Abstract
Biological differences in male and female sexuality are obvious in the behavioral domain, but the central mechanisms that might explain these behavioral gender differences remain unclear. In this study, we merged two earlier positron emission tomography data sets to enable systematic comparison of the brain responses in heterosexual men and women during sexual tactile genital (penile and clitoral) stimulation and during orgasm. Gender commonalities were most evident during orgasm, a phase which demonstrated activations in the anterior lobe of the cerebellar vermis and deep cerebellar nuclei, and deactivations in the left ventromedial and orbitofrontal cortex in both men and women. During tactile genital stimulation, deactivations in the right amygdala and left fusiform gyrus were found for both genders. Marked gender differences were seen during this phase: left fronto‐parietal areas (motor cortices, somatosensory area 2 and posterior parietal cortex) were activated more in women, whereas in men, the right claustrum and ventral occipitotemporal cortex showed larger activation. The only prominent gender difference during orgasm was male‐biased activation of the periaqueductal gray matter. From these results, we conclude that during the sexual act, differential brain responses across genders are principally related to the stimulatory (plateau) phase and not to the orgasmic phase itself. These results add to a better understanding of the neural underpinnings of human sexuality, which might benefit treatment of psychosexual disorders. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: gender, orgasm, genitalia, PET
INTRODUCTION
Gender differences in sexuality and sexual behavior are often subject to humor and ridicule. Nevertheless, differences in the way the male and female brain produce a sexual response may be clinically relevant, because there is a large group of gender‐specific sexual disorders without a clear somatic cause (psycho‐ or neurogenic sexual disorders). As these sexual disorders are associated with high occurrences of health and relationship problems [Laumann et al.,1999; Lloyd,2005], gaining knowledge about the functional neurobiology underlying human sexual behavior is important. This process is delayed by the lack of neurobiological studies that are actually carried out in human subjects, especially pertaining to more progressed stages of the sexual response, like genital stimulation and orgasm. In previous studies, we have identified regions in the human brain associated with tactile genital stimulation and orgasm in men [Georgiadis and Holstege,2005; Holstege et al.,2003; Georgiadis et al.,2007] and women [Georgiadis et al.,2006]. An outstanding question is how the brain reponses in both gender groups compare during these crucial sexual phases.
From similar animal studies conducted in male [Baum and Everitt,1992; Coolen et al.,1996; Kollack‐Walker and Newman,1997; Marson et al.,1993] and female [Coolen et al.,1996; Marson,1995; Marson and Murphy,2006], rodents a picture emerges of a shared neuronal substrate for the processing of genital information and the control of genital responses comprising regions in the thalamus, the amygdala, the midbrain, and the hypothalamus. Bridging the gap between animal studies and human sexual activity is not straightforward. For example, animal models exist for the neuronal substrate underlying penile erection and ejaculation, but not for the subjective experience of sexual arousal and orgasm. Other concerns with the relevant animal studies are that the vast majority of them focuses on male sexual behavior, and that the cerebral cortex is usually not investigated despite insights gained from neurological patients about its importance for the integrity of human sexual behavior [Aloni and Katz,1999; Sandel et al.,1996].
Gender differences in human brain function have been demonstrated for emotional tasks: a meta‐analysis of 65 neuroimaging studies revealed that compared to men, women more frequently activate midline limbic structures, including the subcallosal anterior cingulate, the thalamus, the midbrain, and the cerebellum. Men, on the other hand, showed more hemispheric lateralization and more activation in occipital and left inferior frontal cortices [Wager et al.,2003]. For visually evoked sexual arousal, men had more activation in amygdala [Hamann et al.,2004] and hypothalamus [Hamann et al.,2004; Karama et al.,2002].
With our previous within‐gender analyses, we have shown that during stimulation of the erect penis, the right claustrum, insula, secondary somatosensory cortex, and occipitotemporal cortex were recruited [Georgiadis and Holstege,2005], whereas during clitoral stimulation, the main activations were in the left somatosensory area 2 as well as in the left primary somatosensory cortex [Georgiadis et al.,2006]. The amygdala was deactivated in men and in women (on the right in men, on the left in women), as was the area of the left inferior temporal gyrus/fusiform gyrus. For orgasm, the patterns of activation and deactivation were largely similar between both gender groups (activation in the anterior medial cerebellum, deactivation in the left ventromedial and orbitofrontal cortex), except for involvement of the left rostral midbrain and adjacent ventral thalamus in men, which was not found in women [Georgiadis et al.,2006,2007]. As interesting as these observations may be, they lack direct statistical inference about both gender commonalities and differences. The aim of the present study was to perform a formal group comparison using these data sets.
We hypothesized that the former within‐gender observations would be statistically confirmed as gender differences or commonalities with this new analysis. We also expected that the subcortical network related to sexual tactile genital stimulation in male and female animals (thalamus, hypothalamus, amygdala, and midbrain) would appear as a shared gender effect in humans.
MATERIALS AND METHODS
Participants
Male and female subjects included in the present analysis had originally been included in studies exploring within‐gender brain activation patterns during sexual genital stimulation [Georgiadis and Holstege,2005; Georgiadis et al.,2006] and orgasm [Georgiadis et al.,2006,2007; Holstege et al.,2003]. For the present between‐gender analysis 11 male (mean age 33, range 19–45) and 12 female (mean age 32, range 21‐47) subjects were included. All subjects were healthy, right‐handed, heterosexual, and without a history of psychiatric or sexual disorders. The subjects' partners also participated in the experiment by manually performing the genital stimulation necessary for the subjects to reach a state of sustained sexual arousal and eventually orgasm. This set‐up ensured a sexually salient context with some degree of familiarity for the subject. All participants gave written informed consent according to the Declaration of Helsinki and the procedures were approved by the internal ethics committee of the University Medical Center Groningen.
PET Protocol
Per subject, eight PET scans (63 planes; axial field of view of 15.5 cm) were made in 3D‐mode using an Ecat Exact HR+ (CTI/Siemens, Knoxville, TN, USA) camera with a spatial resolution of 4–5 mm FWHM in all three directions. The radiotracer [15O]‐H2O was used as a measure of regional cerebral blood flow (rCBF). Per scan 500 MBq of activity, dissolved in 32 ml of 0.9% saline was administered intravenously into the right forearm at 8 ml/s. After injection of the radiotracer, data were collected for 120 s. Consecutive scans were made with intervals of approximately eight minutes offset‐to‐onset. The subjects were asked to keep their eyes closed during scanning, and their head was maintained in position with a head‐restraining adhesive band. The couples were allowed to communicate verbally between successive scans. After the experiment, the subjects did not report important differences between their sexual experience under normal circumstances and that inside the scanner. A scan specific calculated attenuation correction was performed to minimize inter‐scan displacement induced variance [Reinders et al.,2002]. Data acquisition was in multiple 10‐s frames (identical for men and women). Scan frames were reconstructed using a filtered back projection procedure and were corrected for background radiation, before being summed to 120s rCBF‐images.
