Abstract
It remains controversial whether the left inferior frontal gyrus subserves syntactic processing or short‐term memory demands. Here we devised a novel picture‐sentence matching task involving Japanese sentences with different structures to clearly contrast syntactic reanalysis processes. Using event‐related functional magnetic resonance imaging (fMRI), activations under three main conditions were directly compared: a canonical/subject‐initial active sentence (AS), a noncanonical/subject‐initial passive sentence (PS), and a noncanonical/object‐initial scrambled sentence (SS). We found that activation in the dorsal region of the left inferior frontal gyrus (dF3t) was enhanced more by the noncanonical processing under the PS and SS conditions than by the canonical processing under the AS condition, and this enhancement was independent of domain‐general factors, such as general memory demands and task difficulty. Moreover, the left posterior superior/middle temporal gyrus (pSTG/MTG) showed more enhanced responses to object‐initial sentences under the SS condition than to subject‐initial sentences under the AS and PS conditions, which were not significantly affected by task difficulty. Furthermore, activation in the left lateral premotor cortex (LPMC) increased under the AS, PS, and SS conditions, in that order. It is possible that task difficulty affects the left LPMC, but the three distinct activations patterns suggest that these frontal and temporal regions work in concert to process syntactic structures, with their respective contributions dynamically regulated by linguistic requirements. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, frontal cortex, temporal cortex, sentence processing, syntax
INTRODUCTION
Sentences convey not only lexico‐semantic information for each word but also sentence meaning based on syntactic structures. In many languages, the same sentence meaning can often be expressed using different sentence structures. Even structurally complex sentences are sometimes preferred to simple active sentences, especially when a particular topic is intended to be emphasized in a certain context. The more complex syntactic structures are, the more difficult sentence comprehension generally becomes. Previous functional magnetic resonance imaging (fMRI) studies tested the effects of object‐initial scrambled sentences in German and Hebrew, and reported enhanced activation in the left inferior frontal gyrus (IFG) and posterior superior/middle temporal gyrus (pSTG/MTG) [Ben‐Shachar et al., 2004; Bornkessel et al., 2005; Fiebach et al., 2005; Röder et al., 2002]. It is true that the sentences used in many of these studies make it difficult to know the cause of increased activation, since processing syntactically complex sentences is inevitably confounded by task difficulty, which may simply explain the increased activation, as indicated earlier. For example, Fiebach et al. (2005) have concluded that left IFG activation reflects “syntactic working memory load” (i.e., the maintenance of linguistic material that has not yet been integrated and is held active in short‐term memory) rather than syntactic integration costs (i.e., computational processes of integrating each incoming word into the phrase structure representation); this conclusion was based on their observation that left IFG activation was enhanced as a function of the distance between a dislocated verb phrase and its canonical position. It is not yet clear, however, whether syntactic working memory is separable from general memory demands and task difficulty, and the debate about the degree of specialization in language‐related activity is still ongoing. Recent imaging studies have shown that a part of the left IFG engages in the processing of language‐specific linearization rules that include not only the rule of subject‐before‐object but the rules of pronoun‐before‐nonpronominal argument [Grewe et al., 2005] and animate‐before‐inanimate subject [Grewe et al., 2006]. It would thus be necessary to clarify whether the left IFG activation is modulated by syntactic processing without the influence of domain‐general factors if this region indeed specializes in syntactic processing.
The goal of the present study was to directly address these important issues, thereby equating domain‐general factors among various types of canonical and noncanonical sentences as much as possible. To minimize the effect of general memory demands, a whole sentence of a minimal length (i.e., two nouns and one verb; see Table I) was visually presented for a longer time than was needed to respond, and the numbers of syllables and letters were strictly controlled among all conditions. The sentences we employed are thus more advantageous than those used in previous studies with sequentially presented stimuli that involve memorization. The task difficulty associated with syntactically complex sentences might be also alleviated by providing an appropriate context for each sentence. Indeed, a self‐paced reading study with Finnish sentences showed that the processing of noncanonical/object‐initial structures was significantly facilitated when the presence of appropriate contexts alleviated the usual difficulty associated with noncanonical constructions [Kaiser and Trueswell, 2004]. In the present study, participants processed a sentence based on a context provided by a picture. A picture representing an action consisted of two stick figures, which were distinguished by “head” forms (without facial expressions), each represented by a symbol: a circle (○), a square (□), or a triangle (▵) (Fig. 1). This picture further excluded the involvement of pragmatic information about word use (e.g., “An officer chases a thief” is more acceptable than “A thief chases an officer.”). Half of the pictures depicted action occurring from left to right, the other half depicted action from right to left. The pictures were similar to those used in previous lesion studies [Maher et al., 1995; Schwartz et al., 1980], but the simultaneous presentation of one picture and one sentence is unique and advantageous to the present study.
Table I.
Sentences used in this study
| Stimulus | English translation |
|---|---|
| Sentence control (SC) condition | |
| ○‐to □‐ga neteiru | ○ and □ lie |
| ○‐to □‐ga tatteru | ○ and □ stand |
| ○‐to □‐ga aruiteru | ○ and □ walk |
| ○‐to □‐ga hashitteru | ○ and □ run |
| ○‐to □‐ga koronderu | ○ and □ tumble |
| ○‐to □‐ga naiteru | ○ and □ cry |
| Active sentence (AS) condition | |
| ○‐ga □‐o hiiteru | ○ pulls □ |
| ○‐ga □‐o oshiteru | ○ pushes □ |
| ○‐ga □‐o shikatteru | ○ scolds □ |
| ○‐ga □‐o ketteru | ○ kicks □ |
| ○‐ga □‐o tataiteru | ○ hits □ |
| ○‐ga □‐o yonderu | ○ calls □ |
| Passive sentence (PS) condition | |
| □‐ga ○‐ni hikareru | □ is affected by ○'s pulling it |
| □‐ga ○‐ni osareru | □ is affected by ○'s pushing it |
| □‐ga ○‐ni shikarareru | □ is affected by ○'s scolding it |
| □‐ga ○‐ni kerareru | □ is affected by ○'s kicking it |
| □‐ga ○‐ni tatakareru | □ is affected by ○'s hitting it |
| □‐ga ○‐ni yobareru | □ is affected by ○'s calling it |
| Scrambled sentence (SS) condition | |
| □‐o ○‐ga hiiteru | As for □,○ pulls it |
| □‐o ○‐ga oshiteru | As for □,○ pushes it |
| □‐o ○‐ga shikatteru | As for □,○ scolds it |
| □‐o ○‐ga ketteru | As for □,○ kicks it |
| □‐o ○‐ga tataiteru | As for □,○ hits it |
| □‐o ○‐ga yonderu | As for □,○ calls it |
Only typical examples are shown here; other combinations among the symbols ○, □, and ▵ were also used. ga, nominative marker; o, accusative marker; ni, dative marker; and to, conjunction “and.”
Figure 1.
