Abstract
The concept of ‘willed’ actions has attracted attention during the last few years. Free choices have been associated with activations on the medial frontal surface, the dorsolateral prefrontal cortex and the parietal lobe. Self‐paced movements and free selection between various motor responses were typically used to investigate voluntary behavior. The aim of the present study was to determine neural correlates of voluntary motor responses and the voluntary inhibition of motor responses in a group of healthy subjects. Hence, a go/nogo/voluntary selection paradigm was used. In the voluntary selection condition subjects decided freely whether or not to respond with a button press after stimulus presentation. Functional MRI data and event‐related potentials were acquired simultaneously in order to reliably investigate spatial and temporal characteristics of these responses. The results showed decision‐related enhanced neural responses predominantly in the medial frontal gyrus/supplementary motor area, lateral frontal brain regions and the inferior parietal gyrus. Additional activations associated with voluntary movements were detected in the frontal eye field as well as brain regions directly linked to motor responses (e.g. somatosensory cortical areas). Altogether, decision processes were shown to be relatively independent of the kind of response chosen. Hum Brain Mapp 2009. © 2009 Wiley‐Liss, Inc.
Keywords: EEG‐fMRI, executive functioning, behavioral control, neural correlates, decision making
INTRODUCTION
Executive functioning describes a set of cognitive abilities that control and regulate other abilities necessary for goal‐directed behavior. Various aspects are included, e.g. the ability to initiate and stop actions, to monitor and evaluate performance in relation to goals, to flexibly change and revise behavior as needed, and to solve problems [Jurado and Rosselli,2007; Lezak,1983; Ylvisaker and DeBonis,2000]. When tasks regarding cognitive control processes were presented in brain imaging studies, functional variations appeared in a network of brain regions including anterior cingulate cortex (ACC)/pre‐supplementary motor area (pre‐SMA), dorsolateral prefrontal cortex (DLPFC), inferior frontal junction, anterior insular cortex, dorsal pre‐motor cortex, and posterior parietal cortex [Brass et al.,2005; Cabeza and Nyberg,2000; Duncan and Owen,2000]. One aspect of cognitive control are ‘willed actions’: actions are said to be ‘willed’, if we consciously pay attention to their selection [Frith et al.,1991] and if we can choose whether or not to execute them, respectively [Passingham,1995]. Several studies focusing on the examination of neural representations of intention compared self‐paced movements of subjects to finger movements triggered by an external cue [Jahanshahi et al.,1995; Jenkins et al.,2000]. In free movement selection tasks a readiness potential was shown in electrophysiological investigations [Cunnington et al.,2005; Dirnberger et al.,1998] as well as increased hemodynamic responses in the DLPFC (BA 46) compared to tasks in which responses were externally specified [Deiber et al.,1991; Hyder et al.,1997; Jahanshahi et al.,1995; Playford et al.,1992; Spence et al.,1998]. Hunter et al. [2003] revealed activity in the primary motor cortex, which was preceded by responses of the left PFC and SMA [Hunter et al.,2003].
Another approach to investigate ‘willed’ actions refers to the choice between two or more response alternatives [Deiber et al.,1991; Frith et al.,1991; Lau et al.,2004b]: in these tasks subjects can decide which task they want to perform and the required response is not fully determined by external parameters (e.g. task instructions). It is assumed that extra mental effort is needed to make a deliberate choice between different response possibilities [Frith et al.,1991]. In addition, two active processes between task alternatives are suggested during voluntary decisions: the decision about which task to perform and the subsequent reconfiguration of the respective task set [Arrington and Logan,2004; Logan and Gordon,2001]. The supposition that cued and uncued reactions may involve the same processes has been disproved: cells in the cingulate motor area were found to be active when monkeys made a voluntary shift in response, but not when the response shift was cued externally [Shima and Tanji,1998]. Functional neuroimaging studies provided some evidence that ACC and DLPFC activations [Deiber et al.,1991; Frith et al.,1991; Hyder et al.,1997; Jueptner et al.,1997; Lau et al.,2004b; Walton et al.,2004] as well as responses of the superior parietal lobule and the posterior part of the intraparietal sulcus [Forstmann et al.,2006] were increased when subjects were able to freely select a response. Further evidence for the involvement of medial frontal structures in free choices comes from lesion studies: damage of the medial frontal cortex leads to severe difficulties in spontaneously initiating actions [Laplane et al.,1977]. Lau et al. [2004b] suggested that the DLPFC activations were associated with attention to selection of action but did not play a unique role in the generation of internally initiated actions. By contrast, the pre‐SMA seemed to be involved in the endogenous generation of responses when the responses were underdetermined without sufficient environmental constraints [Lau et al.,2004b]. Then again, pre‐SMA responses are also tightly associated with response conflict [Ullsperger and von Cramon,2001]. Voluntary responses, too, may lead to an enhanced conflict between different action plans, particularly when no response alternative is preferable to another [Nachev et al.,2005]. Thus, functional variations in tasks related to response conflict and free choice, respectively, could overlap [Nachev et al.,2005]. Nachev et al. [2005] aimed at the dissociation of neural responses, which can be seen when tasks dealing with voluntary responses and conflict‐related behavior, are examined. Their results suggested that conflict revealed activation in the rostral pre‐SMA. In addition, free voluntary action did not itself engender conflict. The generation of volitional plans seemed to engage a more caudal region of the pre‐SMA. By contrast, another study confirmed the hypothesis of an involvement of the pre‐SMA in the endogenous generation of action, whereas response conflict seemed to be associated with ACC responses [Lau et al.,2006].
Apart from medial frontal areas, the DLPFC was thought to be involved in free selection, when there is no external cue to guide action [Hadland et al.,2001]. However, the function of the DLPFC is not clear; possibly the DLPFC is primarily related to the working memory (WM) capacity [Barch et al.,1997; Manoach et al.,1997; Ranganath and D'Esposito,2001], which are also required in most selection tasks. The study of Hadland et al. [2001] examined the effect of repetitive transcranial magnetic stimulation (rTMS) to the DLPFC. The results indicated that response selection depends on the DLPFC and medial frontal cortex even when WM requirement is low [Hadland et al.,2001].
It should be noted that direct or causal associations between cognitive processes and specific electrophysiological as well as hemodynamic responses have not been proved yet. Still, certain cognitive functions seem to be particularly associated with certain brain regions as well as neuronal responses. However, the results are often inconsistent and are characterized by a high inter‐ and intraindividual variability.
We used an adapted go/nogo‐paradigm in a simultaneous EEG‐fMRI study in order to further investigate ‘willed’ actions. In these tasks a well‐learned reflexive action is placed in opposition to another action that is less accurately specified by the environmental circumstances that prompt it [Nachev et al.,2005]. Apart from the go task (button press required) and the nogo task (no response required), a voluntary selection condition was included in which subjects could choose whether or not they wanted to press the response button. The aim of the study was to distinguish neural reactivity with a voluntary behavioral response from those responses which appear when the motor response is withheld voluntarily. Furthermore, decision‐related neural responses are to be separated from those during forced responses. Thereby, the combination of EEG and functional MRI allows for high‐spatial and temporal acquisition of mental processes and may contribute to a more comprehensive understanding of neural correlates of perception and cognition [Debener et al.,2005,2006; Eichele et al.,2005; Mulert et al.,2004,2005a]. We hypothesized decision‐related activations especially in medial frontal brain regions would be demonstrated when comparing voluntary and forced responses (go, nogo). We assumed that neural responses in frontal brain regions are comparable in voluntary selection tasks whether subjects decided freely to press the button or not.
METHODS
Subjects
Fourteen healthy subjects (11 men; aged between 24 and 49 years; average age: 36.5 ± 8.0 years; years of education between 11 and 20 years, 16.5 ± 3.0 years on average) took part in the simultaneous EEG‐fMRI study. A standardized questionnaire was used to exclude neurologic and psychiatric disorders or hearing problems. According to the modified version of the Edinburgh Inventory of Handedness [Oldfield,1971], thirteen subjects were right‐handed, one was left‐handed. After all procedures had been fully explained, written informed consent was obtained from each participant. The study was approved by the ethical committee of the University of Munich and was carried out in accordance with the Declaration of Helsinki. Each volunteer was paid €25 for participating in the study.