Experimental Design
Gender differences in the human sexual response were taken into account when the male and female experiments were designed. For instance, women typically reach orgasm most easily through clitoral stimulation, and not via penetration [Hite,1976; Lloyd,2005]. In addition, women are far more likely to stay aroused after orgasm than men [Mah and Binik,2001]. Therefore, women had three and men two attempts to reach orgasm. An overview of the experimental tasks that were performed by both sexes and the order in which these were scanned is given in Figure 1. For more detailed descriptions of the male and female experimental design, we refer to Holstege et al. [2003] or Georgiadis et al. [2006, 2007], respectively.
Figure 1.
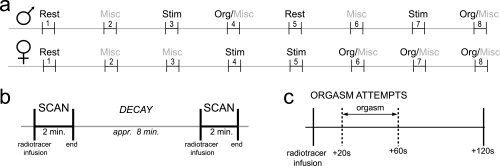
Schematic overview of the experimental set‐up of the male and female studies. A: The scan order differed between men and women. Note that in women the three orgasm attempts were scheduled at the end of the experiment (scans 6–8), whereas men attempted to attain orgasm twice, namely once in the middle (scan 4) and once at the end (scan 8) of the experiment. B: The rhythm of the [15O]‐H2O PET‐protocol. During the eight‐minute interscan interval there was sufficient time to prepare orgasm. C: Orgasm was aimed at the period 20–60 s after tracer injection (indicated by the dashed lines). Misc, miscellaneous (for details, see Materials and Methods); Org, orgasm attempt; Stim, sexual genital stimulation.
Data Preprocessing and Data Modeling
Statistical Parametric Mapping (SPM5) software (http://www.fil.ion.ucl.ac.uk/spm/) was used for spatial transformation and statistical analysis of the data [Friston et al.,1995a,b]. The data were realigned, stereotactically normalized, and smoothed using an isotropic Gaussian kernel of 12 mm (full width at half maximum). Subsequently, the data were fitted to a general linear model for variance estimation using the flexible factorial design set‐up in SPM5. Three factors (Subject, Gender and Condition) were defined, which were incorporated in the model as one main effect (Subject) and one interaction effect (Gender x Condition). The factor “Condition” had four levels: passive non‐sexual resting state (REST), sexual tactile genital stimulation (STIM), orgasm (ORG), and miscellaneous (MISC). The latter category contained the scans of “erection without stimulation” and “failed orgasm attempts” in men, and of “imitation of orgasm” and “failed orgasm attempt” in women. Differences in global activity between scans were corrected for by proportional scaling, with a grand mean set to 50 ml/100g/min−1. For each scan, voxels with a value less than 80% of the mean voxel value were removed to exclude voxels outside of the brain.
Statistical Analysis
Within‐gender, regionally specific condition effects were tested by comparing the parameter estimates using linear compounds or contrasts. The resulting set of voxel values for these contrasts constituted the associated statistical parametric map of the t statistics. These within‐gender t contrasts were calculated for (i) sexual tactile genital stimulation (activations: STIM > REST; deactivations: REST > STIM) and (ii) orgasm (activations: ORG > STIM; deactivations: STIM > ORG).
The main objective of this study was to explore gender commonalities and differences. Due to the lack of studies on this topic, we accepted and report those brain areas that survive a statistical threshold of P < 0.001, uncorrected for multiple comparisons [Friston et al.,1991]. To investigate rCBF changes occurring in men and women for stimulation or orgasm respectively, we performed conjunction analyses using statistical parametric maps of the minimal t statistic, P < 0.001 uncorrected, over “male” and “female” t contrasts (conjunction null hypothesis, see: Friston et al.,2005; Nichols et al.,2005]. These effects are called “gender commonalities.” The mutual subtraction of the appropriate male versus female t contrasts rendered differential contrasts for sexual genital stimulation and orgasm, which are called “gender differences.” Parameter estimates were calculated to gain knowledge about how brain regions in men and women responded during the different phases of the experiment. We used MarsBar (http://marsbar.sourceforge.net/), a region of interest tool that can be implemented in SPM5. Two separate extended models were designed to test for possible confounding effects of differences across genders in scan order and orgasm repetition. The influence of these effects was tested by two independent analyses of covariance (scan order: 1–8; orgasm repetition order: 1, 2, 3) using an F test (P < 0.05, corrected). Brain regions were identified using three stereotactic atlasses: Schmahmann et al. [1999] for foci in the cerebellum, Mai et al. [1997] for foci in the subcortical gray matter, and the WFU‐pickatlas tool [Maldjian et al.,2003,2004] with incorporated Talairach Daemon [Lancaster et al.,2000] for gyral‐ and Brodmann‐labeling of foci in the telencephalic cortical coverings.
RESULTS
Conjunction Analyses
rCBF changes occurring in men and women during tactile sexual genital stimulation
There was conjoint activation in the left primary and secondary somatosensory cortices. Shared deactivation was found in the right amygdala, bilaterally in the fusiform gyrus, and on the ventral aspect of the temporal lobe (Table I and Fig. 2).
Table I.
Comparing brain activation of men and women during sexual genital stimulation
| Side | Brain regions | BA | Voxels | x | y | z | t |
|---|---|---|---|---|---|---|---|
| Common activations for men and women | |||||||
| L | Inferior parietal lobule (SII) | 40 | 142 | −54 | −22 | 28 | 3.84 |
| L | Postcentral gyrus (SI) | 3 | 40 | −20 | −38 | 64 | 3.69 |
| Common deactivations for men and women | |||||||
| R | Amygdala | − | 379 | 20 | −2 | −24 | 4.88a , b |
| R | Fusiform gyrus | 20 | 75 | 40 | −20 | −32 | 4.29 |
| L | Fusiform gyrus | 20 | 526 | −54 | −32 | −28 | 4.06b |
| R | Middle temporal gyrus | 21 | 68 | 54 | 10 | −20 | 3.72 |
| L | Inferior temporal gyrus | 37 | 24 | −56 | −62 | −16 | 3.57 |
| Men > women | |||||||
| R | Claustrum | – | 121 | 32 | −18 | −4 | 4.61a , b |
| L | Cerebellar vermis, posterior lobe | – | 56 | −10 | −80 | −16 | 4.11 |
| R | Middle temporal gyus (VOT) | 37 | 82 | 58 | −64 | −2 | 4.07b |
| L | Cerebellar vermis, Posterior lobe | – | 101 | −12 | −82 | −36 | 3.70 |
| Women > men | |||||||
| L | Inferior parietal lobule (posterior parietal cortex) | 40 | 367 | −34 | −56 | 44 | 4.90a |
| L | Postcentral gyrus (somatosensory area 2) | 2 | 349 | −52 | −24 | 40 | 4.64a , b |
| L | Precentral gyrus/middle frontal gyrus (MI/PMC) | 4/6 | 484 | −40 | −24 | 62 | 4.54a |
| R | Middle frontal gyrus (PMC) | 6 | 210 | 30 | 6 | 64 | 4.35c |
| R | Middle frontal gyrus (PMC) | 6 | 121 | 4 | −8 | 52 | 4.01 |
| R | Inferior parietal lobule (posterior parietal cortex) | 40 | 26 | 48 | −70 | 40 | 3.48c |
| L | Precuneus | 7 | 21 | −20 | −74 | 46 | 3.37 |
Within‐gender, brain activation during sexual genital stimulation was compared to brain activation of a non‐sexual passive resting state (STIM vs. REST). Gender commonalities were found by conjunction and gender differences by mutual subtraction of these within‐gender T contrasts. The statistical threshold for brain regions listed in the table is P < 0.001, uncorrected for multiple comparisons (t = 3.15). Coordinates refer to the MNI (Montréal Neurological Institute) coordinate system.
