Abstract
Lesion studies on nonhuman primates utilizing recognition memory tests have shown that the orbitofrontal cortex is critical for the encoding of novel information, and anatomical studies have shown that the orbitofrontal cortex forms part of a mnemonic circuit that connects limbic medial temporal areas with higher‐order lateral frontal cortical regions. Furthermore, functional neuroimaging studies have demonstrated increased activity in the orbitofrontal cortex of the human brain during the encoding of novel visual and auditory information. The present positron emission tomography study examined brain activity related to the encoding of tactile information. Cerebral blood flow (CBF) in normal human subjects during the tactile exploration of novel stimuli from a related set of textures and patterns, as well as from a set of aversive tactile stimuli, was compared with CBF during a control condition involving familiar tactile stimuli. The results demonstrate that the right rostral orbitofrontal cortex is involved in the active encoding of novel tactile information, while a more caudal region of the orbitofrontal cortex, which is more closely connected with limbic and autonomic regions of the brain, was activated when subjects explored novel aversive tactile stimuli. These results suggest that the orbitofrontal cortex, through its connections with the limbic areas of the medial temporal lobe, influences the processing of incoming information and thus contributes to its encoding. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: somatosensory, tactile tasks, memory, positron emission tomography, human
INTRODUCTION
At the cortical level, tactile information is first coded in the primary somatosensory cortex (SI) as shown by lesion [Randolph and Semmes, 1974] and electrophysiological studies [Nelson et al., 1980] in the monkey. Further processing takes place in the secondary somatosensory cortex (SII) [Murray and Mishkin, 1984; Ridley and Ettlinger, 1976], as well as in the insular cortex [Friedman et al., 1986], leading to the perception of touch. Several investigators have described a cortico‐limbic pathway for tactile memory, linking SI to insular areas via SII and then the insula to limbic structures in the medial temporal lobe, such as the entorhinal and perirhinal cortex, the amygdala, and the hippocampus [Friedman et al., 1986; Mishkin, 1979]. What areas of the frontal lobe play a direct role in the encoding of tactile information in memory? Furthermore, if tactile information deviates significantly from expectation and is therefore slightly aversive, are those frontal areas that are most closely related to the limbic system involved? Research on the monkey utilizing recognition memory tests suggests that the orbitofrontal cortex is as important to the encoding of information [Bachevalier and Mishkin, 1986; Meunier et al., 1997] as the well known memory structures of the medial temporal region [Milner, 1972; Mishkin, 1982; Squire and Zola‐Morgan, 1991; Zola et al., 2000].
Anatomical studies have demonstrated that the majority of connections between the medial temporal lobe and the frontal lobe are with the orbitofrontal cortex [Barbas and Blatt, 1995; Insausti et al., 1987]. In line with these facts, recent functional neuroimaging studies have demonstrated that the orbitofrontal cortex is involved in the encoding of visual [Frey and Petrides, 2000, 2002, 2003; Petrides et al., 2002], and auditory information [Frey et al., 2000, 2004]. Several functional imaging studies have also implicated the orbitofrontal cortex in various aspects of somatosensory processing [Francis et al., 1999; Hagen et al., 2002, Rolls et al., 2003]. In addition, the posterior agranular and dysgranular region of the orbitofrontal cortex has strong bidirectional connections with structures such as the amygdala [Amaral and Price, 1984; Barbas and de Olmos, 1990; Carmichael and Price, 1995; Pandya and Yeterian, 2001; Porrino et al., 1981] and the hypothalamus [Öngür et al., 1998; Rempel‐Clower and Barbas, 1998] that are known to regulate emotion. Consistent with these anatomical findings, functional neuroimaging data show that more posterior areas of the orbitofrontal cortex are activated when subjects are exposed to visual and auditory stimuli that deviate significantly from expectation, rendering them slightly aversive [Frey et al., 2000; Petrides et al., 2002]. In addition, electrical stimulation of the posterior orbitofrontal region has shown that this region can influence autonomic responses, such as blood pressure and heart rate in both monkeys and humans [Bailey and Sweet, 1940; Delgado and Livingston, 1948; Hall and Cornish, 1977; Hall et al., 1977; Livingston et al., 1948]. Thus, one can infer that the caudal orbitofrontal region, via its connections with the hypothalamus [Öngür et al., 1998; Rempel‐Clower and Barbas, 1998], is in a position to regulate autonomic responses to stimuli that might induce emotional reactions.
Given the abovementioned facts, this study examined activity within the right orbitofrontal region using positron emission tomography (PET) during the encoding of novel tactile stimuli and tactile stimuli that deviate significantly from the subjects' expectations. To find the ideal tactile stimuli for this purpose, a psychophysiological experiment was carried out prior to the PET study, in a separate group of subjects, to evaluate the emotional significance of the tactile stimuli. We reasoned that more aversive tactile stimuli would lead to elevated galvanic skin responses.
MATERIALS AND METHODS
Subjects
Nine female volunteer subjects (20–31 yr of age; mean age 25.6 yr) participated in the PET experiment. There was no history of neurological or psychiatric illness in any of the subjects, and none had any neurological deficits. Informed consent was obtained from the subjects after the nature and possible risks of the study were explained to them.
Eight right handed female subjects (21–29 yr of age; mean age 25 yr), different from the subjects that participated in the PET scanning session, were tested in a psychophysiological experiment that examined their skin conductance response to the tactile stimuli. The subjects from both the PET and psychophysiological experiment were right‐handed according to the Edinburgh handedness inventory [Oldfield, 1971]. This study was approved by the Ethics Committee of the Montreal Neurological Institute.