Task
The subjects performed an adapted auditory go/nogo paradigm comprising four different conditions (see Fig. 1). The auditory stimuli consisted of a sinus tonus (duration: 50 ms, pressure level: 100 dB) of three differential pitches delivered binaurally via headphones. The tones were presented in pairs at intervals of 1,000 ms. The tone with the middle frequency [1,000 Hz] served as cue indicating that a button press was required when it was directly followed by the high frequency tone [1,300 Hz; go condition]. The subject's objective was to press a button with their right index finger for the go condition. Subjects were instructed to respond as quickly as possible after the stimuli were presented, while minimizing errors. The prepared behavioral response was to be inhibited if the cue was followed by the tone with a low frequency [800 Hz; nogo condition]. In the voluntary selection condition [selection], participants were instructed to freely decide whether to press the response button [selection+] or not [selection−]. The participants were asked to decide separately at each trial of the voluntary selection task whether they wanted to respond or not. Subjects were told that the ratio selection+/selection− did not matter as long as it was approximately equally often and in random order. Subjects were asked not to count how often they pressed the button. Only subjects who responded in each trial of the voluntary selection condition or did not respond at all were not included in the study, because it could not be guaranteed that they had understood the instructions; these data were not analyzed. In addition, there were two control conditions starting with the low‐frequency tone indicating that no response was required irrespective of the second tone (passive listening). The conditions were presented in pseudo‐randomized order. The go condition was presented 160 times, the other conditions were presented 80 times with an interstimulus interval of 3 sec. All subjects received a practice block of at least 10 min to introduce the different response rules.
Figure 1.
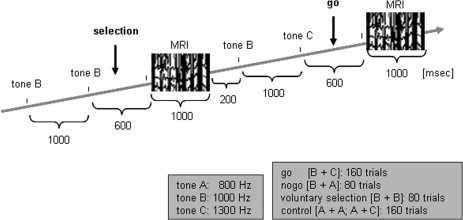
Auditory go/nogo/voluntary selection paradigm.
Behavioral and EEG Data Analysis
Regarding behavioral data reaction times, errors of omission and commission were computed separately for the go and voluntary selection task. Any response delayed by more than 1,000 ms after the stimulus was counted as error. A MANOVA was used to test the difference between reaction times and the quotient of responses, respectively, and between the go condition and the selection+ task.
Evoked potentials were recorded during the acquisition of functional MRI images by 61 Ag/AgCl electrodes placed on the scalp according to the international 10‐10 system using an electrode cap set (Easycap, Germany). All electrodes referred to Cz. Eye movements were recorded from a channel placed beneath the right eye. The ECG was recorded with three electrodes placed on the back of each participant. EEG was continuously recorded and digitized at 5,000 Hz without any filtering during acquisition. Impedances were usually maintained below 10 kΩ. The EEGs were acquired with an amplifier designed for inside scanner recordings (Brain Products, Munich). Participants were asked to stay calm and keep their eyes shut during the task. Eye movements and eye‐blinks as well as cardioballistic artifacts were excluded using a spatial filters algorithm. Common to spatial filter techniques is the decomposition of the EEG into components which, ideally, model either artifact or brain activity [Ille et al.,2002]. A suitable decomposition is achieved when artifact activity can be reconstructed as the product of artifact topographies and waveforms. Here, the ‘surrogate’ algorithm was used which is implemented in BESA software package (MEGIS Software GmbH, Gräfelfing, Germany): brain activity is modeled using a dipole configuration with dipoles that are placed at strategic positions of the brain. This method was already used for the removal of cardioballistic artifacts [Karch et al.,2008; Mulert et al.,2005b; Siniatchkin et al.,2006]. Further analyses were done with Analyzer Software (Brain Products, Munich). The data were re‐referenced to an average reference. The EEG data were filtered with a 20 Hz low‐pass filter (slope 48 dB/oct) and segmented into 750 ms epochs time‐locked to the onset of the second stimulus of each pair of tones, separately for the different conditions (voluntary selection, go, nogo, control). The sampling epoch commenced 150 ms before the presentation of the second tone indicating which task was to be performed. The pre‐stimulus interval was used for baseline correction. Epochs containing artifacts (amplitude higher than ±90 μV) were rejected. The artifact detection was done on Fz, F3, F4, FCz, Cz, C3, C4, and Pz. Trials with incorrect responses (button press after the nogo or control tasks; no response after the go task) were rejected prior to averaging. The ERPs of subjects with less than 30 trials remaining after artifact rejection were excluded from the analyses (three subjects both for the voluntary selection task with button press and the voluntary selection task without button press). The N2 and P3 ERPs were examined at the midline fronto‐centro‐parietal scalp electrodes (Fz, FCz, Cz, Pz). NoGo‐related electrophysiological variations in these locations were determined a priori from previous studies [Bekker et al.,2004; Bruin et al.,2001; Falkenstein et al.,1999; Kamarajan et al.,2005; Karch et al.,2008; Kopp et al.,1996; Pfefferbaum et al.,1985].
Image Acquisition and Procedure
Imaging was performed using a 1.5 Tesla Siemens Sonata MR scanner. For anatomical registration of the functional data high‐resolution anatomical data sets were collected using 3D T1‐weighted sequence. During the functional imaging session 10 T2*‐weighted images were obtained with gradient echo EPI sequence in the same position as the 3D data set (TR = 3 sec; TE = 53 msec; matrix: 64 × 64; FOV: 192 × 192; slice thickness: 8 mm; interslice‐gap: 0.4 mm; interleaved slice acquisition). Data were acquired in temporal synchrony to the task.
We used an interleaved design where the tones were presented during the intervals MR acquisition to reduce the influence of the scanner noise on stimulus presentation and to diminish MR provoked artifacts on the EEG acquisition: the scanner noise took about 1,000 ms. After 1,200 ms, the first tone was presented for 50 ms, the second tone was presented after 2,250 ms. After additional 700 ms, the next volume was measured. Four hundred and eighty‐five image volumes (160 go condition; 80 nogo condition; 80 voluntary selection condition; 160 control conditions; 5 baseline at the beginning) were acquired in total; the experiment lasted about 25 min.
Participants were scanned in one continuous measurement session. During the image acquisition, participants were asked to keep their eyes closed. Participants were positioned comfortably on the scanner bed with their heads cushioned tightly in place to reduce head movement. The tones were presented via headphones. Auditory stimuli were generated on a PC outside the MR environment using the BrainStim software package (Brain Products, Munich) and conducted via a pair of plastic tubes into a set of headphones placed over the subjects' ears. During the adapted go/nogo‐task participants kept their right index finger mounted on a response box.
Analysis of MRI Data
Image preprocessing and statistical analysis were performed using the BrainVoyager Software package version 4.96 (Brain Innovation, Maastricht, Netherlands). Five images at the beginning of each session were cancelled due to inhomogenities of the magnetic field. The preprocessing of the functional images included slice scan time correction and a 3D motion correction. A Gaussian filter with FWHM 8.0 mm was applied. The functional images were transferred to a standard Talairach brain.
Image data for each participant were analyzed individually at the first level using the general linear model (GLM) as implemented in BrainVoyager. Trials were classified according to the four conditions: [1] voluntary selection, [2] nogo, [3] go, [4] control. Significant fMRI activity was determined by cross‐correlation of MR image pixel intensity with an expected hemodynamic response function.
For group analysis, a second level random effects analysis (voluntary selection; nogo; go; control) was computed, thresholded at P < 0.001 uncorrected for multiple comparisons (confidence range T: 4.3–8). A second GLM was computed in which the voluntary selection condition was divided according to the selected response during the voluntary selection condition (selection+; selection−; nogo; go; control; random effects analysis; threshold: P < 0.001 uncorrected for multiple comparisons; confidence range T: 4.3–8). For visualization, regions with significant activations were thresholded at P (uncorrected) < 0.001 and overlayed on a talairachized T1‐weighted image of a single subject. Furthermore, a factorial analysis was done within the factors: 1. ‘voluntariness’ (free vs. forced), 2. ‘response’ (no response vs. button press) in order to demonstrate brain regions needed for voluntary responses and separate these functions from behavioral responses.