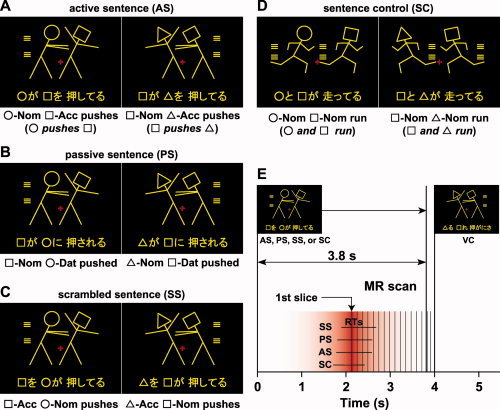
The four sentence conditions used in the picture‐sentence matching task and the timing of slice acquisition. Each stimulus consisted of one picture (top) and one sentence (bottom). Pictures depicting actions consisted of two stick figures, and each stick figure was distinguished by one of three “head” symbols: a circle (○), a square (□), or a triangle (▵). The participants judged whether or not the meaning of each sentence matched the action scene given in the corresponding picture. Two samples are shown for each condition. A: Under the active sentence (AS) condition, canonical/subject‐initial active sentences were presented (left, “○‐ga □‐o oshiteru”; right, “□‐ga ▵‐o oshiteru”). Below each example, a word‐by‐word translation in English is shown. Nom, nominative case; Acc, accusative case; Dat, dative case. See Table I for the stimulus list. B: Under the passive sentence (PS) condition, noncanonical/subject‐initial passive sentences were presented (left, “□‐ga ○‐ni osareru”; right, “▵‐ga □‐ni osareru”). C: Under the scrambled sentence (SS) condition, noncanonical/object‐initial scrambled sentences were presented (left, “□‐o ○‐ga oshiteru”; right, “▵‐o □‐ga oshiteru”). The sentence stimuli were all grammatical and commonly used in Japanese. An identical picture set was used under the AS, PS, and SS conditions. D: Under the sentence control (SC) condition, canonical/subject‐initial active sentences with two agents and one verb were presented (left, “○‐to □‐ga hashitteru”; right, “□‐to ▵‐ga hashitteru”). E: Each stimulus pair was presented for 3,800 ms with 200‐ms interstimulus intervals. The trial events of the picture‐sentence matching task occurred with variable intertrial intervals, in which four, five, or six visual control (VC) trials were presented. The first slice acquisition occurred 2,100 ms after the stimulus onset, and the acquisition timing of each slice was corrected using the first slice as a reference for the functional imaging data. A red‐graded bar schematically denotes a canonical homodynamic response function centered on the first slice (90% sensitivity at ±1 s). The range of RTs (mean ± SD) is also shown under each of the SC, AS, PS, and SS conditions.
We tested the picture‐sentence matching task under three main conditions for event‐related fMRI sessions (Fig. 1A–C): a canonical/subject‐initial active sentence (AS), a noncanonical/subject‐initial passive sentence (PS), and a noncanonical/object‐initial scrambled sentence (SS). The same set of scenes depicted by pictures and sentences was used under each of these three conditions. Under the AS condition, transitive verbs were used with the SOV (S, subject; O, object; V, verb) canonical sentence type (e.g., “○‐ga □‐o oshiteru”; “○ pushes □”). Under the PS condition, noncanonical passive sentences were presented with the SOV sentence type (“□‐ga ○‐ni osareru”; “□ is affected by ○'s pushing it”; this sentence type is further explained in the next paragraph). Under the SS condition, object‐initial scrambled sentences were presented with the OSV noncanonical sentence type (“□‐o ○‐ga oshiteru”; “As for □,○ pushes it”). The OSV type is grammatical and frequently used in conversation and writing in Japanese. Note that the sentences under the PS and SS conditions thus required different types of noncanonical processing. Moreover, sentence comprehension under these conditions explicitly required analysis of both dependency relations (i.e., which is the subject of a verb, and which is its object?) and semantic (thematic) roles (i.e., who initiates the action, and who is affected by it?). In Japanese syntax, the grammatical relations are first marked by case markers (e.g., nominative and accusative), which in turn allow the assignment of semantic roles (e.g., agent and patient), whereas passiveness is also marked in the verb morphology.
The passive sentences in Japanese tested here are noncanonical/subject‐initial and require syntactic reanalysis for the following reasons. In most Japanese sentences, a noun phrase with the nominative case marker ga is associated with the semantic role of an agent (an initiator of an action, an actor), and a noun phrase with the accusative case marker o is associated with the semantic role of a patient (a person affected by an action). For passive sentences with ni direct passive that were used in the present study, however, a noun phrase with the nominative case marker ga is associated with the semantic role of an experiencer (a person experiencing a situation), and a noun phrase with the dative marker ni is associated with an agent [Hoshi, 1994; Kuroda, 1992]. Actually, there are two types of passivization in Japanese: ni direct passive and ni yotte passive. The following three examples indicate this distinction [Hoshi, 1994].
-
(a)
Mary‐ga John‐o nagutta.
‘Mary punched John.’
-
(b)
John‐ga Mary‐ni nagurareta. (ni direct passive)
‘John was affected by Mary's punching him.’
-
(c)
John‐ga Mary‐ni yotte nagurareta. (ni yotte passive)
‘John was punched by Mary.’
According to Kuroda (1992), the ni direct passive form carries the affective connotation and no noun‐phrase (NP) movement takes place, while the ni yotte passive form does not carry that connotation and NP movement occurs as is the case in English. Therefore, the sentences with the ni direct passive construction are noncanonical and subject‐initial.
As stated earlier, the picture stimuli were exactly the same under the AS, PS, and SS conditions. If the visual analysis of pictures was also enhanced by noncanonical processing, then activation in visual areas would be observed. Visual factors alone cannot explain any condition‐selective frontal and temporal activation without activation in the visual areas. Moreover, the pictures provided the same additional contextual information for the three conditions, and thus such semantic information cannot explain any differential activation, either. To identify how task difficulty potentially affects cortical activations in the present paradigm, we also included a sentence control (SC) condition with canonical/subject‐initial active sentences in which intransitive verbs were used (Fig. 1D). The SC condition basically required matching between words (symbols and verbs) and pictures alone, and it did not require the analysis of dependency relations in a sentence. Thus, the SC condition is syntactically less complex and easier to comprehend than other conditions. By using an event‐related fMRI, we focused on the final process of syntactic reanalysis just before responses were produced (Fig. 1E; see MATERIALS AND METHODS). Direct comparisons among the AS, PS, and SS conditions reveal activations that represent syntactic reanalysis per se, which is closely associated with different types of canonical and noncanonical processing for sentence comprehension.
MATERIALS AND METHODS
Participants
Fourteen right‐handed native Japanese speakers (ten males and four females, aged 20–31 years) participated in the present study. Handedness was determined by the Edinburgh inventory [Oldfield, 1971]. None of the participants had any neurological or psychiatric symptoms. Informed consent was obtained from each participant after the nature and possible consequences of the studies were explained. Approval for the experiments was obtained from the institutional review board of the University of Tokyo, Komaba.
