Abstract
Sex differences in emotional responding have been repeatedly postulated but less consistently shown in empirical studies. Because emotional reactions are modulated by cognitive appraisal, sex differences in emotional responding might depend on differences in emotion regulation. In this study, we investigated sex differences in emotional reactivity and emotion regulation using a delayed cognitive reappraisal paradigm and measured whole‐brain BOLD signal in 17 men and 16 women. During fMRI, participants were instructed to increase, decrease, or maintain their emotional reactions evoked by negative pictures in terms of cognitive reappraisal. We analyzed BOLD responses to aversive compared to neutral pictures in the initial viewing phase and the effect of cognitive reappraisal in the subsequent regulation phase. Women showed enhanced amygdala responding to aversive stimuli in the initial viewing phase, together with increased activity in small clusters within the prefrontal cortex and the temporal cortex. During cognitively decreasing emotional reactions, women recruited parts of the orbitofrontal cortex, the anterior cingulate, and the dorsolateral prefrontal cortex to a lesser extent than men, while there was no sex effect on amygdala activity. In contrast, compared to women, men showed an increased recruitment of regulatory cortical areas during cognitively increasing initial emotional reactions, which was associated with an increase in amygdala activity. Clinical implications of these findings are discussed. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: emotion regulation, sex differences, cognitive reappraisal, amygdala, functional magnetic resonance imaging
BACKGROUND
It is well known that women show an increased prevalence of affective disorders [Kessler et al.,1993] and tend to report more depressive symptoms than men [Nolen‐Hoeksema,2001]. Hypothetically, a generally enhanced emotional responding has been proposed as a predisposing factor for enhanced stress levels in women, which might contribute to the higher rate of depression [Hammen,2005]. Indeed, in stereotypical terms, it is presumed that men and women generally differ in their emotional responses, and in particular that women show enhanced emotional expressiveness [Grossman and Wood,1993]. A number of empirical studies have been conducted to explicitly investigate sex differences in emotional responding, and have reported surprisingly mixed results, which are probably due to the level of emotional responding under study (behavior, psychophysiology, and brain activity) and the stimuli used (positive vs. negative).
Studies involving self‐reports of emotional experiences indicate that women show enhanced emotional responding [Bradley et al.,2001; Fujita et al.,1991; Wild et al.,2001] and more detailed representations of their own emotions [Barrett et al.,2000]. In addition, some studies support the idea that women are indeed more emotionally expressive [Grossman and Wood,1993; Hess et al.,2000] and tend to better recall affective everyday life events [Seidlitz and Diener,1998]. Because retrospective data and self‐report may be contaminated with stereotypical biases [Barrett et al.,1998; Grossman and Wood,1993; Robinson et al.,1998], other studies used more objective measures to assess sex differences. Research using physiological measures of emotional arousal and attention also suggests that women are more reactive to emotional stimuli than men [Bradley et al.,2001; Grossman and Wood,1993; Kemp et al.,2004; Orozco and Ehlers,1998]. Interestingly, a recent study provided evidence that enhanced emotional arousal during the processing of aversive pictures in women occurs relatively late in the time course of stimulus processing [Gard and Kring,2007], pointing to possible gender differences in the regulation of the emotional response.
A meta‐analysis including 65 functional neuroimaging studies on the neural basis of emotional processing published between 1999 and 2002 revealed enhanced emotional responding of women in the subcallosal anterior cigulate cortex (ACC), the thalamus, and the midbrain, whereas men showed enhanced activation in the inferior frontal cortex and posterior areas [Wager et al.,2003]. However, to date, only a handful of studies have explicitly investigated the underlying neural circuitry of gender differences in the processing of emotional stimuli. Some of these studies showed that females are more susceptible to the influence of emotional prosody on semantic processing [Schirmer et al.,2004], while others reported attenuated amygdala responding to fear‐inducing stimuli [Schienle et al.,2005], to a sadness‐inducing procedure [Schneider et al.,2000], and to sexually arousing stimuli [Sabatinelli et al.,2004; Wrase et al.,2003] in women compared to men. Other investigations demonstrated that men and women mainly differ in the pattern of cortical activations during emotional processing, suggesting that men and women predominantly differ in terms of their cognitive strategies, with men evaluating affective stimuli based on the recall of past experiences [Lee et al.2002,2005].
On the whole, the literature on sex differences in behavioral and neural responding to emotional stimuli is surprisingly inconsistent, and it is not possible to draw a coherent picture of the differences. One reason for the observed inconsistencies might be the fact that most studies were unable to differentiate between emotional reactivity and the cognitive regulation of emotional responses. Emotional responding on the behavioral as well as the physiological level has been conceptualized as a product of both emotional reactivity and cognitive processes of emotion regulation, including reappraisal and suppression [Gross1998,2002]. According to this process model of emotion regulation, cognitive reappraisal is stimulus‐associated, and compared to suppression, which is reaction‐associated, it occurs relatively early on the processing of emotional events. Cognitive reappraisal involves changing the meaning of the event so as to decrease or increase its emotional impact, a process that has been referred to as “downregulation” and “upregulation” of the emotional response to the stimulus. Thus, sex differences in emotional responding might be attributed to both enhanced emotional reactivity and/or reduced capabilities to cognitively regulate in terms of reappraisal. In turn, the absence of sex differences in emotional responding in some studies might be due to the fact that distinctions in emotional reactivity might have been masked by differences in emotion regulation.
Cognitive reappraisal is a powerful strategy to regulate emotional responses [Ochsner and Gross,2005] and has been shown to modulate the self‐reported emotional experience [Gross,1998] and the emotion‐modulated startle [Eippert et al.,2007; Jackson et al.,2000]. A number of studies have explored the brain areas involved in the cognitive regulation of emotional responses, and showed enhanced activity in the dorsolateral prefrontal cortex (dlPFC), the orbito‐frontal cortex (OFC), and the ACC during the cognitive regulation of emotional reactions [Eippert et al.,2007; Kim and Hamann,2007; Ochsner et al.,2002,2004; Phan et al.,2005]. Prefrontal top‐down processes are involved in cognitive control and executive functions such as conflict‐detection and interference resolution mediated by the anterior cingulate cortex (ACC). They are also involved in altering and updating the context‐sensitive motivational relevance of stimuli, processed in the orbitofrontal (OFC) and ventromedial prefrontal cortex (vmPFC), as well as in mental attribution processes of the medial prefrontal cortex (mPFC) and in goal‐directed behavior, including working memory mediated in the dorsolateral prefrontal cortex (dlPFC) [Beauregard et al.,2001, Levesque et al.,2003; Ochsner et al.,2002,2005; Badre and Wagner,2004, Phan et al.,2005, Miller,2000]. Critically, it has been asserted that cognitive reappraisal modulates not only the self‐reported emotional experience but also the activity in limbic areas, in particular the amygdala [Ochsner et al.,2004; Eippert et al.,2007], a structure in the anterior medial temporal cortex that has been associated with processing of emotional, in particular aversive, stimuli (for a review: Phan et al.,2002]. So far, most of the studies have focused on the downregulation of emotional responses. However, Ochsner et al. [2004] and Eippert et al. [2007] have demonstrated significant effects of cognitive upregulation on peripheral physiologic as well as neural responses. Thus, we decided to investigate the effects of both cognitive down and upregulation of emotional responses to emotional stimuli.