In order to directly compare BOLD responses with behavioral data and electrophysiological activity, a region of interest (ROI) analysis was included. For that purpose, the anatomical ROI definitions of BrainVoyager 2000 for the cingulate gyrus (CG), the inferior frontal gyrus (IFG), the middle frontal gyrus (MFG), the superior frontal gyrus (SFG), the medial frontal gyrus (medial PFC), and the precentral gyrus were used. For each subject, the average T‐value of the activated voxels (t‐score: 2.6–8; P < 0.01 (uncorrected for multiple comparisons)) were determined separately for selection+, selection−, nogo and go. Comparisons were done with the Pearson correlation coefficient.
Statistics
Statistics were obtained using the routines in the SPSS 14.0.1 program. The significance level was 0.05, P values between 0.05 and 0.1 were marked as a trend.
The N1 was defined as the relative minimum of the ERP in the search window of 70–230 msec. The N2 was defined as the largest relative minimum of the ERP in the search window of 170–230 msec. The P3 was defined as the largest relative maximum of the ERP 230–550 msec after presentation of the respective task. In order to test for the significance of each effect, MANOVAs with repeated measurements was run on the maximum ERP‐amplitude in each search window (N1, N2, P3) with two repeated‐measures factor of task (voluntary selection, nogo, go, control) and electrode position (Fz, FCz, Cz, Pz). In the case of a significant Mauchly‐test the Greenhouse‐Geisser correction was used. In addition, post‐hoc t‐tests were used. Based on 4 × 4 task conditions, 16 different tests were performed. Therefore, using Bonferroni‐correction, all tests were performed with a two‐sided P < 0.0031; P values smaller than 0.00625 were marked as trend. Furthermore, the ERP‐amplitudes (N1, N2, P3) of selection+ were compared to the selection− responses using MANOVAs with repeated measurements. Post‐hoc t‐tests were Bonferroni‐corrected with a two‐sided P < 0.00625 (trend level: P < 0.0125).
Pearson correlations were calculated between go‐associated behavioral performance (reaction time; percentage of correct responses) and ERP‐responses in Fz, FCz, Cz and Pz during this task. In addition, the behavioral responses during voluntary selection condition were related to the respective electrophysiological responses (selection+ task). In addition, correlations between the average BOLD response in the regions of interest (CG, MFG, IFG, SFG, medial PFC) and the N2 amplitudes of Fz, FCz and Cz as well as P3 amplitudes of Fz, FCz, Cz and Pz were calculated separately for the different conditions (selection+, selection−, go, nogo). The ROI information of selection+ and go were also compared to the respective reaction times.
RESULTS
Behavioral Results
Behavioral data are shown in Figure 2. The group average for the mean response times were significantly longer in voluntary selection trials [M = 689.5 ± 187.54] than in go trials [M = 473.9 ± 142.23; F(1,13) = 74.641; P < 0.001]. In addition, the percentage of responses differed significantly between go (M = 98.0 ± 1.25%) and voluntary selection trials [M = 58.7 ± 13.65%; F(1,13) = 106.224; P < 0.001].
Figure 2.
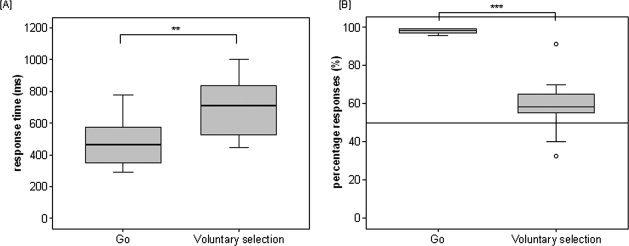
Behavioral data. Boxplots show mean response time [A] and percentage responses [B] for go and voluntary selection trials. Asterisks indicate level of significance: ***p < .001; **p < .01.
ERP Results
ERP results are shown in Figures 3 and 4. Regarding the N1‐amplitude, we found a significant main effect of electrode position [F(3,39) = 26.695; P < 0.001]. Apart from this, the interaction between task and electrode position revealed to be significant [F(4.296,55.849) = 3.244; P = 0.016]. The task effect was not significant [F(3,39) = 0.866; P = 0.467]. Post‐hoc tests showed enhanced N1‐amplitudes in Fz compared to Cz (p = 0.028) and Pz (p < 0.001), enhanced amplitudes in FCz compared to Cz (P = 0.002) and Pz (P < 0.001) as well as Cz and Pz (P = 0.004).
Figure 3.
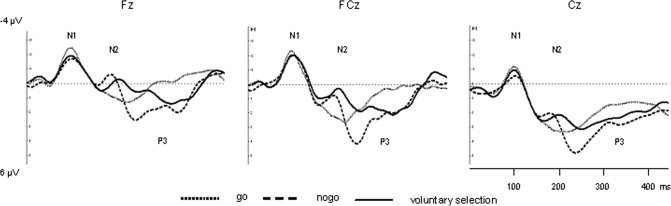
ERP waveform during go, nogo and voluntary selection condition at frontal and central electrode positions.
Figure 4.
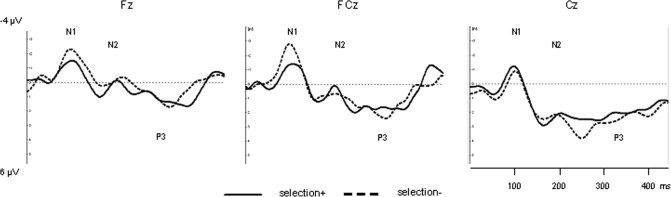
ERP waveform during voluntary selection without behavioural response [selection‐] compared to the selection to respond with a button press [selection+] at frontal and central electrode positions.
Furthermore, regarding the N2‐amplitude, we found a significant main effect of task [F(3,39) = 7.169; P = 0.001] and of electrode position [F(1.525,19.829) = 9.269; P = 0.003]. There was also a significant interaction effect (task × electrode position) [F(4.543,59.057) = 5.003; P = 0.001]. The post‐hoc comparisons revealed significant smaller N2‐amplitudes in the go task compared to the nogo condition (P = 0.049), voluntary selection (P = 0.009) and the control condition (P = 0.002). Regarding the electrode positions, the N2 amplitudes revealed to be significantly more pronounced in Fz compared to Cz (P = 0.009) as well as FCz compared to Cz (P = 0.009). Cz N2 amplitudes were also smaller than those at Pz (P = 0.001).
Concerning the P3 amplitude, significant main effects of task [F(3,39) = 18.834; P < 0.001] and electrode position [F(3,39) = 4.950; P = 0.005] were found. The interaction between task and electrode was also significant [F(9,117) = 3.385; P = 0.001]. Pairwise comparisons revealed significant increased P3‐amplitude associated with the nogo condition compared to go (P = 0.001), voluntary selection (P = 0.032), and control (P < 0.001). The P3 amplitudes related to the control task were also significantly reduced compared to go (P = 0.015) and voluntary selection (P = 0.009). The analysis of electrode position revealed significantly increased P3 amplitudes in Cz compared to Fz (P = 0.021).
The comparison of N1‐amplitudes of the selection+ compared to selection− revealed a significant main effect of electrode position [F(1.796,23,349) = 20.697; P < 0.001] but not of task [F(1,13) = 0.551; P = 0.471]. The interaction effect (task × electrode position) failed to be significant [F(1.731,22.499) = 1.897; P = 0.146]. Post‐hoc comparisons revealed significant enhanced N1‐amplitudes in Fz compared to Pz (P = 0.001), in FCz compared to Cz (P = 0.007) and compared to Pz (P < 0.001) as well as Cz compared to Pz (P = 0.002).
The main task‐effect for N2‐amplitudes [F(1,13) = 0.031; P = 0.862] and P3‐amplitudes [F(1,13) = 0.008; P = 0.931] as well as interaction effects (N2: [F(1.845,23.983) = 0.432; P = 0.731]; P3: [F(3,39) = 1.675; P = 0.188]) were not significant. The main effect of electrode position was significant in N2‐amplitudes [F(1.860,24.181) = 9.543; P = 0.001] but not in P3‐amplitiudes [F(3,39) = 2.605; P = 0.065]. Post‐hoc tests revealed clearly more negative values in the N2‐interval in Fz compared to Cz (P = 0.019) and Pz (P = 0.027), as well as FCz compared to Cz (P = 0.013).
