Abstract
Early infantile epileptic encephalopathy (EIEE) is a heterogeneous group of severe forms of age-related developmental and epileptic encephalopathies with onset during the first weeks or months of life. The interictal electroencephalogram (EEG) shows a “suppression burst” (SB) pattern. The prognosis is usually poor and most children die within the first two years or survive with very severe intellectual disabilities. EIEE type 3 is caused by variants affecting function, in SLC25A22, which is also responsible for epilepsy of infancy with migrating focal seizures (EIMFS). We report a family with a less severe phenotype of EIEE type 3. We performed exome sequencing and identified two unreported variants in SLC25A22 in the compound heterozygous state: NM_024698.4: c.[813_814delTG];[818 G>A] (p.[Ala272Glnfs*144];[Arg273Lys]). Functional studies in cultured skin fibroblasts from a patient showed that glutamate oxidation was strongly defective, based on a literature review. We clustered the 18 published patients (including those from this family) into three groups according to the severity of the SLC25A22-related disorders. In an attempt to identify genotype–phenotype correlations, we compared the variants according to the location depending on the protein domains. We observed that patients with two variants located in helical transmembrane domains presented a severe phenotype, whereas patients with at least one variant outside helical transmembrane domains presented a milder phenotype. These data are suggestive of a continuum of disorders related to SLC25A22 that could be called SLC25A22-related disorders. This might be a first clue to enable geneticists to outline a prognosis based on genetic molecular data regarding the SLC25A22 gene.
Subject terms: Disease genetics, Medical genetics
Introduction
Early infantile epileptic encephalopathy (EIEE) is a large group of the most severe forms of age-related developmental and epileptic encephalopathic diseases. It is characterized by erratic refractory seizures, usually myoclonic, with an onset during the first weeks or months of life associating with severe developmental delay, often evolving into West syndrome. There are about 70 types of EIEE classified based on the gene involved. In the majority of EIEE, the interictal electroencephalogram (EEG) shows typically a “suppression burst” (SB) pattern with high-voltage bursts of slow waves mixed with multifocal spikes alternating with isoelectric suppression phases [1]. In most cases, brain structural abnormalities are observed [1]. The prognosis is usually poor and most children die within the first two years of life or survive with very severe intellectual disabilities [1]. The EIEE type 3 (OMIM #609304) is characterized by myoclonic seizures, severe global hypotonia, microcephaly, SB pattern, and abnormal electroretinogram (ERG) [2]. EIEE type 3 is caused by variants affecting function in the solute carrier family 25 member 22 gene (SLC25A22, OMIM *609302) [2, 3]. SLC25A22 encodes a 323 amino acids mitochondrial glutamate/H+ symporter that is particularly expressed in the brain [4]. Initially, seven patients from three consanguineous families were reported [2, 3, 5]. These seven patients presented a similar severe phenotype: early-onset seizures, severe hypotonia, microcephaly, abnormalities on brain imaging, SB pattern, and died at 8-year-old age or were reported in a vegetative state. Recently, Reid et al. reported six additional children from three families, presenting variants in SLC25A22 and milder phenotypes than the previous reported patients [6]. In addition, SLC25A22 is also involved in epilepsy of infancy with migrating focal seizures (EIMFS, previously migrating partial seizures in infancy [7]), a severe epileptic encephalopathy with onset in infancy. It was identified in two children who had hemiconvulsive seizures with an onset in the first weeks of life and died before 4 years of age [8].
We report here three additional patients from a multiplex family with variants in SLC25A22 and a less severe phenotype of EIEE type 3 combined with functional studies. We suggest a delineation of the SLC25A22-related disorders group as well as a genotype–phenotype correlation.