Scanning Procedures
PET was the method of choice since it is difficult to image the orbitofrontal area with functional magnetic resonance imaging (fMRI) because of field inhomogeneities near the sinus cavities [Devlin et al., 2000; Lipschutz et al., 2001; Ojemann et al., 1997; Stenger, 2006]. The subjects were scanned for 60 s with PET under each condition of testing. PET scans were obtained with a CTI/Siemens HR+ 63‐slice tomograph operated in a three‐dimensional acquisition mode. In addition to the PET scans, each subject underwent a high‐resolution magnetic resonance imaging (MRI) study which was used to align data sets stereotactically for within‐ and between‐subject averaging of the functional data obtained with PET. The distribution of cerebral blood flow (CBF) was measured during the 60 s scan by means of the water bolus H2 15O methodology [Raichle et al., 1983]. In each scan 10 mCi of 15O‐labeled H2O was injected into the left antecubital vein.
Testing
General testing procedure
Three conditions, tactile encoding, aversive tactile, and tactile control, constituted this study and were part of a larger 10‐scan PET session designed to examine the function of the frontal cortex. Subjects were blindfolded for the duration of the experiment and therefore never saw the tactile stimuli. Each subject was scanned once per condition. Their right arm was supported on an arm rest that was attached to the PET scanner bed. The arm rest was adjusted for each subject so that their fingertips extended over the edge, facing downwards (Fig. 1). In all conditions (except for five stimuli in the tactile aversive condition described later), the stimuli consisted of pieces of abstract patterned rubber or carpet (12.7 cm width × 7.62 cm length) that were mounted on cardboard squares (12.7 cm × 12.7 cm). The thickness of the stimuli ranged in height from 2 to 10 mm off the cardboard backing. The subjects had no task decisions to make during the scan and were instructed simply to touch each item that was presented (one at a time) at their finger tips and to remember it for future recall. The experimenter was in charge of placing and removing the different stimuli (every 4 s) during each condition. Each stimulus was present for 4 s during which time the subject explored with the fingers the stimulus. A total of 15 stimuli were presented during each 60 s PET scan. The stimulus material used in this experiment was made as abstract as possible in order to prevent verbalization and semantic associations that might result from touching familiar objects and which would thus activate regions of the frontal cortex, such as the left inferior frontal language area. Verbalization of the material might have also set in motion other processes of the lateral frontal lobe involved in higher‐order executive memory functions but not specifically needed for active encoding [Petrides, 2000]. A recognition test for the stimuli presented during the tactile encoding condition and the aversive tactile condition was administered several minutes after the end of the scan session while the subjects were still inside the scanner. During the recognition test, the blindfolded subjects were presented, one at a time, with seven tactile stimuli that they had touched during the PET scan along with seven novel stimuli in a random order. The subjects responded verbally (yes/no) whether they had recognized the tactile stimulus as one that had been presented during the PET scanning.
Figure 1.
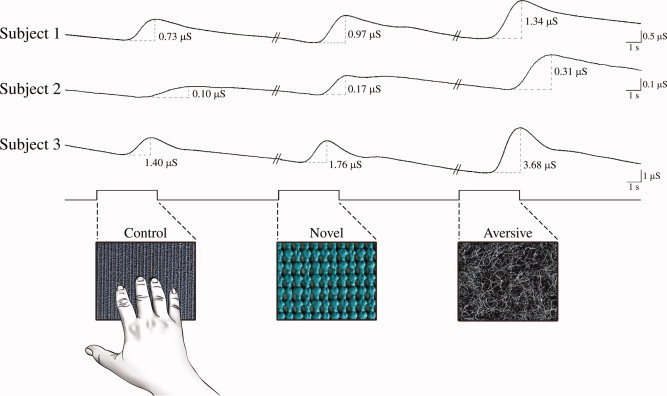
Examples of individual galvanic skin responses (GSR) to the different types of tactile stimuli during the psychophysiological experiment. Baseline GSR rates were recorded in microsiemens (μS) and were first obtained by presenting the subjects with familiar control stimuli (Control). Novel: GSRs to non‐aversive tactile stimuli used in the PET novel tactile encoding condition. Aversive: GSRs to aversive stimuli used in the PET aversive tactile condition. Significant differences were observed between the aversive and the non‐aversive tactile stimuli (paired t‐test, P < 0.05).
Tactile Control Condition
Three familiar stimuli were used in this condition. One of the three familiar tactile stimuli was presented to the blindfolded subjects' fingertips for 4 s and then another stimulus for the same period until the end of the scanning period. The subjects had been simply instructed to touch the tactile stimulus presented. The stimulus to be presented was randomly selected from the three familiar stimuli throughout the scanning period. The stimuli were familiar to the subjects because the subjects were allowed to touch each stimulus three times just before scanning began. Lying in the scanner, the blindfolded subjects were presented with the three stimuli once, then once more in a different order, and in a different order for a final time. Scanning began soon afterwards. All the subjects were informed that this was the control condition and only one scan was performed per subject.
Tactile Encoding Condition
This condition was designed to test the hypothesis that the encoding of tactile information would selectively activate the right rostral orbitofrontal cortex (area 11). The blindfolded subjects were presented with 15 novel tactile stimuli during the scanning session and were specifically asked to try to remember them. Although each stimulus was unique, the stimuli had similar properties to those used in the control condition, i.e. they were different patterns of rubber or carpet. Prior to scanning, the subjects were informed that a recognition test would be administered at the completion of the encoding PET condition. Each one of the 15 stimuli was presented once for 4 s. The total number of stimulus presentations and the method of presentation was the same as in the tactile control condition, the only difference being that the subjects were asked to memorize the tactile information.
Aversive Tactile Condition
This condition was designed to test the hypothesis that attending to tactile stimuli that significantly deviated from the normal set and are therefore slightly aversive would activate more caudal regions of the orbitofrontal cortex (area 13). Prior to scanning, the blindfolded subjects were allowed to explore with their fingers three new tactile stimuli. These three stimuli were similar in design to the three stimuli that were used in the tactile control condition (i.e. they were abstract patterned rubber or carpet), and again, the subjects were able to familiarize themselves with these stimuli by touching them three times just before scanning began, as in the control condition. During scanning, five aversive stimuli, mounted on similar cardboard squares (see testing procedure ) were inserted into the presentation sequence in this condition. Thus, the subjects experienced standard familiar tactile stimuli interspersed with novel aversive stimuli. The five aversive stimuli used were: sandpaper, steel wool, tiny plastic spikes, a tacky gelatinous solution, and tacky gummy “worms.” The subjects were told simply to attend to the randomly presented stimuli and to touch them as they had previously. Without informing the subjects, every third stimulus presented was an aversive stimulus. In other words, during the scan, the subjects were presented with two familiar stimuli followed by an aversive stimulus. Apart from the aversive stimuli that were inserted into the presentation sequence of stimuli, all other aspects of the tactile aversive condition were identical to the tactile control condition. This condition was always presented last in the scanning session.