Cluster with peak voxel value of P FWE < 0.05.
Region included in a priori hypothesis.
Region symmetrical to contralateral region with P FWE < 0.05.
BA, Brodmann's area; P FWE, family‐wise error corrected for multiple comparisons; L, left hemisphere; PMC, premotor cortex; R, right hemisphere; t, t value; SII, secondary somatosensory cortex; VOT, ventral occipitotemporal cortex; x, distance (mm) relative to midsagittal plane (+, right; −, left); y, distance (mm) relative to anterior commissure (+, anterior; −, posterior); z, distance (mm) relative to intercommissural line (+, dorsal; −, ventral).
Figure 2.
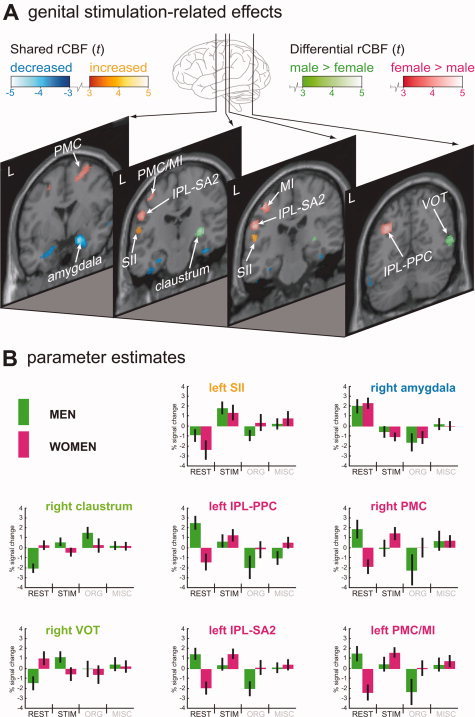
Men and women compared during tactile sexual genital stimulation. A: Genital stimulation‐related effects were assessed by comparing scans of sexual tactile genital stimulation with scans of a non‐sexual passive resting state. Green shading indicates clusters more activated in men than in women and red shading clusters more activated in women than in men. Shared activations are depicted in “hot” metal shading, shared deactivations in blue shading. The threshold for all rCBF changes depicted in the figure is P < 0.001 (uncorrected for multiple comparisons), corresponding to t = 3.15. Sections are lined‐up from anterior (left section) to posterior (right section), with the distance to the anterior commissure (in mm) indicated in the right top corner. B: Parameter estimates were calculated for identified clusters using the region of interest analysis tool MarsBar (http://marsbar.sourceforge.net/). Each boxplot depicts the percentage signal change in a particular region relative to its mean signal over all conditions for men (green) and women (pink). The color of the anatomical label indicates, for the comparison STIM vs. REST, whether a region was activated more in men than in women (green), more in women than in men (pink), or showed an effect in both gender groups (orange, activation; blue, deactivation). Variance is indicated by 90% confidence interval bars. IPL‐PPC, inferior parietal lobule, posterior parietal cortex; IPL‐SA2, inferior parietal lobule, somatosensory area 2; L, left hemisphere; MI/PMC, primary motor cortex and premotor cortex; MISC, miscalleneous condition; ORG, orgasm condition; PMC, premotor cortex; rCBF, regional cerebral blood flow; REST, non‐sexual resting state condition; t, t value; SII, secondary somatosensory cortex; STIM, sexual tactile genital stimulation condition; VOT, ventral occipitotemporal cortex.
rCBF changes occurring in men and women during orgasm
Shared rCBF increases were present in the left anterior lobe of the cerebellar vermis. More caudally there was conjoint activation in the region of the deep cerebellar nuclei, and this cluster extended anteriorly to include the pontine tegmentum. Decreases in rCBF common to both gender groups were found in the prefrontal cortex (right medial orbitofrontal, left lateral orbitofrontal, left dorsolateral) and in the left temporal lobe (fusiform gyrus, superior temporal gyrus: Table II and Fig. 3).
Table II.
Comparing brain activation of men and women during orgasm
| Side | Brain regions | BA | Voxels | x | y | z | t |
|---|---|---|---|---|---|---|---|
| Common activations for men and women | |||||||
| L | Cerebellar vermis, anterior lobe | – | 279 | −6 | −56 | −8 | 4.31a |
| M | Deep cerebellar nuclei/pons | – | 249 | 0 | −40 | −26 | 3.94a , b |
| Common deactivations for men and women | |||||||
| R | Gyrus rectus (medial orbitofrontal cortex) | 11 | 594 | 2 | 34 | −22 | 4.28a |
| L | Inferior frontal gyrus (lateral/middle orbitofrontal cortex) | 47 | 100 | −34 | 32 | −18 | 4.09a |
| L | Middle frontal gyrus (frontal pole) | 10 | 395 | −34 | 56 | −4 | 4.08a |
| L | Superior frontal gyrus | 10 | 93 | −26 | 46 | 28 | 3.81 |
| L | fusiform gyrus | 20 | 33 | −46 | −32 | −30 | 3.72 |
| L | Superior temporal gyrus | 22 | 38 | −44 | −16 | 8 | 3.57 |
| L | Medial frontal gyrus (frontal pole) | 10 | 27 | −10 | 60 | −18 | 3.45a |
| L | Inferior frontal gyrus | 47 | 13 | −40 | 22 | −4 | 3.44a |
| L | Middle frontal gyrus | 9 | 26 | −34 | 24 | 38 | 3.36 |
| Men > women | |||||||
| L | Dorsal midbrain/periaqueductal gray matter (PAG) | – | 40 | 0 | −32 | −2 | 3.95b |
| L | Lingual gyrus | 19 | 23 | −26 | −56 | −4 | 3.54 |
| Women > men | |||||||
| R | Insula | 13 | 230 | 40 | −20 | 14 | 4.33b |
Within‐gender, brain activation during orgasm was compared to brain activation of sexual genital stimulation (ORG vs. STIM). Gender commonalities were found by conjunction and gender differences by mutual subtraction of these within‐gender t contrasts. The statistical threshold for brain regions listed in the table is P < 0.001, uncorrected for multiple comparisons (t = 3.15). Coordinates refer to the MNI (Montréal Neurological Institute) coordinate system.