Correlations Between Behavioral Data and ERPs
Fast behavioral responses during the go condition were related to increased go‐associated N1‐amplitudes in Pz (correlation coefficient [CC] = −0.740**, P = 0.002) as well as enhanced P3‐amplitudes in FCz (CC = −0.583, P = 0.029), Cz (CC = −0.592, P = 0.026) and Pz (CC = −0.745, P = 0.002). A high percentage of correct responses during the go task correlated with increased N1‐amplitudes during go in Pz (CC = 0.536, P = 0.048).
Fast responses during the voluntary selection task correlated with increased ERP amplitudes in Cz (CC = −0.669, p = 0.009) and Pz (CC = −0.629, P = 0.016). The results are also shown in Figure 5.
Figure 5.
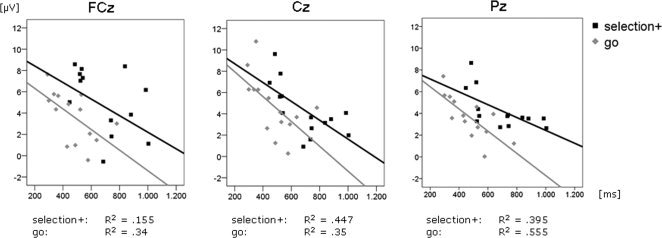
Correlation between P3‐amplitudes [μV] of FCz, Cz and Pz and the corresponding reaction times [ms] for the selection+ and go task.
Functional MRI Results
Voluntary selection versus control task
The results are summarized in Table I and Figure 6. Increased BOLD responses were demonstrated during the voluntary selection task compared to the control condition, especially in frontal brain regions including the superior and medial frontal gyrus (BA 6/8), the left and right middle frontal gyrus (BA6/8/9/46) and the left insular cortex (BA 13). Furthermore, the voluntary selection task led to an enhanced contribution of the postcentral gyrus (BA 3), the inferior parietal lobule (BA 40) and limbic/subcortical regions (e.g. thalamus, caudate body/putamen). Slightly increased functional responses associated with the control task were shown in the occipital lobe (left and right middle occipital gyrus, left lingual gyrus (BA 19/37/18)), the right superior parietal lobule (BA 5/7), the precuneus (BA 7) as well as small areas in the right precentral gyrus and right middle temporal gyrus.
Table I.
Significant activations during voluntary selection compared to the control condition
| Cerebral region | BA | Side | Avg t‐score | Max t‐score | Size | Center of mass | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Voluntary selection > control | ||||||||
| Frontal lobe | ||||||||
| Superior frontal gyrus/medial frontal gyrus | 6/8 | R | 5.478 | 10.309 | 17264 | 2 | 13 | 49 |
| Cingulate gyrus | 23/24 | R/L | 5.237 | 8.947 | 826 | 0 | −22 | 34 |
| Middle frontal gyrus | 9 | L | 4.774 | 6.285 | 941 | −36 | 47 | 27 |
| 8 | L | 4.728 | 5.510 | 281 | −37 | 31 | 42 | |
| 6 | R | 4.529 | 5.345 | 243 | 44 | 12 | 46 | |
| 46 | R | 4.667 | 5.525 | 54 | 46 | 53 | 8 | |
| Inferior frontal gyrus | R | 4.605 | 5.660 | 358 | 35 | 28 | 12 | |
| Parietal lobe | ||||||||
| Postcentral gyrus | 3 | L | 4.680 | 5.974 | 1045 | −40 | −22 | 55 |
| Inferior parietal lobule | 40 | L | 4.654 | 6.363 | 2658 | −47 | −54 | 40 |
| 40 | R | 4.516 | 5.086 | 1124 | 50 | −44 | 45 | |
| 40 | L | 4.682 | 5.790 | 206 | −33 | −37 | 39 | |
| Precuneus | 7 | L | 4.725 | 5.948 | 850 | −2 | −68 | 42 |
| Sub‐cortical/limbic area | ||||||||
| Insula | 13 | L | 4.814 | 6.206 | 2342 | −35 | 18 | 7 |
| Thalamus/ventral lateral nucleus | R | 5.223 | 8.349 | 1221 | 12 | −11 | 12 | |
| Thalamus/medial dorsal nucleus | L | 4.790 | 5.910 | 409 | −7 | −12 | 12 | |
| Caudate body/putamen | R | 5.270 | 7.882 | 1196 | 16 | 7 | 11 | |
| Putamen | L | 5.057 | 6.932 | 1362 | −17 | 9 | 8 | |
| Control > voluntary selection | ||||||||
| Frontal lobe | ||||||||
| Precentral gyrus | R | 4.673 | 5.594 | 193 | 26 | −20 | 59 | |
| Temporal lobe | ||||||||
| Middle temporal gyrus | R | 4.650 | 5.858 | 173 | 39 | −60 | 11 | |
| Occipital lobe | ||||||||
| Middle occipital gyrus | 19 | L | 4.861 | 7.505 | 2301 | −33 | −84 | 20 |
| 19/37 | R | 4.547 | 5.017 | 138 | 43 | −68 | 8 | |
| Lingual gyrus | 18 | L | 5.334 | 9.394 | 619 | −26 | −77 | −3 |
| Parietal lobe | ||||||||
| Superior parietal lobule | 5/7 | R | 5.456 | 9.564 | 3454 | 26 | −85 | 19 |
| 5/7 | R | 6.576 | 5.047 | 197 | 26 | −45 | 61 | |
| Precuneus | 7 | R | 5.041 | 4.546 | 63 | 10 | −81 | 50 |
Figure 6.
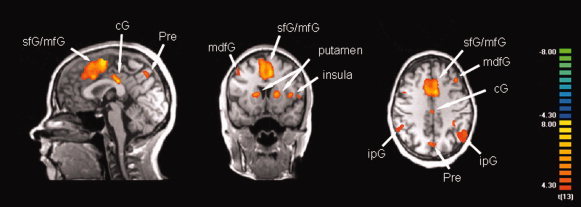
Functional MRI responses to the voluntary selection task compared to the control condition (random effects analysis thresholded at p(uncor) < .001; confidence range T: 4.3−8; xyz: 0 10 40). [Abbreviations: sfG: superior frontal gyrus, mfG: medial frontal gyrus, cG: cingulate gyrus, Pre: Precuneus; mdfG: middle frontal gyrus; ipG: inferior parietal gyrus]. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Voluntary selection with button press [selection+] versus go task
Table II and Figure 7 indicate location and spatial extent of activations related to voluntary motor responses compared to go responses. The selection+ task led to enhanced hemodynamic responses compared to the go task, especially in medial (superior frontal gyrus (BA 6/8), medial frontal gyrus (BA 9)) and lateral parts of the frontal cortex (left and right middle frontal gyrus (BA 6/8/9)). Apart from frontal differences, BOLD responses were mainly increased in parietal areas (inferior parietal lobule (BA 40), superior parietal lobe (BA 19), precuneus (BA 7), supramarginal gyrus) during the selection+ task compared to the go task. However, slightly decreased responses were shown in the cuneus/middle occipital gyrus (BA 6), the insular cortex and the precentral gyrus (BA 4/6).
Table II.
Significant activations during voluntary selection with button press [selection+] compared to the go task
| Cerebral region | BA | Side | Avg t‐score | Max t‐score | Size | Center of mass | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Voluntary selection with button press > Go | ||||||||
| Frontal lobe | ||||||||
| Superior frontal gyrus | 6/8 | L/R | 4.811 | 6.332 | 4981 | 0 | 17 | 53 |
| Middle frontal gyrus | 8/9 | R | 4.812 | 7.295 | 4372 | 46 | 19 | 39 |
| L | 4.703 | 5.582 | 938 | −43 | 22 | 40 | ||
| 6 | L | 4.725 | 6.164 | 905 | −44 | 2 | 43 | |
| 6 | R | 4.455 | 4.794 | 51 | 32 | 14 | 57 | |
| Medial frontal gyrus | 9 | L | 4.773 | 5.943 | 295 | −7 | 31 | 33 |
| 9 | R | 4.688 | 6.027 | 118 | 9 | 47 | 19 | |
| Inferior frontal gyrus/insula | 13/45 | L | 4.765 | 5.968 | 810 | −39 | 19 | 4 |
| Parietal lobe | ||||||||
| Inferior parietal lobule | 40 | L | 5.692 | 11.720 | 2380 | −44 | −46 | 44 |
| Superior parietal lobe/precuneus | 19/7 | L | 4.429 | 4.836 | 81 | −31 | −63 | 43 |
| Precuneus | 7 | R | 4.969 | 6.608 | 1374 | 8 | −66 | 42 |
| Supramarginal gyrus | R | 4.474 | 5.160 | 63 | 51 | −46 | 36 | |
| Subcortical | ||||||||
| Corpus callosum | R | 4.598 | 5.742 | 97 | 9 | −17 | 20 | |
| Go > voluntary selection with button press | ||||||||
| Cuneus/middle occipital gyrus | 6 | R | 4.772 | 5.545 | 375 | 1 | −92 | 30 |
| Insula | L | 4.577 | 6.033 | 104 | −37 | −15 | 23 | |
| Precentral gyrus | 6/4 | R | 4.753 | 6.531 | 66 | 61 | −7 | 39 |
Figure 7.