Materials and methods
Clinical data
Patient one is a 15-year-old girl of Caucasian origin. She is the first child of unrelated and healthy parents. She had one healthy brother and one affected brother. She was born full term after a normal pregnancy with birth weight (BW) 3865 g (70th centile), birth length (BL) 53 cm (75th centile), and birth head circumference (BHC) 35 cm (50th centile). Epilepsy started on her 3rd day of life with clonic seizures followed by generalized myoclonic seizures, and hypertonic seizures, initially refractory to treatment. Seizures were transiently controlled with two medications from 5 years: levetiracetam and clobazam, but the generalized myoclonic seizures recurred at 11 years. The interictal EEG, at 3 months, was normal and, at 1 year, did not show SB but there was slow electrogenesis and sharp waves. From the first months of life, she had severe axial and peripheral hypotonia. At 1 year, she had a kinetic and static ataxia, an important axial hypotonia and frequent dyskinesia interfering with the gripping of objects. Behavioral disturbances with inconsolable crying, sleep disturbances, and self-mutilation disturbances started on 18 months. She had severe global developmental delay from the first months of life (head holding at 8 months, siting posture at 2 years, and crawl at 8 years). Her motor development deteriorated at 11 years, and she did not crawl at 13 years. At 15 years, she had severe global developmental delay, sleep disturbances, and mostly seizures. She had no language and could not sit still. Occipito-frontal circumference (OFC) is 54 cm (20th centile). Brain MRI, at 6 months, showed an expansion of peri-cerebral fluid spaces, then at 18 months, a cortical-subcortical atrophy predominant in frontal regions. A brain MRI control, at 13 years, did not find any anomalies. Heart and kidney ultrasound and visual evoked potentials (VEP) were normal. Array CGH (Agilent; Affymetrix snp6.0) did not detect any abnormality (arr(1-22,X)x2). Metabolic assays were within normal range, including blood count, liver enzymes, lactic acid blood and very long chain fatty acids blood concentrations, analysis for congenital disorders of glycosylation, and blood amino acids chromatography.
Patient 2 is the 10-year-old brother of patient 1. He was born full term after a normal pregnancy, with BW at 4570 g (98th centile), BL at 52 cm (50th centile), and BHC at 37 cm (96th centile). Epilepsy started at 1 month with generalized myoclonic and tonic-clonic seizures, also initially refractory to treatment. His seizures were partially controlled from 3 years, but the generalized myoclonic seizures recurred at 6 years and were treated by valproate, levetiracetam, and phenobarbital at 10 years. The interictal EEG, at 1 month, was normal and, at 1 year, showed slow electrogenesis but no SB. From the first months of life, he was severe axial and peripheral hypotonia and dyskinetic movements started. Behavioral disturbances with sleep disturbances started on 3 years. He was severely globally delayed. He held his head temporarily at 18 months, but he presented a motor regression after 6 years. He neither walked nor spoke. Physical examination at 9 years revealed axial and peripheral hypotonia. He had dystonic and dyskinetic movements, scoliosis, and bilateral cryptorchidism. His OFC was 52 cm (20th centile). Brain MRI showed increased anterior peri-cerebral spaces, at 3 months, and showed cerebral atrophy, at 18 months. Heart and kidney ultrasound and VEP were normal. Array CGH (Agilent; Affymetrix snp6.0) did not detect any abnormality (arr(1-22)x2,(X,Y)x1). Metabolic assays were normal, including blood count, liver enzymes, lactic acid blood and very long chain fatty acids blood concentrations, analysis for congenital disorders of glycosylation, and blood amino acids chromatography.
Patient 3 is patients 1 and 2’s first-degree cousin: their mothers are sisters and their fathers are distant cousins (Fig. 1b). She is a Caucasian 5-year-old girl, the only child of healthy unrelated parents. She was born full term after a normal pregnancy. She had a BW at 3770 g (50th centile), a BL at 51 cm (50th centile), and a BHC at 35 cm (50th centile). Her seizures started aged 6 weeks with generalized myoclonic seizures, clonic seizures, and cyanotic episodes of uncertain cause. Epilepsy was controlled by clobazam and valproate from 2 years. The interictal EEG, at 4 years, showed a poor and disorganized background and several sharp waves. She had severe global hypotonia, developmental delay, and sleep disturbances that appeared at 18 months and aggravated at 5 years, for which she has been taking melatonin from 18 months. At 5 years, she does not crawl and has no language and she starts to hold her head. Her weight was 19 kg (85th centile), her length was 110 cm (85th centile), and her OFC was 49 cm (18th centile). Brain MRI at 1 year was normal. STXBP1, KCNQ2 targeted sequencing, and array CGH (Agilent; Affymetrix snp6.0) were normal (arr(1-22,X)x2).