Data Analysis
The CBF images were reconstructed and blurred with a Gaussian filter to a resolution of 12‐mm, normalized for differences in global CBF, and coregistered with the individual MRIs [Woods et al., 1993]. The MRI volumes were then transformed into the Montreal Neurological Institute (MNI) standardized stereotaxic space that is based on the Talairach space [Talairach and Tournoux, 1988] by means of an automated feature‐matching algorithm [Collins et al., 1994]. The statistical significance of focal changes was tested with a method based on 3D Gaussian random field theory. For an exploratory search involving all peaks within the grey matter volume of 600 ml, the threshold for reporting a peak as significant was set at t = 4.41, corresponding to a corrected probability of P < 0.05. For a priori regions of interest within the orbitofrontal region, the threshold for significance was set at t = 3.10, corresponding to a corrected probability of P < 0.05 based on a 2 cm diameter sphere that was centered over the orbitofrontal cortex in the right hemisphere [Worsley et al., 1996].
Psychophysiological Experiment: Behavioral Testing to Select Stimuli for the PET Scanning Session
Recall that different subjects were used for the behavioral testing experiment in which the stimuli for the PET scanning study were screened for selection. During this experiment, the subjects were blindfolded and skin conductance was continuously monitored using silver electrodes that were attached to the palmar surface of the left index and middle fingers, and the signal was measured via a galvanic skin conductance (GSR) apparatus (ADInstruments, Australia). The GSR signal was amplified and sampled at 100 Hz, and the filtered data were displayed online and recorded digitally using Chart 4.1 software (ADInstruments, Australia) and a PowerBook G4 computer. The subjects were initially presented with the three stimuli that were used in the PET tactile control condition to familiarize the subjects with the type of stimuli to be used and to obtain baseline GSR rates which were recorded in microsiemens (μS). The quantitative analysis of the GSR traces was based on the difference between the maximum of the skin conductance signal and a period of 2 s following stimulus onset (see Fig. 1). The subjects were presented with the different patterned rubber and carpet stimuli used in the PET tactile encoding condition, as well as with the aversive stimuli used in the PET tactile aversive condition. All the testing procedures and the stimuli were the same as those described in the PET general testing procedure except that after the 4 s presentation of each stimulus, 8 s of additional time was allowed to elapse in order to allow for the GSR traces to return to baseline levels. The subjects were not informed that aversive stimuli were to be used. A recognition test was administered several minutes after each test condition, i.e. the tactile novel and the tactile aversive conditions. Each subject was presented, one at a time, with half of the stimuli they had been exposed to during the test condition along with an equal number of novel stimuli. The order of presentation of the familiar and the novel stimuli was randomly determined. The subjects responded verbally (yes/no) after each stimulus presentation to indicate whether they had recognized each stimulus as having been shown during the previous testing condition.
RESULTS
Psychophysiological Experiment
Responses to tactile stimuli
After an initial baseline GSR trace was established, all subjects exhibited deviations from this baseline state that are characteristic of typical traces observed from sympathetic electrodermal activity (see Fig. 1). Although the responses varied across stimuli and subjects, significant statistical differences were found between all of the aversive tactile stimuli and five of the selected stimuli from the nonaversive tactile condition (paired t‐test, t = 1.92, df = 7, P < 0.05). These findings imply that the aversive tactile stimuli led to elevated skin conductance levels and were likely more emotionally charged in comparison to the novel tactile stimuli. In the psychophysiological experiment, performance in the recognition memory test for the nonaversive tactile stimuli was 71.5% correct, sd = 19.9, and for the aversive tactile stimuli was 88.5% correct, sd = 6.4 (paired t‐test, t = 2.46, df = 7, P < 0.05).
PET Experiment
Novel tactile encoding
When activity in the tactile control condition was subtracted from that in the tactile encoding condition, a significant peak of increased activity was noted in the right rostral orbitofrontal cortex (Table I, Fig. 2). This peak was bounded by the medial and lateral orbital sulci and lay in front of the transverse orbital sulcus. Comparative architectonic studies in the monkey and human have shown this region of the human orbitofrontal cortex to be comparable to macaque monkey area 11 [Petrides and Pandya, 1994]. To examine which other areas of the brain were interacting with area 11 during the encoding of the tactile stimuli; regional CBF in the area 11 peak was correlated with CBF in the rest of the brain. Positive correlations were observed with CBF in the right precuneus/cuneus (x = 12, y = −70, z = 26, t = 4.49), the right caudate nucleus (x = 16, y = 15, z = 3, t = 4.49), and the right hippocampal formation (x = 25, y = −16, z = −12, t = 3.97), although the latter activation was below statistical significance.
Table I.
Regional brain activity for all subjects and conditions
| Brain area | Side | t Statistic | Stereotaxic coordinates (x, y, z) |
|---|---|---|---|
| Novel tactile encoding condition compared with the control condition | |||
| Orbitofrontal cortex (area 11) | R | 3.13 | 28, 32, – 6 |
| Frontal lobe (area 6/8) | L | 4.46 | −50, 10, 32 |
| Frontal lobe (area 6) | R | 4.15 | 20, 10, 57 |
| Aversive tactile condition compared with the control condition | |||
| Orbitofrontal cortex (area 13) | R | 3.14 | 27, 30, −5 |
| Somatosensory cortex (area ½) | R | 4.22 | 43, −25, 54 |
| Frontal lobe (area 6) | L | 3.77 | −13, −19, 59 |
| Subcortical (thalamus/hypothalamus) | R | 3.61 | 8, −9, 0 |
| Novel tactile encoding condition compared with aversive tactile condition | |||
| Orbitofrontal cortex (area 11) | R | 2.97 | 36, 53, −9 |
The MNI standardized stereotaxic coordinates (mm) representing changes in cerebral blood flow. x, medial‐to‐lateral distance relative to the midline (positive, right); y, anterior‐to‐posterior distance relative to the anterior commissure (positive, anterior); z, superior‐to‐inferior distance relative to the anterior commissure‐posterior commissure line (positive, superior).