Stimuli
Each visual stimulus consisted of a picture at the top and a Japanese sentence at the bottom as a single stimulus set (Fig. 1A–D). The sentences describing actions were written in a combination of the “hiragana” and “kanji” writing systems, and each sentence included two noun phrases and one verb. For example, a noun phrase (□‐ga) consisted of a symbol (□) and a hiragana (ga). Two sets of Japanese verbs (six transitive verbs: pull, push, scold, kick, hit, and call; and six intransitive verbs: lie, stand, walk, run, tumble, and cry) were used, each of which, including the passive forms, had either four or five syllables (Table I). Note that the verb “call” is used as a transitive verb in Japanese. There was no significant frequency difference between the two sets of verbs (t‐test, t(10) = 0.68, P = 0.52), according to the Japanese lexical database (NTT database series, “Nihongo‐no Goitokusei” (Lexical Properties of Japanese); Nippon Telegraph and Telephone Corporation Communication Science Laboratories, Tokyo, Japan, 2003). The pictures used in the AS, PS, and SS conditions were identical (the number of lines used in a picture, mean ± SD: 14 ± 2.4, n = 6), and equally complex pictures were used under the SC condition (14 ± 2.5, n = 6). In the pictures, the use of symbols was also counterbalanced for both sides.
In each 4‐s trial of the fMRI experiments, all stimuli were presented visually in yellow against a dark background. Each stimulus was presented for 3,800 ms followed by a 200‐ms blank interval (Fig. 1E). For fixation, a red cross was always shown at the center of the screen. Stimulus presentation and behavioral data collection were controlled using the LabVIEW software and interface (National Instruments, Austin, TX). The participants wore earplugs and an eyeglass‐like MRI‐compatible display (resolution, 800 × 600, within the visual angle of 7°) (VisuaStim XGA; Resonance Technology, Northridge, CA).
Tasks
Under the AS, PS, and SS conditions, all mismatched sentences were made by exchanging two symbols in the original sentences, e.g., “□ pushes ○” instead of “○ pushes □”. Under the SC condition, both symbol‐mismatched sentences and action‐mismatched ones were presented equally often, which required the sentences to be read completely to arrive at a judgment. Under all of these conditions, the participants read a sentence covertly and judged whether or not the sentence matched the action scene depicted by the picture by pressing one of two buttons.
In addition to the picture‐sentence matching task, we used a visual control (VC) task as a baseline control task, which required neither word nor sentence processing. In the VC task, the same sets of pictures used in the picture‐sentence matching task were presented, together with a string of jumbled letters taken from a single sentence (Fig. 1E, inset) in which the symbols and “kanji” appeared at the same positions in the string as in the picture‐sentence matching task. The participants were asked to judge whether or not all the symbols in a letter string were the same as those in the picture, irrespective of the order of the symbols. General cognitive factors such as visual perception of the stimuli, response selection, and motor response were controlled by the VC task.
A single run contained 12 “trial events” of the picture‐sentence matching task (three times each for the SC, AS, PS, and SS conditions), with variable intertrial intervals of 16, 20, and 24 s (four, five, and six VC trials, respectively), pseudorandomized within a run. Since letter strings were presented throughout the VC task while sentences were presented only in the trial events, the participants could switch from the VC task to the trial events according to the stimulus type. The order of the SC, AS, PS, and SS conditions was pseudorandomized in each run to prevent any condition‐specific strategy. Half of the stimuli consisted of matched picture‐sentence pairs, and the other half consisted of mismatched pairs. Sixteen runs were tested in two days, with eight runs per day, and the participants did not encounter the same sentence twice in a single day. The participants underwent practice sessions before scanning to become fully familiarized with the tasks.
fMRI Data Acquisition and Analyses
The fMRI scans were conducted on a 1.5 T scanner (Stratis II, Premium; Hitachi Medical Corporation, Tokyo, Japan). We scanned 16 horizontal slices, each 6‐mm thick and having a 1‐mm gap, covering the range of z = −49 to 62 mm from the AC‐PC line, with a gradient echo echo‐planar imaging sequence (repetition time (TR) = 4 s, acquisition time (TA) = 1.85 s, echo time = 50.5 ms, flip angle = 90°, field of view = 192 × 192 mm2, resolution = 3 × 3 mm2). In a single scanning run, we obtained 77 volumes following three dummy images, which allowed for the rise of the blood oxygenation‐level‐dependent signals.
We performed group analyses using SPM2 statistical parametric mapping software [Friston et al., 1995] (Wellcome Department of Cognitive Neurology, London, UK), on MATLAB (Math Works, Natick, MA). Because the mean accuracy was more than 92% under all the conditions (Table II), all events including correct and incorrect answers were used in the analyses. We realigned the functional volume data in multiple runs and removed runs that included data with a translation of >2 mm in any of the three directions and with a rotation of >1.4°. The acquisition timing of each slice was corrected using the first slice as a reference. Each individual brain was spatially normalized to the standard brain space as defined by the Montreal Neurological Institute (MNI) and was resampled every 3 mm using sinc interpolation. These data were then smoothed using an isotropic Gaussian kernel of 9 mm full‐width at half maximum. Low‐frequency noise and global changes in activity were further removed.
Table II.
Behavioral data for each task condition
| Condition | Accuracy (%) | RT (ms) |
|---|---|---|
| SC | 93.8 ± 4.2 | 2010 ± 289 |
| AS | 93.2 ± 4.5 | 2156 ± 283 |
| PS | 92.0 ± 4.8 | 2166 ± 308 |
| SS | 93.2 ± 4.2 | 2197 ± 287 |
Data are shown as mean ± SD.
According to behavioral data, reaction times (RTs) under the SC, AS, PS, and SS conditions were ∼2,100 ms (Fig. 1E, Table II), indicating that there was little time to rehearse a sentence more than once. Based on the pilot data, we set the timing of the first slice acquisition to 2,100 ms after the stimulus onset, and the acquisition of 16 slices triggered the presentation of a visual stimulus with a 50‐ms delay. The acquisition timing of each slice was corrected using the first slice as a reference for the functional imaging data, thereby specifying both TR = 4 s and TA = 1.85 s in the analyses. By using an impulse function for event‐related hemodynamic responses, we focused on the final process of syntactic reanalysis just before responses were produced. When one condition (e.g., AS) was compared with the VC task, one covariate was set that corresponded to event‐related responses from the baseline level of the VC task. For random effects analyses, a contrast image was generated for each participant and used for intersubject comparisons. The statistical threshold was set to P < 0.05, corrected for multiple comparisons (false discovery rate correction) in the entire brain with no cluster threshold. Among the significantly activated regions under each of the AS, PS, and SS conditions, further comparisons were established at a Z‐score > 3.1 (uncorrected P < 0.001) with a volume of more than five voxels. To exclude false‐positive activations, we used a small volume correction (SVC) at the level of corrected P = 0.05 (9‐mm radius) for SS–AS, (SS–AS) vs. (AS–SC), and PS–AS. The MNI coordinates (x, y, z) for the SVC were (−54, 27, 21) and (−57, 9, 9) for the left IFG, (−39, 3, 42) for the left lateral premotor cortex (LPMC) and (−52, −46, 6) for the left pSTG/MTG, which were reported in the previous studies of syntactic processing [Fiebach et al., 2005; Hashimoto and Sakai, 2002; Suzuki and Sakai, 2003]. For the anatomical identification of activated regions, we used the Anatomical Automatic Labeling method [Tzourio‐Mazoyer et al., 2002].