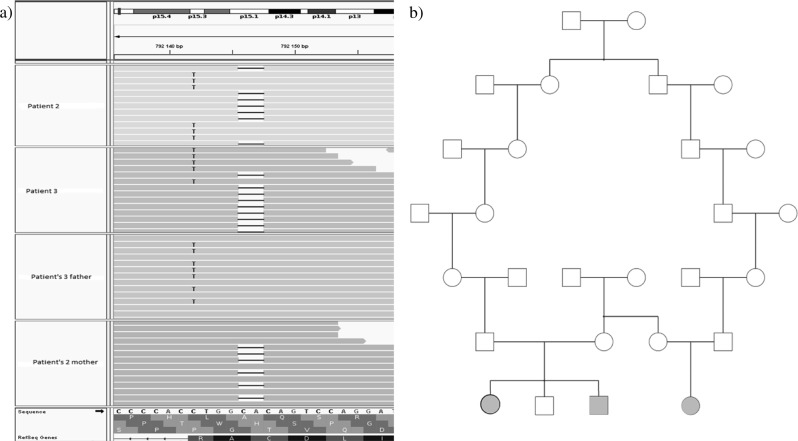
Fig. 1.
a Visualization of variants by integrative genomics viewer (IGV). The sequence is the complementary sequence of genomic DNA. The first two lines correspond to patients 2 and 3. They shared compound heterozygous variants NM_0246989.4: c.[813_814delTG];[818G>A]. The third line corresponds to the father of patient 2 who carries the variant c.818G>A. The fourth line corresponds to the mother of patient 3 who carries the variant c.813_814delTG. b Pedigree
Patients’ features from this report and the literature are summarized in the Table 1.
Table 1.
Clinical features of individuals with variants in SLC25A22 and EIEE phenotype, including updates of the published patients (Molinari et al. 2005 [patients 12–15]; Molinari et al. 2009 [patient 16]; Cohen et al. 2014 [patients 17 and 18]; Poduri et al. 2014 [patients 10 and 11]; Reid et al. 2017 [patients 4–9]). Patients 1– 3 are reported here
| Group | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | MPSI | MPSI | Severe | Severe | Severe | Severe | Severe | Severe | Severe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | P. 1 | P. 2 | P. 3 | P. 4 | P. 5 | P. 6 | P. 7 | P. 8 | P. 9 | P.10 | P.11 | P. 12 | P. 13 | P. 14 | P. 15 | P. 16 | P. 17 | P. 18 | Total |
| Sex | F | M | F | M | M | M | F | F | F | M | F | M | F | M | F | M | F | M | 9F/9M |
| Ethnic group | Caucasian | Caucasian | Caucasian | Arab | Arab | Arab | Arab | Arab | Caucasian | Arab | Arab | Arab | Arab | Arab | Arab | Arab | Arab | Arab | 4 Caucasian/14 Arab |
| Parental consanguinity | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 14 Parental consanguinity (78%) |
| Age of onset seizure | 3 days | 1 month | 6 weeks | 6 weeks | 16 weeks | 16 weeks | First month | 7 years | 6 weeks | 1 week | 2 weeks | Birth | Birth | Birth | Birth | 5 days | First months | 2 weeks | 15/17 before 2 months (88%) |
| Neurological examination | Hypotonia and severe developmental delay | Hypotonia, severe developmental delay, and dystonic and dyskinetic movement | Hypotonia and severe developmental delay | Severe developmental delay, global hypotonia, and dystonic movement | Axial hypotonia, developmental stasis, and no head control | Axial hypotonia, developmental stasis, and no head control | Developmental delay and global hypotonia | Developmental delay (ten words), hypotonia, hypermetropia, and astigmatism | Developmental delay, hypotonia, poor head control, and no visual fixation | Hypotonia and developmental delay | Hypotonia and developmental delay | Hypotonia | Hypotonia | Hypotonia | Hypotonia and later spastic | Hypotonia, no psychomotor acquisition, weak suction, and incomplete archaic reflexes | Hypotonia later spastic and no psychomotor acquisition | Peripheral hypotonia and no psychomotor acquisition | 18/18 Hypotonia (100%); 18/18 developmental delay (100%) |
| Microcephaly | No | No | No | Unknown | Unknown | Unknown | Unknown | Yes | Unknown | Unknown | Unknown | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8/11 (72%) |
| Seizure type | Myoclonic | Myoclonic | Myoclonic and cyanotic seizures | Myoclonic and tonic-clonic seizures | Flexor spasm-like and tonic-clonic seizures | Flexor spasm-like and tonic-clonic seizures | Febrile illness seizures and brief and tonic seizures | Absence seizure and febrile illness | Focal seizures and asymetric clonic | Migrating partial seizures | Migrating partial seizures | Myoclonic | Myoclonic | Myoclonic | Myoclonic | Focal, secondary generalization epileptic spasms | Erratic myoclonic and focal seizures | Focal seizures | 9/17 presenting myoclonic seizure (53%) |