Figure 2.
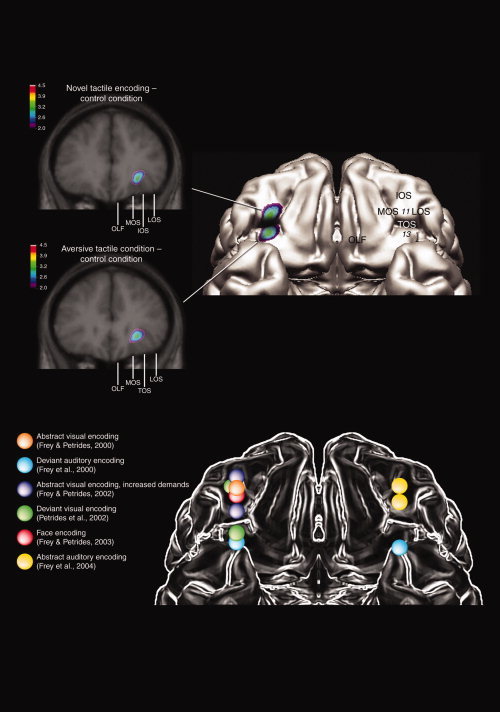
Merged PET‐MRI sections illustrating CBF activations in the right orbitofrontal cortex when the control condition was subtracted from the novel tactile encoding condition and the aversive tactile condition. Composite diagram (right) indicating the location of the orbitofrontal peaks in the present study. Note that the activity in the two conditions was separated by the transverse orbital sulcus (TOS) which separates the rostral orbitofrontal cortex from the caudal orbitofrontal cortex. Site comparison schematic (bottom) indicating the approximate location of the orbitofrontal peaks from earlier‐published visual and auditory encoding experiments in our laboratory. IOS, intermediate orbital sulcus; LOS, lateral orbital sulcus; MOS, medial orbital sulcus; OLF, olfactory sulcus.
The mean recognition performance for the novel tactile stimuli was 89.7% correct (sd = 11.4) although most subjects stated that the task was quite difficult when debriefed at the completion of the experiment.
Aversive tactile encoding
When activity in the tactile control condition was subtracted from that in the aversive tactile condition, a peak of significantly increased activity was noted in the right orbitofrontal cortex (Table I, Fig. 2). This activity peak was bounded by the medial and lateral orbital sulcus and was lying behind the transverse orbital sulcus, i.e. in the caudal orbitofrontal region where area 13 is located [Petrides and Mackey, 2006; Petrides and Pandya, 1994]. This posterior orbitofrontal area is known to be strongly connected to limbic medial temporal areas [Amaral and Price, 1984; Barbas and de Olmos, 1990; Carmichael and Price, 1995; Öngür et al., 1998; Pandya and Yeterian, 2001; Porrino et al., 1981; Rempel‐Clower and Barbas, 1998].
Recall, that the aversive stimuli were randomly interspersed with three non‐aversive familiar stimuli during this PET scanning condition. Although the subjects were not told that an aversive stimulus would be introduced at every third stimulus presentation, the stimuli were sufficiently unique, as compared to the tactile stimuli used in the tactile encoding condition, that the subjects automatically encoded them. The mean recognition performance for the aversive tactile stimuli was 96.1% correct (sd = 6.2).
It is interesting to note that when activity in the aversive tactile condition was subtracted from the novel encoding condition, a single activation peak was located within the right rostral orbitofrontal area 11 (x = 36, y = 53, z = −9). Although this peak just missed statistical significance (t = 2.9), the direct comparison between these two conditions suggests that the rostral orbitofrontal cortex was more active when subjects were specifically trying to memorize the novel tactile information when compared with simply touching aversive stimuli unknowingly. Subtracting the novel encoding condition from the aversive tactile condition did not result in any activation peaks within the orbitofrontal cortex. There were no significant regional CBF correlations with the area 13 activation focus and the rest of the brain.
DISCUSSION
Regardless of the sensory modality within which a stimulus is presented, the brain must be able to evaluate whether the stimulus is familiar or novel, pleasant or aversive, expected or unexpected. Exposure to a novel stimulus will lead to encoding of the information regardless of whether the subject is explicitly asked to remember the stimulus or is simply attending to it. It is also known that if the stimulus is unexpected (unique) or it has an affective quality, the information will be much easier to remember. One of the hypotheses put forth and tested in the present investigation is that the orbitofrontal cortex participates, in combination with limbic medial temporal lobe areas, in the encoding of such information in memory.
In the tactile encoding condition, the subjects were specifically asked to memorize novel tactile information. In comparison to the tactile control condition, there was a selective increase of activity in the orbitofrontal area 11 in the right hemisphere during the tactile encoding condition (Table I, Fig. 2). This activation in area 11 is in agreement with other neuroimaging studies that examined activation related to the encoding of novel information in other sensory modalities, such as vision [Frey and Petrides, 2000, 2002, 2003; Petrides et al., 2002] and audition [Frey et al., 2000, 2004].