Region included in a priori hypothesis.
Region with proven relevance for sexual behavior.
BA, Brodmann's Area; L, left hemisphere; R, right hemisphere; t, t value; x, distance (mm) relative to midsagittal plane (+, right; −, left); y, distance (mm) relative to anterior commissure (+, anterior; −, posterior); z, distance (mm) relative to intercommissural line (+, dorsal; −, ventral).
Figure 3.
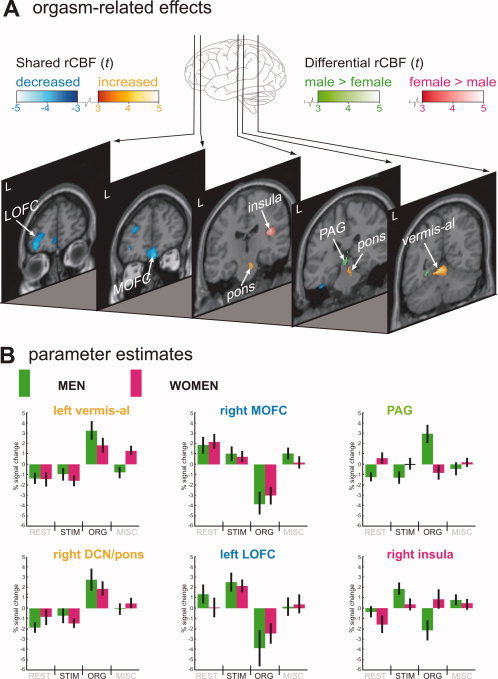
Men and women compared during orgasm. A: Orgasm‐related effects were assessed by comparing scans of orgasm with scans of sexual tactile genital stimulation. Green shading indicates clusters more activated in men than in women and red shading clusters more activated in women than in men. Shared activations are depicted in “hot” metal shading, shared deactivations in blue shading. The threshold for all rCBF changes depicted in the figure is P < 0.001 (uncorrected), corresponding to t = 3.15. Sections are lined‐up from anterior (left section) to posterior (right section), with the distance to the anterior commissure (in mm) indicated in the right top corner. B: Parameter estimates were calculated for identified clusters using the region of interest analysis tool MarsBar (http://marsbar.sourceforge.net/). Each boxplot depicts the percentage signal change in a particular region relative to its mean signal over all conditions for men (green) and women (pink). The color of the anatomical label indicates, for the comparison ORG vs. STIM, whether a region was activated more in men than in women (green), more in women than in men (pink), or showed an effect in both gender groups (orange, activation; blue, deactivation). Variance is indicated by 90% confidence interval bars. DCN, deep cerebellar nuclei; L, left hemisphere; LOFC, lateral orbitofrontal cortex; MISC, miscellaneous condition; MOFC, medial orbitofrontal cortex; ORG, orgasm condition; PAG, periaqueductal gray matter; rCBF, regional cerebral blood flow; REST, non‐sexual resting state condition; STIM, sexual tactile genital stimulation condition; t, t value; vermis‐al, anterior lobe of cerebellar vermis.
Differential Contrasts
Gender differences during sexual tactile genital stimulation: men > women
In men there was more activation in the right posterior claustrum, the ventral occipitotemporal region, and the posterior lobe of the cerebellar vermis (Table I and Fig. 2).
Gender differences during sexual tactile genital stimulation: women > men
In women parietal areas in the left hemisphere were more involved. Most notably, there was differential activation in the posterior parietal cortex (PPC) and, more anteriorly, somatosensory area 2. Additional larger activation in women was found in the left primary motor cortex, and the right premotor cortex. Other clusters of larger activation in women were seen in the right PPC and the left precuneus (Table I and Fig. 2).
Gender differences during orgasm: men > women
In men, the midbrain and the left lingual gyrus demonstrated larger activation during orgasm (Table II and Fig. 3). The midbrain location corresponded with the periaqueductal gray matter (PAG).
Gender differences during orgasm: women > men
In women larger activation during orgasm was found in the right insula (Table II and Fig. 3).
Scan order and orgasm repetition effects
No variance was explained by scan order or orgasm repetition
DISCUSSION
This study aimed to investigate gender differences and commonalities with respect to cerebral blood flow changes during two phases of human sexual behavior, namely orgasm and the preceding phase of tactile genital stimulation. The main result of this study was that gender differences were most prominent during tactile genital stimulation, while gender similarities were observed during orgasm.
It is highly unlikely that differences measured during sexual tactile genital stimulation were caused by sexual dimorphism of cerebral structures. First of all, our spatial brain normalization entailed iterative non‐linear warping which effectively obliterates inter‐individual structural variance. Second, although structural gender differences exist in amygdala, hippocampus and prefrontal cortex [Allen et al.,2003; Cahill,2006; Luders et al.,2006], none of these areas were identified in our study as being functionally different between genders. In contrast, we found shared rCBF changes in both the amygdala and prefrontal cortex. Interestingly, we observed in our study that part of the occipital lobe was activated more in men than in women, a finding which has been demonstrated previously for emotional tasks [Wager et al.,2003].
Sexual Tactile Genital Stimulation
Gender commonalities
The conjunction analysis confirmed results of our prior publications demonstrating little overlap between penile and clitoral stimulation with regard to activated brain regions. Only two regions showed a tendency towards activation in both gender groups, the left primary (SI) and secondary (SII) somatosensory cortex. However, the conjunction analysis did not corroborate animal studies which suggest a shared neuronal substrate for the processing of male and female genital afferent information during sexual activity at the level of the brainstem and the diencephalon [Baum and Everitt,1992; Coolen et al.,1996; Kollack‐Walker and Newman,1997; Marson,1995; Marson and Murphy,2006; Marson et al.,1993]. This is intriguing because penis and clitoris derive from the same embryonic tissue and share a common peripheral somatic innervation via the pudendal nerve. Taking this into account, one might assume that common pathways in humans must exist at the subcortical level for the sensory information from the genitalia to reach the cerebral cortex. However, the present study depicts discrepancies which may be based on physiological differences between rodents and humans. Conflicting results may also be due to the experimental set‐up or the restrictions of the scanning technique used.
Consistent with our previous publications we show activity decreases in the medial temporal lobe in both men and women, most notably in the right amygdala and left fusiform gyrus. Our results are also in line with the well‐established inverse relationship, demonstrated both by clinical [Aloni and Katz,1999; Baird et al.,2002] and neuroimaging [Bocher et al.,2001; Dimpfel et al.,2003; Moulier et al.,2006; Redouté et al.,2000; Tiihonen et al.,1994] studies, between activity in the temporal lobe and the degree of sexual arousal. The present results strengthen this concept, and, most importantly, show specific sites within the temporal lobe in men and women, the right amygdala and left fusiform gyrus, that might restrain sexual arousal during a non‐sexual resting state.