A recent study explored the possibility that women mainly differ from men in terms of emotion regulation rather than emotional reactivity, thus potentially explaining the heterogeneous results of previous studies. In this study, groups of women and men were required to voluntarily decrease their emotions while viewing aversive pictures. The authors report clusters of enhanced activity during reappraisal in women in the ventral striatum, the rostral ACC and medial PFC. In addition, women showed an attenuated decrease of amygdala reactivity compared to men [Mcrae et al.,2008]. Comparing the neural response to negative and neutral pictures, the authors did not find any sex differences in amygdala reactivity.
In this study, we aimed to further investigate sex differences in emotional reactivity and cognitive emotion regulation encompassing both decreasing and increasing initial emotional responses. To assess both the neural activity associated with initial emotional responding and the subsequent effortful cognitive regulation, a delayed reappraisal paradigm was used [Eippert et al.,2007], which is a variant of a widely used emotion regulation paradigm [Jackson et al.,2000; Ochsner et al.,2002,2004]. We hypothesized that women show enhanced initial emotional responding to aversive stimuli compared to men, associated with enhanced activity in emotionally relevant brain areas, in particular the amygdala. In addition, women were expected to show attenuated activity in the areas subserving the cognitive regulation of emotional responses, namely in the dlPFC, OFC, and ACC when attempting to decrease their initial emotional reactions to negative stimuli.
MATERIALS AND METHODS
Participants
A sample of 33 right‐handed adults with an age range of 22–29 years, consisting of 16 men (age, mean ± SD: 25.2 ± 1.9) and 17 women (age, mean ± SD: 24.6 ± 1.6), was recruited from the University of Rostock. The groups did not differ with regard to subclinical symptoms of depression (Beck Depression Inventory, mean ± s.d., men: 3.56 ± 4.66; women: 3.24 ± 2.71; t 31 = 0.36, P = 0.72) and trait anxiety (State Trait Anxiety Inventory, mean ± s.d., men: 34.06 ± 6.06, women: 33.29 ± 6.05; t 31 = 0.25, P = 0.81) and general intellectual abilities as measured with the short German version of the Wechsler Adult Intelligence Scale ‐ revised (mean ± s.d. IQ, men: 134 ± 11; women: 129 ± 9; t 30 = 1.57, P > 0.10). All participants had normal or corrected‐to‐normal vision. Potential participants were excluded if they were left‐handed, had an IQ < 80, reported a history of head trauma, had a current or past diagnosis of neurological or psychiatric disorder, currently used psychoactive medication or had any non‐MRI‐compatible conditions (e.g. metal in body, pregnancy). Each participant provided written informed consent prior to participation and received monetary compensation for participating in the study. The study was approved by the institutional review board of the Medical Faculty of the University of Rostock.
Experimental Design
Participants were scanned while they were being asked to regulate their emotional reactions to emotionally laden pictures [Jackson et al.,2000]. We used a modified version of this paradigm which allowed neural processes related to initial emotional reactivity and cognitive emotion regulation to be distinguished [Eippert et al.,2007]. At the beginning of each trial, a picture appeared on the screen for 3 s and the participants were instructed to view and understand the content of the picture and let their emotional reaction occur (initial phase). Subsequently, a single‐word instruction appeared in the center of the picture for 1 s, asking participants to maintain, increase, or decrease their initial emotional reaction. During increase trials, participants were asked to increase their emotional reaction by thinking that they or a close relative were involved in the depicted situation, while in the decrease trials, they were asked to imagine that the situation was not real or that they were a detached observer. Maintain trials required attentive viewing of the pictures without trying to alter the affective reaction. Participants had 8 s to implement the instruction (regulation phase), which was followed by a black screen and a relaxation phase of an additional 8 s.
To ensure the correct use of reappraisal strategies, all participants completed a practice session prior to scanning. The experimenter gave an overview of the task ahead, and explained and trained the reappraisal strategies, followed by nine practice trials. The practice pictures were not used in the experiment. If there was any indication that the participant used different reappraisal strategies (e.g. exchanging negative with positive emotions), the experimenter corrected the participant and helped with the correct use of the strategies. The participants were further instructed to look directly at the picture and to not avert their gaze or close their eyes.
The stimuli were selected from the International Affective Picture System on the basis of normative ratings [Lang et al.,2008]. The final picture set consisted of 72 negative and 24 neutral pictures. Neutral pictures were exclusively presented with the instruction to maintain, while negative pictures were randomly assigned to the three regulation conditions. In total, there were four runs, each consisting of 24 trials. The number of conditions within each run was counterbalanced and the order of the conditions was randomized. All participants provided postscan ratings of valence and arousal of the pictures.
Magnetic Resonance Imaging
Imaging data were acquired using a 1.5 T scanner (Magnetom Avanto, Siemens, Erlangen, Germany) equipped with a standard head coil. Head movements were minimized using foam cushions. Visual stimuli were presented via a pair of stereoscopic MRI‐compliant goggles. Functional images were obtained by a T2*‐weighted echo‐planar imaging (EPI) sequence (TR = 2,550 ms, TE = 40 ms, flip angle = 90°, FoV = 192 mm, matrix = 64 × 64). Each volume comprised 34 interleaved measured axial slices (thickness = 3 mm, gap = 1 mm). Data were recorded in four runs of 192 volumes (490 s). At the end of the experiment, a structural scan was acquired, using a high‐resolution T1‐weighted sequence (MPRAGE, TR = 1160 ms, TE = 4.17 ms, flip angle = 15°, FoV = 256 mm, matrix = 256 × 256) recording 160 sagittal 1 mm slices.
Image Analysis
Image data processing was conducted using Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neuroscience, London, UK), implemented in MATLAB 7.0.4 (Mathworks, Sherborn, MA). The first four scans of each session were discarded in order to reduce T1‐saturation effects. The remaining functional images of each participant were slicetime corrected, realigned to the first image of the first session. Subsequently, the functional EPI scans were co‐registered with the individual anatomical images, spatially normalized to MNI space using the T1‐template implemented in SPM5 and smoothed with a 3D Gaussian kernel (FWHM 12 mm).
For each participant, a GLM first‐level analysis was conducted. For each of the four experimental conditions, two regressors were modeled using a convolution of the hemodynamic response function (HRF) with boxcar functions. The first regressor was modeled with the onsets of the picture presentations and durations of 3 s, the second with the onsets of the regulation phases and durations of 8 s. Regressors of no interest were realignment parameters and scanning session constants. Effects at the voxel level were estimated by a least square algorithm, and the resulting contrast images for each condition were then entered into group analyses.