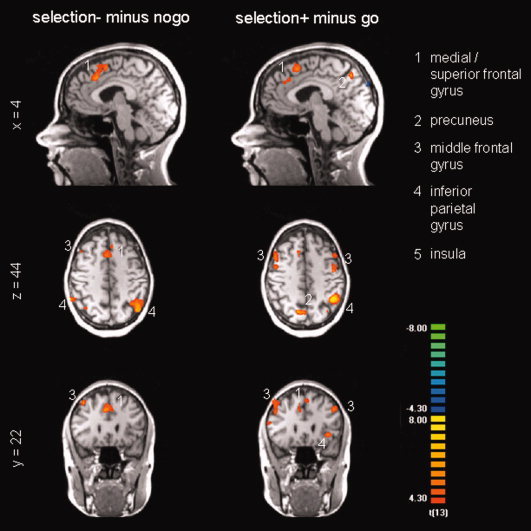
Functional MRI responses to the voluntary selection task without behavioural response [selection‐] compared to the nogo condition and the voluntary selection with behavioural response [selection+] compared to the go condition, respectively (random effects analysis thresholded at p(uncor) < .001; confidence range T: 4.3−8; xyz: 4 22 44). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Voluntary selection without button press [selection−] versus nogo task
The decision in the voluntary selection task [selection−] revealed increased hemodynamic responses in the medial frontal gyrus (BA 6/8), the left and right inferior parietal lobule (BA 7/40) and the supramarginal gyrus compared to the nogo task. Inhibition‐related increases in BOLD‐responses were small (right precentral gyrus (BA 4/6), right postcentral gyrus (BA 4), right cuneus/middle occipital gyrus (BA 6); see Table III, Fig. 7).
Table III.
Significant activations during voluntary selection without button press [selection−] compared to the nogo task
| Cerebral region | BA | Side | Avg t‐score | Max t‐score | Size | Center of mass | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Voluntary selection without button press > NoGo | ||||||||
| Frontal lobe | ||||||||
| Medial frontal gyrus | 6/8 | R | 4.799 | 6.589 | 3867 | 2 | 19 | 48 |
| 8 | R | 4.764 | 7.032 | 417 | 39 | 26 | 49 | |
| Parietal lobe | ||||||||
| Inferior parietal lobule | 40 | L | 5.091 | 7.453 | 2622 | −43 | −55 | 44 |
| 40 | R | 4.933 | 6.102 | 525 | 55 | −47 | 42 | |
| 7 | R | 4.698 | 5.733 | 282 | 36 | −60 | 48 | |
| Supramarginal gyrus | R | 4.631 | 5.771 | 98 | 47 | −37 | 37 | |
| NoGo > voluntary selection without button press | ||||||||
| Precentral gyrus | 6/4 | R | 4.867 | 6.718 | 215 | 58 | −4 | 14 |
| Postcentral gyrus | 4 | R | 4.606 | 5.309 | 105 | 13 | −34 | 57 |
| Cuneus/middle occipital gyrus | 6 | R | 4.413 | 4.946 | 86 | 27 | −79 | 18 |
Brain regions associated to voluntary selection and response (factorial analysis)
The results of the main factor ‘voluntariness’ demonstrated the involvement of medial frontal brain regions, e.g. the superior frontal gyrus and the medial frontal gyrus (BA 8/6) for voluntary responses. In addition, pronounced BOLD responses were demonstrated in left and right lateral frontal regions including the middle frontal gyrus (R > L), the inferior frontal gyrus and the insula (BA 6/9/8/47/45/13) as well as parietal regions. Smaller responses were demonstrated in temporal brain regions (BA 21/22/41). The main factor ‘response’ was associated with pronounced BOLD responses predominantly in the inferior parietal lobe (BA 40) as well as the pre‐ and postcentral gyrus (BA 4, 43), the left insula (BA 13) and the SMA (BA 6). Smaller responses were demonstrated in the subcortical regions (caudate body, putamen). The interaction between these two factors failed to show any significant brain response (see Table IV, Fig. 8).
Table IV.
Significant activations associated with ‘voluntariness’ and ‘response’ in a factorial analysis
| Cerebral region | BA | Side | Avg t‐score | Max t‐score | Size | Center of mass | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Voluntariness | ||||||||
| Frontal lobe | ||||||||
| Superior/medial frontal gyrus | 8/6 | R | 6.003 | 11.916 | 11808 | 1 | 22 | 49 |
| Superior/middle frontal gyrus | 6 | R | 5.055 | 6.500 | 467 | 23 | 9 | 64 |
| 10 | L | 4.607 | 5.383 | 82 | −24 | 50 | 0 | |
| Middle frontal gyrus | 8/6/9 | R | 5.720 | 13.028 | 21622 | 45 | 20 | 41 |
| 10/46 | R | 4.945 | 6.747 | 1577 | 45 | 54 | 4 | |
| 10/46 | L | 4.490 | 4.880 | 109 | −41 | 49 | 14 | |
| Middle/inferior frontal gyrus/insula | 9/8/47 | L | 5.187 | 8.041 | 16619 | −42 | 21 | 28 |
| Inferior frontal gyrus | 45 | R | 5.039 | 6.576 | 539 | 49 | 29 | 3 |
| Parietal lobe | ||||||||
| Inferior parietal lobule | 7/40 | R | 5.151 | 8.857 | 15829 | 35 | −57 | 44 |
| 40 | L | 5.225 | 8.319 | 8510 | −39 | −54 | 43 | |
| Temporal lobe | ||||||||
| Superior temporal gyrus | 22/41 | R | 4.807 | 5.846 | 65 | 51 | −29 | 5 |
| Middle temporal gyrus | 21 | L | 4.626 | 5.492 | 259 | −56 | −29 | −3 |
| Response | ||||||||
| Medial frontal gyrus | 6 | R | 4.464 | 4.733 | 119 | 1 | −6 | 60 |
| Precentral gyrus | 4 | L | 4.408 | 4.749 | 145 | −35 | −23 | 56 |
| R | 4.721 | 5.841 | 516 | 51 | 6 | 9 | ||
| Postcentral gyrus | R | 4.696 | 5.496 | 466 | 58 | −17 | 22 | |
| 43 | L | 4.777 | 6.361 | 319 | −61 | −14 | 16 | |
| Insula | 13 | L | 5.183 | 7.700 | 1495 | −43 | 2 | 11 |
| 13 | R | 4.420 | 4.712 | 23 | 40 | 9 | 14 | |
| Inferior parietal lobe | 40 | L | 4.986 | 6.793 | 4825 | −51 | −29 | 45 |
| 40 | R | 5.107 | 6.902 | 851 | 51 | −56 | 39 | |
| Caudate body | L | 4.709 | 6.381 | 474 | −17 | 8 | 13 | |
| Putamen | R | 4.454 | 4.806 | 21 | 17 | 9 | 7 | |
Figure 8.