RESULTS
Behavioral Data
Behavioral data on the accuracy and RT are shown in Table II. The effect of context on the accuracy, together with the differences between the conditions, was examined by comparing the trials of matched and mismatched sentences. A repeated‐measures analysis of variance (ANOVA) with two factors (condition [SC, AS, PS, SS] × context [matched, mismatched]) showed that neither a main effect of condition (F(3, 39) = 0.53, P = 0.67) nor that of context (F(1, 13) = 2.9, P = 0.11) was significant, and an interaction of condition by context was not significant (F(3, 39) = 0.17, P = 0.92), either. Regarding RTs, there was a significant main effect of condition (F(3, 39) = 15, P < 0.001), while neither a main effect of context (F(1, 13) = 2.5, P = 0.13) nor an interaction of condition by context was significant (F(3, 39) = 0.23, P = 0.88). Paired t‐tests showed that the RTs were significantly longer under the AS, PS, and SS conditions than under the SC condition (SC vs. AS: t(13) = 3.0, P = 0.011; SC vs. PS: t(13) = 2.8, P = 0.015; SC vs. SS: t(13) = 3.7, P = 0.0027), indicating that the SC condition was easier than the other conditions. Under the three main conditions, the RTs of AS and SS alone were significantly different (AS vs. PS: t(13) = 0.44, P = 0.67; AS vs. SS: t(13) = 3.0, P = 0.0094; PS vs. SS: t(13) = 1.7, P = 0.11). These results indicate that the SS condition was more demanding than the AS condition, while the difficulty under the AS and PS conditions, as well as that under the PS and SS conditions, was balanced.
The skewnesses of the accuracy distribution for the SC, AS, PS, and SS conditions were −0.95, −0.70, −0.38, and −0.46, respectively, and those of the RT distribution for these conditions were 1.10, 0.52, 0.60, and 0.51, respectively. The accuracy and RT distributions were more skewed under the SC condition than under the other three conditions, indicating a ceiling effect under the SC condition. Since there was no main effect of context among the four conditions, we treated the trials of matched and mismatched sentences together in the following fMRI analyses.
Commonalities and Differences in Cortical Activation Among the Sentence Conditions
To examine the commonalities and differences in cortical activation among the SC, AS, PS, and SS conditions, we first compared each condition with the VC task. As shown in Figure 2, the overall patterns of activation were similar among the four conditions (see also Table III for activation foci). These results showed the reproducibility and consistency of activation in the cortical language areas among these conditions. Activations in the IFG (Brodmann's areas (BAs) 44/45), LPMC (BAs 6/8/9), and pSTG/MTG (BAs 22/21/37) were most prominent and dominant in the left hemisphere. The activation in the left IFG was localized in the left pars opercularis (F3op, BA 44) and pars triangularis (F3t, BA 45) across the anterior vertical ramus, but this region (F3op/F3t) did not extend to the pars orbitalis (F3O, BA 47). Moreover, activation in the left dorsal F3t (dF3t) was more prominent under the PS and SS conditions than under the SC and AS conditions, a finding that we analyze in detail later. Other consistent activation was observed in the presupplementary motor area (pre‐SMA, BA 6), one or both of the heads of the caudate (the coronal sections in Fig. 2), and the inferior parietal/occipital gyrus (BAs 7/19/39) that did not include the main visual cortex (BAs 17/18). The left intraparietal sulcus (IPS, BAs 7/39/40) was activated under the SC, PS, and SS conditions. The right insula activation was observed only under the SS condition.
Figure 2.
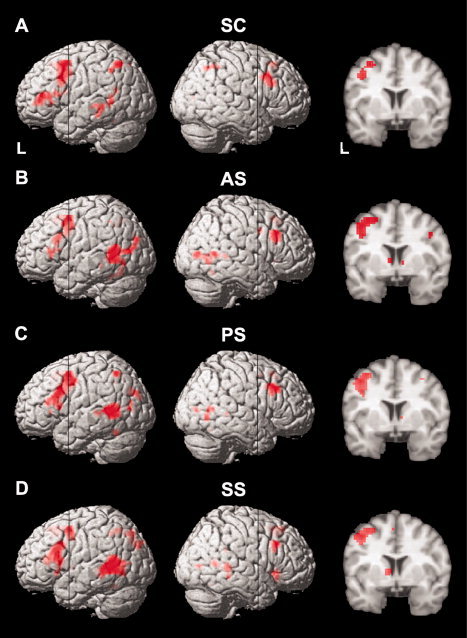
Activation patterns elicited by each condition. A–D: Cortical regions identified by the contrasts of SC, AS, PS, and SS, respectively, which were projected onto the left (L) and right lateral surfaces of a surface‐rendered standard brain. Coronal sections [y = 4 at the black lines] are also shown. For each condition (e.g., AS), one covariate was set that corresponded to event‐related responses from the baseline level of the VC task. The overall patterns of significant activation were similar among the four conditions. Significant activation was also observed in one or both heads of the caudate (see the coronal sections). The thresholds were established at P < 0.05, corrected for multiple comparisons. See Table III for the stereotactic coordinates of the activation foci.
Table III.
Cortical regions identified by condition
| Brain Region | BA | Side | x | y | z | Z | x | y | z | Z | x | y | z | Z | x | y | z | Z |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC | AS | PS | SS | |||||||||||||||
| LPMC | 6/8/9 | L | −36 | 12 | 51 | 4.3 | −45 | 6 | 48 | 4.4 | −39 | −3 | 45 | 4.4 | −42 | 3 | 48 | 4.4 |
| F2/F3op/F3t | 8/44/45 | R | 45 | 15 | 36 | 4.6 | 48 | 21 | 33 | 4.6 | 45 | 18 | 36 | 4.7 | 48 | 21 | 33 | 4.4 |
| dF3t | 45 | L | −54 | 18 | 12 | 3.9 | −51 | 18 | 27 | 3.9 | −51 | 21 | 24 | 4.4 | −51 | 21 | 18 | 5.4 |
| F3op/F3t | 44/45 | L | −45 | 39 | 9 | 4.7 | −51 | 27 | 6 | 4.1 | −54 | 27 | 3 | 4.0 | −48 | 18 | 0 | 3.8 |
| Insula | R | 42 | 21 | −6 | 3.6 | |||||||||||||
| pre‐SMA | 6 | M | −3 | 24 | 48 | 3.8 | −3 | 18 | 51 | 4.5 | 3 | 18 | 51 | 3.3 | −3 | 18 | 48 | 4.8 |
| Caudate | L | −6 | 3 | 3 | 3.8 | −9 | 0 | 3 | 4.2 | |||||||||
| R | 9 | 3 | 0 | 3.5 | 9 | 3 | 3 | 3.3 | ||||||||||
| pSTG/MTG | 22/21/37 | L | −54 | −51 | 6 | 4.0 | −54 | −54 | 6 | 4.5 | −54 | −54 | 6 | 4.8 | −60 | −48 | 9 | 5.2 |
| R | 51 | −54 | 6 | 4.1 | 51 | −60 | 9 | 3.9 | 54 | −36 | 3 | 4.0 | ||||||
| IPS | 7/39/40 | L | −48 | −60 | 51 | 4.0 | −39 | −57 | 54 | 3.8 | −36 | −57 | 51 | 3.9 | ||||
| R | 42 | −63 | 36 | 3.6 | ||||||||||||||
| Inferior parietal/occipital g | 7/19/39 | L | −24 | −78 | 33 | 3.5 | −30 | −81 | 30 | 4.0 | −30 | −84 | 30 | 4.7 | ||||
| R | 45 | −78 | 6 | 4.1 | 42 | −78 | 6 | 3.8 | 45 | −75 | 6 | 3.5 | ||||||
Stereotactic coordinates (x, y, z) in the MNI space are shown for each activation peak of Z values. The threshold is set at corrected P < 0.05 for the voxel level, and clusters smaller than five voxels were removed from the table for brevity. BA, Brodmann's area; L, left hemisphere; R, right hemisphere; M, medial; g, gyrus; F2, middle frontal gyrus; F3op and F3t, opercular and triangular parts of inferior frontal gyrus; d, dorsal; LPMC, lateral premotor cortex; pre‐SMA, presupplementary motor area; pSTG/MTG, posterior superior temporal gyrus and middle temporal gyrus; IPS, intraparietal sulcus.