| Response to AEDs | Yes | Yes | Yes | Yes | Unknown | Unknown | No | – | No | Partially | No | Unknown | Unknown | Unknown | Unknown | No | Unknown | Unknown | |
| EEG | No SB, electrogenesis slow down, and sharp waves | No SB and electrogenesis slow down | No SB, poor and disorganized background, and sharp waves | Unknown | Multifocal abnormalities and no SB | Multifocal abnormalities and no SB | Symmetrical diffuse irregular slow wave activity and no SB | Normal | No SB and semidiscontinuous pattern | Delta brush pattern, positive spikes, and high-voltage focal spikes | Independent focal spikes in a multifocal distribution | SB | Unknown | Unknown | SB | SB and hypsarrhythmia | SB | SB | 5/14 presenting SB (36%) |
| VEP | Normal | Normal | Unknown | Unknown | Unknown | Unknown | Unknown | Normal | Unknown | Normal | Abnormal | Abnormal | Abnormal visual nerve-conduction velocity | Unknown | Abnormal conduction and retinal function | Abnormal | Normal | Normal | 5/11 (45%) |
| ERG | Normal | Normal | Unknown | Unknown | Unknown | Unknown | Unknown | Normal | Unknown | Normal | Normal | Unknown | Normal | Unknown | Abnormal | Abolition of macular and peripheral responses and progressive alteration | Unknown | Unknown | 2/8 (25%) |
| Brain imaging | Normal | Subarachnoïd enlargement | Normal | Frontotemporal hypoplasia, globally delayed myelination, prominent cerebellar folia, and small splenium | Unknown | Unknown | Temporal pole myelination delay, prominent cerebellar folia, and small splenium | Temporal pole myelination delay, prominent cerebellar folia, and small splenium | Normal | Normal | Delayed myelination pattern and diffuse thinning of the CC | Brain atrophy | Brain atrophy | Unknown | Subarachnoïd enlargement | Cerebellar hypoplasia, abnormal CC, abnormal gyration of temporo-parietal regions, and abnormal myelinisation of temporal poles | Thin CC and brain atrophy | Thin CC, subarachnoid enlargement, and brain atrophy | 11/15 abnormal imaging (73%) |
| Metabolic evaluation | Normal | Normal | Unknown | Hyperprolinaemia | Hyperprolinaemia | Hyperprolinaemia | Hyperprolinaemia | Normal | Hyperprolinaemia | Normal | Normal | Unknown | Unknown | Unknown | Normal | Normal | Normal | Normal | 5/14 (36%) |
| Mutation | c.818G>A; p.(Arg273Lys) c.813_814delTG; p.(Ala272Glnfs*144) | c.818G>A; p.(Arg273Lys) c.813_814delTG; p.(Ala272Glnfs*144) | c.818G>A; p.(Arg273Lys) c.813_814delTG; p.(Ala272Glnfs*144) | c.166A>C; p.(Thr56Pro) | c.166A>C; p.(Thr56Pro) | c.166A>C; p.(Thr56Pro) | c.886G>A; p.(Ala296Thr) | c.886G>A; p.(Ala296Thr) | c.235G>A; p.(Glu79Lys) c.746T>A; p.(Val249Glu) | c.328G>C; p.(Gly110Arg) | c.328G>C; p.(Gly110Arg) | c.617C>T; p.(Pro206Leu) | c.617C>T; p.(Pro206Leu) | c.617C>T; p.(Pro206Leu) | c.617C>T; p.(Pro206Leu) | c.706G>T; p.(Gly236Trp) | c.617C>T; p.(Pro206Leu) | c.617C>T; p.(Pro206Leu) | – |
| Death or vegetative state | No | No | No | Unknown | Unknown | Unknown | No | No | No | Death at 14 months of life | Death at 4-year-old age | Unknown | Unknown | Death at 8-year-old age | Unknown | Vegetative state at 10-year-old age | Vegetative state at 6-year-old age | Vegetative state at 4-year-old age | 6/12 (50%) |
P patient, F female, M male, AEDs antiepileptic drugs, EEG electroencephalogram, VEP visual evoked potential, ERG electroretinogram, SB suppression burst, CC corpus callosum
Exome sequencing
