Abstract
While functional neuroimaging studies have helped elucidate major regions implicated in word recognition, much less is known about the dynamics of the associated activations or the actual neural processes of their functional network. We used intracerebral electroencephalography recordings in 10 patients with epilepsy to directly measure neural activity in the temporal and frontal lobes during written words' recognition, predominantly in the left hemisphere. The patients were presented visually with consonant strings, pseudo‐words, and words and performed a hierarchical paradigm contrasting semantic processes (living vs. nonliving word categorization task), phonological processes (rhyme decision task on pseudo‐words), and visual processes (visual analysis of consonant strings). Stimuli triggered a cascade of modulations in the gamma‐band (>40 Hz) with reproducible timing and task‐sensitivity throughout the functional reading network: the earliest gamma‐band activations were observed for all stimuli in the mesial basal temporal lobe at 150 ms, reaching the word form area in the mid fusiform gyrus at 200 ms, evidencing a superiority effect for word‐like stimuli. Peaks of gamma‐band activations were then observed for word‐like stimuli after 400 ms in the anterior and middle portion of the superior temporal gyrus (BA 38 and BA 22 respectively), in the pars triangularis of Broca's area for the semantic task (BAs 45 and 47), and in the pars opercularis for the phonological task (BA 44). Concurrently, we observed a two‐pronged effect in the prefrontal cortex (BAs 9 and 46), with nonspecific sustained dorsal activation related to sustained attention and, more ventrally, a strong reflex deactivation around 500 ms, possibly due to semantic working memory reset. Hum Brain Mapp, 2008. © 2007 Wiley‐Liss, Inc.
Keywords: word recognition, semantic processing, phonological processing, gamma band, intracerebral EEG
INTRODUCTION
Word recognition is believed to involve a series of cognitive processes including visual analysis of letters, word forms and letter strings, conversion of graphemic word forms into corresponding phonological word forms, and access to the semantic representations of words [Joubert et al., 2004].
Over the years, neuropsychological assessment of patients with acquired brain damage and a great number of neuroimaging studies have identified small sets of cardinal brain areas associated with each of these processes. Although contradictions have been evidenced in the literature [Demonet et al., 2005; Jobard et al., 2003], a broad consensus seems to emerge in favor of a functional network predominantly in the left hemisphere, involving in (a) visual analysis: early visual areas (BA 17, 18, and 19) and midfusiform gyrus (BA 19, 37) [Binder et al., 2006; Cohen et al., 2000; Devlin et al., 2006; Gaillard et al., 2006; James et al., 2005; Mechelli et al., 2007; Vigneau et al., 2005], (b) phonological processing: portions of the superior temporal (BA 22), supramarginalis (BA 40) gyri and pars opercularis (BAs 44 and 45) of Broca's area [Demonet et al., 2005; Fiez, 1997; Poldrack et al., 1999; Zatorre et al., 1996], and (c) semantic processing: the pars triangularis of Broca's area (BA 47) and the posterior part of the middle temporal gyrus (BA 21) [Demb et al., 1995; Devlin et al., 2003; Petersen et al., 1988; Roskies et al., 2001; Thompson‐Schill et al., 1999]. Recent studies have sought to refine this identification of basic networks by investigating possible subspecializations for example in processing inflected versus unmarked verbs [i.e. Yokoyama et al., 2006]. The neuroimaging literature on this subject is quite extensive and has been competently reviewed several times in recent years [Demonet et al., 2005; Fiez and Petersen, 1998; Jobard et al., 2003; Price, 2000].
The dynamics of neural activations within the implicated networks have for their part been partially described in a parallel literature centering on the fine temporal resolution afforded by electroencephalography (EEG) and magnetoencephalography (MEG). EEG/MEG studies have revealed that the presentation of written words triggers a cascade of overlapping cortical activations from 30 to 900 ms poststimulus, propagating along a posterior–anterior axis [Dhond et al., 2007; Hauk et al., 2006; Holcomb and Grainger, 2006; Marinkovic et al., 2003]. In terms of EEG/MEG signals, those activations produce a series of event‐related potentials/fields (ERP/F) consistently associated with processes sequentially ranging from early visual analysis before 150 ms [Tarkiainen et al., 2002], orthographic processing around 200 ms [Cornelissen et al., 2003; Tarkiainen et al., 2002], phonological and semantic analysis around 400 ms [Bentin et al., 1999; Kutas and Hillyard, 1980; Proverbio et al., 2004], accessing semantic memory [Kutas and Federmeier, 2000], and assessing emotional valence [Kissler et al., 2006]. Thus, ERP/ERF studies have yielded temporal coordinates for most of the subprocesses involved in individual word reading [reviewed in Salmelin, 2007]. Arguably, the main finding from EEG/MEG studies is a negative ERP/F component elicited by incongruous words in the context of a sentence, which peaks around 400 ms after word presentation [Pylkkanen and Marantz, 2003]. This so‐called N400 is probably the electrophysiological response most often associated with language processing, since it was first reported in 1980 [Kutas and Hillyard, 1980]. The N400 is observed over temporofrontal regions and appears predominantly related to depth of semantic analysis: for instance, the N400 elicited by words during sentence reading decreases with their predictability [Dambacher et al., 2006], it is sensitive to semantic priming [Chwilla et al., 1995] and smaller for abstract words than concrete words [Dhond et al., 2007]. Source analysis techniques have revealed generators of the N400 in the superior temporal lobe and the frontal and lateral prefrontal cortices [Frishkoff et al., 2004; Halgren et al., 2002; Helenius et al., 1998; Makela et al., 2001; Pylkkanen and Marantz, 2003; Pylkkanen et al., 2002; Salmelin et al., 1996; Simos et al., 1997]. More directly, sources of the N400, and of the other ERP components of reading, have also been identified with direct intracranial electrophysiological recordings in epileptic patients, providing a comprehensive view of the neural events evoked by written words [Fried et al., 1981; Guillem et al., 1995, 1999; Halgren et al., 1994a, 1994b, 2006; Heit et al., 1990; McCarthy et al., 1995; Nobre and McCarthy, 1995; Nobre et al., 1994; Ojemann and Schoenfield‐McNeill, 1999; Ojemann et al., 1989].
In addition to ERP/ERFs, the presentation of written words also evokes strong spectral energy fluctuations in EEG/MEG and intracranial EEG signals in various frequency bands ranging between 0.1 and 150 Hz. The analysis of language‐related oscillatory brain activations is still an emerging field, with relatively few studies as compared with the rich ERP/ERF literature (as acknowledged in a recent review [Bastiaansen and Hagoort, 2006]). However, analysis of cortical oscillations may prove necessary to reveal crucial aspects of the neural dynamics of reading not visible in ERP/F studies. Such oscillations have indeed been postulated as a mechanism subserving local and long‐distance neural communication between neural populations [Fries, 2005; Pulvermuller, 1999; Salmelin and Kujala, 2006; Varela et al., 2001].
Evoked changes in spectral power in response to word presentation have been reported in all the major EEG frequency bands (theta, 4–7 Hz, alpha, 8–13 Hz, beta, 15–30 Hz, and gamma, above 40 Hz). Effects observed in the alpha and beta ranges are mostly power decreases [Klimesch et al., 1997; Rohm et al., 2001], that is, an interruption of ongoing oscillatory activity [Bastiaansen et al., 2005]. In contrast, theta and gamma oscillations are usually enhanced during reading, in frequent association with memory processes [Bastiaansen et al., 2005; Fell et al., 2003; Sederberg et al., 2003; Summerfield and Mangels, 2005; Van Strien et al., 2005], but also with syntactic and semantic operations [Bastiaansen and Hagoort, 2006; Lutzenberger et al., 1994; Pulvermuller et al., 1999]. For instance, theta and gamma‐band responses equivalent to the N400 (in terms of latency and task sensitivity) have been evidenced in EEG/MEG recordings [Braeutigam et al., 2001; Hald et al., 2006; Weiss and Mueller, 2003].
Our understanding of gamma‐band oscillations, in particular, has benefited from a recent stream of intracranial EEG studies describing, with high temporal and anatomical precision, their emergence and task‐sensitivity in relation to several major brain functions such as memory [Fell et al., 2002, 2003; Howard et al., 2003; Mainy et al., 2007; Sederberg et al., 2003], visual attention and perception [Brovelli et al., 2005; Lachaux et al., 2000; Tallon‐Baudry et al., 2005; Tanji et al., 2005], motor processes [Crone et al., 1998; Lachaux et al., 2006; Pfurtscheller et al., 2003; Szurhaj et al., 2005], or specifically language [Crone et al., 2001; Sinai et al., 2005; Tanji et al., 2005]. These observations appear congruent with the suggestion, partially demonstrated in animal studies, that gamma oscillations effect local synchronization phenomena mediating communication between neighbouring neurons in brain areas critical to the process at hand [Fries, 2005].
With respect to reading, the prediction would be that rapid local neural communication via gamma‐band synchronization should be observed within all functional brain areas involved in word recognition. The high frequency of gamma oscillations renders them particularly well‐suited to subserving the rapid sequence of processes involved in word reading, which does not exceed a couple of hundreds of milliseconds in duration.