To analyze sex differences with regard to the effects of picture valence and emotion regulation on amygdala activity, we employed random‐effects region of interest analyses. On the basis of previous studies [Eippert et al.,2007; Ochsner et al.,2004], we restricted our analysis to the contrasts which reveal enhanced activity evoked by negative pictures (contrast: negative > neutral), and to the attenuating effects of decreasing (maintain > decrease) and the enhancing effects of increasing emotions (increase > maintain) by means of reappraisal. For each of these contrasts, the individual peak voxels within the left and the right amygdala according to the templates provided by the Automatic Anatomical labeling (AAL) software [Tzourio‐Mazoyer et al.,2002] were determined using the algorithms provided by the rfxplot toolbox [Glascher,2009]. To account for individual structural differences, the mean beta values within a sphere (3 mm radius) centered on these peak voxels were calculated for the corresponding regressors for subsequent analyses of variance. To address the issue of circularity in data analysis, the individual peak voxel was identified from three of the four sessions and the individual beta value was extracted from the remaining session. This procedure was repeated for each of the four sessions, and the beta values were then averaged for further analyses. We then calculated two‐way repeated measures ANOVAs to test for the main effects of group and the valence‐by‐group interaction in the initial viewing phase on amygdala activity. In addition, two‐way ANOVAs were calculated to test for main effects of group and regulation‐by‐group interactions on amygdala activity. Statistical significance for the ROI analyses was set to P < 0.05.
For whole‐brain group analyses, we employed a full factorial design (Sex × Phase × Condition) and used a random‐effects model implemented in SPM5, which accounts for intersubject variance [Worsley et al.,2002]. For the main effects of picture valence and regulation instruction, we applied a statistical threshold of P < 0.05, family‐wise error (FWE) corrected for the entire brain according to the random field theory [Worsley et al.,2004]. For the valence‐by‐sex interaction and the instruction‐by‐sex interaction, we applied a statistical threshold of P < 0.001 and an extent threshold of k > 10 adjunctive voxels.
Coordinates of statistical maxima are given according the Montreal Neurological Institute (MNI) standard brain and were labeled according to the AAL software [Tzourio‐Mazoyer et al.,2002]. For visualization of activations, statistical parametric maps are displayed on slices of a normalized high‐resolution T1 image. Effect sizes within activated clusters were calculated as percent signal change using the “rfxplot” toolbox [Glascher,2009].
RESULTS
Ratings of Valence and Arousal
Postscan ratings of valence and arousal (see Table I) were tested for effects of picture category (collapsing over regulation instructions) and sex, calculating two separate two‐way repeated‐measures ANOVAs. As expected, negative pictures were rated as less positive (F[1,31] = 351.63; P < 0.001) and more arousing (F[1,31] = 393.38; P < 0.001) than neutral pictures. There were no main effects of sex (valence: F[1,31] = 0.57; P = 0.454; arousal: F[1,31] = 0.72; P = 0.402) or a category by sex interaction (valence: F[1,31] = 0.75; P = 0.749; arousal: F[1,31] = 0.05; P = 0.834).
Table I.
Descriptive statistics for (a) valence and (b) arousal ratings of the presented pictures across sexes
| Women | Men | Total | ||||
|---|---|---|---|---|---|---|
| m | SD | m | SD | m | SD | |
| a. Valence | ||||||
| Neutral | 6.30 | 1.19 | 6.57 | 1.08 | 6.44 | 1.13 |
| Negative | 2.94 | 0.53 | 3.09 | 0.89 | 3.01 | 0.73 |
| Maintain | 2.98 | 0.53 | 3.15 | 0.98 | 3.07 | 0.79 |
| Decrease | 2.97 | 0.61 | 3.10 | 0.93 | 3.05 | 0.78 |
| Increase | 2.86 | 0.56 | 3.01 | 0.81 | 2.94 | 0.69 |
| b. Arousal | ||||||
| Neutral | 1.45 | 0.42 | 1.74 | 0.60 | 1.60 | 0.53 |
| Negative | 5.78 | 1.59 | 5.98 | 1.10 | 5.88 | 1.34 |
| Maintain | 5.67 | 1.52 | 5.90 | 1.23 | 5.79 | 1.36 |
| Decrease | 5.78 | 1.63 | 5.91 | 1.19 | 5.85 | 1.40 |
| Increase | 5.89 | 1.67 | 6.12 | 1.00 | 6.01 | 1.35 |
Inspecting for effects of emotion regulation on postscan ratings of the negative pictures, we conducted two‐way repeated‐measures ANOVAs. For the valence ratings, the main effect of the regulation condition showed a statistical trend (F[2,31] = 2.64; P = 0.079), whereas the sex (F[1,31] = 0.357; P < 0.554) and the regulation‐by‐sex interaction (F[2,31] = 0.073; P = 0.930) were not significant. For the arousal ratings, we found a significant main effect of regulation (F[2,31] = 3.76; P = 0.029). Post‐hoc single comparison indicated that this global effect was driven by the enhanced arousal for items that were presented with the instruction to increase the emotions. The main effect of sex (F[1,31] = 0.175; P = 0.679) and the sex by regulation interaction (F[2,31] = 0.23; P = 0.754) were not significant.
Brain Activity in the Initial Viewing Phase
Region‐of‐interest analyses of amygdala activity
Region‐of‐interest analyses of amygdala activity in the initial viewing phase revealed that women showed enhanced reactivity of the bilateral amygdala to both picture categories in terms of a main effect (left amygdala: F[1,31] = 4.50; P = 0.042; right amygdala: F[1,31] = 6.96; P = 0.013) and were more reactive to negative than to neutral pictures as compared to men (sex‐by‐valence interaction; left amygdala: F[1,31] = 4.75; P = 0.037; right amygdala: F[1,31] = 7.72; P = 0.009), Fig. 1.
Figure 1.
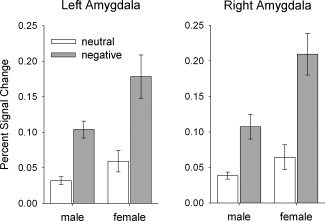
Region‐of‐interest analyses. Effects of stimuli valence on amygdala activity in the initial viewing phase. A significant interaction of valence and group was observed in the left and the right amygdala: Women showed enhanced activity to negative pictures compared to neutral pictures, whereas in males this difference was smaller. The bars depict the mean percent signal change ± s.e.m.
Whole brain analyses
In the initial viewing phase, negative pictures were associated with a significantly higher BOLD response compared to neutral pictures in both the left amygdala (−21, −3, −12; Z = 6.06; P < 0.0001 [SVC]) and the right amygdala (21, 0, −12; Z = 4.01; P < 0.001 [SVC]). Testing for differential effects of sex in the initial viewing phase, we found that compared to men, women showed enhanced activity in the left amygdala when contrasting BOLD responses to negative vs. neutral pictures (−30, 0, −21; Z = 2.74; P < 0.05 [SVC]). This effect was even more pronounced when contrasting the groups for the simple main effect to solely negative pictures (left amygdala: −24, 3, −18; Z = 3.35; P < 0.01 [SVC]; right amygdala: 24, 0, −12; Z = 2.78; P < 0.05 [SVC]).
In addition, enhanced activations in response to negative compared to neutral pictures were found in the initial viewing phase in a number of cortical and subcortical areas (Table II). These areas comprised clusters in the bilateral middle and inferior temporal gyrus, bilateral supramarginal gyrus/temporo‐parietal junction, the right ventro‐medial gyrus, the caudal ACC, and the right inferior frontal gyrus.