DNA from patient 2 and his mother and patient 3 and her father were studied using exome sequencing (Centre National de Recherche en Génomique Humaine, Institut de Biologie François Jacob, CEA). After complete DNA quality control for each sample (duplicate quantification, evaluating DNA integrity/quality, and checking for absence of PCR inhibitors), genomic DNA (3 µg) was captured by using an in-solution enrichment method (Human All Exon v5–50 Mb, Agilent Technologies, Santa Clara, CA, USA). Library preparation and exome enrichment (~20.000 targeted genes) was performed automatically by using NGSx (Perkin Elmer, MA, USA) and Bravo (Agilent Technologies), respectively, according to the manufacturer’s instructions (SureSelect, Agilent Technologies). After normalization and quality control, exome-enriched libraries were sequenced by using the Illumina HiSeq2000 system (Illumina, CA, USA) as paired-end 100-bp reads. Samples were sequenced as pools of four samples per lane, to obtain an average coverage of 70–80×, with at least 80% of the target nucleotides covered at 30×. Image analysis and base calling involved use of the Illumina Real Time Analysis Pipeline. Sequence-quality parameters were assessed daily during the 12 days of sequencing. The standard bioinformatics analysis of sequencing data was based on the Illumina pipeline (CASAVA1.8.2) to generate a FASTQ file for each sample.
Sanger sequencing
SLC25A22 was sequenced by direct Sanger sequencing to confirm next-generation sequencing results as described by Barat-Houari et al. (BDT3.1 technology in a ABI3130XL sequencer) [9] on a genomic DNA sample from probands and their relatives.
Cell respiration and enzyme activities [2]
Standard conditions were used to grow cultured skin fibroblasts [10]. Cells of patient 3 could grow on glutamine in the absence of glucose. Respiration and mitochondrial substrate oxidation in the presence of either glutamate or succinate were polarographically studied in fibroblast suspensions before and after permeabilization by digitonin [11]. Frozen cell pellets were thawed and used for spectrophotometric determination of respiratory chain [12] and glutamate dehydrogenase activities [13, 14].
Results
We identified using exome sequencing and confirmed by Sanger sequencing two variants in SLC25A22 shared by the three patients: NM_024698.4:c.[813_814delTG];[818G>A], (p.[Ala272Glnfs*144];[Arg273Lys]) (Fig. 1a). Familial segregation shows that p.(Ala272Glnfs*144) is inherited from their mothers, whereas p.(Arg273Lys) is inherited from their fathers, who were found to be distantly related (Fig. 1b). The variant c. 813_814delTG is absent from the control populations database (dbSNP, ExAC, GnomAD). The frequency in GnomAD of the variant c.818G>A is 4.124e−6 (rs 1195505218). These two variants are classified “pathogenic” according to ACMG criteria (Supplementary Table 1). We have submitted the information of the c.813_814delTG and c.818G>A variants to ClinVar database in NCBI (www.ncbi.nlm.nih.gov/clinvar/; accession number: SCV000882718 and SCV000898499, respectively).
We investigated the oxidation of glutamate in cultured skin fibroblasts from patient 3 (Fig. 2) [2]. Under standard conditions, polarographic studies showed normal cell respiration by intact cells, indicating that glutamate does not represent a major respiratory substrate. However, after permeabilization of cell membranes by digitonin in the presence of amino oxyacetate, a specific inhibitor of amino-aspartate transferase, the patient’s cells failed to oxidize glutamate normally in comparison with controls, whereas oxidation of another substrate (succinate) was normal in the patient’s cells. These results provide evidence that the patient’s cells show a clear defect in mitochondrial glutamate metabolism, which is consistent with the involvement of the variants in SLC25A22 in the disease of the children.