The aim of the present study was to investigate, by means of direct intracranial EEG recordings, local gamma‐band synchronization in language areas during reading and to reveal new aspects of the fine underlying spatio‐temporal neural dynamics. Our work is a direct continuation of a previous study by Crone et al. [ 2001], who reported intra‐cranially recorded gamma‐band responses to written words in the temporal lobe. From their observations, they proposed the intracranial mapping of gamma‐band activations as a new and promising approach for the study of language processes. Their results were comforted by a recent intracranial study, also based on temporal lobe recordings [Tanji et al., 2005]. The novelty of the present study is to take this approach one step further and to study gamma‐band activities associated with word recognition throughout the reading network, with intracranial EEG recordings of both frontal and temporal structures, and to evidence dissociations between activities evoked by visual, phonological, and semantic processing.
We recorded from 10 patients as they performed a classic hierarchical paradigm, emphasizing either one of the three processes studied. We measured and contrasted the energy modulations in the gamma‐band associated with each condition in order to identify brain areas evidencing gamma‐band modulations and to compare the dynamics of those gamma‐band responses across tasks and regions. Given the assumed relationship between gamma and BOLD signals [Kayser et al., 2004; Niessing et al., 2005; Lachaux et al., (in press)], our main prediction was that gamma‐band energy modulations would occur during word recognition in regions related to reading processes with fMRI. In addition, we also anticipated that the time‐course of these modulations would differ between regions, providing new information concerning the precise neural implementation of the various component processes of word recognition.
MATERIALS AND METHODS
Participants
The 10 patients (P1–P10) suffered from drug‐resistant partial epilepsy and were candidates for surgery. There were eight females and two males, aged 17–40 years (mean: 25 years). Brain magnetic resonance imaging (MRI) showed various lesions in eight patients: hippocampal sclerosis (HcS) in five patients (P1‐P2‐P4‐P5‐P6), HcS with posterior parietal atrophy in one patient (P3), HcS with temporal lobe atrophy in one patient (P9), temporal neocortical ganglioma with cortical dysplasia in one patient (P8)), and no lesion in the remaining two patients (P7‐P10). Because the location of the epileptic foci could not be identified with sufficient precision using noninvasive methods, the patients underwent intracerebral EEG recordings by means of stereotactically implanted multilead depth electrodes (SEEG) [for explanation of this methodology, see Kahane et al., 2004]. These recordings evidenced that the epileptogenic zone was left temporal in seven patients (P1‐P2‐P3‐P4‐P5‐P9), left temporal extending to the lateral temporo‐occipital junction in one patient (P8), left premotor frontal in one patient (P10), and right temporal in one patient (P6).
Implantation sites were selected on purely clinical grounds, with no reference to the present experimental protocol. However, patients were selected for this protocol because their implantation sampled regions classically associated with language and word recognition processes. The patients performed the task 4 days after the implantation of the electrodes, and all had previously given written informed consent to participate in the experiment.
Electrode Implantation
11 to 14 semi‐rigid depth electrodes (not cortical grids) were implanted per patient, in cortical areas that varied depending on the suspected origin of seizures (Fig. 1). In the 10 patients, a total number of 344 sites were recorded: 310 in the left hemisphere and 34 in the right. Each electrode had a diameter of 0.8 mm and comprised 10 or 15 leads of 2 mm length, 1.5 mm apart (Dixi, Besançon, France), depending on the target region. Thus, various mesial and lateral cortical areas were evaluated, including the sulcal cortex. The electrode contacts were identified on each individual stereotactic scheme, and then anatomically localized using the proportional atlas of Talairach and Tournoux [ 1988]. In addition, the computer‐assisted matching of a postimplantation CT‐scan with a preimplantation 3D MRI provided a direct visualization of the electrode contacts with respect to the brain anatomy of each patient (Activis, Lyon, France).
Figure 1.

Anatomical and task‐sensitivity of gamma band responses. The 3D brain plot displays the entry points of all the depth electrodes implanted in the left hemisphere (across nine patients), on a reconstruction of the mni‐single subject MRI template. Electrodes are orthogonal to this sagittal plane. Open squares show anatomical clusters where gamma band responses to visual stimuli were observed. Time‐frequency maps show representative responses for one site in each cluster (see Table II for the Talairach coordinates), in the three experimental conditions; the energy is expressed in units of the standard deviation of the [−500 ms:−100 ms] prestimulus period (a Z‐score, using the same mean and standard deviation values to normalize the three conditions). Maps show the 2 s following stimulus presentation and frequencies between 1 and 200 Hz. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Paradigm
Patients were recorded under four different experimental conditions: two control tasks (VISUAL) and (SYMBOL), a phonological task (PHONO), and a semantic task (SEMAN). In the VISUAL condition, participants were shown a series of five or six‐letter consonant strings, which could not be read according to the conventional graphotactic rules of the French language (i.e., “xwxqn”). Their task was to indicate whether the string contained the same letter twice. In the SYMBOL condition, both conditions and task were identical except that the letters were replaced by symbols unknown to the participant, namely unreadable, white, Karalyn Pattersen false font characters [examples of which can be seen in Price, 2000, page 344]. In the PHONO condition, participants had to silently read five‐ and six‐letter pseudo‐words, pronounceable according to the phonotactic conventions of the French language (i.e., “picron”) but with no obvious resemblance to known words. Their task was to decide whether the final phoneme sounded like a vowel (like “picron”, which does in French but not in English) or like a consonant (like ‘pouque’, the final “e” being silent in French). In the SEMAN task, participants had to judge whether five or six‐letter words represented living things or not. Since data analyses revealed no differences between the VISUAL and SYMBOL condition, the results of this last condition are not presented in the manuscript.
The experiment itself consisted of 12 runs. In each run, participants were presented with four blocks of stimuli, one for each condition. The order of the four conditions was counterbalanced between runs. The patients could take a short break and relax between the runs. All stimuli were presented foveally on a 17′′ computer screen, as black lower‐case letters (courier font) on a light gray background. The study used a stimulus presentation software “stimulat” designed at the Cognitive Neuroscience and Brain Imaging laboratory (LENA, CNRS UPR640). Between stimuli, a black cross‐hair fixation point was presented in the center of the screen. Each block consisted of 20 string stimuli (10 “yes” and 10 “no” answers). Strings were presented for 2000 ms, with a random interstimulus interval of between 2800 and 3200 ms. Participants were instructed to respond by pressing a key with the index finger of their left hand. Responses were recorded and task performance evaluated offline. Participants were asked not to move their tongue or lips during the tasks.
Recordings
Intracerebral recordings were obtained using an audio‐video‐EEG monitoring system (Micromed, Treviso, Italy), which allows for the simultaneous recording of 63 depth‐EEG channels sampled at 512 Hz (0.1–200 Hz bandwidth) during the experimental paradigm. The chosen reference, one of the contact sites in the white matter, had the same impedance as the other contact sites, and was located in a region with no or few electrical field sources nor was it contaminated by eye‐movement artefacts or electromyographic activity from subtle muscle contractions. Before analysis, all signals were rereferenced to their nearest neighbor on the same electrode, 3.5 mm away, yielding a bipolar montage. Recording sites showing clear epileptiform activities were excluded from analysis. Among the remaining sites, both raw and high‐pass filtered (above 15 Hz) monopolar and bipolar data were systematically inspected, and any trial showing epileptic spikes in was discarded.
Time‐frequency analysis
For each single trial, bipolar derivations computed between adjacent electrode contacts were analyzed in the time‐frequency (TF) domain by convolution with complex Gaussian Morlet's wavelets [Tallon‐Baudry et al., 1996] thus providing a TF power map
 , where w(t,f) was, for each time t and frequency f, a complex Morlet's wavelet
, where w(t,f) was, for each time t and frequency f, a complex Morlet's wavelet  , with
, with  and
and  and
and  a function of the frequency f:
a function of the frequency f: 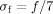 .
.
Significant stimuli‐evoked spectral modulations were evidenced by use of a Wilcoxon nonparametric test which compared the total energy in a given time‐frequency “tile” during the trials with that of a tile of similar frequency extent recorded during a prestimulus baseline period (from −500 to −100 ms) (in this study, to detect gamma responses, the typical frequency extent of tiles was 40–200 Hz). Significant responses threshold was set at P‐values of less than 0.001.
Comparisons between TF responses measured in two different conditions (e.g. in response to words in the SEMAN condition versus in response to pseudo‐words in the PHONO condition) were carried out by means of a Mann‐Whitney nonparametric analysis, using the raw time‐frequency values of energy for a set of time‐frequency tiles (100 ms × 30 Hz) covering a (0: 2,000 ms) × (5: 200 Hz) domain (one test per tile comparing the values obtained for all the trials in the two conditions).
Note that EEG signals were evaluated with the software package for electrophysiological analysis (ELAN‐Pack) developed in the INSERM U821 laboratory.