Table II.
Significant clusters of neural activation associated with the processing of aversive pictures during the initial viewing phase
| Region | BA | Coordinatesa | Size | Z | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Contrast: negative > neutral pictures | |||||||
| Mid. temporal gyrus | R | 37 | 51 | −63 | 0 | 695 | >8.00 |
| Inf. temporal gyrus | R | 20 | 48 | −39 | −21 | LM | 6.82 |
| Inf. temporal gyrus | R | 37 | 45 | −51 | −12 | LM | 5.72 |
| Mid. temporal gyrus | L | 37 | −54 | −69 | 6 | 567 | 7.60 |
| Inf. temporal gyrus | L | 20 | −45 | −39 | −21 | LM | 6.10 |
| Supramarginal gyrus | L | 2 | −66 | −27 | 33 | 289 | 6.62 |
| Supramarginal gyrus | L | 40 | −33 | −45 | 48 | LM | 6.08 |
| Supramarginal gyrus | L | 2 | −45 | −33 | 39 | LM | 4.71 |
| Ventromedial PFC | R | 10 | 6 | 60 | 27 | 194 | 6.54 |
| Ventromedial PFC | R | 9 | 6 | 54 | 39 | LM | 5.84 |
| Supramarginal gyrus | R | 2 | 54 | −27 | 36 | 110 | 6.31 |
| Caudal ACC | R | 24 | 3 | 15 | 27 | 394 | 6.13 |
| Caudal ACC | L | 24 | −6 | 18 | 27 | LM | 5.97 |
| Caudal ACC | L | 32 | −9 | 27 | 30 | LM | 5.85 |
| Amygdala | L | 34 | −21 | −3 | −12 | 703 | 6.06 |
| Putamen | R | 21 | 15 | 3 | LM | 5.91 | |
| Thalamus | R | 12 | −9 | 6 | LM | 5.84 | |
| Inf. frontal gyrus | R | 48 | 51 | 15 | 21 | 135 | 6.06 |
| Sup. parietal gyrus | R | 40 | 36 | −45 | 57 | 160 | 5.91 |
| Precentral gyrus | R | 6 | 54 | 0 | 48 | 36 | 5.43 |
| Mid. frontal gyrus | L | 6 | −24 | −9 | 48 | 26 | 5.42 |
| Precuneus | L | 7 | −3 | −66 | 36 | 37 | 4.92 |
| Inf. frontal gyrus | R | 45 | 54 | 36 | 6 | 15 | 4.81 |
| Inf. frontal gyrus | R | 45 | 42 | 36 | 9 | LM | 4.60 |
| Contrast: neutral > negative pictures | |||||||
| Parahippocampal g. | R | 37 | 30 | −42 | −6 | 57 | 6.86 |
| Parahippocampal g. | L | 37 | −30 | −45 | −6 | 38 | 5.89 |
| Calcarine fissure | R | 17 | 21 | −57 | 15 | 21 | 5.34 |
Cluster with P < 0.05 (FWE corrected).
BA, Brodmann area; R, right hemisphere; L, left hemisphere; PFC, prefrontal cortex; ACC, anterior cingulate cortex; LM, local maximum.
Coordinates are given in MNI space.
Regarding further sex differences in brain activation to negative vs. neutral pictures (contrast: femalenegative>neutral > malenegative>neutral), we found enhanced activation in the female group in the right mid‐frontal gyrus, right dorsolateral PFC, and left mid‐temporal gyrus. For the reverse contrast (malenegative>neutral > femalenegative>neutral), there were no significant voxels on the same threshold (P < 0.001 uncorrected) (Table III).
Table III.
Significant sex differences regarding the neural correlates associated with the processing of aversive pictures during the initial viewing phase
| Region | BA | Coordinatesa | Size | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Contrast: femalenegative>neutral > malenegative>neutral | ||||||
| Dorsolateral LPFC | 48 | 27 | 3 | 30 | 73 | 3.84 |
| Dorsolateral PFC | 6 | 24 | 3 | 48 | LM | 3.75 |
| Dorsolateral PFC | 32 | 15 | 21 | 48 | 10 | 3.55 |
| Mid. temporal gyrus | 37 | −51 | −69 | 6 | 12 | 3.31 |
| Contrast: malenegative>neutral > femalenegative>neutral | ||||||
| No suprathreshold voxels | ||||||
Cluster with P < 0.001 (uncorrected) and an extent threshold of k > 10 voxels.
BA, Brodmann area; R, right hemisphere; L, left hemisphere; PFC, prefrontal cortex; ACC, anterior cingulate cortex; LM, local maximum.
Coordinates are given in MNI space.
Modulation of Brain Activity During the Regulation Phase
Region‐of‐interest analyses of amygdala activity
For the regulation phase, region‐of‐interest analyses of amygdala activity revealed that men showed enhanced differential activity in the left amygdala when cognitively increasing the emotional response compared to maintaining the initial response, as compared to women (left amygdala: F[1,31] = 6.80; P = 0.014; right amygdala: F[1,31] = 1.47; P = 0.235), Fig. 2a. The group main effects were not significant (left amygdala: F[1,31] = 0.01; P = 0.921; right amygdala: F[1,31] < 0.01; P = 0.958). When cognitively decreasing the emotional response, there was no significant group‐by‐instruction interaction (left amygdala: F[1,31] = 0.01; P = 0.923; right amygdala: F[1,31] = 2.48; P = 0.125) and no significant group main effect (left amygdala: F[1,31] = 0.60; P = 0.446; right amygdala: F[1,31] = 0.02; P = 0.892), Fig. 2b.
Figure 2.
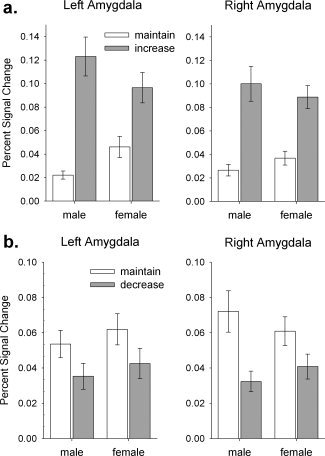
Effects of regulation instruction on amygdala activity in the regulation phase. (a) When increasing the emotional response, a significant group‐by‐instruction interaction was found in the left amygdala: men showed enhanced differential activity compared to women. (b) When decreasing the emotional response, there was no significant group effect on amygdala activity. The bars depict the mean percent signal change ± s.e.m.
Whole brain analyses
In the whole‐brain analysis, there was no effect in the amygdala for the contrast maintain > decrease, but significantly enhanced activation for the contrast increase > maintain (left amygdala: −21, −6, −12; Z = 5.71; P < 0.001 [SVC]; right amygdala: 24, 0, −12; Z = 5.44; P < 0.001 [SVC]). Testing for differential effects of sex in the regulation phase (maleincrease>maintain > femaleincrease>maintain), we found a significant effect in the left amygdala (−21, −6, −15, t = 4.21, Z = 4.13, P < 0.001 [SVC]) and a trend towards an effect in the right amydala (30, −3, −12, t = 2.53, Z = 2.51, P = 0.068 [SVC]), indicating enhanced amygdala activity in male participants during the increase of their emotional response compared to women.