Orbitofrontal area 11 has strong connections with the medial temporal region [Barbas and Blatt, 1995; Carmichael and Price, 1996; Insausti et al., 1987; Lavenex et al., 2002], damage to which leads to a severe recognition memory disorder in the monkey [Meunier et al., 1993; Suzuki et al., 1993] and in human subjects [Milner, 1972; Mishkin, 1982; Squire and Zola‐Morgan, 1991; Zola et al., 2000]. Note that, in this study, CBF in the orbitofrontal region (area 11), which showed increased activity during the encoding of new tactile information, was positively correlated with activity in the right hippocampal formation, demonstrating that area 11 was in close functional interaction with this critical memory structure. It is interesting that the caudate nucleus in the right hemisphere was also active along with the hippocampus and area 11 while subjects encoded the tactile stimuli, consistent with suggestions from work in rodents that this basal ganglia structure is involved in learning [Packard and Knowlton, 2002]. Finally, the correlation between activity in area 11 and activation in the precuneus/cuneus region is consistent with anatomical studies showing connections between these two regions [Petrides and Pandya, 2002a] and may be related to mental imagery involved in encoding information for subsequent recall, as has been described by other groups [Fletcher et al., 1996].
The findings of this study are also in agreement with monkey lesion data demonstrating that the orbitofrontal cortex is an important frontal region for recognition memory [Bachevalier and Mishkin, 1986; Meunier et al., 1997]. These studies have demonstrated that bilateral lesions to the orbitofrontal cortex produce as severe memory deficits as those produced by bilateral medial temporal lobe damage [Mishkin, 1982; Squire and Zola‐Morgan, 1991; Zola et al., 2000]. Evidence from patients with bilateral lesions of the orbitofrontal cortex is much less clear due to the large variability of the cortical damage, although some patients have shown impaired performance on memory tasks [e.g. Alexander and Freedman, 1984; Bechara et al., 1998; Damasio et al., 1985; Gade, 1982; Petrides, 2000; Philips et al., 1987; Talland et al., 1967].
The present neuroimaging data, which demonstrates a strong relationship between the orbitofrontal cortex and the attempt to encode tactile information, is consistent with numerous anatomical studies that show strong bidirectional links between the orbitofrontal cortex and structures in the limbic medial temporal region [Barbas and Blatt, 1995; Insausti et al., 1987; Petrides and Pandya, 2002b]. The anatomical facts suggest that novel information processed in the medial temporal lobe can be accessed directly by the orbitofrontal cortex. Interestingly, several studies in the monkey have described neurons within the rostral orbitofrontal cortex that respond to novel visual information [Rolls et al., 2005, 2006; Tremblay and Shultz, 2000].
The observation that posterior areas of the dorsal premotor cortex (areas 6, 6/8, Table I) showed increased activity in the present conditions is consistent with the numerous studies that have noted activations in these areas during working memory conditions [Cabeza and Nyberg, 2000]. It is possible that our blindfolded subjects were forming some mental representation of the tactile stimuli while they performed the task, thus causing a signal increase in these areas that are heavily connected to visuospatial areas of the brain (see Petrides and Pandya, 1999).
In the aversive tactile condition, without informing the subjects, aversive stimuli were presented throughout the scan along with ordinary familiar tactile stimuli, i.e. stimuli presented prior to the scan. When activity in the tactile control condition was subtracted from that in the aversive tactile condition, there was a significant focus of increased activity again in the right orbitofrontal region but somewhat more posterior (Table I, Fig. 2). It should be noted that the major difference between the aversive tactile condition and the other two scanning conditions was the fact that aversive tactile stimuli were mixed with the other stimuli. The aversive stimuli most likely led the subjects to experience an affective reaction to the aversive stimuli, as suggested by the greater GSR response observed in the psychophysiological data. It is of interest in this regard that the activation was located somewhat more posteriorly in the right orbitofrontal cortex suggesting greater involvement of area 13 which has a proisocortical, limbic‐type architecture [Petrides and Mackey, 2006; Petrides and Pandya, 1994] and is strongly linked to the amygdala [Amaral and Price, 1984; Barbas and de Olmos, 1990; Carmichael and Price, 1995; Pandya and Yeterian, 2001; Porrino et al., 1981] and the hypothalamic region [Öngür et al., 1998; Rempel‐Clower and Barbas, 1998]. Note also that there was an activation bordering the thalamus/hypothalamus in this subtraction in the right hemisphere (Table I), suggesting a close functional interaction with the posterior orbitofrontal cortex and the medial limbic structures. The focus of activation in area 13 in relation to aversive stimuli is in agreement with other functional imaging studies from our laboratory that demonstrated activation of the posterior orbitofrontal cortex in response to emotionally laden visual and auditory information [Frey et al., 2000, Petrides et al., 2002].
It is known that, when electrically stimulated, the caudal orbitofrontal cortex which is heavily connected with limbic brain regions [Aggleton et al., 1980; Amaral et al., 1992; Barbas and de Olmos, 1990; Carmichael and Price, 1995; Pandya and Yeterian, 2001; Petrides and Pandya, 2002b; Rempel‐Clower and Barbas, 1998] can produce autonomic changes, such as modifications of heart rate, respiration, and gastric motility [Bailey and Sweet, 1940; Delgado and Livingston, 1948; Hall and Cornish, 1977; Hall et al., 1977; Livingston et al., 1948]. Furthermore, there is a large body of evidence from lesion and electrophysiological studies demonstrating that the orbitofrontal cortex is involved in the processing of emotionally charged stimuli [Rolls, 2000]. In particular, there are orbitofrontal neurons that respond to deviations from expectation of reward [Rosenkilde et al., 1981; Thorpe et al., 1983; Tremblay and Schultz, 2000] which would be important in alerting the organism to potentially harmful stimuli. Additionally, there are studies which link lesions of the orbitofrontal cortex in monkeys [Butter, 1964; Butter et al., 1970; Izquierdo et al., 2005; Ruch and Shenkin, 1943] and patients [Hornak et al., 1996; Rolls et al., 1994] with abnormal behavioral responses to emotional stimuli, and functional neuroimaging data linking the orbitofrontal cortex in the human brain with emotional responses to stimuli [Angrilli et al., 1999; Blood et al., 1999; Dolan et al., 1996; Sarazin et al., 1998].