RESULTS
As shown in Table I, the percentage of correct responses was generally high in all three tasks (SEMAN: 94.8% (std = 5.0); PHONO: 76.9% (24.2); VISUAL: 92.1% (4.1)). The PHONO task proved to be the hardest. An analysis of reaction times showed that in general responses were faster in the SEMAN task (1,027 ms (495 ms)), followed by the PHONO task (1,227 ms (428 ms)) and the VISUAL task (1,365 ms (415 ms)).
Table I.
Behavioral results for the 10 patients in the three conditions
| Patient | Sem (% c.r.) | Pho (% c.r.) | Vis (% c.r.) | Sem (RT) | Pho (RT) | Vis (RT) |
|---|---|---|---|---|---|---|
| P1 | 100 | 65 | 86 | 660 (90) | 1,137 (305) | 1,037 (448) |
| P2 | 90 | 94 | 92 | 982 (271) | 1,180 (311) | 1,346 (308) |
| P3 | 95 | 95 | 95 | 876 (161) | 1,141 (288) | 1,168 (300) |
| P4 | 95 | 85 | 90 | 995 (484) | 1,410 (578) | 1,334 (483) |
| P5 | 100 | 95 | 90 | 743 (145) | 788 (208) | 1,298 (416) |
| P6 | 89 | 50 | 89 | 799 (266) | 1,057 (221) | 872 (256) |
| P7 | 100 | 95 | 95 | 850 (171) | 1,349 (295) | 1,334 (312) |
| P8 | 89 | 70 | 95 | 740 (125) | 1,306 (294) | 1,233 (325) |
| P9 | 90 | 25 | 89 | 1,284 (519) | 661 (86) | 1,634 (669) |
| P10 | 100 | 95 | 100 | 2,345 (92) | 2,247 (559) | 2,397 (123) |
c.r., correct response; RT, mean reactions times in ms (standard deviation are shown in parenthesis).
Time‐frequency analysis of intracerebral EEG signals revealed that consonant strings induced transient and focal energy variations in a broad (40–150 Hz) gamma frequency range (Fig. 2) (Significant energy variations were detected using a Wilcoxon nonparametric comparison with a [500 ms:−100 ms] prestimulus period, see methods). These energy modulations occurred in a limited number of recording sites, clustered in specific brain areas including the anterior and middle portions of the superior temporal gyrus (STG), inferior frontal gyrus (IFG), the ventral and dorsal lateral prefrontal cortex, and the fusiform gyrus (see Table II for the list of activated sites with their Talairach coordinates).
Figure 2.

Mode of graphical representation of the gamma band responses. This figure explains the mode of display used in this article to depict stimulus‐induced energy modulations and their differences across experimental conditions. The three curves (named S, P, and V) were obtained by averaging in the frequency domain TF maps such as in Figure 1, in the gamma band, for conditions Seman, Phono, and Visual respectively. Those three curves are superimposed over three statistical TF maps, showing TF regions with statistical differences between conditions (Kruskal‐Wallis, P < 0.001) (white “clouds”) in the gamma band. The top (resp. middle and bottom) TF map shows significant differences between the Seman and Phono conditions (resp. Seman and Visual, and Phono and Visual). Gray vertical lines indicate latencies, with one short tick every 250 ms and one long tick every 500 ms (from 0 to 200 ms following stimulus presentation).
Table II.
| Patient no. | Location | Hemisphere | x | y | z | IES |
|---|---|---|---|---|---|---|
| IFG | ||||||
| P1 | R'8 | L | −62 | 0 | 19 | No |
| P2 | R'8 | L | −55 | 4 | 23 | Yes |
| P3 | R'7 | L | −57 | 0 | 17 | N/A |
| P3 | Q'6 | L | −50 | 23 | 11 | Yes |
| P4 | Q'7 | L | −51 | 12 | 9 | No |
| P7 | R'6 | L | −64 | 5 | 19 | Yes |
| DLPFC | ||||||
| P4 | G'12 | L | −46 | 38 | 12 | No |
| P6 | G14 | R | 47 | 28 | 19 | No |
| P7 | G'14 | L | −56 | 37 | 12 | No |
| P9 | Y'14 | L | −22 | 44 | 18 | No |
| VLPFC | ||||||
| P3 | O'7 | L | −30 | 46 | −11 | No |
| P3 | G'12 | L | −47 | 37 | −5 | No |
| P4 | O'9 | L | −37 | 49 | −6 | No |
| P5 | O'6 | L | −25 | 39 | −14 | No |
| P5 | G'13 | L | −51 | 36 | 6 | No |
| P7 | O'12 | L | −49 | 49 | 0 | No |
| Anterior STG | ||||||
| P2 | T'6 | L | −56 | −8 | −1 | Yes |
| P5 | T'6 | L | −60 | −4 | −4 | No |
| P10 | T'6 | L | −67 | −8 | 5 | N/A |
| Posterior STG | ||||||
| P8 | U'9 | L | −60 | −42 | 12 | No |
| P9 | U'9 | L | −63 | −26 | 11 | Yes |
| Fus. Gyrus | ||||||
| P2 | F'10 | L | −53 | −49 | −7 | No |
| P6 | F2 | R | 27 | −58 | −4 | No |
| P8 | F'2 | L | −25 | −51 | −9 | No |
| P10 | F'8 | L | −59 | −57 | −8 | N/A |
x, y, and z refer to the Talairach coordinates (not the MNI coordinates) of the sites, in millimeters. These values were converted to MNI coordinates to plot the sites onto the MNI single subject MRI (IFG, inferior frontal gyrus; BA 44, 45, 47; DLPFC, dorsolateral prefrontal cortex (BA 9, 10, 46); VLPFC, ventrolateral prefrontal cortex (BA 11); STG, superior temporal gyrus (BA 22); Fus. Gyrus, fusiform gyrus); IES, interference of intracranial electrical stimulations (50 Hz to 3 mA) with language functions (yes/no or not available).
As can be seen in Figure 1, the strength and timing of responses varied across experimental conditions, in an anatomically‐dependant fashion. The analysis was focused on sites showing different gamma‐band responses when word‐like stimuli (real words presented during PHONO and SEMAN conditions) were contrasted with nonword‐like stimuli (consonant strings presented during VISUAL condition), that is, sites showing significant differences in gamma energy for the PHONO versus VISUAL contrast, and for the SEMAN versus VISUAL contrast (those sites as identified by two nonparametric Kruskal‐Wallis TF statistical tests corresponding to each contrast).
Figures 3, 4, 5, 6, 7, 8 illustrate gamma energy modulations in five anatomical clusters where word‐like stimuli elicited a specific response. Energy time‐profiles and statistical differences between conditions are displayed in the [40–150 Hz] range as per the representation convention outlined in Figure 2. As is apparent in these figures, time‐profiles were consistent within clusters, despite the fact that they were recorded in different individuals and from slightly different anatomical locations.
Figure 3.

Gamma‐band responses observed in the IFG. The displays follow the organization explained in Figure 2. The anatomical picture shows the locations of the corresponding sites reconstructed on the MNI single‐subject MRI.
Figure 4.

Gamma‐band responses observed in the dorsal lateral prefrontal cortex. The displays follow the organization explained in Figures 2 and 3. The two bottom graphs represent energy time courses averaged relative to the patients responses (“button press”) to illustrate the activity preceding that event.
Figure 5.

Gamma‐band responses observed in the Ventral Lateral Prefrontal Cortex. The displays follow the organization explained in Figures 2 and 3.
Figure 6.

Gamma‐band responses observed in the Ventral Lateral Prefrontal Cortex. The displays on the left follow the organization explained in Figures 2 and 3. The three displays on the right show the Evoked Potentials for the same conditions in the corresponding sites (same color code as for the left graphs, potentials expressed in microVolts).
Figure 7.

Gamma‐band responses observed in the middle STG. The displays follow the organization explained in Figures 2 and 3.
Figure 8.

Gamma‐band responses observed in the Fusiform gyrus. The displays follow the organization explained in Figures 2 and 3.
The majority of sites in the IFG showed a common pattern of activation (Fig. 3) displaying particularly strong activation in the PHONO condition. This activation reached a peak around 400 ms poststimulus followed by a progressive decay over the next second and a half. This pattern did not occur in the VISUAL condition where energy values were significantly lower than for the PHONO condition at all sites. Furthermore, the response reached higher values in the PHONO condition than in the SEMAN condition at five sites (P1, r'8; P4 q'7; P2 r'8; P7 r'6; P3 r'7), either at the peak or during the return to baseline level. Response was not systematic in the SEMAN condition, where responses exceeded the VISUAL condition at only two of the previously quoted sites (P7 r'6 and P3 r'7). Interestingly, one additional IFG site (P3 q'6) showed an opposite response pattern, with a strong peak around 400 ms during the SEMAN condition and no equivalent activity during the PHONO or VISUAL conditions. This activation, the strongest we observed in the one patient implanted at this site, was markedly different to the activity recorded just 20 mm posterior in the same subject (P3 r'7).