Gender differences
During tactile genital stimulation we observed higher activation of the right posterior claustrum in men than in women. The claustrum has extensive and mostly reciprocal connections with the neocortex [for review, see Crick and Koch,2005], and functionally, has been linked to cross‐modal matching [Hadjikhani and Roland,1998; Horster et al.,1989] and multisensory integration [Naghavi et al.,2007]. Studies using visual sexual stimulation (VSS) have shown a positive correlation between claustrum activity levels and the degree of penile turgidity [Arnow et al.,2002; Redouté et al.,2000,2005]. Arnow and colleagues proposed that claustrum involvement reflects cross‐modal transfer of visual input to imagined tactile (penile) stimulation [Arnow et al.,2002]. Following this line of thinking, claustrum activation during sexual tactile genital stimulation with eyes closed could point to cross‐modal transfer of genital sensory information to a visually imagined situation. Support comes from anatomical studies, showing that the posterior portion of the claustrum is connected to visual cortical areas, at least in cat [Narkiewicz,1964; Olson and Graybiel,1980] and monkey [Pearson et al.,1982]. Although no data were collected from our subjects that could confirm stronger visual imagery in men during tactile genital stimulation than in women, the idea remains attractive: (i) next to the claustrum, men also had stronger activation in the right ventral occipitotemporal region, a visual area that is activated during visual imagery [Ishai et al.,2000; Roland and Gulyas,1995]. This is also consistent with the meta analysis by Wager et al. which speculated about the a higher sensitivity and attention to visual stimuli in men in emotional paradigms [Wager et al.,2003]. (ii) behavioral observations have shown that men have a greater interest in visual sexual stimuli than women [Laumann et al.,1994].
Higher levels of activation in women than in men was found in clusters spread over the convexity of the left parietal lobe and the adjacent posterior part of the left frontal lobe. Expectedly, differential activation in somatosensory area 2 (SA2), a somatosensory association area in the postcentral gyrus, was observed with higher activation in women. A novel finding was strong differential activation (P < 0.05, corrected) in the posterior parietal cortex (PPC), the posterior portion of the inferior parietal lobule. In addition to multi‐sensory integration, other functions have been ascribed to this region such as shifting attention, stimulus selection, and movement planning. As such, the PPC constitutes a key interface between sensory cortex and motor areas in the frontal lobes, performing intermediate operations for sensory‐motor transformation [for review, see: Andersen et al.,1997]. Indeed, motor regions in the frontal lobe (left primary motor cortex and bilateral premotor cortices) were also more strongly activated during clitoral than during penile stimulation.
The question arises as to why tactile sexual genital stimulation elicits such marked differences in frontoparietal activity between men and women. One explanation could be that the stimulation was not identical for both genders due to the different physical properties of penis and clitoris. For instance, in women often the entire vaginal vestibulum was stimulated, and not only the clitoris. It is quite possible that in a region like SA2, where somatotopy is still highly preserved [Young et al.,2004], such physical differences could lead to divergent activation across genders. Another explanation is provided by mirror neuron theory, which holds that certain neurons activate both during the execution of an action and the perception of the same action performed by another person. This may be a potential neural basis for individuals to understand each other's actions [Rizzolatti and Craighero,2004]. Main elements of this system, the premotor cortex, the inferior parietal lobule, and the posterior parietal cortex, showed female‐biased activation in the present study. Individuals with a high capacity to sense the perspective of others, a trait that is more often associated with women than men [Baron‐Cohen et al.,2005], display stronger activation in “mirror areas” [Gazzola et al.,2006]. One might therefore assume that our female subjects had a stronger “motor embodiment” of their partner's action (i.e. performing genital stimulation). Left‐lateralized primary motor and premotor activation was consistent with right‐handed stimulation of the clitoris performed by the partner. Although premotor activation in response to the sight of a hand action has been reported to be more ventral than the location we presently show (e.g. Buccino et al.,2001], dorsal premotor activation similar to ours was found in response to heard, but unseen hand actions [Gazzola et al.,2006]. Recall that our subjects were unable to see their partner perform the stimulation. Within the inferior parietal lobule the activated clusters were located posteriorly, corresponding to simulation of hand/arm or possibly even leg/foot or pelvis actions [Buccino et al.,2001]. We want to stress that this explanation remains speculative in the absence of data on perspective taking.
Orgasm
Gender commonalities
Gender commonalities in brain activation during orgasm were much more prominent than during the preceding phase of sexual genital stimulation. Consistent with previous results [Georgiadis et al.,2006,2007] there was profound deactivation in the anterior part of the orbitofrontal cortex (OFC) in men and women alike.
The OFC consists of functionally distinct regions [Kringelbach,2005; Kringelbach and Rolls,2004; Öngür et al.,2003] and here we adopt the tripartite terminology of Kringelbach [2005] for the anterior OFC: LOFC—lateral OFC, MiOFC—middle OFC and MOFC—medial OFC. We found deactivated clusters on the border of LOFC and MiOFC and also in the MOFC.
The LOFC has been extensively discussed in our previous work [Georgiadis et al.,2006,2007], primarily because of its role in urge suppression and behavioral release [Beauregard et al.,2001; Dougherty et al.,1999; Small et al.,2001], but the more medial areas have received less attention.
The MiOFC is believed to specifically encode hedonic experience, becoming activated with increasing satiation and subjective pleasantness, and deactivated with feelings of satiety [Kringelbach et al.,2003; Rolls,2000]. Although the temporal resolution of PET is relatively low, the orgasm‐related MiOFC deactivation probably reflects satiety and not the hedonic experience of orgasm.
The MOFC constitutes a crucial part of a neural network underlying self‐monitoring and self‐referential thought [Gusnard et al.,2001; Northoff et al.,2006]. Another part of this network, the amygdala [Gusnard and Raichle,2001], was deactivated during tactile genital stimulation relative to the non‐sexual resting state, but did not show further deactivation during orgasm (see parameter estimates for the right amygdala in Fig. 2B). Deactivation of this network encompassing MOFC and amygdala is generally associated with a more carefree state of mind [Hariri et al.,2000]. The correspondence with subjective descriptions of orgasm is striking indeed [Mah and Binik,2002].
The anterior lobe of the cerebellum was also activated across genders. For detailed discussion of cerebellar involvement in orgasm we refer to previous work [Georgiadis et al.,2006,2007]. A novel orgasm‐related effect was the activation of the pontine extension of the cerebellar cluster. In this part of the brainstem cerebellum‐projecting nuclei are located, but it is also the site for cardiovascular and respiratory control centers, as well as for centers controlling sympathetic tone. This corresponds well with the peak state of cardiovascular arousal [Exton et al.,1999; Krüger et al.,1998] and the high level of norepinephrine in the cerebrospinal fluid [Krüger et al.,2006] that are found during orgasm. Consistent with this finding is a study on the neuronal correlates of mental and physical exercise in humans, which showed that the pons and anterior lobe of the cerebellar vermis were positively correlated with cardiovascular arousal [Critchley et al.,2000].