Whole brain analysis of the contrast decrease > maintain emotions revealed enhanced activity bilaterally in the inferior prefrontal cortex, the supplementary motor area, the precentral gyrus, the inferior PFC, the ventrolateral PFC, and the left superior temporal sulcus (Table IV and Fig. 3). The reverse contrast revealed no significant voxels. Sex differences in decreasing emotions by cognitive reappraisal were tested calculating the contrast maledecrease>maintain > femaledecrease>maintain and the reverse contrast.
Table IV.
Significant clusters for the contrast of interest during the regulation phase
| Region | BA | Coordinatesa | Size | Z | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Contrast: decrease > maintain emotions | |||||||
| Suppl. motor area | L | 6 | −3 | 12 | 60 | 1321 | >8.00 |
| Suppl. motor area | R | 6 | 12 | 3 | 66 | LM | 6.96 |
| Precentral gyrus | L | 6 | −45 | 0 | 51 | LM | 6.58 |
| Inf. Frontal gyrus | L | 38 | −54 | 21 | −3 | 631 | >8.00 |
| Inf. Frontal gyrus | L | 44 | −60 | 15 | 24 | LM | 5.50 |
| Insula | L | 48 | −36 | 15 | 3 | LM | 5.42 |
| Inf. Frontal gyrus | R | 38 | 54 | 27 | −6 | 383 | 6.82 |
| Inf. Frontal gyrus | R | 45 | 60 | 24 | 6 | LM | 6.05 |
| Inf. Frontal gyrus | R | 38 | 60 | 15 | −3 | LM | 5.97 |
| Supramarginal gyrus | R | 48 | 57 | −42 | 27 | 89 | 6.12 |
| Ventrolateral PFC | R | 46 | 33 | 42 | 27 | 78 | 5.73 |
| Ventrolateral PFC | R | 46 | 33 | 30 | 33 | LM | 5.38 |
| Sup. temporal gyrus | L | 22 | −63 | −51 | 21 | 234 | 5.62 |
| Mid. temporal gyrus | L | 21 | −63 | −33 | 0 | LM | 5.45 |
| Mid. temporal gyrus | L | 21 | −66 | −48 | 6 | LM | 5.30 |
| Ventrolateral PFC | L | 46 | −30 | 48 | 27 | 27 | 5.07 |
| Precentral gyrus | R | 6 | 51 | 3 | 48 | 13 | 4.78 |
| Contrast: maintain > decrease emotions | |||||||
| No suprathreshold voxels | |||||||
| Contrast: increase > maintain emotions | |||||||
| Suppl. motor area | − | 6 | 0 | 6 | 63 | 1793 | >8.00 |
| Dorsal ACC | L | 24 | −3 | 18 | 39 | LM | >8.00 |
| Dorsal ACC | L | 24 | −6 | 12 | 24 | LM | >8.00 |
| Inf. Frontal gyrus | L | 48 | −51 | 12 | 0 | 848 | >8.00 |
| Inf. Frontal gyrus | L | 44 | −60 | 15 | 24 | LM | 6.01 |
| Thalamus | L | 27 | −12 | −33 | 3 | 840 | 7.53 |
| Precuneus | R | 27 | 6 | −45 | 3 | LM | 7.26 |
| Hippocampus | L | 35 | −18 | −12 | −12 | LM | 7.11 |
| Inf. frontal gyrus | R | 48 | 45 | 12 | 6 | 176 | 7.15 |
| Cerebellum | R | 48 | −54 | −36 | 116 | 7.00 | |
| Cerebellum | R | 36 | −51 | −33 | LM | 6.02 | |
| Supramarginal gyrus | L | 48 | −57 | −42 | 24 | 129 | 6.72 |
| Precuneus | L | 7 | −6 | −66 | 63 | 53 | 6.52 |
| Precuneus | L | 7 | −6 | −75 | 57 | LM | 6.47 |
| Precentral gyrus | R | 6 | 57 | 0 | 48 | 24 | 6.45 |
| Cerebellum | L | −3 | −63 | −15 | 52 | 6.37 | |
| Midbrain | R | 25 | 9 | 0 | −6 | 21 | 6.25 |
| Dorsolateral PFC | L | 9 | −12 | 57 | 36 | 18 | 6.22 |
| Mid. frontal gyrus | L | 46 | −33 | 48 | 30 | 41 | 6.01 |
| Hippocampus | R | 21 | −24 | −9 | 11 | 5.66 | |
| Contrast: maintain > increase emotions | |||||||
| No suprathreshold voxels | |||||||
Cluster with P < 0.05 (FWE corrected).
BA, Brodmann area; R, right hemisphere; L, left hemisphere; PFC, prefrontal cortex; ACC, anterior cingulate cortex; LM, local maximum.
Coordinates are given in MNI space.
Figure 3.
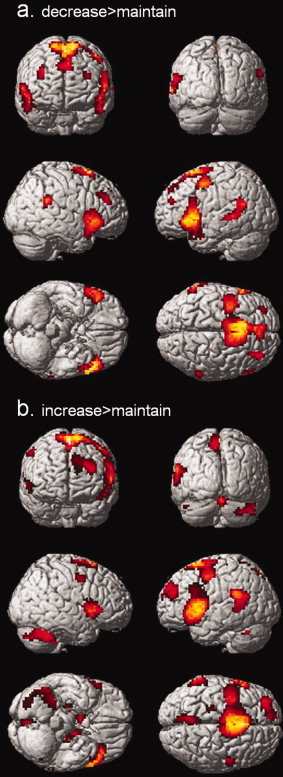
Whole brain analysis for the regulation phase. Statistical parametric maps of the contrast (a) decrease > maintain emotion and (b) increase > maintain rendered on the single‐subject template; P < 0.05 (FWE‐corrected); extent threshold k > 10 voxels.
We found sex differences during decreasing emotions in terms of men recruiting lateral parts of the right OFC, the posterior section of the right dlPFC, the right rostral ACC, and parts of the left superior temporal lobe to a greater extent than women. The reverse contrast revealed no significant voxels (Table V).
Table V.