Strong gamma energy modulations were also observed in more anterior sites distributed within the left and right dorsolateral prefrontal cortex (DLPFC) (P4, g'12; P6, g14; P7, g'14; P9, y'14; Fig. 4). Although the effects of the different conditions on these modulations were not consistent across patients, reproducible response profiles were observed at three sites (P4, g'12; P6, g14; P9, y'14), with an early and steep increase within the first 400 ms following stimulus presentation for all conditions, followed by decreases of various slopes, according to task. To investigate whether these differences in slope could be attributed to the differences in reaction time between conditions, we computed the mean temporal profile of the gamma‐band energy averaged with respect to the patient's responses (instead of the stimulus). As illustrated in Figure 4, this proved to be the case for two sites: evoked gamma energy returned to baseline levels at the latency of the subject's response, with a similar profile for all three conditions. Interestingly, the patient with the earliest peak in the gamma response (P6) was found to have the fastest response times, at least in the visual condition (Table I).
Responses elicited by the consonant strings were very different in the Ventrolateral Prefrontal Cortex, VLPFC (P3, o'7 and g'12; P4, o'9; P5, o'6 and g'13; P7, o'12; Fig. 5). In this large region, and during each condition, stimulus presentation induced a decrease in gamma energy, following a very consistent time course across the six recording sites; to wit an initial decrease, peaking at around 500 ms, followed by a gradual return to baseline levels within the next 500 ms. In some of these sites, the amplitude of the decrease varied across conditions (Fig. 5) but such effects were inconsistent across recording sites.
In the temporal lobe, we observed three clusters of gamma responses. In the anterior portion of the STG, three sites from three patients (P2, t'6; P5, t'6; P10, t'6; Fig. 6) generated consistently reproducible response patterns, specifically in the SEMAN and PHONO conditions, with no significant difference between these two conditions. Gamma‐band energy rose from baseline levels after 200 ms to reach a maximum at around 400 ms, followed by a rapid return to baseline within the next 300 ms. These responses were specific to word‐like stimuli and were not observed during the VISUAL condition. More posterior, in the middle portion of the STG, we observed similar response patterns during SEMAN condition for two patients (but not during VISUAL condition) (P8, u'9; P9, u'9); Fig. 7). However, the two sites displayed markedly different effects during the PHONO condition: while response profile at one site (P8, u'9) reproduced the response observed in the IFG sites, gamma‐band response was virtually absent at the other site (P9, u'9) and did not differ from the VISUAL condition response. It is important to note that this last patient had not performed the PHONO task correctly (25% correct responses).
Finally, all stimuli generated strong gamma responses within associative visual regions with time‐profiles characteristic (in three out of the four patients) of these regions (Fig. 8). These responses, originating from the left and right fusiform gyri, were characterized by an abrupt increase reaching maximal energy around 200–300 ms, followed by gradual decay. In three instances (P2, f'10; P8, f'2; P10, f'8), we observed that the peak amplitudes of these responses were stronger in the SEMAN and PHONO conditions than in the VISUAL condition. Also, in two inferior temporal gyrus sites, one in the right (P6, f2) and another in the left (P8, f'2) hemisphere, response was more prolonged in the VISUAL condition, suggesting that this task required more in depth or longer visual inspection of the character strings. Interestingly, the response patterns in one of these two sites (P6, f2) mirrored the temporal patterns observed within DLPFC (P6, g14; Fig. 4) in the same patient.
Note that, although this study focused solely on the (40–150 Hz) frequency band, responses in the lower frequency bands and ERPs were also systematically studied. The dominant effects in the lower frequency ranges were energy decreases in the alpha (8–12 Hz) and beta (12–30 Hz) ranges (often found in cognitive paradigms and termed event‐related desynchronizations). As is often the case [Brovelli et al., 2005], these event‐related desynchronizations were observed concurrently with gamma‐band energy increases (see Fig. 1). Although a detailed analysis of the low frequency responses would extend beyond the scope of this paper, the amplitude of observed variations in decreases across conditions appeared less systematic than variations in gamma‐band responses. Statistical comparisons of the ERPs across the different conditions revealed a large variety of effects, distributed in all recorded brain areas (but not necessarily in all recorded areas of each individual patient) including the amygdala and the hippocampus. In general, ERP responses lacked the reproducibility of gamma‐band responses. For comparison purposes, Figure 6 displays the most reproducible ERPs observed. This figure illustrates the common observation that effects observed in the ERPs and in the gamma‐band are related, but not identical.
DISCUSSION
Our hypothesis was that word recognition is associated with transient and local neural synchronization in the gamma frequency range, within those language‐functional brain areas previously described in fMRI and PET studies. We largely confirmed this hypothesis, showing reproducible task‐sensitive gamma‐band responses in several of these areas, including the inferior temporal, superior temporal, and inferior frontal cortices. Therefore, this study was successful in directly observing some of the actual neural phenomena underlying reading, traces of which had been only indirectly measured with functional neuroimaging.
The reproducibility of those observed gamma‐band responses, both in terms of timing and task‐sensitivity, makes it possible to reach robust conclusions regarding the dynamics of neural activations within the word recognition network, provided a clear understanding of how the tasks used in this study relate to the various cognitive processes involved in reading.
Task Analysis
These processes include (a) low and high level visual processing (letter and letter strings identification) and orthographic processing (of visual words and word‐like forms), (b) phonological processing (conversion of graphemic representations into corresponding phonological representations and phono‐articulatory programs), and (c) semantic processing (access to and activation of lexical representations) [Herbster et al., 1997; Joubert et al., 2004]. Our main contrast (SEMAN vs. PHONO) compared a semantic category decision with a phonological decision task, and was chosen to maximize the separation between semantic and phonological processing.
Two elements must be considered when interpreting this contrast: first, that implicit or explicit semantic and phonological processing are likely to occur during both tasks [Binder et al., 2003; Price et al., 1997], and second, that the two conditions used different stimuli (words in the semantic task and pseudo‐words in the phonological task). This choice was deliberate and aimed to minimize automatic semantic processing during the phonological task. Still, one should bear in mind that normal adult readers have two different cognitive strategies to convert written words and pseudo‐words into their corresponding phonological forms (thus possibly two different networks or “routes” [Coltheart et al., 2001; Jobard et al., 2003]). A global, whole‐word recognition route is predominantly involved in processing usual words, while the other involves an analytical procedure based on grapheme to phoneme conversion rules and is predominantly used in the processing of novel or pseudo‐words [Proverbio et al., 2004]). Thus, the SEMAN condition should recruit the global route to access and activate lexical representations while the PHONO condition should implicate the analytical route of grapho‐phonological conversion specific to pseudo‐words and phonological‐articulatory processing.
In the light of this analysis, responses triggered by each task can be discussed region by region both (a) individually, better to understand the dynamics of both word processing and pseudo‐word phonological analysis networks, and (b) in comparison with each other, better to evidence similarities and differences in their spatio‐temporal organization.
Pars Opercularis of Broca's Area
The most reliable gamma responses were found in the posterior part of the left IFG (pars opercularis of Broca's area). Gamma power was stronger and a faster return to baseline was observed, in the PHONO condition than in the other conditions, in line with previous reports of stronger IFG responses to pseudo‐words than to words (e.g. [Dietz et al., 2005]).
This frontal activation appears related to the grapho‐phonological conversion of word‐like stimuli, the distinctively specific process of the PHONO condition. This is consistent with several neuroimaging studies associating clearly the pars opercularis of Broca's area with phonological processing and/or pseudo‐words reading [Demonet et al., 2005; Fiez, 1997; Fiez and Petersen, 1998; Poldrack et al., 1999; Zatorre et al., 1996]. However, as a response was also evoked in the SEMAN condition, it appears this region is not solely devoted to the phonological conversion of pseudo‐words. This finding, and the similar patterns of responses found for words and pseudo‐words in other brain regions (with the exception of Broca pars triangularis, see below), is in line with a recent meta‐analysis of 35 PET and fMRI studies [Jobard et al., 2003] evidencing that the grapho‐phonological conversion of words and pseudo‐words implicate largely overlapping networks.
The posterior part of Broca's area is recruited by all pronounceable letter strings, possibly in relation to subvocal articulatory processes, or to the formation of phonological forms. In support of both hypotheses, covert articulatory rehearsal has been shown to activate regions in the left precentral gyrus adjacent to, or overlapping with, the posterior part of Broca [Fiez, 1997]. Congruently with this, in the VISUAL condition, nonpronounceable consonant strings yielded no response. Both interpretations also fit with the longer response times observed in the PHONO condition because (a) sustained mental pronunciation was encouraged in that condition by presentation times of up to 1500 ms for six letters pseudo‐words and (b) according to the dual‐route model, grapho‐phonological conversion is longer for words than for pseudo‐words. Recent findings from our group support the putative correspondence between gamma activations in the pars opercularis of Broca's area and the formation of phonological forms: in a separate study [Mainy et al., 2007] in which a different group of patients had to remember short lists of letters over the short‐term (a Sternberg verbal memory paradigm), we found a steady energy increase in the gamma‐band as the letters, presented sequentially, were stored in memory. This finding was interpreted, in line with Baddeley's working memory model [Baddeley, 2003], as a direct trace of the phonological loop serving to rehearse mentally a list of verbal items. If pseudo‐words are read as lists of syllables, phonological conversion should involve the phonological loop and therefore gamma activity in the pars opercularis of Broca's area. Further, gamma‐band increases appeared in response to the first letter of the list, possibly explaining why, in the present study, words which are not thought to be converted as lists of multiple syllables also triggered a gamma‐band response in this region. Such gamma‐band responses to words would suggest a fixed timing window for grapho‐phonological conversion of words of between 400 and 1000 ms postword presentation. Although speculative, our interpretation is sufficiently precise to predict that long pseudo‐words should trigger a longer gamma‐band response in the pars opercularis of Broca's area than short ones, while long and short words should yield responses of equal duration (like monosyllabic pseudo‐words).