Fig. 2.

Respiration and mitochondrial substrate oxidation in cultured skin fibroblasts from one patient and one control. After polarographic measurement of intact cell respiration, digitonin-permeabilized cells were loaded with adenosine diphosphate (ADP) and amino oxyacetate. This compound inhibits the aspartate-amino transferase enzyme activity. A subsequent addition of glutamate allowed estimation of mitochondrial glutamate oxidation under phosphorylation conditions (i.e., presence of ADP). Note the lack of glutamate-triggered oxygen uptake in the patient’s cells. A similar succinate oxidation rate was measured in control’s and patient’s cells. The dotted line corresponds to the speed of consumption of O2 by cells. After addition of glutamate, the speed rises by 41% in the control’s cells and decreases by 12% in the patient’s cells. Numbers along the traces are nmol O2 consumed per minute per milligram of protein. AOA = amino oxyacetate; Asp = aspartate; Glut = glutamate; α-KG = α-ketoglutarate; OAA = oxaloacetate. Experimental conditions were as described in the “Materials and methods” section
Discussion
EIEE type 3 is a rare and severe genetic disorder due to variants affecting the functioning of protein SLC25A22. The SLC25A22 protein is highly expressed in brain, particularly in areas dedicated to motor coordination (red nuclei, olivary complexes, pontocerebellar nuclei, and substantia nigra). SLC25A22 is more abundant in astrocytes, where it controls glutamate uptake [15, 16]. The protein is localized in the inner mitochondrial membrane and catalyzes glutamate/H+ symporter for the transport of glutamate into the mitochondria. The absence of functional SLC25A22 protein would lead to the dysregulation of extracellular glutamate and activation of the extrasynaptic glutamate receptors [16, 17]. This could impair the cAMP response element binding protein shut-off pathway that would be responsible for long-lasting protection against apoptosis to neural death induction [18], and consequently, inactivation of the prosurvival extracellular signal regulated kinase [19] and neuronal excitability [2].
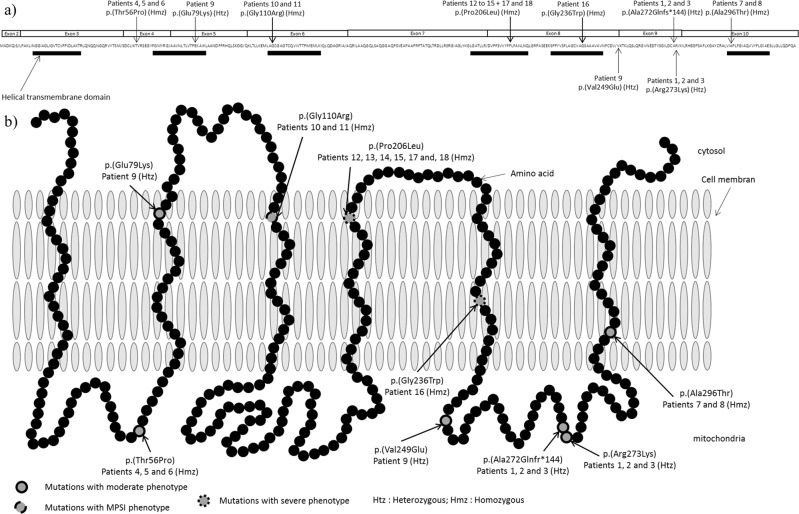
The first patients reported in the literature either died during the first year of life or survived in a persistent vegetative state [2, 3, 5]. However, recently, Reid et al. described six additional patients with variants in SLC25A22 and a milder phenotype [6]. To date, six different variants affecting function of protein have been described (Fig. 3a).
Fig. 3.