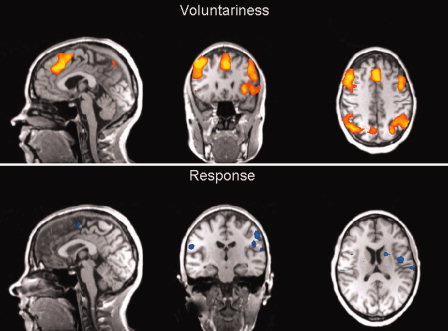
Brain responses associated with voluntary behaviour compared to forced behaviour (factorial analysis (“voluntariness” and “response”); random effects analysis thresholded at p(uncor) < .001; confidence range T: 4.3−8). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Correlations Between ROIs and Behavioral Data
Subjects who responded fast during the go condition showed slightly enhanced BOLD responses in the IFG (correlation coefficient [CC] = −0.541*, P < 0.046). Fast responses during the voluntary selection task were also associated with increased hemodynamic responses in the IFG (CC = −0.559*, P = 0.038). Furthermore, the association between response time and BOLD responses in the medial PFC reached significance (CC = −0.524, P = 0.054) during selection+.
Correlations Between ROIs and Electrophysiological Responses
The N2 amplitudes at Fz during selection+ correlated with the average T value of the cingulate gyrus (CC = −0.745**, P = 0.002): the N2 amplitude was more pronounced in subjects with increased responses of the cingulate gyrus. The association of N2 at Fz to the MFG (CC = −0.523, P = 0.055) and the N2 at FCz to the medial PFC (CC = −0.448, P = 0.109) reached trend level. In addition, the P3 amplitude in Fz was correlated to increased responses of the MFG.
We did not find any significant association between ERPs and ROI information in the selection−, go and nogo task.
DISCUSSION
The present study aimed at investigating ‘willed’ actions and neuronal responses occurring when tasks are presented requiring voluntary responses. Therefore, a go/nogo/voluntary selection paradigm was used. In the voluntary selection condition subjects decided freely whether or not to respond with a button press after stimulus presentation [selection+, selection−]. The neural correlates related to voluntary responses were associated with those during the motor responses during go and the behavioral inhibition during the nogo condition to distinguish voluntary and forced aspects of behavior. Functional MRI data and event‐related potentials [ERPs] were acquired simultaneously in order to reliably investigate temporal and spatial characteristics of the responses.
The functional MRI data revealed increased responses in the voluntary selection task compared to the control task [passive listening] in lateral and medial frontal regions, e.g. the pre‐SMA/ACC (BA 6/8), and the DLPFC (BA 6/8/9/46). These results are consistent with those of neuroimaging studies on the voluntary selection between two or more motor response alternatives [Deiber et al.,1991; Frith et al.,1991; Hyder et al.,1997; Jueptner et al.,1997]. Apart from frontal regions, enhanced responses were found in areas which are essential for guidance of limb movement (e.g. inferior parietal lobule (BA 40)), primary somatosensory cortical areas (postcentral gyrus (BA 3)), precuneus (BA 7) as well as subcortical/limbic regions (thalamus, caudate, putamen, insular cortex (BA 13)). Parietal activations have already been demonstrated to be of importance for voluntary selection tasks [Forstmann et al.,2006]. In addition, patients with parietal lesions demonstrated difficulties regarding conscious monitoring of voluntary actions [Sirigu et al.,2004]. Thalamic responses, too, have been linked with multiple functions, especially sensory and motor systems. Altogether, the brain regions affected are assumed to be of importance for intentional responses and the generation of movements, respectively.
The electrophysiological data revealed enhanced P3 amplitudes during the voluntary selection condition compared to the control task. Positive deflections in the interval of 300–600 msec after stimulus presentation [P3/P300], especially in parietal areas, have often been associated with cognitive functioning in decision making, attentional processes, information processing and context updating, respectively [Donchin and Coles,1988; Kramer and Strayer,1988; Polich and Kok,1995]. More frontal responses in the respective interval, for instance, were related to response inhibition, the so‐called nogo‐P3 [Bekker et al.,2004; Bruin et al.,2001; Kamarajan et al.,2005; Kopp et al.,1996; Pfefferbaum et al.,1985]. In the present study, response inhibition assumedly influenced at least some of the voluntary selection trials, e.g. when the subjects decided not to press the response button. Concerning selection+ trials, P3 amplitudes might have been influenced by movement‐related potentials. Contrary to our results, former studies reported an enhanced readiness potential influenced by movement selection [Dirnberger et al.,1998]. The reason for these somewhat contradictory results could be that tasks during the preparation interval did not differ. A differentiation between tasks was possible as soon as the second tone was presented.
Regarding event‐related potentials, P3 amplitudes were largest in the nogo condition, especially in frontocentral sites (FCz, Cz). The P3 amplitudes of the voluntary selection and go task were significantly decreased compared to the nogo condition but increased in relation to the control task. Accordant variations are assumed to be influenced by attention [Donchin and Coles,1988; Polich and Kok,1995]. Inhibitory processes (nogo, selection−) as well as movement‐related changes (go, selection+) seem to contribute to this process. Furthermore, electrophysiological data were influenced by behavioral performance: frontocentral and parietal P3 amplitudes, related to the go condition and the voluntary selection task, were higher in subjects who responded fast compared to those with slow responses.
Task‐related electrophysiological variations were also demonstrated concerning the N2 amplitude: the N2 response was enhanced in voluntary selection as well as the nogo task compared to the go task. The N2 has been associated with the top‐down inhibition processes in order to suppress the incorrect tendency to respond [Falkenstein et al.,1999; Kim et al.,2007]. More recently, it has also been related to response conflict caused by the necessity to respond to low‐frequency stimuli, regardless of the kind of response requested [Bartholow et al.,2005; Donkers and van Boxtel,2004; Nieuwenhuis et al.,2003]. Some core assumptions of this hypothesis are that nogo ‘responses’ compete with overt responses [Ruchsow et al.,2008]. In the current study nogo tasks and selection tasks were presented equally often, whereas the go condition was presented twice as often. Response conflict induced by the frequency of task presentation might have influenced the N2 amplitude.
The N1 component was not influenced by the task condition. These results match reports that the N1 seems to be influenced mainly by early sensory processes. Earlier sensory processes seemed to be unrelated to task instruction.
In order to further distinguish voluntary aspects of behavior from those elicited by behavior per se, ‘willed’ actions were compared to forced responses. The results revealed that subjects responded significantly slower in the voluntary selection task than in the go task. These results match those of another study revealing costs associated with a voluntary task switch, where subjects had to actively control the choice of task to be performed [Arrington and Logan,2004].
The comparison of hemodynamic responses during selection+ and those during the forced responses in the go condition revealed selection‐related BOLD responses in frontal areas (e.g. superior frontal gyrus, medial frontal gyrus, middle frontal gyrus (BA 6/8/9)), in the inferior and superior parietal lobe (BA 19/40), the precuneus, and the supramarginal gyrus. Parietal differences were more pronounced in the left hemisphere than the right hemisphere. These brain regions were related to ‘willed’ action, conflict, and decision‐making on the one hand [Botvinick et al.,2001; Deiber et al.,1991; Forstmann et al.,2006; Garavan et al.,2003; Goldberg et al.,2006; Hyder et al.,1997; Jahanshahi et al.,1995; Nachev et al.,2005; Playford et al.,1992; Spence et al.,1998], and motor responses on the other hand. Increased left lateral responses of the premotor cortex/frontal eye field for example, have also been demonstrated in tasks with voluntary saccades and initiation of eye movements, respectively [Berman et al.,1999; Milea et al.,2007]. Go‐related increases in neural activity were demonstrated in brain regions associated with the default mode of the brain (cuneus). The enhanced activation of the right precentral gyrus may indicate that the contralateral motor system is more involved during go tasks than selection tasks. Overall, go‐associated compared to selection‐related increases in BOLD responses were very small. The main differences between tasks indicated increased choice‐related BOLD responses, particularly in frontal and parietal brain regions.
Similar networks of brain regions were involved when BOLD responses selection− were compared to nogo‐responses. However, increases in frontal activations were less pronounced during the voluntary and forced inhibition of responses when compared to voluntary and forced motor responses.
The comparison of ERPs and behavioral responses, too, indicated partly overlapping neural responses during go and selection+: both forced and voluntary responses were related to the inferior frontal gyrus. Beyond, the speed of voluntary responses also tended to be associated with medial frontal BOLD responses.