A further prediction of our interpretation is that IFG gamma‐band responses should be observed simultaneously with responses in other brain regions known to be involved in inner speech. Auditory areas in the STG the planum temporale in particular have been shown to be active during the silent rehearsal of speech or silent language tasks on words [Buchsbaum et al., 2005; Hickok et al., 2003]. Indeed, we found a response pattern in the auditory cortex of patient P8 (u'9) similar to that observed in several other patients in the IFG, which results are consistent with a frontal‐temporal network underlying conversion into phonological representation and inner speech.
Pars Triangularis of Broca's Area
An increasing number of neuroimaging studies suggest that the most anterior part of Broca's area (pars triangularis) is preferentially involved in semantic processing rather than phonological processing as is the posterior part [i.e. Demb et al., 1995; Devlin et al., 2003; Vigneau et al., 2005]. It has been suggested that, during sentence comprehension, word information might be retrieved and combined into larger units in that region [Hagoort, 2005]. Data from patient P3 provide a striking support for this semantic‐phonology distinction within Broca's area, with one site in the anterior part of Broca's area (q'6) responding only in the SEMAN condition and a second site 2 cm more posterior (r'7) responding more strongly to the PHONO condition. Interestingly, the response to words (in the SEMAN condition) occurred at similar latencies (with a peak around 400 ms) in the two sites, indicating parallel semantic and phonological processes, as suggested by previous studies [Salmelin, 2007].
Dorsal and Ventral Lateral Prefrontal Cortex
Gamma‐band responses were also observed bilaterally in all conditions in the dorsal part of the prefrontal cortex (DLPFC), anterior to Broca's area. The activity was similar across all conditions, and determined by the timing of the participant's mental effort, since return to baseline level occurred when participants gave their response after completing the visual, phonological or semantic analysis tasks. We suggest the timing and nonspecificity of this sustained activity is compatible with a role in the attribution of nonspecific attention, as proposed for instance by Burgund et al. [ 2003]. The anatomical origin of those responses also coincides with a region (Talaraich: –50, 27, 13) found active during the inhibition of emotional distraction, in a study of the effect of distraction by emotional pictures during a working memory task [Dolcos and McCarthy, 2006]. Consistent with this interpretation, in the VISUAL condition which required a detailed and sustained visual analysis of the strings, we observed in one patient (P6) that the time course of the gamma response in the lateral prefrontal site (g14) mirrored the time course of the response observed in the ventral visual pathway (f2).
There was a sharp dissociation between these activations and a strong gamma deactivation observed slightly more ventrally in the ventral lateral prefrontal cortex (VLPFC) around 500 ms. To our knowledge, this is the first report of task‐induced gamma‐band deactivations in a high‐order brain structure. Previously, such deactivations have been reported in the periphery of the primary visual cortex in response to visual stimuli presented foveally [Lachaux et al., 2005]. The exact functional role of the deactivation observed in the present study remains unclear. In fMRI studies, it has been suggested that deactivations correspond to the attenuation of neural activity in regions presumably supporting processes unrelated or irrelevant to the task at hand [Fox et al., 2005]. This portion of the left VLPFC has been repeatedly associated with semantic memory processes [Fletcher and Henson 2001; Otten et al., 2001]. So why would there be a deactivation in tasks, which may involve semantic analysis? Moreover, why would this deactivation be transient, and not sustained like the more dorsal activation? Quite speculatively, we offer that this region may act as a semantic memory buffer. The presentation of new items might then trigger a reflex reset of the semantic buffer, prior to its updating with the newly presented item. A consequently testable prediction is that normal subjects would perform word recognition tasks better after a transient inactivation of this portion of the VLPFC, for instance with Trancranial Magnetic Stimulation, within the first 500 ms after word presentation.
Inferior Temporal Lobe
Finally, we observed gamma‐band responses in the mid basal temporal cortex and a functional dissociation between mesial and lateral sites, with response in mesial sites P6 P8 (f2 f'2) starting earlier, at around 150 ms and showing no initial difference between word‐like and nonword‐like stimuli at this latency, and lateral responses peaking after 200 ms and being stronger for word‐like stimuli.
This apparent propagation from medial to lateral part in the inferior temporal lobe reproduced previous MEG observations [Pammer et al., 2004]. Our results are also consistent several ERPs/fields studies reporting a left lateralized occipito‐temporal activation around 150 ms that does not differentiate between words, non words and consonant strings [Cornelissen et al., 2003; Salmelin et al., 1996; Wydell et al., 2003]. This region has been proposed to be the first stage of visual analysis specific to letter strings [Tarkiainen et al., 2002].
The lateral differentiation, in the mid fusiform gyrus, between word‐like and non word‐like stimuli after 200 ms is also in line with previous non invasive studies, showing a bilateral occipito‐temporal activation at about 200 ms postword presentation [Cornelissen et al., 2003; Pammer et al., 2004; Salmelin et al., 1996, 2000]. A similar effect, congruent with respect to timing and location has previously been reported from intracranial recordings [Nobre et al., 1994]; in particular a gamma‐band response to written words has been already reported in the same region and at the same latency by Crone et al. [Crone et al., 2001].
This mid fusiform region has been proposed to be a “Visual Word Form Area” (Talairach coordinates: [–43,–54,–12]) involved in prelexical [Cohen et al., 2000] and possibly early phonological processing of words and word‐like stimuli [Dietz et al., 2005]. Indeed,1 this region has been shown to respond to meaningless letter strings as a function of their approximation to conventional English orthography [Binder et al., 2006] and lesions of this area have been observed to produce selective reading deficits [Gaillard et al., 2006] [but see Devlin et al., 2006; Mechelli et al., 2003]. In our study, words and pseudo‐words elicited similar responses in this area, confirming that it is not solely devoted to processing words. In that sense, we failed to confirm that the inferior temporal gyrus sustains semantic associations related to words and objects, as suggested by several studies [McCarthy et al., 1995; Nobre and McCarthy, 1995; Thompson‐Schill et al., 1999], but this apparent discrepancy may be due to differences in recording position.
Superior Temporal Lobe
The lateral mid‐fusiform responses we found were mirrored by gamma activations in the anterior part of the STG. These gamma‐band responses were also specific to word‐like stimuli (in the SEMAN and PHONO conditions), with no response to consonant strings (VISUAL condition). Their timing reproduced the midfusiform responses with a 100 ms delay. Such parallelism of responses could provide support to the recent suggestion by Cohen et al [Cohen et al., 2004] that this region (Talairach: –60, –8, –4) might serve as a functional homolog of the Visual Word Form Area in the auditory stream, that is, a region responding selectively to speech‐like auditory stimuli (two out of three recording sites fell within 5 mm of the coordinates provided in the Cohen et al. study while the third site was 1 cm away). This interpretation matches well with the known implication of this area in speech perception, demonstrated in situations contrasting vocal vs. non vocal sounds [Belin et al., 2000, 2002], or human vs. animal vocal sounds [Fecteau et al., 2004]. The observed responses would then evidence reactivity to covert speech.
This interpretation is in apparent discordance with several EEG/MEG studies locating a sustained activation at 200–600 ms differentiating between words and nonwords in the STG [Salmelin et al., 1996; Wilson et al., 2005; Wydell et al., 2003], presumably associated with reading comprehension, rather than covert speech and related to the N400 [Halgren et al., 2002; Helenius et al., 1998; Pylkkanen and Marantz, 2003; Pylkkanen et al., 2002; Simos et al., 1997]. This discordance may simply be due to the fact that intracranial electrodes in our study largely undersampled this brain region and may have missed semantic functional structures. Alternatively, the N400 component may in fact include a contribution from the inferior frontal lobe (where we observed a clear semantic‐phonological dissociation) unseen by the source reconstruction technique used in those studies (dipole modeling). Indeed, when two different methods of MEG source localization (dipole vs. distributed source modeling) were used to reconstruct the generators of this semantic‐specific N400 component, generators in the inferior frontal lobe were found with the later method, but not with the former [Halgren et al., 2002].