Significant sex effects for the contrasts of interest during the regulation phase
| Region | H | BA | Coordinatesa | Size | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Contrast: femaledecrease>maintain > maledecrease>maintain | |||||||
| No suprathreshold voxels | |||||||
| Contrast: maledecrease>maintain > femaledecrease>maintain | |||||||
| Sup. temporal gyrus | L | 22 | −60 | −21 | 3 | 49 | 4.13 |
| Sup. temporal pole | L | 48 | −57 | 6 | 0 | 25 | 3.55 |
| Lateral OFC | R | 38 | 36 | 21 | −21 | 26 | 3.54 |
| Rostral ACC | R | 32 | 6 | 42 | 12 | 14 | 3.39 |
| Caudal DLPFC | R | 6 | 18 | −15 | 57 | 10 | 3.30 |
| Contrast: femaleincrease>maintain > maleincrease>maintain | |||||||
| No suprathreshold voxels | |||||||
| Contrast: maleincrease>maintain > femaleincrease>maintain | |||||||
| Postcentral gyrus | L | 4 | −18 | −33 | 72 | 2101 | 5.58 |
| Paracentral lobule | R | 4 | 9 | −36 | 75 | LM | 4.80 |
| Suppl. motor area | L | 6 | −6 | −9 | 72 | LM | 4.69 |
| Postcentral gyrus | L | 43 | −57 | −6 | 24 | 328 | 4.40 |
| Inf. frontal gyrus | L | 6 | −63 | 12 | 9 | LM | 4.16 |
| Insula | L | 48 | −39 | 15 | −6 | LM | 3.52 |
| Amygdala | L | −18 | −6 | −15 | 67 | 4.25 | |
| Sup. parietal gyrus | L | 7 | −24 | −69 | 51 | 57 | 3.81 |
| Sup. parietal gyrus | L | 7 | −12 | −75 | 51 | LM | 3.20 |
| Mid. frontal gyrus | L | 46 | −27 | 36 | 24 | 38 | 3.81 |
| Mid. frontal gyrus | L | 46 | −33 | 45 | 18 | LM | 3.22 |
| Lingual gyrus | R | 30 | 15 | −48 | 0 | 117 | 3.78 |
| Lingual gyrus | R | 17 | 6 | −60 | 6 | LM | 3.32 |
| Inf. frontal gyrus | R | 48 | 30 | 18 | 21 | 13 | 3.64 |
| Cerebellum | R | 30 | −36 | −36 | 21 | 3.60 | |
| Mid. temporal gyrus | R | 21 | 63 | −51 | −3 | 33 | 3.59 |
| Hippocampus | R | 20 | 27 | −21 | −9 | 19 | 3.58 |
| Mid. frontal gyrus | R | 9 | 27 | 30 | 39 | 20 | 3.55 |
| Mid. frontal gyrus | R | 46 | 36 | 36 | 36 | LM | 3.20 |
| Sup. temporal gyrus | R | 42 | 57 | −39 | 18 | 11 | 3.54 |
| Inf. frontal gyrus | R | 45 | 60 | 27 | 6 | 33 | 3.52 |
| Inf. temporal gyrus | L | 37 | −45 | −54 | −9 | 24 | 3.50 |
| Mid. temporal gyrus | L | 22 | −54 | −18 | −3 | 12 | 3.50 |
| Thalamus | L | −12 | −15 | 9 | 20 | 3.45 | |
| Thalamus | L | −18 | −12 | 15 | LM | 3.29 | |
| Fusiform gyrus | L | 37 | −30 | −33 | −27 | 25 | 3.45 |
| Mid. temporal gyrus | L | 37 | −57 | −60 | 0 | 28 | 3.36 |
| Sup. temporal gyrus | R | 21 | 63 | −9 | −6 | 13 | 3.35 |
| Precentral gyrus | L | 6 | −45 | 3 | 39 | 10 | 3.33 |
| Precentral gyrus | L | 6 | −36 | 0 | 45 | LM | 3.21 |
| Precuneus | R | 7 | 12 | −81 | 48 | 23 | 3.24 |
| Sup. occipital gyrus | R | 7 | 24 | −72 | 45 | LM | 3.17 |
Cluster with P < 0.001 (uncorrected) and an extent threshold of k > 10 voxels.
BA, Brodmann area; R, right hemisphere; L, left hemisphere; PFC, prefrontal cortex; ACC, anterior cingulate cortex; LM, local maximum.
Coordinates are given in MNI space.
The contrast increase > maintain revealed brain activation specific for cognitively increasing emotional responding in areas comparable to those observed during decreasing emotions: a huge cluster comprising the supplementary motor area, bilateral parts of the posterior dlPFC, and the caudal ACC. In addition, the contrast revealed significant clusters in the inferior frontal gyrus, the precuneus, the left temporo‐parietal junction, the left thalamus, a cluster in the midbrain, the right hippocampus, and parts of the cerebellum. Again, there were no significant voxels when calculating the reverse contrast using the same threshold (Table IV).
Searching for sex differences by calculating the contrast maleincrease>maintain > femaleincrease>maintain, we found enhanced activity during increasing emotional responding in the male group in a huge cluster stretching across parts of the medial paracentral lobule and the supplementary motor area. In addition, there were significant clusters bilaterally in the inferior frontal gyrus, the mid‐frontal gyrus, the superior temporal sulcus, the left fusiform gyrus, the left insula, the left hippocampus, the right lingual gyrus, and the left thalamus (Table V and Fig. 4). Again, the reverse contrast showed no significant voxels.
Figure 4.
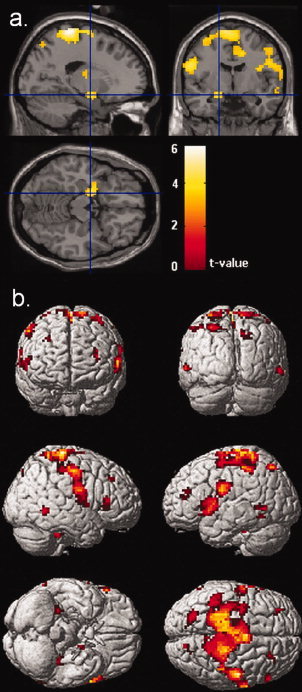
Whole brain analysis for the contrast maleincrease>maintain > femaleincrease>maintain (a) Sections with the statistical parametric map superimposed on a single‐subject structural template illustrating the effect in the left amygdala. (b) Renderings of the statistical parametric map on a single‐subject structural template, illustrating cortical areas more strongly activated in the male group compared to the female group while upregulating emotional responses to aversive pictures; P < 0.001 (uncorr); extent threshold: k > 10.
DISCUSSION
In this study, we were able to closely replicate previous findings from studies using similar approaches to investigate the neural basis of emotion regulation (e.g. Eippert et al.,2007; Ochsner et al.,2004]. In brief, negative pictures evoked bilateral amygdala responses and enhanced activity in mid‐temporal, superior temporal, inferior frontal cortical areas, and the caudal part of the ACC. Emotion regulation, in turn, was associated with activity in the dorsomedial and dorsolateral parts of the PFC and in inferior frontal regions. In addition, our data show that amygdala activity was enhanced during the upregulation and attenuated during the downregulation of the emotional responses to negative stimuli.
The dlPFC has been implicated in the effortful processing of information [Cabeza and Nyberg,2000], for example working memory [Bunge et al.,2002] and the control of attention to emotional stimuli [Bishop et al.,2004]. Several studies have consistently shown activation of the bilateral dlPFC during the downregulation [Eippert et al.,2007; Levesque et al.,2003; Ochsner et al.,2002,2004] as well as the upregulation of emotional responses [Eippert et al.,2007; Ochsner et al.,2004]. The study nicely replicates these results as the data show strong overlapping activations in the bilateral dlPFC during both up and downregulation of emotional responses. A possible interpretation might be that the cognitive up and downregulation of emotional response both involve the reallocation of attention to the cognitive process away from the stimulus itself and might therefore recruit the dlPFC to a comparable extent. It has been put forward that although the dlPFC does not have direct connections to the amygdala, it might modulate amygdala activity through connections via the OFC and the ACC [Meyer‐Lindenberg and Zink,2007; Ochsner and Gross,2005]. In this study, we found activations during the regulation in the inferior frontal cortex extending to the orbital part, possibly reflecting the mediating role of this region between dlPFC and amygdala activity.