Potential Effects of Task Difficulty and Syntax on Cortical Activations
As mentioned earlier, the processing of syntactically complex sentences such as scrambled sentences is inevitably confounded by task difficulty. Therefore, we first identified how task difficulty potentially affects cortical activations. We examined activation in AS–SC and SS–AS (Fig. 3A,B), since both sentence complexity (Table I) and RTs (Table II) increased under the SC, AS, and SS conditions in that order. Because an intransitive verb and a transitive verb were used in sentences under the SC and AS conditions, respectively, in which their frequency was matched (see MATERIALS AND METHODS), the AS condition required the analysis of dependency relations, but the SC condition did not, as noted earlier. This difference in verb type may be one reason for the enhanced task difficulty under the AS condition when compared with the SC condition.
Figure 3.
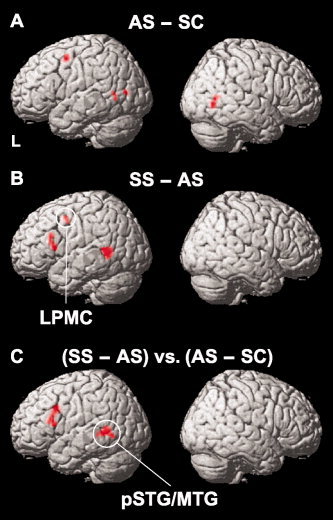
Potential effects of task difficulty and syntax on cortical activations. The sentence stimuli became more complex under the SC, AS, and SS conditions in that order (Table I). According to the behavioral data (Table II), RTs also increased significantly in the same order. A: Cortical regions identified by AS–SC. The contrast of AS (Fig. 2; uncorrected P < 0.001) was used as an inclusive mask to eliminate any deactivation due to SC. The threshold was established at a Z‐score >3.1. Note that the left inferior frontal gyrus (IFG) was not significantly activated in this comparison. B: Cortical regions identified by SS–AS. The contrast of SS (Fig. 2; uncorrected P < 0.001) was used as an inclusive mask. The threshold was established at a Z‐score >3.1 (corrected P < 0.05 after SVC) with a volume of more than five voxels. Note the significant activation in the left LPMC as well as in the left dF3t and left pSTG/MTG. C: Cortical regions identified by (SS–AS) vs. (AS–SC). The threshold was established at a Z‐score >3.1 (corrected P < 0.05 after SVC). Note the significant activation in the left dF3t and left pSTG/MTG. See Table IV for the stereotactic coordinates of the activation foci.
As shown in Figure 3A and Table IV, prominent activations in AS–SC were observed in the left LPMC (−39, 0, 54) and bilateral pSTG/MTG [(−57, −60, 0) and (54, −66, 0)]. Among the brain regions equally activated under the AS, PS, and SS conditions, the left inferior parietal/occipital gyrus (−51, −75, 6) also showed activation in this comparison, whereas the pre‐SMA and the heads of caudate were not significantly activated in this comparison and thus were not significantly affected by task difficulty. In contrast, the most prominent activation in SS–AS was observed in the left dF3t (−52, 21, 21) and this activated region extended to the left F3op/F3t (Fig. 3B), whereas the left IFG was not significantly activated in AS–SC. Significant activation was also observed in the left LPMC (−39, 0, 45) and left pSTG/MTG (−54, −54, 3), which were activated in AS–SC as well. However, the activated region of the left pSTG/MTG in SS–AS was much wider than that in AS–SC, indicating that syntactic processing still affects the activation of this region.
Table IV.
Cortical regions identified by each comparison
| Brain region | BA | Side | x | y | z | Z | Voxels |
|---|---|---|---|---|---|---|---|
| AS – SC | |||||||
| LPMC | 6/8/9 | L | −39 | 0 | 54 | 4.0 | 20 |
| pSTG/MTG | 22/21/37 | L | −57 | −60 | 0 | 3.8 | 11 |
| R | 54 | −60 | 0 | 5.8 | 19 | ||
| Inferior parietal/occipital g | 7/19/39 | L | −51 | −75 | 6 | 3.7 | 6 |
| SS – AS | |||||||
| LPMC | 6/8/9 | L | −39 | 0 | 45 | 4.8 | 7 |
| dF3t | 45 | L | −52 | 21 | 21 | 4.7 | 11 |
| pSTG/MTG | 22/21/37 | L | −54 | −54 | 3 | 4.8 | 29 |
| (SS – AS) vs. (AS – SC) | |||||||
| dF3t | 45 | L | −51 | 21 | 18 | 4.1 | 26 |
| pSTG/MTG | 22/21/37 | L | −51 | −51 | 3 | 4.0 | 29 |
| PS – AS | |||||||
| dF3t | 45 | L | −48 | 24 | 21 | 3.8 | 10 |
Stereotactic coordinates (x, y, z) in the MNI space and cluster size are shown for each activation peak of Z values. The threshold is set at uncorrected P < 0.001 with a volume of more than five voxels. To exclude false‐positive activations, we used an SVC (9 mm radius) at the level of corrected P = 0.05 for SS – AS, (SS – AS) vs. (AS – SC), and PS – AS.
It is possible that the ceiling effect under the SC condition might have weakened the AS–SC result. Therefore, we carefully examined the difference in task difficulty between AS–SC and SS–AS. The difference in RTs of AS–SC (146 ± 134 ms) was about 100 ms longer than that of SS–AS (41 ± 50 ms). There was also a significant difference between these two data (paired‐t, t(13) = 2.7, P = 0.018), indicating a greater behavioral difference in difficulty for AS–SC than for SS–AS. We performed a more direct and compelling test of the syntactic processing by task difficulty interaction, in which the results of SS–AS and AS–SC for each participant were submitted to a paired t‐test. As shown in Figure 3C and Table IV, this analysis revealed greater activation differences in SS–AS than in AS–SC for the left dF3t (−51, 21, 18) and left pSTG/MTG (−51, −51, 3). The opposite pattern of results across these two types of analyses suggest that activation in the left dF3t and left pSTG/MTG is modulated more by syntactic differences than by task difficulty differences.
Left Frontal Activation Enhanced by Noncanonical Syntactic Reanalysis
Next we examined activation in PS–AS, thereby directly contrasting the noncanonical and canonical conditions, where both conditions included subject‐initial structures and where their difference in RTs was not significant. To further control the effect of task difficulty, an analysis of covariance (ANCOVA) was conducted with the difference in RTs as a covariate, in which the variability of RTs among participants was removed (Fig. 4, Table IV). This analysis resulted in a significant activation in the left dF3t alone (−48, 24, 21), which matched with the left dF3t activation observed in SS–AS (Fig. 3B,C, Table IV). This result demonstrated that the left dF3t is specifically recruited for the noncanonical processing of syntactic reanalysis without the influence of domain‐general factors, including general memory demands and task difficulty.