SUMMARY
In conclusion, we believe that the global pattern of gamma‐band responses lends itself to the following summary (see supplementary Fig. 2): after low‐level visual analysis common to all stimuli, the midfusiform gyrus differentiates between word‐like and nonword‐like stimuli at around 200 ms poststimulus presentation. Semantic and phonological processing occur maximally 200 ms later, around 400 ms, at least partly in the anterior and posterior portions of Broca's area respectively, with longer grapho‐phonological conversion for pseudo‐words than for words. Simultaneously, neuronal populations in the mid and anterior STG would activate transiently in relation to mental pronunciation. Finally, all stimuli trigger a two‐pronged response pattern in the lateral prefrontal cortex, with a reflex deactivation of its more ventral part, peaking at 500 ms postpresentation, and a sustained dorsal activation throughout the task possibly corresponding to a nonspecific allocation of attention. Most of these activations largely overlap in time, in agreement with previous EEG/MEG findings [e.g. Pammer et al., 2004].
This summary does not reveal the dynamic of the entire word recognition network, since large parts of the brain were left unexplored, and since some of the explored regions were sparsely sampled. However, our findings were remarkably consistent across patients, which was surprising given the well‐known inter‐subject variability of the language systems [Sinai et al., 2005]; and clearly compatible with previous EEG/MEG and functional imaging studies. The timing of the activations we observed is compatible with the dynamics identified in EEG and MEG studies [Salmelin, 2007; Salmelin and Kujala, 2006], although gamma‐band responses and ERP/Fs comparisons should be done with care, as both types of responses correspond to different neural mechanisms [Tallon‐Baudry et al., 2005]. The compatibility of gamma‐band activation results with fMRI functional maps is no longer a surprise, considering recent but replicated findings of strong correlations between the BOLD signal and gamma‐band electrophysiological activity in both human and monkey [Kayser et al., 2004; Logothetis et al., 2001; Niessing et al., 2005]. Indeed, we established the spatial correspondence between gamma‐band and BOLD activations in this particular paradigm in three patients recorded in the same task in both fMRI and SEEG [Lachaux et al., (in press)]. This indicates that our study results may be equivalent to a time‐resolved fMRI study of word recognition. The finer temporal information gained from this study enabled us not only to infer cerebral activation dynamics, but also to distinguish between alternative functional interpretations. Temporal information also allows to test for potential interactions between distant brain regions [Pulvermuller, 1999; Salmelin and Kujala, 2006], but none were found in the present context, at least not in the form of phase‐synchronization in the gamma‐band [Lachaux et al., 2002], possibly because the methods used to detect phase‐synchrony are not well‐suited for wide‐band gamma‐band responses. Nevertheless, our results convincingly demonstrate the usefulness of mapping task‐induced spectral energy modulations of intracranial EEG signals for fine functional brain mapping. This approach, we termed dynamic spectral imaging, is still in its infancy, but it has great potential in further identifying the fine functions carried by brain tissues, not least when evaluating the risk of neurological damage following surgery for intractable epilepsy [Sinai et al., 2005].
Supporting information
Additional Supporting Information may be found in the online version of this article.
Figure S1: Event related potentials (ERPs) for the selected clusters represented on figure 1. The ERPs shown for each anatomical cluster correspond to the same data used to compute the TF representations in figure 1. Colors code for the three experimental conditions.
Figure S2: schematic time‐line of gamma band responses within the word recognition network. Colored lines indicate the timing of gamma activation (or deactivation) for the three conditions (blue = Seman, red = Phono, green = Visual). Faded colors indicate strongly reduced responses. Those should be taken as timing indications only and do not exclude contributions from brain regions not recorded in the present study.
Acknowledgements
We thank Valérie Balle, Patricia Boschetti, Carole Chatelard, Véronique Dorlin, Eliane Gamblin, Martine Juillard for their invaluable help. Laurent Hugueville designed the stimulus presentation software used in this study. Jessica Foxton gave very precious comments on an earlier version of this manuscript.
REFERENCES
- Baddeley A ( 2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4: 829–839. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M,Hagoort P ( 2006): Oscillatory neuronal dynamics during language comprehension. Prog Brain Res 159: 179–196. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC,van der Linden M,Ter Keurs M,Dijkstra T,Hagoort P ( 2005): Theta responses are involved in lexical‐semantic retrieval during language processing. J Cogn Neurosci 17: 530–541. [DOI] [PubMed] [Google Scholar]
- Belin P,Zatorre RJ,Lafaille P,Ahad P,Pike B ( 2000): Voice‐selective areas in human auditory cortex. Nature 403: 309–312. [DOI] [PubMed] [Google Scholar]
- Belin P,Zatorre RJ,Ahad P ( 2002): Human temporal‐lobe response to vocal sounds. Brain Res Cogn Brain Res 13: 17–26. [DOI] [PubMed] [Google Scholar]
- Bentin S,Mouchetant‐Rostaing Y,Giard MH,Echallier JF,Pernier J ( 1999): ERP manifestations of processing printed words at different psycholinguistic levels: Time course and scalp distribution. J Cogn Neurosci 11: 235–260. [DOI] [PubMed] [Google Scholar]
- Binder JR,Medler DA,Westbury CF,Liebenthal E,Buchanan L ( 2006): Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage 33: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR,McKiernan KA,Parsons ME,Westbury CF,Possing ET,Kaufman JN,Buchanan L ( 2003): Neural correlates of lexical access during visual word recognition. J Cogn Neurosci 15: 372–393. [DOI] [PubMed] [Google Scholar]
- Braeutigam S,Bailey AJ,Swithenby SJ ( 2001): Phase‐locked gamma band responses to semantic violation stimuli. Brain Res Cogn Brain Res 10: 365–377. [DOI] [PubMed] [Google Scholar]
- Brovelli A,Lachaux JP,Kahane P,Boussaoud D ( 2005): High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage 28: 154–164. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR,Olsen RK,Koch PF,Kohn P,Kippenhan JS,Berman KF ( 2005): Reading, hearing, and the planum temporale. Neuroimage 24: 444–454. [DOI] [PubMed] [Google Scholar]
- Burgund ED,Lugar HM,Miezin FM,Petersen SE ( 2003): Sustained and transient activity during an object‐naming task: A mixed blocked and event‐related fMRI study. Neuroimage 19: 29–41. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ,Brown CM,Hagoort P ( 1995): The N400 as a function of the level of processing. Psychophysiology 32: 274–285. [DOI] [PubMed] [Google Scholar]
- Cohen L,Jobert A,Le Bihan D,Dehaene S ( 2004): Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage 23: 1256–1270. [DOI] [PubMed] [Google Scholar]
- Cohen L,Dehaene S,Naccache L,Lehericy S,Dehaene‐Lambertz G,Henaff MA,Michel F ( 2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123(Part 2): 291–307. [DOI] [PubMed] [Google Scholar]
- Coltheart M,Rastle K,Perry C,Langdon R,Ziegler J ( 2001): DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev 108: 204–256. [DOI] [PubMed] [Google Scholar]
- Cornelissen P,Tarkiainen A,Helenius P,Salmelin R ( 2003): Cortical effects of shifting letter position in letter strings of varying length. J Cogn Neurosci 15: 731–746. [DOI] [PubMed] [Google Scholar]
- Crone NE,Miglioretti DL,Gordon B,Lesser RP ( 1998): Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event‐related synchronization in the gamma band. Brain 121(Part 12): 2301–2315. [DOI] [PubMed] [Google Scholar]
- Crone NE,Hao L,Hart J Jr,Boatman D,Lesser RP,Irizarry R,Gordon B ( 2001): Electrocorticographic gamma activity during word production in spoken and sign language. Neurology 57: 2045–2053. [DOI] [PubMed] [Google Scholar]
- Dambacher M,Kliegl R,Hofmann M,Jacobs AM ( 2006): Frequency and predictability effects on event‐related potentials during reading. Brain Res 1084: 89–103. [DOI] [PubMed] [Google Scholar]
- Demb JB,Desmond JE,Wagner AD,Vaidya CJ,Glover GH,Gabrieli JD ( 1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet JF,Thierry G,Cardebat D ( 2005): Renewal of the neurophysiology of language: Functional neuroimaging. Physiol Rev 85: 49–95. [DOI] [PubMed] [Google Scholar]
- Devlin JT,Matthews PM,Rushworth MF ( 2003): Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 15: 71–84. [DOI] [PubMed] [Google Scholar]