Several functional imaging studies have investigated the orbitofrontal cortex during somatosensory processing and have reported bilateral activations throughout the orbitofrontal cortex [Francis et al., 1999; Hagen et al., 2002; Rolls et al., 2003]. Although the findings of the above studies were not related to mnemonic functioning but to attending to tactile stimuli, some of which contained hedonic information (e.g., pleasant and painful stimuli; see Francis et al., 1999; Rolls et al., 2003), these activity changes in the orbitofrontal cortex are consistent with our present results, suggesting that the orbitofrontal cortex is involved in the regulation of emotionally charged information. Hagen et al. [2002] argue that their stimuli, von Frey hairs applied to four body locations, had no hedonic qualities; however, all their subjects were asked to monitor or keep track of the number of times there was a pause during the touch stimulation. Although the experimenters applied somatosensory stimulation without interruption throughout the scans, it is possible that the orbitofrontal cortex was in a heightened state as the subjects carefully scrutinized the stimulation, expecting breaks when in fact none occurred. These results are also in agreement with this study as well as a previous study in our laboratory [Petrides et al., 2002], suggesting that the orbitofrontal cortex responds to expectations of behaviorally significant information.
The present neuroimaging study suggested functional differentiation between the rostral and caudal areas of the orbitofrontal cortex during exposure to different aspects of tactile information. Other imaging studies have investigated comparable issues in vision and audition, and those results corroborate the present ones [Frey and Petrides, 2000, 2002, 2003; Frey et al., 2000, 2004; Petrides et al., 2002]. It can therefore be concluded that area 11 in the rostral orbitofrontal region is involved in processing underlying the encoding of novel information, whereas caudal orbitofrontal area 13 is more active in the processing of stimuli that deviate significantly from expectations, most probably because such significant deviations are threatening and thus aversive. If stimuli deviate too much from what is expected (i.e. the tactile aversive condition), this information must be evaluated quickly in order to judge its potential for harm to the organism. The process of attending to these unexpected or emotionally salient stimuli, which results in activation of the more caudal area of the orbitofrontal cortex, leads to excellent encoding of the information as shown by the high mnemonic performance (96.1% correct) of the subjects.
Clearly, both the rostral and the caudal orbitofrontal cortex respond to novel stimulation. Nevertheless, it appears that relative activation of the orbitofrontal areas 11 and 13 is influenced by the degree of motivational/emotional significance that is attached to the incoming information. In the tactile encoding condition, the subjects were asked to try to remember tactile stimuli that were novel but rather ordinary (in terms of the subjects' experience) and this requirement led to increased activity in area 11 in the rostral part of the orbitofrontal cortex. On the other hand, greater activity was observed more caudally in area 13 when aversive tactile stimuli (aversive tactile condition) were presented. Thus, the caudal part of the orbitofrontal cortex, which is more strongly related to the autonomic system than its rostral part, is engaged when more emotionally laden information is presented to the organism.
Direct subtraction of activity in the aversive tactile condition from that in the tactile encoding condition revealed a single peak of activation (t = 2.9) within the right rostral orbitofrontal cortex (area 11). This weak peak suggests that, despite the fact that both the rostral and the caudal orbitofrontal regions are involved in the encoding of novel information, the rostral orbitofrontal cortex may be engaged to a greater extent due to the greater effort needed to encode ordinary compared with aversive stimuli.
It should be noted that areas 11 and 13 are heavily connected to all the other areas of the orbitofrontal cortex [Carmichael and Price, 1996] as well as many areas of the lateral and medial prefrontal cortex [Barbas, 1993; Carmichael and Price, 1996]. These orbitofrontal areas, therefore, are in a unique position to mediate between the higher level control processes that depend on lateral prefrontal cortex (e.g. monitoring of information in working memory or active retrieval of information from memory) and the memory systems centered in the medial temporal lobe region [Petrides, 1996].
REFERENCES
- Aggleton JP,Burton MJ,Passingham RE ( 1980): Cortical and subcortical afferents to the amygdala of the rhesus monkey (macaca mulatta). Brain Res 190: 347–368. [DOI] [PubMed] [Google Scholar]
- Alexander MP,Freedman M ( 1984): Amnesia after anterior communicating artery aneurysm rupture. Neurology 34: 752–757. [DOI] [PubMed] [Google Scholar]
- Amaral DG,Price JL ( 1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230: 465–496. [DOI] [PubMed] [Google Scholar]
- Amaral DG,Price JL,Pitkänen A,Carmichael ST ( 1992): Anatomical organization of the primate amygdaloid complex In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Willey‐Liss; pp 1–66. [Google Scholar]
- Angrilli A,Palomba D,Cantagallo A,Maietti A,Stegagno L ( 1999): Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. Neuroreport 10: 1741–1746. [DOI] [PubMed] [Google Scholar]
- Bachevalier J,Mishkin M ( 1986): Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Res 20: 249–261. [DOI] [PubMed] [Google Scholar]
- Bailey P,Sweet WH ( 1940): Effects on respiration, blood pressure and gastric motility of stimulation of orbital surface of frontal lobe. J Neurophysiol 3: 276–281. [Google Scholar]
- Barbas H ( 1993): Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience 56: 841–864. [DOI] [PubMed] [Google Scholar]
- Barbas H,Blatt GJ, ( 1995): Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5: 511–533. [DOI] [PubMed] [Google Scholar]