Figure 4.
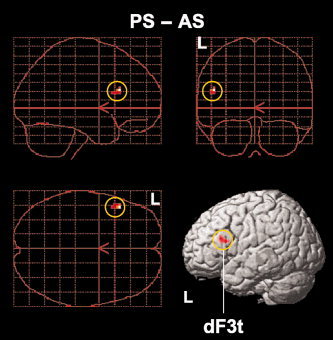
Left frontal activation enhanced by noncanonical syntactic reanalysis. A single region was identified by PS–AS, which is projected in three orthogonal planes (sagittal, coronal, and horizontal from the top left panel) and onto a left surface‐rendered standard brain. The threshold was established at a Z‐score >3.1 (corrected P < 0.05 after SVC). Note the significant activation in the left dF3t (yellow circles). See Table IV for the stereotactic coordinates of the activation focus.
Distinct Activation Patterns in the Left Frontal and Temporal Regions
Next we examined the relative contributions of the frontal and temporal regions to the cognitive processes required under the AS, PS, and SS conditions. For this purpose, the amplitudes of fitted hemodynamic responses (6.1 s after each trial event) were calculated under each condition at the local maxima of the left dF3t (−48, 24, 21), left LPMC (−39, 0, 45), and left pSTG/MTG (−51, −51, 3) (Fig. 5). A repeated‐measures ANOVA with two factors (region [dF3t, LPMC, pSTG/MTG] × condition [AS, PS, SS]) revealed a significant main effect of region (F(2, 26) = 4.9, P = 0.016) and that of condition (F(2, 26) = 15, P < 0.001), together with a significant interaction of region by condition (F(4, 52) = 2.6, P = 0.045). These results indicated that each of these three regions is differentially modulated by task‐related factors under the AS, PS, and SS conditions.
Figure 5.
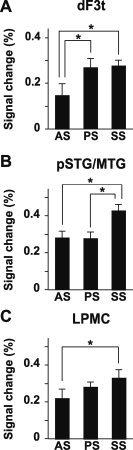
Distinct activation patterns in the left frontal and temporal regions. The amplitudes of fitted hemodynamic responses for the AS, PS, and SS conditions are shown with reference to the SC condition. A: Histograms for the amplitudes of fitted hemodynamic responses at the local maxima of the left dF3t. Error bars indicate the SEM of participants, and asterisks denote the significance level of P < 0.05. Signal changes under the SS and PS conditions were significantly larger than those under the AS condition. B: Histograms for the left pSTG/MTG. Signal changes under the SS condition were significantly larger than those under the AS and PS conditions. C: Histograms for the left LPMC. The SS condition elicited significantly greater responses than the AS condition.
Finally, we examined the effects of condition on the amplitudes of fitted hemodynamic responses for each region. As shown in Figure 5A, the left dF3t activation was enhanced more under the PS and SS conditions than under the AS condition (PS vs. AS: t(13) = 2.4, P = 0.033; SS vs. AS: t(13) = 2.5, P = 0.029), whereas there was no significant difference in response between the PS and SS conditions (t(13) = 0.88, P = 0.15). Moreover, the left pSTG/MTG showed a distinct activation pattern separable from the left dF3t, such that the SS condition elicited significantly greater responses than either the AS or PS condition (SS vs. AS: t(13) = 10, P < 0.001; SS vs. PS: t(13) = 11, P < 0.001) (Fig. 5B), whereas there was no significant difference in amplitude between the AS and PS conditions (t(13) = 1.1, P = 0.31). Furthermore, activation in the left LPMC increased under the AS, PS, and SS conditions in that order (Fig. 5C). The SS condition elicited significantly greater responses than the AS condition (t(13) = 2.3, P = 0.040), whereas there was no significant difference in amplitude between the other conditions (PS vs. AS: t(13) = 1.1, P = 0.27; SS vs. PS: t(13) = 0.93, P = 0.37). These results confirm the unique contribution of the left dF3t to noncanonical syntactic processing (Fig. 4), as well as the supportive roles of the left pSTG/MTG and left LPMC (Fig. 3B), the former of which was not significantly affected by task difficulty (Fig. 3C).
DISCUSSION
The present study with the picture‐sentence matching task successfully established for the first time that the left dF3t activation was enhanced more by the noncanonical processing under the PS and SS conditions than by the canonical processing under the AS condition, and that this enhancement was independent of domain‐general factors such as general memory demands and task difficulty. Moreover, the left pSTG/MTG showed more enhanced responses to object‐initial sentences under the SS condition than to subject‐initial sentences under the AS and PS conditions, which were not significantly affected by task difficulty. Furthermore, activation in the left LPMC increased under the AS, PS, and SS conditions, in that order. It is possible that task difficulty affects the left LPMC, but the three distinct activation patterns suggest that these frontal and temporal regions work in concert to process syntactic structures, with their respective contributions dynamically regulated by linguistic requirements.
It has been reported that noncanonical sentence structures are inherently more difficult to process than canonical ones, as shown by the significant differences in accuracy and RT in the processing of scrambled and active sentences in Japanese [Tamaoka et al., 2005] as well as in German [Fiebach et al., 2005]. On the other hand, the present study showed no significant difference in accuracy among the sentences under the AS, PS, or SS conditions, and the RTs for the sentences under the AS and PS conditions did not differ significantly. One possibility for the absence of any significant difference in accuracy is that the present task did not require memorization, since the whole sentence of a minimal length was visually presented for a longer time than was needed to respond. As a result, we successfully showed the distinct contribution of each region to syntactic processing.
A number of previous imaging studies on verbal short‐term memory have reported greater activation of the left frontal regions including the left IFG; this might reflect general task difficulty stemming from memory load [Braver et al., 1997; Clark et al., 2000; Cohen et al., 1997; Rypma and D'Esposito, 1999]. A recent fMRI study examined the dependency of left IFG activity on the nature of a behavioral task during syntactic processing and suggested that left IFG activation is related to task parameters such as mnemonic and other integrative functions [Love et al., 2006]. Other studies have also suggested that the left IFG activation reflects short‐term memory demands [Cooke et al., 2002; Fiebach et al., 2005]. In our previous study, however, we directly compared the cortical activations in syntactic decision tasks with those in verbal short‐term memory tasks and observed that the left dF3t showed selective activation for syntactic decisions in sentences, and that this activation was much greater than would be expected merely due to task difficulty and verbal short‐term memory demands [Hashimoto and Sakai, 2002]. In the present study, we first examined activation in AS–SC (dependency relations due to verb types) and SS–AS (noncanonical/object‐initial sentences) to clarify the effect of task difficulty coupled with sentence complexity. Despite evidence for a significantly larger behavioral effect for the difference between AS and SC than for SS and AS, we demonstrated the opposite pattern in the activation data, such that the activation in SS–AS is significantly greater in the left dF3t than that in AS–SC (Fig. 3C). The following cognitive factors were further strictly equated among the AS, PS, and SS conditions: visual perception, word recognition, verbal memory load (the number of verbal items held in the phonological store), semantic information, and response selection. Therefore, the remaining factor that can explain the selective activation of the left dF3t in PS–AS should be syntactic reanalysis per se. Indeed, we have previously reported that a selective priming effect on syntactic decisions, but not on semantic decisions, was induced when event‐related transcranial magnetic stimulation was administered to the left IFG with a specific timing [Sakai et al., 2002]. Previous neuroimaging studies have also reported syntactic complexity effects on left IFG activation in English sentences [Dapretto and Bookheimer, 1999; Stromswold et al., 1996], as well as an effect of grammatical rules contrasted with linguistically illegal rules [Musso et al., 2003] or an effect of the language‐specific linearization rules for arguments [Grewe et al., 2005, 2006] as noted earlier. The present study further clarifies a specific and independent role of the left dF3t during the noncanonical processing of syntactic reanalysis.