The dorsal ACC has been previously associated with cognitive control [Bush et al., 2000] and has been repeatedly shown to be active during the regulation of emotional responses [Beauregard et al.,2001; Eippert et al.,2007; Ochsner et al.,2004]. In this study, we found strong activations in the dorsal ACC during the upregulation of emotional responses, which concurs with the results of Eippert et al. [2007], who found stronger dorsal ACC activation in the upregulation compared to the downregulation of emotional responses.
Sex Differences
With regard to sex differences, and in line with our first hypothesis, we found that compared to men, women showed enhanced activity in the amygdala in response to negative pictures in the initial viewing phase. Contrasting BOLD responses in the amygdala for negative pictures to neutral pictures in the ROI analyses, this effect appeared to be present in the bilateral amygdala. Compared to men, women also showed enhanced amygdala activity in response to neutral pictures. It might be speculated that women appraised the neutral pictures as more negative or arousing and might therefore have displayed an emotional response. However, given the lack of sex differences in the post‐scan ratings for the neutral pictures, this explanation seems unlikely. An alternative explanation for the enhanced amygdala activity in response to neutral pictures might be that women were more likely to expect negative pictures in the waiting phase before a specific picture was shown. Previous studies have shown that the anticipation of aversive stimuli evokes emotional responses [e.g. Nitschke et al.,2002,2006] and that there are sex differences in amygdala activity in the anticipation of aversive events [Mackiewicz et al.,2006]. Because the data from our study are not conclusive with respect to this point, further studies could explicitly address this hypothesis of enhanced anticipatory negative emotions in women. A third explanation refers to the possibility that women show higher levels of anxious or depressive symptoms than men, which might account for the enhanced amygdala responding regardless of picture valence. However, in this study, there were no differences with regard to self‐reported levels of anxiety and depression. In addition to enhanced initial amygdala responding in the female group, we found enhanced activity in the medial PFC and in small clusters within the dlPFC during the initial phase. As pointed out earlier, the recruitment of prefrontal areas during the initial processing might reflect effortful cognitive processing, such as the allocation of attentional resources to the emotional aspects of the stimulus, which have been enhanced in women compared to men. Alternatively, women might have attempted to decrease their emotions as soon as the aversive stimuli appeared. However, as women showed enhanced amygdala responding in the initial viewing phase, these attempts might have been less effective compared to men.
With regard to the regulation phase, the data only partially confirmed our second hypothesis: although we found some small clusters within the caudal ACC, the lateral OFC and the inferior PFC that were more active in male compared to female participants, no sex effects on amygdala activity were found during down‐regulation. Thus, although there were small clusters within the (pre‐) frontal cortex, which women recruited to a lesser extent than men during downregulation, amygdala activity appeared to be unaffected by this sex difference.
In addition, we found a widespread network of areas known to be involved in emotion regulation that men recruited to a greater extent than women during the upregulation of emotional responses. These areas comprised the bilateral inferior PFC, the paracentral lobe, the supplementary area, and mid‐temporal gyrus, which have previously been associated with the cognitive processes during reappraisal in general rather than with increasing negative affect in particular [Eippert et al.,2007; Ochsner et al.,2004]. Men also showed enhanced activity in areas traditionally associated with the generation of emotional responses: amygdala, insula, and fusiform gyrus [Phan et al.,2002]. This pattern of activations might suggest that men used cognitive strategies that recruited regulatory areas to a greater extent than women and thereby enhanced the activity in emotion processing areas more efficiently. It might also be speculated that men were more willing to follow the instruction to increase their emotional response. In turn, it could be possible that women were hesitant to further enhance emotional response because of the initially increased emotional responding. Finally, enhanced amygdala activity in the initial viewing phase in women might have produced a ceiling effect and thus compromised further enhancement in the regulation phase.
Clinical Implications
Enhanced amygdala reactivity [Drevets,2000; Whalen et al.,2002] as well as attenuated activation in the PFC during the cognitive control of emotional reactions [Beauregard et al.,2006] and a reduced as well as a dysfunctional coupling of medial PFC and amygdala [Johnstone et al.,2007] have been reported in depression. The differential brain activity to emotional stimuli and during the cognitive control of emotional responses shown in women in the present study concur with the observations in depression and might in part correspond to the enhanced prevalence of affective disorders in women [Kessler et al.,1993]. This being said, our data in part support the idea that women may be more vulnerable to depression because they tend to be more reactive to emotional stimuli and are less effective in regulating their emotional response.
The present finding of increased amygdala activity to negative stimuli in men during cognitively increasing emotional responses might also have implications for the understanding of the neural basis of maladaptive behaviors associated with enhanced emotional responding to aversive interpersonal stimuli that is more prevalent in men, e.g. aggressive behavior. The present results suggest that emotionally laden aggressive behavior might not only be due to difficulties in impulse control, but might also be promoted by the relative ease of voluntary emotional upregulation in men.
Limitations and Future Directions
The present study has some limitations with regard to the conclusions that can be drawn from the results. Although we explicitly tried to distinguish between the emotional reaction and the process of emotion regulation through the inclusion of an initial viewing phase, we cannot rule out the possibility that the cognitive regulation was spontaneously initiated even before the verbal cue for a specific regulation direction was presented. In addition, although all participants were thoroughly trained in using reappraisal strategies, we also cannot rule out that some participants did not follow the instructions or used regulation strategies other than reappraisal to modify their emotions. Since reappraisal and suppression, for example, seem to differentially alter activity in the amygdala and the insula [Goldin et al.,2008], future studies should control for the possibility that men and women employ different regulation strategies. These studies could also control for differences in trait emotion regulation abilities and trial‐by‐trial task performance success. In addition, future studies could measure gaze pattern using eye‐tracking to control for differences in visual attention.
The results of this study do not allow causal interpretations to be drawn about the interaction of different brain regions. The interpretations regarding the role of specific brain regions as “reactive” or “regulatory” is indirect, and it is based on the literature on cognition and emotion regulation. An initial study investigated whether amygdala activity is differentially predicted by the activity in prefrontal regions during decreasing as compared to maintaining emotional reactions to negative stimuli. As predicted, the authors found an enhanced coupling between OFC, dlPFC, and subgenual ACC activity and amygdala activity during decreasing emotions [Banks et al.,2007]. Future studies should focus on connectivity between prefrontal areas and limbic structures in order to gain a better understanding of the functional relationship between these areas using dynamic causal modeling [Stephan et al.,2007].
Furthermore, we do not provide data on the consequences of emotion regulation differences on the behavioral and physiological level. Measuring additional variables of emotional arousal, such as skin conductance or heart rate, could be a promising approach to further characterize emotion regulation efficiency on the physiological level.
Finally, we did not control for variations in menstrual cycle status in the female group, which might have added a certain amount of variance regarding emotional reactivity and emotion regulation. Future studies could control for these effects by thoroughly assessing the menstrual cycle phase.