Visualization of variants in SLC25A22. a Linear visualization of patient’s variants. Htz = heterozygote; Hmz = homozygote. b 2-D visualization of variants in SLC25A22 protein
SLC25A22 is also involved in EIMFS [8], a severe developmental and epileptic encephalopathy with onset in early infancy and characterized by migrating partial seizures on EEG. The two patients, described by Poduri et al., presented a similar phenotype to EIEE type 3: they developed seizures between 1 and 2 weeks of life, hypotonia, severe developmental delay, and died before 4-year-old age. The brain MRI of the second patient revealed a delayed myelination pattern. The only difference between phenotypes concerns the type of seizures with partial seizures for EIMFS.
The patients described here present a EIEE type 3 phenotype with a milder course of the disease, like patients reported previously by Reid et al. [6], (absence of SB, some acquisitions, no microcephaly, normal VEP, and still alive at 15-year-old age for the oldest) (Table 1). The functional studies allowed us to confirm the involvement of SLC25A22 gene in our family.
The identification of the genetic variations involved in the disease of the patients allowed us to propose a targeted research screening of the causative genetic variations for the direct family members and a complete screening of the SLC25A22 gene for unrelated members of future unions.
We clustered the 18 patients and the variants they carried into three groups according to the severity of the SLC25A22-related disorders. In an attempt to identify genotype–phenotype correlations, we compared the variants according to the location depending on the protein domains (Table 2 and Fig. 3b).
Table 2.
Severe versus mild phenotype comparison
EIEE early infantile epilepsy encephalopathy, EIMFS epilepsy of infancy with migrating focal seizures, SB suppression burst, MPS migrating partial seizures, VEP visual evoked potential, ERG electroretinogram
The variants c.166A>C; p.(Thr56Pro), c.235G>A; p.(Glu79Lys), c.746T>A; p.(Val249Glu), c.813_814delTG; p.(Ala272Glnfs*144), c.818G>A; p.(Arg273Lys), and c.886G>A; p.(Ala296Thr), involved in a milder phenotype (patients 1–9 in Table 1), were located in exons 4, 5, 9, and 10 (NG_023407.1), and were not in helical transmembrane domains except for the two of them: p.(Glu79Lys) and p.(Ala296Thr). However, in patient 9, the p.(Glu79Lys) variant was associated with the p.(Val249Glu) variant (c. [235G>A]; [746T>A]), which did not affect the helical domain (patient 9). The p.(Ala296Thr) variant was in the homozygous state in patients 7 and 8. These patients had atypical phenotypes with febrile and absence seizures. This variant was present once in the heterozygous state in GnomAD and ExaC database. In silico pathogenicity prediction by SIFT and PolyPhen2 was “damaging”, but functional studies were not performed. This variant involved the last helical transmembrane domain (Fig. 3b). We speculate that its consequences on the three-dimensional conformation and channel function are less severe than those of the other helical transmembrane domain variants. This should be tested by functional studies. The variants c.617C>T; p.(Pro206Leu) and c.706G>T; p.(Gly236Trp), involved in the severe phenotype (patients 12–18 in Table 1), were located in exon 8 (NG_023407.1), and affected the helical transmembrane domains, essential for the protein conformation [20]. The variant c.328G>C; p.(Gly110Arg) found in patients with severe EIMFS (patients 10 and 11 in Table 1) was located in exon 6 (NG_023407.1) and affected also a helical transmembrane domain. This could directly compromise the channel function and explain the more severe phenotype. The criteria of pathogenicity for each variant according to ACMG criteria are summarized in Supplementary Table 1.
In summary, we found that patients with two variants affecting function of protein localized in helical transmembrane domains were likely to present with severe phenotypes, whereas patients with at least one variant outside the helical domains presented milder phenotypes. This could explain the phenotypic difference among patients (severe versus mild, Table 2 and Fig. 3).
In conclusion, we defined three groups in SLC25A22-related disorders: EIEE type 3 with severe outcome, EIMFS with severe outcome, and EIEE type 3 with milder outcome, and suggest an association between the location of the variants and the variability of the severity of the disease. Additional studies of patients with EIEE type 3 and EIMFS will be required to further delineate the full phenotypic range associated with SLC25A22 variants and genotype–phenotype correlations.
Supplementary information
Criteria of pathogenicity of each variant and classification according to ACMG criteria
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The first two authors contributed equally: Camille Lemattre, Marion Imbert-Bouteille
Supplementary information
The online version of this article (10.1038/s41431-019-0433-2) contains supplementary material, which is available to authorized users.