- Barbas H,de Olmos J ( 1990): Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol 300: 549–571. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio H,Tranel D,Anderson SW ( 1998): Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 18: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ,Zatorre RJ,Bermudez P,Evans AC ( 1999): Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci 2: 382–387. [DOI] [PubMed] [Google Scholar]
- Butter CM ( 1964): Habituation of responses to novel stimuli in monkeys with selective frontal lesions. Science 144: 313–315. [DOI] [PubMed] [Google Scholar]
- Butter CM,Snyder DR,McDonald JA ( 1970): Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. J Comp Physiol Psychol 72: 132–144. [DOI] [PubMed] [Google Scholar]
- Cabeza R,Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cognit Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Carmichael ST,Price JL ( 1995): Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641. [DOI] [PubMed] [Google Scholar]
- Carmichael ST,Price JL ( 1996): Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371: 179–207. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in stardardized Talairach space. J Comput Assit Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Damasio AR,Graff‐Radford NR,Eslinger PJ,Damasio H,Kassell N ( 1985): Amnesia following basal forebrain lesions. Arch Neurol 42: 263–271. [DOI] [PubMed] [Google Scholar]
- Delgado JMR,Livingston RB ( 1948): Some respiratory, vascular and thermal responses to stimulation of orbital surface of frontal lobe. J Neurophysiol 11: 39–55. [DOI] [PubMed] [Google Scholar]
- Devlin JT,Russell RP,Davis MH,Price CJ,Wilson J,Moss HE,Matthews PM,Tyler LK ( 2000): Susceptibility‐induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage 11: 589–600. [DOI] [PubMed] [Google Scholar]
- Dolan RJ,Fletcher P,Morris J,Kapur N,Deakin JF,Frith CD ( 1996): Neural activation during covert processing of positive emotional facial expressions. Neuroimage 4: 194–200. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Shallice T,Frith CD,Frackowiak RSJ,Dolan RJ ( 1996): Brain activity during memory retrieval. The influence of imagery and semantic cueing. Brain 119: 1587–1596. [DOI] [PubMed] [Google Scholar]
- Francis S,Rolls ET,Bowtell R,McGlone F,O'Doherty J,Browning A,Clare S,Smith E ( 1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport 10: 453–459. [DOI] [PubMed] [Google Scholar]
- Frey S,Petrides M ( 2000): Orbitofrontal cortex: A key prefrontal region for encoding information. Proc Natl Acad Sci USA 97: 8723–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S,Petrides M ( 2002): Orbitofrontal cortex and memory formation. Neuron 36: 171–176. [DOI] [PubMed] [Google Scholar]
- Frey S,Petrides M ( 2003): Greater orbitofrontal activity predicts better memory for faces. Eur J Neurosci 17: 2755–2758. [DOI] [PubMed] [Google Scholar]
- Frey S,Kostopoulos P,Petrides M ( 2000): Orbitofrontal involvement in the processing of unpleasant auditory information. Eur J Neurosci 12: 3709–3712. [DOI] [PubMed] [Google Scholar]
- Frey S,Kostopoulos P,Petrides M ( 2004): Orbitofrontal contribution to auditory encoding. Neuroimage 22: 1384–1389. [DOI] [PubMed] [Google Scholar]
- Friedman DP,Murray EA,O'Neill JB,Mishkin M ( 1986): Cortical connections of the somatosensory fields of the lateral sulcus of macaques: Evidence for a corticolimbic pathway for touch. J Comp Neurol 252: 323–347. [DOI] [PubMed] [Google Scholar]
- Gade A ( 1982): Amnesia after operations on aneurysms of the anterior communicating artery. Surg Neurol 18: 46–49. [DOI] [PubMed] [Google Scholar]
- Hagen MC,Zald DH,Thornton TA,Pardo JV ( 2002): Somatosensory processing in the human inferior prefrontal cortex. J Neurophysiol 88: 1400–1406. [DOI] [PubMed] [Google Scholar]
- Hall RE,Cornish K ( 1977): Role of the orbital cortex in cardiac dysfunction in unanesthetized rhesus monkey. Exp Neurol 56: 289–297. [DOI] [PubMed] [Google Scholar]
- Hall RE,Livingston RB,Bloor CM ( 1977): Orbital cortical influences on cardiovascular dynamics and myocardial structure in conscious monkeys. J Neurosurg 46: 648–653. [PubMed] [Google Scholar]
- Hornak J,Rolls ET,Wade D ( 1996): Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 34: 247–261. [DOI] [PubMed] [Google Scholar]
- Insausti R,Amaral DG,Cowan WM ( 1987): The entorhinal cortex of the monkey. II. Cortical afferents. J Comp Neurol 264: 356–395. [DOI] [PubMed] [Google Scholar]
- Izquierdo A,Suda RK,Murray EA ( 2005): Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24: 7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P,Suzuki WA,Amaral DG ( 2002): Perirhinal and parahippocampal cortices of the macaque monkey: Projections to the neocortex. J Comp Neurol 447: 394–420. [DOI] [PubMed] [Google Scholar]
- Lipschutz B,Friston KJ,Ashburner J,Turner R,Price CJ ( 2001): Assessing study‐specific regional variations in fMRI signal. NeuroImage 13: 392–398. [DOI] [PubMed] [Google Scholar]
- Livingston RB,Chapman WP,Livingston KE,Kraintz L ( 1948): Stimulation of orbital surface of man prior to frontal lobotomy. Res Publ Ass Nerv Ment Dis 27: 421–432. [PubMed] [Google Scholar]
- Meunier M,Bachevalier J,Mishkin M,Murray EA ( 1993): Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 13: 5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M,Bachevalier J,Mishkin M ( 1997): Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia 35: 999–1015. [DOI] [PubMed] [Google Scholar]
- Milner B ( 1972): Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–446. [DOI] [PubMed] [Google Scholar]
- Mishkin M ( 1979): Analagous neural models for tactual and visual learning. Neuropsychologia 17: 139–151. [DOI] [PubMed] [Google Scholar]
- Mishkin M ( 1982): A memory system in the monkey. Phil Trans R Soc Lond B 298: 85–95. [DOI] [PubMed] [Google Scholar]
- Murray EA,Mishkin M ( 1984): Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res 11: 67–83. [DOI] [PubMed] [Google Scholar]
- Nelson RJ,Sur M,Felleman DJ,Kaas JH ( 1980): Representations of the body surface in postcentral parietal cortex of Macaca fascicularis . J Comp Neurol 192: 611–643. [DOI] [PubMed] [Google Scholar]