One way to interpret the fact that gender commonalities dominate the picture for orgasm is that the orgasmic experience is largely similar for both gender groups. Support for this notion is provided by sexological research showing that written orgasmic experiences of men and women could not be differentiated by gynaecologists, medical students, and psychologists [Vance and Wagner,1976].
Gender differences
Orgasm‐related gender differences in brain activity were sparse, but interesting: in particular, men had stronger activation than women in the periaqueductal gray matter (PAG). The PAG has a prominent role in the control of reproductive behavior, at least in experimental animals [Sakuma and Pfaff,1979a,b]. Our data for the first time indicate that the PAG may also be involved in human sexual activity. Besides its role in reproductive reflexes, the PAG is also known to be involved in vocalization, cardiovascular and respiratory arousal, and pain suppression [for review see Holstege et al.,1996]. Our finding also corresponds with the observation in male human opiate addicts that the PAG was activated after intravenous administration of heroin [Sell et al.,1999], which could point to orgasm‐related, male‐biased endogeneous opiate release in the PAG. Although the differential involvement of the PAG in male and female sexual orgasm cannot be explained at this point, it is potentially interesting and certainly merits further research.
Stronger activation in women than in men was seen in the right posterior insula, but this effect appeared to be driven exclusively by a deactivation in men (see parameter estimates for the right insula in Fig. 3B). Therefore, we do not consider this to be an effect that truly reflects female‐biased brain activation related to orgasm.
Comparison with gender differences found in response to visual sexual stimulation
Most studies concerning the neurobiology of human sexuality have used visual sexual stimulation (VSS) to elicit a state of sexual desire and arousal in subjects. Gender differences in brain responses to VSS were investigated in only two studies, which basically showed that men activated the hypothalamus and amygdala more than women [Hamann et al.,2004; Karama et al.,2002]. As we have demonstrated here, these effects were not present during later stages of the sexual response. It is important to understand, however, that VSS paradigms differ fundamentally from the paradigm we used, both with respect to the sexual phase under investigation (sexual desire vs. sexual “consumption”) and the type of sexual stimulation used (visual vs. tactile).
In rodents activation of the amygdala is important for identifying sexual salience in distal olfactory and visual signals [Newman,1999; Parfitt and Newman,1998], after which sexual desire can be established. This is consistent with amygdala activation demonstrated with VSS paradigms [Beauregard et al.,2001; Ferretti et al.,2005; Gizewski et al.,2006]. However, the present data clearly demonstrate that amygdala activity decreases once the sexual act commences. Supporting our finding are studies showing that similar attenuated amygdala activity is characteristic of euphoric mental states such as cocaine rush [Breiter et al.,1997] and romantic love [Bartels and Zeki,2004]. This change in amygdala activity may be crucial for prolonged sexual arousal during the sexual act.
In order to understand the absence of a gender effect in the hypothalamus it is important to consider recent work by Ferretti et al. [2005], who demonstrated that the hypothalamus was activated during the onset of penile erection, but not when sustaining it. In other words, once penile erection has been established it can be sustained without further marked activation of the hypothalamus, provided that there is erotic stimulation.
Limitations and Considerations
Different experimental designs for men and women
The experimental design for men and women was not identical (see Fig. 1) to accommodate for natural/biological differences between male and female sexuality. For one, slightly different tasks were performed. These were pooled in the model as a separate mixed condition called “Misc.” As Figures 2B and 3B show, this mixed condition explained little or no variance in brain activation. In addition, the scan order and the occurrence of multiple orgasms differed between men and women. Potential biases of these factors on gender related brain activation were tested with separate extended models using covariates of interest. No significant rCBF effects were found when testing the scan order or orgasm repetition effects independently.
Considerations concerning the orgasm condition
Orgasms are relatively short events so that orgasm scans in the present study included peri‐orgasmic events, e.g. preorgasmic sexual arousal and postorgasmic satiety. Because there was random variability in orgasm timing, the relative contribution of pre‐orgasmic vs postorgasmic phenomena to the measurement was also variable.
It is likely that there was random variability in the duration of orgasms and hence in the contribution of orgasmic phenomena to the measurement. Finally, orgasm duration could be gender biased, but neither of these issues can be addressed with the present data or with the PET technique in its present form.
Concluding, brain regions reported for orgasm in this paper must be considered orgasm‐related rather than orgasm‐specific.
Effect size
Because of the lack of studies on sexual genital stimulation and orgasm, we decided to use an uncorrected statistical threshold of P < 0.001 to increase the sensitivity of our analysis. Caution should be taken, because of an associated increased risk of false positives. We made sure, however, to focus on the strongest effects (P < 0.05, corrected for multiple comparisons), on effects that were expected beforehand, and on effects with proven biological relevance for sexual behavior. Other studies can hopefully benefit from our reports.
Absence of behavioral data
An important limitation is the lack of behavioral data. Although in women rectal pressure measurements were made and ratings about the perceived level of sexual arousal were collected, no behavioral data were collected in men. As a consequence, there is a possibility that the reported gender effects are due to systematic differences in experienced intensity or quality of stimulation and orgasm.
CONCLUSION
Gender differences in brain activity were particularly marked during tactile genital stimulation. Specifically, in men the right claustrum and ventral occipitotemporal cortex were activated more strongly, and in women left fronto‐parietal areas. Gender commonalities were sparse during this phase. For the orgasm phase a different picture emerged: The gender commonalities dominated the picture, most notably in the orbitofrontal cortex, whereas the only prominent gender difference was observed in the midbrain PAG. Translating these neuroimaging results to actual sexual behavior, this could mean that men and women have different ways to reach orgasm while undergoing tactile stimulation, but that their orgasmic experience is largely similar. These results could potentially benefit treatment strategies for psychosexual problems.
Acknowledgements
The authors are grateful to the staff and personnel at the Department of Nuclear Medicine & Molecular Imaging for their magnanimous cooperation. J.R.G. thanks H.E. Glover for helpful and inspiring discussions. The work was performed at the University Medical Center Groningen, The Netherlands.