References
- 1.Ohtahara S, Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J Clin Neurophysiol. 2003;20:398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Molinari F, Raas-Rothschild A, Rio M, Fiermonte G, Encha-Razavi F, Palmieri L, et al. Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclinic epilepsy. Am J Hum Genet. 2005;76:334–9. doi: 10.1086/427564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molinari F, Kaminska A, Fiermonte G, Boddaert N, Raas-Rothschild A, Plouin P, et al. Mutations in the mitochondrial glutamate carrier SLC25A22 in neonatal epileptic encephalopathy with suppression bursts. Clin Genet. 2009;76:188–94. doi: 10.1111/j.1399-0004.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 4.Fiermonte G, Palmieri L, Todisco S, Agrimi G, Palmieri F, Walker JE. Identification of the mitochondrial glutamate transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem. 2002;277:19289–94. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
- 5.Cohen R, Basel-Vanagaite L, Goldberg-Stern H, Halevy A, Shuper A, Feingold-Zadok M, et al. Two siblings with early infantile myoclonic encephalopathy due to mutation in the gene encoding mitochondrial glutamate/H+ symporter SLC25A22. Eur J Paediatr Neurol. 2014;18:801–5. doi: 10.1016/j.ejpn.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Reid ES, Williams H, Anderson G, Benatti M, Chong K, James C, et al. Mutations in SLC25A22: hyperprolinaemia, vacuolated fibroblasts and presentation with developmental delay. J Inherit Metab Dis. 2017;40:385–94. doi: 10.1007/s10545-017-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poduri A, Heinzen EL, Chitsazzadeh V, Lasorsa FM, Elhosary PC, LaCoursiere CM, et al. SLC25A22 is a novel gene for migrating partial seizures in infancy. Ann Neurol. 2013;74:873–82. doi: 10.1002/ana.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barat-Houari M, Dumont B, Fabre A, Them FT, Alembik Y, Alessandri JLA, et al. The expanding spectrum of COL2A1 gene variants in 136 patients with a skeletal dysplasia phenotype. Eur J Hum Genet. 2016;24:992–1000. doi: 10.1038/ejhg.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brines ML, Sundaresan S, Spencer DD, de Lanerolle NC. Quantitative autoradiographic analysis of ionotropic glutamate receptor subtypes in human temporal lobe epilepsy: up-regulation in reorganized epileptogenic hippocampus. Eur J Neurosci. 1997;9:2035–44. doi: 10.1111/j.1460-9568.1997.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, et al. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res. 2003;143:33–46. doi: 10.1016/S0165-3806(03)00097-X. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeron T, Chretien D, Rotig A, Munnich A, Rustin P. Fate and expression of the deleted mitochondrial DNA differ between human heteroplasmic skin fibroblast and Epstein-Barr virus-transformed lymphocyte cultures. J Biol Chem. 1993;268:19369–76. [PubMed] [Google Scholar]
- 13.Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JK. Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied bu 13C and 31P nuclear magnetic resonance. J Exp Bot. 2001;52:37–45. [PubMed] [Google Scholar]
- 15.Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF. Mitochondrial transport proteins of the brain. J Neurosci Res. 2007;85:3367–77. doi: 10.1002/jnr.21500. [DOI] [PubMed] [Google Scholar]
- 16.Goubert E, Mircheva Y, Lasorsa FM, Melon C, Profilo E, Sutera J, et al. Inhibition of the mitochondrial glutamate carrier SLC25A22 in astrocytes leads to intracellular glutamate accumulation. Front Cell Neurosci. 2017;11:149. doi: 10.3389/fncel.2017.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trabelsi Y, Amri M, Becq H, Molinari F, Aniksztejn L. The conversion of glutamate by glutamine synthase in neocortical astrocytes from juvenile rat is important to limit glutamate spillover and peri/extrasynaptic activation of NMDA receptors. Glia. 2017;65:401–15. doi: 10.1002/glia.23099. [DOI] [PubMed] [Google Scholar]
- 18.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shutoff and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov A, Pellegrino C, Rama S, Dumalska I, SalyhaY, Ben-Ari Y, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–98. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Asp Med. 2013;34:465–84. doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Criteria of pathogenicity of each variant and classification according to ACMG criteria