- Devlin JT,Jamison HL,Gonnerman LM,Matthews PM ( 2006): The role of the posterior fusiform gyrus in reading. J Cogn Neurosci 18: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP,Witzel T,Dale AM,Halgren E ( 2007): Spatiotemporal cortical dynamics underlying abstract and concrete word reading. Hum Brain Mapp 28: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz NA,Jones KM,Gareau L,Zeffiro TA,Eden GF ( 2005): Phonological decoding involves left posterior fusiform gyrus. Hum Brain Mapp 26: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F,McCarthy G ( 2006): Brain systems mediating cognitive interference by emotional distraction. J Neurosci 26: 2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S,Armony JL,Joanette Y,Belin P ( 2004): Is voice processing species‐specific in human auditory cortex? An fMRI study. Neuroimage 23: 840–848. [DOI] [PubMed] [Google Scholar]
- Fell J,Klaver P,Elger CE,Fernandez G ( 2002): The interaction of rhinal cortex and hippocampus in human declarative memory formation. Rev Neurosci 13: 299–312. [DOI] [PubMed] [Google Scholar]
- Fell J,Klaver P,Elfadil H,Schaller C,Elger CE,Fernandez G ( 2003): Rhinal‐hippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? Eur J Neurosci 17: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Fiez JA ( 1997): Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp 5: 79–83. [PubMed] [Google Scholar]
- Fiez JA,Petersen SE ( 1998): Neuroimaging studies of word reading. Proc Natl Acad Sci USA 95: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC,Henson RN ( 2001): Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124(Part 5): 849–881. [DOI] [PubMed] [Google Scholar]
- Fox MD,Snyder AZ,Vincent JL,Corbetta M,Van Essen DC,Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I,Ojemann GA,Fetz EE ( 1981): Language‐related potentials specific to human language cortex. Science 212: 353–356. [DOI] [PubMed] [Google Scholar]
- Fries P ( 2005): A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480. [DOI] [PubMed] [Google Scholar]
- Frishkoff GA,Tucker DM,Davey C,Scherg M ( 2004): Frontal and posterior sources of event‐related potentials in semantic comprehension. Brain Res Cogn Brain Res 20: 329–354. [DOI] [PubMed] [Google Scholar]
- Gaillard R,Naccache L,Pinel P,Clemenceau S,Volle E,Hasboun D,Dupont S,Baulac M,Dehaene S,Adam C,Cohen L ( 2006): Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 50: 191–204. [DOI] [PubMed] [Google Scholar]
- Guillem F,N'Kaoua B,Rougier A,Claverie B ( 1995): Intracranial topography of event‐related potentials (N400/P600) elicited during a continuous recognition memory task. Psychophysiology 32: 382–392. [DOI] [PubMed] [Google Scholar]
- Guillem F,Rougier A,Claverie B ( 1999): Short‐ and long‐delay intracranial ERP repetition effects dissociate memory systems in the human brain. J Cogn Neurosci 11: 437–458. [DOI] [PubMed] [Google Scholar]
- Hagoort P ( 2005): On Broca, brain, and binding: A new framework. Trends Cogn Sci 9: 416–423. [DOI] [PubMed] [Google Scholar]
- Hald LA,Bastiaansen MC,Hagoort P ( 2006): EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang 96: 90–105. [DOI] [PubMed] [Google Scholar]
- Halgren E,Baudena P,Heit G,Clarke JM,Marinkovic K,Chauvel P,Clarke M ( 1994a): Spatio‐temporal stages in face and word processing. II. Depth‐recorded potentials in the human frontal and Rolandic cortices. J Physiol Paris 88: 51–80. [DOI] [PubMed] [Google Scholar]
- Halgren E,Baudena P,Heit G,Clarke JM,Marinkovic K,Clarke M ( 1994b): Spatio‐temporal stages in face and word processing. I. Depth‐recorded potentials in the human occipital, temporal and parietal lobes [corrected]. J Physiol Paris 88: 1–50. [DOI] [PubMed] [Google Scholar]
- Halgren E,Dhond RP,Christensen N,Van Petten C,Marinkovic K,Lewine JD,Dale AM ( 2002): N400‐like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage 17: 1101–1116. [DOI] [PubMed] [Google Scholar]
- Halgren E,Wang C,Schomer DL,Knake S,Marinkovic K,Wu J,Ulbert I ( 2006): Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage 30: 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O,Davis MH,Ford M,Pulvermuller F,Marslen‐Wilson WD ( 2006): The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage 30: 1383–1400. [DOI] [PubMed] [Google Scholar]
- Heit G,Smith ME,Halgren E ( 1990): Neuronal activity in the human medial temporal lobe during recognition memory. Brain 113(Part 4): 1093–1112. [DOI] [PubMed] [Google Scholar]
- Helenius P,Salmelin R,Service E,Connolly JF ( 1998): Distinct time courses of word and context comprehension in the left temporal cortex. Brain 121(Part 6): 1133–1142. [DOI] [PubMed] [Google Scholar]
- Herbster AN,Mintun MA,Nebes RD,Becker JT ( 1997): Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp 5: 84–92. [DOI] [PubMed] [Google Scholar]
- Hickok G,Buchsbaum B,Humphries C,Muftuler T ( 2003): Auditory‐motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. J Cogn Neurosci 15: 673–682. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ,Grainger J ( 2006): On the time course of visual word recognition: An event‐related potential investigation using masked repetition priming. J Cogn Neurosci 18: 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW,Rizzuto DS,Caplan JB,Madsen JR,Lisman J,Aschenbrenner‐Scheibe R,Schulze‐Bonhage A,Kahana MJ ( 2003): Gamma oscillations correlate with working memory load in humans. Cereb Cortex 13: 1369–1374. [DOI] [PubMed] [Google Scholar]
- James KH,James TW,Jobard G,Wong AC,Gauthier I ( 2005): Letter processing in the visual system: Different activation patterns for single letters and strings. Cogn Affect Behav Neurosci 5: 452–466. [DOI] [PubMed] [Google Scholar]
- Jobard G,Crivello F,Tzourio‐Mazoyer N ( 2003): Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage 20: 693–712. [DOI] [PubMed] [Google Scholar]
- Joubert S,Beauregard M,Walter N,Bourgouin P,Beaudoin G,Leroux JM,Karama S,Lecours AR ( 2004): Neural correlates of lexical and sublexical processes in reading. Brain Lang 89: 9–20. [DOI] [PubMed] [Google Scholar]
- Kahane P,Minotti L,Hoffmann D,Lachaux J,Ryvlin P ( 2004): Invasive EEG in the definition of the seizure onset zone: Depth electrodes In: Rosenow F,Lüders HO, editors. Handbook of Clinical Neurophysiology, Vol. 3: Presurgical Assessment of the Epilepsies with Clinical Neurophysiology and Functional Neuroimaging. Amsterdam: Elsevier Science; 109–133 pp. [Google Scholar]
- Kayser C,Kim M,Ugurbil K,Kim DS,Konig P ( 2004): A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cereb Cortex 14: 881–891. [DOI] [PubMed] [Google Scholar]
- Kissler J,Assadollahi R,Herbert C ( 2006): Emotional and semantic networks in visual word processing: Insights from ERP studies. Prog Brain Res 156: 147–183. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Pachinger T,Russegger H ( 1997): Event‐related desynchronization in the alpha band and the processing of semantic information. Brain Res Cogn Brain Res 6: 83–94. [DOI] [PubMed] [Google Scholar]
- Kutas M,Hillyard SA ( 1980): Event‐related brain potentials to semantically inappropriate and surprisingly large words. Biol Psychol 11: 99–116. [DOI] [PubMed] [Google Scholar]
- Kutas M,Federmeier KD ( 2000): Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci 4: 463–470. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,Rodriguez E,Martinerie J,Adam C,Hasboun D,Varela FJ ( 2000): A quantitative study of gamma‐band activity in human intracranial recordings triggered by visual stimuli. Eur J Neurosci 12: 2608–2622. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,Lutz A,Rudrauf D,Cosmelli D,Le Van Quyen M,Martinerie J,Varela F ( 2002): Estimating the time‐course of coherence between single‐trial brain signals: An introduction to wavelet coherence. Neurophysiol Clin 32: 157–174. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,George N,Tallon‐Baudry C,Martinerie J,Hugueville L,Minotti L,Kahane P,Renault B ( 2005): The many faces of the gamma band response to complex visual stimuli. Neuroimage 25: 491–501. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,Hoffmann D,Minotti L,Berthoz A,Kahane P ( 2006): Intracerebral dynamics of saccade generation in the human frontal eye field and supplementary eye field. Neuroimage 30: 1302–1312. [DOI] [PubMed] [Google Scholar]
- Lachaux JP,Fonlupt P,Kahane P,Minotti L,Hoffmann D,Bertrand O,Baciu M: Relationship between task‐related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. Hum Brain Mapp 2007. Feb 1; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK,Pauls J,Augath M,Trinath T,Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W,Pulvermuller F,Birbaumer N ( 1994): Words and pseudowords elicit distinct patterns of 30‐Hz EEG responses in humans. Neurosci Lett 176: 115–118. [DOI] [PubMed] [Google Scholar]
- Mainy N,Kahane P,Minotti L,Hoffmann D,Bertrand O,Lachaux JP ( 2007): Neural correlates of consolidation in working memory. Hum Brain Mapp 28: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela AM,Makinen V,Nikkila M,Ilmoniemi RJ,Tiitinen H ( 2001): Magnetoencephalographic (MEG) localization of the auditory N400m: Effects of stimulus duration. Neuroreport 12: 249–253. [DOI] [PubMed] [Google Scholar]