REFERENCES
- Allen JS,Damasio H,Grabowski TJ,Bruss J,Zhang W( 2003): Sexual dimorphism and asymmetries in the gray‐white composition of the human cerebrum. Neuroimage 18: 880–894. [DOI] [PubMed] [Google Scholar]
- Aloni R,Katz S ( 1999): A review of the effect of traumatic brain injury on the human sexual response. Brain Inj 13: 269–280. [DOI] [PubMed] [Google Scholar]
- Andersen RA,Snyder LH,Bradley DC,Xing J ( 1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330. [DOI] [PubMed] [Google Scholar]
- Arnow BA,Desmond JE,Banner LL,Glover GH,Solomon A,Polan ML,Lue TF,Atlas SW ( 2002): Brain activation and sexual arousal in healthy, heterosexual males. Brain 125: 1014–1023. [DOI] [PubMed] [Google Scholar]
- Baird AD,Wilson SJ,Bladin PF,Saling MM,Reutens DC ( 2002): Hypersexuality after temporal lobe resection. Epilepsy Behav 3: 173–181. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S,Knickmeyer RC,Belmonte MK ( 2005): Sex differences in the brain: implications for explaining autism. Science 310: 819–823. [DOI] [PubMed] [Google Scholar]
- Bartels A,Zeki S ( 2004): The neural correlates of maternal and romantic love. Neuroimage 21: 1155–1166. [DOI] [PubMed] [Google Scholar]
- Baum MJ,Everitt BJ ( 1992): Increased expression of c‐fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience 50: 627–646. [DOI] [PubMed] [Google Scholar]
- Beauregard M,Levesque J,Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher M,Chisin R,Parag Y,Freedman N,Meir Weil Y,Lester H,Mishani E,Bonne O ( 2001): Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O‐H2O PET study in heterosexual men. Neuroimage 14: 105–117. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Gollub RL,Weisskoff RM,Kennedy DN,Makris N,Berke JD,Goodman JM,Kantor HL,Gastfriend DR,Riorden JP,Mathew RT,Rosen BR,Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Buccino G,Binkofski F,Fink GR,Fadiga L,Fogassi L,Gallese V,Seitz RJ,Zilles K,Rizzolatti G,Freund HJ ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Cahill L ( 2006): Why sex matters for neuroscience. Nat Rev Neurosci 7: 477–484. [DOI] [PubMed] [Google Scholar]
- Coolen LM,Peters HJ,Veening JG ( 1996): Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res 738: 67–82. [DOI] [PubMed] [Google Scholar]
- Crick FC,Koch C ( 2005): What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 360: 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD,Corfield DR,Chandler MP,Mathias CJ,Dolan RJ ( 2000): Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523 (Pt 1): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimpfel W,Wedekind W,Keplinger I ( 2003): Gender difference in electrical brain activity during presentation of various film excerpts with different emotional content. Eur J Med Res 8: 192–198. [PubMed] [Google Scholar]
- Dougherty DD,Shin LM,Alpert NM,Pitman RK,Orr SP,Lasko M,Macklin ML,Fischman AJ,Rauch SL ( 1999): Anger in healthy men: a PET study using script‐driven imagery. Biol Psychiatry 46: 466–472. [DOI] [PubMed] [Google Scholar]
- Exton MS,Bindert A,Kruger T,Scheller F,Hartmann U,Schedlowski M ( 1999): Cardiovascular and endocrine alterations after masturbation‐induced orgasm in women. Psychosom Med 61: 280–289. [DOI] [PubMed] [Google Scholar]
- Ferretti A,Caulo M,Del Gratta C,Di Matteo R,Merla A,Montorsi F,Pizzella V,Pompa P,Rigatti P,Rossini PM,Salonia A,Tartaro A,Romani GL ( 2005): Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 26: 1086–1096. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Liddle PF,Frackowiak RS ( 1991): Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11: 690–699. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Ashburner J,Poline J‐B,Frith CD,Heather JD,Frackowiak RS ( 1995a): Spatial registration and normalisation of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ,Worsley KJ,Holmes AP,Poline J‐B,Frith CD,Frackowiak RS ( 1995b): Statistical parametric mapping in functional imaging: a general approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ,Penny WD,Glaser DE ( 2005): Conjunction revisited. Neuroimage 25: 661–667. [DOI] [PubMed] [Google Scholar]
- Gazzola V,Aziz‐Zadeh L,Keysers C ( 2006): Empathy and the somatotopic auditory mirror system in humans. Curr Biol 16: 1824–1829. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR,Holstege G ( 2005): Human brain activation during sexual stimulation of the penis. J Comp Neurol 493: 33–38. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR,Kortekaas R,Kuipers R,Nieuwenburg A,Pruim J,Reinders AATS,Holstege G ( 2006): Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci 24: 3305–3316. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR,Reinders AA,van der Graaf FH,Paans AM,Kortekaas R ( 2007): Brain activation during human male ejaculation revisited . Neuroreport 18: 553–557. [DOI] [PubMed] [Google Scholar]
- Gizewski ER,Krause E,Karama S,Baars A,Senf W,Forsting M ( 2006): There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: a fMRI study. Exp Brain Res 174: 101–108. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Raichle ME ( 2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Gusnard DA,Akbudak E,Shulman GL,Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N,Roland PE ( 1998): Cross‐modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. J Neurosci 18: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S,Herman RA,Nolan CL,Wallen K ( 2004): Men and women differ in amygdala response to visual sexual stimuli. Nature Neurosci 7: 411–416. [DOI] [PubMed] [Google Scholar]
- Hariri AR,Bookheimer SY,Mazziotta JC ( 2000): Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11: 43–48. [DOI] [PubMed] [Google Scholar]
- Hite S ( 1976): The Hite report: A Nationwide Survey of Female Sexuality. New York: MacMillan. [Google Scholar]
- Holstege G,Bandler R,Saper CB ( 1996): The emotional motor system. Prog Brain Res 107: 3–6. [DOI] [PubMed] [Google Scholar]
- Holstege G,Georgiadis JR,Paans AM,Meiners LC,van der Graaf FH,Reinders AA ( 2003): Brain activation during human male ejaculation. J Neurosci 23: 9185–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster W,Rivers A,Schuster B,Ettlinger G,Skreczek W,Hesse W ( 1989): The neural structures involved in cross‐modal recognition and tactile discrimination performance: an investigation using 2‐DG. Behav Brain Res 33: 209–227. [DOI] [PubMed] [Google Scholar]
- Ishai A,Ungerleider LG,Haxby JV ( 2000): Distributed neural systems for the generation of visual images. Neuron 28: 979–990. [DOI] [PubMed] [Google Scholar]
- Karama S,Lecours AR,Leroux JM,Bourgouin P,Beaudoin G,Joubert S,Beauregard M ( 2002): Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack‐Walker S,Newman SW ( 1997): Mating‐induced expression of c‐fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol 32: 481–501. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML ( 2005): The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML,Rolls ET ( 2004): The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML,O'Doherty J,Rolls ET,Andrews C ( 2003): Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 13: 1064–1071. [DOI] [PubMed] [Google Scholar]