CONCLUSIONS
Our results extend previous research [Mcrae et al.,2008], as we show that men and women differ in terms of brain activity associated with the initial appraisal of negative stimuli and during the voluntary control of emotional responses to aversive stimuli. In particular, we provide evidence that women show enhanced amygdala reactivity to negative stimuli, as well as differences in areas in the PFC during downregulation of emotional responses. In addition, marked differences mainly appeared in the upregulation condition, i.e. men showed enhanced activity in a widespread network of brain areas previously associated with emotion regulation when attempting to increase their emotional reactions by means of reappraisal.
Acknowledgements
We are grateful to Christoph Berger and Juliane Nantke for assistance with data analysis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Badre D, Wagner AD ( 2004): Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41: 473–487. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL ( 2007): Amygdala‐frontal connectivity during emotion regulation. Social Cogn Affective Neurosci 2: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Robin L, Pietromonaco PR, Eyssell KM ( 1998): Are women the “more emotional” sex? Evidence from emotional experiences in social context. Cogn Emotion 12: 555–578. [Google Scholar]
- Barrett LF, Lane RD, Sechrest L, Schwartz GE ( 2000): Sex differences in emotional awareness. Personality Social Psychol Bull 26: 1027–1035. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J ( 2006): Dysfunction in the neural circuitry of emotional self‐regulation in major depressive disorder. Neuroreport 17: 843–846. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD ( 2004): Prefrontal cortical function and anxiety: Controlling attention to threat‐related stimuli. Nat Neurosci 7: 184–188. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ ( 2001): Emotion and motivation II: Sex differences in picture processing. Emotion 1: 300–319. [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD ( 2002): Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Drevets WC ( 2000): Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126: 413–431. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S ( 2007): Regulation of emotional responses elicited by threat‐related stimuli. Hum Brain Mapp 28: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita F, Diener E, Sandvik E ( 1991): Gender differences in negative affect and well‐being: The case for emotional intensity. J Pers Soc Psychol 61: 427–434. [DOI] [PubMed] [Google Scholar]
- Gard MG, Kring AM ( 2007): Sex differences in the time course of emotion. Emotion 7: 429–437. [DOI] [PubMed] [Google Scholar]
- Glascher J ( 2009): Visualization of group inference data in functional neuroimaging. Neuroinformatics 7: 73–82. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ ( 2008): The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry 63: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ ( 1998): Antecedent‐ and response‐focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol 74: 224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ ( 2002): Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology 39: 281–291. [DOI] [PubMed] [Google Scholar]
- Grossman M, Wood W ( 1993): Sex differences in intensity of emotional experience: A social role interpretation. J Pers Soc Psychol 65: 1010–1022. [DOI] [PubMed] [Google Scholar]
- Hammen C ( 2005): Stress and depression. Annu Rev Clin Psychol 1: 293–319. [DOI] [PubMed] [Google Scholar]
- Hess U, Senecal S, Kirouac G, Herrera P, Philippot P, Kleck RE ( 2000): Emotional expressivity in men and women: Stereotypes and self‐perceptions. Cogn Emotion 14: 609–642. [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ ( 2000): Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 37: 515–522. [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ ( 2007): Failure to regulate: counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. J Neurosci 27: 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Silberstein RB, Armstrong SM, Nathan PJ ( 2004): Gender differences in the cortical electrophysiological processing of visual emotional stimuli. Neuroimage 21: 632–646. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB ( 1993): Sex and depression in the National Comorbidity Survey. I. Lifetime prevalence, chronicity and recurrence. J Affect Disord 29: 85–96. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S ( 2007): Neural correlates of positive and negative emotion regulation. J Cogn Neurosci 19: 776–798. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8. Gainesville, FL: University of Florida. [Google Scholar]
- Lee TM, Liu HL, Hoosain R, Liao WT, Wu CT, Yuen KS, Chan CC, Fox PT, Gao JH ( 2002): Gender differences in neural correlates of recognition of happy and sad faces in humans assessed by functional magnetic resonance imaging. Neurosci Lett 333: 13–16. [DOI] [PubMed] [Google Scholar]
- Lee TM, Liu HL, Chan CC, Fang SY, Gao JH ( 2005): Neural activities associated with emotion recognition observed in men and women. Mol Psychiatry 10: 450–455. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M ( 2003): Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53: 502–510. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB ( 2006): The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci USA 103: 14200–14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcrae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ ( 2008): Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Process Intergroup Relat 11: 143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Zink CF ( 2007): Imaging genetics for neuropsychiatric disorders. Child Adolesc Psychiatr Clin N Am 16: 581–597. [DOI] [PubMed] [Google Scholar]
- Miller EK ( 2000): The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJ, Ruffalo D, Mackiewicz KL, Gray SM, Victor E, Davidson RJ ( 2002): Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology 39: 254–258. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ ( 2006): Functional neuroanatomy of aversion and its anticipation. Neuroimage 29: 106–116. [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema S ( 2001): Gender differences in depression. Curr Direct Psychol Sci 10: 173–176. [Google Scholar]
- Ochsner KN, Gross JJ ( 2005): The cognitive control of emotion. Trends Cogn Sci 9: 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD ( 2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL ( 1998): Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry 44: 281–289. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME ( 2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57: 210–219. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Johnson JT, Shields SA ( 1998): The gender heuristic and the database: Factors affecting the perception of gender‐related differences in the experience and display of emotions. Basic Appl Soc Psychol 20: 206–219. [Google Scholar]
- Sabatinelli D, Flaisch T, Bradley MM, Fitzsimmons JR, Lang PJ ( 2004): Affective picture perception: Gender differences in visual cortex? Neuroreport 15: 1109–1112. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Vaitl D ( 2005): Gender differences in the processing of disgust‐ and fear‐inducing pictures: An fMRI study. Neuroreport 16: 277–280. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, Yves von Cramon D ( 2004): Gender differences in the activation of inferior frontal cortex during emotional speech perception. Neuroimage 21: 1114–1123. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Salloum JB, Posse S ( 2000): Gender differences in regional cerebral activity during sadness. Hum Brain Mapp 9: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlitz L, Diener E ( 1998): Sex differences in the recall of affective experiences. J Pers Soc Psychol 74: 262–271. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ ( 2007): Dynamic causal models of neural system dynamics: Current state and future extensions. J Biosci 32: 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF ( 2003): Valence, gender, and lateralization of functional brain anatomy in emotion: A meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H ( 2002): Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry 7: 234–242. [DOI] [PubMed] [Google Scholar]
- Wild B, Erb M, Bartels M ( 2001): Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: Quality, quantity, time course and gender differences. Psychiatry Res 102: 109–124. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC ( 2002): A general statistical analysis for fMRI data. Neuroimage 15: 1–15. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J ( 2004): Unified univariate and multivariate random field theory. Neuroimage 23 ( Suppl 1): S189–S195. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, Braus DF, Heinz A ( 2003): Gender differences in the processing of standardized emotional visual stimuli in humans: A functional magnetic resonance imaging study. Neurosci Lett 348: 41–45. [DOI] [PubMed] [Google Scholar]