- Ojemann JG,Akbudak E,Snyder AZ,McKinstry RC,Raichle ME,Conturo TE ( 1997): Anatomic localization and quantitative analysis of gradient refocused echo‐planar fMRI susceptibility artifacts. Neuroimage 6: 156–167. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Öngür D,An X,Price JL ( 1998): Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 401: 480–505. [PubMed] [Google Scholar]
- Packard MG,Knowlton BJ ( 2002): Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25: 563–593. [DOI] [PubMed] [Google Scholar]
- Pandya DN,Yeterian EH ( 2001): The anatomical substrates of emotional behavior: The role of the cerebral cortex In: Boller F,Grafman J, editors.Handbook of Neuropsychology, 2nd ed.,Vol. 5 Amsterdam: Elsevier; pp 49–87. [Google Scholar]
- Petrides M ( 1996): Specialized systems for the processing of mnemonic information within the primate frontal cortex. Phil Trans R Soc Lond B 351: 1455–1462. [DOI] [PubMed] [Google Scholar]
- Petrides M ( 2000): Frontal lobes and memory In: Boller F,Grafman J, editors.Handbook of Neuropsycholgy,2nd ed.,vol. 2 Amsterdam: Elsevier; pp 67–84. [Google Scholar]
- Petrides M,Pandya DN ( 1994): Comparitive architectonic analysis of the human and the macque frontal cortex In: Boller F,Grafman J, editors. Handbook of Neuropsychology. Amsterdam, Elsevier; pp 17–58. [Google Scholar]
- Petrides M,Pandya DN ( 1999): Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11: 1011–1036. [DOI] [PubMed] [Google Scholar]
- Petrides M,Pandya DN ( 2002a): Comparative architectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16: 291–310. [DOI] [PubMed] [Google Scholar]
- Petrides M,Pandya DN ( 2002b): Association pathways of the prefrontal cortex and functional observations In: Stuss DT,Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; pp 31–50. [Google Scholar]
- Petrides M,Mackey S ( 2006): The orbitofrontal cortex: sulcal and gyral morphology and architecture In: Zald DH,Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; pp 19–37. [Google Scholar]
- Petrides M,Alivisatos B,Frey S ( 2002): Differential activation of the human orbital, mid‐ventrolateral, and mid‐dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci USA 99: 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A,Sangalang V,Sterns G ( 1987): Basal forebrain infarction, a clinicopathologic correlation. Arch Neurol 44: 1134–1138. [DOI] [PubMed] [Google Scholar]
- Porrino LJ,Crane AM,Goldman‐Rakic PS ( 1981): Direct and indirect pathways form the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 198: 121–136. [DOI] [PubMed] [Google Scholar]
- Raichle ME,Martin WRW,Herscovitch P,Mintun MA,Markham J ( 1983): Brain blood flow measured with intravenous H2 15O. II. Implementation and validation. J Nucl Med 24: 790–798. [PubMed] [Google Scholar]
- Randolph M,Semmes J ( 1974): Behavioral consequences of selective subtotal ablations in the postcentral gyrus of macaca mulatta. Brain Res 70: 55–70. [DOI] [PubMed] [Google Scholar]
- Rempel‐Clower NL,Barbas H ( 1998): Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol 398: 393–419. [DOI] [PubMed] [Google Scholar]
- Ridley RM,Ettlinger G ( 1976): Impaired tactile learning and retention after removals of the second sensory projection cortex (SII) in the monkey. Brain Res 109: 656–660. [DOI] [PubMed] [Google Scholar]
- Rolls ET ( 2000): The orbitofrontal cortex and reward. Cerb Cortex 10: 284–294. [DOI] [PubMed] [Google Scholar]
- Rolls ET,Hornak J,Wade D,McGrath J ( 1994): Emotion‐related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 57: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET,O'Doherty J,Kringelbach ML,Francis S,Bowtell R,McGlone F ( 2003): Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13: 308–317. [DOI] [PubMed] [Google Scholar]
- Rolls ET,Browning AS,Inoue K,Hernadi I ( 2005): Novel visual stimuli activate a population of neurons in the primate orbitofrontal cortex. Neurobiol Learn Mem 84: 111–123. [DOI] [PubMed] [Google Scholar]
- Rolls ET,Critchley HG,Browning AS,Inoue K ( 2006): Face‐selective and auditory neurons in the primate orbitofrontal cortex. Exp Brain Res 170: 74–87. [DOI] [PubMed] [Google Scholar]
- Rosenkilde CE,Bauer RH,Fuster JM ( 1981): Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res 209: 375–394. [DOI] [PubMed] [Google Scholar]
- Ruch TC,Shenkin HA ( 1943): The relation of area 13 on orbital surface of frontal lobes to hyperactivity and hyperphagia in monkeys. J Neurophysiol 6: 349–360. [Google Scholar]
- Sarazin M,Pillon B,Giannakopoulos P,Rancurel G,Samson Y,Dubois B ( 1998): Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology 51: 142–148. [DOI] [PubMed] [Google Scholar]
- Squire LR,Zola‐Morgan S ( 1991): The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Stenger VA ( 2006): Technical considerations for BOLD fMRI of the orbitofrontal cortex In: Zald DH,Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; pp 423–446. [Google Scholar]
- Suzuki WA,Zola‐Morgan S,Squire LR,Amaral DG ( 1993): Lesions of the perirhinal and parahippocampal cortices in the monkey produce long‐lasting memory impairment in the visual and tactual modalities. J Neurosci 13: 2430–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System––An Approach to Cerebral Imaging. Stuttgart: Thieme Medical Publishers. [Google Scholar]
- Talland GA,Sweet WH,Ballantine HT ( 1967): Amnesic syndrome with anterior communicating artery aneurysm. J Nerv Ment Dis 145: 179–192. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ,Rolls ET,Maddison S ( 1983): The orbitofrontal cortex: Neuronal activity in the behaving monkey. Exp Brain Res 49: 93–115. [DOI] [PubMed] [Google Scholar]
- Tremblay L,Schultz W ( 2000): Modifications of reward expectation‐related neuronal activity during learning in primate orbitofrontal cortex. J Neurophysiol 83: 1877–1885. [DOI] [PubMed] [Google Scholar]
- Woods RP,Mazziotta JC,Cherry SR ( 1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]
- Worsley KJ,Marret S,Neelin P,Vandal AC,Friston KJ,Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zola SM,Squire LR,Teng E,Stefanacci L,Buffalo EA,Clark RE ( 2000): Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci 20: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]