- Marinkovic K,Dhond RP,Dale AM,Glessner M,Carr V,Halgren E ( 2003): Spatiotemporal dynamics of modality‐specific and supramodal word processing. Neuron 38: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G,Nobre AC,Bentin S,Spencer DD ( 1995): Language‐related field potentials in the anterior‐medial temporal lobe. I. Intracranial distribution and neural generators. J Neurosci 15: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A,Gorno‐Tempini ML,Price CJ ( 2003): Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. J Cogn Neurosci 15: 260–271. [DOI] [PubMed] [Google Scholar]
- Mechelli A,Josephs O,Lambon Ralph MA,McClelland JL,Price CJ ( 2007): Dissociating stimulus‐driven semantic and phonological effect during reading and naming. Hum Brain Mapp 28: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J,Ebisch B,Schmidt KE,Niessing M,Singer W,Galuske RA ( 2005): Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309: 948–951. [DOI] [PubMed] [Google Scholar]
- Nobre AC,McCarthy G ( 1995): Language‐related field potentials in the anterior‐medial temporal lobe. II. Effects of word type and semantic priming. J Neurosci 15: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC,Allison T,McCarthy G ( 1994): Word recognition in the human inferior temporal lobe. Nature 372: 260–263. [DOI] [PubMed] [Google Scholar]
- Ojemann GA,Fried I,Lettich E ( 1989): Electrocorticographic (ECoG) correlates of language. I. Desynchronization in temporal language cortex during object naming. Electroencephalogr Clin Neurophysiol 73: 453–463. [DOI] [PubMed] [Google Scholar]
- Ojemann GA,Schoenfield‐McNeill J ( 1999): Activity of neurons in human temporal cortex during identification and memory for names and words. J Neurosci 19: 5674–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ,Henson RN,Rugg MD ( 2001): Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across‐ and within‐task comparisons. Brain 124(Part 2): 399–412. [DOI] [PubMed] [Google Scholar]
- Pammer K,Hansen PC,Kringelbach ML,Holliday I,Barnes G,Hillebrand A,Singh KD,Cornelissen PL ( 2004): Visual word recognition: The first half second. Neuroimage 22: 1819–1825. [DOI] [PubMed] [Google Scholar]
- Petersen SE,Fox PT,Posner MI,Mintun M,Raichle ME ( 1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Graimann B,Huggins JE,Levine SP,Schuh LA ( 2003): Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self‐paced movement. Clin Neurophysiol 114: 1226–1236. [DOI] [PubMed] [Google Scholar]
- Poldrack RA,Wagner AD,Prull MW,Desmond JE,Glover GH,Gabrieli JD ( 1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197(Part 3): 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ,Moore CJ,Humphreys GW,Wise RJS ( 1997): Segregating semantic from phonological processes during reading. J Cogn Neurosci 9: 727–733. [DOI] [PubMed] [Google Scholar]
- Proverbio AM,Vecchi L,Zani A ( 2004): From orthography to phonetics: ERP measures of grapheme‐to‐phoneme conversion mechanisms in reading. J Cogn Neurosci 16: 301–317. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F ( 1999): Words in the brain's language. Behav Brain Sci 22: 253–279; discussion 280–336. [PubMed] [Google Scholar]
- Pulvermuller F,Lutzenberger W,Preissl H ( 1999): Nouns and verbs in the intact brain: Evidence from event‐related potentials and high‐frequency cortical responses. Cereb Cortex 9: 497–506. [DOI] [PubMed] [Google Scholar]
- Pylkkanen L,Marantz A ( 2003): Tracking the time course of word recognition with MEG. Trends Cogn Sci 7: 187–189. [DOI] [PubMed] [Google Scholar]
- Pylkkanen L,Stringfellow A,Marantz A ( 2002): Neuromagnetic evidence for the timing of lexical activation: An MEG component sensitive to phonotactic probability but not to neighborhood density. Brain Lang 81: 666–678. [DOI] [PubMed] [Google Scholar]
- Rohm D,Klimesch W,Haider H,Doppelmayr M ( 2001): The role of theta and alpha oscillations for language comprehension in the human electroencephalogram. Neurosci Lett 310: 137–140. [DOI] [PubMed] [Google Scholar]
- Roskies AL,Fiez JA,Balota DA,Raichle ME,Petersen SE ( 2001): Task‐dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci 13: 829–843. [DOI] [PubMed] [Google Scholar]
- Salmelin R ( 2007): Clinical neurophysiology of language: The MEG approach. Clin Neurophysiol 118: 237–254. [DOI] [PubMed] [Google Scholar]
- Salmelin R,Kujala J ( 2006): Neural representation of language: Activation versus long‐range connectivity. Trends Cogn Sci 10: 519–525. [DOI] [PubMed] [Google Scholar]
- Salmelin R,Service E,Kiesila P,Uutela K,Salonen O ( 1996): Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann Neurol 40: 157–162. [DOI] [PubMed] [Google Scholar]
- Salmelin R,Schnitzler A,Schmitz F,Freund HJ ( 2000): Single word reading in developmental stutterers and fluent speakers. Brain 123(Part 6): 1184–1202. [DOI] [PubMed] [Google Scholar]
- Sederberg PB,Kahana MJ,Howard MW,Donner EJ,Madsen JR ( 2003): Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23: 10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG,Basile LF,Papanicolaou AC ( 1997): Source localization of the N400 response in a sentence‐reading paradigm using evoked magnetic fields and magnetic resonance imaging. Brain Res 762: 29–39. [DOI] [PubMed] [Google Scholar]
- Sinai A,Bowers CW,Crainiceanu CM,Boatman D,Gordon B,Lesser RP,Lenz FA,Crone NE ( 2005): Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain 128(Part 7): 1556–1570. [DOI] [PubMed] [Google Scholar]
- Summerfield C,Mangels JA ( 2005): Coherent theta‐band EEG activity predicts item‐context binding during encoding. Neuroimage 24: 692–703. [DOI] [PubMed] [Google Scholar]
- Szurhaj W,Bourriez JL,Kahane P,Chauvel P,Mauguiere F,Derambure P ( 2005): Intracerebral study of gamma rhythm reactivity in the sensorimotor cortex. Eur J Neurosci 21: 1223–1235. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain: 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Tallon‐Baudry C,Bertrand O,Delpuech C,Pernier J ( 1996): Stimulus specificity of phase‐locked and non‐phase‐locked 40 Hz visual responses in human. J Neurosci 16: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry C,Bertrand O,Henaff MA,Isnard J,Fischer C ( 2005): Attention modulates gamma‐band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15: 654–662. [DOI] [PubMed] [Google Scholar]
- Tanji K,Suzuki K,Delorme A,Shamoto H,Nakasato N ( 2005): High‐frequency gamma‐band activity in the basal temporal cortex during picture‐naming and lexical‐decision tasks. J Neurosci 25: 3287–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkiainen A,Cornelissen PL,Salmelin R ( 2002): Dynamics of visual feature analysis and object‐level processing in face versus letter‐string perception. Brain 125(Part 5): 1125–1136. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL,Aguirre GK,D'Esposito M,Farah MJ ( 1999): A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia 37: 671–676. [DOI] [PubMed] [Google Scholar]
- Van Strien JW,Hagenbeek RE,Stam CJ,Rombouts SA,Barkhof F ( 2005): Changes in brain electrical activity during extended continuous word recognition. Neuroimage 26: 952–959. [DOI] [PubMed] [Google Scholar]
- Varela F,Lachaux JP,Rodriguez E,Martinerie J ( 2001): The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2: 229–239. [DOI] [PubMed] [Google Scholar]
- Vigneau M,Jobard G,Mazoyer B,Tzourio‐Mazoyer N ( 2005): Word and non‐word reading: What role for the Visual Word Form Area? Neuroimage 27: 694–705. [DOI] [PubMed] [Google Scholar]
- Weiss S,Mueller HM ( 2003): The contribution of EEG coherence to the investigation of language. Brain Lang 85: 325–343. [DOI] [PubMed] [Google Scholar]
- Wilson TW,Leuthold AC,Lewis SM,Georgopoulos AP,Pardo PJ ( 2005): The time and space of lexicality: A neuromagnetic view. Exp Brain Res 162: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydell TN,Vuorinen T,Helenius P,Salmelin R ( 2003): Neural correlates of letter‐string length and lexicality during reading in a regular orthography. J Cogn Neurosci 15: 1052–1062. [DOI] [PubMed] [Google Scholar]
- Yokoyama S,Miyamoto T,Riera J,Kim J,Akitsuki Y,Iwata K,Yoshimoto K,Horie K,Sato S,Kawashima R ( 2006): Cortical mechanisms involved in the processing of verbs: An fMRI study. J Cogn Neurosci 18: 1304–1313. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ,Meyer E,Gjedde A,Evans AC ( 1996): PET studies of phonetic processing of speech: Review, replication, and reanalysis. Cereb Cortex 6: 21–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Figure S1: Event related potentials (ERPs) for the selected clusters represented on figure 1. The ERPs shown for each anatomical cluster correspond to the same data used to compute the TF representations in figure 1. Colors code for the three experimental conditions.
Figure S2: schematic time‐line of gamma band responses within the word recognition network. Colored lines indicate the timing of gamma activation (or deactivation) for the three conditions (blue = Seman, red = Phono, green = Visual). Faded colors indicate strongly reduced responses. Those should be taken as timing indications only and do not exclude contributions from brain regions not recorded in the present study.


