman DJ, Ashburner J, Josephs O, Friston KJ ( 1999): The critical relationship between the timing of stimulus presentation and data acquisition in blocked designs with fMRI. Neuroimage 10: 36–44. [DOI] [PubMed] [Google Scholar]
- Steinlin M ( 2007): The cerebellum in cognitive processes: Supporting studies in children. Cerebellum 6: 237–241. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Connors L, Shuster J, Knezevic B, Parks S ( 2008): Review of cognitive, cognitive‐behavioral, and neural‐based interventions for Attention‐Deficit/Hyperactivity Disorder (ADHD). Clin Psychol Rev 28: 801–823. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD ( 1998): Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci USA 95: 14494–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Ent D, Lehn H, Derks EM, Hudziak JJ, Van Strien NM, Veltman DJ, De Geus EJ, Todd RD, Boomsma DI ( 2007): A structural MRI study in monozygotic twins concordant or discordant for attention/hyperactivity problems: Evidence for genetic and environmental heterogeneity in the developing brain. Neuroimage 35: 1004–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ ( 2002): Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol 12: 557–566. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, Pelham WE Jr ( 2004): Using stimulants in children with ADHD. Science 306: 1473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Materials.