We also found a localized activation in the left pSTG/MTG (−51, −51, 3), which showed significant activation in (SS–AS) vs. (AS–SC). A previous fMRI study reported activation in the left posterior superior temporal sulcus as well as other regions, such as the premotor cortex or occipitotemporal sulcus, which were activated by object‐initial vs. subject‐initial sentences [Bornkessel et al., 2005]. We previously reported that a focal region in the left pSTG/MTG (−54, −42, 3), proximal to the present activation, was differentially activated by sentences containing either syntactic or semantic anomalies when compared with normal sentences [Suzuki and Sakai, 2003]. Although there were no anomalies in the sentences used in the present study, it is interesting to note that the left pSTG/MTG activation was modulated by the syntactic processing of scrambled sentences. It is thus likely that this activation reflects the syntactic reanalysis required by anomalous sentences or scrambled sentences that were more confusing than the subject‐initial sentences used under the AS and PS conditions. A recent intraoperative electrocorticography study in humans showed bidirectional connectivity between the left IFG and the left pSTG/MTG [Matsumoto et al., 2004], and additional evidence for this connectivity has been reported in studies using MRI to investigate structural connectivity [Catani et al., 2005; Friederici et al., 2006]. Therefore, it is possible that this network subserves syntactic integration, thereby combining multiple linguistic information.
The identification of linguistic information processed in the left pSTG/MTG has been one of the main issues addressed by recent neuroimaging studies. A recent study with German transitive sentences (nominative‐accusative structures), in which an animate subject acts on either an inanimate object (a natural, i.e., unmarked, construction) or an animate object (a more complex, i.e., marked, construction), showed that such a deviation leads to increased activation in the left STG/MTG [Grewe et al., 2007]. This result is consistent with our present finding of the enhanced activation of this region due to noncanonical/object‐initial scrambled sentences, where a more detailed analysis of marked structural relationships between two animate arguments is required. On the other hand, the right pSTG/MTG has been implicated in the processing of nonlinguistic information about visual cues such as the movement of eyes, hands, and mouth [Frith and Frith, 1999; Pelphrey et al., 2005; Wright et al., 2003]. In the present study, the activation of the right pSTG/MTG was significant only in AS–SC (Fig. 3A) and not in the other comparisons (Figs. 3B,C, and 4). Although equally complex pictures were used, the pictures under the AS, PS, and SS conditions involved more visual cues than those under the SC conditions regarding two interacting stick figures (Fig. 1A–D). These results suggest that the left and right pSTG/MTG play functionally separable roles while receiving and interpreting relevant information.
The present study also indicated that the activation of the pre‐SMA and bilateral caudate was enhanced in the AS, PS, and SS conditions when compared with the VC task (Fig. 2), but not in the direct comparisons among the AS, PS, and SS conditions nor in AS–SC (Figs. 3 and 4). This finding suggests that these regions play a general role in linguistic processing. Matsumoto et al. (2004) suggested a possible indirect pathway between the left IFG and left pSTG/MTG via subcortical regions. An anatomical study in primates showed a pre‐SMA projection to the head of the caudate [Inase et al., 1999]; this projection may form the pre‐SMA‐basal ganglia loop used in word generation [Crosson et al., 2003]. Previous studies based on an inherited speech disorder also suggested that an inferior frontal‐basal ganglia loop is a critical neural circuit for speech production [Vargha‐Khadem et al., 2005]. On the other hand, it has been proposed that the basal ganglia plays a role in the application of grammatical rules by which morphemes are combined into words (e.g., talk‐ed, talk‐ing) [Ullman, 2001], and a recent neuroimaging study has indicated that in bilinguals the head of the left caudate may control the language in use at a given moment [Crinion et al., 2006]. Although many studies have outlined the importance of the basal ganglia in cognitive operations such as planning and monitoring [Middleton and Strick, 2000], these previous studies and the present study suggest that the pre‐SMA and bilateral caudate have a more specific role in the language faculties, because such general cognitive factors are completely controlled in our paradigm.
Previous lesion studies have reported aphasics with deficits in sentence comprehension despite adequate lexico‐semantic knowledge, i.e., “agrammatic comprehension” [Maher et al., 1995; Schwartz et al., 1980]. These studies used a two‐choice picture‐pointing task, in which the demand of lexico‐semantic factors was well controlled, incorporating stick‐figure characterizations of circles and squares as protagonists in the stimulus pictures. The aphasics judged which of the two pictures correctly depicted an action scene described by a spoken sentence, such as “The square is shooting the circle” or “The circle is shot by the square,” but they were not always able to interpret those sentences. The aphasics may have more difficulty in understanding or producing syntactically complex sentences, such as passive sentences, than canonical active sentences. Our present results provide further supporting evidence for agrammatism, such that grammatical comprehension is separable from domain‐general factors.
CONCLUSIONS
The present paradigm utilizing a novel picture‐sentence matching task (Fig. 1) successfully demonstrated the syntactic‐selective activation in the left dF3t and left pSTG/MTG, which also excluded the possible involvement of general cognitive factors, since these factors cannot explain the results of direct comparisons between noncanonical and canonical syntactic processing in both SS–AS and PS–AS (Figs. 3B,C, and 4). It is a question for future research with a design parametrically manipulating task difficulty, whether general short‐term memory demands or task difficulty have an effect in dF3t and pSTG/MTG if the task were made more difficult. In this regard, the present design could be applied to aphasics, since individuals with agrammatism would presumably perform at a much lower level relative to the normal individuals studied here. The multiple sentence conditions would also be useful for successfully dissociating any deficits in syntactic processing for aphasics. Although the agrammatism reported by Schwartz et al. (1980) was due to extensive lesions, the differential contributions of multiple regions found in the present study may allow for more precise identification and interpretation of linguistic disorders. Conversely, if a patient's responses to the various sentence conditions are similar to one of the three patterns shown in the bar graphs in Figure 5, it would be possible to narrow down the critical regions that could be responsible for such a deficit. The use of multiple sentence conditions will thus provide, in the near future, a powerful tool for assessing and screening aphasics, as well as for designing rehabilitation programs for them.
Acknowledgements
We thank M. Koizumi for his suggestion regarding linguistic issues, N. Saeki for her technical assistance, and S. Matsukura for her administrative assistance.