Overall, a network of brain regions was involved when ‘willed’ actions were compared to forced responses. The neural responses differed comparatively little between voluntary movements and the free selection not to respond. These results were supported by the results of the factorial analysis, which did not show any significant interaction effect between the factors voluntariness and response. This may indicate that a behavioral response and the inhibition of a response are hierarchically equivalent. The importance of medial frontal and right parietal areas for decision related processes were strengthened with a factorial analysis: pronounced medial and lateral frontal as well as parietal BOLD responses were demonstrated to be related to voluntariness whereas the type of response was predominantly related to the left inferior parietal lobe.
The comparison of ERPs and BOLD responses indicated that during selection+ medial frontal hemodynamic responses were especially related to the N2 amplitude whereas associations to the frontal P3 were less pronounced. These results are in favor of the specific association of medial frontal regions and the N2 during selection processes. An association between N2 and medial frontal areas has been demonstrated in a study using scalp topographies and LORETA [Tian et al.,2008]. However, there were no significant associations between medial frontal responses and N2/P3 during the selection− condition. Reason for these results could be a higher inter‐subject variability in brain responses related to the voluntary inhibition of behavioral responses.
The event‐related N1, N2 and P3 amplitudes of selection+ did not differ significantly from those evoked during the selection− task. Hence, the neural responses seemed to be relatively independent from the kind of decision that was made but rather associated with the decision process per se. To some degree, these results match the functional MRI results of free decision making. Frontal BOLD responses which have been frequently associated with voluntary movements and related WM processes [Deiber et al.,1991; Frith et al.,1991; Hadland et al.,2001; Hyder et al.,1997; Jueptner et al.,1997; Lau et al.,2004a] seemed to be relatively independent of the kind of decision made. Though, the selection+ condition additionally led to increased responses in areas which are essential for finger movements (e.g. inferior parietal lobule; primary somatosensory cortical areas). Motor response‐related differences were more pronounced in the left hemisphere compared to the right hemisphere since all subjects were asked to respond with their right index finger.
Overall, an enhanced working memory load in the voluntary selection task compared to the go and nogo condition could also be held responsible for the existing neural alterations. However, studies looking at neuronal responses associated with working memory capacities revealed that a whole network of brain regions is involved, mainly concerning the dorsolateral prefrontal cortex as well as temporo‐parietal brain regions. Medial frontal brain regions also have been demonstrated to contribute to these processes. However, alterations have been found mainly in the anterior cingulate cortex. The superior and medial frontal gyrus (BA 8/9), which were predominantly affected in the actual study, did not seem to contribute significantly to these processes. Considering these results, we assume that the brain responses we found were not primarily the expression of an enhanced working memory load during the voluntary selection task but are associated with the selection process itself.
CONCLUSION
Altogether, the voluntary decision task elicited enhanced responses, especially in the medial frontal regions, compared to forced responses. Apart from medial frontal regions, voluntariness‐related BOLD responses in lateral frontal and parietal areas were demonstrated. Taking into account the prolonged responses during these tasks, these results suggest that there might be additional cognitive processes driven by the absence of constraints related to ‘willed’ actions [Lau et al.,2006]. These processes were comparatively independent of the kind of response chosen. Significant differences between the response alternatives (motor response, inhibition) were only demonstrated in brain regions which are directly related to motor responses, e.g. somatosensory cortical areas. The electrophysiological results partially supported these results. Inconsistencies between ERPs and BOLD responses may indicate that comparable electrophysiological responses might be related to variations in differing brain structures in different tasks.
Acknowledgements
Parts of this work were prepared in the context of the MD thesis of Tobias Thalmeier at the Faculty of Medicine, Ludwig‐Maximilians‐University, Munich. We thank Mije Hartmann who assisted with the proof‐reading of the manuscript.
All authors declare that they have no conflict of interest. There were no study sponsors, and no funding was supplied.
REFERENCES
- Arrington CM,Logan GD( 2004): The cost of a voluntary task switch. Psychol Sci 15: 610–615. [DOI] [PubMed] [Google Scholar]
- Barch DM,Braver TS,Nystrom LE,Forman SD,Noll DC,Cohen JD ( 1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Bartholow BD,Pearson MA,Dickter CL,Sher KJ,Fabiani M,Gratton G( 2005): Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology 42: 33–42. [DOI] [PubMed] [Google Scholar]
- Bekker EM,Kenemans JL,Verbaten MN ( 2004): Electrophysiological correlates of attention, inhibition, sensitivity and bias in a continuous performance task. Clin Neurophysiol 115: 2001–2013. [DOI] [PubMed] [Google Scholar]
- Berman RA,Colby CL,Genovese CR,Voyvodic JT,Luna B,Thulborn KR,Sweeney JA ( 1999): Cortical networks subserving pursuit and saccadic eye movements in humans: An FMRI study. Hum Brain Mapp 8: 209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM,Braver TS,Barch DM,Carter CS,Cohen JD ( 2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Brass M,Derrfuss J,Forstmann B,von Cramon DY ( 2005): The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9: 314–316. [DOI] [PubMed] [Google Scholar]
- Bruin KJ,Wijers AA,van Staveren AS ( 2001): Response priming in a go/nogo task: Do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clin Neurophysiol 112: 1660–1671. [DOI] [PubMed] [Google Scholar]
- Cabeza R,Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cunnington R,Windischberger C,Moser E ( 2005): Premovement activity of the pre‐supplementary motor area and the readiness for action: Studies of time‐resolved event‐related functional MRI. Hum Mov Sci 24: 644–656. [DOI] [PubMed] [Google Scholar]
- Debener S,Ullsperger M,Siegel M,Fiehler K,von Cramon DY,Engel AK ( 2005): Trial‐by‐trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25: 11730–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S,Ullsperger M,Siegel M,Engel AK ( 2006): Single‐trial EEG‐fMRI reveals the dynamics of cognitive function. Trends Cogn Sci 10: 558–563. [DOI] [PubMed] [Google Scholar]
- Deiber MP,Passingham RE,Colebatch JG,Friston KJ,Nixon PD,Frackowiak RS ( 1991): Cortical areas and the selection of movement: A study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Dirnberger G,Fickel U,Lindinger G,Lang W,Jahanshahi M ( 1998): The mode of movement selection. Movement‐related cortical potentials prior to freely selected and repetitive movements. Exp Brain Res 120: 263–272. [DOI] [PubMed] [Google Scholar]
- Donchin E,Coles MGH ( 1988): Is the P300 component a manifestation of context updating? Behav Brain Sci 11: 355–372. [Google Scholar]
- Donkers FC,van Boxtel GJ ( 2004): The N2 in go/no‐go tasks reflects conflict monitoring not response inhibition. Brain Cogn 56: 165–176. [DOI] [PubMed] [Google Scholar]
- Duncan J,Owen AM ( 2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483. [DOI] [PubMed] [Google Scholar]
- Eichele T,Specht K,Moosmann M,Jongsma ML,Quiroga RQ,Nordby H,Hugdahl K ( 2005): Assessing the spatiotemporal evolution of neuronal activation with single‐trial event‐related potentials and functional MRI. Proc Natl Acad Sci USA 102: 17798–17803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M,Hoormann J,Hohnsbein J ( 1999): ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 101: 267–291. [DOI] [PubMed] [Google Scholar]
- Forstmann BU,Brass M,Koch I,von Cramon DY ( 2006): Voluntary selection of task sets revealed by functional magnetic resonance imaging. J Cogn Neurosci 18: 388–398. [DOI] [PubMed] [Google Scholar]