- Krüger T,Exton MS,Pawlak C,zur Muhlen A,Hartmann U,Schedlowski M ( 1998): Neuroendocrine and cardiovascular response to sexual arousal and orgasm in men. Psychoneuroendocrinology 23: 401–411. [DOI] [PubMed] [Google Scholar]
- Krüger TH,Schiffer B,Eikermann M,Haake P,Gizewski E,Schedlowski M ( 2006): Serial neurochemical measurement of cerebrospinal fluid during the human sexual response cycle. Eur J Neurosci 24: 3445–3452. [DOI] [PubMed] [Google Scholar]
- Lancaster JL,Woldorff MG,Parsons LM,Liotti M,Freitas CS,Rainey L,Kochunov PV,Nickerson D,Mikiten SA,Fox PT ( 2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann EO,Gagnon JH,Michael RT,Michaels S ( 1994): The Social Organization of Sexuality: Sexual Practices in the United States. Chicago and London: The University of Chicago Press. [Google Scholar]
- Laumann EO,Paik A,Rosen RC ( 1999): Sexual dysfunction in the United States: prevalence and predictors. JAMA 281: 537–544. [DOI] [PubMed] [Google Scholar]
- Lloyd EA ( 2005): The case of the female orgasm: Bias in the Science of Evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- Luders E,Narr KL,Thompson PM,Rex DE,Woods RP,Deluca H,Jancke L,Toga AW ( 2006): Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp 27: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah K,Binik YM ( 2001): The nature of human orgasm: a critical review of major trends. Clin Psychol Rev 21: 823–856. [DOI] [PubMed] [Google Scholar]
- Mah K,Binik YM ( 2002): Do all orgasms feel alike? Evaluating a two‐dimensional model of the orgasm experience across gender and sexual context. J Sex Res 39: 104–113. [DOI] [PubMed] [Google Scholar]
- Mai JK,Assheuer J,Paxinos G ( 1997): Atlas of the Human Brain. San Diego: Academic Press. [Google Scholar]
- Maldjian JA,Laurienti PJ,Kraft RA,Burdette JH ( 2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Maldjian JA,Laurienti PJ,Burdette JH ( 2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21: 450–455. [DOI] [PubMed] [Google Scholar]
- Marson L ( 1995): Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett 190: 41–44. [DOI] [PubMed] [Google Scholar]
- Marson L,Murphy AZ ( 2006): Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol 291, R419–R428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L,Platt KB,McKenna KE ( 1993): Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience 55: 263–280. [DOI] [PubMed] [Google Scholar]
- Moulier V,Mouras H,Pelegrini‐Issac M,Glutron D,Rouxel R,Grandjean B,Bittoun J,Stoleru S ( 2006): Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage 33: 689–699. [DOI] [PubMed] [Google Scholar]
- Naghavi HR,Eriksson J,Larsson A,Nyberg L ( 2007): The claustrum/insula region integrates conceptually related sounds and pictures. Neurosci Lett 422: 77–80. [DOI] [PubMed] [Google Scholar]
- Narkiewicz O ( 1964): Degenerations in the claustrum after regional neocortical ablations in the cat. J Comp Neurol 123: 335–355. [DOI] [PubMed] [Google Scholar]
- Newman SW ( 1999): The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877: 242–257. [DOI] [PubMed] [Google Scholar]
- Nichols T,Brett M,Andersson J,Wager T,Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de GM,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Olson CR,Graybiel AM ( 1980): Sensory maps in the claustrum of the cat. Nature 288: 479–481. [DOI] [PubMed] [Google Scholar]
- Öngür D,Ferry AT,Price JL ( 2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Parfitt DB,Newman SW ( 1998): Fos‐immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav 34: 17–29. [DOI] [PubMed] [Google Scholar]
- Pearson RC,Brodal P,Gatter KC,Powell TP ( 1982): The organization of the connections between the cortex and the claustrum in the monkey. Brain Res 234: 435–441. [DOI] [PubMed] [Google Scholar]
- Redouté J,Stoleru S,Gregoire MC,Costes N,Cinotti L,Lavenne F,Le Bars D,Forest MG,Pujol JF ( 2000): Brain processing of visual sexual stimuli in human males. Hum Brain Mapp 11: 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redouté J,Stoleru S,Pugeat M,Costes N,Lavenne F,Le Bars D,Dechaud H,Cinotti L,Pujol JF ( 2005): Brain processing of visual sexual stimuli in treated and untreated hypogonadal patients. Psychoneuroendocrinology 30: 461–482. [DOI] [PubMed] [Google Scholar]
- Reinders AATS,Willemsen ATM,Georgiadis JR,Hovius M,Paans AMJ,den Boer JA ( 2002): Interscan displacement‐induced variance in PET activation data is excluded by a scan‐specific attenuation correction. Neuroimage 17: 1844–1853. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Craighero L ( 2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Roland PE,Gulyas B ( 1995): Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: functional anatomy by positron emission tomography. Cereb Cortex 5: 79–93. [DOI] [PubMed] [Google Scholar]
- Rolls ET ( 2000): The orbitofrontal cortex and reward. Cereb Cortex 10: 284–294. [DOI] [PubMed] [Google Scholar]
- Sakuma Y,Pfaff DW ( 1979a): Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am J Physiol 237: R278–R284. [DOI] [PubMed] [Google Scholar]
- Sakuma Y,Pfaff DW ( 1979b): Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol 237: R285–R290. [DOI] [PubMed] [Google Scholar]
- Sandel ME,Williams KS,Dellapietra L,Derogatis LR ( 1996): Sexual functioning following traumatic brain injury. Brain Inj 10: 719–728. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD,Doyon J,McDonald D,Holmes C,Lavoie K,Hurwitz AS,Kabani N,Toga A,Evans A,Petrides M ( 1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10: 233–260. [DOI] [PubMed] [Google Scholar]
- Sell LA,Morris J,Bearn J,Frackowiak RS,Friston KJ,Dolan RJ ( 1999): Activation of reward circuitry in human opiate addicts. Eur J Neurosci 11: 1042–1048. [DOI] [PubMed] [Google Scholar]
- Small DM,Zatorre RJ,Dagher A,Evans AC,Jones‐Gotman M ( 2001): Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124: 1720–1733. [DOI] [PubMed] [Google Scholar]
- Tiihonen J,Kuikka J,Kupila J,Partanen K,Vainio P,Airaksinen J,Eronen M,Hallikainen T,Paanila J,Kinnunen I ( 1994): Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neurosci Lett 170: 241–243. [DOI] [PubMed] [Google Scholar]
- Vance EB,Wagner NN ( 1976): Written descriptions of orgasm: a study of sex differences. Arch Sex Behav 5: 87–98. [DOI] [PubMed] [Google Scholar]
- Wager TD,Phan KL,Liberzon I,Taylor SF ( 2003): Valence, gender, and lateralization of functional brain anatomy in emotion: a meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Young JP,Herath P,Eickhoff S,Choi J,Grefkes C,Zilles K,Roland PE ( 2004): Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci 24: 5391–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]