REFERENCES
- Ben‐Shachar M,Palti D,Grodzinsky Y ( 2004): Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336. [DOI] [PubMed] [Google Scholar]
- Bornkessel I,Zysset S,Friederici AD,von Cramon DY,Schlesewsky M ( 2005): Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage 26: 221–233. [DOI] [PubMed] [Google Scholar]
- Braver TS,Cohen JD,Nystrom LE,Jonides J,Smith EE,Noll DC ( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Catani M,Jones DK,Ffytche DH ( 2005): Perisylvian language networks of the human brain. Ann Neurol 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Clark CR,Egan GF,McFarlane AC,Morris P,Weber D,Sonkkilla C,Marcina J,Tochon‐Danguy HJ ( 2000): Updating working memory for words: A PET activation study. Hum Brain Mapp 9: 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD,Perlstein WM,Braver TS,Nystrom LE,Noll DC,Jonides J,Smith EE ( 1997): Temporal dynamics of brain activation during a working memory task. Nature 386: 604–608. [DOI] [PubMed] [Google Scholar]
- Cooke A,Zurif EB,De Vita C,Alsop D,Koenig P,Detre J,Gee J,Pinãngo M,Balogh J,Grossman M ( 2002): Neural basis for sentence comprehension: Grammatical and short‐term memory components. Hum Brain Mapp 15: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J,Turner R,Grogan A,Hanakawa T,Noppeney U,Devlin JT,Aso T,Urayama S,Fukuyama H,Stockton K,Usui K,Green DW,Price CJ ( 2006): Language control in the bilingual brain. Science 312: 1537–1540. [DOI] [PubMed] [Google Scholar]
- Crosson B,Benefield H,Cato MA,Sadek JR,Moore AB,Wierenga CE,Gopinath K,Soltysik D,Bauer RM,Auerbach EJ,Gökçay D,Leonard CM,Briggs RW ( 2003): Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J Int Neuropsychol Soc 9: 1061–1077. [DOI] [PubMed] [Google Scholar]
- Dapretto M,Bookheimer SY ( 1999): Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ,Schlesewsky M,Lohmann G,von Cramon DY,Friederici AD ( 2005): Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp 24: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD,Bahlmann J,Heim S,Schubotz RI,Anwander A ( 2006): The brain differentiates human and non‐human grammars: Functional localization and structural connectivity. Proc Natl Acad Sci USA 103: 2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline J‐P,Frith CD,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Frith CD,Frith U ( 1999): Cognitive psychology—Interacting minds—A biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Grewe T,Bornkessel I,Zysset S,Wiese R,von Cramon DY,Schlesewsky M ( 2005): The emergence of the unmarked: A new perspective on the language‐specific function of Broca's area. Hum Brain Mapp 26: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe T,Bornkessel I,Zysset S,Wiese R,von Cramon DY,Schlesewsky M ( 2006): Linguistic prominence and Broca's area: The influence of animacy as a linearization principle. Neuroimage 32: 1395–1402. [DOI] [PubMed] [Google Scholar]
- Grewe T,Bornkessel‐Schlesewsky I,Zysset S,Wiese R,von Cramon DY,Schlesewsky M ( 2007): The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. Neuroimage 35: 343–352. [DOI] [PubMed] [Google Scholar]
- Hashimoto R,Sakai KL ( 2002): Specialization in the left prefrontal cortex for sentence comprehension. Neuron 35: 589–597. [DOI] [PubMed] [Google Scholar]
- Hoshi H ( 1994): Theta‐role assignment, passivization, and excorporation. J East Asian Linguist 3: 147–178. [Google Scholar]
- Inase M,Tokuno H,Nambu A,Akazawa T,Takada M ( 1999): Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: Comparison with the input zones from the supplementary motor area. Brain Res 833: 191–201. [DOI] [PubMed] [Google Scholar]
- Kaiser E,Trueswell JC ( 2004): The role of discourse context in the processing of a flexible word‐order language. Cognition 94: 113–147. [DOI] [PubMed] [Google Scholar]
- Kuroda S‐Y ( 1992): What can Japanese say about government and binding? In: Kuroda S‐Y,editor. Japanese syntax and semantics: Collected papers. Dordrecht: Kluwer Academic; pp 240–252. [Google Scholar]
- Love T,Haist F,Nicol J,Swinney D ( 2006): A functional neuroimaging investigation of the roles of structural complexity and task‐demand during auditory sentence processing. Cortex 42: 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher LM,Chatterjee A,Rothi LJG,Heilman KM ( 1995): Agrammatic sentence production: The use of a temporal‐spatial strategy. Brain Lang 49: 105–124. [DOI] [PubMed] [Google Scholar]
- Matsumoto R,Nair DR,LaPresto E,Najm I,Bingaman W,Shibasaki H,Lüders HO ( 2004): Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain 127: 2316–2330. [DOI] [PubMed] [Google Scholar]
- Middleton FA,Strick PL ( 2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Rev 31: 236–250. [DOI] [PubMed] [Google Scholar]
- Musso M,Moro A,Glauche V,Rijntjes M,Reichenbach J,Büchel C,Weiller C ( 2003): Broca's area and the language instinct. Nat Neurosci 6: 774–781. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA,Morris JP,Michelich CR,Allison T,McCarthy G ( 2005): Functional anatomy of biological motion perception in posterior temporal cortex: An fMRI study of eye, mouth and hand movements. Cereb Cortex 15: 1866–1876. [DOI] [PubMed] [Google Scholar]
- Röder B,Stock O,Neville H,Bien S,Rösler F ( 2002): Brain activation modulated by the comprehension of normal and pseudo‐word sentences of different processing demands: A functional magnetic resonance imaging study. Neuroimage 15: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Rypma B,D'Esposito M ( 1999): The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proc Natl Acad Sci USA 96: 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai KL,Noguchi Y,Takeuchi T,Watanabe E ( 2002): Selective priming of syntactic processing by event‐related transcranial magnetic stimulation of Broca's area. Neuron 35: 1177–1182. [DOI] [PubMed] [Google Scholar]
- Schwartz MF,Saffran EM,Marin OSM ( 1980): The word order problem in agrammatism. I. Comprehension. Brain Lang 10: 249–262. [DOI] [PubMed] [Google Scholar]
- Stromswold K,Caplan D,Alpert N,Rauch S ( 1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Suzuki K,Sakai KL ( 2003): An event‐related fMRI study of explicit syntactic processing of normal/anomalous sentences in contrast to implicit syntactic processing. Cereb Cortex 13: 517–526. [DOI] [PubMed] [Google Scholar]
- Tamaoka K,Sakai H,Kawahara J,Miyaoka Y,Lim H,Koizumi M ( 2005): Priority information used for the processing of Japanese sentences: Thematic roles, case particles or grammatical functions? J Psycholinguist Res 34: 281–332. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N,Landeau B,Papathanassiou D,Crivello F,Etard O,Delcroix N,Mazoyer B,Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ullman MT ( 2001): A neurocognitive perspective on language: The declarative/procedural model. Nat Rev Neurosci 2: 717–726. [DOI] [PubMed] [Google Scholar]
- Vargha‐Khadem F,Gadian DG,Copp A,Mishkin M ( 2005): FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci 6: 131–138. [DOI] [PubMed] [Google Scholar]
- Wright TM,Pelphrey KA,Allison T,McKeown MJ,McCarthy G ( 2003): Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex 13: 1034–1043. [DOI] [PubMed] [Google Scholar]