- Frith CD,Friston K,Liddle PF,Frackowiak RS ( 1991): Willed action and the prefrontal cortex in man: A study with PET. Proc Biol Sci 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Garavan H,Ross TJ,Kaufman J,Stein EA ( 2003): A midline dissociation between error‐processing and response‐conflict monitoring. Neuroimage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Goldberg II,Harel M,Malach R ( 2006): When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 50: 329–339. [DOI] [PubMed] [Google Scholar]
- Hadland KA,Rushworth MF,Passingham RE,Jahanshahi M,Rothwell JC ( 2001): Interference with performance of a response selection task that has no working memory component: An rTMS comparison of the dorsolateral prefrontal and medial frontal cortex. J Cogn Neurosci 13: 1097–1108. [DOI] [PubMed] [Google Scholar]
- Hunter MD,Farrow TF,Papadakis NG,Wilkinson ID,Woodruff PW,Spence SA ( 2003): Approaching an ecologically valid functional anatomy of spontaneous “willed” action. Neuroimage 20: 1264–1269. [DOI] [PubMed] [Google Scholar]
- Hyder F,Phelps EA,Wiggins CJ,Labar KS,Blamire AM,Shulman RG ( 1997): “Willed action”: A functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc Natl Acad Sci USA 94: 6989–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille N,Berg P,Scherg M ( 2002): Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol 19: 113–124. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M,Jenkins IH,Brown RG,Marsden CD,Passingham RE,Brooks DJ ( 1995): Self‐initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement‐related potentials in normal and Parkinson's disease subjects. Brain 118(Pt 4): 913–933. [DOI] [PubMed] [Google Scholar]
- Jenkins IH,Jahanshahi M,Jueptner M,Passingham RE,Brooks DJ ( 2000): Self‐initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123(Pt 6): 1216–1228. [DOI] [PubMed] [Google Scholar]
- Jueptner M,Stephan KM,Frith CD,Brooks DJ,Frackowiak RS,Passingham RE ( 1997): Anatomy of motor learning. I. Frontal cortex and attention to action J Neurophysiol 77: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Jurado MB,Rosselli M ( 2007): The elusive nature of executive functions: A review of our current understanding. Neuropsychol Rev 17: 213–233. [DOI] [PubMed] [Google Scholar]
- Kamarajan C,Porjesz B,Jones KA,Choi K,Chorlian DB,Padmanabhapillai A,Rangaswamy M,Stimus AT,Begleiter H ( 2005): Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a Go/No‐Go task. Biol Psychol 69: 353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S,Jager L,Karamatskos E,Graz C,Stammel A,Flatz W,Lutz J,Holtschmidt‐Taschner B,Genius J,Leicht G,Pogarell O,Born C,Moller HJ,Hegerl U,Reiser M,Soyka M,Mulert C ( 2008): Influence of trait anxiety on inhibitory control in alcohol‐dependent patients: Simultaneous acquisition of ERPs and BOLD responses. J Psychiatr Res 42: 734–745. [DOI] [PubMed] [Google Scholar]
- Kim MS,Kim YY,Yoo SY,Kwon JS ( 2007): Electrophysiological correlates of behavioral response inhibition in patients with obsessive‐compulsive disorder. Depress Anxiety 24: 22–31. [DOI] [PubMed] [Google Scholar]
- Kopp B,Mattler U,Goertz R,Rist F ( 1996): N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming Electroencephalogr Clin Neurophysiol 99: 19–27. [DOI] [PubMed] [Google Scholar]
- Kramer AF,Strayer DL ( 1988): Assessing the development of automatic processing: An application of dual‐task and event‐related brain potential methodologies. Biol Psychol 26: 231–267. [DOI] [PubMed] [Google Scholar]
- Laplane D,Talairach J,Meininger V,Bancaud J,Orgogozo JM ( 1977): Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci 34: 301–314. [DOI] [PubMed] [Google Scholar]
- Lau HC,Rogers RD,Haggard P,Passingham RE ( 2004a): Attention to intention. Science 303: 1208–1210. [DOI] [PubMed] [Google Scholar]
- Lau HC,Rogers RD,Ramnani N,Passingham RE ( 2004b): Willed action and attention to the selection of action. Neuroimage 21: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Lau HC,Rogers RD,Passingham RE ( 2006): Dissociating response selection and conflict in the medial frontal surface. Neuroimage 29: 446–451. [DOI] [PubMed] [Google Scholar]
- Lezak MD ( 1983): Neuropsychological assessment, 2nd edition New York: Oxford University Press. [Google Scholar]
- Logan GD,Gordon RD ( 2001): Executive control of visual attention in dual‐task situations. Psychol Rev 108: 393–434. [DOI] [PubMed] [Google Scholar]
- Manoach DS,Schlaug G,Siewert B,Darby DG,Bly BM,Benfield A,Edelman RR,Warach S ( 1997): Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport 8: 545–549. [DOI] [PubMed] [Google Scholar]
- Milea D,Lobel E,Lehericy S,Leboucher P,Pochon JB,Pierrot‐Deseilligny C,Berthoz A ( 2007): Prefrontal cortex is involved in internal decision of forthcoming saccades. Neuroreport 18: 1221–1224. [DOI] [PubMed] [Google Scholar]
- Mulert C,Jager L,Schmitt R,Bussfeld P,Pogarell O,Moller HJ,Juckel G,Hegerl U ( 2004): Integration of fMRI and simultaneous EEG: Towards a comprehensive understanding of localization and time‐course of brain activity in target detection. Neuroimage 22: 83–94. [DOI] [PubMed] [Google Scholar]
- Mulert C,Jager L,Propp S,Karch S,Stormann S,Pogarell O,Moller HJ,Juckel G,Hegerl U ( 2005a): Sound level dependence of the primary auditory cortex: Simultaneous measurement with 61‐channel EEG and fMRI. Neuroimage 28: 49–58. [DOI] [PubMed] [Google Scholar]
- Mulert C,Menzinger E,Leicht G,Pogarell O,Hegerl U ( 2005b): Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56: 65–80. [DOI] [PubMed] [Google Scholar]
- Nachev P,Rees G,Parton A,Kennard C,Husain M ( 2005): Volition and conflict in human medial frontal cortex. Curr Biol 15: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S,Yeung N,van den Wildenberg W,Ridderinkhof KR ( 2003): Electrophysiological correlates of anterior cingulate function in a go/no‐go task: Effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci 3: 17–26. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Passingham RE ( 1993): The frontal lobes and voluntary action. Oxford: Oxford University Press. [Google Scholar]
- Pfefferbaum A,Ford JM,Weller BJ,Kopell BS ( 1985): ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol 60: 423–434. [DOI] [PubMed] [Google Scholar]
- Playford ED,Jenkins IH,Passingham RE,Nutt J,Frackowiak RS,Brooks DJ ( 1992): Impaired mesial frontal and putamen activation in Parkinson's disease: A positron emission tomography study. Ann Neurol 32: 151–161. [DOI] [PubMed] [Google Scholar]
- Polich J,Kok A ( 1995): Cognitive and biological determinants of P300: An integrative review. Biol Psychol 41: 103–146. [DOI] [PubMed] [Google Scholar]
- Ranganath C,D'Esposito M ( 2001): Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31: 865–873. [DOI] [PubMed] [Google Scholar]
- Ruchsow M,Groen G,Kiefer M,Buchheim A,Walter H,Martius P,Reiter M,Hermle L,Spitzer M,Ebert D,Falkenstein M ( 2008): Response inhibition in borderline personality disorder: Event‐related potentials in a Go/Nogo task. J Neural Transm 115: 127–133. [DOI] [PubMed] [Google Scholar]
- Shima K,Tanji J ( 1998): Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282: 1335–1338. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M,Boor R,Jacobs J,Wolff S,Jansen O,Stephani U,Scherg M ( 2006): Correction of ballistocardiogram artefacts from EEG acquired in the MRI scanner using spatial filters based on artefact and brain signal topographies. Neuroimage 31: S86. [Google Scholar]
- Sirigu A,Daprati E,Ciancia S,Giraux P,Nighoghossian N,Posada A,Haggard P ( 2004): Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci 7: 80–84. [DOI] [PubMed] [Google Scholar]
- Spence SA,Hirsch SR,Brooks DJ,Grasby PM ( 1998): Prefrontal cortex activity in people with schizophrenia and control subjects. Evidence from positron emission tomography for remission of ‘hypofrontality’ with recovery from acute schizophrenia. Br J Psychiatry 172: 316–323. [DOI] [PubMed] [Google Scholar]
- Tian Y,Yao D ( 2008): A study on the neural mechanism of inhibition of return by the event‐related potential in the Go/Nogo task. Biol Psychol 79: 171–178. [DOI] [PubMed] [Google Scholar]
- Ullsperger M,von Cramon DY ( 2001): Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- Walton ME,Devlin JT,Rushworth MF ( 2004): Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci 7: 1259–1265. [DOI] [PubMed] [Google Scholar]
- Ylvisaker M,DeBonis D ( 2000): Executive function impairment in adolescence: TBI and ADHD. Top Lang Disord 20: 29–57. [Google Scholar]


